ABSTRACT
The differences in physiological and immunological parameters and pathological damage to organ tissues exposed to chronic heat stress provide the basis for evaluating heat resistance of different chicken breeds (white recessive rock [WRR] and The Lingshan [LS]). Ninety broilers of each breed were divided equally into a chronic heat stress group and a no heat stress group. The effects of chronic heat stress on the physiological and immunological parameters of broilers were analyzed using flow cytometry, ELISA, RT-qPCR, etc. Under heat stress conditions: (1) H and H/L values were significantly increased (P < 0.01) in the 2 breeds, and were higher in the WRR broilers than in the LS broilers at a late stage (P < 0.05). Although the corticosterone levels were also significantly increased (P < 0.01) in both breeds, they were lower in the 49 d WRR broilers than in the LS broilers (P < 0.01). The number of leukocytes were significantly increased in the 49 d WRR broilers (P < 0.01), whereas the number of CD3+, CD8+ cells, and erythrocytes were significantly reduced (P < 0.05). A significantly (P < 0.01) lower number of CD3+, CD4+ T-lymphocytes, and CD4+/CD8+ were present in WRR compared to that in the LS broilers. (2) The HSP70 transcript was significantly increased in the WRR broilers (P < 0.01), and was higher than the level in the LS broilers. The expression level of HSP70 protein was significantly (P < 0.05) increased in WRR broilers. (3) The WRR broilers developed cardiac and leg muscle inflammatory cellular hyperplasia and local inflammatory lesions, as well as cerebral meningitis and inflammatory hyperplasia of the brain tissue. The LS broilers developed mild cerebral inflammatory hyperplasia and mild inflammatory cellular proliferation in the leg muscle. In conclusion, under heat stress conditions, the relative physiological and immunological parameters were worse in the WRR broilers than in the LS broilers. The WRR broilers showed poor heat tolerance as evidenced from the expression of HSP70 and the extent of histopathological damages.
Keywords: broiler, heat stress, physiological and immunological parameters, HSP70 gene, pathological damage
INTRODUCTION
The recessive white feathered or white recessive rock (WRR) chicken is a fast-growing broiler breed introduced to China. The Lingshan (LS) chicken is a local quality broiler breed. In hot Chinese summers, temperatures can be very high, and the difference in the response of these 2 breeds to heat stress is not clear. When ambient temperatures rise above 32°C, the body temperatures of broilers exceed the normal range. As a result, their respiratory rate rises from 20 times/min to 120–160 times/min, and there is an increase in the heart rate. Under these conditions, the broilers typically show shortness of breath, stretched wings, and reduced feed intake (Lisanne et al., 2016). Mignon-Grasteau et al. (2015) showed that the intensity of chronic heat stress is relatively weak, the duration of stress is relatively long, and that such instances occur at a higher frequency. Chronic heat stress is common and frequent in livestock and poultry production.
Studies have shown that chronic heat stress can lead to changes in the physiology of poultry, which can affect the production (Van Goor et al., 2015). Such stress results in decreased appetite, slowed growth, and increased morbidity and mortality. Heat stress affects immune function, number of T cells, secretion of antibodies and cytokines, immunoglobulin concentrations, and proliferation of lymphocytes (do Amaral et al., 2010; Wieten et al., 2010). It causes neurological damage and cerebral dysfunction (Lemiale et al., 2008), and also causes physiological dysfunctions, such as an increase in the plasma corticosterone concentration (Chen et al., 2013). In addition, heat stress can cause irreversible pathological damage to organs (Zhu et al., 2009). Buttari et al. (2015) studied erythrocyte and immune system, according to which, under normal physiological conditions, the content of blood cells in an animal is maintained in a dynamic balance by constant depletion and supplementation; this plays an important role as an immune barrier. The physiological parameters of erythrocyte and leukocytes may, therefore, serve as a reference in determining whether the immune system of the animal is affected (Miao and Xiao, 2018).
Currently, the heat shock protein 70 kDa gene (HSP70) is known to encode one of the most well-understood HSPs of the HSP family. When a broiler is subjected to heat stress, HSPs are rapidly induced and their contents are increased, which rapidly enhances the heat tolerance capacity of the body (Das et al., 2010). In addition, HSPs play important roles in cell growth, development, differentiation, and protein synthesis (Jason et al., 2014), and also play a central regulatory role in the stress response at the level of both tissues and cells. It has been suggested that the highly conserved evolutionary features of HSPs and their ability to directly sense the changes in the extracellular environment may serve as a biological marker for stress response (Khalil et al., 2011; Manjari et al., 2015).
In the present study, we systematically analyzed the differences in the main physiological and immunological parameters between 2 breeds of broilers. We also assessed the pathological damages sustained by tissues and organs of the broilers exposed to heat stress to determine the basis for heat resistance of the 2 varieties and to establish a foundation for the prevention and control of heat stress.
MATERIALS AND METHODS
Experimental Animals and Rearing Conditions
Artificial stress test. Guangdong Wens Food Group Co. Ltd. (Xinxing county, Yunfu city, Guangdong province, China) provided 1 d LS broilers (n = 90, female) and WRR broilers (n = 90, female). These were reared to 28 d in artificial chambers (Canada Conviron, PGV36: Height 260 cm × Width 241 cm × Depth 341 cm). Of the 90 broilers of each breed, 45 were randomly assigned to a heat stress group and 45 to a no heat stress group. Broilers were transferred to and maintained in cages (9 per cage: H 40 cm × W 100 cm × D 80 cm) in an artificial chamber, under a relative humidity of more than 65%, and were able to feed and drink ad libitum. The room temperature was maintained at 34 ± 1°C, from 9:00 to 18:00 h, and 26 ± 1°C, from 18:00 to 9:00 h for the heat stress group, and maintained at a constant 26 ± 1°C for the no heat stress group.
