ABSTRACT
A 35-d experiment was conducted in broilers to study the effect of supplementation of sulfate or hydroxychloride forms of Zn and Cu at 2 supplemental Zn levels on growth performance, meat yield, and tissue levels of Zn. On day 0, 900 male Ross 308 broiler chicks (45 ± 1.10 g) were allocated to 4 treatments in a randomized complete block design and 2 × 2 factorial arrangement of treatments. The factors were 2 sources (sulfate or hydroxychloride) of Zn and Cu and 2 levels (low or high) of Zn. The Zn sources were zinc sulfate monohydrate (ZSM) or hydroxychloride Zn. Copper sources were copper (II) sulfate pentahydrate or hydroxychloride Cu. Each of the 4 treatments had 15 replicates and 15 birds per replicate. Birds were weighed on days 0, 21, and 35 for growth performance. On day 35, left tibia bone, liver, and blood were collected from 4 randomly selected birds per pen. In addition, 7 birds per pen were used for carcass evaluation. There was no significant source × level interaction on any of the growth performance response. Broiler chickens receiving hydroxychloride Zn and Cu had greater (P < 0.05) gain: feed, whereas broiler chickens receiving lower Zn level had greater (P < 0.01) weight gain. There was no source × level interaction on meat yield. Broiler chickens receiving hydroxychloride Zn and Cu had greater (P < 0.05) % breast yield than those receiving sulfate Zn and Cu. Higher level of Zn, irrespective of source, produced greater (P < 0.01) tibia and plasma Zn levels, whereas liver Cu was greater (P < 0.05) in broiler chickens receiving hydroxychloride Zn and Cu. It was concluded that hydroxychloride Zn and Cu were more efficacious than sulfate Zn and Cu in promoting growth performance and enhancing meat yield in the current study.
Keywords: zinc, copper, broiler, meat yield, plasma minerals
INTRODUCTION
Trace minerals are very important in maintaining healthy and productive birds. Zinc is a component of more than 200 metalloenzymes (Prasad, 1984) and hence it is very important in many physiological processes taking place in the body. Copper plays critical roles as a co-factor in many enzymes (Davis and Mertz, 1987) relevant to maintaining proper body functions. Because of its many roles, Zn is vitally important in supporting bone and tissue development (Sahraei et al., 2012), egg shell quality (Zhang et al., 2017), as well as in proper functioning of the immune system (Feng et al., 2009; Jarosz et al., 2017; Perez et al., 2017). In pigs, Zn has been reported to help alleviate post-weaning diarrhea (Zhang and Guo, 2009) and high levels of the Zn and Cu have pharmacological relevance in the swine and poultry industries (Pettigrew, 2006; Zhang and Guo, 2007).
Various sources of supplemental Zn and Cu, such as oxide, citrate, or sulfate, are added to the diets to supplement what is provided by plant-based feedstuffs. However, many of these sources have poor bioavailability, may cause irritation of the intestinal mucosa, or lead to increased trace mineral excretion to the environment (Miles et al., 1998; Cao et al., 2002, Mwangi et al., 2017). Organic Zn and Cu sources are popular for poultry because they have greater bioavailability and do not suffer the same negative consequence resulting from feeding inorganic trace minerals (Ma et al., 2011, 2018). Hydoxychloride Zn and Cu do not have the same limitations as the sources listed previously. Hydroxychloride copper is reported to have bioavailability equal to, or greater, than that of copper sulfate (Miles et al., 1998; Luo et al., 2005). On the basis of growth performance response of broiler chicks, Batal et al. (2001) reported that Zn relative bioavailability of hydroxychloride Zn was greater than that for feed grade ZnSO4·H2O. Part of the reasons for greater efficacy of hydroxychloride Zn and Cu is reported to be due to the fact that only marginal quantity of their trace minerals is soluble in water but completely soluble in weak acid (Pang and Applegate, 2006; Zhang and Guo, 2007).
Most studies on hydroxychloride minerals (Zn, Cu, and Mn) have studied them in isolation. However, trace minerals are included in diets in a premix. Because hydroxychloride trace minerals (Zn, Mn, and Cu) are now widely used in poultry, it is vital to understand how their use in a trace mineral premix as well as use of varying levels of Zn impacts chicken growth performance as well as the effects on carcass yield and deposition of the minerals in tissues. There is a dearth of information regarding the latter. Consequently, the objective of the current experiment was to compare the effect of dietary inclusion of sulfate or hydroxychloride forms of Zn and Cu as well as influence of varying levels of Zn on broiler chicken performance and carcass yield.
MATERIALS AND METHODS
All the animal experiment procedures used in this study were approved by the Scotland's Rural College's Animal Experiment Committee.
Diets and Experimental Design
A total of 900 Ross 308 male broilers at 0 day old (45 ± 1.10 g) were allocated to 4 dietary treatments in a 2 × 2 factorial arrangement. The factors were 2 sources of Zn and Cu (sulfate or hydroxychloride) and 2 levels (low or high) of Zn. Each of the 4 treatments had 15 replicates and 15 birds per replicate. The wheat-soybean meal basal diet was supplemented with trace mineral premix that was devoid of Zn or Cu (basal Zn and Cu levels were 34.3 and 7.6 ppm, respectively). Diets 1 and 2 were supplemented with analytical grade zinc sulfate monohydrate (ZSM; Acro Organics, Belgium) and copper sulfate pentahydrate (Sigma Aldrich, United Kingdom). Diets 3 and 4 were supplemented with hydroxychloride Zn (Selko IntelliBond Zn, Trouw Nutrition, Netherlands) and hydroxychloride Cu (Selko IntelliBond C, Trouw Nutrition, Netherlands). Both hydroxychloride Zn and Cu are produced through a reactive crystallization process (EFSA, 2011, 2012).
