ABSTRACT
To better understand how relevant intensive systems’ characteristics simultaneously affect the performance and welfare of broiler chickens, a meta-analysis of recent literature was carried out. The study determined the effects of gender, genetics, experimental initial age (EIA, d), stocking density (SD; kg/m2), group size (GS; n), bedding material (yes/no), duration of photoperiod (DP; h), divided scotoperiod (yes/no), feeding phases (1/2/3/>3), environmental control (EC; yes/no), environmental enrichment (yes/no), use of vaccines and other medications (yes/no), experimental duration (d), and relevant 2-way interactions on average daily gain (g/d), average daily feed intake (g/d), FCR (g: g), mortality (%), behavior (%), and gait score (mean value). Predictive equations for response variables were calculated using multiple regression models including a random experiment effect. Among other results, EIA × SD interaction indicated that relatively high SD may improve FCR at older ages, but parallel increased mortality would pose concerns about the actual productive benefits and welfare. Combining large GS and relatively low SD seem to improve performance and decrease flock disturbance. They would also increase leg problems, and so their actual benefits on welfare remain unclear. A gradual increase in FCR seems to occur with longer DP at older EIA (EIA × DP interaction), highlighting the importance of adapting light programs to flock age to optimize performance. The SD × DP and GS × DP interactions predicted increased FCR for longer DP at low SD or large GS, that is, with more effective space available. Longer DP combined with low SD or large GS would overall promote enhanced leg conditions, and therefore welfare. Predictions would not support scotoperiod division from both performance and welfare perspectives. The SD × EC interaction indicated that EC would benefit chicken performance at low SD, although EC would seem to increase leg problems. Our study highlights the complex, interactive nature of production systems’ characteristics on broiler chicken performance and welfare.
Keywords: broiler, intensive systems, meta-analysis, performance, welfare
INTRODUCTION
Broiler chickens are one of the main animal protein sources for human consumption, with about 6.2 × 1010 produced/slaughtered chickens in the world during 2014 (FAOSTAT, 2017). Thus, genetic selection and management efforts are applied to maximize bird performance, and have been the main driving forces of the chicken industry in the past decades (Zuidhof et al., 2014). To maximize productive efficiency, the vast majority of global broiler chicken production is carried out in intensive indoor systems (Robins and Phillips, 2011). Although this has allowed fulfillment of consumer demands at affordable prices, there exists the perception that a degradation of the welfare status of chickens has occurred in parallel. Animal welfare is nowadays a well-established dimension of animal production, with strong implications on systems’ long-term sustainability and on food quality as perceived by consumers (Broom, 2010). From this standpoint, intensive production systems are a matter of intense debate. Therefore, some re-orientation of production towards upgraded welfare standards that bear in mind profitability would certainly have a positive impact on systems’ long-term sustainability and on perceived product quality. But combining system productivity and improved animal welfare is difficult, and therefore sensible and effective decisions to improve broiler chicken welfare are highly relevant. These informed decisions should be, in consequence, based on an integrated analysis of current scientific knowledge aiming at determining how intensive production systems affect the performance and welfare of broiler chickens.
Literature addressing the effects of different housing and management aspects of intensive systems on the performance and welfare of broiler chickens is abundant (see for instance the reviews by Bessei, 2006; Humphrey, 2006; Estevez, 2007). Nevertheless, information is atomized in multiple, independent experiments, each of them controlling for a specific set of housing/management variables, but with many other variables remaining uncontrolled, and with their effects therefore unknown. Beyond classic literature reviews, global quantitative information is still lacking, and this should be particularly welcome in order to base informed, objective decisions. For this, the application of meta-analytical tools to the existing scientific information is a useful approach. In the context of broiler chicken production meta-analysis of information may allow to gain a deeper understanding about interactions of different housing and management aspects that influence bird performance and welfare beyond the scope of each individual experiment.
Given their usefulness, meta-analysis is a widespread tool used in many scientific disciplines. Regarding the performance and welfare of animal production species, meta-analytical techniques have been successfully applied to examine the effect of housing systems on growing-finishing pigs (Gonyou et al., 2006; Averós et al., 2010a,b, 2012; Douglas et al., 2015), gestating sows (Douglas et al., 2014), and fattening rabbits (Sommerville et al., 2017). In poultry, meta-analyses have been used to determine the effect of bacterial infection on performance (Remus et al., 2014), factors affecting selenium accumulation in broilers (Zoidis et al., 2014), and to determine the impact of heat stress on the performance of laying hens (Mignon-Grasteau et al., 2015). To our knowledge, no studies dealing with the effect of housing systems by combining performance and welfare indicators have been carried out in broiler chickens.
The housing environment of intensively reared broiler chickens involves the simultaneous action of multiple stressors, such as high densities or inadequate lighting programs, with negative implications for their welfare. Intensive genetic selection for fast growth and efficiency has also led to leg problems and lameness, which are stressful and are detrimental for bird welfare (Kestin et al., 1992; Sorensen, 1992). Genetic selection for slow-growing lines has partially reversed this trend (Fanatico et al., 2008). To effectively cope with stressors, broiler chickens will activate different response mechanisms aiming at maintenance of homeostasis (Moberg, 2000). Among these, behavior modifications are relatively inexpensive coping mechanisms (Rushen, 2000). But if all activated response mechanisms to a stressor are ineffective, basic biological functions may be impaired, such as growth in young animals (Moberg, 2000). Beyond welfare concerns, impaired growth and performance will have obvious, negative implications for systems’ efficiency and productivity. In most serious cases, failure to cope with stressors may even cause death.
Given that, as suggested by Broom (1991) a reliable, robust welfare assessment must be based on the combination of different indicators, the combined use of performance, mortality, behavior, and leg disorders causing walking difficulties appears to be a suitable approach to a general evaluation of intensive systems that integrates productivity and efficiency aspects with welfare. An objective insight about how all these indicators will be affected by modifying housing and management conditions on the way toward upgraded welfare standards and bird performance simultaneously would be feasible. Therefore, the present meta-analysis aims at quantifying the simultaneous effects of relevant animal and housing factors on the performance, mortality, behavior, and leg problems/gait score of broiler chickens, with the ultimate objective of determining the consequences of intensive systems on the performance and welfare of broiler chickens.
MATERIALS AND METHODS
The followed methodology is based on that proposed by St-Pierre (2001) and Sauvant et al. (2008), which has been applied in Averós et al., 2010a,b; 2012), Douglas et al. (2014, 2015), and Sommerville et al. (2017). The steps described by Hamer and Simpson (2002) have been followed.
Data Collection
The aim of the study was to quantify the influence of the rearing environment and animal traits on different performance and welfare indicators of broiler chickens, considering availability and homogeneity of the reported information. Information was extracted from peer-reviewed papers published between 2000 and August 2016. This time span limitation was imposed to minimize any potential bias due to the rapid evolution of chickens’ genetics in time (Havenstein et al., 2003). Literature research was carried out through the online ISI Web of Knowledge using combinations of terms chicken* and welfare*, broiler* and welfare*, chicken* and performance*, and broiler* and performance*. References from these different searches were pooled together and duplicates removed, which resulted in a first list of 12,401 potentially relevant references. Given that one of our focuses was broiler chicken performance, studies testing feed additives and/or feed restriction as well as studies evaluating diets differing in their composition were omitted to avoid interference of dietary treatments. Studies carried out under extensive or semi-extensive conditions and those testing slow-growing breeds were also discarded. After applying these filters, the number of potentially relevant references dropped to 382. These references underwent a second revision to check for completeness and correctness of reported information, and this further reduced the number of candidate papers to 134 references. These were then scrutinized for inclusion in the database.
For final inclusion in the database papers had to report, for each experimental treatment, or for each time interval within experimental treatment in case of experiments reporting repeated measures, information about gender (female/male/mixed), genetics (Aviagen/Aviagen × Cobb Vantress/Cobb Vantress/Group Grimaud/other), stocking density (kg/m2), group size (n), use of bedding material (yes/no), duration of photoperiod (h), division of scotoperiod (yes/no), number of feeding phases (1/2/3/>3), use of environmental control (yes/no), use of environmental enrichment (yes/no), and explicit information about the use of vaccines or other medication (yes/no). Lighting type and ventilation type were initially considered as an inclusion criteria as well, but were finally discarded because of the elevated number of papers that did either not report this information or reported it in a confusing way. Our study was controlling for the simultaneous effects of stocking density and group size. When stocking density is changed and group size remains constant, the enclosure size unavoidably changes, and the same occurs when group size changes keeping stocking density constant (Leone and Estevez, 2008; Leone et al., 2010). Therefore, it must be noted that in our study the effect of enclosure size was not controlled, and remained confounded with that of stocking density and group size. Duration of the photoperiod referred to the total number of hours per day during which lights were on, irrespective of whether the photoperiod was divided or not. Division of scotoperiod referred to whether total dark hours per day were divided into different periods or not, irrespective of the number of divisions. The fact that most of treatments where bedding material was absent were carried out in cages resulted in the confusion of the effect of bedding material with that of caging chickens.
For each experimental treatment, information about the number of replicates (i.e., experimental units to which a treatment was applied) was collected. Models also included chicken age at the beginning of each experimental period, or time interval within each experimental period in case of repeated measures (experimental initial age, d), as well as the duration of the experimental period, or time interval within the experimental period in case of repeated measures (experimental duration, d). These parameters were included to account for the fact that mean values of independent variables were strongly dependent on the duration of the experimental. When building the database, each paper, experiment within paper, and experimental treatment within experiment were identified using a different, unique numeric code. In addition to previous requirements, each paper had to report, at least, information regarding one of the following response variables to maximize the number of manuscripts available for further analysis: average daily gain (ADG, g/d), average daily feed intake (ADFI, g/d), FCR (g: g), mortality (% at the end of experimental period), behavior (frequency of scans (%) in which birds were observed resting, standing inactive, walking, eating, or drinking), or gait score (mean score collected as defined by Kestin et al., 1992). For behavioral variables, duration of measurements was also collected to account for any potential bias in behavioral output. Finally, 62 experiments were available for estimation of ADG, 58 experiments were available for estimation of ADFI, 59 experiments were available for estimation of FCR, 40 experiments were available for estimation of mortality, 7 experiments were available for resting, 5 experiments were available for standing inactive, 6 experiments were available for walking, 7 experiments were available for eating, 7 experiments were available for drinking, and 9 experiments were available for estimation of gait score (descriptive statistics for all continuous variables are shown in Table 1). Most of references included in final models reported controlled experiments, conditions which may differ from commercial conditions. This was the particular case of group size, for which the range of values was largely below commercial values.
Table 1.