Sample Preparation
The heterophil (H), heterophil/lymphocyte (H/L) value, and the physiological parameter of blood, and the content of serum corticosterone were measured at 35, 42, and 49 d broilers in the venous blood drawn from under the wings of the broilers. Eight samples of blood were used for total RNA extraction and RT-qPCR detection of the HSP70 gene at 42 and 49 d broilers. Protein extraction was performed from 3 blood samples chosen randomly from these 8 samples. The HSP70 protein was detected by western blotting. The concentrations of CD3+, CD4+, and CD8+ were also measured at 49 d broilers. The brain, leg muscle, and cardiac tissue samples were harvested at 49 d from both the groups and were used for histological analysis. The histopathological changes in the different organs were observed before and after the heat stress.
Determination of H and H/L Values
Venous blood samples were collected, and EDTA was added to the blood as an anticoagulant. Wright-Giemsa complex staining was performed to prepare the blood samples for microscopy analysis. The cells were classified and assayed by Guangzhou Exons Biological Technology Co. Ltd., following the techniques and calculations described by Campo and Davila (2002).
Detection of CD3+, CD4+, and CD8+ T Cells
The venous blood samples were collected from the broilers, and heparin was added to them as an anticoagulant. Flow cytometry was performed to detect the levels of CD3+, CD4+, and CD8+ T cells in the blood samples by Guangzhou Exons Biological Technology Co. Ltd. The procedure used for the experiment was as follows: 5 μL of CD3+ and CD8+ and 3 μL of CD4+ antibodies were added to the bottom of labeled flow tubes. The heparinized whole blood (50 μL) was added to each tube and mixed thoroughly by oscillation. The mixture was incubated for 15 min in the dark. Thereafter, hemolysis was performed by adding 2 mL of erythrocyte lysis buffer to each tube and mixing the contents by oscillation. The mixture was incubated for another 15 min in the dark. The mixture was then washed with 2 mL of PBS and centrifuged at 120 × g for 5 min. The supernatant was discarded, the cells were resuspended in 2 mL of PBS, and centrifuged again at 120 × g for 5 min. The supernatant was discarded again and the cells were resuspended in 500 μL of PBS and filtered, and were subsequently used for flow cytometry. Cell-Quest software (FACSCalibur) was used to obtain and analyze the data. The flow cytometry gating was performed according to the flow principle. The lymphocyte groups were delineated and gated, and each cell group was analyzed.
Determination of Corticosterone Content
The serum was separated after centrifugation of the coagulated venous blood samples. The corticosterone levels in the different serum samples were determined using enzyme linked immunosorbent assay (ELISA) kits by Guangzhou Exons Biological Technology Co. Ltd. Following the instructions of the ELISA kit, serial dilutions of the standard corticosterone solution were added to a row of 7 wells (0.1 mL in each well). The different samples (100 μL) from each group were added to the individual wells. The enzyme plates were covered and incubated at 37°C for 90 min. Thereafter, the plates were washed by using an automatic plate washer. Subsequently, 0.1 mL of biotinylated antibody solution was added to each well, and allowed to bind at 37°C for 60 min. The wells were then washed once with 0.01 M TBS or thrice with 0.001 M PBS, each time for 1 min. The ABC solution (0.1 mL) was then added to each well, and allowed to react at 37°C for 30 min. The wells were then washed once with 0.01 M TBS or 5 times with 0.001 M PBS. Finally, 0.1 mL of equilibrated (i.e., at 37°C for 30 min) tetramethylbenzidine chromogenic liquid was added to each well and the plate was incubated at 37°C in the dark. The addition of the stop solution (0.1 mL per well) was started when a blue-color gradient was distinguishable to the naked eye in the first 3 to 4 wells of the standard but no such difference in color was obvious in the other wells (the color reaction was not allowed to proceed for more than 30 min). The absorbance was measured at 450 nm with a microplate reader (BIORAD 680). The concentration of corticosterone in each sample was determined using the calibration curve prepared with the absorbance values for the different dilutions of the standard solution.
Determination of Physiological Parameter of the Blood Samples
The venous blood samples were collected, and EDTA was added as an anticoagulant. The different physiological parameters of the blood were determined using the Blood Cell Analyzer by Guangzhou Exons Biological Technology Co. Ltd.
Expression Analysis of HSP70
Eight blood samples were randomly taken from each of the treatment groups of both broiler breeds. The corresponding wing number (from which the blood sample was collected) was recorded. RNA was extracted from each sample. The quantified RNA solution was stored at –80°C. The cDNA was prepared by reverse transcription using PrimeScript® RT reagent Kit with gDNA Eraser (Takara Biomedical Technology (Beijing) Co., Ltd.). A pair of primers (Table 1) was designed based on the HSP70 cDNA sequences (accession number: J02579) in GenBank (http://www.ncbi.nlm.nih.gov/) using Primer 5.0 software (Premier Biosoft, Palo Alto, CA). The amplification of HSP70 gene was performed and a 259-bp product was obtained. The expression level was determined by a 2-step quantitative real-time PCR (RT-qPCR). The reaction mixture was prepared in 96-well real-time PCR plates and consisted of 1 μL of cDNA, 0.4 μL of the upstream and downstream primers (20 μM), 10 μL of SYBR® Green Realtime PCR Master Mix, and 8.6 μL of ddH2O. Each sample was analyzed in triplicate. If the Ct values of the same sample differed by more than 0.04, 2 additional reactions were performed. The cycling conditions used for RT-qPCR were as follows: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s and 60°C for 20 s. A melting curve analysis was performed over a temperature range of 65 to 95°C (in increments of 0.5°C).
Table 1.