The target supplementary dietary Cu level was 15 ppm. The target supplementary low dietary Zn level was 20 ppm and 80 ppm for high Zn level. The supplemental rates of the sulfate and hydroxychloride minerals to the diets are shown in Table 1. The broiler chicks received the experimental diets in 2 phases from day 0 to 21 (starter phase) and day 21 to 35 (finisher phase). The diets were provided as crumbled pellets during the first 7 d and as pellets for the remainder of the study. The diets were provided ad libitum throughout the entire study. The compositions of the experimental diets are shown in Table 2. Birds and feed were weighed on days 0, 21, and 35 for determination of growth performance.
Table 1.
Supplementation rates (g/ton) of zinc and copper sources for the dietary treatments.
| Diet number | Zn and Cu source | Zn level | ZSM | CSP | Hydroxychloride Zn | Hydroxychloride Cu |
|---|---|---|---|---|---|---|
| 1 | Sulfate | High | 220.0 (80 ppm) | 59.0 (15 ppm) | – | – |
| 2 | Low | 55.0 (20 ppm) | 59.0 (15 ppm) | – | – | |
| 3 | Hydroxychloride | High | – | – | 145.5 (80 ppm) | 27.8 (15 ppm) |
| 4 | Low | – | – | 36.4 (20 ppm) | 27.8 (15 ppm) |
Values in parentheses are the added Zn and Cu levels.
ZSM—analytical grade zinc sulfate monohydrate.
CSP—analytical grade copper (II) sulfate pentahydrate.
Hydroxychloride Zn (IntelliBond Zn).
Hydroxychloride Cu (IntelliBond Cu).
Table 2.
| Starter | Grower | |
|---|---|---|
| (day 0 to 21) | (day 21 to 35) | |
| Wheat | 468.8 | 545.8 |
| Barley | 150 | 150 |
| Soybean meal | 330 | 240 |
| Soybean oil | 15 | 35 |
| DL-Methionine | 1.6 | 1.4 |
| L-Lysine | 1.6 | 1.8 |
| L-Threonine | 0.15 | 0.18 |
| Calcium carbonate | 9.5 | 8.0 |
| Dicalcium phosphate | 14 | 8.5 |
| Salt (NaCl) | 2.5 | 2.4 |
| Sodium bicarbonate | 2.8 | 2.8 |
| VTE Premix3 | 4.0 | 4.0 |
| Phytase (Quantum Blue, 500 FTU/kg) | 0.1 | 0.1 |
| Total | 1,000 | 1,000 |
| Total amino acids, g/kg | ||
| Arg | 14.9 | 12.2 |
| His | 5.8 | 4.8 |
| Ile | 9.5 | 7.8 |
| Leu | 16.7 | 13.9 |
| Lys | 13.1 | 10.8 |
| Met | 4.9 | 4.2 |
| Cys | 3.9 | 3.5 |
| Phe | 8.1 | 5.9 |
| Tyr | 5.9 | 4.3 |
| Thr | 8.3 | 6.9 |
| Trp | 2.9 | 2.5 |
| Val | 10.5 | 8.8 |
1AME was 3,159 and 3,238 kcal/kg for starter and grower diets, respectively.
2To achieve the required dietary levels of Cu (15 ppm) analytical grade copper (II) sulfate pentahydrate or hydroxychloride Cu (IntelliBond Cu) were added at the rates of 59.0 or 27.8 g/ton, respectively. To achieve the required low Zn level (20 ppm), analytical grade zinc sulfate monohydrate (ZSM) and hydroxychloride Zn (IntelliBond Zn) were added at the rates of 55 or 36.4 g/ton, respectively. To achieve the high Zn level (80 ppm), ZSM and hydroxychloride Zn were added at the rates of 220.0 or 145.5 g/ton, respectively.
3Vitamin and trace mineral premix supplied the following per kilogram of diet: vitamin A, 5484 IU; vitamin D3, 2643 ICU; vitamin E, 11 IU; menadione sodium bisulfite, 4.38 mg; riboflavin, 5.49 mg; d-pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 μg; biotin, 55.2 μg; thiamine mononitrate,2.2 mg; folic acid, 990 μg; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg; Mn, 66.06 mg; Fe, 44.1 mg; Se, 250 μg. The premix was Cu- and Zn-free.
Footpad Scoring
Footpad scoring was done on day 35 on 5 randomly selected birds per pen. The scoring was done by visual inspection of the footpad of each of the selected birds. Five scores were assigned with scores ranging from 1, where skin of the footpad and digital pads appeared normal and there was no reddening, to 5, if there were significant lesions covering more than a third of the footpad. Total footpad score (TFPS) was calculated as: 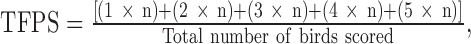 where the numbers indicate the score assigned to each bird and n indicates the number of birds assigned the particular score in each pen. A lower score is associated with better footpad quality.
where the numbers indicate the score assigned to each bird and n indicates the number of birds assigned the particular score in each pen. A lower score is associated with better footpad quality.
Litter Scoring
Litter scoring was done at the end of the study after birds had been removed. Litter scoring was done by visual assessment of each pen and assigning scores ranging from 1, if the litter was friable with no capping or compaction, to 5, if litter was wet and dough-like. The average litter score (LS) was calculated as follows: 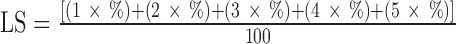 where the numbers 1 to 5 were the scores as described previously and the % was the percentage of the pen area corresponding to each score in each pen. A lower score is associated with better litter quality.
where the numbers 1 to 5 were the scores as described previously and the % was the percentage of the pen area corresponding to each score in each pen. A lower score is associated with better litter quality.