References used in the meta-analysis, specifying the models in which they were included (complete references can be found in the Supplementary Material).
| Model inclusion | Model inclusion | ||
|---|---|---|---|
| Brito et al. (2016) | 1,2,3,4 | Villagra et al. (2010) | 1,4 |
| Olanrewaju et al. (2016) | 1,2,3,4 | Alvino et al. (2009) | 5,6,7,8,9 |
| Benyi et al. (2015) | 1,2,3 | Blatchford et al. (2009) | 1 |
| Ohara et al. (2015) | 1,2,3,4 | Calvet et al. (2009) | 7,8,9 |
| Shao et al. (2015) | 1,2,3,4,10 | Karakaya et al. (2009) | 1,2,3 |
| Coban et al. (2014) | 1,2,3,4 | Simsek et al. (2009) | 1,2,3,4 |
| De Jong et al. (2014) | 1,2,3,4,10 | Skrbic et al. (2009) | 1,10 |
| Olanrewaju et al. (2014) | 1,2,3,4 | Torok et al. (2009) | 1,2,3 |
| Petek et al. (2014) | 3,4 | Zhao et al. (2009) | 1,2,3 |
| Schwean-Lardner et al. (2014) | 5,6,7,8,9 | Gutierrez et al. (2008) | 1,2,3 |
| Abudabos et al. (2013) | 1,2,3 | Lewis et al. (2008) | 1,2,3,4 |
| Altan et al. (2013) | 1,2,3,4 | Lien et al. (2008) | 1,2,3,4 |
| Bailie et al. (2013) | 5,7,8,9,10 | Meluzzi et al. (2008) | 1,2,3,4 |
| Deep et al. (2013) | 1,2,3,4 | Turkyilmaz (2008) | 1,2,3,4 |
| Zhao et al. (2013) | 1,2,3,8,9 | Atapattu and Wickramasinghe (2007) | 1,2,3,4 |
| Schwean-Lardner et al. (2013) | 4 | Fortomaris et al. (2007) | 1,2,3 |
| Bayraktar et al. (2012) | 1,2,3,4 | Lewis and Gous (2007) | 1,2,3,4 |
| Deep et al. (2012) | 5,6,7,8,9 | Lien et al. (2007) | 1,2,3,4 |
| Nielsen (2012) | 1,2,3 | Onbasilar et al. (2007) | 1,2,3 |
| Olanrewaju et al. (2012) | 1,2,3 | de Oliveira et al. (2006a) | 1,2,3 |
| Passini et al. (2012) | 1 | de Oliveira et al. (2006b) | 1,2,3 |
| Shim et al. (2012) | 1,2,3,4 | Dozier, III et al. (2006) | 1,2,3,4 |
| Benyi et al. (2012) | 1,2,3,4 | Ipek and Sahan (2006) | 1,2,3,4 |
| Schwean-Lardner et al. (2012) | 5,6,7 | Kristensen et al. (2006) | 10 |
| Tong et al. (2012) | 1,2,3 | Mogyca Leandro et al. (2006) | 1,2,3,4 |
| Van Harn et al. (2012) | 1,2,3,4 | Segura et al. (2006) | 1,2,3,4 |
| Wei et al. (2012) | 1,2,3 | Dozier, III et al. (2005) | 1,2,3,4 |
| Zhao et al. (2012) | 1,2,3,8,9 | Thomas et al. (2004) | 1,2,3,4,10 |
| Duve et al. (2011) | 1,2,3 | Arnould and Faure (2003) | 5,6 |
| Guardia et al. (2011) | 1,2,3 | Bizeray et al. (2002) | 1,2,3,4,10 |
| Nowaczewski et al. (2011) | 1,2,3,4 | McLean et al. (2002) | 1,2,3,4,10 |
| Olanrewaju et al. (2011) | 1,2,3,4 | Hall (2001) | 4 |
| Thomas et al. (2011) | 5 | Lana et al. (2001) | 1,2,3 |
| Zuowei et al. (2011) | 1,2,3 | Ingram and Hatten III (2000) | 1,2,3,4 |
| Deep et al. (2010) | 1,2,3,4 | Ohtani and Leeson (2000) | 1,2,3 |
| Lewis et al. (2010) | 1,2,3 | Pedersen and Thomsen (2000) | 1,2,3,4 |
| Toghyani et al. (2010) | 1,2,3,4 | Sørensen et al. (2000) | 10 |
| Ventura et al. (2010) | 1,2,3,4 |
For each reference, its use is shown according to inclusion in models: (1) ADG (g/day); (2) ADFI (g/d); (3) FCR (g: g); (4) mortality (%); (5) resting (%); (6) standing (%); (7) walking (%); (8) eating (%); (9) drinking (%); (10) gait score (mean treatment value).
Statistical Analysis
Predictive equations for ADG, ADFI, FCR, mortality, behavior, and gait score were calculated using multiple regression, generalized linear mixed models (GLIMMIX procedure; SAS Institute, 2011). Given that available information, as imposed by previously explained criteria, differed depending on response variables, the chosen data modeling approach also differed for each response variable.
ADG, ADFI, FCR, and Mortality
Models included the main fixed effects gender (female/male/mixed), genetics (Aviagen/Aviagen × Cobb Vantress/Cobb Vantress/Group Grimaud/other), experimental initial age (d), experimental duration (d), stocking density (kg/m2), group size (n), bedding material (yes/no), duration of the photoperiod (h), division of scotoperiod (yes/no), feeding phases (1/2/3/>3), environmental control (yes/no), use of environmental enrichment (yes/no), and use of vaccines or other medication (yes/no). Quadratic terms of experimental initial age, experimental duration, stocking density, and group size were tested in all models to account for potentially existing non-linear relationships with response variables, although they only remained in final models if they showed statistical significance (P < 0.05). Initial, full models included all fixed effects and 2-way interactions. Final models were calculated using a stepwise backward procedure in which non-significant interactions were gradually removed from the model using the highest P-value as the removal criterion. Final models retained all significant interactions (P < 0.05). Models also accounted for the experiment as a random effect (St-Pierre, 2001; Sauvant et al., 2008), and for an additional period nested within experiment random effect to account for repeated measures experiments. Weighting observations according to experimental variability is advisable to account for interexperiment variance heterogeneity (Sauvant et al., 2008), but this was not always possible given the heterogeneous way in which variability was reported across experiments. To overcome this, sample size was proposed as an indirect variance estimate (Lipsey and Wilson, 2000) which was group size and was already included in models. Although unweighted observations have also been proposed (see Schmidely et al., 2008), number of replicates/treatment, as a measure of each experiment's statistical power (Thomas and Juanes, 1996), was used as the weighting criterion for each observation. Root mean square error (RMSE) of each model was calculated as an estimate of model prediction accuracy.
Gait Score
For this variable available information was more limited with respect to performance and mortality. Models were simpler, and the modeling approach also differed. Model considered the main fixed effects of gender (female/male/mixed), experimental initial age (d), experimental duration (d), stocking density (kg/m2), group size (n), duration of photoperiod (h), duration of scotoperiod (yes/no), feeding phases (2/3/>3 only available in this case), environmental control (yes/no), use of environmental enrichment (yes/no), and use of vaccines or other medication (yes/no). Initial full models included all fixed effects, and a forward stepwise procedure was performed in which 2-way interactions were tested and remained in the model according to a smallest P-value inclusion criterion. Final model only included the significant stocking density × duration of photoperiod interaction, since the addition of more interactions resulted in models failing to converge. Models also accounted for the experiment as random effect, and for an additional period nested within experiment random effect to account for repeated measures experiments. The number of replicates/treatment was used as a weighting criterion for each observation. Model prediction accuracy was also estimated using RMSE.
Behavior
Available information regarding performance and mortality models was limited. Therefore, the model construction was similar to that described for gait score. In this case, models considered the main fixed effects of gender (male/mixed birds only available in this case), experimental initial age (d), experimental duration (d), stocking density (kg/m2), group size (n), duration of photoperiod (h), duration of scotoperiod (yes/no; only included for resting), use of environmental enrichment (yes/no; included for all behaviors except for stand inactive due to limited information availability), and use of vaccines or other medication (yes/no). Bedding material was also discarded from the model due to the mentioned information availability. Duration of behavioral measurements was tested in all models, but did not show any significant effect and was therefore not included. Initial full models included all fixed effects, and a forward stepwise procedure was performed in which 2-way interactions were tested and remained in the model according to a smallest P-value inclusion criterion. Final model only included the significant stocking density × group size for resting, since addition of more interactions resulted in models failing to converge. Models also accounted for the experiment as random effect, and for an additional period nested within experiment random effect to account for repeated measures experiments. The number of replicates/treatment was used as the weighting criterion for each observation. Model prediction accuracy was also estimated using RMSE.
RESULTS
Descriptive statistics of the studied variables for all experiments included in the study are shown in Table 2.
Table 2.
Descriptive data for all continuous variables included in the analysis.