Primers used for the RT-qPCR detection of the HSP70 gene the chicken-specific GAPDH gene.
| Gene | Primer | Sequence (5΄-3΄) | Temperature |
|---|---|---|---|
| HSP70 | Forward | CGATCTGGCTGCAATTACG | 60°C |
| Reverse | ATTTCCAGAAGCTGCATTGC | ||
| GAPDH | Forward | CGTTGACGTGCAGCAGGAACACT | 60°C |
| Reverse | CTTTGCCAGAGAGGACGGCAGC |
Western Blot Analysis
Eight blood samples were randomly taken from each of the treatment groups of both the broiler breeds. Of these, 3 samples were randomly selected for total protein extraction. The HSP70 protein was detected by western blotting. The cells were lysed by proper mixing with the RIPA lysis buffer (Solarbio, consisting 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.1% SDS), and the homogenate was centrifuged at 12,000 × g for 3 to 5 min. The supernatant was collected and the protein concentration was determined immediately using a BCA protein quantification kit. The protein samples were mixed with 5X SDS protein loading buffer, boiled at 100°C for 5 min, and electrophoresed on a 5% polyacrylamide gel. After transferring the protein bands to PVDF membrane, the membrane was incubated in the HSP70 monoclonal antibody (1:1000, Abcam) and secondary HRP-conjugated IgG (1:30,000, Abcam) sequentially. The blot was developed using enhanced chemiluminescence reagent to detect the protein expression.
Pathological Sections
Samples from brain, leg muscle, and cardiac tissues were harvested from individuals from each of the treatment groups for both the breeds of broilers (WRR: n = 3, LS: n = 3) when the broilers were 49 d. The samples were embedded in paraffin and 5-μm serial sections were cut and stained with hematoxylin and eosin. The sections were sequentially immersed in xylene twice for 10 min, each time. The sections were then dewaxed and placed in absolute ethanol twice, each time for 3 min. The sections were thereafter rinsed with water for 5 min and stained with hematoxylin for 5 to 15 min. The sections were then rinsed with water for another 5 min and placed in 0.5% hydrochloric acid–alcohol mixture for 10 s. The sections were rinsed with water again and stained with eosin for 30 to 60 s. Finally, the sections were decolored in water for 5 min and dehydrated in a graded series of alcohol—75% ethanol, 85% ethanol, twice in 95% ethanol, and twice in anhydrous ethanol—each time for 3 min. The sections were then made transparent by immersing them in xylene twice, each time for 3 min. Neutral resin was used to seal the slices, which were left to dry naturally. The sections were visualized under a microscope and photographed.
Data Analysis
The t-test was performed using the SAS 9.0 software (version 9.0, SAS Institute, Inc., Cary, NC) for comparing the differences in heat tolerance between the different breeds under different conditions.
RESULTS
Comparison of H/L Values
The trends of variation in the H and H/L values for both the breeds of broilers were similar under heat stress and no heat stress conditions. The trend showed that initially these values were significantly (P < 0.05, or P < 0.01) lower in WRR than in LS, but became significantly (P < 0.01) higher than those in the LS breed later on in the experiment; the H and the H/L values of the 2 types of broilers under heat stress were significantly (P < 0.05, or P < 0.01) higher than those in the no heat stress (Figure 1a and c). In the WRR broilers, this difference became obvious in the 35-day-old broilers, whereas the difference became apparent only in 42-day-old broilers in the LS broiler breed.
Figure 1.
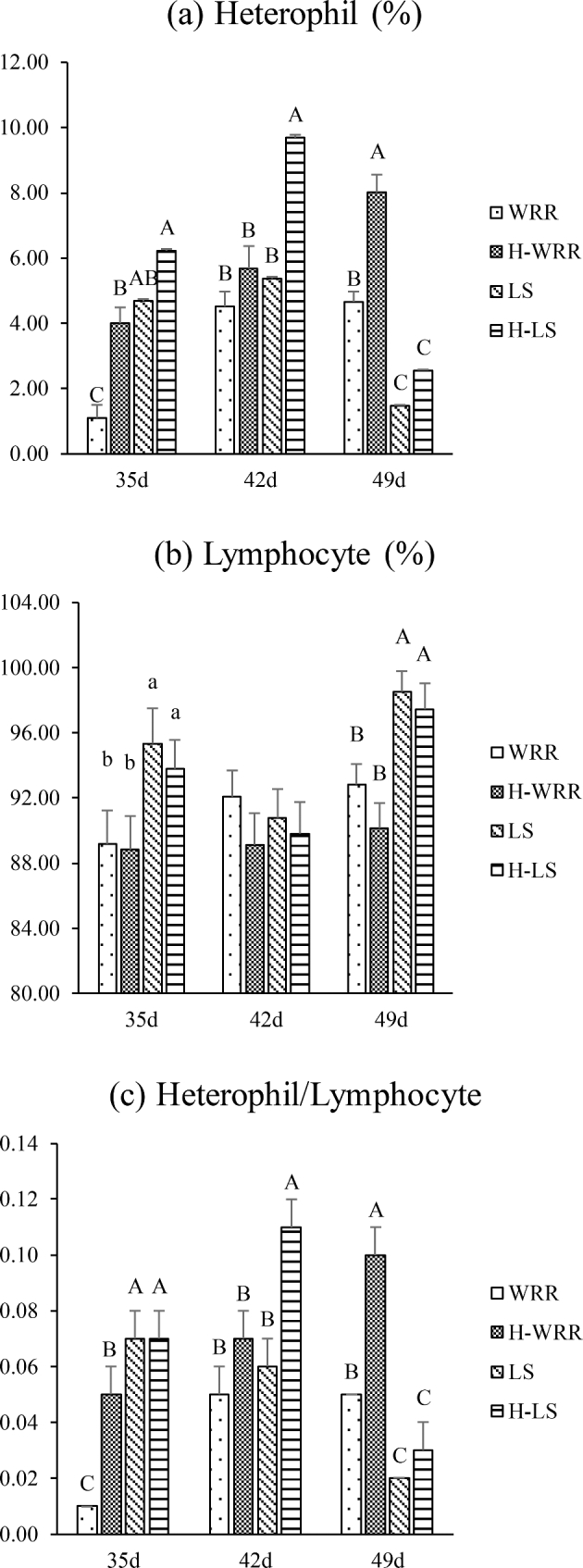
Comparison of the H, L, and H/L values in blood samples from 2 different broiler breeds (WRR and LS) and under different conditions (H-WRR or H-LS means broilers under heat stress). (a) Means the percentage of heterophil; (b) means the percentage of lymphocyte; (c) means the ratio of heterophil to lymphocyte. Significant differences exist in the values with lower case letters in the same cluster, of the same breed, at the same age (P < 0.05). Highly significant differences exist in the values with upper case letters (P < 0.01).