Carcass Evaluation
Carcass evaluation was done on day 35 on 7 representative birds per pen. Carcass evaluation was done at the Carcass Evaluation Unit of Scotland’ Rural College. The procedure for killing birds and carcass evaluation conformed to EU legislation (1099/2009). For evisceration, the neck and crop were removed from each carcass before being re-hung onto the evisceration line. The abdominal fat was removed and weighed along with the eviscerated carcass weight (eviscerated carcass yield). After chilling with ambient temperature of –4°C (overnight), the eviscerated carcass was portioned (and weighed) into the following components: breast (breast yield), drumstick, thigh, wings, and the remaining back and ribs.
Blood and Tissues Analysis
Four randomly selected birds per pen were used for blood and tissue analysis on day 35. Pooled blood from 4 birds per pen was collected into specialized tubes for trace mineral analysis (S-Monovette, Sarstedt, Germany). The blood was kept on ice and later centrifuged at 2,000 × g for 30 min to separate the plasma, which was subsequently frozen at –20°C prior to analysis for Zn and Cu.
The entire liver and the left tibia were also obtained from each of the 4 selected birds per pen. The tibias were cleaned of adhering flesh. The liver and tibia were immediately stored frozen on dry ice and subsequently stored frozen at –20°C prior to chemical analysis.
Chemical Analysis
Diets were analyzed (in triplicates) for dry matter, nitrogen, ether extract, acid hydrolyzed fat, crude fiber, ash, and minerals. Plasma samples were analyzed for plasma Zn content using inductively coupled plasma—mass spectroscopy (ICP-MS). The tibias were ashed overnight at 500°C to determine the ash content, followed by solubilization in concentrated hydrochloric acid and ICP-MS analysis to determine the Zn content in tibia ash. Each liver sample was ashed at 500°C and solubilized in hydrochloric acid, followed by analysis in ICP-MS to determine the Cu content.
Dry matter was determined by drying the samples in a drying oven at 100°C for 24 h (Method 934.01, AOAC, 2006). Nitrogen was determined by the combustion method (Method 968.06, AOAC, 2006). Ether extraction was done in Soxhlet apparatus (Method 920.39, AOAC, 2006). Acid hydrolyzed fat analysis was done using AOAC method 925.32 (AOAC, 2006). Crude fiber was analyzed using the Ankom nylon bag technique (Ankom 220 Analyzer). Ash content was determined in a muffle furnace by ashing the sample overnight at 600°C (Method 942.05, AOAC, 2006). Minerals content was determined using inductively coupled plasma—optical emission spectroscopy (AOAC, 2006) following digestion, in turn, in concentrated HNO3 and HCl.
Statistical Analysis
The data were analyzed by the MIXED procedure of SAS as appropriate for a randomized complete block design and a factorial treatment arrangement (the pen was the experimental unit). Because of the hierarchical arrangement of factorial treatment arrangement, only the simple effects are discussed for responses in which the interaction was significant, whereas main effects means are discussed in cases where the interaction was not significant. When interactions were not significant, the interaction term was removed from the model and the data reanalyzed. Significantly different means (for simple effects) were separated using Tukey. Significance was declared when P ≤ 0.05.
RESULTS
Table 3 shows the results of the analysis of the experimental diets. The analysis showed that the expected nutrient content of the diets were met. More importantly, the difference in low and high levels of Zn in the diets were achieved both with the use of sulfate and hydroxychloride Zn sources.
Table 3.
Analyzed composition (%, as fed basis) of the experimental diets.
| Zn and Cu source1 | Zn Level | DM | CP | EE | AHF | CF | Ash | Ca | Na | Cu, ppm | Mn, ppm | Zn, ppm | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter (day 0 to 21) | |||||||||||||
| Sulfate | High | 88.1 | 22.8 | 3.6 | 4.38 | 2.8 | 5.2 | 0.91 | 0.18 | 18 | 119 | 131 | 0.66 |
| Low | 87.8 | 22.0 | 3.2 | 3.80 | 2.5 | 5.3 | 0.85 | 0.17 | 17 | 117 | 72 | 0.64 | |
| Hydroxychloride | High | 87.6 | 22.8 | 3.0 | 3.75 | 2.6 | 5.5 | 0.85 | 0.16 | 18 | 110 | 105 | 0.63 |
| Low | 87.5 | 22.9 | 3.1 | 3.63 | 2.6 | 5.4 | 0.92 | 0.18 | 20 | 125 | 69 | 0.68 | |
| Grower (day 21 to 35) | |||||||||||||
| Sulfate | High | 88.6 | 18.3 | 4.5 | 5.11 | 2.6 | 4.1 | 0.66 | 0.14 | 24 | 111 | 120 | 0.48 |
| Low | 88.5 | 19.1 | 4.3 | 5.21 | 2.8 | 4.6 | 0.77 | 0.17 | 26 | 131 | 87 | 0.52 | |
| Hydroxychloride | High | 88.5 | 18.9 | 4.7 | 5.02 | 2.7 | 4.6 | 0.76 | 0.18 | 21 | 125 | 142 | 0.52 |
| Low | 88.4 | 19.0 | 4.5 | 5.00 | 2.8 | 4.5 | 0.81 | 0.19 | 23 | 136 | 78 | 0.54 | |
CP—crude protein; EE—ether extract; AHF—acid hydrolyzed fat; CF—crude fiber.
1Sulfate Zn and Cu were analytical grade zinc sulfate monohydrate and copper sulfate pentahydrate, respectively. Hydroxychloride Zn and Cu were IntelliBond Zn and IntelliBond Cu, respectively.
