ABSTRACT
Campylobacter jejuni (C. jejuni) is one of the most important zoonotic pathogens worldwide. In Europe, the majority of the cases are caused by consuming contaminated poultry meat. The objective of the present study was to investigate potential effects of different crude protein levels in complete diets for broilers on infection dynamics of C. jejuni after experimental infection. In total, 300 commercial broilers line Ross 308 were divided into 4 different groups, including 5 replications of 15 chickens each. The chickens were fed a conventional diet (212 g CP/kg DM) and a protein-reduced test diet (190 g CP/kg DM) supplemented with essential amino acids. This resulted simultaneously in lower amino-acid concentrations preferentially utilized by C. jejuni, such as aspartate, glutamate, proline, and serine. One group of each feeding concept was infected artificially with C. jejuni at day 21 by applying an oral C. jejuni inoculum containing 4.17 ± 0.09 log10 cfu of C. jejuni to 3 of 15 chickens, called “seeders.” Feeding the test diet resulted in a significant reduction (P < 0.001) in CP intake (31.5 ± 1.20 g CP/broiler/day and 27.7 ± 0.71 g CP/broiler/day, respectively), a significant decrease (P < 0.05) in crude mucin in excreta (55.7 ± 8.23 g/kg DM and 51.9 ± 7.62 g/kg DM, respectively), and in goblet cell number in cecal crypts (P < 0.05; 15.1 ± 5.71 vs. 13.6 ± 5.91 goblet cells/crypt). In groups receiving the test diet, the excretion of C. jejuni was significantly reduced in seeders by 1.9 log10 cfu/g excreta at day 23 (3.38a ± 2.55 vs. 1.47b ± 2.20; P = 0.033). At day 25, prevalence of C. jejuni in cloacal swabs amounted to 53.3% in the group fed the test diet and 75.7% in the control group, respectively (P < 0.05). In summary, a definite amino acid pattern in the broiler diets could contribute to a development of an effective feeding strategy to reduce the prevalence of C. jejuni infection in chickens (Patent No 17187659.2-1106).
Keywords: amino acid, Campylobacter jejuni, mucus
INTRODUCTION
Campylobacteriosis is one of the world's most important diarrheal diseases (Devleesschauwer et al., 2017). Campylobacter jejuni (C. jejuni) is the leading cause of bacterial enteritis in humans (Sedat, 2015). In Europe, consistently rising numbers of Campylobacter-associated diarrheal diseases are caused in the overwhelming majority of the cases by the consumption of contaminated poultry meat (EFSA, 2013). The extent of contamination shows a positive correlation between the counts of Campylobacter in the cecal content and the number of bacteria on the carcass and the pectoral muscle (Reich et al., 2008). A whole series of control strategies has been tested so far. In particular, feed additives were of interest. (Metcalf et al., 2011; Kurekci et al., 2014; Awad et al., 2016; Gracia et al., 2016; Guyard-Nicodème et al., 2016; Schneitz and Hakkinen, 2016; Khattak et al., 2018). The success of these measures was heterogeneous.
In contrast to most other intestinal bacteria, the genus Campylobacter uses carbohydrates only to a very limited extent as substrate for its metabolism (Epps et al., 2013). The metabolism of C. jejuni relies on the utilization of amino acids (Epps et al., 2013), mainly on serine, aspartate, glutamate, and proline, which are preferred in this order (Guccione et al., 2008; Wright et al., 2009). These amino acids are also involved in the formation of the mucin glycoproteins of the intestinal mucus layer (Ravindran and Bryden, 1999; Adedokun et al., 2011) and thus are most frequently included in both the mucus layer and the excreta of poultry (Parsons, 1984).
The crude protein supply influences the thickness and composition of the intestinal mucus layer (Lemme et al., 2004; Ravindran et al., 2009). The main constituents of the intestinal mucus layer are the mucins, which can account for 1 to 10% of the layer (Allen, 1981) and are produced by the goblet cells (Allen, 1981; Kim and Khan, 2013). C. jejuni has developed very successful strategies to invade and colonize the mucus layer (Van Deun et al., 2008). The presence of mucins appears to be essential for the survival and growth of C. jejuni (Van Deun et al., 2008). The synthesis of mucins depends on the crude protein content in the ration, whereby a reduced crude protein content leads to reduced mucin production and release into the intestinal tract of the broilers (Ravindran et al., 2009).