| Independent variable | N | Mean | St. Dev. | Minimum | Maximum | Median |
|---|---|---|---|---|---|---|
| Experimental initial age (d) | 749 | 16 | 13 | 1 | 44 | 14 |
| Experimental duration (d) | 749 | 17 | 12 | 6 | 61 | 13 |
| Stocking density (kg/m2) | 749 | 21 | 13 | 0.6 | 62 | 20 |
| Group size (n) | 749 | 284 | 1,879 | 4 | 23,000 | 60 |
| Duration of photoperiod (h) | 749 | 20 | 4 | 6 | 24 | 22 |
| Response variable | ||||||
| ADG (g/d) | 679 | 64.88 | 24.23 | 11.65 | 123.53 | 65.29 |
| ADFI (g/d) | 664 | 120.18 | 58.57 | 16.99 | 321.10 | 117.62 |
| FCR (g:g) | 670 | 1.80 | 0.40 | 0.91 | 3.65 | 1.75 |
| Mortality (%) | 334 | 2.95 | 2.75 | 0.00 | 18.79 | 2.22 |
| Gait score (mean) | 39 | 1.27 | 0.72 | 0.01 | 3.0 | 1.31 |
| Rest (%) | 49 | 66.12 | 14.47 | 20.96 | 86.58 | 70.91 |
| Eat (%) | 49 | 12.51 | 6.91 | 5.16 | 27.90 | 10.64 |
| Drink (%) | 49 | 6.24 | 1.68 | 1.99 | 11.28 | 6.14 |
| Stand (%) | 25 | 4.17 | 1.73 | 1.39 | 8.38 | 3.85 |
| Walk (%) | 27 | 3.27 | 2.58 | 0.50 | 9.24 | 2.36 |
Performance
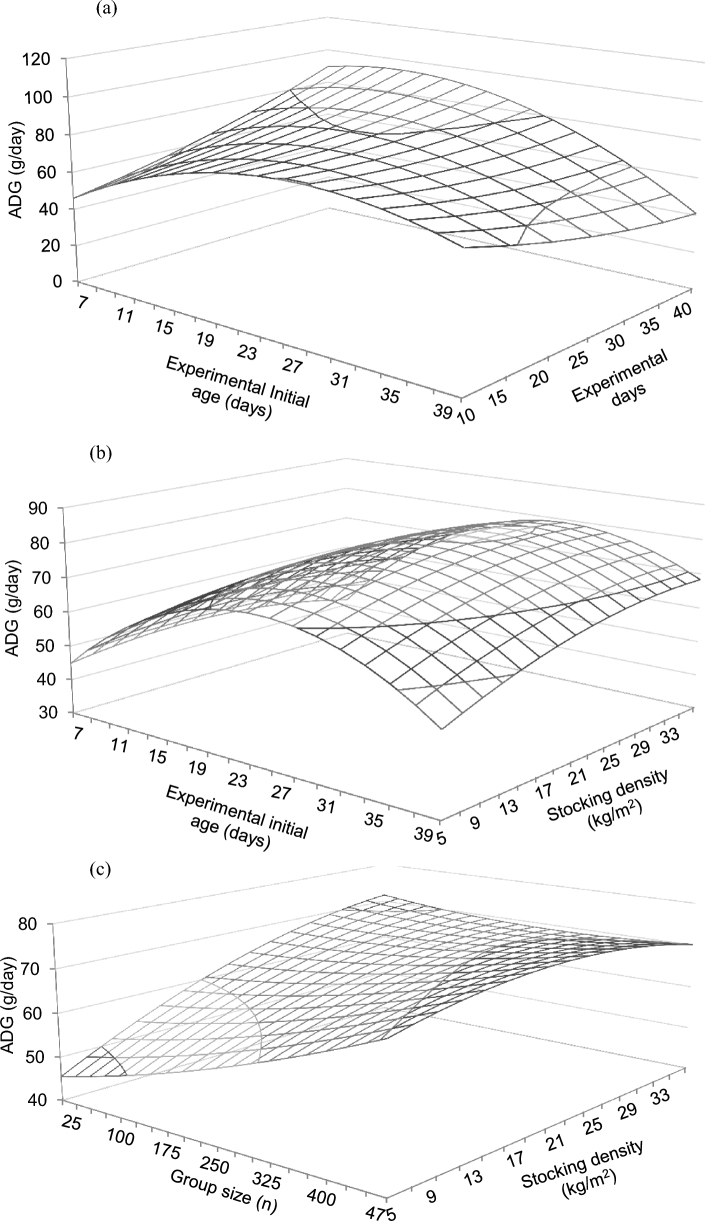
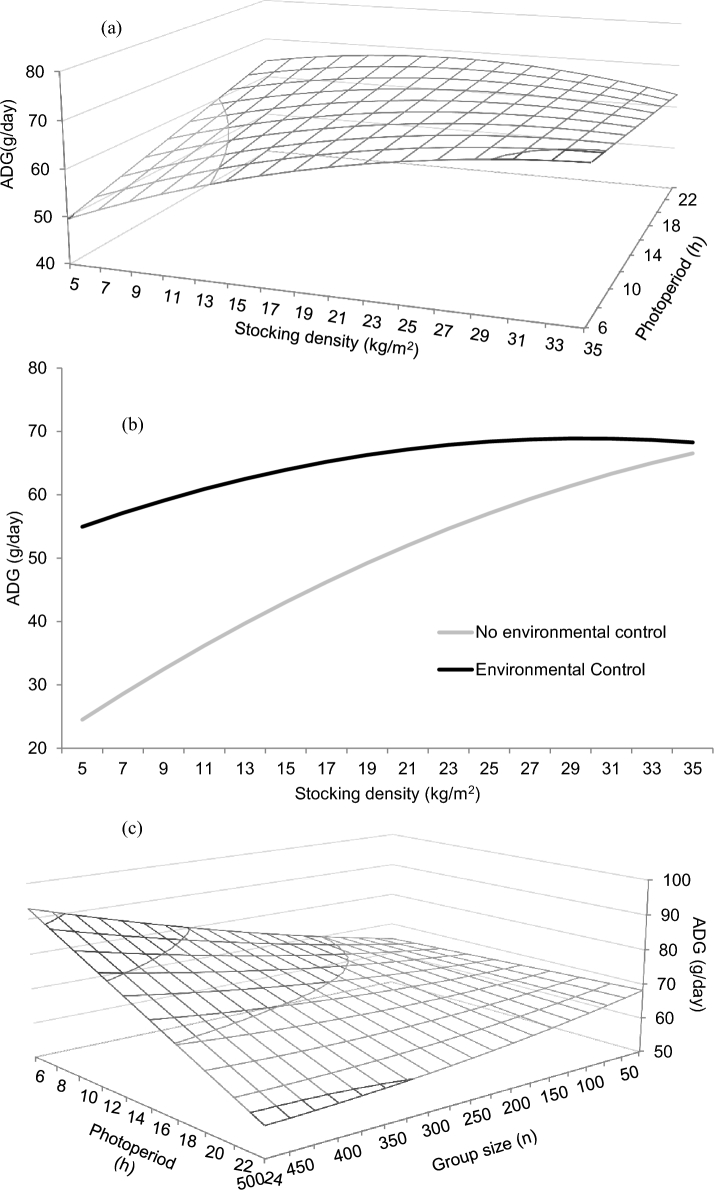
Multivariate performance models are shown in Table 3, together with their RMSE. Given the amount of predicted significant results found for these variables, only significant interactions will be commented. Otherwise, significant main effects are shown in Table 3. An experimental initial age × experimental duration interaction was predicted for ADG (Figure 1a) that increased for longer experimental periods and young birds, and with changes due to experimental period being minor at older ages. The experimental initial age × stocking density interaction predicted a peak in ADG around 29 d of age, and a positive effect of relatively high stocking density that was only apparent at older ages (Figure 1b). A stocking density × group size interaction was found on ADG (Figure 1c), with models predicting increased ADG at large stocking density and small group size, but a gradual disappearance of stocking density effects with larger group size. A stocking density × gender interaction was predicted on ADG, with a negative coefficient found for males, that was smaller than that for females as compared to mixed genders (Table 3). A stocking density × number of feeding phases interaction was predicted on ADG, with a positive coefficient for 1 feeding phase, and with negative coefficients for 2 and 3 feeding phases regarding more than 3 feeding phases. A stocking density × duration of photoperiod interaction was predicted on ADG (Figure 2a), which increased markedly as duration of photoperiod increased and stocking density remained low, and with the opposite effect predicted at relatively high stocking density. The stocking density × environmental control interaction predicted on ADG (Figure 2b) indicated an increase of the variable with stocking density in the absence of environmental control, but a smaller effect of stocking density in the presence of environmental control. A group size × gender interaction was predicted on ADG, with the coefficient being higher for males than for females. A group size × bedding material interaction was predicted on ADG, with a positive coefficient for absence of bedding material with respect to its presence. A group size × duration of photoperiod interaction was predicted on ADG (Figure 2c), with values remaining low and constant as photoperiod duration increased when group size was small, but gradually decreasing as photoperiod duration increased when group size was large. A group size × environmental control interaction was found on ADG, with a negative coefficient being found in the absence of environmental control with respect to its presence (Table 3).
Table 3.
Parameter estimates and standard error for the models quantifying the effect of the different rearing system characteristics on the ADG, ADFI, and FCR of broiler chickens.
| ADG | ADFI | FCR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable1 | (g/d; n = 679) | P-value | (g/d; n = 664) | P-value | (g:g; n = 670) | P-value | ||||
| Intercept | −25.70 | 13.03 | 0.0536 | −109.14 | 33.75 | 0.0021 | 0.965 | 0.201 | <0.0001 | |
| ED | 1.02 | 0.355 | 0.0044 | 0.271 | 0.783 | 0.7294 | 0.002 | 0.006 | 0.6959 | |
| ED × ED | 0.019 | 0.006 | 0.0020 | 0.094 | 0.013 | <0.0001 | 0.0004 | 0.0001 | <0.0001 | |
| IA | 4.89 | 0.35 | <0.0001 | 7.66 | 0.75 | <0.0001 | −0.012 | 0.005 | 0.4333 | |
| IA × IA | −0.077 | 0.004 | <0.0001 | −0.064 | 0.008 | <0.0001 | 0.00073 | 0.00007 | <0.0001 | |
| Breed | Aviagen | −4.29 | 8.71 | 0.3454 | 3.40 | 23.45 | 0.7686 | −0.0002 | 0.1261 | 0.6975 |
| Aviagen × Cobb Vantress | −63.26 | 25.36 | −177.10 | 65.64 | −0.560 | 0.331 | ||||
| Cobb Vantress | −8.94 | 9.32 | −5.46 | 24.83 | −0.006 | 0.138 | ||||
| Group Grimaud | 4.93 | 10.83 | −4.28 | 28.15 | −0.130 | 0.161 | ||||
| Others | 0 | – | 0 | – | 0 | – | ||||
| Sex | Females | −8.08 | 5.61 | <0.0001 | −23.38 | 13.57 | <0.0001 | −0.133 | 0.099 | 0.3865 |
| Males | 10.05 | 5.26 | 16.61 | 12.89 | −0.116 | 0.089 | ||||
| Mixed | 0 | – | 0 | – | 0 | – | ||||
| FP | 1 | 14.68 | 14.05 | 0.1397 | 72.58 | 37.09 | 0.1033 | 0.113 | 0.172 | 0.5799 |
| 2 | −6.50 | 7.26 | 8.88 | 20.52 | 0.151 | 0.110 | ||||
| 3 | 7.54 | 7.56 | 37.48 | 21.32 | 0.122 | 0.112 | ||||
| >3 | 0 | - | 0 | - | 0 | - | ||||
| SD | 2.33 | 0.36 | <0.0001 | 5.06 | 0.78 | <0.0001 | 0.014 | 0.007 | 0.1075 | |
| SD × SD | −0.023 | 0.004 | <0.0001 | −0.042 | 0.009 | <0.0001 | NS | NS | NS | |
| GS | −0.081 | 0.086 | 0.3719 | −0.124 | 0.222 | 0.3521 | −0.0003 | 0.0002 | 0.1965 | |
| GS × GS | 0.00004 | 0.00002 | 0.0028 | 0.00008 | 0.00003 | 0.0242 | −3.08E-8 | 0.01E-10 | 0.3983 | |
| BM | No | −13.48 | 6.84 | 0.0493 | −33.87 | 15.52 | 0.0295 | −0.027 | 0.081 | 0.7370 |
| Yes | 0 | – | 0 | – | 0 | – | ||||
| Enrichment | No | −2.00 | 2.05 | 0.3297 | −4.16 | 4.36 | 0.3403 | 0.006 | 0.8933 | |
| Yes | 0 | – | 0 | – | 0 | |||||
| DP | 1.53 | 0.26 | <0.0001 | 4.50 | 0.57 | <0.0001 | 0.006 | 0.2658 | ||
| DS | No | −4.50 | 2.45 | 0.0671 | −10.89 | 5.22 | 0.0375 | 0.002 | 0.055 | 0.9660 |
| Yes | 0 | – | 0 | – | 0 | – | ||||
| EC | No | −9.53 | 7.92 | 0.2293 | 0.548 | 21.27 | 0.9795 | 0.314 | 0.121 | 0.0095 |
| Yes | 0 | – | 0 | – | 0 | – | ||||
| MU | No | −6.59 | 6.59 | 0.3175 | −26.72 | 19.40 | 0.1689 | −0.020 | 0.103 | 0.8474 |
