Abstract
Small, 1st and 2nd-order, headwater streams and ponds play essential roles in providing natural flood control, trapping sediments and contaminants, retaining nutrients, and maintaining biological diversity, which extend into downstream reaches, lakes and estuaries. However, the large geographic extent and high connectivity of these small water bodies with the surrounding terrestrial ecosystem makes them particularly vulnerable to growing land-use pressures and environmental change. The greatest pressure on the physical processes in these waters has been their extension and modification for agricultural and forestry drainage, resulting in highly modified discharge and temperature regimes that have implications for flood and drought control further downstream. The extensive length of the small stream network exposes rivers to a wide range of inputs, including nutrients, pesticides, heavy metals, sediment and emerging contaminants. Small water bodies have also been affected by invasions of non-native species, which along with the physical and chemical pressures, have affected most groups of organisms with consequent implications for the wider biodiversity within the catchment. Reducing the impacts and restoring the natural ecosystem function of these water bodies requires a three-tiered approach based on: restoration of channel hydromorphological dynamics; restoration and management of the riparian zone; and management of activities in the wider catchment that have both point-source and diffuse impacts. Such activities are expensive and so emphasis must be placed on integrated programmes that provide multiple benefits. Practical options need to be promoted through legislative regulation, financial incentives, markets for resource services and voluntary codes and actions.
Keywords: Streams, Ponds, Headwaters, Anthropogenic pressures, Remediation, Ecosystem services
Graphical abstract
Highlights
-
•
Small Water Bodies (SWB) provide a suite of vital ecosystem services.
-
•
Hydromorphology of SWBs makes them highly vulnerable to anthropogenic pressures.
-
•
Land-use and environmental changes are disrupting the ecosystem functions of SWBs.
-
•
3-tier restoration is needed: channel, riparian and wider catchment management.
-
•
Success will require government prioritization, expert advice, and stakeholder buy-in.
1. Introduction
Pressures on freshwater ecosystems in Great Britain and Ireland (GB&I) have inevitably increased with human population growth and resource demands. While impacts were focused historically around larger population centres, particularly on estuaries and the lower reaches of rivers, concern is now growing for the condition of upstream tributaries as more intensive land-uses such as agriculture and silviculture have expanded (Chesterton, 2009). Risks from emerging stressors such as invasive non-native species and climate change are also growing (Di Matteo et al., 2017; Rahel and Olden, 2008). The legacy of centuries of waterway degradation from navigation, power generation, water supply, industrial pollution and waste disposal are now joined by pressures from the intensive use of catchment land for agriculture, commercial forestry, industry, housing and transport (Mainstone et al., 2016; Raven et al., 1998). Ponds are also important foci for biodiversity, supporting more (and rare) species than other freshwater ecosystems (Céréghino et al., 2008), but pond numbers in the UK decreased by about 75% between the 19th century and 1980s. The creation of golf courses, nature reserves, gardens and allotments is now reversing this trend (Jeffries, 2012), but pond condition has continued to deteriorate (Carey et al., 2008).
The present review reappraises the importance of small, natural and man-made standing and running freshwater habitats in GB&I including small streams, ditches and ponds, which we collectively refer to as small water bodies (SWBs). Although SWBs have been under-represented by freshwater science, there is growing evidence of their significance in the structure and function of freshwater ecosystems (Biggs et al., 2017; MacDonald and Coe, 2007), their influence on conditions in downstream river reaches (Alexander et al., 2007; Dodds and Oakes, 2008) and their contribution to biodiversity (Clarke et al., 2008; Finn et al., 2011). We use case studies in GB&I to demonstrate the importance of and threats to SWBs. This information will be of relevance outside of these islands, although related problems in more mountainous areas may be notably different.
Several recent reviews have considered the effects of the degradation of SWBs in GB&I on salmonid fish (IBIS and AST, 2012) and freshwater fish stocks in general (IFM, 2013), or have focused on research needs (Biggs et al., 2017). Here, we take a holistic perspective, embracing most groups of organisms that inhabit SWBs and the interactions between their physico-chemical and biological character. We review the ecology and condition of SWBs to assess how they have become impaired and provide clear evidence of adverse impacts from land management practices that occur frequently with little cognisance of downstream consequences and external costs. We consider how the degradation of SWBs is affecting the natural hydrological and chemical processes that operate in catchments, threatening the species they support (Hayhow et al., 2016) and disrupting natural ecosystem function, and we propose a systematic approach for remediation and associated policy action.
While there is extensive legislation to support the societal benefits of protecting freshwater ecosystems, the large number and widespread nature of SWBs makes them difficult to monitor and manage, and they are often omitted from such programmes (Baattrup-Pedersen et al., 2018). Despite their ecological importance, we postulate that SWBs are under-represented in freshwater monitoring and research to an extent that could jeopardise management and restoration programmes.
Growing the rural economy (The Rural Coalition, 2010), sustaining food security and generating energy from renewable sources (DECC, 2014) are all important national policy commitments that may increase the impacts on natural ecosystems within SWBs. Unless properly managed, these objectives will conflict with the need to manage water in a way that reduces flood risk and water stress, and that delivers wider environmental benefits and wildlife protection (Anon, 2016; Royal Geographical Society and IBG, 2012). We therefore suggest that SWB are an urgent priority for management, restoration and policy development with more extensive application than hitherto.
2. Definition of SWBs
SWBs encompass a range of small standing and running freshwater ecosystems. The term ‘small’ stream is often used interchangeably with ‘headwater’, with no consensus on a definition of either. Although not all small streams are headwaters (Moore and Richardson, 2003; Ovenden and Gregory, 1980), most lie within headwater reaches. Furse (2000) defined headwaters as streams within 2.5 km from the source, and most identify headwaters as zero, first or second-order water courses (e.g. Barmuta et al., 2009; Callanan et al., 2008; Clarke et al., 2008; Finn et al., 2011; Meyer et al., 2007a), and this is the approach adopted here. In England and Wales, the total length of first and second order streams is 126,338 km, or 73.4% of the total running water network. A similar figure of 77% has been estimated for Ireland (McGarrigle, 2014). Headwater streams are short (mean length of 1st order = 833 m, and of 2nd order = 723 m), narrow (<3 m), have a large bank length/channel area ratio, and a low width/depth ratio.
SWBs also include ditches, both man-made or modified natural drainage channels (Williams et al., 2004), and may be either seasonal (Kavanagh and Harrison, 2014) or permanently wet and like headwater streams, ditches have a high bank/bed area ratio, and low width/depth ratio, being narrow and deep. Brown et al. (2006) estimated that there are 600,000 km of ditches in UK.
Ponds are small standing waters varying in size from 1 m2 to between 2 and 5 ha and may be permanent or seasonal, man-made or natural (Brown et al., 2006; Céréghino et al., 2008; Collinson et al., 1995; Pond Conservation Group, 1993). In Great Britain, there are around 0.5 million ponds, excluding those in gardens (Williams et al., 2010).
3. Physical and chemical processes in SWBs
3.1. Hydromorphology
Headwater streams are recruitment areas for sediments and organic matter, and connect the downstream river network to sediment sources on the catchment surface. Inputs of water, sediment and large woody debris interact with the river bed, bank, and riparian vegetation to form the channel (Sear et al., 2010). The valley form determines the degree of coupling between the channel system and the valley slopes (Harvey, 2002), and the space available for development of braiding and meandering.
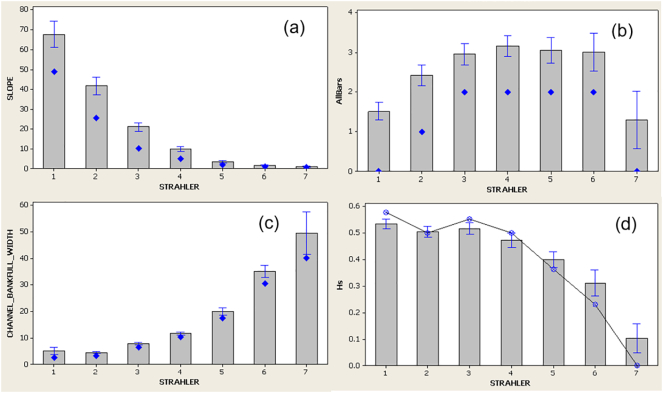
In steep channels, sediment supply is low and episodic, and mainly generated by slope processes such as land-sliding. The contribution of slope-derived materials results in smaller watercourses having the highest substratum diversity in the river network (Fig. 1). As slope decreases and sediment supply increases, the channel morphology transitions into step-pool and plane bed channel types. Wood debris dams modify channel morphology, these are more common in headwaters because of the strong coupling between stream and valley and the narrow channel width relative to log length which results in low rates of transfer (Fig. 1).
Fig. 1.
Mean and 95% Cl for 483 semi-natural River Habitat Survey (RHS) sites across the UK. Small steams (Strahler (1957) stream order 1 and 2), are characterised by, a) the steepest slopes (%), b) relatively few sediment storage features (bars) per unit length of channel (no/500 m), c) smallest bankfull channel widths (metres) and, d) highest substratum diversity within the river network (Shannon diversity index). Original Data from the Environment Agency, RHS database.
Less is known about lowland small stream systems, although in principle the same processes will apply. Given lower slopes and reduced incidence of land-sliding, the morphological responses are likely to be dominated by finer sediment accumulation and transport.
3.2. Hydrological and thermal variability
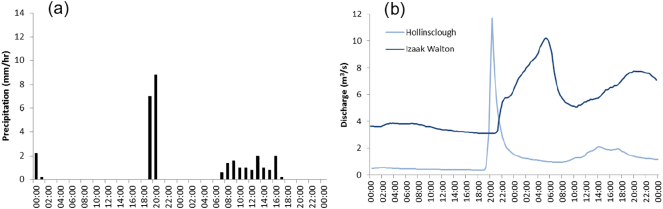
Variation in headwater discharge and pond water levels are governed by the balance between precipitation, evaporation, changes in soil moisture and groundwater storage. Upland headwaters have higher annual precipitation, greater accumulation and duration of snow cover, smaller absolute discharge, but greater and more rapid variations in peak discharge per unit area than sites downstream (Fig. 2). Headwater streams, therefore, have marked discharge intermittence compared with middle and lower reaches, and hyporheic zones often act as refuges for fauna during low discharge and/or high temperature episodes (Wood et al., 2010).
Fig. 2.
Hourly precipitation (a), and discharge (b) in the River Dove, UK at Hollinsclough (8 km2 headwater catchment) and Izaak Walton (≈30 km from source, draining 83 km2 catchment) on 5–6 July 2012, illustrates rapid hydrological change in a headwater stream following an intense rain storm relative to the delayed and protracted response lower in the catchment.
Water temperature is a key hydro-chemical variable that regulates the metabolism of ectothermic organisms. Headwater stream temperatures vary over daily and annual cycles due to seasonal patterns of solar forcing and river discharge. Thermal budgets of SWBs are dominated by catchment controls of water temperature such as stream aspect, topography and geology, which govern solar energy receipt, precipitation and thermal regime of surface and spring discharge (Evans et al., 1998; Johnson and Wilby, 2015; Johnson et al., 2014). Sub-daily variations in SWB water temperature may be caused by cloud cover, pulses of snow and ice melt or intense summer precipitation (Wilby et al., 2015). Over annual- to decadal time-scales, climate cycles such as the North Atlantic Oscillation (NAO), influence runoff and heat fluxes (Basarin et al., 2016) to the extent that winter temperatures in UK headwaters can vary by 3–6 °C depending on NAO phases (Bradley and Ormerod, 2001; Elliott et al., 2000).
3.3. Chemical processes
Headwater streams provide biogeochemical connectivity between terrestrial and aquatic ecosystems, controlling the supply and transport of nutrients (nitrogen, N, and phosphorus, P) to downstream reaches (Alexander et al., 2007; Nadeau and Rains, 2007). As such, they act as ‘hotspots’ of nutrient and organic matter processing (Withers and Jarvie, 2008), contributing cumulatively to water quality and the functional integrity of the downstream aquatic ecosystem (Alexander et al., 2007; Armstrong et al., 2012; Nadeau and Rains, 2007).
In SWBs, the large benthic area relative to water volume and high connectivity with riparian and hyporheic zones promotes nutrient uptake and exchange at reactive surfaces (Bernal et al., 2015; Lassaletta et al., 2010; Triska et al., 2007). Biogeochemical cycling of N and P occurs in periphyton and biofilms attached to surfaces (Battin et al., 2016; Jarvie et al., 2002; Ziegler and Lyon, 2010), in fine-grained sediments deposited on the stream bed (Ballantine et al., 2009; House and Denison, 1998; Jarvie et al., 2005) and within the hyporheic and riparian zones (Bernal et al., 2015; Lapworth et al., 2011; Triska et al., 1993a, Triska et al., 1993b; Williams et al., 2015). Processes controlling biogeochemical cycling vary according to the stream environment and the supply of autochthonous and allochthonous organic matter and C, N and P inputs (Benstead et al., 2009; Hoellein et al., 2007; Rodriguez-Cardona et al., 2016). Also, N and P supplies are varied in timing of delivery and composition, and this can have an important influence on how and where they are assimilated, and whether ecological impacts are localised in the headwater or propagated downstream (Withers and Jarvie, 2008).
In small streams, nutrient processing and spiralling (Mullholland et al., 1985; Newbold et al., 1983) are controlled by the interactions between water discharge and contact with the reactive surfaces (Nadeau and Rains, 2007; Ziegler and Lyon, 2010).The hydrology and geomorphology of SWBs influence nutrient cycling, by controlling residence times in ‘transient storage’ (i.e. pools, eddies, and the hyporheic zone) at the reactive surfaces enhancing biogeochemical nutrient cycling (Gonzalez-Pinzon et al., 2014). Nutrient retention and cycling in headwaters provides an important ecosystem service, by regulating downstream delivery of nutrients (Alexander et al., 2007) and reducing impacts of acute loadings to downstream ecosystems at times of eutrophication risk (Jarvie et al., 2013; Triska et al., 2007).
It is clear that ponds are biogeochemical hotspots in carbon cycles (e.g. Holgerson and Raymond, 2016). Although there is considerable heterogeneity in the biogeochemical cycling and nutrient balance of ponds, they have been categorized using water quality indicators such as pH, chlorophyll α, turbidity and sediment quality (Biggs et al., 2000).
4. Effects of physical, chemical and biological pressures on SWBs
4.1. Drainage and sediment
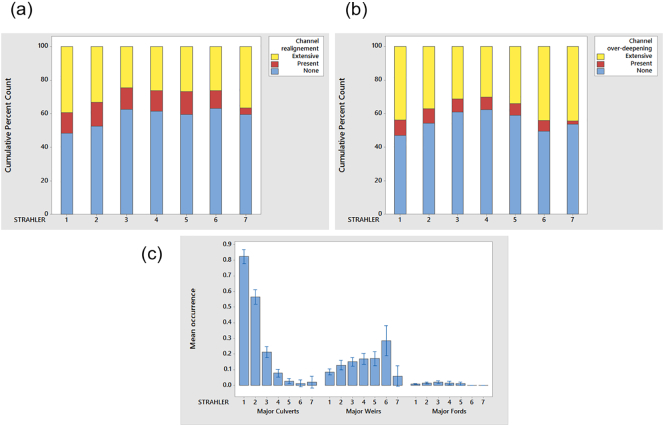
The natural physical processes in headwater stream networks have been modified through their extension to create drainage systems for agriculture and forestry (Ovenden and Gregory, 1980; Sear et al., 2000), culverting for urban development, and dredging to increase freeboard for drainage. These activities usually include removal of large woody debris, a key factor in the rate of run-off and in-channel sediment trapping, which has implications for downstream transport of sediment and organic matter, and vulnerability to changing discharge and temperature regimes. Analysis of over 22,000 river reaches demonstrates the legacy of modification on water courses in England and Wales (Fig. 3: Raven et al., 1998).
Fig. 3.
Channel modifications based on River Habitat Survey (RHS) data. Headwater streams (Strahler (1957) orders 1 and 2) have higher proportions of channel modifications: a) channel planform modifications through realignment; b) cross-section modification through dredging; c) major in-channel structures (bars are 95% CI for the mean).
Agriculture contributes 72–76% of the total fine-grained sediment delivered to all water courses across England and Wales (Collins and Anthony, 2008; Collins et al., 2009a, Collins et al., 2009b; Zhang et al., 2014). Livestock density and grazing pressures contribute to elevated erosion and sediment loss in headwater catchments (Evans et al., 1998; Harrod et al., 2000; McHugh, 2002) resulting in widespread soil compaction which reduces rainfall infiltration leading to accelerated runoff and particle entrainment. Cattle access to streams for drinking and loafing also removes protective vegetation cover, causes poaching and enhances river bank erosion (Belsky et al., 1999; Collins et al., 2010a, Collins et al., 2013). It is likely that ponds are also negatively affected by the increased loss of sediment from the land, along with other diffuse pollutants, and this may have contributed to declining pond quality in the period 1996–2007 (Williams et al., 2010).
Commercial forest management also elevates soil erosion at different phases of the forest rotation (Collins et al., 1997; Leeks and Roberts, 1987). Brash management, soil cultivation, the construction of drains and roads, and heavy machinery use, increases erosion of forest soils (Clarke et al., 2015; Forestry Commission, 1988, Forestry Commission, 1993; Marks, 1994). Fine-grained sediment mobilised by these activities can be transported directly to the channel system (Leeks, 1992), leading to suspended sediment concentrations above pre-afforestation levels (Francis and Taylor, 1989).
Although no measurements exist, we can surmise that removal of large wood and logjams by land-use change and stream management has reduced the sediment storage capacity of headwater river networks. A recent survey of 230 largely headwater streams for which agriculture was the dominant sediment pressure found that the quantity of both infiltrated fine sediments and that accumulated as a surface drape were predominantly related to stream power (Naden et al., 2016). Other studies in the UK have highlighted the importance of fine-grained sediment storage in lowland headwater catchments (e.g. Collins and Walling, 2007a, Collins and Walling, 2007b; Walling et al., 1998). At small scales, on-line ponds may also trap substantial sediment loads in farmed landscapes, but the catchment or landscape scale significance of these processes remains little explored (Ockenden et al., 2014).
4.2. Hydrology and water temperature pressures
SWBs are typically under-represented by sampling networks for discharge (Bradford and Marsh, 2003) and water temperature (Orr et al., 2015), although there are some important long-term records from established observatories, such as at Llyn Brianne (1981–present; Durance and Ormerod, 2007). No such sites are located on ponds.
In uplands, conversion of grassland to coniferous forests tends to increase canopy interception loss and evapotranspiration, and reduce soil moisture recharge and stream discharge (Marc and Robinson, 2007), while associated ditching and road networks may enhance peak discharges (Telzlaff et al., 2007). Water regulation for hydropower and water resources modifies the annual discharge cycle (Birkel et al., 2014). Upland headwater streams are vulnerable to modest changes in air temperature, which alter snowmelt and the winter/spring discharge regime. In lowland streams, water-level variations arise from irrigation, flood control, weirs, dredging and weed cutting (Old et al., 2014). Expansion of field drainage systems can dewater the landscape whilst reducing times to peak after heavy rainfall (Harrigan et al., 2014).