Essential amino acids are of crucial importance for growth (Baker and Han, 1994). By targeting these growth-limiting amino acids, the crude protein content can be reduced in broilers' compound feeds without adversely affecting animal growth (Mack et al., 1999). Indirectly, the content of the amino acids in the ration can be reduced which are essential for the metabolism of C. jejuni. The concentration of these amino acids in the ceca is also diminished by a smaller passage of these amino acids into the lower parts of the intestine (Lemme et al., 2004).
The hypothesis of the study was that C. jejuni prevalence and excretion can be reduced by a particular amino acid pattern in the diet in 2 ways: first, by a lower availability of amino acids from the diet at the end of the small intestine; second, by a lower mucin availability for the growth of Campylobacter.
MATERIAL AND METHODS
Animal experiments were performed in accordance with the German rules and regulations and approved by the Ethics Committee of Lower Saxony for Care and Use of Laboratory Animals LAVES (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit; reference: 33.19–42,502-05–15A540).
Animals and Housing
Newly hatched chickens (day 0) of both sexes (N = 300; ROSS 308) were obtained from a commercial hatchery (BWE-Brüterei Weser Ems, PHW Gruppe/LOHMANN & Co. AG, Visbek, Germany).
Day of hatch chicks were randomly divided into 4 groups, housed in 4 identical floor pens with wood shavings, and fed identical diets starter and grower diets for 14 d. Feed and water was offered ad libitum. Starting with a temperature of about 34 to 36°C, the temperature was lowered by about 1°C every 2 d, reaching a minimum temperature of about 20°C. The photoperiod beginning from day 4 was 16 h of light and 8 h of darkness including periods of diminution of light during the whole trial applying to the German regulation on Animal welfare in livestock production (Tierschutz-Nutztierhaltungsverordnung).
After the 14-d rearing phase, the animals were transferred from the rearing shed to the infection unit of the animal house (security level 2). At this time, the animals were randomly subdivided into 20 subgroups in a 2 × 2 factorial design with 2 different diets and a different infections modus (SPCN—standard protein, Campylobacter negative; LPCN low protein, Campylobacter negative; SPCP—standard protein, Campylobacter positive; LPCP—low protein, Campylobacter positive).
The animals were kept on solid floor pens littered with wood shavings (1 kg/m2) in groups of 15 animals (“subgroup”) up to the end of the experimental period (day 42) or rather up to dissection (day 43 to 45). Each subgroup had an unrestricted available floor space (total area minus the area under the trough) of 1.45 m².
Feeding Regime and Feed Analysis
This rearing phase was divided into a 1-wk starter phase with a conventional pelleted starter diet (starter) and a subsequent 7-d phase with a commercially available pelleted grower diet (grower; Best 3 Geflügelernährung GmbH, Twistringen, Germany). All diets were offered ad libitum.
Diets for the main experimental period (day 14 to 42) were produced in cooperation with Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany) and a special manufacturer for trial diets (Research Diet Services BV, Wijk bij Duurstede, the Netherlands; Table 1). The standard diet (SP diet) was designed in accordance with a commercially available standard fattening diet concerning ingredients and composition (Table 2) exceeding, to a certain amount, the recommendations for the energy and nutrient supply of the laying hens and fowls (broilers) of the Committee for Needs Standards of the Society for Nutritional Physiology (GfE, 1999). The low-protein diet (LP diet) contained a smaller amount of soybean meal and therefore had a reduced crude protein content. The growth-limiting amino acids lysine, methionine, threonine, L-isoleucine, valine, and L-arginine were added to the LP diet in higher proportions than to the SP diet. All diets were offered ad libitum. Circular feeding troughs were used during the whole trial (Crown Poultry Feeders, Nelson, New Zealand). Water was offered ad libitum in double-cylinder plastic bell drinkers in the rearing phase, and later via drinking lines equipped with Top Nipples with a drinking cup (Big Dutchman International GmbH, Vechta-Calveslage, Germany). The water was treated with chlorine dioxide (Virbac Clean Pipe, VIRBAC Tierarzneimittel GmbH, Bad Oldesloe, Germany) at a concentration of 0.3 mg ClO2/L to kill any C. jejuni in the drinking water.