The growth performance response of the broiler chickens to provision of 2 sources of Zn and Cu and 2 Zn levels is shown in Table 4. There was no significant source × level interaction on any of the growth performance response except for a trend for interaction for gain to feed ratio (G: F) for day 21 to 35 and overall periods. The interaction shows that G: F tended to be lower when higher level of ZSM was used in the diet and the reverse was the case when hydroxychloride Zn was used. There was significant (P < 0.05) main effect of Zn and Cu source on G: F in the grower period and overall and a significant (P < 0.05) main effect of Zn level on weight gain in the grower period and overall. Broiler chickens receiving hydroxychloride Zn and Cu had greater (P < 0.05) G: F, whereas broiler chickens receiving lower Zn level had greater (P < 0.01) weight gain than those receiving the higher level of Zn supplementation. In addition, chickens receiving diets containing hydroxychloride Zn and Cu tended to have greater (P < 0.10) weight gain in the grower period.
Table 4.
Growth performance of broilers receiving sulfate or hydroxychloride zinc and copper at 2 supplemental levels of zinc.1
| Starter (day 0 to 21) | Grower (day 21 to 35) | Overall (day 0 to 35) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn and Cu source2 | Zn Level | IBW, g | BWG, g | FI, kg | G:F | BWG, g | FI, kg | G:F | BWG, g | FI, kg | G:F |
| Sulfate | High | 46.0 | 948.6 | 1.20 | 790.5 | 1443 | 2.43 | 593.8 | 2391 | 3.62 | 660.5 |
| Low | 46.1 | 947.0 | 1.19 | 795.7 | 1512 | 2.46 | 614.6 | 2459 | 3.64 | 675.5 | |
| Hydroxychloride | High | 46.0 | 922.7 | 1.16 | 795.4 | 1501 | 2.40 | 625.4 | 2424 | 3.52 | 688.6 |
| Low | 45.7 | 948.2 | 1.18 | 804.0 | 1538 | 2.49 | 617.7 | 2486 | 3.65 | 681.1 | |
| Pooled SEM | 0.283 | 13.7 | 0.018 | 8.45 | 22.2 | 0.035 | 7.35 | 30.0 | 0.049 | 6.17 | |
| Source × level | – | 0.330 | 0.137 | 0.770 | 0.461 | 0.403 | 0.066 | 0.922 | 0.262 | 0.071 | |
| Main effect of source | |||||||||||
| Sulfate | 46.0 | 947.8 | 1.20 | 789.2 | 1477 | 2.45 | 603.0 | 2425 | 3.63 | 668.0 | |
| Hydroxychloride | 45.8 | 935.5 | 1.17 | 800.0 | 1519 | 2.45 | 620.1 | 2455 | 3.59 | 683.8 | |
| Pooled SEM | 0.200 | 9.71 | 0.013 | 5.98 | 15.7 | 0.025 | 5.20 | 21.2 | 0.035 | 4.36 | |
| P-value for source | – | 0.372 | 0.633 | 0.365 | 0.065 | 0.982 | 0.025 | 0.330 | 0.438 | 0.017 | |
| Main effect of level | |||||||||||
| High | 46.0 | 935.7 | 1.18 | 792.9 | 1472 | 2.42 | 608.1 | 2407 | 3.57 | 674.3 | |
| Low | 45.9 | 947.6 | 1.19 | 796.3 | 1525 | 2.48 | 614.9 | 2473 | 3.64 | 679.3 | |
| SEM | 0.200 | 9.71 | 0.013 | 5.98 | 15.7 | 0.025 | 5.20 | 21.2 | 0.035 | 4.36 | |
| P-value for level | – | 0.389 | 0.483 | 0.641 | 0.021 | 0.093 | 0.397 | 0.036 | 0.132 | 0.594 | |
1Means for simple effects are with n of 15 replicate pens per treatments, whereas means for main effects are with n of 30 replicate pens per treatment (15 broiler chickens per replicate pen).
2Sulfate Zn and Cu were analytical grade zinc sulfate monohydrate and copper sulfate pentahydrate, respectively. Hydroxychloride Zn and Cu were IntelliBond Zn and IntelliBond Cu, respectively.
IBW—initial body weight; WG—body weight gain; FI—average feed intake per bird; G: F—gain to feed ratio (g/kg).
The carcass yield data for the broiler chickens receiving the experimental diets are shown in Table 5. There was no source × level interaction. Broiler chickens receiving hydroxychloride Zn and Cu had greater % breast (P < 0.05) and lower % back and ribs than those receiving sulfate Zn and Cu. In addition broiler chickens receiving the lower Zn level had greater % yield and breast (P < 0.05). There was significant (P < 0.05) source × level interaction for litter score. Litter score was greater (P < 0.05) in broiler chickens receiving lower level of ZSM but there was no difference in litter score of broiler chickens receiving hydroxychloride Zn and Cu (Table 6). In addition, footpad score was marginally greater in broiler chickens receiving hydroxychloride Zn and Cu. It must be stated that both footpad and litter scores were very low in the current experiment.
Table 5.
Carcass yield (%) of broilers sulfate or hydroxychloride zinc and copper at 2 supplemental levels of zinc.1
| Zn and Cu source2 | Zn level | % yield | % breast | % drum | % thigh | % back and ribs | % wing | % abdominal fat |
|---|---|---|---|---|---|---|---|---|
| Sulfate | High | 68.5 | 29.1 | 13.4 | 18.3 | 28.4 | 10.2 | 1.349 |
| Low | 69.1 | 29.6 | 13.3 | 18.2 | 28.2 | 10.1 | 1.243 | |
| Hydroxychloride | High | 67.9 | 30.0 | 13.5 | 18.2 | 27.9 | 10.1 | 1.240 |
| Low | 69.4 | 30.2 | 13.3 | 17.8 | 28.0 | 10.0 | 1.246 | |
| SEM | 0.308 | 0.153 | 0.088 | 0.141 | 0.165 | 0.052 | 0.048 | |
| Source × level | 0.170 | 0.504 | 0.281 | 0.511 | 0.369 | 0.311 | 0.252 | |
| Sulfate | 68.8 | 29.4 | 13.4 | 18.3 | 28.3 | 10.1 | 1.296 | |
| Hydroxychloride | 68.6 | 30.1 | 13.4 | 18.0 | 27.9 | 10.1 | 1.243 | |
| SEM | 0.218 | 0.108 | 0.063 | 0.099 | 0.117 | 0.037 | 0.034 | |
| P-value for source | 0.628 | <0.001 | 0.681 | 0.079 | 0.030 | 0.103 | 0.277 | |
| High | 68.2 | 29.6 | 13.4 | 18.2 | 28.2 | 10.2 | 1.295 | |
| Low | 69.2 | 29.9 | 13.3 | 18.0 | 28.1 | 10.1 | 1.244 | |
| SEM | 0.218 | 0.108 | 0.063 | 0.099 | 0.117 | 0.037 | 0.034 | |
| P-value for level | 0.001 | 0.028 | 0.130 | 0.095 | 0.703 | 0.061 | 0.305 |
1Means for simple effects are with n of 15 replicate pens per treatments, whereas means for main effects are with n of 30 replicate pens per treatment (7 broiler chickens per replicate pen).