SWBs are also vulnerable to artificial influences on water temperature. For example, reservoir discharges tend to increase temperature in winter but depress it in summer, relative to ambient temperatures in downstream waters (Webb, 1995). Other thermal impacts on SWBs can arise from runoff from paved surfaces entering urban drainage networks (Herb et al., 2008), or by changing land cover in uplands. Hannah et al. (2008) reported that the maximum temperature in moorland streams may be 6 °C warmer than in forest streams.
Predicted rising air temperatures and altered precipitation patterns under climate change could further adversely affect the discharge and thermal regimes of fresh waters (Hannaford, 2015; Hannah and Garner, 2015; Watts et al., 2015). The vulnerability of SWBs to these pressures may be exacerbated by abstraction and discharge regulation, which affect at least 85% of gauged river discharge records in the UK (Marsh, 2010). The effects of surface and groundwater abstraction become most apparent during severe droughts when water is transferred out of the catchment or used for irrigation (Agnew et al., 2000).
Indirect thermal impacts may arise from the management of riparian areas and wetland drainage as heavy summer rainfall flushes in additional heat from warm near-surface soil moisture and pools (Langan et al., 2001). Clear-felling of forests has been reported to increase maximum temperature in forest drains by up to 13 °C (Moore et al., 2005). Even modest changes of temperature can aggregate into markedly different annual degree-days, with impacts on sensitive species like the mayfly Ephemera danica (Everall et al., 2015). Little is known of pond thermal regimes, but early studies indicate substantial daily and seasonal variation, therefore considerable heterogeneity between ponds seems likely.
4.3. Chemical pressures
4.3.1. Nutrients
Across Great Britain, nutrient pressures in headwater streams are generally lower relative to large river systems (Jarvie et al., 2018). Some 23% of headwater streams were P-impaired (P concentrations greater than ecologically-limiting thresholds), compared with 51% of the rivers monitored; and 52% of headwater streams were N-impaired, compared with 87% of the rivers monitored. Nutrient pressures were highest in the lowland-high-alkalinity headwater streams, where 41% were P-impaired and 78% were N-impaired, linked to higher intensity of agricultural land-use and population pressures. Nutrient pressures were generally lowest in the upland-low-alkalinity headwater streams, where 8% were P impaired and 15% were N-impaired.
Degradation of SWBs tends to reduce their capacity to retain and cycle nutrients in both particulate and dissolved phase, resulting in increased delivery of nutrients downstream (Alexander et al., 2007), which can be further exacerbated by climate drivers. Drought exacerbates low baseflow dilution capacity and can result in increased anoxia in streambed sediments, thus reducing their capacity to retain P, and increasing dissolved P remobilisation (Withers and Jarvie, 2008). And greater magnitude and frequency of high discharge events increases stream scouring and reduces nutrient processing and retention capacity (Alexander et al., 2007), mobilises ‘legacy’ nutrient stores within the catchment, and increases the loadings of P, N and C inputs from terrestrial stores (Sharpley et al., 2015). Increases in temperature extend the seasonal time-window for biological activity, promoting higher rates of primary production and microbial activity, and amplify soil wetting and drying cycles, resulting in greater rates of organic matter mineralization and greater nutrient loadings to streams (Whitehead et al., 2009). High nutrient loadings can saturate N and P uptake processes, further reducing the efficiency of nutrient retention in the headwaters, and exacerbating greater downstream transport (Alexander et al., 2007). Less detailed information is available on pond nutrient regimes with most data limited to snap-shot surveys of nutrient status at different spatial scales (Biggs et al., 2005, Biggs et al., 2014; Williams et al., 2010).
4.3.2. Contaminants
Contaminants of SWBs include agricultural and amenity pesticides, veterinary and human medicines, personal care products, biocides, heavy metals and polyaromatic hydrocarbons (PAHs). Pesticides are the most important and widespread organic contaminants in SWBs and a major risk to aquatic ecosystems (Brown et al., 2007). Extensive contamination of ditches and headwater streams can be inferred from statutory monitoring for larger UK water bodies and from many field experiments that quantify edge-of-field concentrations in sub-surface drainage (Brown et al., 2006; Brown and Van Beinum, 2009). Pesticide transfer to water from treated areas occurs via surface and sub-lateral flows, including sub-surface drains (Williams et al., 1995). Contamination is dominated by autumn-applied herbicides with peak concentrations frequently in the 10s and exceptionally in the 100 s of μg L−1 at edge of field. Direct entry of pesticide via spray drift can cause short-lived peaks in concentrations in SWBs (Maltby and Hills, 2008), and point sources such as from farmyards following sprayer mixing and cleaning activities can be significant contributors to total contamination (Mason et al., 1999).
A small number of studies have used in situ bioassays to assess ecological impacts of pesticides in streams under field conditions (Crane et al., 1995; Matthiessen et al., 1995; Thomas et al., 2001). Recently, bioindicators have been applied to isolate the impacts of pesticides from those of other stressors in agricultural landscapes such as dredging, sediment, nutrients and changes in riparian vegetation. The most common approach is SPEAR (Liess and Beketov, 2011; Liess and von der Ohe, 2005), which uses sensitivity to pesticides and ecological traits to identify species at risk from pesticide contamination.
Disposal of spent sheep dip has been a localised source of contamination of streams, primarily due to runoff after disposal on land. Pesticides Forum (2012) reported monitoring data for 2009–2011, which showed that exceedances of environmental quality standards (EQSs) for pesticides in SWBs were primarily associated with the sheep dip actives diazinon (organophosphate) and cypermethrin (pyrethroid), or with legacy pesticides including dichlorvos, dieldrin, aldrin and endrin (organochlorines).
Road runoff can be a pathway for contamination of streams and ponds with heavy metals, hydrocarbons, including PAHs and de-icing salt. Maltby et al. (1995) showed clear effects on diversity and composition of macroinvertebrate assemblages in streams receiving road runoff; the dominant PAHs were phenanthrene, pyrene, and fluoranthene, whilst dominant metals were zinc, cadmium, chromium, and lead.
Microplastics are emerging as a significant concern for freshwater systems. Current monitoring only occurs in larger rivers (Horton et al., 2017a), however a recent study in NW England indicated that they are found throughout the catchment (Hurley et al., 2018), suggesting that SWBs are likely to be contaminated elsewhere. As well as discharge of effluent from sewage treatment (Murphy et al., 2016), sources of microplastics to SWBs may include: runoff of agricultural fertilisers derived from sewage sludge, road runoff, and the use and fragmentation of agricultural plastics (Horton et al., 2017b).
Discharge from abandoned metal mines is a major pressure on water quality and accounts for 8% of Water Framework Directive (WFD) failures in England and Wales (Jones et al., 2017b). Iron is the most important pollutant in coal mine waters, whilst metal mine discharges result from oxidative dissolution of metal sulphide minerals with arsenic, cadmium, copper, lead, tin and zinc the primary pollutants of concern. Mine discharge water can be highly acidic, resulting in elevated concentrations of aluminium. Historical mining was particularly associated with headwater river catchments in the UK, where problems can be particularly acute because dilution potential is restricted (Jones et al., 2017b). Legacy contamination with metals is often highly heterogenous due to control by fluvial processes of sediment erosion and deposition (Dennis et al., 2009). Jones et al. (2017b) found that >2 km downstream of abandoned metal mines, 3–24% (by mass) of the riverbed sediment was derived from the mine facilities indicating continued release of arsenic, cadmium, copper, lead, tin and zinc >100 years after the closure of the mines.
4.3.3. Acidification
The acidification phenomenon illustrates that some pressures affecting SWBs originate at scales beyond individual catchments and can only be addressed effectively by large-scale initiatives. Long-term acidification, at its peak during the 1970s and 1980s, affected large, base-poor areas of Europe, including the British Isles. Acidification particularly affected SWBs in the uplands, and half the stream length in Wales, around 12,000 km, was acidified. Sulphate loading depleted catchment soils of base-cations and mobilised aluminium at pH values, typically around 4.0–5.7, that maximised its toxicity to freshwater organisms (Edwards et al., 1990). Afforestation exacerbated acidification by enhancing local S and N deposition by >40% (Ormerod et al., 1989).
Since the early 1970s, industrial decline coupled with the regulatory control of emissions have led to a reduction in acid deposition, and there have been signs of chemical recovery (Battarbee et al., 2014; Kernan et al., 2010; Malcolm et al., 2014; Ormerod and Durance, 2009). However, biological recovery is often incomplete, due to acid episodes which still occur in vulnerable catchments (Murphy et al., 2014). In Great Britain, these are driven by a combination of base-cation dilution at high flow coupled with the release of mineral acidity and aluminium (Kowalik et al., 2007; Kowalik and Ormerod, 2006). In Ireland, organic acidity from peat soils is a key driver of episodic acidification, particularly in afforested catchments (Feeley et al., 2013). Current debate revolves around the additional requirements to engender biological recovery, and whether the reduction of deposition should be supplemented by interventions such as base-cation addition, i.e. liming (Mant et al., 2013).
4.4. Biological pressures
The deliberate introduction of aquatic non-native species has a long history in GB&I (Copp et al., 2006; Pinder et al., 2005). Although small streams are less likely to contain non-native species (Jones et al., 2018), some may become invasive and exacerbate pressures on native pond species. Well known invaders of SWBs include Himalayan balsam Impatiens glandulifera, Japanese knotweed Fallopia japonica, Canadian pondweed Elodea canadensis and goldfish Carassius auratus. The estimated cost to control invasive pondweeds in Great Britain is over £11.6 m y−1 (Oreska and Aldridge, 2011).
Bioinvasions are often facilitated by other negative pressures on the invaded ecosystem. For example, Nuttall's pondweed Elodea nuttallii benefits from eutrophic conditions in which it can displace Canadian pondweed in 1–3 years and adversely impact native species (Dadds and Bell, 2008; Simpson and Duenas, 2011). Environmental disturbance appears to be a key factor in bioinvasions of non-native fishes (Moyle, 1986) and recent studies have shown invasions to be facilitated by river regulation (Almeida et al., 2009) and management actions. Pond dredging, intended to remove invasive plants had the unexpected result of creating a population explosion by pumpkinseed Lepomis gibbosus (Van Kleef et al., 2008). Food web structures can be impacted by the introduction of omnivorous species (Sievers, 2012), which force native fish to shift their foraging behaviour (Copp et al., 2017a) and invasive predators, such as non-native crayfish, which affect the recruitment of native fish (Copp et al., 2017b; Edmonds et al., 2011) and reduce richness of invertebrates and aquatic plants (Jones et al., 2018). The invasive American mink Neovison vison, can exert enormous predation pressure on small riparian mammals (e.g. water vole Arvicola amphibius) and aquatic birds (Barrat et al., 2010; MacDonald and Harrington, 2003), although it consumes fewer fish than the native Eurasian otter Lutra lutra (Chanin, 1981).
5. Deterioration in biological communities of SWBs
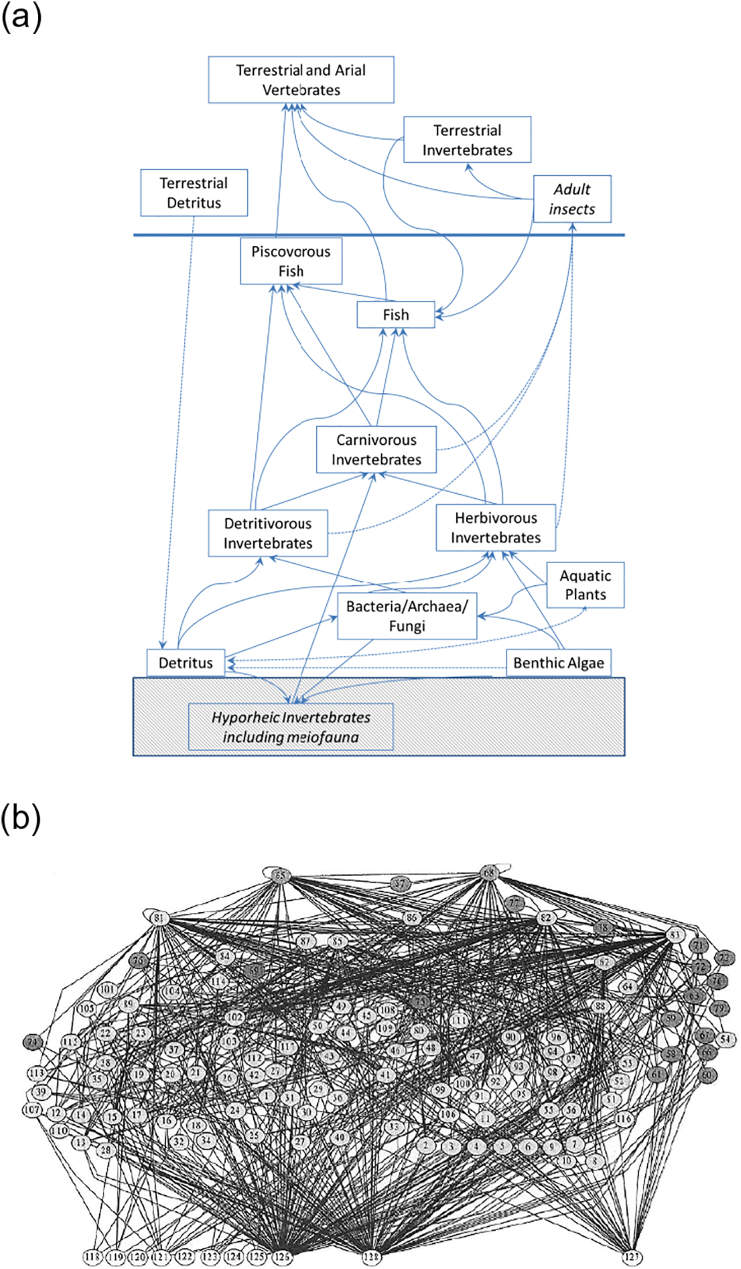
In this section, we consider how the pressures on SWBs described in Section 4 affect individual species, groups of organisms and communities. A food web summarises the feeding links between organisms. In even the smallest SWBs the food web would form a complex network of hundreds of species interactions (e.g. Hildrew, 2009; Fig. 4a and b). These interactions largely determine ecosystem function (Reiss et al., 2009), a topic we revisit at the end of this section.
Fig. 4.
a) A highly simplified food web for a small stream showing the main feeding links (solid arrows) and non-feeding inputs (dashed arrows), and b) a real food web from a small, fishless stream without plants or terrestrial links (each numbered node represents a different food item/species which, with the exception of basal resources (unfilled circles), are all invertebrates: full details in Fig. 9 Schmid-Araya et al., 2002).
5.1. Macrophytes and phytobenthos
Aquatic plants play a central role in the dynamics of SWB ecosystems, linking soil, water and atmosphere, influencing the quality of the aquatic environment and providing propagule sources for adjacent water bodies. Submerged higher plants occur throughout the lowland river network, but are often rare in steep, upland streams due to lack of appropriate rooting substratum, whereas mosses and liverworts are common in such streams (Meyer et al., 2007b; Weekes et al., 2014). Phytobenthos are found in all types of running water.
Elevated sediment levels reduce primary productivity and biodiversity of macrophytes and phytobenthos due to reduced light penetration for photosynthesis and abrasion by suspended and deposited sediment (Jones et al., 2012a, Jones et al., 2014; Wood and Armitage, 1997). Phytobenthos may be more susceptible to the effects of sedimentation than macrophytes; Lawler et al. (2017) report severe reductions in biomass which impacted secondary production. Conversely, sediments bring particulate nutrients into streams and increased deposits of fine sediments are associated with changes in macrophyte (Jones et al., 2012a, Jones et al., 2017c) and phytobenthos (Jones et al., 2014, Jones et al., 2017a) communities typical of the response to eutrophication.
Responses of macrophytes to nutrient enrichment are well documented and are the basis for their use as indicators of eutrophication (Holmes et al., 1999; Szoszkiewicz et al., 2006). Similar responses have been documented for phytobenthos (Kelly et al., 2008). Acidification usually results in reduced macrophyte richness and dominance of liverwort taxa (Ormerod et al., 1987), and changes in the diatom assemblage composition (Juggins et al., 2016).
Ponds provide a substantial habitat for aquatic macrophytes and are generally the richest part of the SWB network (e.g. Biggs et al., 2014; Williams et al., 2004). Ponds are often the refuge for endangered water plants, particularly those eliminated from larger water bodies by nutrient pollution.
5.2. Invertebrates
Small streams provide extensive habitat for aquatic invertebrates and up to 29% of river invertebrate species are unique to headwaters (Callanan et al., 2014; Feeley et al., 2012; Furse, 1995) including specialised habitats for several rare and important species of water beetle (Foster et al., 2009; Foster, 2010), stonefly (MacAdam, 2015) and caddis fly (Wallace, 2016). Although typically less species-rich than large rivers (Davy-Bowker et al., 2008), stream beta diversity is high, and collectively the small stream network makes a large contribution to regional biodiversity (Callanan et al., 2014; Clarke et al., 2008). Adult aquatic invertebrates generally stay within 10 m of the stream and provide a food source for terrestrial predators (spiders, beetles, birds, bats and reptiles), which occur in higher populations along stream margins (Baxter et al., 2005).
Most invertebrate monitoring is focussed on higher order reaches. Nevertheless, the UK Countryside Surveys indicated that, 46%, 43% and 35% of sites in England, Wales and Scotland respectively, failed to achieve WFD targets in 2007, and provided an indication of change in headwater invertebrate communities through time (1990, 1998, 2007: Dunbar et al., 2010). With the exception of the agri-environment scheme in Wales (Anthony et al., 2012; Centre for Ecology and Hydrology, 2017), there has been no large-scale assessment of the condition of UK headwaters since. In Ireland the Small Streams Risk Score is used to assess risk of pollution (McGarrigle, 2014); 76% of sites in 2006 were assessed as ‘at risk’ or ‘probably at risk’ (McGarrigle, pers. comm.) of impact from pollution. Degradation of invertebrate communities has been associated with arable and improved grassland and river channel management in southeast England (Dunbar et al., 2010), inputs of sediment and pesticides in Wales (Jones et al., 2017c), and agricultural land-use impacts on water quality in Ireland (Baars and Kelly-Quinn, 2005; Bradley et al., 2015). Among other pressures, the increased inputs of fine sediment from agriculture has marked impacts on invertebrate communities (Jones et al., 2012b; Murphy et al., 2015b), including the systematic loss of larger, long-lived invertebrates and certain feeding guilds (Larsen and Ormerod, 2014; Murphy et al., 2017).
Acidification impacts on invertebrates are well documented for Scotland (Helliwell et al., 2014), Wales (Ormerod et al., 1993; Reynolds et al., 1995) and Ireland (Kelly-Quinn et al., 2016), often exacerbated under non-native conifer forests on base-poor soils (Ormerod and Durance, 2009). Impacts may also arise from nutrients and sediment inputs during tree harvesting (Kelly-Quinn et al., 2016; Reynolds et al., 1995) and windrowing in preparation for replanting (Clarke et al., 2015).