Table 1.
Ingredient composition of the standard protein diet and the low protein diet for the main experimental period.
| Ingredients (in %) | SP diet1 | LP diet |
|---|---|---|
| Wheat | 41.4 | 47.1 |
| Corn | 25.0 | 25.0 |
| Soybean meal2 | 24.1 | 18.0 |
| Soybean oil | 5.16 | 4.43 |
| Monocalciumphosphate | 1.37 | 1.38 |
| Calcium carbonate | 1.28 | 1.72 |
| Premix “Blank Poultry”3 | 0.50 | 0.50 |
| L-Lysin-HCl®4 | 0.26 | 0.45 |
| Sodium bicarbonate | 0.25 | 0.35 |
| MetAMINO®5 | 0.25 | 0.30 |
| Sodium chloride | 0.20 | 0.13 |
| ThreAMINO®6 | 0.10 | 0.18 |
| L-Isoleucin | 0.06 | 0.16 |
| ValAMINO®7 | 0.06 | 0.16 |
| L-Arginin | 0.00 | 0.17 |
SP diet = standard protein diet; LP diet = low protein diet.
1The sum of all the ingredients does not add up to 100 due to rounding differences.
248% crude protein.
3Carrier: cornflour; content per kg: iron (16,000 mg), copper (2400 mg), manganese (17,000 mg), zinc (12,000 mg), iodine (160 mg), selenium (30 mg), vitamin A (2000,000 IU), vitamin D3 (500,000 IU), vitamin E (10,000 IU), vitamin K3 (300 mg), vitamin B1 (400 mg), vitamin B2 (1,500 mg), vitamin B6 (700 mg), vitamin B12 (4,000 μg), niacin (7,000 mg), D-pantothenic acid (2,400 mg), choline chloride (92,000 mg), folic acid (200 mg), biotin (40 mg).
478.0% L-Lysin (Evonik Nutrition & Care GmbH, Hanau-Wolfgang, Germany).
599.0% DL-Methionin (Evonik Nutrition & Care GmbH, Hanau-Wolfgang, Germany).
698.5% L-Threonin (Evonik Nutrition & Care GmbH, Hanau-Wolfgang, Germany).
798.0% L-Valin (Evonik Nutrition & Care GmbH, Hanau-Wolfgang, Germany).
Table 2.
Energy content and concentrations of ingredients in the standard protein diet and the low protein diet for broilers in the experimental period (analyzed values; days 21 to 42).
| Item | SP diet | LP diet | Item | SP diet | LP diet | |
|---|---|---|---|---|---|---|
| Dry matter (g/kg diet) | 884 | 884 | Arginine (g/kg DM) | 13.5 | 13.2 | |
| Crude ash (g/kg DM) | 57.6 | 57.6 | Cysteine | 3.36 | 3.24 | |
| Crude fat | 81.0 | 71.7 | Isoleucine | 8.71 | 8.60 | |
| Crude fiber | 27.1 | 25.0 | Leucine | 16.4 | 13.7 | |
| Crude protein | 212 | 190 | Lysine | 13.3 | 12.6 | |
| Nitrogen-free extract1 | 622 | 655 | Methionine | 4.62 | 5.28 | |
| Starch | 455 | 499 | Phenylalanine | 10.2 | 8.36 | |
| Sugar | 50.7 | 45.6 | Threonine | 8.69 | 8.34 | |
| Calcium | 10.2 | 12.1 | Valine | 9.39 | 9.44 | |
| Phosphorus | 7.68 | 6.59 | Alanine | 9.53 | 8.09 | |
| Potassium | 8.66 | 8.34 | Aspartic acid | 20.6 | 17.0 | |
| Sodium | 1.85 | 1.96 | Glutamic acid | 45.1 | 39.0 | |
| Chloride | 2.77 | 2.79 | Glycine | 8.87 | 7.49 | |
| Magnesium | 1.92 | 1.80 | Histidine | 5.33 | 4.61 | |
| Sulfur | 3.22 | 2.87 | Proline | 13.2 | 10.8 | |
| AME (MJ/kg DM)2 | Serine | 11.1 | 9.22 | |||
| 14.3 | 14.3 | Tyrosine | 6.89 | 5.93 | ||
SP diet = standard protein diet; LP diet = low protein diet.