Physiological Parameter of Blood
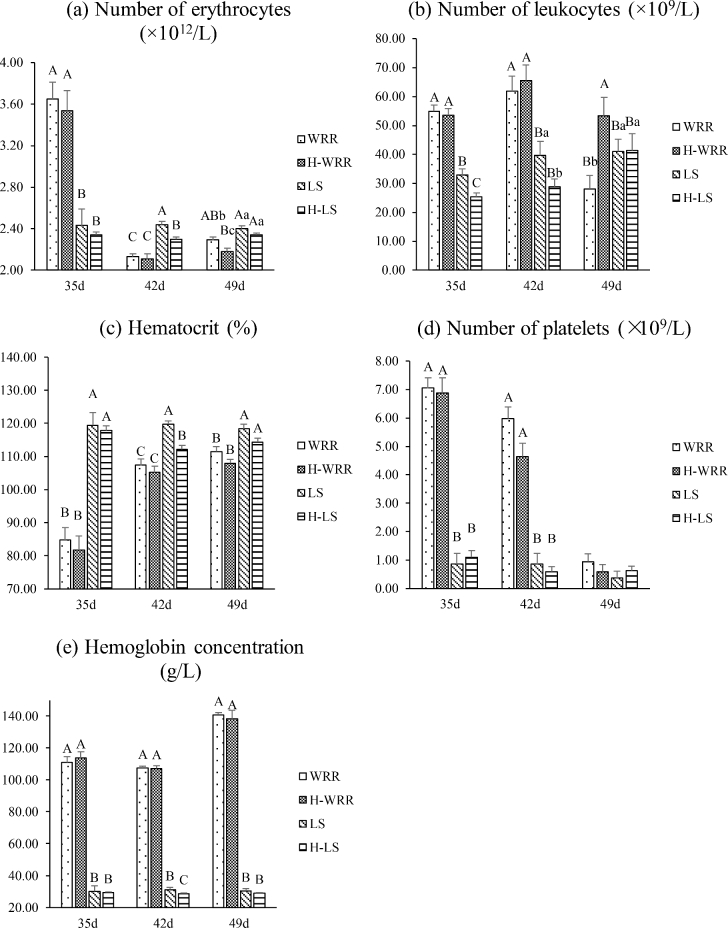
The trends of variation in the physiological parameter of blood under heat stress and non-stress conditions were similar for the 2 breeds of broilers. In response to heat stress, the WRR broilers showed a significant decrease in the erythrocytes count (P < 0.05) and an increase in leukocytes count (P < 0.01) in the 49 d individuals (Figure 2a and b). The LS broilers showed a significant decrease in the erythrocytes count, leukocytes count, hematocrit, and hemoglobin concentrations in the 35 and 42 d individuals but no significant difference in 49 d (Figure 2a, b, c, and e).
Figure 2.
Comparison of the physiological parameter in blood samples from 2 different broilers and under different conditions. (a) Means the number of erythrocytes; (b) means the number of leukocytes; (c) means the percentage of hematocrit; (d) means the number of platelets; (e) means the concentration of hemoglobin. Significant differences exist in the values with lower case letters in the same cluster, of the same breed, at the same age (P < 0.05). Highly significant differences exist in the values with upper case letters (P < 0.01).
Detection of CD3+, CD4+, and CD8+ T Cells
The trend of variations in the subsets of T-lymphocytes present in both the breeds of 49 d broilers were similar under heat stress and no stress conditions; the number of CD3+, CD8+ cells, and erythrocytes were significantly reduced (P < 0.05) under heat stress, however, a significantly (P < 0.01) lower number of CD3+, CD4+ T-lymphocytes, and CD4+/CD8+ were present in WRR compared to that in the LS broilers (Figure 3).
Figure 3.