Although poorly documented, there is great potential for degradation of invertebrate communities in urban streams and ponds, from a range of hydromorphological alterations, effluent discharges and inappropriate pesticide use (Wittmer et al., 2011). Contaminated sediments from past mining activities are likely to constrain biological recovery resulting in impoverished invertebrate communities (Jones et al., 2017b). Bass et al. (2008) demonstrated the ecological impacts of past metal mining activity on macroinvertebrates to aluminium, zinc, acidity and copper.
Ponds support a large proportion of the aquatic invertebrate species found in networks of SWBs (Davies et al., 2008; Williams et al., 2004). Regional and national studies suggest that pond invertebrate assemblages are exposed to most, if not all, of the stressors seen in the stream network, although responses are mediated by site history.
5.3. Fish
In GB&I, small streams support important fish communities, but with considerable diversity between reaches (Davies et al., 2004). Resident brown trout Salmo trutta dominate upland, high gradient streams, which may also be spawning and nursery areas for anadromous salmonids (sea trout Salmo trutta; Atlantic salmon Salmo salar), and habitat for other species of conservation concern (e.g. brook lamprey Lampetra planeri; European bullhead Cottus gobio; European eel Anguilla anguilla). Small coastal streams and the headwaters and cross-channels of highly braided chalk streams may also contain significant resident and anadromous salmonid populations (Crisp, 2000; Riley et al., 2006). Small lowland streams and ditches generally support a wider diversity of species, such as roach Rutilus rutilus, European dace Leuciscus leuciscus, chub Squalius cephalus, threespine stickleback Gasterosteus aculeatus and European minnow Phoxinus phoxinus, which use the streams for recruitment and refuge (Copp, 1992; Davies et al., 2004).
Fishes in small streams are vulnerable to a wide range of pressures (Friberg et al., 2016), both within and outside the stream channel (IBIS and AST, 2012; IFM, 2013). Silting of spawning gravels and alteration in the timing and magnitude of stream discharge can severely impact salmonid recruitment dynamics (Cowx et al., 2012; Milner et al., 2012). The natural movement of fish into, and within, many small streams has been compromised by construction of barriers (Kemp, 2016; Nunn and Cowx, 2012). Canalisation of many lowland streams, and construction of flood retention obstructions reduces longitudinal and lateral connectivity and consequently impedes fish movement (Peirson et al., 2008; Walton et al., 2016). Emerging issues include the effects of climate change on discharge (Riley et al., 2009a) and temperature (Harrod, 2016), and the increased use of artificial light at night (Riley et al., 2015).
Ponds in GB&I tend to sustain populations of relatively few fish species including native tench Tinca tinca, threespine stickleback, ninespine stickleback Pungitius pungitius and crucian carp Carassius carassius (Copp et al., 2008). Major threats to pond fishes are invasive non-native species (Copp et al., 2005; Sayer et al., 2012), as well as changes in land-use, resulting in anoxic conditions and loss of virtually all species due to heavy shading or surface coverage by duckweeds (Lemnaceae) (Sayer et al., 2011, Sayer et al., 2012).
5.4. Amphibians
In Great Britain, national monitoring programmes, using traditional field surveys, indicate that the common frog (Rana temporaria) occurs in around 60% of ponds, with common toad (Bufo bufo), smooth newt (Lissotriton vulgaris) and palmate newt (Lissotriton helveticus) found respectively in 33%, 28% and 27% of ponds (Wilkinson and Arnell, 2013). In Ireland, the 2010/11 national survey showed frogs densities were generally high in farm ponds and bog pools but were highest in drainage ditches, with 86% of all breeding frogs in this habitat (Reid et al., 2013).
Surveys of the Great crested newts Triturus cristatus (which are specially protected under European nature conservation legislation) in 2015–17 using environmental DNA techniques, indicate that the species occurs in 18–32% of 1 km grid squares in England, and that in the short term this hasn't changed (Ewald et al., 2018). Around 13% of ponds are occupied by Great crested newts within their range in England, with poor quality ponds unlikely to be occupied. Pond occupancy per 1 km grid square was lower than expected. In the majority of 1 km squares (41%) newts were only recorded from between 26 and 50% of the ponds. The only native newt species in Ireland (Lissotriton vulgaris) is dependent on SWBs such as freshwater marshes, ditches and ponds for breeding (Marnell, 1998).
In Great Britain natterjack toads (Epidalea calamita) are rare and found at around 60 mainly coastal sites, with about 1500 spawn strings counted annually (Beebee, 2014). This number has remained fairly constant over the last 20 years. In Ireland, they are also restricted to coastal sites where they breed mainly in ponds, including constructed ponds, and some lakes (Sweeney et al., 2013).
The main threats to amphibians are loss or deterioration of aquatic and terrestrial habitats by: water pollution; the intensification of land use; the demise of gentle grazing of SWBs; the spread of diseases; and for some species (e.g. Great crested newt) the introduction of fish to ponds that would normally be fish-free.
5.5. Birds
Several bird species depend on small streams, notably dipper Cinclus cinclus, grey wagtail Motacilla cinerea, common sandpiper Actitis hypoleucos and kingfisher Alcedo atthis. Ponds can support more species, such as mallard Anas platyrynchos, little grebe Tachybaptus ruficollis, moorhen Gallinula chloropus and sedge warbler Acrocephalus schoenobaenus. However, most species use SWBs and associated features within a broader habitat mosaic (Céréghino et al., 2014; Pickett and Siriwardena, 2011; Santoul et al., 2009). For example, grey heron Ardea cinerea and little egret Egretta garzetta may forage at many SWBs, but cannot subsist on a single such “habitat patch”. Specific habitat features associated with stream channels may also be critical, including sandbanks, which provide nest sites for sand martins Riparia riparia and kingfishers.
Pressures faced by birds inhabiting small streams include changes in water quality and stream channelization, which results in loss of stream, bank and vegetation heterogeneity as well as reduced food availability (Brooker, 1985; Larsen et al., 2010). Livestock poaching threatens bank habitats (Clews et al., 2010), abstraction threatens peripheral habitats by reducing soil moisture/inundation, and increased flooding can destroy local habitat for ground-nesting species and disrupt breeding.
The effects of changing habitat quality will vary according to the nature of the deterioration and some species may actually benefit, although generally not conservation-priority species. For example, ponds overtaken by scrub are poor for aquatic bird species, but provide habitat for various scrub- species, such as warblers (Davies et al., 2016). Similarly, eutrophication could enhance food resources for birds that exploit emergent Chironomidae, although ephemeral flushes of invertebrate food abundance (e.g. Florencio et al., 2014) are unlikely to support local breeding territories. Species more exclusively associated with aquatic habitats are likely to suffer negative effects (e.g. Fernández et al., 2005; Matsunaga et al., 1999).
5.6. Mammals
Several mammal species use SWBs ranging in size from the soprano pipistrelle (Pipistrellus pygmaeus) to the Eurasian otter. Their dependence upon these habitats is a factor of their mobility and the resources available at each site. For instance, otters use small streams as corridors, whereas a water vole colony may be totally reliant on one particular SWB for their entire lives (Telfer et al., 2001). Consequently, the impact of SWB degradation varies depending on the scale at which mammal species operate. Otters may be able to pass a pollution incident and move to another part of the catchment, but less mobile species such as water voles or shrews are at greater risk of localised extinction. Indeed, water voles have suffered the greatest declines in recent decades (Barrat et al., 2010) due to the combined impact of channel management, habitat fragmentation and invasive predators, especially American mink (Barrat et al., 2010.
Bats, which have been in decline in recent decades (Lundy and Montgomery, 2010), use small streams and their riparian borders both as habitat corridors and to forage (Fukui et al., 2006; Vaughan, 1997). Therefore, any loss of habitat connectivity reduces bat diversity and regional biodiversity (Naiman et al., 1993).
5.7. Ecosystem-scale considerations
An ecosystem view is important because it represents the emergent properties of ponds or headwaters, the ecosystem processes that result, and the ecosystem services that are jeopardised without appropriate protection or restoration.
In SWBs, organisms from microbes to vertebrates combine within abiotic constraints to affect production, decomposition, competition, predation and energy flow through food webs, as well as time-critical processes such as recovery from disturbance or succession (Thoms et al., 2006).
The high connectivity of SWBs with their surrounding terrestrial environment results in large exchanges of matter, energy and nutrients across the aquatic-terrestrial ecotone in the form of sediments, solutes and both living and dead organisms, with emerging aquatic insects also subsidising terrestrial food chains (Baxter et al., 2005; Paetzold et al., 2011). These reciprocal subsidies mean that the energetics of SWBs are always in dynamic partition between autochthonous and allochthonous production of varying quality (Marcarelli et al., 2011). Similarly, exports from SWBs in the form of emerging invertebrates vary seasonally to influence the niche use of a wide range of terrestrial organisms in marginal and riparian land, and the gene flow between adjacent water bodies (Petersen et al., 2004).
Terrestrial inputs have also been shown to influence pond food webs, principally through increased autotrophic respiration, particularly where endogenous sources of carbon are low as in temporary (Rubbo et al., 2006) or shaded ponds (Earl and Semlitsch, 2013).
As well as lateral exchanges in streams, longitudinal transport networks route sediments, water, solutes, nutrients, organic matter and migratory organisms through downstream freshwater reaches, floodplains, estuaries and marine ecosystems (Gomi et al., 2002). While these coupled hydrological, physical and biological linkages are well known qualitatively, in some cases, such as for organic carbon transport or some key solutes, quantification of fluxes through terrestrial, freshwater and downstream ecosystems is still rudimentary (Alexander et al., 2007).
Lateral and longitudinal connectivity of SWBs and adjacent or downstream ecosystems can propagate effects that are both positive and negative for resource conservation and management. The ecosystem services provided by intact SWBs are recognised as consumptive use of water for people and livestock, a source of food and energy, and their cultural values for recreation, tourism, education, heritage and as inspiration for arts and religion (Maltby et al., 2011). When mismanaged, however, adverse effects arise when mass-loadings of water, sediments, nutrients and pathogens increase from background levels to affect sensitive organisms, abstracted supply or other human uses. The balance of positive and negative effects arising at ecosystem scales respectively from the protection or degradation of SWBs requires fuller quantification through natural capital accounting. This applies both intrinsically within SWBs, but also for adjacent or downstream ecosystems (Biggs et al., 2017). There are, nevertheless, options for positive management and restoration at scales ranging from the water body to entire catchments.
6. Options for and benefits of restorative action
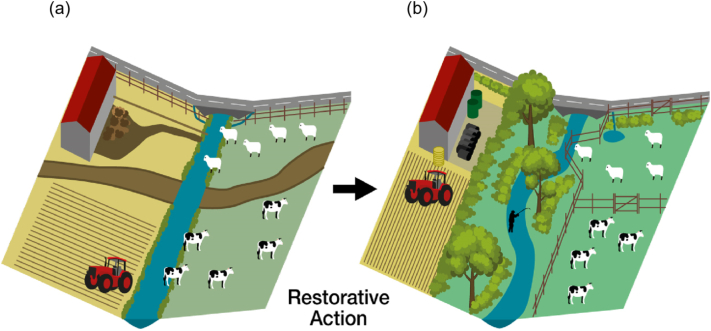
We propose a three-tiered approach to reversing the deterioration of SWBs: (1) restore channel hydromorphological dynamics; (2) restore and manage riparian zones, including the planting of buffers as protection from wider landscape pressures; and (3) manage point-source and diffuse impacts associated with activities in the wider catchment (Fig. 5).
Fig. 5.
Graphical representation of the three-tiered approach to reversing the decline of SWBs from a) degraded, to b) a state of improved resilience following restorative action.
Full restoration of SWBs to pristine conditions, or ‘rewilding’, is only likely to be an option in a restricted range of environments, often where disturbance has been relatively small. More generally, the focus must be on improving the resilience of SWBs in more impacted agricultural and urban catchments, along with managed reductions in human-generated pressures. There is no single approach that can be applied to all SWBs, and applying remedial measures at appropriate scales is expensive even with careful targeting. Approaches must therefore be pragmatic and tailored to local catchment conditions and land uses, and solutions sought that give the greatest multiple benefits for the cost. SWBs are complex biophysical systems and in some cases restorative action may have unintended consequences and there may be a need to accept trade-offs. In all such programmes, it is important to specify clearly the expected outcomes of any remediation measures and then evaluate success, not just in terms of uptake and implementation of recommendations, but in terms of a range of measurable criteria which capture ecosystem recovery/change over appropriate time scales.
Beechie et al. (2010) identified four principles for stream restoration: (1) address the root causes of degradation; (2) ensure restoration is conducted at a scale commensurate with the environmental pressures; (3) ensure restorative actions are well matched with local physical and biological potential, and (4) have clearly articulated expected outcomes and monitor to establish if these have been achieved. Applying these principles will help avoid common pitfalls in river restoration, such as creating habitat types that are outside the range of a site's natural potential, attempting to build static habitats in dynamic environments, constructing habitat features that are ultimately overwhelmed by untreated system drivers (Beechie et al., 2010), or expending scarce resources on rehabilitation work unlikely to enhance the current stream character (Champkin et al., 2018).
6.1. Restoration of channel hydromorphological dynamics
Restoration of headwater streams, and of rivers more generally, has tended to be focussed on biodiversity goals, although its implementation typically involves physical habitat modification. It can provide multiple benefits, most notably the retention and storage of excess runoff, sediments and organic matter (Dixon et al., 2016), and provide natural flood management for downstream communities. Since channel complexity directly influences hydraulics and hyporheic flow, complexity will affect transient storage processes and nutrient processing (Baker et al., 2012; Gooseff et al., 2007). Thus, the habitat complexity of headwater streams results in additional ecosystem benefits including nutrient processing (Weigelhofer et al., 2013); sediment storage (Pollock et al., 2014) and increased carbon sequestration (Rheinhardt et al., 2012).
Since many headwater streams have been modified for land drainage, the resulting channels are often straightened and over-deepened (Sear et al., 2000). Remedial measures range from re-cutting a complex channel pattern, bed level raising and substratum replacement, to small scale installation of structures to increase habitat diversity (Wohl et al., 2005). The key challenge facing such feature-based approaches is that they are expensive to undertake at the scales needed to deliver wider catchment benefits.
The second key principle in headwater stream restoration lies in targeting the treatment appropriately to deliver the required benefits. Opportunistic restoration, which has dominated the practice to date, has been largely ineffective in delivering biodiversity and other ecosystem services (Palmer et al., 2010). Alongside scale, location is important particularly when dealing with delivery of offsite (downstream) benefits such as natural flood management. Dixon et al. (2016) showed how the location of a restoration needs to be carefully considered to optimise the reduction in flood height.
The third principle identified above relates to the effectiveness of a given restoration treatment to mitigate a given pressure. In the case of flooding, the efficiency relates to increases in channel and floodplain roughness and water storage capacity. In woodland planting programmes, roughness is optimised as the woodland matures; a process that may take 25–100 years (Dixon et al., 2016).
Most river restoration projects are implemented over short reaches (<1 km), but restoration measures that are spatially restricted to the reach-scale (Teufl et al., 2013; Weigelhofer et al., 2013) cannot compensate for deficits in the catchment (Bernhardt and Palmer, 2011). If conditions in the catchment remain unchanged, then streams will largely maintain their human-modified sediment, nutrient and organic matter transport functions, thus limiting the potential of stream restoration to improve in-stream habitat and biodiversity. Continued accumulation of nutrient-rich sediments from agricultural or urban catchments limits the effectiveness of stream restoration (Weigelhofer et al., 2013). Thus, reach-scale restoration measures have to be combined with measures in the catchment which reduce nutrient and soil inputs to streams.
Restoration of headwater streams requires spatial planning to target options, delivery over large enough scales to realise the mitigation, and sustainability over longer timescales. Thus, the final challenge lies in persuading the catchment stakeholder communities and riparian owners to undertake stream restoration.
6.2. Restoration and management of the riparian zone
Restoration of riparian zones, including the creation of buffers to protect smaller streams from the wider landscape pressures has the potential to deliver multiple benefits including reduced stream temperatures, increased wood loading and thus channel complexity. However, their effectiveness varies depending on size relative to stream width and vegetation height, and they require management in order to maintain effectiveness because they may become saturated with nutrients and sediments (Osborne and Kovacic, 1993).
Riparian woodland exerts significant control over the physical, chemical and biological environment in small streams (Nislow, 2010), providing a more natural and stable stream structure and morphology, moderating incident light and the thermal regime, reducing sediment and pollution input, and providing sources of food, organic matter and cover. Although riparian woodland in different locations affects different freshwater species in different ways, where the goal is to restore key processes that have been altered by human activity, some general principles apply.
Riparian buffers reduce sediment and nutrient loads entering the stream (see Table 1) and may enhance the capacity of in-stream processes to retain dissolved N and P. Riparian buffers are often promoted to protect stream ecosystems from diffuse pollution in agricultural landscapes (Lowrance et al., 1997; Nisbet et al., 2011), and woodland buffers have been reported as more effective at removing nitrate than grass buffers (Lyons et al., 2000; Mayer et al., 2005; Osborne and Kovacic, 1993). However, Sabater et al. (2003) reported that across Europe, vegetation type was not the dominant factor in the attenuation of nitrate in the riparian zone. Ranalli and Macalady (2010) regard topography of the stream valley rather than vegetation type as the controlling factor on denitrification in the riparian zone, as forest and grass both provide enough organic carbon for the denitrifying bacteria.
Table 1.
Efficacy ranges (combining plot or field scale empirical data and elicitation of expert judgement) for reductions in nutrient and sediment loss at plot or field scale for a range of mitigation measures targeting both riparian management and farming activities in the wider catchment.
| Mitigation measure | Efficacy ranges for pollutant reductions |
||
|---|---|---|---|
| Nitrate | Soluble reactive phosphorus | Sediment | |
| Riparian relevant measures | |||
| Locate out-wintered stock away from watercourses | 0–10 | 0–10 | 0–10 |
| Establish and maintain artificial wetlands - steading runoff | 10–50 | 25–80 | |
| Site solid manure heaps away from watercourses/field drains | 2–25 | 2–25 | |
| Establish 6 m riparian grass buffer strips | −30–95 | −83–95 | 2–98 |
| Measures for wider catchment | |||
| Farm track management | 0–10 | 0–10 | 0–10 |
| Reduce field stocking rates when soils are wet | 2–25 | 2–25 | 2–25 |
| Move feeders at regular intervals | 2–25 | 2–25 | 2–25 |
| Establish cover crops in the autumn | 25–80 | 25–80 | 50–95 |
| Fertiliser spreader calibration | 0–10 | 0–10 | |
| Do not apply manufactured fertiliser to high-risk areas | 2–25 | 10–50 | |
| Increase the capacity of farm slurry stores to improve timing of slurry applications | 2–25 | 2–25 | |
| Do not apply P fertilisers to high P index soils | 10–50 | ||
| Loosen compacted soil layers in grass fields | 10–50 | 10–50 | 10–50 |
| Allow grassland field drainage systems to deteriorate | 0–10 | 0–10 | 0–10 |
| Re-site gateways away from high risk areas | 2–25 | 2–25 | 2–25 |
Gaps indicate mitigation measure does not impact on the pollutant; – indicates risk of increased losses.