| Yes | 0 | – | 0 | – | 0 | – | ||||
| EIA × ED | −0.068 | 0.009 | <0.0001 | −0.100 | 0.020 | <0.0001 | NS | NS | NS | |
| EIA × Breed | Aviagen | 0.079 | 0.114 | <0.0001 | 0.010 | 0.241 | <0.0001 | 0.005 | 0.002 | 0.0002 |
| Aviagen × Cobb Vantress | 0 | – | 0 | – | 0 | – | ||||
| Cobb Vantress | 0.097 | 0.152 | 0.257 | 0.326 | 0.012 | 0.003 | ||||
| Group Grimaud | −1.07 | 0.19 | −1.65 | 0.45 | 0.015 | 0.004 | ||||
| Others | 0 | – | 0 | – | 0 | – | ||||
| EIA × SD | 0.020 | 0.004 | <0.0001 | 0.035 | 0.008 | <0.0001 | −0.00024 | 0.00007 | 0.0005 | |
| EIA × DP | −0.017 | 0.010 | 0.0872 | −0.052 | 0.021 | 0.0153 | 0.0006 | 0.0002 | 0.0040 | |
| SD × GS | −0.0019 | 0.0002 | <0.0001 | −0.0032 | 0.0005 | <0.0001 | 9.09E-6 | 4.11E-6 | 0.0275 | |
| SD × Sex | Females | −0.086 | 0.133 | <0.0001 | 0.270 | 0.286 | 0.0002 | 0.007 | 0.003 | 0.0432 |
| Males | −0.380 | 0.096 | −0.594 | 0.207 | 0.004 | 0.002 | ||||
| Mixed | 0 | – | 0 | – | 0 | – | ||||
| SD × FP | 1 | 0.483 | 0.628 | <0.0001 | −1.06 | 1.61 | <0.0001 | NS | NS | NS |
| 2 | −0.280 | 0.114 | −0.657 | 0.246 | NS | NS | NS | |||
| 3 | −0.425 | 0.094 | −0.950 | 0.203 | NS | NS | NS | |||
| >3 | 0 | – | 0 | – | NS | NS | NS | |||
| SD × DP | −0.041 | 0.014 | 0.0024 | −0.100 | 0.029 | 0.0006 | −0.0006 | 0.003 | 0.0427 | |
| SD × EC | No | 0.957 | 0.132 | <0.0001 | 1.36 | 0.30 | <0.0001 | −0.012 | 0.003 | <0.0001 |
| Yes | 0 | – | 0 | – | 0 | – | ||||
| GS × Breed | Aviagen | 0.176 | 0.071 | 0.0541 | 0.349 | 0.174 | 0.0537 | NS | NS | NS |
| Aviagen × Cobb Vantress | 0.657 | 0.291 | 1.19 | 0.62 | NS | NS | NS | |||
| Cobb Vantress | 0.184 | 0.072 | 0.378 | 0.178 | NS | NS | NS | |||
| Group Grimaud | 0.189 | 0.109 | 0.682 | 0.282 | NS | NS | NS | |||
| Others | 0 | – | 0 | – | NS | NS | NS | |||
| GS × Sex | Females | 0.002 | 0.035 | <0.0001 | 0.028 | 0.088 | <0.0001 | NS | NS | NS |
| Males | 0.023 | 0.035 | 0.068 | 0.089 | NS | NS | NS | |||
| Mixed | 0 | – | 0 | – | NS | NS | NS | |||
| GS × BM | No | 0.365 | 0.151 | 0.0161 | 0.787 | 0.337 | 0.0198 | NS | NS | NS |
| Yes | 0 | – | 0 | – | NS | NS | NS | |||
| GS × DP | −0.005 | 0.002 | 0.0208 | −0.012 | 0.005 | 0.0183 | NS | NS | NS | |
| GS × EC | No | −0.514 | 0.090 | <0.0001 | −1.21 | 0.21 | <0.0001 | NS | NS | NS |
| Yes | 0 | – | 0 | – | NS | NS | NS | |||
| RMSE | 0.25 | 0.51 | 0.01 | |||||||
1ED: duration of the experimental period (d); EIA: experimental initial age (d); FP: feeding phases; SD: stocking density (kg/m2); GS: group size (n); BM: bedding material; DP: duration of the photoperiod (h); DS: divided scotoperiod (h); EC: environmental control; MU: use of vaccines and other medications.
Figure 1.
Predicted effects of the interactions between experimental initial age and experimental days (a), initial age and stocking density (b), and group size and stocking density (c) on the ADG of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.25).
Figure 2.
Predicted effects of the interactions between stocking density and photoperiod duration (a), stocking density and environmental control (b), and photoperiod duration and group size (c) on the ADG of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.25).
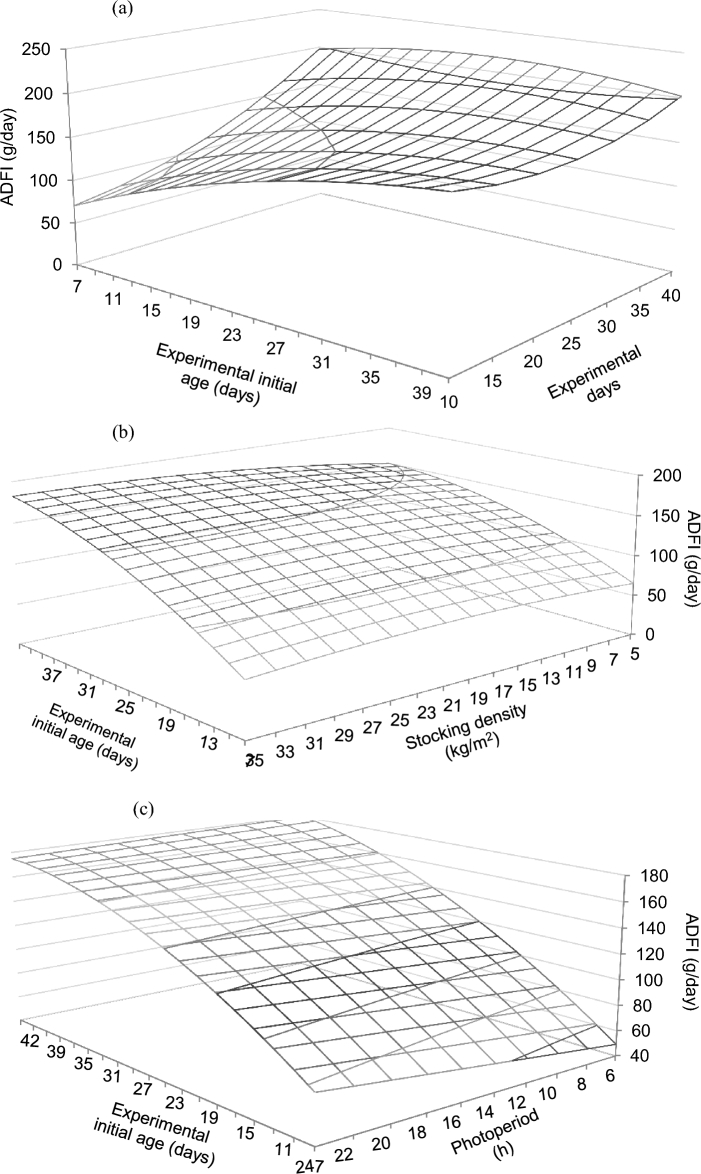
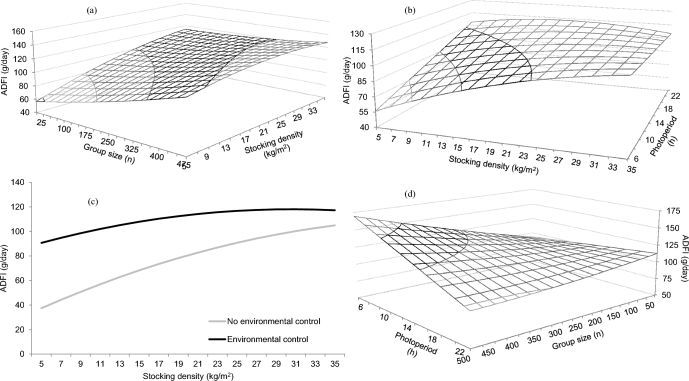
An experimental initial age × experimental duration interaction was predicted for ADFI (Figure 3a), which increased with longer experimental periods for young birds, but with a less marked effect predicted at older ages. An experimental initial age × stocking density interaction affected ADFI, with models predicting a more evident increment with age at higher stocking density (Figure 3b). Models predicted an experimental initial age × duration of photoperiod interaction on ADFI, which increased with longer photoperiod at young experimental initial age, but remained high and invariable as experimental initial age increased (Figure 3c). A stocking density × group size interaction was predicted on ADFI (Figure 4a), with increased ADFI at large stocking density and small group size, but with the stocking density effect gradually disappearing as group size became larger. A stocking density × gender interaction was predicted on ADFI, with a negative coefficient detected for males, and a positive coefficient detected for females with respect to mixed sexes (Table 3). A stocking density × number of feeding phases interaction was predicted on ADFI, with negative coefficients for 1, 2, and 3 feeding phases with respect to more than 3 feeding phases. A stocking density × duration of photoperiod interaction was predicted on ADFI (Figure 4b), which increased markedly as duration of photoperiod increased and stocking density remained low, but with the opposite effect at relatively high stocking density. A stocking density × environmental control interaction was predicted on ADFI (Figure 4c), which increased with stocking density in the absence of environmental control, but with a less apparent effect of stocking density in its presence. A group size × gender interaction was predicted on ADFI, with coefficients being higher for males than for females. A group size × bedding material interaction was predicted on ADFI, with positive coefficients in the absence of bedding material with respect to its presence. A group size × duration of photoperiod interaction was predicted for ADFI (Figure 4d), with values remaining low and constant as photoperiod duration increased when group size was small, but gradually decreasing as photoperiod duration increased when group size was large. A group size × environmental control interaction was detected on ADFI, with a negative coefficient being found in the absence of environmental control with respect to its presence.
Figure 3.
Predicted effects of the interactions between experimental initial age and experimental duration (a), experimental initial age and stocking density (b), and experimental initial age and photoperiod duration (c) on the ADFI of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.51).
Figure 4.
Predicted effects of the interactions between group size and stocking density (a), stocking density and photoperiod duration (b), stocking density and environmental control (c), and photoperiod duration and group size (d) on the ADFI of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.51).