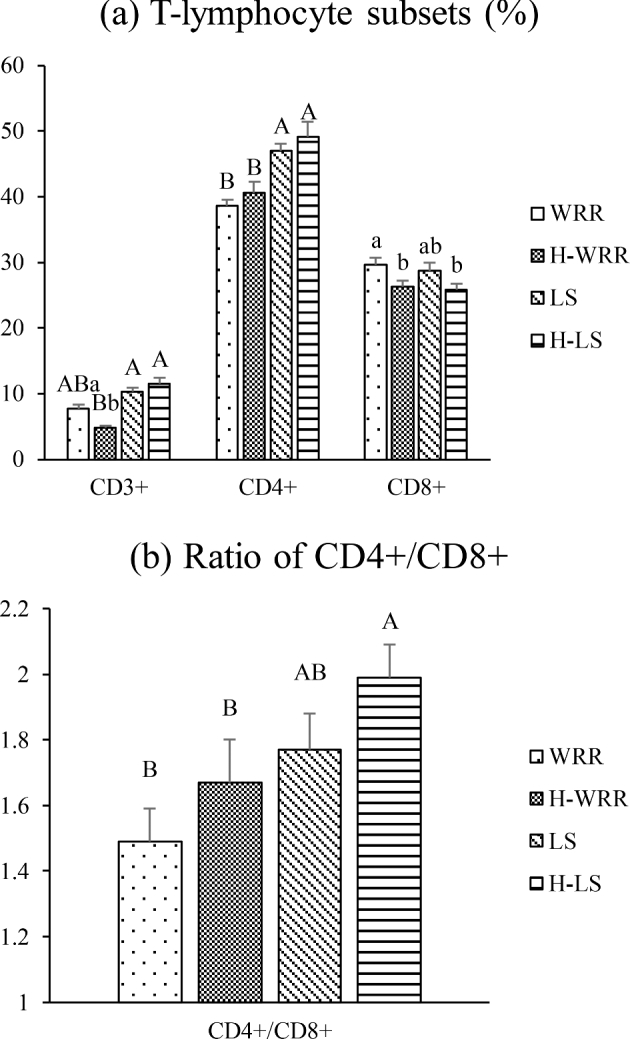
Comparison of the differences in the immunological parameter in 49-day-old broilers and under different conditions. (a) means the percentage of T-lymphocyte subsets; (b) means the ratio of CD4+ to CD8+. Significant differences exist in the values with lower case letters in the same cluster, of the same breed, at the same age (P < 0.05). Highly significant differences exist in the values with upper case letters (P < 0.01).
Comparison of Corticosterone Levels
The trends of variations in the corticosterone concentration in individuals exposed to heat stress and no heat stress varied between the 2 breeds of broilers. Under no heat stress, the corticosterone levels in the WRR broilers were significantly (P < 0.01) higher than those in the LS broilers of 35 and 42 d, but lower in 49 d; however, under heat stress, the corticosterone levels in the WRR broilers were significantly (P < 0.01) lower than those in the LS broilers; moreover; under heat stress, the cortisol concentrations in both the breeds of broilers were significantly higher than the respective concentrations under no heat stress (Figure 4a).
Figure 4.
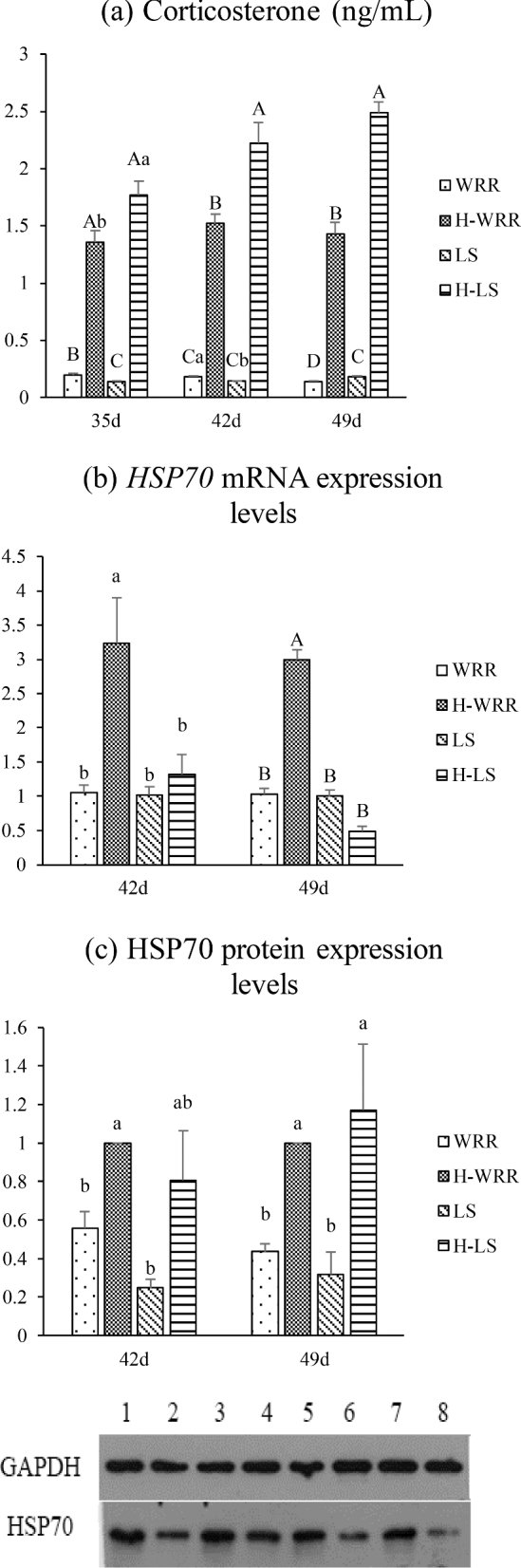
Comparison of the serum corticosterone levels (ng/mL) and HSP70 expression in the different broilers and under different conditions. (a) Means content of serum corticosterone; (b) means the HSP70 mRNA expression levels; (c) means the HSP70 protein expression levels, and in the bottom, lane 1: 42-day-old H-WRR, lane 2: 42-day-old WRR. lane 3: 49-day-old H-WRR, lane 4: 49-day-old WRR, lane 5: 42-day-old H-LS, Lane 6: 42-day-old WRR, lane 7: 49-day-old H-LS, lane 8: 49-day-old LS. Significant differences exist in the values with lower case letters in the same cluster, of the same breed, at the same age (P < 0.05). Highly significant differences exist in the values with upper case letters (P < 0.01).