Riparian woodland buffers have been reported as more effective than grassland buffers at mitigating downstream nutrient transport (Hall et al., 2002; Weigelhofer et al., 2012). Tracer experiments at the Hubbard Brook experimental forest (Hall et al., 2002) demonstrated that the current velocities are slower in forested streams, and Weigelhofer et al. (2012) reported significantly higher hydrologic retention times in forested stream reaches compared with adjacent open reaches. Furthermore, nutrient uptake was enhanced in-forest streams where flow obstructions, such as submerged roots and woody debris, increased hyporheric water exchange and the benthic surface area. The forest streams had greater loads of leaf litter and coarse woody debris trapped in debris dams, which provided additional sources of carbon for heterotrophic communities thereby increasing the biological demand for N and P. However, in intensely agricultural catchments, instream nutrient retention cannot compensate for deficits in riparian nutrient retention where nutrient supply exceeds demand significantly. Unmanaged woodland may become saturated with nutrients and be a less efficient buffer than grass (Osborne and Kovacic, 1993). Therefore, riparian woodland buffers must be designed and managed to maintain ground cover through the year to reduce soil erosion and enhance nutrient uptake, and anthropogenic drainage (e.g. tile drains) should be destroyed. In addition to delivering benefits to aquatic habitats, riparian buffer zones produce linear corridors through the landscape that have the potential to form a coherent ecological network for terrestrial organisms (Lawton et al., 2010).
In ponds, well-planned management action based on a risk assessment (Williams et al., 2010) can play a crucial role in biodiversity conservation by the maintenance of high water quality and gentle grazing pressure, which stimulates natural hydro-biotic relationship. Reversal of ecological succession, such as thinning of riparian vegetation and careful dredging to re-establish an early-to-intermediate stage of ecological succession, can also be beneficial. In pond-rich landscapes, especially those with a high proportion of heavily-terrestrialised ponds, restoration of heterogeneity (i.e. a mosaic of water bodies at different successional stages, e.g. Copp, 1989) can maintain both local and regional biodiversity (Sayer et al., 2012).
The ability to moderate thermal fluctuations will become increasingly important in the face of the predicted effects of climate change (Hammond and Pryce, 2007; Whitehead et al., 2009). Riparian woodland can be used to reduce stream temperatures, particularly summer maxima (Weatherley and Ormerod, 1990). Even local interventions such as installation of revetments to control bank erosion can reduce temperature of micro-habitats by approximately 1 °C when compared with the open mid-channel (Everall et al., 2012). Likewise, removal of instream features such as small weirs and woody debris reduces the water travel time and potential for energy gain. Johnson and Wilby (2015) found that in headwater catchments, 500 m of riparian shade offset water temperature by 1 °C in July, whereas in larger catchments, 25 km downstream of the source, over 1 km of continuous riparian shade was required to achieve the same response. Although riparian shade can provide cool water refugia for thermally sensitive salmonid species, there may be detrimental consequences for fisheries productivity (Hornbach et al., 2015; Riley et al., 2009b; Riley and Pawson, 2010). Nevertheless, the effects of riparian planting could enhance other aspects of resource provision. In upland Wales, streamside deciduous woodlands considerably increase coarse particulate organic matter in streams, associated with increased densities in several macroinvertebrate groups (Thomas et al., 2016), however shading can also limit algal growth (Halliday et al., 2016).
6.3. Management of activities in the wider catchment
While one of the principal aims of stream restoration is to re-establish natural physical and chemical processes, channel restoration and riparian management must be considered in conjunction with activities in the wider catchment. Sear (1994) and Palmer et al. (2010) concluded that the practice of restoring riverine ecosystems by increasing geomorphic complexity at the reach scale is less successful where stream degradation is a function of catchment-scale human impacts rather than direct modification of the channel and/or flood plain.
Conceptually, land-use practices that impact SWBs can be divided into structural land cover (e.g. woodland versus grassland or arable) and specific aspects of farm infrastructure (e.g. yards or fields) and management (e.g. livestock, fertiliser or soil management). Structural land cover change, whilst able to deliver significant environmental benefits by improving sustained soil vegetation cover or removing livestock, remains highly unpopular with farmers and land managers due to income foregone. In this context, and with the need to ensure food security, the concept of the pollutant transfer continuum, i.e. source-mobilisation-delivery-impact (Haygarth et al., 2005; Lemunyon and Gilbert, 1993), has been adopted widely by scientists and practitioners for structuring the assessment of water pollution risk and development of targeted mitigation strategies (Bloodworth et al., 2015; McGonigle et al., 2014; Murphy et al., 2015a; Wall et al., 2011). Given the prohibitive costs associated with blanket implementation of numerous on-farm measures, and the reduced cost-efficiency of such management approaches (Jones et al., 2017c), there is a growing trend towards optimising the selection of on-farm mitigation measures using critical source areas (Doody et al., 2012; Strauss et al., 2007), cost-effectiveness optimisation (Gooday et al., 2014; Zhang et al., 2012) or farmer attitudes to identify industry–preferred measures (Collins et al., 2016). A number of fundamental issues must be borne in mind when devising water pollutant abatement strategies, including: the interaction of multiple measures tackling the pollutant cascade and associated issues of measure dependency, competition and synergism; the risks of pollutant swapping; and the resilience of options under future climate change projections (Collins and McGonigle, 2008; Collins et al., 2016; Ockenden et al., 2016; Schoumans et al., 2014; Stevens and Quinton, 2009; Verspecht et al., 2012).
The list of individual on-farm mitigation measures for targeting the components of the water pollutant cascade is quite extensive, although empirical evidence on cost-effective measures for specific circumstances is rarely comprehensive in terms of environmental drivers, including rainfall and soil types (Angelopoulos et al., 2017). Where empirical evidence does exist, it is largely at plot or field scale, rather than at farm or landscape scale (McGonigle et al., 2014). In many cases, given data gaps, information on efficacy ranges frequently combines empirical evidence and the elicitation of expert opinion (Table 1), especially where mitigation scenarios are modelled (Gooday et al., 2014; Collins et al., 2016; Zhang et al., 2017). The components of the diffuse water cascade can be used to group individual measures into those dealing with sources, mobilisation and delivery (Zhang et al., 2017). Table S1 provides a more comprehensive list of on-farm measures matched to components of the water pollution cascade from farm yard or slope to stream channel. In many situations, given the low efficacy of some of the individual measures and common configurations of risk that can be identified on different farm types (e.g. Collins et al., 2010b), it is advisable to devise so-called ‘treatment-trains’ whereby a sequence of measures is aligned to provide cumulative pollutant reduction. Where on-farm measures are targeted appropriately and ‘treatment-trains’ are developed, the high connectivity indices in headwater stream catchments can facilitate improvements in water quality, compared to water bodies where connectivity between land and streams is lower. However, such progress is hampered by low uptake rates of measures on farms, poor maintenance of those measures that are implemented and the downscaling of efficacy associated with cross-sector source apportionment. With regards to the latter, progress may be impaired by pollutant inputs from non-agricultural sources, including point sources, residential areas and atmospheric deposition (Zhang et al., 2014).
Another challenge is the emerging evidence from longer-term monitoring programmes that longer-term, large-scale hydro-climatic variability can override the benefits of on-farm measures for pollution control (Mellander et al., 2018), further underscoring the need for empirical data to be based on longer-term sustained campaigns to deliver a robust and compelling evidence base to catchment stakeholders. Where landscape impacts have been reported, they tend to be for short duration programmes, and hence interpretation is open to question. The requirement for longer-term monitored data to support more robust conclusions on the impacts of intervention strategies at landscape scale, means that computer models are used to explore technically feasible outcomes as a means of engaging stakeholders in the shorter-term who are required to implement changes on the ground. A major gap in the current empirical evidence base is the potential for cumulative benefits (or trade-offs) arising from interactions between targeted on-farm interventions and in-channel restorative measures.
7. Incentives, instruments and governance
While the previous Section indicates that practical options are available for the protection, adaptation or restoration of SWBs, a key question is how these might be promoted. Policy drivers vary from the global (e.g. UN Convention on Biological Diversity; Aichi Targets), through the European (reform of the Common Agricultural Policy, CAP) to the national or regional (e.g. agri-environment schemes and devolved legal responsibility). Moreover, opportunities vary among political contexts, and much depends on constraints develop under fluctuating political climates, such as the UK's likely exit from the European Union (EU).
In GB&I, some large-scale land-users, notably the forestry sector, operate a voluntary code of practice that encourages methods intended to protect standing and running waters, including SWBs (Forestry Commission, 2011). Large land owners in the civil society sector (e.g. National Trust/National Trust for Scotland, Wildlife Trusts, Royal Society for the Protection of Birds), as well as some in the private sector, have also taken opportunities for sensitive land management that can deliver downstream benefits, for example through land management for nature conservation, landscape-scale restoration or ‘rewildling’. Although only limited geographical areas are involved, opportunities for the expansion of voluntary schemes to other sectors, such as National Parks, should be encouraged because these schemes act as valuable pathfinders and demonstration projects.
Voluntary schemes may be enhanced by developments to regulatory instruments such as more targeted upstream and/or riparian actions under the WFD (Feld et al., 2018), or financial incentives. While there are concerns about the long-term effectiveness of incentives for local conservation, at a national level, payments to the agriculture industry has beneficial effects for aquatic systems, with spatially targeted schemes providing the best use of public money (Jones et al., 2017c). Current agricultural subsidies often incentivise damaging practices and outweigh the effects of agri-environment schemes; agri-environment payments accounted for only around 5% of the EU CAP budget for 2007–2013 (EC, 2015; European Court of Auditors, 2011). There are opportunities and proposals under CAP reform from 2021 to allow some local flexibility in increasing this sum, but critically in the UK there is uncertainty over how exit from the EU will: (1) allow alignment with Europe; (2) provide sufficient funding for environmental action, and (3) incentivise actions for freshwaters. Current incentives to protect SWBs under EU agricultural Pillar I provide a viable model that could be expanded including drivers such as the Cross Compliance and Nitrate Vulnerable Zone (NVZ) programme rules, which must be adhered to if farmers wish to claim for the Basic Payment Scheme. Schemes funded by EU Pillar II for agri-environment include the new Countryside Stewardship Scheme in England, Glastir in Wales and the Green, Low carbon, Agri-environment Scheme (GLAS) in Ireland. Specific initiatives designed to promote measure uptake such as Catchment Sensitive Farming (www.gov.uk/guidance/catchment-sensitive-farming-reduce-agricultural-water-pollution) cover fertilisers and sediments in the main, but geographical coverage is currently limited as is the funding (capital grant scheme requiring matching from farmers) for incentivisation of measure implementation in the aftermath of one-to-one advice. However, simply offering schemes is insufficient, and a fundamental shift in identities, normative behavioural beliefs and social norms is also required by farmers (Inman et al., 2018). Nevertheless, working with the mitigation options preferred by farmers can produce substantial environmental and economic benefits (Collins et al., 2016). In addition, recent research using the Demonstration Test Catchments (Collins et al., 2018) has been used to underpin the new ‘water rules for farming’ (www.gov.uk/government/publications/farming-rules-for-water-from-april-2018/farming-rules-for-water-overview), introducing ‘basic measures applicable to all farmers’ in England. Key future needs in the UK include expansion, robust evaluation of effectiveness and co-benefits, and continuation of financial incentives in the absence of EU funding. There is also a need to switch funding from subsidies to agri-environment incentives, although care is needed to ensure that this does not lead to increased impacts elsewhere if a shift to more benign practices reduces yields.
Interest is growing in mechanisms to recognize and fund the protection of SWBs and their catchments through natural capital accounting and payment for ecosystem services (PES). Most such schemes are currently developmental or experimental, and attempt to fund services such as the protection of drinking water catchments, natural flood management, drought resilience, climate change adaptation or carbon storage (see details of an EU cost action project on PES for water quality protection: https://riojournal.com/article/13828/). Water companies have so far been closely involved with scheme development, often in partnership with responsible agri-food businesses or environmental charities as large landowners or brokers. For example, the Demonstration Test Catchment programme has been monitoring the impact of the Upstream Thinking initiative (www.upstreamthinking.org/): a PES scheme funded by South West Water, UK and delivered by Westcountry Rivers Trust, UK. While seen as an attractive option because markets for ecosystem services potentially link multiple benefits to multiple stakeholders, key needs are to demonstrate market viability and environmental benefits at scale, to develop better funding links between beneficiaries and providers, and to encourage the flow of resources from investors seeking environmental or social impact as well as financial return.
Opportunities might also arise through improved regulation, although this is likely to be slow to implement and may not address the wide diversity of issues. SWBs are currently excluded from the implementation of the WFD (Baattrup-Pedersen et al., 2018; EC, 2000; UK TAG www.wfduk.org/stakeholders/uktag) because of resource constraints, but our review indicates that this is an important oversight because better upstream control could be used to deliver improvements in downstream ecological status. The Habitats Directive (EC, 2002) lists few organisms typical of headwaters, although the importance of small headwater streams to β (among-site) and γ (landscape-scale) diversity has been shown (Callanan et al., 2014; Finn et al., 2011). Greater emphasis should therefore be applied to conservation species, such as migratory salmonids, in upstream reaches. Some SWBs in the UK are notified under planning regulations in their own right or because they add interest to terrestrial reserves, but coverage is well below 1% of stream or pond networks, and much lower than in terrestrial environments (e.g. Wildlife & Countryside Act, 1981: www.legislation.gov.uk/ukpga/1981/69). The UK Biodiversity Action Plan (1994: http://jncc.defra.gov.uk/PDF/UKBAP_Action-Plan-1994.pdf) has attempted to expand the recognition of headwaters, but with little or no traction or regulatory effect. While stronger protection could offer opportunity, a key constraint is that new regulatory approaches are unlikely under current political climates.
8. Conclusions
SWBs comprise about three-quarters of the total running water network and their close connectivity with the terrestrial environment makes them vulnerable to physical and chemical pressures that can have major impacts on conditions downstream. In this review, GB&I are used as case studies. Although this information will be of relevance outside of these islands, related problems in more mountainous areas may be notably different.
In GB&I, adverse impacts on SWBs from channel management, land-use practices, environmental change, and invasive species are affecting the natural hydrological, chemical and biological processes that operate in catchments, threatening the native species they support, and disrupting natural ecosystem function.
The greatest pressure on the physical processes in SWBs has been their extension and modification for agricultural and forestry drainage, resulting in highly modified flow and temperature regimes which have implications for flood and drought control downstream. The extensive length of the small stream network exposes them to a wide range of inputs, including nutrients, pesticides, heavy metals, sediment, and emerging contaminants (e.g. medicines and personal care products). SWBs have also been affected by invasions of non-native species, which along with the physical and chemical pressures, have affected most groups of organisms with consequent implications for the wider biodiversity within the catchment.
We propose a three-tiered approach to reduce these impacts, improve the resilience of SWBs and restore their natural functioning to secure cumulative downstream benefits: (1) restoration of channel hydromorphological dynamics; (2) restoration and management of the riparian zone, and (3) management of activities in the wider catchment.
No single approach can be applied to all SWBs, and remedial action must be pragmatic and tailored to local catchment conditions and land uses. However, in all programmes it is important to specify clearly the expected outcomes and then evaluate success, not just in terms of uptake and implementation of recommendations, but in terms of a range of measurable criteria that capture ecosystem recovery/change over appropriate time scales.
Effective restorative action will be expensive and so emphasis must be placed on integrated programmes that provide multiple benefits. Therefore, successful outcomes will necessitate: government prioritization; industry partnership; third-sector collaboration; applied communication of expert advice; wider communication of the impacts of degradation, the high connectivity between aquatic and terrestrial ecosystems, and the benefits of restorative action; and stakeholder buy-in. Practical options will ideally be adopted on a voluntary basis, but can also be promoted through, financial incentives, markets for resource services, and legislative regulation.
The following is the supplementary data related to this article.
Example on-farm mitigation measures targeting different components of the water pollution cascade.
Acknowledgements
Production of this paper has been supported in part by the UK Department for Environment, Food and Rural Affairs (Defra) under contract SF0272; Rothamsted Research receives strategic funding from the UK Biotechnology and Biological Sciences Research Council and ALC was funded by BBSRC grant BBS/E/C/000I0330; HPJ was funded by the Natural Environment Research Council (National Capability Research project NEC05966: The Strategic Importance of Headwaters for UK Water Resources).
The authors are particularly grateful to members of the Defra Expert Group on SWBs both past and present, including: S. Aubrey (Natural Resources Wales); B. Bendall (Rivers Trust); A. Champneys (Nottingham Trent University); D. Dyne, J. Piercy & R. Velterop (Defra); P. Gaskell (Wild Trout Trust); J. Gee (Aberystwyth University); J. Gurnell (Queen Mary University of London); D. Holliday (Country Land & Business Association, CLA); R. Howells, N. Dunn & G. Righton (National Farmers Union); P. Knight & J. Gray (Salmon & Trout Association); J. Letts (Forestry Commission); M. Owen (Angling Trust); I. Russell & J. Gillson (Cefas); G. Storey & I. Dolben (Environment Agency); and K. Whelan & I. Llewelyn (Atlantic Salmon Trust).