1Nitrogen-free extract = DM—(crude ash + crude fat + crude fiber + crude protein).
2AME (MJ/kg DM) = 0.1551 × % crude protein+0.3431 × % crude fat+0.1669 × % starch+0.1301 × % sugar.
Diets were analyzed by standard procedures in accordance with the official methods of the VDLUFA (Naumann and Bassler, 2012). The analyses were always performed in duplicate. The DM content was determined by drying to the weight constancy at 103°C. The raw ash was analyzed by means of incineration in the muffle furnace at 600°C for 6 h. The total nitrogen content was determined by means of the analyzer Vario Max® (Elementar, Hanau, Germany), which operates according to the principle of a catalytic tube combustion (DUMAS combustion method). The crude fat content was determined after acid digestion in the soxleth apparatus. The content of crude fiber was determined after washing in dilute acids and alkalis using established methods. Starch determination was carried out polarimetrically (Polatronic E, Schmidt und Haensch GmbH & Co., Berlin, Germany). The sugar content was analyzed by the method in accordance with Luff-Schoorl by titration with sodium thiosulfate. The mineral content was determined in accordance with the official methods (Naumann and Bassler, 2012) by atomic absorption spectrometry (Unicam Solaar 116, from Thermo, Dreieich, Germany). Amino acids were determined by ion-exchange chromatography (AA Analyzer LC 3000, Biotronic, Maintal, Germany).
Experimental Infection and Sampling
The experimental infection with C. jejuni took place at day 21 in all subgroups of the groups (n = 5 for groups SPCP and LPCP each) SPCP and LPCP. In each subgroup, 3 of 15 broilers in a box (n = 3 per subgroup) were administered orally with a C. jejuni suspension. A field strain of C. jejuni was used for experimental infection (Jansen et al., 2014). This isolate had been identified as C. jejuni both culturally and mass-spectrometrically (MALDI-TOF MS) in an accredited laboratory (AniCon Labor GmbH, Höltinghausen, Germany). The conserved strain for experimental infection was recultivated on solid selective culture media (mCCD agar; Oxoid Germany GmbH, Wesel, Germany). After incubation at 41.5°C in a microaerobic atmosphere for 43.5 h, the infection strain was used in its stationary growth phase (24 to 48 h) for preparing the infection solution. C. jejuni was resuspended in normal saline to prepare the inoculum. Therefore, an isotonic 0.9% sodium chloride solution was used as the basis for the bacterial suspension for infection (about 10,000 CFU/2 mL). For the experimental infection, 3 of 15 animals were randomly selected. Infection solution was administered orally by means of a button cannula (single-button cannula, sterile, 1.0 × 100 mm, Meiser Medical GmbH, Neuenstein, Germany). Analogous to the groups with experimental infection, 3 of 15 animals were randomly selected in the non-infected control groups (SPCN and LPCN). These animals were administered 2 mL of a sterile sodium chloride solution by means of a button cannula.
At day 18 a sample of 5 animals per subgroup and at day 21 each individual animal (N = 300) were tested by means of a cloacal swab (Cary Blair smear test system, Süsse Labortechnik GmbH & Co. KG, Gudensberg, Germany) regarding a possible excretion of C. jejuni to ensure that there is no Campylobacter colonization prior to Campylobacter challenge in birds. In the same way, a sampling of all animals in the groups SPCP and LPCP (n = 75/group) was carried out at days 22, 23, 24, 25, 28, 35, and 42. In the groups SPCN and LPCN regular spot-checks were carried out on the excretion of C. jejuni. For this purpose, 5 randomly selected animals per subgroup were examined by means of cloacal swabs and further cultural Campylobacter detection. At the end of the trial (day 42), animals from all groups were qualitatively tested concerning their Campylobacter status according to DIN EN ISO 10272–1:2006 (see section “Bacterological Analyses”). In addition to these qualitative studies, determining the colony-forming units of C. jejuni in excreta of seeder birds in groups SPCP und LPCP was done at days 23, 32, and 38. For excreta collection, the corresponding animals were individually placed in purified, disinfected 10 L plastic bucket (ϕ 26.5 cm) to collect fresh dropped excreta. All birds in the study were individually tagged by wing-tags.