RT-qPCR Detection of HSP70
Under heat stress, the expression level of HSP70 mRNA was significantly (P < 0.05) higher in the 42 d WRR broilers compared to the level in the LS broilers under the same conditions; significant expression levels were, however, not observed in both the breeds of broilers under no heat stress; under heat stress, the expression levels of HSP70 mRNA in the 42 d WRR broilers were significantly (P < 0.01) higher than the levels under no heat stress; in contrast, the changes in the expression levels were not significant (P > 0.05) in the LS broilers (Figure 4b).
Western Blot Analysis of HSP70 Expression in the Blood Cells of Different Broilers Under Heat Stress
The results of the western blotting, to determine the expression of HSP70 in the blood cells, were consistent with those of the RT-qPCR experiment. Under heat stress, HSP70 expression level in LS broilers was increased with no significant. For the WRR broilers at different ages, the expression level of HSP70 in the experimental group was significantly (P < 0.05) higher than that under no heat stress (Figure 4c).
Pathological Manifestation of Chronic heat Stress on Broilers
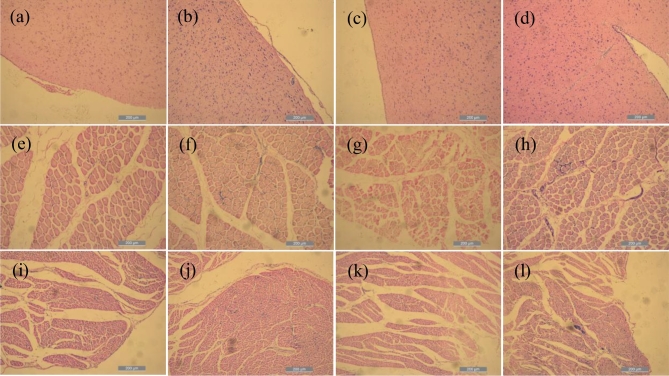
The observation of the histological sections revealed that the LS broilers under heat stress had mild inflammatory cellular hyperplasia in the brain (Figure 5a and b), leg muscles (Figure 5e and f), and cardiac (Figure 5i and j). The WRR broilers in the experimental group had cardiac and leg muscle inflammatory cellular hyperplasia and local inflammatory lesions (Figure 5g, h, k, l), as well as cerebral meningitis and inflammatory hyperplasia of brain tissue (Figure 5c and d).
Figure 5.
Histopathological section visualized by hematoxylin-eosin (HE) staining (×100). (a) Means the brain tissue of LS, (b) means the brain tissue of H-LS, (c) means the brain tissue of WRR, (d) means the brain tissue of H-WRR, (e) means the crosscut leg muscle tissue of LS, (f) means the crosscut leg muscle tissue of H-LS, (g) means the crosscut leg muscle tissue of WRR, (h) means the crosscut leg muscle tissue of H-WRR, (i) means the crosscut cardiac tissue of LS, (j) means the crosscut cardiac tissue of H-LS, (k) means the crosscut cardiac tissue of WRR, and (l) means the crosscut cardiac tissue of H-WRR.
DISCUSSION
The Level of Corticosterone Suggests the Degree of Heat Stress
Corticosterone is a glucocorticoid, secreted by the adrenal cortex. It can inhibit glucose uptake by muscle and adipose tissues, which leads to the decomposition of muscle protein and adipose tissue. This phenomenon promotes gluconeogenesis, which provides sufficient energy for the body to resist stress. This process improves the heat stress resistance of the body. An increase in the plasma corticosterone concentration, therefore, indicates higher stress resistance. Our results show that the corticosterone concentration of WRR broilers was lower than LS broilers under heat stress, and the concentration of 49 d WRR broilers was significantly (P < 0.01) decreased under no heat stress. This result indicated significant breed-specific differences that the stress resistance of WRR broilers had become weak in 49 d. Under heat stress, the serum concentrations of corticosterone in the WRR and LS broilers were significantly (P < 0.01) higher than in the broilers that were not exposed to stress, at 35, 42, and 49 d. Under heat stress, the broilers breathed with opened beaks and were prone to diarrhea, which resulted in the loss of a large quantity of water and led to dehydration. Vodyanoy (2015) observed a structural transformation of human and rat erythrocytes at elevated temperatures. As heat stress is prolonged, in vivo endotoxin can directly damage the erythrocytes, and cause liver and kidney damages (Bouaziz et al., 2007). This phenomenon inhibits the production of erythropoietin, which leads to a decline in the number of erythrocytes. Heat stress can easily lead to imbalances in the intestinal flora. Mucosal damages can cause a large number of bacteria and endotoxins to enter the blood stream, and cause a reduction in the number of leukocytes. When the heat stress is further prolonged, the immune system can be gradually stimulated to generate more leukocytes. The increase in the hematocrit and hemoglobin concentration is related to vomiting, diarrhea, and other similar symptoms. We observed significant differences in the physiological parameter of blood in the 2 breeds of broilers under heat stress and no heat stress, which indicated that significant differences exist between the 2 breeds. Under different conditions, the difference in the physiological parameter of blood appeared at 49 d in the WRR broilers, whereas such difference appeared at 35 and 42 d in the LS broilers. The results indicated that WRR broilers were still in the heat stress state at 49 d, whereas the change in the physiological parameter of blood had become more stable in the LS broilers, indicating a level of physiological adaption to the conditions. These results show that the LS broilers have stronger resistance and tolerance to heat stress than the WRR broilers.