Editor: Jay Gan
References
- Agnew C.T., Clifford N.J., Haylett S. Identifying and alleviating low flows in regulated rivers: the case of the rivers Bulbourne and Gade, Hertfordshire, UK. Regul. Rivers Res. Manag. 2000;16:245–266. [Google Scholar]
- Alexander R.B., Boyer E.W., Smith R.A., Schwarz G.E., Moore R.B. The role of headwater streams in downstream water quality. J. Am. Water Resour. Assoc. 2007;43:41–59. doi: 10.1111/j.1752-1688.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida D., Almodóvar A., Nicola G.G., Elvira B. Feeding tactics and body condition of two introduced populations of pumpkinseed Lepomis gibbosus: taking advantages of human disturbances? Ecol. Freshw. Fish. 2009;18:15–23. [Google Scholar]
- Angelopoulos N.V., Cowx I.G., Buijse A.D. Integrated planning framework for successful river restoration projects: upscaling lessons learnt from European case studies. Environ. Sci. Pol. 2017;76:12–22. [Google Scholar]
- Anon National Flood Resilience Review. 2016. www.gov.uk/government/publications
- Anthony S., Jones I., Naden P., Newell-Price P., Jones D., Taylor R., Gooday R., Hughes G., Zhang Y., Fawcett D., Simpson D., Turner D., Murphy J., Arnold A., Blackburn J., Duerdoth C., Hawczak A., Pretty J., Scarlett P., Liaze C., Douthwright T., Newell-Price P., Lathwood T., Jones M., Peers D., Kingston H., Chauhan M., Williams D., Rollett A., Roberts J., Edwards-Jones G. Welsh Government, Agri-Environment Monitoring and Technical Services Contract Lot 3: Soil, Water and Climate Change (Ecosystems), No. 183/2007/08, Final Report. 2012. Contribution of the Welsh agri-environment schemes to the maintenance and improvement of soil and water quality, and to the mitigation of climate change. (477 pp) [Google Scholar]
- Armstrong A., Stedman R.C., Bishop J.A., Sullivan P.J. What's a stream without water? Disproportionality in headwater regions impacting water quality. Environ. Manag. 2012;50:849–860. doi: 10.1007/s00267-012-9928-0. [DOI] [PubMed] [Google Scholar]
- Baars J.-R., Kelly-Quinn M. Monitoring freshwater diversity in streams draining catchments under intensive agricultural activities in Ireland. Tearmann: Ir. J. Agri-Environ. Res. 2005;4:91–102. [Google Scholar]
- Baattrup-Pedersen A., Larsen S.E., Andersen D.K., Jepsen N. Headwater streams in the EU Water Framework Directive: evidence-based decision support to select streams for river basin management plans. Sci. Total Environ. 2018;613–614:1048–1054. doi: 10.1016/j.scitotenv.2017.09.199. [DOI] [PubMed] [Google Scholar]
- Baker D.W., Bledsoe B.P., Price J.M. Stream nitrate uptake and transient storage over a gradient of geomorphic complexity, north-central Colorado, USA. Hydrol. Process. 2012;26:3241–3252. [Google Scholar]
- Ballantine D.J., Walling D.E., Collins A.L., Leeks G.J.L. The content and storage of phosphorus in fine-grained channel bed sediment in contrasting lowland agricultural catchments in the UK. Geoderma. 2009;151:141–149. [Google Scholar]
- Barmuta L.A., Watson A., Clarke A., Clapcott J.E. National Water Commission; Canberra: 2009. The Importance of Headwater Streams. Waterlines Report. (75 pp) [Google Scholar]
- Barrat J., Richomme C., Moinet M. The accidental release of exotic species from breeding colonies and zoological collections. Rev. Sci. Tech. 2010;29:113–122. doi: 10.20506/rst.29.1.1968. [DOI] [PubMed] [Google Scholar]
- Basarin B., Lukić T., Pavić D., Leščešen I., Wilby R.L. Trends and multi-annual variability of water temperatures in the River Danube, Serbia. Hydrol. Process. 2016;30:3315–3329. [Google Scholar]
- Bass J.A.B., Blust R., Clarke R.T., Corbin T.A., Davison W., de Schamphelaere K.A.C., Janssen C.R., Kalis E.J.J., Kelly M.G., Kneebone N.T., Lawlor A.J., Lofts S., Temminghoff E.J.M., Thacker S.A., Tipping E., Vincent C.D., Warnken K.W., Zhang H. Environment Agency; Almondsbury, UK: 2008. Environmental Quality Standards for Trace Metals in the Aquatic Environment. Science Report SC030194; p. 190. [Google Scholar]
- Battarbee R.W., Shilland E.M., Kernan M., Monteith D.T., Curtis C.J. Recovery of acidified surface waters from acidification in the United Kingdom after twenty years of chemical and biological monitoring (1988–2008) Ecol. Indic. 2014;37B:267–273. [Google Scholar]
- Battin T.J., Besemer K., Bengtsson M.M., Romani A.M., Packmann A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016;14:251–263. doi: 10.1038/nrmicro.2016.15. [DOI] [PubMed] [Google Scholar]
- Baxter C., Fausch V.K.D., Saunders W.C. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 2005;50:201–220. [Google Scholar]
- Beebee T.J.C. Amphibian conservation in Britain: a 40-year history. J. Herpetol. 2014;48:2–12. [Google Scholar]
- Beechie T.J., Sear D.A., Olden J.D., Pess G.R., Buffington J.M., Moir H., Roni P., Pollock M.M. Process-based principles for restoring river ecosystems. Bioscience. 2010;60:209–222. [Google Scholar]
- Belsky A.J., Matzke A., Uselman S. Survey of livestock influences on stream and riparian ecosystems in the western United States. J. Water Soil Conserv. 1999;54:419–431. [Google Scholar]
- Benstead J.P., Rosemond A.D., Cross W.F., Wallace J.B., Eggert S.L., Suberkropp K., Gulis V., Greenwood J.L., Tant C.J. Nutrient enrichment alters storage and fluxes of detritus in a headwater stream ecosystem. Ecology. 2009;90:2556–2566. doi: 10.1890/08-0862.1. [DOI] [PubMed] [Google Scholar]
- Bernal S., Lupon A., Ribot M., Sabater F., Marti E. Riparian and in-stream controls on nutrient concentrations and fluxes in a headwater forested stream. Biogeosciences. 2015;12:1941–1954. [Google Scholar]
- Bernhardt E.S., Palmer M.A. River restoration: the fuzzy logic of repairing reaches to reverse catchment scale degradation. Ecol. Appl. 2011;21:1926–1931. doi: 10.1890/10-1574.1. [DOI] [PubMed] [Google Scholar]
- Biggs J., Williams P., Whitfield M., Fox G., Nicolet P. Pond Action (2000). Proceedings of the Ponds Conference. Pond Action; Oxford: 2000. Factors affecting the nature conservation value of ponds: results of the National Pond Survey; p. 1998. [Google Scholar]
- Biggs J., Williams P., Whitfield M., Nicolet P., Weatherby A. 15 years of pond assessment in Britain: results and lessons learned from the work of Pond Conservation. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2005;15:693–714. [Google Scholar]
- Biggs J., Stoate S., Williams P., Brown C., Casey A., Davies S., Grijalvo Diego I., Hawczak A., Kizuka T., McGoff E., Szczur J. Freshwater Habitats Trust, Oxford, and Game and Wildlife Conservation Trust, Fordingbridge, UK. 2014. Water Friendly Farming. Results and practical implications of the first 3 years of the programme. (83 pp.) [Google Scholar]
- Biggs J., von Fumetti S., Kelly-Quinn M. The importance of small waterbodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia. 2017;793:3–39. [Google Scholar]
- Birkel C., Soulsby C., Ali G., Telzlaff D. Assessing the cumulative impacts of hydropower regulation on the flow characteristics of a large Atlantic salmon river system. River Res. Appl. 2014;30:456–475. [Google Scholar]
- Bloodworth J.W., Holman I.P., Burgess P.J., Gillman S., Frogbrook Z., Brown P. Developing a multi-pollutant conceptual framework for the selection and targeting of interventions in water industry catchment management schemes. J. Environ. Manag. 2015;161:153–162. doi: 10.1016/j.jenvman.2015.06.050. [DOI] [PubMed] [Google Scholar]
- Bradford R.B., Marsh T.J. Defining a network of benchmark catchments for the UK. P. I. Civ. Eng. Water. 2003;156:109–116. [Google Scholar]
- Bradley D.C., Ormerod S.J. Community persistence among stream invertebrates tracks the North Atlantic Oscillation. J. Anim. Ecol. 2001;70:987–996. [Google Scholar]
- Bradley C., Byrne C., Craig M., Free G., Gallagher T., Kennedy B., Little R., Lucey J., Mannix A., McCreesh P., McDermott G., McGarrigle M., Ní Longphuirt S., O'Boyle S., Plant C., Tierney D., Trodd W., Webster P., Wilkes R., Wynne C. Environmental Protection Agency; Wexford: 2015. Water Quality in Ireland 2010–2012. (173 pp.) [Google Scholar]
- Brooker M.P. The ecological effects of channelization. Geogr. J. 1985;151:63–69. [Google Scholar]
- Brown C.D., Van Beinum W. Pesticide transport via sub-surface drains in Europe. Environ. Pollut. 2009;157:3314–3324. doi: 10.1016/j.envpol.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Brown C.D., Turner N.L., Hollis J.M., Bellamy P.H., Biggs J., Williams P.J., Arnold D.J., Pepper T., Maund S.J. Morphological and physico-chemical properties of British aquatic habitats potentially exposed to pesticides. Agric. Ecosyst. Environ. 2006;113:307–319. [Google Scholar]
- Brown C.D., Holmes C., Williams R., Beulke S., van Beinum W., Pemberton E., Wells C. How does crop type influence risk from pesticides to the aquatic environment? Environ. Toxicol. Chem. 2007;26:1818–1826. doi: 10.1897/06-498R.1. [DOI] [PubMed] [Google Scholar]
- Callanan M., Baars J.-R., Kelly-Quinn M. Critical influence of seasonal sampling on the ecological quality assessment of small headwater streams. Hydrobiologia. 2008;610:245–255. [Google Scholar]
- Callanan M., Baars J.-R., Kelly-Quinn M. Macroinvertebrate communities of Irish headwater streams: contribution to catchment biodiversity. Biol. Environ. Proc. R. Ir. Acad. 2014;114B:143–162. [Google Scholar]
- Carey P.D., Wallis S., Emmett B.A., Maskell L.C., Murphy J., Norton L.R., Simpson I.C., Smart S.M. NERC/Centre for Ecology & Hydrology; 2008. Countryside Survey: UK headline Messages From 2007. (30 pp.) [Google Scholar]
- Centre for Ecology and Hydrology Glastir Monitoring and Evaluation Programme. 2017. https://gmep.wales/
- Céréghino R., Biggs J., Oertli B., Declerck S. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia. 2008;597:1–6. [Google Scholar]
- Céréghino R., Boix D., Cauchie H.M., Martens K., Oertli B. The ecological role of ponds in a changing world. Hydrobiologia. 2014;723:1–6. [Google Scholar]
- Champkin J.D., Copp G.H., Sayer C.D., Clilverd H.M., George L., Vilizzi L., Godard M.J., Clarke J., Walker A.M. Responses of fishes and lampreys to the re-creation of meanders in a small English chalk stream. River Res. Appl. 2018;34:34–43. [Google Scholar]
- Chanin P. The diet of the otter and its relations with the feral mink in two areas of southwest England. Acta Theriol. 1981;26:83–95. [Google Scholar]
- Chesterton C. Natural England Research Report NERR030. 2009. Environmental impacts of land management. (175 pp.) [Google Scholar]
- Clarke A., MacNally R., Bond N., Lake P.S. Macroinvertebrate diversity in headwater streams: a review. Freshw. Biol. 2008;53:1707–1721. [Google Scholar]
- Clarke J., Kelly-Quinn M., Blacklocke S., Bruen M. The effect of windrowing on physico-chemical water quality in Ireland. Sci. Total Environ. 2015;514:155–169. doi: 10.1016/j.scitotenv.2015.01.107. [DOI] [PubMed] [Google Scholar]
- Clews E., Vaughan I.P., Ormerod S.J. Evaluating the effects of riparian restoration on a temperate river-system using standardized habitat survey. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2010;20(S1):S96–S104. [Google Scholar]
- Collins A.L., Anthony S.G. Assessing the likelihood of catchments across England and Wales meeting ‘good ecological status’ due to sediment contributions from agricultural sources. Environ. Sci. Pol. 2008;11:163–170. [Google Scholar]
- Collins A.L., McGonigle D.F. Monitoring and modelling diffuse pollution from agriculture for policy support: UK and European experience. Environ. Sci. Pol. 2008;11:97–101. [Google Scholar]
- Collins A.L., Walling D.E. The storage and provenance of fine sediment on the channel bed of two contrasting lowland permeable catchments, UK. River Res. Appl. 2007;23:429–450. [Google Scholar]
- Collins A.L., Walling D.E. Fine-grained bed sediment storage within the main channel systems of the Frome and Piddle catchments, Dorset, UK. Hydrol. Process. 2007;21:1448–1459. [Google Scholar]
- Collins A.L., Walling D.E., Leeks G.J.L. Sediment sources in the Upper Severn catchment: a fingerprinting approach. Hydrol. Earth Syst. Sci. 1997;1:509–521. [Google Scholar]
- Collins A.L., Anthony S.G., Hawley J., Turner T. Predicting potential change in agricultural sediment inputs to rivers across England and Wales by 2015. Mar. Freshw. Res. 2009;60:626–637. [Google Scholar]
- Collins A.L., Anthony S.G., Hawley J., Turner T. The potential impact of projected change in farming by 2015 on the importance of the agricultural sector as a sediment source in England and Wales. Catena. 2009;79:243–250. [Google Scholar]
- Collins A.L., Walling D.E., McMellin G.K., Zhang Y., Gray J., McGonigle D., Cherrington R. A preliminary investigation of the efficacy of riparian fencing schemes for reducing contributions from eroding channel banks to the siltation of salmonid spawning gravels across the south west UK. J. Environ. Manag. 2010;91:1341–1349. doi: 10.1016/j.jenvman.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Collins A.L., Zhang Y., Walling D.E., Black K. Sediment Dynamics for a Changing Future. International Association of Hydrological Sciences Publication No. 337; Wallingford, UK: 2010. Apportioning sediment sources in a grassland dominated agricultural catchment in the UK using a new tracing framework; pp. 68–75. [Google Scholar]
- Collins A.L., Zhang Y.S., Duethmann D., Walling D.E., Black K.S. Using a novel tracing-tracking framework to source fine-grained sediment loss to watercourses at sub-catchment scale. Hydrol. Process. 2013;27:959–974. [Google Scholar]
- Collins A.L., Zhang Y.S., Winter M., Inman A., Jones J.I., Johnes P.J., Cleasby W., Vrain E., Lovett A., Noble L. Tackling agricultural diffuse pollution: what might uptake of farmer-preferred measures deliver for emissions to water and air? Sci. Total Environ. 2016;547:269–281. doi: 10.1016/j.scitotenv.2015.12.130. [DOI] [PubMed] [Google Scholar]
- Collins A.L., Newell Price J.P., Zhang Y., Gooday R., Naden P.S., Skirvin D. Assessing the potential impacts of a revised set of ‘basic’ on-farm nutrient and sediment ‘basic’ control measures for reducing agricultural diffuse pollution across England. Sci. Total Environ. 2018;621:1499–1511. doi: 10.1016/j.scitotenv.2017.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N.H., Biggs J., Corfield A., Hodson M.J., Walker D., Whitfield M., Williams P.J. Temporary and permanent ponds: an assessment of the effects of drying out on the conservation value of aquatic macroinvertebrate communities. Biol. Conserv. 1995:125–133. [Google Scholar]
- Copp G.H. The habitat diversity and fish reproductive function of floodplain ecosystems. Environ. Biol. Fish. 1989;26:1–26. [Google Scholar]
- Copp G.H. An empirical model for predicting the microhabitat of 0+ juveniles in lowland streams. Oecologia. 1992;91:338–345. doi: 10.1007/BF00317621. [DOI] [PubMed] [Google Scholar]
- Copp G.H., Wesley K.J., Vilizzi L. Pathways of ornamental and aquarium fish introductions into urban ponds of Epping Forest (London, England): the human vector. J. Appl. Ichthyol. 2005;21:263–274. [Google Scholar]
- Copp G.H., Stakėnas S., Davison P.I. The incidence of non-native fishes in water courses: example of the United Kingdom. Aquat. Invasions. 2006;1:72–75. [Google Scholar]
- Copp G.H., Warrington S., Wesley K.J. Management of an ornamental pond as a conservation site for a threatened native fish species, crucian carp Carassius carassius. Hydrobiologia. 2008;597:149–155. [Google Scholar]
- Copp G.H., Britton J.R., Guo Z., Edmonds-Brown V., Pegg J., Vilizzi L., Davison P.I. Trophic consequences of non-native pumpkinseed Lepomis gibbosus for native fishes on pond fishes. Biol. Invasions. 2017;19:25–41. [Google Scholar]
- Copp G.H., Godard M.J., Vilizzi L., Ellis A., Riley W.D. Predation by invasive signal crayfish on early-life stages of European barbel may be limited. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2017;27:1056–1060. [Google Scholar]
- Cowx I.G., Noble R.A., Nunn A.D., Bolland J., Walton S., Peirson G., Harvey J.P. Flow requirements of non-salmonids. Fish. Manag. Ecol. 2012;19:548–556. [Google Scholar]
- Crane M., Delaney P., Mainstone C., Clarke S. Measurement by in-situ bioassay of water quality in an agricultural catchment. Water Res. 1995;29:2441–2448. [Google Scholar]
- Crisp D.T. Blackwell Science; Oxford: 2000. Trout and Salmon: Ecology, Conservation and Rehabilitation. [Google Scholar]
- Dadds N., Bell S. Plantlife Royal Botanic Garden Edinburgh Scottish Natural Heritage, Scottish Environment Protection Agency, Scottish Water; 2008. Invasive Non-native Plants Associated With Fresh Waters: A Guide to Their Identification. [Google Scholar]
- Davies C.E., Shelley J., Harding P., McLean I., Gardiner R., Peirson G. Harley Books. Colchester; UK: 2004. Freshwater fishes in Britain: The species and their distribution. [Google Scholar]
- Davies B.R., Biggs J., Williams P., Whitfield M., Nicolet P., Sear D., Bray S., Maund S. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agric. Ecosyst. Environ. 2008;25:1–8. [Google Scholar]
- Davies S.R., Sayer C.D., Greaves H., Siriwardena G.M., Axmacher J.C. A new role for pond management in farmland bird conservation. Agric. Ecosyst. Environ. 2016;233:179–191. [Google Scholar]
- Davy-Bowker J., Clarke R., Corbin T., Vincent H., Pretty J., Hawczak A., Blackburn J., Murphy J., Jones I. SNIFFER project WFD72C; Edinburgh, Scotland, UK: 2008. River Invertebrate Classification Tool. Scotland and Northern Ireland Forum for Environmental Research. (268 pp.) [Google Scholar]