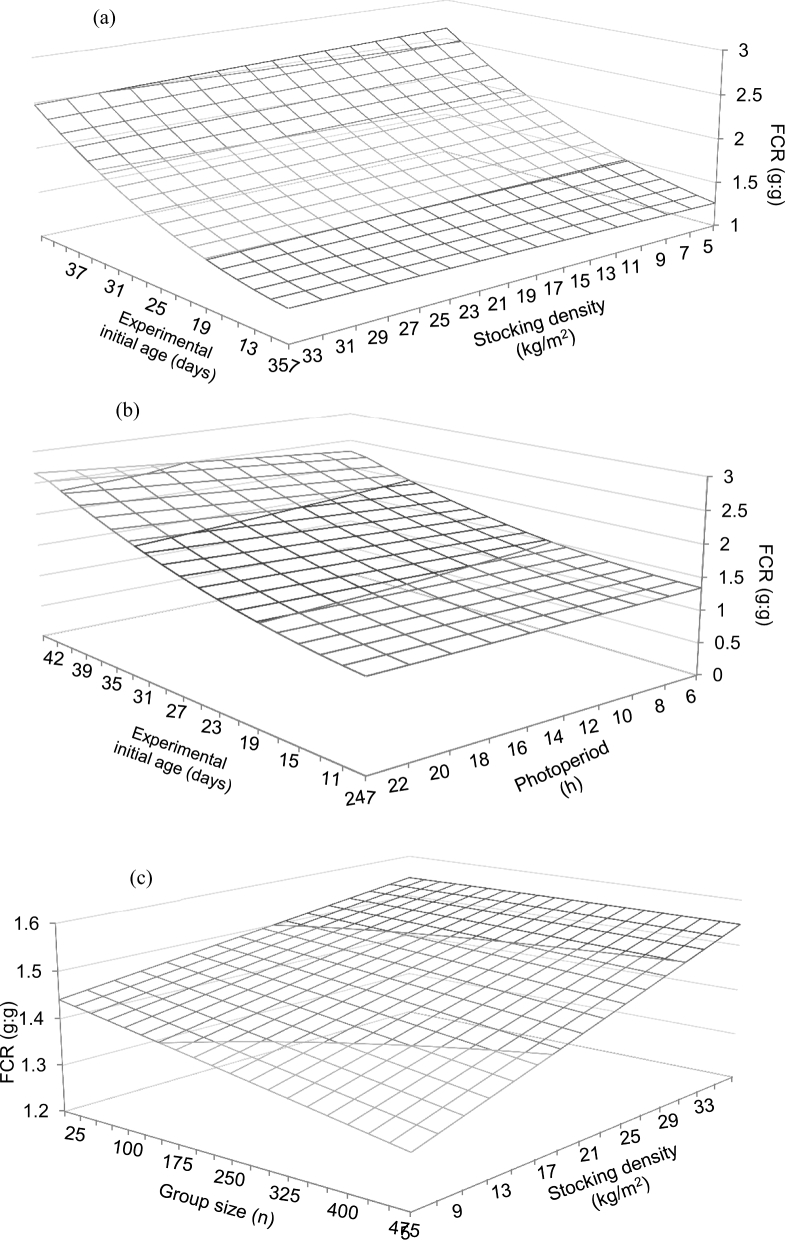
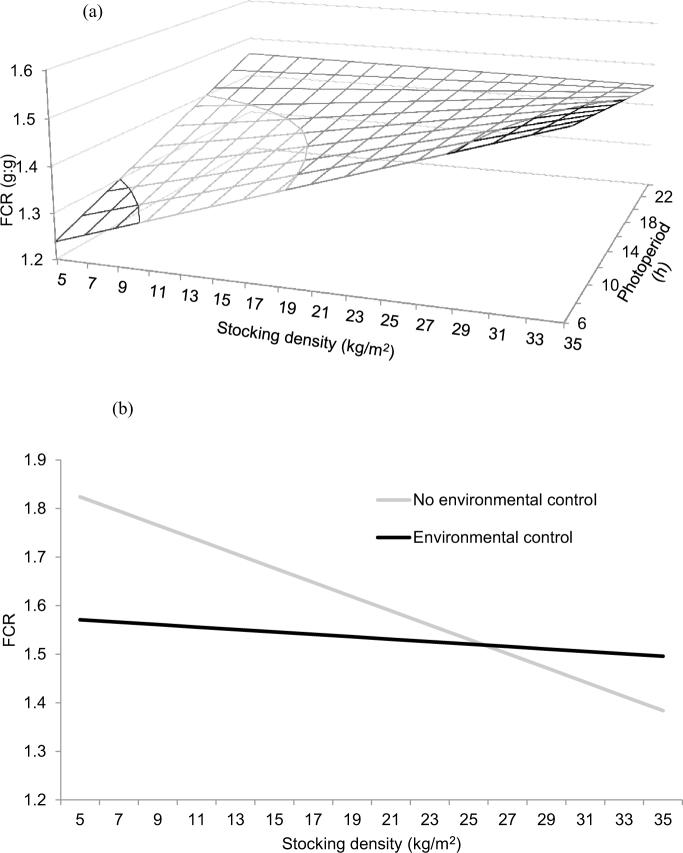
The experimental initial age × stocking density interaction predicted an increase in FCR with age that was moderate at relatively high stocking density as compared to smaller stocking density (Figure 5a). Models predicted an experimental initial age × duration of photoperiod interaction on FCR (Figure 5b), which remained relatively low and invariable with longer photoperiod at young experimental initial age, and became gradually larger as both photoperiod duration and experimental initial age increased. A stocking density × group size interaction was predicted on FCR (Figure 5c), with largest values predicted at highest stocking density independent from group size. A stocking density × gender interaction was predicted on FCR, with coefficient for females being slightly higher than that of males when compared to mixed sexes. A stocking density × duration of photoperiod interaction was predicted on FCR (Figure 6a), which showed a marked increase as photoperiod duration increased and stocking density remained low, but with the opposite prediction when stocking density was relatively high. The stocking density × environmental control interaction on FCR (Figure 6b) predicted a decrease in FCR as stocking density increased in the absence of environmental control, whereas values remained relatively constant in the presence of environmental control.
Figure 5.
Predicted effects of the interactions between experimental initial age and stocking density (a), experimental initial age and photoperiod duration (b), and group size and stocking density (c) on the FCR of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.01).
Figure 6.
Predicted effects of the interactions between stocking density and photoperiod duration (a), and stocking density and environmental control on the FCR of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.01).
Mortality
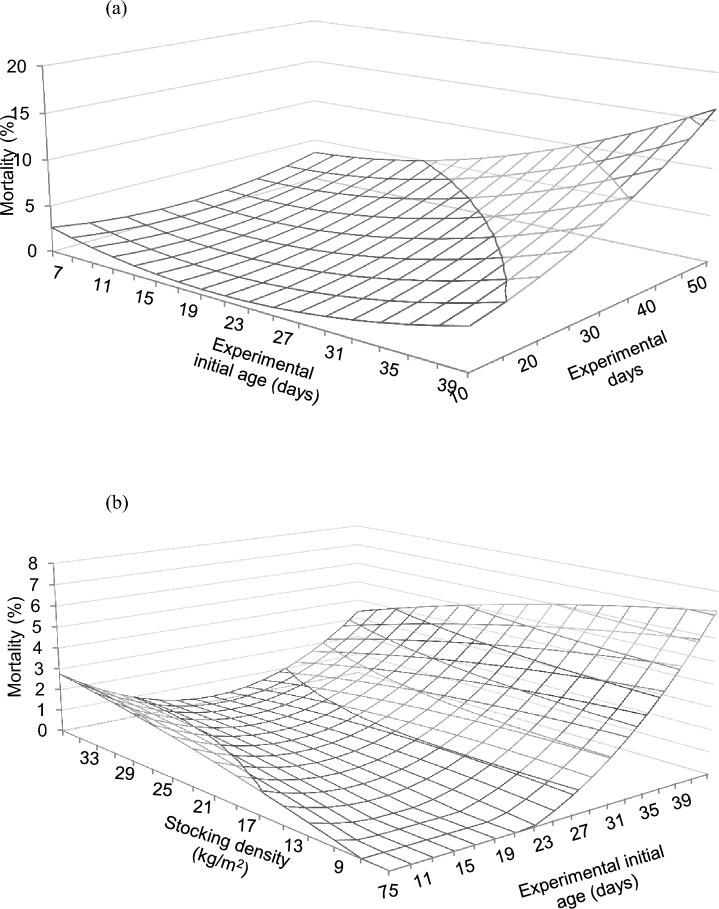
Multivariate model and RMSE for mortality are shown in Table 4. Mortality was predicted to differ between genders, with a negative coefficient for females and a positive for males with respect to mixed flocks. An experimental initial age × experimental duration interaction was predicted (Figure 7a), with mortality remaining low and invariable at younger experimental initial age, but increasing as experimental duration increased for older experimental initial age. An experimental initial age × stocking density interaction was predicted (Figure 7b), so that a gradual increase in mortality was predicted from 23 d of experimental initial age onward at low stocking densities. In contrast, at relatively high stocking densities mortality was predicted to be minimum at about 21 to 23 d of experimental initial age, and then to gradually increase at older experimental initial ages.
Table 4.
Parameter estimates and standard error for the models quantifying the effect of the different rearing system characteristics on the mortality of broiler chickens.
| Mortality | ||||
|---|---|---|---|---|
| Variable1 | (%; n = 334) | P-value | ||
| Intercept | 3.73 | 2.74 | 0.1828 | |
| ED | −0.288 | 0.108 | 0.0082 | |
| ED × ED | 0.004 | 0.002 | 0.0190 | |
| EIA | −0.332 | 0.069 | <0.0001 | |
| EIA × EIA | 0.010 | 0.001 | <0.0001 | |
| Breed | Aviagen | −0.368 | 1.322 | 0.4346 |
| Aviagen × Cobb Vantress | 0.396 | 2.949 | ||
| Cobb Vantress | −0.927 | 1.361 | ||
| Group Grimaud | 4.08 | 4.04 | ||
| Others | 0 | - | ||
| Sex | Females | −1.33 | 1.01 | 0.0017 |
| Males | 0.602 | 0.898 | ||
| Mixed | 0 | - | ||
| FP | 1 | −0.712 | 3.432 | 0.8071 |
| 2 | −0.135 | 1.201 | ||
| 3 | −0.719 | 1.141 | ||
| >3 | 0 | - | ||
| SD | 0.114 | 0.090 | 0.2066 | |
| SD × SD | −0.001 | 0.002 | 0.1563 | |
| GS | −0.0010 | 0.0007 | 0.1571 | |
| BM | No | −0.043 | 1.402 | 0.9754 |
| Yes | 0 | - | ||
| Enrichment | No | −0.074 | 0.472 | 0.8755 |
| Yes | 0 | - | ||
| DP | −0.026 | 0.065 | 0.6868 | |
| DS | No | 0.539 | 1.770 | 0.7611 |
| Yes | 0 | - | ||
| EC | No | 0.882 | 1.187 | 0.4584 |
| Yes | 0 | - | ||
| MU | No | 0.784 | 1.305 | 0.5485 |
| Yes | 0 | - | ||
| EIA × ED | 0.008 | 0.003 | 0.0010 | |
| EIA × SD | −0.007 | 0.001 | <0.0001 | |
| SD × GS | 0.00004 | 0.00002 | 0.0528 | |
| SD × DP | 0.005 | 0.003 | 0.0660 | |
| RMSE | 0.06 | |||
1ED: duration of the experimental period (d); EIA: experimental initial age (d); FP: feeding phases; SD: stocking density (kg/m2); GS: group size (n); BM: bedding material; DP: duration of the photoperiod (h); DS: divided scotoperiod (h); EC: environmental control; MU: use of vaccines and other medications.