2Sulfate Zn and Cu were analytical grade zinc sulfate monohydrate and copper sulfate pentahydrate, respectively. Hydroxychloride Zn and Cu were IntelliBond Zn and IntelliBond Cu, respectively.
Table 6.
Litter and footpad scores of broilers receiving sulfate or hydroxychloride zinc and copper at 2 supplemental levels of zinc.1
| Zn and Cu source2 | Zn level | Litter score | Footpad score3 |
|---|---|---|---|
| Sulfate | High | 1.34b | 1.01 |
| Low | 1.67a | 1.04 | |
| Hydroxychloride | High | 1.54a,b | 1.08 |
| Low | 1.52a,b | 1.15 | |
| SEM | 0.087 | 0.037 | |
| Source × level | 0.044 | 0.591 | |
| Main effect of source | |||
| Sulfate | 1.504 | 1.027 | |
| Hydroxychloride | 1.528 | 1.113 | |
| SEM | 0.062 | 0.026 | |
| P-value for source | 0.788 | 0.024 | |
| Main effect of level | |||
| High | 1.438 | 1.047 | |
| Low | 1.594 | 1.093 | |
| SEM | 0.062 | 0.026 | |
| P-value for level | 0.082 | 0.213 | |
a,bMeans in a column, with different superscripts, are significantly different (P < 0.05).
1Means for simple effects are with n of 15 replicate pens per treatments, whereas means for main effects are with n of 30 replicate pens per treatment.
2Sulfate Zn and Cu were analytical grade zinc sulfate monohydrate and copper sulfate pentahydrate, respectively. Hydroxychloride Zn and Cu were IntelliBond Zn and IntelliBond Cu, respectively.
3Five broiler chickens per replicate pen were used in footpad scoring.
Tibia, plasma, and liver mineral contents in response to the experimental diets are shown in Table 7. Broiler chickens receiving higher level of Zn, irrespective of source, had greater (P < 0.01) tibia and plasma Zn levels. On the other hand, liver Cu was greater (P < 0.05) in broiler chickens receiving hydroxychloride Zn and Cu.
Table 7.
Zinc and copper composition in different tissues of broilers receiving sulfate or hydroxychloride zinc and copper at 2 supplemental levels of zinc.1
| Zn and Cu source2 | Level | Tibia Zn, ppm | Tibia ash, % | Plasma Cu, ppm | Plasma Zn, ppm | Liver Cu, ppm |
|---|---|---|---|---|---|---|
| Sulfate | High | 313.2 | 22.4 | 0.185 | 1.808 | 3.402 |
| Low | 303.4 | 22.7 | 0.187 | 1.693 | 3.437 | |
| Hydroxychloride | High | 319.2 | 22.8 | 0.213 | 2.073 | 3.545 |
| Low | 299.8 | 22.7 | 0.190 | 1.684 | 3.573 | |
| SEM | 3.35 | 0.184 | 0.012 | 0.122 | 0.057 | |
| Source × level | 0.156 | 0.171 | 0.300 | 0.266 | 0.959 | |
| Main effect of source | ||||||
| Sulfate | 308.3 | 22.6 | 0.186 | 1.751 | 3.419 | |
| Hydroxychloride | 309.5 | 22.7 | 0.202 | 1.878 | 3.559 | |
| SEM | 2.37 | 0.130 | 0.008 | 0.086 | 0.040 | |
| P-value for source | 0.729 | 0.292 | 0.386 | 0.301 | 0.019 | |
| Main effect of level | ||||||
| High | 316.2 | 22.6 | 0.199 | 1.940 | 3.473 | |
| Low | 301.6 | 22.7 | 0.189 | 1.689 | 3.505 | |
| SEM | 2.37 | 0.130 | 0.008 | 0.086 | 0.040 | |
| P-value for level | <0.001 | 0.680 | 0.300 | 0.045 | 0.579 | |
1Means for simple effects are with n of 15 replicate pens per treatments, whereas means for main effects are with n of 30 replicate pens per treatment (4 broiler chickens per replicate pen).
2Sulfate Zn and Cu were analytical grade zinc sulfate monohydrate and copper sulfate pentahydrate, respectively. Hydroxychloride Zn and Cu were IntelliBond Zn and IntelliBond Cu, respectively.
DISCUSSION
The objective of the current experiment was to study the influence of 2 sources of Zn and Cu (sulfate or hydroxychloride) at 2 levels of Zn on growth performance, carcass yield, and mineral content of bone and liver in broiler chickens. There are environmental concerns regarding the use of inorganic sources of trace minerals. When minerals from these sources reach the upper digestive tract, they tend to dissociate and react with other minerals and thus have reduced availability (Underwood and Suttle, 1999; Yan and Waldroup, 2006). On the other hand, only marginal quantity of hydroxychloride Zn and Cu, respectively, are soluble in water but completely soluble in weak acid (Zhang and Guo, 2007). The crystalline structure (Hawthorne and Sokolova, 2002) of the minerals makes them dissolve slowly in the digestive tract. Thus, they can be expected to promote more optimal growth performance in broilers.