- DECC . Department of Energy and Climate Change; London: 2014. Community Energy Strategy: Full Report. (108 pp.) [Google Scholar]
- Dennis I.A., Coulthard T.J., Brewer P., Macklin M.G. The role of floodplains in attenuating contaminated sediment fluxes in formerly mined drainage basins. Earth Surf. Process. Landf. 2009;34:453–466. [Google Scholar]
- Di Matteo L., Dragoni W., Maccari D., Piacentini S.M. Climate change, water supply and environmental problems of headwaters: the paradigmatic case of the Tiber, Savio and Marecchia rivers (Central Italy) Sci. Total Environ. 2017;598:733–748. doi: 10.1016/j.scitotenv.2017.04.153. [DOI] [PubMed] [Google Scholar]
- Dixon S.J., Sear D.A., Odoni N., Sykes T.R., Lane S.N. The effects of river restoration on catchment scale flood risk and flood hydrology. Earth Surf. Process. Landf. 2016;41:997–1008. [Google Scholar]
- Dodds W.K., Oakes R.M. Headwater influences on downstream water quality. Environ. Manag. 2008;41:367–377. doi: 10.1007/s00267-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody D.G., Archbold M., Foy R.N., Flynn R. Approaches to the implementation of the Water Framework Directive: targeting mitigation measures at critical source areas of diffuse phosphorus in Irish catchments. J. Environ. Manag. 2012;93:225–234. doi: 10.1016/j.jenvman.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Dunbar M., Murphy J., Clarke R., Baker R., Davies C., Scarlett P. 2010. Countryside Survey: Headwater Streams Report From 2007. CS Technical Report No. 8/07 NERC/Centre for Ecology & Hydrology (CEH Project Number: C03259) (67 pp.) [Google Scholar]
- Durance I., Ormerod S.J. Climate change effects on upland stream macroinvertebrates over a 25-year period. Glob. Chang. Biol. 2007;13:942–957. [Google Scholar]
- Earl J.E., Semlitsch R.D. Spatial subsidies, trophic state and community structure: examining the effects of leaf litter input on ponds. Ecosystems. 2013;16:639–665. [Google Scholar]
- EC (European Commission) European Commission; Brussels: 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23rd October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Official Journal of the European Communities, L327/1. [Google Scholar]
- EC (European Commission) Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities. 2002;L206:7–50. [Google Scholar]
- EC (European Commission) 2015. EU Agriculture Spending Focused on Results. (Brussels 8 pp.) [Google Scholar]
- Edmonds N.J., Riley W.D., Maxwell D.L. Predation by Pacifastacus leniusculus on the intra-gravel embryos and emerging fry of Salmo salar. Fish. Manag. Ecol. 2011;18:521–524. [Google Scholar]
- Edwards R.W., Gee A.S., Stoner J.H. Kluwer Academic Publishers; Dordrecht: 1990. Acid Waters in Wales. -Monographiae Biologicae, BD.66. [Google Scholar]
- Elliott J.M., Hurley M.A., Maberly S.C. The emergence period of sea trout fry in a Lake District stream correlates with the North Atlantic Oscillation. J. Fish Biol. 2000;56:208–210. [Google Scholar]
- European Court of Auditors . 2011. Is Agri-environment Support Well Designed and Managed? Special Report No 7/2011 European Court of Auditors, Luxembourg. (82 pp.) [Google Scholar]
- Evans E.C., McGregor G., Petts G.E. River energy budgets with special reference to river bed processes. Hydrol. Process. 1998;12:575–595. [Google Scholar]
- Everall N.C., Farmer A., Heath A.F., Jacklin T.E., Wilby R.L. Ecological benefits of creating messy rivers. Area. 2012;44:470–478. [Google Scholar]
- Everall N.C., Johnson M.F., Wilby R.L., Bennett C.J. Detecting phenology change in the mayfly Ephemera danica: responses to spatial and temporal water temperature variations. Ecol. Entomol. 2015;40:95–105. [Google Scholar]
- Ewald N., Quinlan L., Case P., Dunn F., Heathcote A., Shaw H., Worker H., Williams P., Biggs J. Freshwater Habitats Trust; Oxford: 2018. People, Ponds and Water Summary Report 2015-2018. (28 pp.) [Google Scholar]
- Feeley H.B., Davis S., Bruen M., Blacklocke S., Kelly-Quinn M. The impact of a catastrophic storm event on benthic macroinvertebrate communities in upland headwater streams and potential implications for ecological diversity and assessment of ecological status. J. Limnol. 2012;71:309–318. [Google Scholar]
- Feeley H.B., Bruen M., Blacklocke S., Kelly-Quinn M. A regional examination of episodic acidification response to reduced acidic deposition and the influence of plantation forests in Irish headwater streams. Sci. Total Environ. 2013;443:173–183. doi: 10.1016/j.scitotenv.2012.10.074. [DOI] [PubMed] [Google Scholar]
- Feld C.K., Rosário Pereira Fernandes M., Fereira M.T., Hering D., Ormerod S.J., Venohr M., Gutiérrez-Cánovas C. Evaluating riparian solutions to multiple stressor problems in river ecosystems—a conceptual study. Water Res. 2018;139:381–394. doi: 10.1016/j.watres.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Fernández J.M., Selma M.A.E., Aymerich F.R., Sáez M.T.P., Fructuoso M.F.C. Aquatic birds as bioindicators of trophic changes and ecosystem deterioration in the Mar Menor lagoon (SE Spain) Hydrobiologia. 2005;550:221–235. [Google Scholar]
- Finn D.S., Bonada N., Murria C., Hughes J.M. Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. J. N. Am. Benthol. Soc. 2011;30:963–980. [Google Scholar]
- Florencio M., Diaz-Paniagua C., Gomez-Rodriguez C., Serrano L. Biodiversity patterns in a macroinvertebrate community of a temporary pond network. Insect Conserv. Divers. 2014;7:4–21. [Google Scholar]
- Forestry Commission . first ed. HMSO; London: 1988. Forests and Water Guidelines. [Google Scholar]
- Forestry Commission . third ed. HMSO; London: 1993. Forests and Water Guidelines. [Google Scholar]
- Forestry Commission . UK Forestry Standard Guidelines. Forestry Commission; Edinburgh: 2011. Forest and Water Guidelines. [Google Scholar]
- Foster G.N. Joint Nature Conservation Committee; Peterborough, UK: 2010. A review of the scarce and threatened Coleoptera of Great Britain Part (3): Water beetles of Great Britain. Species Status 1. [Google Scholar]
- Foster G.N., Nelson B., O'Connor Á. 2009. A Regional Red List for Water Beetles in Ireland. Report to National Parks and Wildlife. Dublin. [Google Scholar]
- Francis I.S., Taylor J.A. The effect of forestry drainage operations on upland sediment yields: a study of two peat-covered catchments. Earth Surf. Process. Landf. 1989;14:73–83. [Google Scholar]
- Friberg N., Angelopoulos N.V., Buijse A.D., Cowx I.G., Kail J., Moe T.F., Moir H., O'Hare M.T., Verdonschot P.F.M., Wolter C. Effective river restoration in the 21st century: from trial and error to novel evidence-based approaches. Adv. Ecol. Res. 2016;55:535–611. [Google Scholar]
- Fukui D., Murakami M., Nakano S., Aoi T. Effect of emergent aquatic insects on bat foraging in a riparian forest. J. Anim. Ecol. 2006;75:1252–1258. doi: 10.1111/j.1365-2656.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- Furse M.T. National Rivers Authority; Bristol, UK: 1995. The Faunal Richness of Headwater Streams: Stage 4—Development of a Conservation Strategy. NRA R&D Note 455. [Google Scholar]
- Furse M.T. The application of RIVPACS procedures in headwater streams – an extensive and important resource. In: Wright J.F., Sutcliffe D.W., Furse M.T., editors. Assessing the Biological Quality of Fresh Waters. RIVPACS and Other Techniques. Freshwater Biological Association; Ambleside, UK: 2000. pp. 79–91. [Google Scholar]
- Gomi T., Sidle R.C., Richardson J.S. Understanding processes and downstream linkages of headwater system. Bioscience. 2002;52:905–916. [Google Scholar]
- Gonzalez-Pinzon R., Haggerty R., Argerich A. Quantifying spatial differences in metabolism in headwater streams. Freshw. Sci. 2014;33:798–811. [Google Scholar]
- Gooday R.D., Anthony S.G., Chadwick D.R., Newell-Price P., Harris D., Duethman D., Fish R., Collins A.L., Winter M. Modelling the cost effectiveness of mitigation measures for multiple pollutants at the farm scale. Sci. Total Environ. 2014;468–469:1198–1209. doi: 10.1016/j.scitotenv.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Gooseff M.N., Hall R.O., Tank J.L. Relating transient storage to channel complexity in streams of varying land use in Jackson Hole, Wyoming. Water Resour. Res. 2007;43 (W01417) [Google Scholar]
- Hall R.O., Bernhardt E.S., Likens G.E. Relating nutrient uptake with transient storage in forested mountain streams. Limnol. Oceanogr. 2002;47:255–265. [Google Scholar]
- Halliday S.J., Skeffington R.A., Wade A.J., Bowes M.J., Read D.S., Jarvie H.P., Loewenthal M. Riparian shading controls instream spring phytoplankton and benthic algal growth. Environ. Sci.: Processes Impacts. 2016;18:677–689. doi: 10.1039/c6em00179c. [DOI] [PubMed] [Google Scholar]
- Hammond D., Pryce A.R. Environment Agency; UK: 2007. Climate Change Impacts and Water Temperature. Science Report: SC060017/SR. (101 pp.) [Google Scholar]
- Hannaford J. Climate-driven changes in UK river flows: a review of the evidence. Prog. Phys. Geogr. 2015;39:29–48. [Google Scholar]
- Hannah D.M., Garner G. River water temperature in the United Kingdom: changes over the 20th century and possible changes over the 21st century. Prog. Phys. Geogr. 2015;39:68–92. [Google Scholar]
- Hannah D.M., Malcolm I.A., Soulsby C., Youngson A.F. A comparison of forest and moorland stream microclimate, heat exchanges and thermal dynamics. Hydrol. Process. 2008;22:919–940. [Google Scholar]
- Harrigan S., Murphy C., Hall J., Wilby R.L., Sweeney J. Attribution of detected changes in streamflow using multiple working hypotheses. Hydrol. Earth Syst. Sci. 2014;18:1935–1952. [Google Scholar]
- Harrod C. Climate Change and Fisheries. In: Craig J.J., editor. Freshwater Fisheries Ecology. Wiley Blackwell; Oxford: 2016. pp. 641–694. [Google Scholar]
- Harrod T.R., McHugh M., Appleby P.G., Evans R., George D.G., Haworth E.Y., Hewitt D., Hornung M., Housen G., Leeks G., Morgan R.P.C., Tipping E. MAFF; London: 2000. Research on the Quantification and Causes of Upland Erosion. Final Report to MAFF. MAFF Project SP0402. [Google Scholar]
- Harvey A.M. Effective timescales of coupling within fluvial systems. Geomorphology. 2002;44:175–201. [Google Scholar]
- Haygarth P.M., Condron L.M., Heathwaite A.L., Turner B.L., Harris G.P. The phosphorus transfer continuum: linking source to impact with an interdisciplinary and multi-scaled approach. Sci. Total Environ. 2005;344:5–14. doi: 10.1016/j.scitotenv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hayhow D.B., Burns F., Eaton M.A., Al Fulaij N., August T.A., Babey L., Bacon L., Bingham C., Boswell J., Boughey K.L., Brereton T., Brookman E., Brooks D.R., Bullock D.J., Burke O., Collis M., Corbet L., Cornish N., De Massimi S., Densham J., Dunn E., Elliott S., Gent T., Godber J., Hamilton S., Havery S., Hawkins S., Henney J., Holmes K., Hutchinson N., Isaac N.J.B., Johns D., Macadam C.R., Mathews F., Nicolet P., Noble D.G., Outhwaite C.L., Powney G.D., Richardson P., Roy D.B., Sims D., Smart S., Stevenson K., Stroud R.A., Walker K.J., Webb J.R., Webb T.J., Wynde R., Gregory R.D. The State of Nature Partnership. 2016. State of nature 2016. (85 pp.) [Google Scholar]
- Helliwell R.C., Aherne J., Nisbet T.R., MacDougall G., Broadmeadow S., Sample J., Jackson-Blake L., Doughty R. Modelling the long-term response of stream water chemistry to forestry in Galloway, Southwest Scotland. Ecol. Indic. 2014;37:396–411. [Google Scholar]
- Herb W.R., Janke B., Mohseni O., Stefan H.G. Thermal pollution of streams by runoff from paved surfaces. Hydrol. Process. 2008;22:987–999. [Google Scholar]
- Hildrew A.G. Sustained research on stream communities: a model system and the comparative approach. Adv. Ecol. Res. 2009;41:175–312. [Google Scholar]
- Hoellein T.J., Tank J.L., Rosi-Marshall E.J., Entrekin S.A., Lamberti G.A. Controls on spatial and temporal variation of nutrient uptake in three Michigan headwater streams. Limnol. Oceanogr. 2007;52:1964–1977. [Google Scholar]
- Holgerson M.A., Raymond P.A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016;9:222–226. [Google Scholar]
- Holmes N.T.H., Newman J.R., Chadd S., Rouen K.J., Saint L., Dawson F.H. Environment Agency of England and Wales; Bristol, UK: 1999. Mean Trophic Rank: A Users Manual. R&D Technical Report E38. [Google Scholar]
- Hornbach D.J., Beckela R., Hustada E.N., McAdama D.P., Roena I.M., Warehama A.J. The influence of riparian vegetation and season on stream metabolism of Valley Creek, Minnesota. J. Freshw. Ecol. 2015;30:569–588. [Google Scholar]
- Horton A.A., Svendsen C., Williams R.J., Spurgeon D.J., Lahive E. Large microplastic particles in sediments of tributaries of the river Thames, UK - abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017;114:218–226. doi: 10.1016/j.marpolbul.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- House W.A., Denison F.H. Phosphorus dynamics in a lowland river. Water Res. 1998;32:1819–1830. [Google Scholar]
- Hurley R., Woodward J., Rothwell J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018 [Google Scholar]
- IBIS (Integrated Aquatic Resource Management Between Ireland, N Ireland and Scotland)., AST (Atlantic Salmon Trust) Report of the workshop held at Carlingford, Co. Louth, Ireland, 27–28 November 2012. 2012. Small streams: Contribution to populations of trout and sea trout.http://ibis-eu-know.weebly.com/nov-2012-small-streams--carlingford.html [Google Scholar]
- IFM (Institute of Fisheries Management) Managing Small Streams for Fish in a Changing Environment. Report of a Workshop Held in York, 6 and 7 March 2013. 2013. https://ifm.org.uk/managing-small-streams-for-fish-in-a-changing-climate/
- Inman A., Winter M., Wheeler R., Vain E., Lovett A., Collins A.L., Jones J.I., Johnes P., Cleasby W. An exploration of individual, social and material factors influencing water pollution mitigation behaviours within the farming community. Land Use Policy. 2018;70:16–26. [Google Scholar]
- Jarvie H.P., Neal C., Warwick A., White J., Neal M., Wickham H.D., Hill L.K., Andrews M.C. Phosphorus uptake into algal biofilms in a lowland chalk river. Sci. Total Environ. 2002;282:353–373. doi: 10.1016/s0048-9697(01)00924-x. [DOI] [PubMed] [Google Scholar]
- Jarvie H.P., Jurgens M.D., Williams R.J., Neal C., Davies J.J.L., Barrett C., White J. Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye. J. Hydrol. 2005;304:51–74. [Google Scholar]
- Jarvie H.P., Sharpley A.N., Withers P.J.A., Scott J.T., Haggard B.E., Neal C. Phosphorus mitigation to control river eutrophication: murky waters, inconvenient truths, and “postnormal” science. J. Environ. Qual. 2013;42:295–304. doi: 10.2134/jeq2012.0085. [DOI] [PubMed] [Google Scholar]
- Jarvie H.P., Smith D.R., Norton L.R., Edwards F., Bowes M.J., King S.M., Scarlett P., Davies S., Dils R., Bachiller-Jareno N. Phosphorus and nitrogen limitation and impairment of headwater streams relative to rivers in Great Britain: a national perspective on eutrophication. Sci. Total Environ. 2018;621:849–862. doi: 10.1016/j.scitotenv.2017.11.128. [DOI] [PubMed] [Google Scholar]
- Jeffries M.J. Ponds and the importance of their history: an audit of pond numbers, turnover and the relationship between the origins of ponds and their contemporary plant communities. Hydrobiologia. 2012;689:11–21. [Google Scholar]
- Johnson M.F., Wilby R.L. Seeing the landscape from the trees: metrics to guide riparian shade management in river catchments. Water Resour. Res. 2015;51:3754–3767. [Google Scholar]
- Johnson M.F., Wilby R.L., Toone J.A. Inferring air-water temperature relationships from river and catchment properties. Hydrol. Process. 2014;28:2912–2928. [Google Scholar]
- Jones J.I., Collins A.L., Naden P.S., Sear D.A. The relationship between fine sediment and macrophytes in rivers. River Res. Appl. 2012;28:1006–1018. [Google Scholar]
- Jones J.I., Murphy J.F., Collins A.L., Sear D.A., Naden P.S., Armitage P.D. The impact of fine sediment on macro-invertebrates. River Res. Appl. 2012;28:1055–1071. [Google Scholar]
- Jones J.I., Duerdoth C.P., Collins A.L., Naden P.S., Sear D.A. Interactions between diatoms and fine sediment. Hydrol. Process. 2014;28:1226–1237. [Google Scholar]
- Jones J.I., Douthwright T.A., Arnold A., Duerdoth C.P., Murphy J.F., Edwards F.K., Pretty J.L. Diatoms as indicators of fine sediment stress. Ecohydrology. 2017;10(5) [Google Scholar]
- Jones J.I., Spencer K., Rainbow P.S., Collins A.L., Murphy J.F., Arnold A., Duerdoth C.P., Pretty J.L., Smith B., Fitzherbert M., O'Shea F.T., Day M.C., Groves S., Zhang Y., Clarke A., Stopps J., McMillan S., Moorhouse A., Aguilera V., Edwards P., Parsonage F., Potter H., Whitehouse P. 2017. The Ecological Impacts of Contaminated Sediment From Abandoned Metal Mines. Final Report Defra Project WT0970. [Google Scholar]
- Jones J.I., Murphy J.F., Anthony S.G., Arnold A., Blackburn J.H., Duerdoth C.P., Hawczak A., Hughes G.O., Pretty J.L., Scarlett P.D., Gooday R.D., Zhang Y.S., Fawcett L.E., Simpson D., Turner A.W.B., Naden P.S., Skates J. Do agri-environment schemes result in improved water quality? J. Appl. Ecol. 2017;54:537–546. [Google Scholar]
- Jones J.I., Murphy J.F., Roy H., Harrower C., Kratina P., Peyton J., Pretty J.L., Rorke S. Environment Agency; UK: 2018. Linking the Presence of Invasive Alien Species to Measures of Ecological Quality. Project Number 22020. [Google Scholar]
- Juggins S., Kelly M., Allott T., Kelly-Quinn M., Monteith D. A water framework directive-compatible metric for assessing acidification in UK and Irish rivers using diatoms. Sci. Total Environ. 2016;568:671–678. doi: 10.1016/j.scitotenv.2016.02.163. [DOI] [PubMed] [Google Scholar]