Figure 7.
Predicted effects of the interactions between experimental initial age and experimental duration (a), and stocking density and experimental initial age (b) on the mortality of broiler chickens. Predictions calculated using the median of the rest of variables included in the models (RMSE = 0.06).
Gait Score
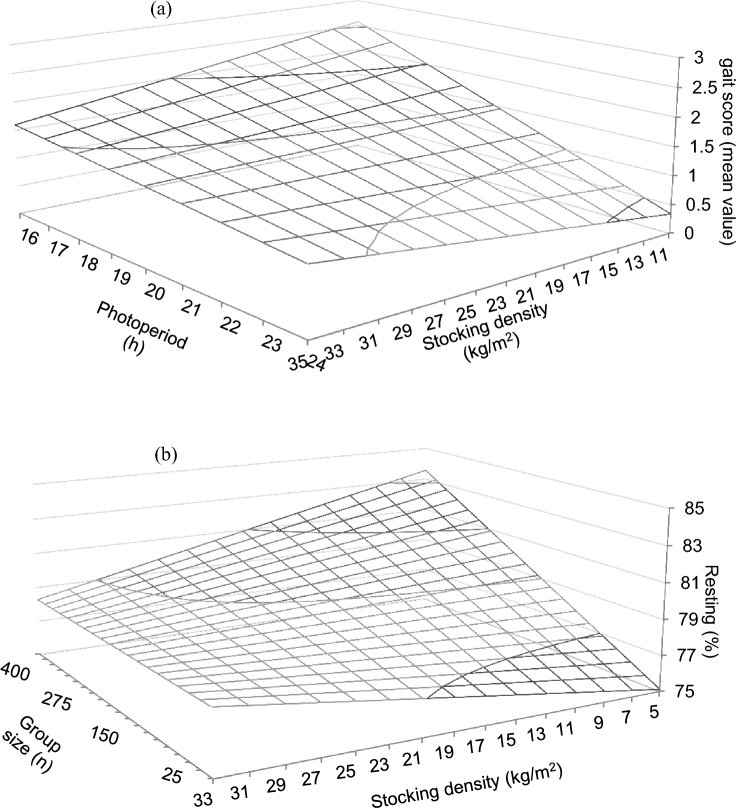
Multivariate model and RMSE are shown in Table 5. A negative coefficient was predicted for experimental initial age. The number of feeding phases was predicted to affect gait score, with the highest positive coefficient found for 3 feeding phases with respect to more than 3 feeding phases. A negative coefficient was predicted when the scotoperiod was not divided with respect to divided scotoperiod division, as well as for the absence of environmental control as compared to its presence. The stocking density × duration of photoperiod interaction predicted a decrease in the mean gait score with increasing stocking density for short photoperiods (Figure 8a).
Table 5.
Parameter estimates and standard error for the models quantifying the effect of the different rearing system characteristics on the gait score of broiler chickens.
| Gait score | ||||
|---|---|---|---|---|
| Variable1 | (mean value; n = 39) | P-value | ||
| Intercept | 10.03 | 2.29 | 0.0221 | |
| EIA | −0.027 | 0.007 | 0.0005 | |
| Sex | Females | 0.113 | 0.259 | 0.3705 |
| Males | 0.252 | 0.259 | ||
| Mixed | 0 | – | ||
| FP | 2 | 0.630 | 0.245 | 0.0317 |
| 3 | 1.169 | 0.412 | ||
| >3 | 0 | – | ||
| SD | −0.186 | 0.069 | 0.0129 | |
| GS | −0.00006 | 0.00003 | 0.0841 | |
| DP | −0.379 | 0.102 | 0.0012 | |
| DS | No | −1.469 | 0.255 | <0.0001 |
| Yes | 0 | – | ||
| EC | No | −0.367 | 0.175 | 0.0464 |
| Yes | 0 | – | ||
| MU | No | 0.608 | 0.409 | 0.1510 |
| Yes | 0 | – | ||
| SD × DP | 0.009 | 0.003 | 0.0057 | |
| RMSE | 0.02 | |||
1EIA: experimental initial age (d); FP: feeding phases; SD: stocking density (kg/m2); GS: group size (n); DP: duration of the photoperiod (h); DS: divided scotoperiod (h); EC: environmental control; MU: use of vaccines and other medications.
Figure 8.
Predicted effects of the interactions between photoperiod duration and stocking density on the gait score (a; model RMSE = 0.02), and stocking density and group size on the resting behavior (b; model RMSE = 0.51) of broiler chickens. Predictions calculated using the median of the rest of variables included in the models.
Behavior
Multivariate models and RMSE are shown in Table 6. Models predicted a positive coefficient for experimental duration and experimental initial age on the resting frequency. Walking frequency was predicted to be positively associated with stocking density, whereas a negative association was predicted between stocking density and drinking and standing inactive frequencies. Standing inactive frequency was predicted to be positively related to group size. Absence of enrichment was predicted to increase drinking frequencies with respect to its presence. Resting frequency was predicted to increase with increasing photoperiod duration, whereas frequencies of the rest of behaviors decreased with increasing photoperiod duration. Resting and walking frequencies were lower, whereas drinking frequency was higher when no vaccines or other medication was used as compared to its use. The stocking density × group size interaction predicted a slight increase in the resting frequency at higher stocking density and small group size, although resting frequency decreased as stocking density increased when group size was large (Figure 8b).
Table 6.
Parameter estimates and standard error for the models quantifying the effect of the different rearing system characteristics on the behavior of broiler chickens.
| Rest | Eat | Drink | Walk | Stand | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable1 | (%; n = 49) | P-value | (%; n = 49) | P-value | (%; n = 49) | P-value | (%; n = 27) | P-value | (%; n = 25) | P-value | ||||||
| Intercept | 5.57 | 13.80 | 0.7136 | 18.91 | 12.16 | 0.2178 | 10.16 | 2.23 | 0.0199 | −41.77 | 16.55 | 0.2401 | 4.90 | 4.66 | 0.4837 | |
| ED | 1.34 | 0.32 | 0.0002 | 0.133 | 0.209 | 0.5269 | −0.011 | 0.042 | 0.7964 | 0.098 | 0.163 | 0.5559 | 0.218 | 0.173 | 0.2258 | |
| EIA | 0.621 | 0.096 | <0.0001 | 0.0003 | 0.0728 | 0.9970 | 0.054 | 0.027 | 0.0527 | −0.001 | 0.063 | 0.9847 | −0.011 | 0.069 | 0.8700 | |
| Sex | Males | 12.03 | 8.20 | 0.1511 | −6.40 | 12.32 | 0.6062 | −0.053 | 0.472 | 0.9118 | 2.08 | 1.69 | 0.2355 | |||
| Mixed | 0 | – | 0 | – | 0 | – | 0 | – | ||||||||
| SD | 0.004 | 0.096 | 0.9668 | −0.089 | 0.061 | 0.1490 | −0.089 | 0.021 | 0.0002 | 2.80 | 0.97 | 0.0103 | −0.117 | 0.036 | 0.0050 | |
| GS | 0.025 | 0.011 | 0.0350 | −0.0002 | 0.0006 | 0.7619 | −4.14E-6 | 0.00003 | 0.8813 | −0.0002 | 0.0001 | 0.2680 | 0.005 | 0.002 | 0.0072 | |
| Enrichment | No | 4.18 | 4.09 | 0.3142 | 0.893 | 0.575 | 0.1290 | 0.833 | 0.261 | 0.0028 | −0.249 | 0.555 | 0.6591 | |||
| Yes | 0 | – | 0 | – | 0 | – | 0 | – | ||||||||
| DP | 2.10 | 0.36 | <0.0001 | −0.618 | 0.216 | 0.0069 | −0.490 | 0.097 | <0.0001 | −0.278 | 0.050 | <0.0001 | −0.280 | 0.070 | 0.0010 | |
| DS | No | −6.45 | 7.17 | 0.3743 | ||||||||||||
| Yes | 0 | – | ||||||||||||||
| MU | No | −17.44 | 8.56 | 0.0492 | 7.82 | 12.42 | 0.5327 | 8.010 | 0.728 | <0.0001 | −43.79 | 14.89 | 0.0091 | 0.532 | 1.556 | 0.7370 |
| Yes | 0 | – | 0 | – | 0 | – | 0 | – | 0 | – | ||||||
| SD × GS | −0.0007 | 0.0003 | 0.0218 | |||||||||||||
| RMSE | 0.51 | 0.22 | 0.11 | 0.11 | 0.16 | |||||||||||
1ED: duration of the experimental period (d); EIA: experimental initial age (d); SD: stocking density (kg/m2); GS: group size (n); DP: duration of the photoperiod (h); DS: divided scotoperiod (h); EC: MU: use of vaccines and other medications.
DISCUSSION
This work aimed at quantifying the influence of animal traits and housing conditions, characterizing intensive systems, on the performance and welfare of broiler chickens. The models included key aspects, such as stocking density, group size, or photoperiod duration, for which interactive effects were predicted. Additional aspects, well represented in the literature such as gender and genetics, were also included to improve model prediction precision (St-Pierre, 2001; Sauvant et al., 2008). The number of papers used in the present study was relatively small compared to existing scientific literature. This came as result of the restrictions that we imposed for manuscript selection in order to guarantee the accuracy of results, and that we acknowledge might have had an influence in the outcomes of the study.
Predictions in the growth model (Figure 1a), besides reflecting the peak of growth at a given age and its decline later on, exemplified in the manuscripts of Goliomytis et al. (2003) and Marcato et al. (2008), also reflected the modulatory effect of stocking density over bird performance with age. Although it is known that broiler performance is compromised at high stocking density (Bessei, 2006; Estevez, 2007), contrary to our expectations the decay of growth with age would appear to be smoother at relatively high stocking density (Figure 1b), at least within the range of studied densities. These predictions were calculated for stocking densities up to 35 kg/m2, which represents moderate density values for commercial conditions. This finding might be explained as an indirect consequence of the barrier effect created by the birds (Newberry and Hall, 1990; Estevez et al., 1997) rather than to stress and associated growth reduction (Moberg, 2000). The barrier effect at moderated to high densities may hinder bird movement (Estevez, 2007) potentially reducing energy expenditure and favoring growth (Lewis and Hurnik, 1990), as long as stocking density is not too high to cause a reduction in growth due to the deterioration of environmental conditions. The effects of variations in stocking density were predicted at constant group size (median group size value), meaning that increased stocking density would be the result of the reduction in enclosure size. Reduction in total enclosure space, even when stocking density remains constant, is documented to increase restriction of movements (Leone and Estevez, 2008), further supporting the idea that a barrier effect might have an impact on growth. Within the normal higher FCR of older birds, our models also predicted a slightly higher FCR when combining older ages and lower stocking density (Figure 5a). This subtle but significant result may respond to the combination of the higher bird mobility detected at lower densities (Leone and Estevez, 2008), which would imply higher energy expenditure during movement. This effect would be particularly relevant for heavier birds (Stojcic and Bessei, 2009) at low, as compared to high densities.