Growth Performance
There have been several reports of positive response of broilers to supplementation of Zn and Cu from various sources (Arias and Koutsos, 2006; Huang et al., 2007; Liu et al., 2015), but others have also reported minimal or no effect (Kidd et al., 1993; Wang et al., 2002). Several factors, individually or in combination, can influence the response observed when trace minerals are supplemented to diets. For example, it has been suggested that response to Zn supplementation is usually lower in corn-SBM diet because the intrinsic Zn in the ingredients may be sufficient to meet birds’ requirement (Mwangi et al., 2017). Wheat-based diets tend to have higher digesta viscosity in the gut (Choct, 2006), resulting in stressing of the gut. Phytase, which usually increases availability of trace minerals in feedstuffs (Yi et al., 1996), may reduce the observable response to their use in diet.
Growth performance at the lower Zn supplemental level was more superior than at higher Zn levels in the current experiment. In most studies that have reported positive effect of Zn supplementation (Huang et al., 2007; Liu et al., 2015), performance had been observable at the lowest supplemental level without significant improvement at higher levels. Feng et al. (2009) observed poorer G: F in broilers receiving 120 ppm Zn on day 42. The poorer growth performance response observed at the higher supplemental Zn level in the current study may indicate a curvilinear response to increase Zn supplemental level. For example, it has been reported that proportional Zn absorption decreases with increase in luminal Zn (Coppen and Davis, 1987) possibly due to shift in uptake location in the digestive tract (Yu et al., 2008). In Huang et al. (2007) study, the expression level of pancreas Zn transporter decreased after 80 ppm. This phenomenon in combination with the slow release of hydroxychloride minerals might explain the differences between high and low Zn levels. Interestingly though, the observation of the effect on G: F indicates that the negative effect of higher supplemental Zn was driven by ZSM, and in fact, broilers receiving higher level of hydroxychloride Zn had marginally better G: F that those receiving the lower level.
The superior performance of birds receiving the diet supplemented with hydroxychloride Zn and Cu may be due to the superior availability of Zn and Cu from these sources (Miles et al., 1998; Batal et al., 2001). Another reason may be improved efficiency of nutrient utilization as observed for Cu-supplemented diet (Arias and Koutsos, 2006; Rochell et al., 2017) or enhanced resistance to disease or stressors. The effect of Zn and Cu supplementation from the 2 sources on meat yield was similar to the effect that the sources and Zn levels had on growth performance. Both percentage eviscerated carcass and breast yields were increased, whereas the percentage back and ribs was reduced. The observation of the positive effects of hydroxychloride relative to sulfate Zn and Cu, and at lower Zn level, indicates that the minerals enhanced the development of the economically important parts of the broiler chickens.
There are few reports of Zn and Cu effects on meat yield. Liu et al. (2011) observed that Zn supplementation increased intramuscular fat in broiler breast meat. Studies with steers showed that Zn supplementation generally improved carcass quality including marbling as well as yield and quality grades (Malcolm et al., 2000; Spears and Kegley, 2002). There will be more need to understand the effect of Zn on meat yield because a better understanding of the effect may help with understanding of whole body energy utilization in view of differential use of dietary energy for deposition of body protein or fat (Olukosi et al., 2008).
Bone, Liver, and Plasma Mineral Content
Liver is the main storage organ for Cu (Suttle, 2010). The liver Cu concentrations in this study were greater in the birds fed hydroxychloride minerals compared to sulfate minerals. The level of Zn fed did not have an effect on liver Cu, indicating there was no clear interaction between Zn and Cu in both mineral sources.
Bones are the functional reserve of Zn (Suttle, 2010) and have been used as sensitive indicator of response to Zn supplementation (Wedekind et al., 1992; Ma et al., 2018). Mwangi et al. (2017) noted that chicks fed low Zn (imprinted diet) caught up on day 21 with those fed normal-level Zn with regard to their liver Zn. This shows the ability of chicks to regulate their Zn utilization to achieve homeostasis when there is low dietary Zn. Liu et al. (2015) reported that supplementation of Zn from various sources increased the level of Zn in the liver, muscle, and breast and upregulated the expression of Zn-containing superoxide dismutase. The effect was increased antioxidant activity in the tissues. The positive effect of Zn was irrespective of Zn source or level. Wang et al. (2012) reported an increase in liver Zn content in piglets. Liu et al. (2015) observed an increase in liver, breast muscle, and thigh Zn content when Zn was supplemented at 60 ppm (dietary level of 90 ppm). The increase in tissue Zn content was not affected by Zn source.
In the current experiment, Zn source had no effect on tibia ash or tibia Zn but tibia Zn was greater in broilers chickens receiving the higher level of Zn supplementation. In Huang et al. (2007) study, bone content of Zn increased in response to Zn supplementation from ZSM up to 80 ppm. Sunder et al. (2008) observed a reduction in bone ash at Zn supplemental level greater than 160 ppm, which occurred alongside an increase in bone content of Zn and a decrease in Ca and P content of bone at the higher Zn supplemental levels. Consequently, it appears that higher Zn level interferes with Ca and P deposition in the bone thus reducing bone mineralization, and potentially growth performance.
Zhang and Guo (2007) observed a linear increase in plasma and bone Zn in piglets receiving up to pharmacological dietary Zn level and reported Zn bioavailability in hydroxychloride Zn, relative to ZnO to be >120%. The plasma Zn content in the current experiment indicates that the higher Zn supplemental level released greater quantity of Zn into the blood, whereas the different sources of Zn produced similar plasma Zn level. It is reasonable that up to a certain minerals concentration level, the greater concentration of luminal Zn will translate into greater absolute quantity absorbed especially because the absorption of the mineral is driven via both active and passive mechanisms (Krebs, 2000).