- Kavanagh J.A., Harrison S.S.C. The contribution of a drainage network to the spatial and temporal patterns of macroinvertebrate diversity across and agricultural headwater catchment. Biol. Environ. Proc. R. Ir. Acad. 2014;114:181–197. [Google Scholar]
- Kelly M.G., Juggins S., Guthrie R., Pritchard S., Jamieson J., Rippey B., Hirst H., Yallop M. Assessment of ecological status in U.K. rivers using diatoms. Freshw. Biol. 2008;53:403–422. [Google Scholar]
- Kelly-Quinn M., Bruen M., Harrison S., Healy M., Clarke J., Drinan T., Feeley H., Finnegan J., Graham C., Regan J., Blacklocke S. Environmental Protection Agency; Wexford, Ireland: 2016. Assessment of the Impacts of Forest Operations on the Ecological Quality of Water (HYDROFOR), Synthesis Report. [Google Scholar]
- Kemp P.S. Meta-analyses, metrics and motivation: mixed messages in the fish passage debate. River Res. Appl. 2016;32:2116–2124. [Google Scholar]
- Kernan M., Battarbee R.W., Curtis C.J., Monteith D.T., Shilland E.M. UK Acid Waters Monitoring Network 20-year Interpretative Report. Report to Defra. Environmental Change Research Centre, University College London; 2010. Recovery of lakes and streams in the UK from the effects of acid rain. (465 pp.) [Google Scholar]
- Kowalik R.A., Ormerod S.J. Intensive sampling and transplantation experiments reveal continued effects of episodic acidification on sensitive stream invertebrates. Freshw. Biol. 2006;51:180–191. [Google Scholar]
- Kowalik R.A., Copper D.M., Evans C.D., Ormerod S.J. Acidic episodes retard the biological recovery of upland British streams from chronic acidification. Glob. Chang. Biol. 2007;13:2439–2452. [Google Scholar]
- Langan S.J., Johnston L., Donaghy M.J., Youngson A.F., Hay D.W., Soulsby C. Variation in river water temperatures in an upland stream over a 30-year period. Sci. Total Environ. 2001;265:195–207. doi: 10.1016/s0048-9697(00)00659-8. [DOI] [PubMed] [Google Scholar]
- Lapworth D.J., Gooddy D.C., Jarvie H.P. Understanding phosphorus mobility and bioavailability in the hyporheic zone of a chalk stream. Water Air Soil Pollut. 2011;218:213–226. [Google Scholar]
- Larsen S., Ormerod S.J. Anthropogenic modification disrupts species co-occurrence in stream invertebrates. Glob. Chang. Biol. 2014;20:51–60. doi: 10.1111/gcb.12355. [DOI] [PubMed] [Google Scholar]
- Larsen S., Sorace A., Mancini L. Riparian bird communities as indicators of human impacts along Mediterranean streams. Environ. Manag. 2010;45:261–273. doi: 10.1007/s00267-009-9419-0. [DOI] [PubMed] [Google Scholar]
- Lassaletta L., Garcia-Gomez H., Gimeno B.S., Rovira J.V. Headwater streams: neglected ecosystems in the EU Water Framework Directive. Implications for nitrogen pollution control. Environ. Sci. Pol. 2010;13:423–433. [Google Scholar]
- Lawler D., Rymszewicz A., Conroy L., O'Sullivan J.J., Bruen M., Turner J., Kelly-Quinn M. Environmental Protection Agency; Wexford, Ireland: 2017. SILTFLUX Literature Review (2010-W-LS-4). EPA Report No. 176. (112 pp.) [Google Scholar]
- Lawton J.H., Brotherton P.N.M., Brown V.K., Elphick C., Fitter A.H., Forshaw J., Haddow R.W., Hilborne S., Leafe R.N., Mace G.M., Southgate M.P., Sutherland W.J., Tew T.E., Varley J., Wynne G.R. Defra; London, UK: 2010. Making Space for Nature: A Review of England's Wildlife Sites and Ecological Network. [Google Scholar]
- Leeks G.J.L. Impact of plantation forestry on sediment transport processes. In: Billi P., Hey R.D., Thorne C.R., Tacconi P., editors. Dynamics of Gravel-bed Rivers. Wiley; Chichester, UK: 1992. pp. 651–673. [Google Scholar]
- Leeks G.J.L., Roberts G. The effects of forestry on upland streams—with special reference to water quality and sediment transport. In: Good J.E.G., editor. Environmental Aspects of Plantation Forestry in Wales. Institute of Terrestrial Ecology; Grange-over-Sands, UK: 1987. pp. 9–24. [Google Scholar]
- Lemunyon J.L., Gilbert R.G. The concept and need for a phosphorus assessment tool. J. Prod. Agric. 1993;6:483–486. [Google Scholar]
- Liess M., Beketov M. Traits and stress: keys to identify community effects of low levels of toxicants in test systems. Ecotoxicology. 2011;20:1328–1340. doi: 10.1007/s10646-011-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liess M., von der Ohe P.C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005;24:954–965. doi: 10.1897/03-652.1. [DOI] [PubMed] [Google Scholar]
- Lowrance R., Altier L.S., Newbold J.D., Schnabel R.R., Groffman P.M., Denver J.M., Correll D.L., Gilliam J.W., Robinson J.L., Brinsfield R.B., Staver K.W., Lucas W., Todd A.H. Water quality functions of riparian forest buffers in Chesapeake Bay watersheds. Environ. Manag. 1997;21:687–712. doi: 10.1007/s002679900060. [DOI] [PubMed] [Google Scholar]
- Lundy M., Montgomery I. Summer habitat associations of bats between riparian landscapes and within riparian areas. Eur. J. Wildl. Res. 2010;56:385–394. [Google Scholar]
- Lyons J., Thimble S.W., Paine L.K. Grass versus trees: managing riparian areas to benefit streams of central North America. J. Am. Water Resour. Assoc. 2000;36:919–930. [Google Scholar]
- MacAdam C. 2015. A review of the stoneflies (Plecoptera) of Great Britain Species Status No.20. Natural England, Commissioned Report NECR174. [Google Scholar]
- MacDonald L.H., Coe D. Influence of headwater streams on downstream reaches in forested areas. For. Sci. 2007;53:148–168. [Google Scholar]
- MacDonald D.W., Harrington L.A. The American mink: the triumph and tragedy of adaptation out of context. N. Z. J. Zool. 2003;30:421–441. [Google Scholar]
- Mainstone C., Hall R., Diack I. Natural England Research Reports, Number 064. 2016. A narrative for conserving freshwater and wetland habitats in England. (117 pp.) [Google Scholar]
- Malcolm I.A., Bacon P.J., Middlemas S.J., Fryer R.J., Shilland E.M., Collen P. Relationships between hydrochemistry and the presence of juvenile brown trout (Salmo trutta) in headwater streams recovering from acidification. Ecol. Indic. 2014;37:351–364. [Google Scholar]
- Maltby L., Hills L. Spray drift of pesticides and stream macroinvertebrates: experimental evidence of impacts and effectiveness of mitigation measures. Environ. Pollut. 2008;156:1112–1120. doi: 10.1016/j.envpol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Maltby L., Forrow D.M., Boxall A.B.A., Calow P., Betton C.I. The effects of motorway runoff on freshwater ecosystems. 1. Field study. Environ. Toxicol. Chem. 1995;14:1079–1092. [Google Scholar]
- Maltby E., Ormerod S., Acreman M., Dunbar M., Jenkins A., Maberly S., Newman J., Blackwell M., Ward R. UNEP-WCMC; Cambridge, UK: 2011. Freshwaters: Openwaters, Wetlands and Floodplains, in: UK National Ecosystem Assessment: Understanding nature's Value to Society. Technical Report; pp. 295–360. [Google Scholar]
- Mant R.C., Jones D.L., Reynolds B., Ormerod S.J., Pullin A.S. A systematic review of the effectiveness of liming to mitigate impacts of river acidification on fish and macro-invertebrates. Environ. Pollut. 2013;179:285–293. doi: 10.1016/j.envpol.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Marc V., Robinson M. The long-term water balance (1972–2004) of upland forestry and grassland at Plynlimon, mid-Wales. Hydrol. Earth Syst. Sci. 2007;11:44–60. [Google Scholar]
- Marcarelli A.M., Baxter C.V., Mineau M.M., Hall R.O. Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology. 2011;92:1215–1225. doi: 10.1890/10-2240.1. [DOI] [PubMed] [Google Scholar]
- Marks S.D. Fluvial particulate inputs from afforested catchments: origins and impacts. In: Gibbins C.N., Turnbull D.A., editors. Human Impact on Freshwater Systems. Department of Environment, University of Northumbria; UK: 1994. pp. 1–17. [Google Scholar]
- Marnell F. Discriminant analysis of the terrestrial and aquatic habitat determinants of the smooth newt (Triturus vulgaris) and the common frog (Rana temporaria) in Ireland. J. Zool. 1998;244:1–6. [Google Scholar]
- Marsh T.J. Tenth Symposium on Stochastic Hydraulics; Fifth International Conference on Water Resources and Environment Research, 5–7 July 2010, Quebec City, Canada. 2010. The UK benchmark network – designation, evolution and application; p. 14. [Google Scholar]
- Mason P.J., Foster I.D.L., Carter A.D., Walker A., Higginbotham S., Jones R.L., Hardy I.A.J. Relative importance of point source contamination of surface waters: River Cherwell catchment monitoring study. In: Del Re A.A.M., Brown C., Capri E., Errera G., Evans S.P., Trevisan M., editors. Human and Environmental Exposure to Xenobiotics. La Goliardica Pavese, Pavia, Italy. 1999. pp. 405–412. [Google Scholar]
- Matsunaga H., Harada K.I., Senma M., Ito Y., Yasuda N., Ushida S., Kimura Y. Possible cause of unnatural mass death of wild birds in a pond in Nishinomiya, Japan: sudden appearance of toxic cyanobacteria. Nat. Toxins. 1999;7:81–84. doi: 10.1002/(sici)1522-7189(199903/04)7:2<81::aid-nt44>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Matthiessen P., Sheahan D., Harrison R.M., Kirby M., Rycroft R., Turnbull A., Volkner C., Williams R. Use of a Gammarus pulex bioassay to measure the effects of transient carbofuran runoff from farmland. Ecotoxicol. Environ. Saf. 1995;30:111–119. doi: 10.1006/eesa.1995.1013. [DOI] [PubMed] [Google Scholar]
- Mayer P.M., Reynolds S.K., Canfield T.J., McCutchen M.D. U.S. Environmental Protection Agency; Washington, D.C.: 2005. Riparian buffer width, vegetative cover, and nitrogen removal effectiveness: a review of current science and regulations. (EPA/600/R-05/118) [Google Scholar]
- McGarrigle M. Assessment of SWBs in Ireland. Biol. Environ. Proc. R. Ir. Acad. 2014;114B:119–128. [Google Scholar]
- McGonigle D.F., Burke S.P., Collins A.L., Gartner R., Haft M.R., Harris R.C., Haygarth P.M., Hedges M.C., Hiscock K.M., Lovett A.A. Developing Demonstration Test Catchments as a platform for transdisciplinary land management research in England and Wales. Environ. Sci.: Processes Impacts. 2014;16:1618–1628. doi: 10.1039/c3em00658a. [DOI] [PubMed] [Google Scholar]
- McHugh M. Defra; London: 2002. NSI Soil Erosion Survey. Final Report to Defra. Defra Project SP0407. [Google Scholar]
- Mellander P.-E., Jordan P., Bechmann M., Fovet O., Shore M.M., McDonald N., Gascuel-Odoux C. Intregratred climate-chemical indicators of diffuse pollution from land to water. Sci. Rep. 2018;8:944. doi: 10.1038/s41598-018-19143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Beilfuss R., Kaplan L.A., Carpenter Q., Newbold D., Semlitsch R., Strayer D.L., Watzin M.C., Woltemade C.J., Zedler P.H., Zedler J.B. American Rivers and the Sierra Club; Washington, D.C.: 2007. Where Rivers are Born: The Scientific Imperative for Defending Small Streams and Wetlands. (26 pp.) [Google Scholar]
- Meyer J.L., Strayer D.L., Wallace J.B., Eggert S.L., Helfman G.S., Leonard N.E. The contribution of headwater streams to biodiversity in river networks. J. Am. Water Resour. Assoc. 2007;43:86–103. [Google Scholar]
- Milner N.J., Cowx I.G., Whelan K. Salmonids and flows: a perspective on the state of the science and its application. Fish. Manag. Ecol. 2012;19:445–450. [Google Scholar]
- Moore D., Richardson J.S. Progress towards understanding the structure, function, and ecological significance of small stream channels and their riparian zones. Can. J. For. Res. 2003;33:1349–1351. [Google Scholar]
- Moore R.D., Spittlehouse D.L., Story A. Riparian microclimate and stream temperature response to forest harvesting: a review. J. Am. Water Resour. Assoc. 2005;41:813–834. [Google Scholar]
- Moyle P.B. Fish introductions into North America: patterns and ecological impact. In: Mooney H.A., Drake J.A., editors. Ecology of Biological Invasion of North America and Hawaii. Ecological Studies. Vol. 58. Springer-Verlag; New York: 1986. pp. 27–43. [Google Scholar]
- Mullholland P.J., Newold J.D., Elwood J.W., Ferren L.A., Webster J.R. Phosphorus spiralling in woodland stream seasonal variations. Ecology. 1985;66:1012–1023. [Google Scholar]
- Murphy J.F., Winterbottom J.H., Orton S., Simpson G.L., Shilland E.M., Hildrew A.G. Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: a 20-year time series. Ecol. Indic. 2014;37:330–340. [Google Scholar]
- Murphy P.N.C., Mellander P.-E., Melland A.R., Buckley C., Shore M., Shortle G., Wall D.P., Treacy M., Shine O., Mechan S., Jordan P. Variable response to phosphorus mitigation measures across the nutrient transfer continuum in a diary grassland catchment. Agric. Ecosyst. Environ. 2015;207:192–202. [Google Scholar]
- Murphy J.F., Jones J.I., Pretty J.L., Duerdoth C.P., Hawczak A., Arnold A., Blackburn J.H., Naden P.S., Old G., Sear D.A., Hornby D., Clarke R.T., Collins A.L. Development of a biotic index using stream macroinvertebrates to assess stress from deposited fine sediment. Freshw. Biol. 2015;60:2019–2036. [Google Scholar]
- Murphy F., Ewins C., Carbonnier F., Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;50:5800–5808. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- Murphy J.F., Jones J.I., Arnold A., Pretty J.L., Naden P.S., Sear D.A., Collins A.L. Can macroinvertebrate biological traits indicate fine-grained sediment conditions in streams? River Res. Appl. 2017;33:1606–1617. [Google Scholar]
- Nadeau T.-L., Rains M.C. Hydrological connectivity between headwater streams and downstream waters: how science can inform policy. J. Am. Water Resour. Assoc. 2007;43:118–133. [Google Scholar]
- Naden P.S., Murphy J.F., Old G.H., Newman J., Scarlett P., Harman M., Duerdoth C.P., Hawczak A., Pretty J.L., Arnold A., Laize C., Hornby D.D., Collins A.L., Sear D.A., Jones J.I. Understanding the controls on deposited fine sediment in the streams of agricultural catchments. Sci. Total Environ. 2016;547:366–381. doi: 10.1016/j.scitotenv.2015.12.079. [DOI] [PubMed] [Google Scholar]
- Naiman R.J., Decamps H., Pollock M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993;3:209–212. doi: 10.2307/1941822. [DOI] [PubMed] [Google Scholar]
- Newbold J.D., Elwood J.W., Oneill R.V., Sheldon A.L. Phosphorus dynamics in a woodland stream ecosystem – a study of nutrient spiralling. Ecology. 1983;64:1249–1265. [Google Scholar]
- Nisbet T., Silgram M., Shah N., Morrow K., Broadmeadow S. Forest Research, Surrey; UK: 2011. Woodland for Water: Woodland Measures for Meeting Water Framework Directive Objectives. Forest Research Monograph, 4. (156 pp.) [Google Scholar]
- Nislow K. Riparian management: alternative paradigms and implications for wild salmon. In: Kemp P., editor. Salmonid Fisheries: Freshwater Habitat Management. Wiley-Blackwell; Oxford: 2010. pp. 164–182. [Google Scholar]
- Nunn A.D., Cowx I.G. Restoring river connectivity: prioritizing passage improvements for diadromous fishes and lampreys. Ambio. 2012;41:402–409. doi: 10.1007/s13280-012-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenden M.C., Quinton J.N., Favaretto N., Deasy C., Surridge B. Reduced nutrient pollution in a rural stream following septic tank upgrade and installation of runoff retention measures. Environ. Sci.: Processes Impacts. 2014;16:1637–1645. doi: 10.1039/c3em00681f. [DOI] [PubMed] [Google Scholar]
- Ockenden M.C., Deasy C.E., Benskin C.McW.H., Beven K.J., Burke S., Collins A.L., Evans R., Falloon P.D., Forber K.J., Hiscock K.M., Kahana R., Macleod C.J.A., Reaney S.M., Snell M.A., Villamizar M.L., Wearing C., Withers P.J.A., Zhou J.G., Haygarth P.M. Changing climate and nutrient transfers: evidence from high temporal resolution concentration-flow dynamics in headwater catchments. Sci. Total Environ. 2016;548–549:325–339. doi: 10.1016/j.scitotenv.2015.12.086. [DOI] [PubMed] [Google Scholar]
- Old G.H., Naden P.S., Rameshwaran P., Acreman M.C., Baker S., Edwards F.K., Sorensen J.P.R., Mountford O., Gooddy D.C., Stratford C.J., Scarlett P.M., Newman J.R., Neal M. Instream and riparian implications of weed cutting in a chalk river. Ecol. Eng. 2014;71:290–300. [Google Scholar]
- Oreska M.P., Aldridge D.C. Estimating the financial costs of freshwater invasive species in Great Britain: a standardized approach to invasive species costing. Biol. Invasions. 2011;13:305–319. [Google Scholar]
- Ormerod S.J., Durance I. Restoration and recovery from acidification in upland Welsh streams over 25 years. J. Appl. Ecol. 2009;46:164–174. [Google Scholar]
- Ormerod S.J., Wade K.R., Gee A.S. Macro-floral assemblages in upland Welsh streams in relation to acidity, and their importance to invertebrates. Freshw. Biol. 1987;18:545–557. [Google Scholar]
- Ormerod S.J., Donald A.P., Brown S.J. The influence of plantation forestry on the pH and aluminium concentration of upland Welsh streams: a re-examination. Environ. Pollut. 1989;62:47–62. doi: 10.1016/0269-7491(89)90095-x. [DOI] [PubMed] [Google Scholar]
- Ormerod S.J., Rundle S.D., Lloyd E.C., Douglas A.A. The influence of riparian management on the habitat structure and macroinvertebrate communities of upland streams draining plantation forests. J. Appl. Ecol. 1993;30:13–24. [Google Scholar]
- Orr H.G., Simpson G.L., des Clers S., Watts G., Hughes M., Hannaford J., Dunbar M.J., Laize C.L.R., Wilby R.L., Battarbee R.W., Evans R. Detecting changing river temperatures in England and Wales. Hydrol. Process. 2015;29:752–766. [Google Scholar]
- Osborne L.L., Kovacic D.A. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw. Biol. 1993;29:243–258. [Google Scholar]
- Ovenden J.C., Gregory K.J. The permanence of stream networks in Britain. Earth Surf. Process. 1980;5:47–60. [Google Scholar]