Sample Collection and Histological Investigations
Following the experimental period (day 21 to 42), a dissection of the animals was performed by standard protocol approved by the animal care committee on 3 consecutive days (day 43, 44, and 45). The contents of the 2 ceca were removed under sterile provisos and placed in a screw cup (screw cup 100 mL, PP, Sarstedt AG & Co., Nümbrecht, Germany) for all animals in groups SPCP and LPCP.
For determining the number of goblet cells in the cecal crypts of the experimentally infected groups SPCP and LPCP, an approximately 1 cm long piece was removed from the apex of the right cecum, approximately 1 cm proximal to the apex, and fixed in 4 % formaldehyde for 48 h. After fixation, tissue samples were embedded in paraffin using standard techniques and for histological evaluation, 4 μm sections of all samples were stained with H&E using established protocols (Slaoui and Fiette, 2011). Sections were viewed with a Zeiss Axioskop (Carl Zeiss Jena GmbH, Jena, Germany) and images were digitally captured using an Olympus DP Soft Camera (Olympus Deutschland GmbH, Hamburg, Germany).
Sample analysis was done in accordance with established methods (Horn et al., 2009) with slight modifications. In each of the blinded samples, the depth of 5 complete vertically oriented crypts was measured. The goblet cells of the crypt were counted. Subsequently, an average crypt depth of about 250 μm was calculated from the crypt depths of all the samples examined in both experiments. The mean crypt depth was defined as the standard crypt depth. The number of goblet cells of the individual, measured crypts of each sample was converted to this depth for better comparability.
Excreta and Litter Sampling and Analysis
Samples of fresh excreta of the birds were collected from each box at day 20, 28 (only SPCN and LPCN), 35 (only SPCN and LPCN), and 42 in accordance with established methods (Abd El-Wahab et al., 2012). Within the infection trial, sampling in the infected groups was omitted to minimize the risk of transmission of infection between boxes. The collected excreta were then removed from each box, thoroughly mixed and processed in parts for further analysis (mucin content).
Mucin Content
The content of total mucin was determined in pool samples of excreta. Quantifying the water-soluble and ethanol-precipitable fraction of excreta was carried out in accordance with modified methods (Lien et al., 1997; Horn et al., 2009). Freeze-dried excreta (3 g) were mixed with 20 mL of a cooled NaCl/NH3 solution (0.15 mol NaCl and 0.02 mol NH3 per liter) and then homogenized for 30 s (Disperser Ultra Turrax T 25, IKA Werke GmbH & CO KG, Staufen, Germany). Centrifugation for 30 min at 4°C and 12,000 × g (Biofuge Stratos, Heraeus Holding GmbH, Hanau, Germany) separated the insoluble from the soluble parts of the sample. The soluble supernatant was transferred to a new 50 mL centrifuge tube and the dissolved mucin glycoproteins precipitated overnight at 18°C by addition of 15 mL of chilled pure ethanol. By recentrifugation for 10 min at 4°C and 1,400 × g, the precipitate was separated from soluble, non-precipitable components in ethanol. The precipitate was resuspended in 10 mL of cooled NaCl solution and reprecipitated by adding 15 mL of cooled ethanol again overnight at 18°C. These operations, consisting of centrifugation, resuspension of the precipitate, and renewed precipitation, were repeated until the supernatant was unclouded after centrifugation. The pellet washed in this way was freeze-dried and the proportion of mucins calculated.