Copper was provided along with Zn from hydroxychloride and sulfate sources but plasma Cu level was neither influenced by Cu source nor Zn level although liver Cu was greater with consumption of hydroxychloride Cu. This observation suggests greater availability of Cu in hydroxychloride Cu and mimics effect of higher Cu level on liver Cu content (Ledoux et al., 1987). It can be reasoned that cumulative effects of Cu and Zn in their hydroxychloride form accounted for their superior effects on growth performance and carcass yield observed in the current study.
On the basis of these observations, it can be concluded from the current experiment that hydroxychloride Zn and Cu were more efficacious than their sulfate counterparts in promoting growth performance and enhancing meat yield. The negative effects of higher supplemental Zn level appear to be primarily related to sulfate Zn usage and were not related to Zn deposition in the tissue. Therefore, this aspect will need to be further studied to help understand if greater level of hydroxychloride Zn can offer additional benefits.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the help of Andrew Scott and Jenna Morrison in care of animals and in carcass processing. SRUC receives support from Scottish government (RESAS).
REFERENCES
- Arias V. J., Koutsos E. A.. 2006. Effects of copper source and level on intestinal physiology and growth of broiler chickens. Poult. Sci. 85:999–1007. [DOI] [PubMed] [Google Scholar]
- Batal A. B., Parr T. M., Baker D. H.. 2001. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 80:87–90. [DOI] [PubMed] [Google Scholar]
- Cao J., Henry P. R., Davis S. R., Cousins R. J., Miles R. D., Littell R. C., Ammerman C. B.. 2002. Relative bioavailability of organic zinc sources based on tissue zinc and metallothionein in chicks fed conventional dietary zinc concentrations. Anim. Feed Sci. Technol. 101:161–170. [Google Scholar]
- Choct M. 2006. Enzymes for the feed industry: past, present and future. Worlds Poult. Sci. J. 62:5–16. [Google Scholar]
- Coppen D. E., Davies N. T.. 1987. Studies on the effects of dietary zinc dose on Zn absorption in vivo and on the effects of Zn status on Zn absorption and body loss in young rats. Br. J. Nutr. 57:35–44. [DOI] [PubMed] [Google Scholar]
- Davis G. K., Mertz W.. 1987. Copper. Pages 301–364 in Trace Elements in Human and Animal Nutrition. 5th ed Vol. 1 Mertz W., ed. Academic Press, New York. [Google Scholar]
- European Food Safety Authority (EFSA). 2011. Scientific Opinion on the safety and efficacy of di copper chloride tri hydroxide (tribasic copper chloride, TBCC) as feed additive for all species. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA J. 9:2355. [Google Scholar]
- European Food Safety Authority (EFSA). 2012. Scientific Opinion on the safety and efficacy of tetra-basic zinc chloride for all animal species. EFSA J. 10:2672. [Google Scholar]
- Feng J., Ma W. Q., Niu H. H., Wu X. M., Wang Y., Feng J.. 2010. Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace Elem. Res. 133:203–211. [DOI] [PubMed] [Google Scholar]
- Hawthorne F. C., Sokolova E.. 2002. Simonkolleite, Zn5(OH)8Cl2(H2O), a decorated interrupted-sheet structure of the form [Mϕ2]4. The Canadian Mineralogist 40:939–946. [Google Scholar]
- Huang Y. L., Lu L., Luo X. G., Liu B.. 2007. An optimal dietary zinc level of broiler chicks fed a corn-soybean meal diet. Poult. Sci. 86:2582–2589. [DOI] [PubMed] [Google Scholar]
- Jarosz Ł., Marek A., Grądzki Z., Kwiecień M., Z·ylińska B., Kzczmarek B.. 2017. Effect of feed supplementation with zinc glycine chelate and zinc sulfate on cytokine and immunoglobulin gene expression profiles in chicken intestinal tissue. Poult. Sci. 96:4224–4235. [DOI] [PubMed] [Google Scholar]
- Kidd M. T., Anthony N. B., Newberry L. A., Lee S. R.. 1993. Effect of supplemental zinc in either a corn-soybean or a milo and corn-soybean meal diet on the performance of young broiler breeders and their progeny. Poult. Sci. 72:1492–1499. [Google Scholar]
- Krebs N. F. 2000. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 130:1374S–1377S. [DOI] [PubMed] [Google Scholar]
- Ledoux D. R., Miles R. D., Ammerman C. B., Harms R. H.. 1987. Interaction of dietary nutrient concentration and supplemental copper on chick performance and tissue copper concentrations. Poult. Sci. 66:1379–1384. [DOI] [PubMed] [Google Scholar]
- Liu Z. H., Lu L., Li S. F., Zhang L. Y., Xi L., Zhang K. Y., Luo X. G.. 2011. Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult. Sci. 90:1782–1790. [DOI] [PubMed] [Google Scholar]
- Liu Z. H., Lu L., Wang S. R. L., Lei H. L., Li S. F., Zhang L. Y., Luo X. G.. 2015. Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult. Sci. 94:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. G., Ji F., Lin Y. X., Steward F. A., Lu L., Liu B., Yu S. X.. 2005. Effects of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and oxidation stability of vitamin E in feed. Poult. Sci. 84:888–893. [DOI] [PubMed] [Google Scholar]
- Ma W., Niu H., Feng J., Wang Y., Feng J.. 2011. Effects of zinc glycine chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol. Trace Elem. Res. 142:546–556. [DOI] [PubMed] [Google Scholar]