The Immune Response and Tissue Inflammation Caused by Heat Stress
When chickens are exposed to heat stress, the content of lymphocytes and monocytes in the blood decreases and the number of heterophils increases, i.e., the H/L ratio increases (Aengwanich and Suttajit, 2010; Ruell et al., 2014). The present study showed that the variation in the trend of H and H/L values was similar in the 2 breeds of broilers under heat stress and no heat stress conditions. The difference in the specific values indicated that the breed-specific differences were significant. The H and H/L values in the heat-stressed WRR broilers were significantly higher at 35 and 49 d than in the individuals under no heat stress conditions. The LS broilers under heat stress showed no significant differences in the H and H/L values when compared to individuals that were not stressed at 49 d. These findings indicate that the LS broilers have higher resistance to heat stress. The CD3+ T cells in the peripheral blood represent the overall level of the immune system. The CD4+ T cells are helper lymphocytes, whereas the CD8+ T cells are inhibitory lymphocytes, which have a synergistic effect in the resistance to infections. To some extent, the CD4+/CD8+ ratio reflects the regulatory function of the cellular immune system. The normal CD4+/CD8+ ratio is 1.5. A reduced ratio indicates a decrease in the immune function. A high number of CD4+ T cells indicate a stronger immunity, which is not prone to infection, as the immune response is triggered swiftly by the invasion of the microorganisms. A high level of CD8+ T cells indicates a decrease in immunity and that the chicken requires a vaccine against pathogens (Zhang et al., 2015).
We observed that the trends of variations in the T-lymphocyte subsets for both the breeds of broilers were similar under heat stress and no heat stress; however, significantly lower numbers of cells were observed in WRR compared to those observed in the LS broilers. These results indicate significant breed-specific differences. Under heat stress conditions, only the numbers of CD3+ and CD8+ T cells were significantly lower than those under no heat stress in the WRR at 49 d. However, no significant change was detected in the LS broilers, and however, a significantly (P < 0.01) lower number of CD3+, CD4+ T-lymphocytes, and CD4+/CD8+ were present in WRR compared to that in the LS broilers, indicating that broilers of the LS breed have stronger heat resistance than those of the WRR breed.
With the onset of stress, the sympathetic-adrenal medullary system is stimulated in order to maintain or increase the blood supply to vital organs. The secretion of angiotensin II and aldosterone also increases, which results in an increased resistance to vasoconstriction. These effects lead to cardiac contractions and enhanced heart rate, which can damage the cardiac tissues. We observed inflammatory cellular hyperplasia in the cardiac tissues of the WRR and LS broilers. In addition, local inflammatory lesions were observed in the cardiac tissues of the WRR broilers. Our results showed that the heat stress caused pathological damage to the cardiac tissues of the broilers. Lemiale et al. (2008) demonstrated that heat stress can cause neurological damage and brain dysfunction. We also observed inflammatory cellular hyperplasia in the brain tissues of both the WRR and LS broilers. In addition, the WRR broilers had developed severe meningitis symptoms, which indicated that chronic heat stress caused greater pathological damages to the WRR broilers.
The Role of HSP70 in Inflammation or Immune Response
Heat stress increases the activity of heat stress transcription factors, which result in the rapid increase in the transcription of HSP70 gene. This leads to a high level of HSP70 expression in the cells and an increase in the content of the HSP70 protein (Ogura et al., 2007; Loc et al., 2013; Achary et al., 2014). The high temperature environment induces the production of HSP70 in brain tissues, liver, lung, heart, and leukocytes (Zulkifli et al., 2002; Zhen et al., 2006). Sohn et al. (2013) extracted mRNA from blood and tested the concentration of the HSP70 gene. The mRNA extraction from the blood reduces the harm incurred on the experimental animals and facilitates the ease of sampling. Our results showed that the expression level of HSP70 mRNA was significantly higher in the WRR broilers than in the LS broilers under heat stress. However, no differences were observed in individuals under no heat stress. In WRR broilers under heat stress, the expression level of HSP70 mRNA was significantly higher than that under no heat stress. However, there was no significant difference in the LS broilers. In addition, the western blot results were also consistent with the expression trend observed using RT-qPCR. These results consist with those of the assessment of HSP70 expression in the blood of broilers exposed to chronic heat stress (Sohn et al., 2013), wherein the authors reported elevated levels of the protein.
In conclusion, we observed significant differences in the physiological and immunological parameters between the 2 breeds of broilers under heat stress and no heat stress conditions. In particular, breed-specific differences were more prominent under the heat stress condition. The LS breed showed a stronger resistance to heat stress as revealed by most of the parameter. Chronic heat stress caused histopathological damages in both the breeds of broilers; however, the WRR broilers were found to have sustained more severe tissue damage.
ACKNOWLEDGMENTS
We thank the National Science and Technology Support Program (2014BAD08B08) and the National Natural Science Foundation (30972093) for sponsorship and support.