Bacteriological Analyses
The qualitative bacteriological examination was based on the DIN EN ISO 10272–1:2006, taken from the official collection of analysis methods in accordance with § 64 LFBG. The sample matrix to be examined was incubated in a 1:9 ratio (sample: Bolton-broth) in sterile 5 mL tubes mounted with a vent cap (Sarstedt AG & Co.). According to DIN EN ISO 10272–1:2006 incubation was lasting for 4 h at 37°C followed by 44 h ± 4 h at 41.5°C under a microaerobic atmosphere (oxygen content of 5% ± 2%, carbon dioxide content of 10% ± 3%). After enrichment in Bolton-broth, the samples were streaked onto 2 solid selective culture media (mCCD agar and Karmali agar; Oxoid Germany GmbH) by sterile inoculation loops. The incubation of the inoculated selective culture was carried out again for 44 h ± 4 h at 41.5°C in a microaerophilic atmosphere. Individual colonies were analyzed to confirm the presence of Campylobacter. This was done by phase-contrast microscopy (Distelkamp-Electronic, Kaiserslautern, Germany) and biochemical methods (apiCampy, bioMérieux SA, Marcy-LÈtoile, France).
For quantitative bacteriological examination, a 10-fold dilution series (0.5 g sample material in 4.5 mL of sterile phosphate buffered saline) was made with phosphate buffered saline (Oxoid Germany GmbH). In duplicate, 100 μL of each dilution was plated onto mCCD agar (Oxoid Germany GmbH). After incubation in a microaerophilic atmosphere for 44 h ± 4 h at 41.5°C, the colonies were counted and an average value from the 2 duplicate experiments was taken for calculating the CFU/g intestinal content. In accordance with DIN EN ISO 10272–2:2006, only plates with more than 30 and fewer than 300 colonies were considered.
Performance Parameters
The body weight of the animals was recorded individually at days 7, 14, 21, and 42 (PCE TB 30, PCE Instruments, Meschede, Germany). The feed and water intake were recorded at the level of the box (n = 5 subgroups per group). Feed conversion ratio reflects feed consumption per kilogram of body weight gain. The animal losses were taken into account by adding the increase in body weight until the time of death. Protein efficiency was calculated as the increase in body weight per kilogram of crude protein intake. Animal losses were taken into consideration for this parameter. The feed to water ratio was calculated as the ratio of water intake to feed intake. At the time of dissection (day 43, 44, and 45), the slaughtering weight (body with feathers and without head, feet, gastrointestinal tract, liver, gallbladder, spleen, heart) and the slaughtering exploitations were calculated as a quotient of slaughter weight to the body mass (in %).
Statistical Analyses
The statistical analysis of the collected data was performed using the Statistical Analysis System for Windows the SAS® Enterprise Guide®, version 9.3 (SAS Institute Inc., Cary). For a comparison of mean values, the normal distribution of the residues was tested first with a Shapiro-Wilk test. Normally distributed data were examined for differences in mean values by a 1-factorial analysis of variance for multiple pairwise comparisons (Fisher's smallest significant difference). In the case of non-normalized data, comparisons with a Wilcoxon signed-rank test were carried out in pairs to investigate differences in the mean values. For comparing a sample with a constant, a 1-sample t test was used for normal distributed data. If uniform distribution of the sample was present, 2-dimensional frequency distributions of categorical features were checked for dependency with the Pearson`s Chi-square homogeneity test. Otherwise, the Fisher's exact test was used.
Correlation analyses were carried out on normal distributed data using Pearson's correlation coefficient and on non-normalized data using Spearman's rank correlation coefficient.
At P < 0.05, differences in the mean values or a dependence of the frequency distribution or a correlation were regarded as significant.
RESULTS
The experiment ran completely without complications as regards animal health. Mortality was 2.33%. Five of 300 broilers used in the experiment died during the experiment, 2 had to be euthanized. Six of these animals were excluded from the experiment within the trial period (day 21 to 42) and 1 animal after completion of the trial, but before dissection (losses in %: SPCN: 4.00%, LPNC: 2.67%, SPCP: 1.33%, LPCP: 1.33%).
Histology of the Intestine
For determining the goblet cells in the ceca, histological tissue samples of the experimentally infected experimental groups SPCP and LPCP were examined. This histological examination showed a very uniform crypt depth for the 2 experimental groups (Table 3). The number of goblet cells was significantly higher (P = 0.027) in the SPCP group (15.1 ± 5.71; Table 3; Figure 1) than in the experimental LPCP group (13.6 ± 5.91; Table 3; Figure 2).
Table 3.