Stocking density also modulated changes in mortality with age (Figure 7b). This effect reflected in the higher predicted mortality at relatively high stocking densities and young ages, results that suggest that the negative effects of density may become evident from the very start of the rearing period. The influence of age on mortality was clear although, surprisingly, mortality increased sharply at low stocking density after week 4, perhaps due to the higher body weight potential that birds may reach when reared at low stocking densities. In any case, considering performance and mortality it is clear that relatively high stocking densities do not improve bird efficiency and pose welfare concerns.
Stocking density and group size are frequently confounded (Estevez et al., 2007). It is important to simultaneously account for both, when possible, in order to discern their effects as done in this study. Best FCR was predicted for low stocking densities and large groups (Figure 5c). This result might be attributed to the combined benefits of the larger total space availability and higher social tolerance that characterize large groups, both aspects known to promote bird movement (Estevez et al., 1997). Predictions suggest that relatively low stocking density would enhance growth (Figure 1c) and feed efficiency (Figure 5c) of birds in large flocks. Increased standing and resting frequencies were predicted for larger groups (Table 6), likely due to the larger effective space available. Higher resting frequency was additionally predicted when large groups were combined with low stocking densities (Figure 8b), which is likely related to the lower level of disturbances that normally occur at lower densities and larger space availability (Cornetto et al., 2002). These results show the benefits of the combination of a relatively low stocking density and large group in terms of broiler chicken behavior and welfare.
The influence of stocking density was further modulated by individual traits such as gender (Table 3). ADG and ADFI values were, overall, smaller for females than for males, reflecting the sexual dimorphism of these traits (Mignon-Grasteau et al., 1999). However, both parameters were strongly affected by the effect of density in males showing that males are much more sensitive to the negative effects of increasing stocking density, coinciding with the results of Puron et al. (1995).
The results of ADG, ADFI, and FCR (Figures 2b, 4c, and 6b, respectively) indicate that controlled environmental conditions lead to enhanced performance, in line with the review of Lara and Rostagno (2013). Given that modern chicken breeds have high metabolic activity, and high susceptibility to environmental challenges such as thermal stress (Mendes et al., 1997; Sohail et al., 2012), a good environmental control is critical for good bird performance. Predictions indicate that performance differences between controlled and non-controlled environments are particularly evident at low stocking density. This is surprising, but might be potentially explained by larger fluctuations in environmental conditions when density is low, or alternatively, by a reduction in efficiency of the environmental control system to manage the environment to the ideal parameters at higher densities. The higher gait scores (Kestin et al., 1992) predicted for environmentally controlled conditions (Table 5) may be reflecting the increment of problems due to impaired walking ability, probably as a consequence of the faster growth rates that may be achieved under environmentally control conditions. Given that most of studies included in the meta-analysis were conducted under experimental conditions, extrapolation of these results to commercial conditions should be taken with caution though.
Results also revealed the relevance of photoperiod total duration in modulating the effects of many of the tested variables over the performance and welfare of broiler chickens. Under commercial conditions a relatively wide range of photoperiod durations are used, although no general agreement has still been reached regarding their benefits and drawbacks. This is partly because other aspects of the lighting programs (type of lights, colors, and intensity among other features) are also important. To resolve this issue a systematic approach has been encouraged to determine the ideal lighting conditions (Olanrewaju et al., 2006). Under commercial practice, the need to adapt photoperiod to birds’ age is commonly accepted, and it has been suggested that either short or near-continuous photoperiods should be avoided (Schwean-Lardner and Classen, 2010). Figures 3c and 5b show that the relative improvement of ADFI when combining longer photoperiods and older ages would be accompanied by an increment in FCR, which was inherent to their higher body weight. These results would agree with previous studies in fast- and medium-growth rate meat chicken strains (Classen and Riddell, 1989; Yang et al., 2015), who found similar effects of long photoperiods on chicken performance during later life stages. This negative effect may be explained by the fact that, above an upper threshold, a higher ADFI would be inefficient due to the limitations of the birds’ capacity to digest all the ingesta, or even by longer photoperiods resulting in feed spillage. Longer photoperiods are also likely to be detrimental for bird welfare, as indeed shown by Bayram and Özkan (2010), who reported behavior patterns closer to those under natural conditions, reduced fearfulness, and increased sociality with 16L:8D light patterns compared to continuous lighting programs.
Our models predicted increased frequencies in resting, and decreased eating, drinking, walking, and standing inactive with longer photoperiods (Table 6), which might be interpreted as evidence of decreased bird activity. Long photoperiods are known to disrupt chickens’ normal behavior patterns (Olanrewaju et al., 2006). Previous studies pointed out that photoperiods of nearly 24 h should be avoided as they increase inactivity (Schwean-Lardner et al., 2010). In addition, these authors found a reduction in behavioral and physiological synchrony with photoperiods of nearly 24 h. Therefore, these results provide further support to the extended recommendation that a sufficiently long dark period should be provided to older birds to optimize their performance without compromising welfare.
Stocking density also modulated the influence of photoperiod duration on bird performance, with ADG, ADFI, and FCR markedly increasing with longer photoperiods and low stocking densities (Figures 2a, 4b, and 6a respectively). This may be the result of the combination of more space available and more lighting hours that might had facilitated bird access to the feeders. Longer photoperiods were also predicted to improve gait score of birds housed at low stocking density (Figure 8a), which is contrary to what should be expected. On the contrary, reduced ADG and ADFI were observed for the combined effects of longer photoperiods and larger groups (Figures 2c and 4d). Nevertheless, no effect on FCR was predicted for this interaction, which might be interpreted as an indication that FCR might be affected to a greater extend by stocking density than by the size of the group. No effect on gait score was predicted for this interaction either. Thus, it appears that longer photoperiods will only translate into increased locomotion if sufficient space is provided, and that increasing space availability appears more effective than increasing group size in terms of bird welfare, as the potential benefits of larger effective space in the movement of larger flocks are masked by the presence of more birds, and therefore increased barrier effect.
The use of intermittent lighting programs, with repeated light/dark cycles during a 24-h period, was proposed because of their apparent benefits for the performance and welfare of broiler chickens (Olanrewaju et al., 2006). Intermittent lighting programs appeared to have advantages over continuous lighting programs, such as higher plasma growth hormone concentrations and higher growth rates in male birds (Kühn et al., 1996). However, our results reveal some potential negative effects. We predicted higher ADFI with divided scotoperiods, but only a trend toward increased growth and no FCR improvement was detected (Table 3), suggesting that their use would not actually translate into real advantages for performance. In addition, dividing the scotoperiod also resulted in an unexpected deterioration of leg conditions as shown by the higher gait scores obtained as compared to continuous scotoperiod (Table 5). Provision of a dark period improves the leg condition of birds (Prescott et al., 2004), and this has been associated to greater activity during the light period (Brickett et al., 2007). Nevertheless, according to our results, and given that birds usually eat more after a dark period (de Jong et al., 2005), it seems as if divided scotoperiods would only promote feeding behavior rather than increased overall bird activity. This would explain why we predicted that improved feed intake when using divided scotoperiods would be associated with more leg problems. Thus, our results would not support the use of divided scotoperiods both from the performance and welfare perspectives.
In conclusion, we were able to quantify, through the use of the existing scientific literature, the effects of relevant animal and housing aspects affecting the performance and welfare of intensively reared broiler chickens, and the interactive nature of many of these aspects. Clearly, the use of high stocking densities was shown to have a negative impact on bird performance and welfare. On the contrary, increasing total effective space, either by lowering stocking density or by increasing the size of the groups for a given constant density, would have a beneficial effect on broiler welfare. Long photoperiods when used at older ages did not appear to improve feed efficiency and did appear to disrupt birds’ behavior patterns, compromising their welfare. Our results would not support a scotoperiod division for either performance or welfare perspectives. Large group sizes may benefit broiler welfare, and also performance provided that relatively short photoperiods are used. Environmental control benefits flock performance, particularly at low stocking densities or large groups.
SUPPLEMENTARY DATA
Supplementary data are available at Poultry Science online.
ACKNOWLEDGMENTS
The authors thank the Spanish Ministry of Economy and Competitiveness, General Directorate of Science and Technology, National Research Program “Retos de la sociedad” for the funding the research project “iBOSP—Intelligent software to support sustainable strategies and decisions in the meat chicken production chain” (AGL2013–49173-C2–1-R).