- Paetzold A., Smith M., Warren P.H., Maltby L. Environmental impact 584 propagated by cross-system subsidy: chronic stream pollution controls riparian spider 585 populations. Ecology. 2011;92:1711–1716. doi: 10.1890/10-2184.1. [DOI] [PubMed] [Google Scholar]
- Palmer M.A., Menninger H.L., Bernhardt E. River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshw. Biol. 2010;55:205–222. [Google Scholar]
- Peirson G., Bolland J.D., Cowx I.G. Lateral dispersal and displacement of fish during flood events in lowland river systems in the UK – implications for sustainable floodplain management. Ecohydrol. Hydrobiol. 2008;8:363–373. [Google Scholar]
- Pesticides Forum . Published by the Health and Safety Executive. 2012. Pesticides in the UK. The 2012 report on the impacts and sustainable use of pesticides. (95 pp.) [Google Scholar]
- Petersen I., Masters Z., Hildrew A.G., Ormerod S.J. Dispersal of adult aquatic insects in catchments of differing land use. J. Appl. Ecol. 2004;41:934–950. [Google Scholar]
- Pickett S.R.A., Siriwardena G.M. The relationship between multi-scale habitat heterogeneity and farmland bird abundance. Ecography. 2011;34:955–969. [Google Scholar]
- Pinder A.C., Gozlan R.E., Britton J.R. Dispersal of the invasive topmouth gudgeon, Pseudorasbora parva in the UK: a vector for an emergent infectious disease. Fish. Manag. Ecol. 2005;12:411–414. [Google Scholar]
- Pollock M.M., Beechie T.J., Wheaton J.M., Jordan C.E., Bouwes N., Weber N., Volk C. Using beaver dams to restore incised stream ecosystems. Bioscience. 2014;64:279–290. [Google Scholar]
- Pond Conservation Group . An Agenda for Action. Pond Conservation Group; Oxford: 1993. A future for Britain's ponds. [Google Scholar]
- Rahel F.J., Olden J.D. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 2008;22:521–533. doi: 10.1111/j.1523-1739.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- Ranalli A.J., Macalady D.L. The importance of the riparian zone and in-stream processes in nitrate attenuation in undisturbed and agricultural watersheds – a review of the scientific literature. J. Hydrol. 2010;389:406–415. [Google Scholar]
- Raven P.J., Holmes N.T.H., Dawson F.H., Everard M. Quality assessment using River Habitat Survey data. Aquat. Conserv. Mar. Freshwat. Ecosyst. 1998;8:477–499. [Google Scholar]
- Reid N., Dingerkus S.K., Stone R.E., Pietravalle S., Kelly R., Buckley J., Beebee T.J.C., Wilkinson J.W. Department of Arts, Heritage and the Gaeltacht; Dublin, Ireland: 2013. National Frog Survey of Ireland 2010/11. Irish Wildlife Manuals, No. 58. National Parks and Wildlife Service. [Google Scholar]
- Reiss J., Bridle J., Montoya J.M., Woodward G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009;24:505–514. doi: 10.1016/j.tree.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Reynolds B., Stevens P.A., Hughes S., Parkinson J.A., Weatherley N.S. Stream chemistry impacts of conifer harvesting in welsh catchments. Water Air Soil Pollut. 1995;79:147–170. [Google Scholar]
- Rheinhardt R.D., Brinson M.M., Meyer G.F., Miller K.H. Carbon storage of headwater riparian zones in an agricultural landscape. Carbon Balance Manag. 2012;7:1–5. doi: 10.1186/1750-0680-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley W.D., Pawson M.G. Habitat requirements for juvenile salmonids in chalk streams: how will management best address conflicting interests? In: Kemp P., editor. Salmonid Fisheries: Freshwater Habitat Management. Wiley-Blackwell; Oxford: 2010. pp. 242–262. [Google Scholar]
- Riley W.D., Ives M.J., Pawson M.G., Maxwell D.L. Seasonal variation in habitat use by salmon (Salmo salar L.), trout (Salmo trutta L.) and grayling (Thymallus thymallus L.) in a chalk stream. Fish. Manag. Ecol. 2006;13:221–236. [Google Scholar]
- Riley W.D., Maxwell D.L., Pawson M.G., Ives M.J. The effects of low summer flow on wild salmon (Salmo salar) trout (Salmo trutta) and grayling (Thymallus thymallus) in a small stream. Freshw. Biol. 2009;54:2581–2599. [Google Scholar]
- Riley W.D., Pawson M.G., Qualyle V., Ives M.J. The effect of stream canopy management on macroinvertebrate communities and juvenile salmonid production in a chalk stream. Fish. Manag. Ecol. 2009;16:100–111. [Google Scholar]
- Riley W.D., Davison P.I., Maxwell D.L., Newman R.C., Ives M.J. A laboratory study to determine the dispersal response of Atlantic salmon (Salmo salar) fry to street light intensity. Freshw. Biol. 2015;60:1016–1028. [Google Scholar]
- Rodriguez-Cardona B., Wymore A.S., McDowell W.H. DOC:NO3- ratios and NO3- uptake in forested headwater streams. J. Geophys. Res. Biogeosci. 2016;121:205–217. [Google Scholar]
- Royal Geographical Society (with IBG) RGS–IBG Policy Briefing. 2012. Water policy in the UK: the challenges. (27 pp.) [Google Scholar]
- Rubbo M.J., Cole J.J., Kiesecker J.M. Terrestrial subsidies of organic carbon support net ecosystem production in temporary forest ponds: evidence from an ecosystem experiment. Ecosystems. 2006;9:1170–1176. [Google Scholar]
- Sabater S., Butturini A., Clement J.-C., Burt T., Dowrick D., Hefting M., Maıˆtre V., Pinay G., Postolache C., Rzepecki M., Sabater F. Nitrogen removal by riparian buffers along a European climatic gradient: patterns and factors of variation. Ecosystems. 2003;6:20–30. [Google Scholar]
- Santoul F., Gaujard A., Angélibert S., Mastrorillo S., Céréghino R. Gravel pits support water-bird diversity in an urban landscape. Hydrobiologia. 2009;634:107–114. [Google Scholar]
- Sayer C.D., Copp G.H., Emson D., Zięba G., Godard M.J., Wesley K.J. Towards the conservation of crucian carp Carassius carassius: understanding the extent and causes of decline within part of its native English range. J. Fish Biol. 2011;79:1608–1624. doi: 10.1111/j.1095-8649.2011.03059.x. [DOI] [PubMed] [Google Scholar]
- Sayer C.D., Andrews K., Shilland E., Edmonds N.J., Edmonds-Brown R., Patmore I., Emson D., Axmacher J. The role of pond management for biodiversity conservation in an agricultural landscape. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2012;22:626–638. [Google Scholar]
- Schmid-Araya J.M., Hildrew A.G., Robertson A., Schmid P.E., Winterbottom J. The importance of meiofauna in food webs: evidence from an acid stream. Ecology. 2002;83:1271–1285. [Google Scholar]
- Schoumans O.F., Chardon W.J., Bechmann M.E., Gascuel-Odoux C., Hofman G., Kronvang B., Rubaek G.H., Ulen B., Dorioz J.-M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: a review. Sci. Total Environ. 2014;468:1255–1266. doi: 10.1016/j.scitotenv.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Sear D.A. River restoration and geomorphology. Aquat. Conserv. Mar. Freshwat. Ecosyst. 1994;4:169–177. [Google Scholar]
- Sear D.A., Wilcock D., Robinson M.R., Fisher K.R. Channel modifications and impacts. In: Acreman M., editor. The Changing Hydrology of the UK, Routledge. 2000. pp. 55–81. [Google Scholar]
- Sear D.A., Millington C.E., Kitts D.R., Jeffries R. Logjam controls on channel: floodplain interactions in wooded catchments and their role in the formation of multi-channel patterns. Geomorphology. 2010;116:305–319. [Google Scholar]
- Sharpley A.N., Bergstrom L., Aronsson H., Bechmann M., Bolster C.H., Borling K., Djodjic F., Jarvie H.P., Schoumans O.F., Stamm C., Tonderski K.S., Ulen B., Uusitalo R., Withers P.J.A. Future agriculture with minimized phosphorus losses to waters: research needs and direction. Ambio. 2015;44:S163–S179. doi: 10.1007/s13280-014-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers K. Bournemouth University; Poole, Dorset, UK: 2012. Predicting Ecological Impacts of Climate Change and Species Introductions on a Temperate Chalk Stream in Southern Britain – A Dynamic Food Web Model Approach. PhD Thesis. (235 pp.) [Google Scholar]
- Simpson D., Duenas M. GB Non-native Organism Risk Assessment for Elodea nutallii. 2011. www.nonnativespecies.org
- Stevens C.J., Quinton J.N. Diffuse pollution swapping in arable agricultural systems. Crit. Rev. Environ. Sci. Technol. 2009;396:478–520. [Google Scholar]
- Strahler A.N. Quantitative analysis of watershed geomorphology. Trans. Am. Geophys. Union. 1957;38:913–920. [Google Scholar]
- Strauss P., Leone A., Ripa M.N., Turpin N., Lescot J.-M., Laplana R. Using critical source areas for targeting cost-effective best management practices to mitigate phosphorus and sediment transfer at the watershed scale. Soil Use Manag. 2007;23:144–153. [Google Scholar]
- Sweeney P., Sweeney N., Hurley C. Irish Wildlife Manuals, No. 67. National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht; Dublin, Ireland: 2013. Natterjack toad monitoring project, 2011–2012. [Google Scholar]
- Szoszkiewicz K., Ferreira T., Korte T., Baattrup-Pedersen A., Davy-Bowker J., O'Hare M. European river plant communities: the importance of organic pollution and the usefulness of existing macrophyte metrics. Hydrobiologia. 2006;566:211–234. [Google Scholar]
- Telfer S., Holt A., Donaldson R., Lambin X. Metapopulation processes and persistence in remnant water vole populations. Oikos. 2001;95:31–42. [Google Scholar]
- Telzlaff D., Malcolm I.A., Soulsby C. Influence of forestry, environmental change and climatic variability on the hydrology, hydrochemistry and residence times of upland catchments. J. Hydrol. 2007;346:93–111. [Google Scholar]
- Teufl B., Weigelhofer G., Fuchsberger J., Hein T. Effects of bank and channel morphology on the sediment quality of agricultural low-order streams. Environ. Sci. Pollut. Res. 2013;20:1781–1793. doi: 10.1007/s11356-012-1135-2. [DOI] [PubMed] [Google Scholar]
- The Rural Coalition . Achieving Sustainable Rural Communities for the 21st Century. Town and Country Planning Association; London: 2010. The rural challenge. [Google Scholar]
- Thomas K.V., Hurst M.R., Matthiessen P., Sheahan D., Williams R.J. Toxicity characterisation of organic contaminants in stormwaters from an agricultural headwater stream in South East England. Water Res. 2001;35:2411–2416. doi: 10.1016/s0043-1354(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Thomas S.M., Griffiths S.W., Ormerod S.J. Beyond cool: adapting upland streams for climate change using riparian woodlands. Glob. Chang. Biol. 2016;22:310–324. doi: 10.1111/gcb.13103. [DOI] [PubMed] [Google Scholar]
- Thoms J.H., Delong M.C., Thorp M.D. The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res. Appl. 2006;22:123–147. [Google Scholar]
- Triska F.J., Duff J.H., Avanzino R.J. Patterns of hydrological exchange and nutrient transformation in the hyporheic zone of a gravel-bottom stream – examining terrestrial aquatic linkages. Freshw. Biol. 1993;29:259–274. [Google Scholar]
- Triska F.J., Duff J.H., Avanzino R.J. The role of water exchange between a stream channel and its hyporheic zone in nitrogen cycling at the terrestrial aquatic interface. Hydrobiologia. 1993;251:167–184. [Google Scholar]
- Triska F.J., Duff J.H., Sheibley R.W., Jackman A.P., Avanzino R.J. DIN retention-transport through four hydrologically connected zones in a headwater catchment of the Upper Mississippi River. J. Am. Water Resour. Assoc. 2007;43:60–71. [Google Scholar]
- Van Kleef H., Van der Velde G., Leuven R.S.E.W., Esselink H. Pumpkinseed sunfish (Lepomis gibbosus) invasions facilitated by introductions and nature management strongly reduce macroinvertebrate abundance in isolated water bodies. Biol. Invasions. 2008;10:1481–1490. [Google Scholar]
- Vaughan N. The diets of British bats (Chiroptera) Mammal Rev. 1997;27:77–94. [Google Scholar]
- Verspecht A., Vandermeulen V., Ter Avest E., Van Huylenbroeck G. Review of trade-offs and co-benefits from greenhouse gas mitigation measures in agricultural production. J. Integr. Environ. Sci. 2012;9:147–157. [Google Scholar]
- Wall D., Jordan P., Melland A.R., Mellander P.-E., Buckley C., Reaney S.M., Shortle G. Using the nutrient transfer continuum concept to evaluate the European Union Nitrates Directive National Action Programme. Environ. Sci. Pol. 2011;14:664–674. [Google Scholar]
- Wallace I.D. 2016. A Review of the Status of the Caddis Flies (Trichoptera) of Great Britain Species Status No.27. Natural England Commissioned Report, Number 191. [Google Scholar]
- Walling D.E., Owens P.N., Leeks G.J.L. The role of channel and floodplain storage in the suspended sediment budget of the River Ouse, Yorkshire, UK. Geomorphology. 1998;22:225–242. [Google Scholar]
- Walton S.E., Nunn A.D., Probst W.N., Bolland J.D., Acreman M., Cowx I.G. Do fish go with the flow? The effects of periodic and episodic flow pulses on 0+ fish biomass in a constrained lowland river. Ecohydrology. 2016;10:1–14. [Google Scholar]
- Watts G., Battarbee R., Bloomfield J.P., Crossman J., Daccache A., Durance I., Elliot J., Garner G., Hannaford J., Hannah D.M., Hess T., Jackson C.R., Kay A.L., Kernan M., Knox J., Mackay J., Monteith D.T., Ormerod S., Rance J., Stuart M.E., Wade A.J., Wade S.D., Weatherhead K., Whitehead P.G., Wilby R.L. Climate change and water in the UK – past changes and future prospects. Prog. Phys. Geogr. 2015;39:6–28. [Google Scholar]
- Weatherley N.S., Ormerod S.J. Forests and the temperature of upland streams: a modelling study of the biological consequences. Freshw. Biol. 1990;24:109–122. [Google Scholar]
- Webb B.W. Regulation and thermal regime in a Devon river system. In: Foster I.D.L., Gurnell A.M., Webb B.W., editors. Sediment and Water Quality in River Catchments. John Wiley & Sons; Chichester, UK: 1995. pp. 65–94. [Google Scholar]
- Weekes L., Matson R., Kelly F., Fitz Patrick U., Kelly-Quinn M. Composition and characteristics of macrophyte assemblages in small streams in Ireland. Biol. Environ. Proc. R. Ir. Acad. 2014;114B(3):163–180. [Google Scholar]
- Weigelhofer G., Fuchsberger J., Teufl B., Welti N., Hein T. Effects of riparian forest buffers on in-stream nutrient retention in agricultural catchments. J. Environ. Qual. 2012;41:373–379. doi: 10.2134/jeq2010.0436. [DOI] [PubMed] [Google Scholar]
- Weigelhofer G., Welti N., Hein T. Limitations of stream restoration for nitrogen retention in agricultural headwater streams. Ecol. Eng. 2013;60:224–234. [Google Scholar]
- Whitehead P.G., Wilby R.L., Battarbee R.W., Kernan M., Wade A.J. A review of the potential impacts of climate change on surface water quality. Hydrol. Sci. J. 2009;54:101–123. [Google Scholar]
- Wilby R.L., Johnson M.F., Toone J.A. Thermal shockwaves in an upland river. Weather. 2015;70 [Google Scholar]
- Wilkinson J.W., Arnell A.P. 2013. NARRS Report 2007–2012: Establishing the Baseline (HWM Edition). ARC Research Report 13/01. [Google Scholar]
- Williams R.J., Brooke D.N., Matthiessen P., Mills M., Turnbull A., Harrison R.M. Pesticide transport to surface waters within an agricultural catchment. J. Inst. Water Environ. Manage. 1995;9:72–81. [Google Scholar]
- Williams P., Whitfield M., Biggs J., Bray S., Fox G., Nicolet P., Sear D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2004;115:329–341. [Google Scholar]
- Williams P., Biggs J., Whitfield M., Bryant S., Fox G., Nicolet P. Second ed. Pond Conservation; Oxford: 2010. The Pond Book: A Guide to the Management and Creation of Ponds. [Google Scholar]
- Williams M.R., Buda A.R., Elliott H.A., Singha K., Hamlett J. Influence of riparian seepage zones on nitrate variability in two agricultural headwater streams. J. Am. Water Resour. Assoc. 2015;51:883–897. [Google Scholar]
- Withers P.J.A., Jarvie H.P. Delivery and cycling of phosphorus in rivers: a review. Sci. Total Environ. 2008;400:379–395. doi: 10.1016/j.scitotenv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Wittmer I.K., Scheidegger R., Bader H.P., Singer H., Stamm C. Loss rates of urban biocides can exceed those of agricultural pesticides. Sci. Total Environ. 2011;409:920–932. doi: 10.1016/j.scitotenv.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Wohl E., Angermeier P.L., Bledsoe B., Kondolf G.M., MacDonnell L., Merritt D.M., Palmer M.A., Poff N.L., Tarboton D. River restoration. Water Resour. Res. 2005;41 [Google Scholar]
- Wood P., Armitage P.D. Biological effects of fine sediment in the lotic environment. Environ. Manag. 1997;21:203–217. doi: 10.1007/s002679900019. [DOI] [PubMed] [Google Scholar]
- Wood P.J., Boulton A.J., Little S., Stubbington R. Is the hyporheic zone a refugium for aquatic macroinvertebrates during severe low flow conditions? Fundam. Appl. Limnol. Arch. Hydrobiol. 2010;176:377–390. [Google Scholar]
- Zhang Y., Collins A.L., Gooday R.D. Application of the FARMSCOPER tool for assessing agricultural diffuse pollution mitigation methods across the Hampshire Avon Demonstration Test Catchment, UK. Environ. Sci. Pol. 2012;24:120–131. [Google Scholar]
- Zhang Y., Collins A.L., Murdoch N., Lee D., Naden P.S. Cross sector contributions to river pollution in England and Wales: updating waterbody scale information to support policy delivery for the Water Framework Directive. Environ. Sci. Pol. 2014;42:16–32. [Google Scholar]
- Zhang Y., Collins A.L., Jones J.I., Johnes P.J., Inman A., Freer J.E. The potential benefits of on-farm mitigation scenarios for reducing multiple pollutant loadings in prioritised agri-environment areas across England. Environ. Sci. Pol. 2017;73:100–114. [Google Scholar]
- Ziegler S.E., Lyon D.R. Factors regulating epilithic biofilm carbon cycling and release with nutrient enrichment in headwater streams. Hydrobiologia. 2010;657:71–88. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example on-farm mitigation measures targeting different components of the water pollution cascade.