- Ma Y. L., Lindemann M. D., Webb S. F., Rentfrow G.. 2018. Evaluation of trace mineral source and preharvest deletion of trace minerals from finishing diets on tissue mineral status in pigs. Asian-Australas J. Anim. Sci. 31:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm C. K. J., Duff G. C., Gunter S. A.. 2000. Effects of supplemental zinc concentration and source on performance, carcass characteristics, and serum values in finishing beef steers. J. Anim. Sci. 78:2801–2808. [DOI] [PubMed] [Google Scholar]
- Miles R. D., O’Keefe S. F., Henry P. R., Ammerman C. B., Luo X. G.. 1998. The effect of dietary supplementation with copper sulfate or tribasic copper chloride on broiler performance, relative copper bioavailability, and dietary prooxidant activity. Poult. Sci. 77:416–425. [DOI] [PubMed] [Google Scholar]
- Mwangi S., Timmons J., Ao. T., Paul M., Macalintal L., Pescatore A., Cantor A., Ford M., Dawson K. A.. 2017. Effect of zinc imprinting and replacing inorganic zinc with organic zinc on early performance of broiler chicks. Poult. Sci. 96:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O. A., Cowieson A. J., Adeola O.. 2008. Energy utilization and growth performance of broilers receiving diets supplemented with enzymes containing carbohydrase or phytase activity individually or in combination. Br. J. Nutr. 99:682–690. [DOI] [PubMed] [Google Scholar]
- Pang Y., Applegate T. J.. 2006. Effects of copper source and concentration on in vitro phytate phosphorus hydrolysis by phytase. J. Agric. Food Chem. 54:1792–1796. [DOI] [PubMed] [Google Scholar]
- Perez V., Shanmugasundaram R., Sifri M., Parr T. M., Selvaraj R. K.. 2017. Effects of hydroxychloride and sulfate form of zinc and manganese supplementation on superoxide dismutase activity and immune responses post lipopolysaccharide challenge in poultry fed marginally lower doses of zinc and manganese. Poult. Sci. 96:4200–4207. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. E. 2006. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: Dietary tools, Part 1. Anim. Biotechnol. 17:207–215. [DOI] [PubMed] [Google Scholar]
- Prasad A. S. 1984. Discovery and importance of zinc in human nutrition. Fed. Proc. 43:2829–2834. [PubMed] [Google Scholar]
- Rochell S. J., Usry J. L., Parr T. M., Parsons C. M., Dilger R. N.. 2017. Effects of dietary copper and amino acid density on growth performance, apparent metabolizable energy, and nutrient digestibility in Eimeria acervulina-challenged broilers. Poult. Sci. 96:602–610. [DOI] [PubMed] [Google Scholar]
- Sahraei M., Janmmohamdi H., Taghizadeh A., Cheraghi S.. 2012. Effect of different zinc sources on tibia bone morphology and ash content of broiler chickens. Adv. Biol. Res. 6:128–132. [Google Scholar]
- Spears J. W., Kegley E. B.. 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80:2747–2752. [DOI] [PubMed] [Google Scholar]
- Suttle N. F. 2010. The Mineral Nutrition of Livestock. 4th ed CABI publishing, Oxfordshire, UK. [Google Scholar]
- Sunder G. S., Panda A. K., Gopinath N. C. S., Rama Rao S. V., Raju M. V. L. N., Reddy M. R., Kumar Ch. V.. 2008. Effects of higher levels of zinc supplementation on performance, mineral availability, and immune competence in broiler chickens. J. Appl. Poult. Res. 17:79–86. [Google Scholar]
- Underwood E. J., Suttle N. F.. 1999. The Mineral Nutrition of Livestock. 3rd ed.CABI Publishing, Oxfordshire, UK. [Google Scholar]
- Wang M. Q., Tao W. J., Ye S. S., Du Y. J., Wang C., Shen S. X.. 2012. Effects of dietary pharmacological zinc on growth, liver metallothionein, Cu, Zn-SOD concentration and serum parameters in piglets. J. Anim. Vet. Adv. 11:1390–1394. [Google Scholar]
- Wang X., Fosmire G. J., Gay C. V., Leach R. M.. 2002. Short-Term zinc deficiency inhibits chondrocyte proliferation and induces cell apoptosis in the epiphyseal growth plate of young chickens. J. Nutr. 132:665–673. [DOI] [PubMed] [Google Scholar]
- Wedekind K. J., Hortin A. E., Baker D. H.. 1992. Methodology for assessing zinc bioavailability: efficacy estimates for zinc-methionine, zinc sulfate, and zinc oxide. J. Anim. Sci. 70:178–187. [DOI] [PubMed] [Google Scholar]
- Yan F, Waldroup P. W.. 2006. Evaluation of Mintrex® manganese as a source of manganese for young broilers. Int. J. Poult. Sci. 5:708–713. [Google Scholar]
- Yi Z., Kornegay E. T., Denbow D. M.. 1996. Supplemental microbial phytase improves zinc utilization in broilers. Poult. Sci. 75:540–546. [DOI] [PubMed] [Google Scholar]
- Yu Y., Lu L., Luo X. G., Liu B.. 2008. Kinetics of zinc absorption by in situ ligated intestinal loops of broilers involved in zinc transporters. Poult. Sci. 87:1146–1155. [DOI] [PubMed] [Google Scholar]
- Zhang B., Guo Y.. 2007. Beneficial effects of tetrabasic zinc chloride for weanling piglets and the bioavailability of zinc in tetrabasic form relative to ZnO. Anim. Feed Sci. Technol. 135:75–85. [Google Scholar]
- Zhang B., Guo Y.. 2009. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 102:687–693. [DOI] [PubMed] [Google Scholar]
- Zhang Y. N., Zhang H. J., Wang J., Yue H. Y., Qi X. L., Wu S. G., Qi G. H.. 2017. Effect of dietary supplementation of organic or inorganic zinc on carbonic anhydrase activity in eggshell formation and quality of aged laying hens. Poult. Sci. 96:2176–2183. [DOI] [PubMed] [Google Scholar]