REFERENCES
- Averós X., Brossard L., Dourmad J. Y., de Greef K., Edge H. L., Edwards S. A., Meunier-Salaün M. C.. 2010a. Quantitative assessment of the effects of space allowance, group size and floor characteristics on the lying behaviour of growing-finishing pigs. Animal 4:777–783. [DOI] [PubMed] [Google Scholar]
- Averós X., Brossard L., Dourmad J. Y., de Greef K., Edge H. L., Edwards S. A., Meunier-Salaün M. C.. 2010b. A meta-analysis of the combined effect of housing and environmental enrichment characteristics on the behaviour and performance of pigs. Appl. Anim. Behav. Sci. 127:73–85. [Google Scholar]
- Averós X., Brossard L., Dourmad J. Y., de Greef K. H., Edwards S. A. M. C. Meunier-Salaün. 2012. Meta-analysis on the effects of the physical environment, animal traits, feeder and feed characteristics on the feeding behaviour and performance of growing-finishing pigs. Animal 6:1275–1289. [DOI] [PubMed] [Google Scholar]
- Bayram A, S., Özkan. 2010. Effects of a 16-hour light, 8-hour dark lighting schedule on behavioral traits and performance in male broiler chickens. J. Appl. Poult. Res. 19:263–273. [Google Scholar]
- Bessei W. 2006. Welfare of broilers: a review. Worlds Poult. Sci. J. 62:455–466. [Google Scholar]
- Brickett K. E., Dahiya J. P., Classen H. L., Annett C. B., Gomis S.. 2007. The impact of nutrient density, feed form, and photoperiod on the walking ability and skeletal quality of broiler chickens. Poult. Sci. 86:2117–2125. [DOI] [PubMed] [Google Scholar]
- Broom D. M. 1991. Animal welfare: concepts and measurement. J. Anim. Sci. 69:4167–4175. [DOI] [PubMed] [Google Scholar]
- Broom D. M. 2010. Animal welfare: an aspect of care, sustainability, and food quality required by the public. J. Vet. Med. Educ. 37:83–88. [DOI] [PubMed] [Google Scholar]
- Classen H. L., Riddell C.. 1989. Photoperiodic effects on performance and leg abnormalities in broiler chickens. Poult. Sci. 68:873–879. [DOI] [PubMed] [Google Scholar]
- Cornetto T., Estevez I., Douglass D. W.. 2002. Using artificial cover to reduce aggression and disturbances in domestic fowl. Appl. Anim. Behav. Sci. 75:325–336. [Google Scholar]
- de Jong I. C., Fillerup M., Blokhuis H. J.. 2005. Effect of scattered feeding and feeding twice a day during rearing on indicators of hunger and frustration in broiler breeders. Appl. Anim. Behav. Sci. 92: 61–76. [Google Scholar]
- Douglas S. L., Szyszka O., Stoddart K., Edwards S. A., Kyriazakis I.. 2014. A meta-analysis to identify animal and management factors influencing gestating sow efficiency. J. Anim. Sci. 92:5716–5726. [DOI] [PubMed] [Google Scholar]
- Douglas S. L., Szyszka O., Stoddart K., Edwards S. A., Kyriazakis I.. 2015. Animal and management factors influencing grower and finisher pig performance and efficiency in European systems: a meta-analysis. Animal 9:1210–1220. [DOI] [PubMed] [Google Scholar]
- Estevez I. 2007. Density allowances for broilers: where to set the limits? Poult. Sci. 86:1265–1272. [DOI] [PubMed] [Google Scholar]
- Estevez I., Andersen I.-L., Nævdal E.. 2007. Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 103:185–204. [Google Scholar]
- Estevez I., Newberry R., Arias de Reyna L.. 1997. Broiler chickens: a tolerant social system? Etologia 5:19–29. [Google Scholar]
- Fanatico A. C., Pillai P. B., Hester P. Y., Falcone C., Mench J. A., Owens C. M., Emmert J. L.. 2008. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 87:1012–1021. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. 2017. Statistical Database of the Food and Agriculture Organization. Acccessed July 2017. http://faostat3.fao.org. [Google Scholar]
- Goliomytis M., Panopoulou E., Rogdakis E.. 2003. Growth curves for body weight and major component parts, feed consumption, and mortality of male broiler chickens raised to maturity. Poult. Sci. 82:1061–1068. [DOI] [PubMed] [Google Scholar]
- Gonyou H. W., Brumm M. C., Bush E., Deen J., Edwards S. A., Fangman T., McGlone J. J., Meunier-Salaün M. C., Morrison R. B., Spoolder H., Sundberg P. L., Johnson A. K.. 2006. Application of broken-line analysis to assess floor space requirements of nursery and grower-finisher pigs expressed on an allometric basis. J. Anim. Sci. 84:229–235. [DOI] [PubMed] [Google Scholar]
- Hamer R. M., Simpson O. M.. 2002. SAS® tools for meta-analysis. 250–227 in Proceedings of the Twenty-Seventh Annual SAS® Users Group Int. Conf., April 14–17, Orlando, FL. SAS Inst. Inc., Cary, NC. [Google Scholar]
- Havenstein G. B., Ferket P. R., Qureshi M. A.. 2003. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 82:1500–1508. [DOI] [PubMed] [Google Scholar]
- Humphrey T. 2006. Are happy chickens safer chickens? Poultry welfare and disease susceptibility 1. Br. Poult. Sci. 47:379–391. [DOI] [PubMed] [Google Scholar]
- Kestin S. C., Knowles T. G., Tinch A. E., Gregory N. G.. 1992. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet. Rec. 131:190–194. [DOI] [PubMed] [Google Scholar]
- Kühn E. R., Darras V. M., Gysemans C., Decuypere E., Berghman L. R., Buyse J.. 1996. The use of intermittent lighting in broiler raising.: 2. Effects on the somatotrophic and thyroid axes and on plasma testosterone levels. Poult. Sci. 75:595–600. [DOI] [PubMed] [Google Scholar]
- Lara L. J., Rostagno M. H.. 2013. Impact of heat stress on poultry production. Animals 3:356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone E. H., Christman M. C., Douglass L., Estevez I.. 2010. Separating the impact of group size, density, and enclosure size on broiler movement and space use at a decreasing perimeter to area ratio. Behav. Processes. 83:16–22. [DOI] [PubMed] [Google Scholar]
- Leone E. H., Estevez I.. 2008. Use of space in the domestic fowl: separating the effects of enclosure size, group size and density. Anim. Behav. 76:1673–1682. [Google Scholar]
- Lewis N. J., Hurnik J. F.. 1990. Locomotion of broiler chickens in floor pens. Poult. Sci. 69:1087–1093. [DOI] [PubMed] [Google Scholar]
- Lipsey M. W., Wilson D. B.. 2000. Practical Meta-Analysis. Sage Publications, Thousand Oaks, CA. [Google Scholar]
- Marcato S. M., Sakomura N. K., Munari D. P., Fernandes J. B. K., Kawauchi Í. M., Bonato M. A.. 2008. Growth and body nutrient deposition of two broiler commercial genetic lines. Rev. Bras. Cienc. Avic. 10:117–123. [Google Scholar]
- Mendes A. A., Watkins S. E., England J. A., Saleh E. A., Waldroup A. L., Waldroup P. W.. 1997. Influence of dietary lysine levels and arginine:lysine ratios on performance of broilers exposed to heat or cold stress during the period of three to six weeks of age. Poult. Sci. 76:472–481. [DOI] [PubMed] [Google Scholar]
- Mignon-Grasteau S., Beaumont C., Le Bihan-Duval E., Poivey J. P., De Rochambeau H., Ricard F. H.. 1999. Genetic parameters of growth curve parameters in male and female chickens. Br. Poult. Sci. 40:44–51. [DOI] [PubMed] [Google Scholar]
- Mignon-Grasteau S., Moreri U., Narcy A., Rousseau X., Rodenburg T. B., Tixier-Boichard M., Zerjal T.. 2015. Robustness to chronic heat stress in laying hens: a meta-analysis. Poult. Sci. 94:586–600. [DOI] [PubMed] [Google Scholar]
- Moberg G. P. 2000. Biological response to stress: implications for animal welfare. Pages 1–21 In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. Moberg G. P., Mench J. A., eds. CABI Publishing, Wallingford, Oxon, UK. [Google Scholar]
- Newberry R. C., Hall J. W.. 1990. Use of pen space by broiler chickens: effects of age and pen size. Appl. Anim. Behav. Sci. 25:125–136. [Google Scholar]
- Olanrewaju H. A., Thaxton J. P., Dozier W. A. III, Purswell J., Roush W. B., Branton S. L.. 2006. A review of lighting programs for broiler production. Int. J. Poult. Sci. 5:301–308. [Google Scholar]
- Prescott N. B., Kristensen H. H., Wathes C. M.. 2004. Light. Pages 101–116 In Measuring and Auditing Broiler Welfare, eds. Weeks C., Butterworth A.. CABI Publishing, Wallingford, Oxon, UK. [Google Scholar]
- Puron D., Santamaria R., Segura J. C., Alamilla J. L.. 1995. Broiler performance at different stocking densities. J. Appl. Poult. Res. 4:55–60. [Google Scholar]
- Remus A., Hauschild L., Andretta I., Kipper M., Lehnen C. R., Sakomura N. K.. 2014. A meta-analysis of the feed intake and growth performance of broiler chickens challenged by bacteria. Poult. Sci. 93:1149–1158. [DOI] [PubMed] [Google Scholar]
- Robins A., Phillips C. J. C.. 2011. International approaches to the welfare of meat chickens. Worlds Poult. Sci. J. 67:351–369. [Google Scholar]
- Rushen J. 2000. Some Issues in the Interpretation of Behavioural Responses to Stress. Pages 23–42 In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. Moberg G. P., Mench J. A., eds. CABI Publishing; Wallingford, Oxon, UK. [Google Scholar]
- SAS Institute, 2011. SAS Proprietary Software Release 9.3. SAS Inst., Inc. Cary, NC. [Google Scholar]
- Sauvant D., Schmidely P., Daudin J. J., St-Pierre N. R.. 2008. Meta-analyses of experimental data in animal nutrition. Animal 2:1203–1214. [DOI] [PubMed] [Google Scholar]
- Schmidely P., Glasser F., Doreau M., Sauvant D.. 2008. Digestion of fatty acids in ruminants: a meta-analysis of flows and variation factors. 1. Total fatty acids. Animal 2:677–690. [DOI] [PubMed] [Google Scholar]
- Schwean-Lardner K., Classen H. L.. 2010. Ross Tech - Lighting for broilers. Accessed Apr. 2018. http://es.aviagen.com/assets/Uploads/RossTechLightingforBroilers.pdf. [Google Scholar]
- Schwean-Lardner K., Fancher B. I., Classen H. L.. 2010. Effect of daylength on physiological and behavioral rhythms in broilers. Poult. Sci. 89(E-Suppl. 1):521 (Abstr.) [Google Scholar]
- Sohail M. U., Hume M. E., Byrd J. A., Nisbet D. J., Ijaz A., Sohail A., Shabbir M. Z., Rehman H.. 2012. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 91:2235–2240. [DOI] [PubMed] [Google Scholar]
- Sommerville R., Ruiz R., Averós X.. 2017. A meta-analysis on the effects of the housing environment on the behaviour, mortality, and performance of growing rabbits. Anim. Welf. 26:223–238. [Google Scholar]
- Sorensen P. 1992. The genetics of leg disorders. Pages 213–219 in Bone Biology and Skeletal Disorders in Poultry. Whitehead C. C., ed. Poult. Sci. Symp. 23 Carfax, Abingdon, UK. [Google Scholar]
- St-Pierre N. R. 2001. Invited review: integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 84:741–755. [DOI] [PubMed] [Google Scholar]
- Stojcic M. D., Bessei W.. 2009. The effect of locomotor activity and weight load on bone problems in fast and slow growing chickens. Arch. Geflügelk. 73:242–249. [Google Scholar]
- Thomas L., Juanes F.. 1996. The importance of statistical power analysis: an example from animal behaviour. Anim. Behav. 52:856–859. [Google Scholar]
- Yang Y. F., Jin S. F., Zhong Z. T., Yu Y. H., Yang B., Yuan B., Pan J. M.. 2015. Growth responses of broiler chickens to different periods of artificial light1. J. Anim. Sci. 93:767–775. [DOI] [PubMed] [Google Scholar]
- Zoidis E., Demiris N., Kominakis A., Pappas A. C.. 2014. Meta-analysis of selenium accumulation and expression of antioxidant enzymes in chicken tissues. Animal 8:542–554. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Schneider B. L., Carney V. L., Korver D. R., Robinson F. E.. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult. Sci. 93:2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.