Crypt depth and the number of goblet cells in the ceca of broiler chickens of experimental infected subgroups.
| Diet | |||||
|---|---|---|---|---|---|
| SP diet | LP diet | ||||
| Item | Mean | SD | Mean | SD | P-value |
| Crypt depth (μm; n = 25 or rather n = 24 per diet1) | |||||
| 256 | ±57.7 | 259 | ±59.1 | 0.647 | |
| Goblet cells per standard crypt (μm; n = 25 or rather n = 24 per diet1,2) | |||||
| 15.1a | ±5.71 | 13.6b | ±5.91 | 0.027 | |
SP diet = standard protein diet; LP diet = low protein diet.
1To determine the goblet cells in the ceca, histological tissue samples of the experimentally infected groups were used.
2Determined goblet cell number to 250 μm crypt depth.
a,bValues within a row with different superscripts differ significantly at P < 0.05.
Figure 1.
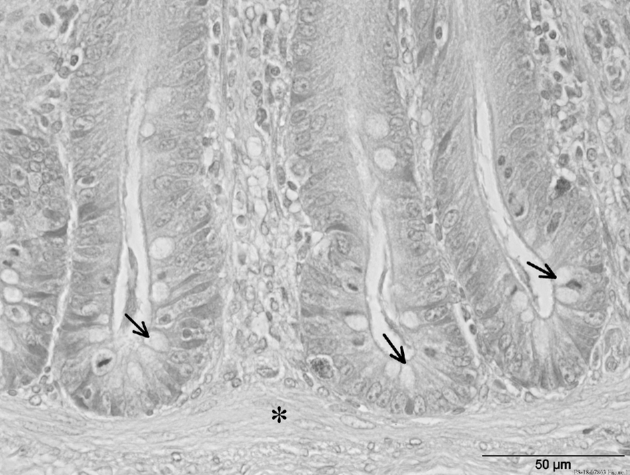
H&E staining from cecal crypts (apex region) of broiler chickens with SP diet. Note especially the goblet cells (arrows) within crypts and the lamina muscularis mucosae (star) underneath. Scale bars = 50 μm.
Figure 2.
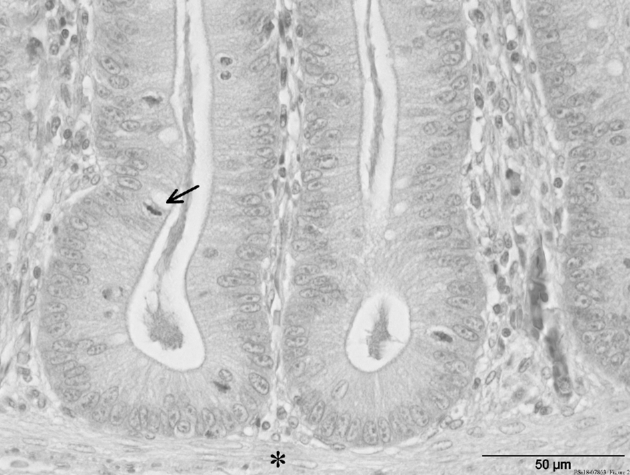
H&E staining from cecal crypts (apex region) of broiler chickens with LP diet. Note especially the goblet cells (arrow) within crypts and the lamina muscularis mucosae (star) underneath. Scale bars = 50 μm.
Mucins in Excreta
The total mucin content in the excreta showed a continuous increase from day 20 to 42 in all groups (Table 4). An influence of the infection of the samples examined at day 42 on the total mucin content of excrements was not apparent (CN: 60.4 ± 7.06 or CP: 59.8 ± 7.74 g total mucin/kg excrements). Significant differences were found in the overall concentrations of mucins in excreta depending on the diet at day 28, 35, and 42. The total mucin content in excreta in control animals was higher. On average, the total mucin content of the excreta in animals receiving the SP diet was 55.7 ± 8.23 g/kg DM. Using the LP diet significantly reduced the mucin content (51.9 ± 7.62 g/kg DM; P = 0.006).
Table 4.