REFERENCES
- Aengwanich W., Suttajit M.. 2010. Effect of polyphenols extracted from Tamarind (Tamarindus indica L.) seed coat on physiological changes, heterophil/lymphocyte ratio, oxidative stress and body weight of broilers (Gallus domesticus) under chronic heat stress. Anim. Sci. J. 81:264–270. [DOI] [PubMed] [Google Scholar]
- Achary B. G., Campbell K. M., Co I. S., Gilmour D. S.. 2014. RNAi screen in Drosophila larvae identifies histone deacetylase 3 as a positive regulator of the hsp70 heat shock gene expression during heat shock. Biochim. Biophys. Acta 1839:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz H., Croute F., Boudawara T., Soleilhavoup J. P., Zeghal N.. 2007. Oxidative stress induced by fluoride in adult mice and their suckling pups. Exp. Toxicol. Pathol. 58:339–349. [DOI] [PubMed] [Google Scholar]
- Buttari B., Profumo E., Riganò R.. 2015. Crosstalk between red blood cells and the immune system and its impact on atherosclerosis. BioMed Res. Int. 2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo J. L., Davila S. G.. 2002. Estimation of heritability for heterophil:lymphocyte ratio in chickens by restricted maximum likelihood. Effects of age, sex, and crossing. Poult. Sci. 81:1448–1453. [DOI] [PubMed] [Google Scholar]
- Chen Z. Y., Gan J. K., Xiao X., Jiang L. Y., Zhang X. Q., Luo Q. B.. 2013. The association of SNPs in Hsp90β gene 5΄ flanking region with thermo tolerance traits and tissue mRNA expression in two chicken breeds. Mol. Biol. Rep. 40:5295–5306. [DOI] [PubMed] [Google Scholar]
- Das S., Pan D., Bera A. K., Rana T., Bandyopadhyay S., De S., Das S. K., Bhattacharya D., Bandyopadhyay S. K.. 2010. Stress inducible heat shock protein 70: a potent molecular and toxicological signature in arsenic exposed broiler chickens. Mol. Biol. Rep. 37:3151–3155. [DOI] [PubMed] [Google Scholar]
- do Amaral B. C, Connor E. E., Tao S., Hayen J., Bubolz J., Dahl G. E.. 2010. Heat stress abatement during the dry period influences prolactin signaling in lymphocytes. Domest. Anim. Endocrinol. 38:38–45. [DOI] [PubMed] [Google Scholar]
- Jason D., Maurie B., Lakshmi G., Antwan G., Jessica H., Brian S. J., Robert L. M.. 2014. High-throughput screen of natural product libraries for Hsp90 inhibitors. Biology(Basel). 3:101–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A. A., Kabapy N. F., Deraz S.F., Smith C.. 2011. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim. Biophys. Acta. 1816:89–104. [DOI] [PubMed] [Google Scholar]
- Lemiale V., Huet O., Vigué B., Mathonnet A., Spaulding C., Mira J. P., Carli P., Duranteau J., Cariou A.. 2008. Changes in cerebral blood flow and oxygen extraction during post-resuscitation syndrome. Resuscitation 76:17–24. [DOI] [PubMed] [Google Scholar]
- Lisanne M. S., Bart A. A., Suzy V. G., Tom V. B., Evelien D. H., Jasper L. T., Frank A. M.. 2016. Opinion of belgian egg farmers on hen welfare and its relationship with housing type. Animals(Basel). 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc N. H., Macrae T. H., Musa N., Bin Abdullah M. D., Abdul Wahid M. E., Sung Y. Y.. 2013. Non-lethal heat shock increased Hsp70 and immune protein transcripts but not Vibrio tolerance in the white-leg shrimp. PLoS One. 8:e73199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjari R., Yadav M., Ramesh K., Uniyal S., Rastogi S. K., Sejian V., Hyder I.. 2015. HSP70 as a marker of heat and humidity stress in Tarai buffalo. Trop. Anim. Health Prod. 47:111–116. [DOI] [PubMed] [Google Scholar]
- Miao H., Xiao C.. 2018. Simultaneous segmentation of leukocyte and erythrocyte in microscopic images using a marker-controlled watershed algorithm. Comput. Math. Method Med. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon-Grasteau S., Moreri U., Narcy A., Rousseau X., Rodenburg T. B., Tixier-Boichard M., Zerjal T.. 2015. Robustness to chronic heat stress in laying hens: a meta-analysis. Poult. Sci. 94:586–600. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Naito H., Tsurukawa T., Ichinoseki-Sekine N., Saga N., Sugiura T., Katamoto S.. 2007. Microwave hyperthermia treatment increases heat shock proteins in human skeletal muscle. Br. J. Sports Med. 41:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruell P. A., Simar D., Périard J. D., Best S., Caillaud C., Thompson M. W.. 2014. Plasma and lymphocyte Hsp72 responses to exercise in athletes with prior exertional heat illness. Amino Acids. 46:1491–1499. [DOI] [PubMed] [Google Scholar]
- Sohn S. H., Subramani V. K., Moon Y. S., Jang I. S.. 2012. Telomeric DNA quantity, DNA damage, and heat shock protein gene expression as physiological stress markers in chickens. Poult. Sci. 91:829–836. [DOI] [PubMed] [Google Scholar]
- Van Goor A., Bolek K. J., Ashwell C. M., Persia M. E., Rothschild M. F., Schmidt C. J., Lamont S. J.. 2015. Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress. Genet. Sel. Evol. 47:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanoy V. 2015. Thermodynamic evaluation of vesicles shed by erythrocytes at elevated temperatures. Colloid Surf. B-Biointerfaces. 133:231–238. [DOI] [PubMed] [Google Scholar]
- Wieten L, van der Zee R., Spiering R., Wagenaar-Hilbers J., van Kooten P., Broere F., van Eden W.. 2010. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheumatol. 62:1026–1035. [DOI] [PubMed] [Google Scholar]
- Zhang W. W., Kong L. N., Zhang D. X., Ji C. L., Zhang X. Q., Luo Q. B.. 2015. Effect of the C.–1 388 A>G polymorphism in chicken heat shock transcription factor 3 gene on heat tolerance. J. Integr. Agric. 14:1808–1815. [Google Scholar]
- Zhen F. S., Du H. L., Xu H. P., Luo Q. B., Zhang X.Q.. 2006. Tissue and allelic-specific expression of hsp70 gene in chickens: basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. Br. Poult. Sci. 47:449–455. [DOI] [PubMed] [Google Scholar]
- Zhu L., Bao E., Zhao R., Hartung J.. 2009. Expression of heat shock protein 60 in the tissues of transported piglets. Cell Stress Chaperones. 14:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I., Che Norma M. T., Israf D. A., Omar A. R.. 2002. The effect of early-age food restriction on heat shock protein 70 response in heat-stressed female broiler chickens. Br. Poult. Sci. 43:141–145. [DOI] [PubMed] [Google Scholar]