Total mucin content of the excreta of broiler chickens.
| Item | SP diet | LP diet | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | Mean | SD | P-value | ||
| Total mucin content excreta (g/kg DM)1 | |||||||
| Day 20 | 10 | 46.6 | 4.28 | 43.6 | 1.66 | 0.062 | |
| Day 28 | 15 | 51.1a | 3.43 | 47.7b | 3.94 | 0.017 | |
| Day 35 | 15 | 53.1a | 3.93 | 49.6b | 4.69 | 0.014 | |
| Day 42 | 30 | 62.3a | 7.40 | 58.0b | 6.75 | 0.022 | |
SP diet = standard protein diet; LP diet = low protein diet.
1Samples of fresh excreta of the birds were collected from each box at day 20 and day 42. On day 28 and day 35 samples were taken only from groups SPCN and LPCN.
a,bValues within a row with different superscripts differ significantly at P < 0.05.
Campylobacter Excretion
The solutions for the experimental infection contained an average of 4.17 ± 0.09 log10 CFU C. jejuni per infection dose (2 mL).
Before and at the time of the experimental infection, all animals in the experiment were Camplylobacter spp. negative in cloacal swabs (N = 300). Due to the experimental infection at day 21, the SPCP and LPCP groups were exposed to a C. jejuni infection. Already 1 day after this infection, an excretion of C. jejuni could be seen with cultural techniques (Table 5). At day 2 post infection, already 12 animals in the group receiving the SPCP diet and 10 animals receiving the LPCP diet were C. jejuni positive in excreta. The prevalence in LPCP animals was numerically lower up to day 29. At day 25, differences in prevalence were significant (P = 0.004). The number of C. jejuni-positive animals continued to increase in both experimental groups until the prevalence nearly reached 100 % at day 29 in both groups. From day 29 until the end of the trial there were no differences.
Table 5.
Prevalence of C. jejuni in cloacal swabs of all animals and counts of C. jejuni in the excreta of the seeder birds (n = 15 per group) at days 23, 32, and 38 as well as total counts of C. jejuni in the cecal content of all animals on the day of dissection.
| Diet | |||||
|---|---|---|---|---|---|
| SP diet | LP diet | ||||
| Item | % positive | n (pos./total) | % positive | n (pos./total) | P-value |
| Prevalence on group level | |||||
| Day 18 | 0.00 | (0/25) | 0.00 | (0/25) | 1.000 |
| Day 21 | 0.00 | (0/75) | 0.00 | (0/75) | 1.000 |
| Day 22 | 1.33 | (1/75) | 1.33 | (1/75) | 1.000 |
| Day 23 | 16.0 | (12/75) | 13.3 | (10/75) | 0.644 |
| Day 24 | 50.7 | (38/75) | 45.3 | (34/75) | 0.513 |
| Day 25 | 75.7a | (56/74) | 53.3b | (40/75) | 0.004 |
| Day 29 | 100 | (74/74) | 97.3 | (73/75) | 0.497 |
| Day 35 | 98.7 | (73/74) | 100 | (74/74) | 1.000 |
| Day 39 | 97.3 | (72/74) | 97.3 | (72/74) | 1.000 |
| Day 42 | 97.3 | (72/74) | 94.6 | (70/74) | 0.405 |
| Quantitative counts C. jejuni excreta seeder birds (log10 cfu/g) | |||||
| Mean | SD | Mean | SD | P-value | |
| Day 23 | 3.38a | 2.55 | 1.47b | ±2.20 | 0.033 |
| Day 32 | 5.60 | 0.97 | 5.59 | ±1.03 | 0.934 |
| Day 38 | 4.67 | 0.91 | 4.02 | ±1.27 | 0.117 |
| Quantitative counts C. jejuni cecal content (log10 cfu/g) | |||||
| Dissection | 7.94 | ±0.60 | 8.09 | ±0.73 | 0.184 |
SP diet = standard protein diet; LP diet = low protein diet.
a,bValues within a row with different superscripts differ significantly at P < 0.05.
At day 23 (P = 0.033) and 38 (P = 0.117), the mean values of log10 CFU C. jejuni in the excreta of the seeder animals (15 animals per group) were lower in group LPCP (Table 5). The mean counts of C. jejuni in the cecal content did not differ between the SPCP and LPCP groups.
The SPCN and LPCN groups were not experimentally infected with C. jejuni. From day 21 onwards, regular random tests of these non-experimentally infected groups were carried out using cloacal swabs. Shortly before the end of the experiment, Campylobacter entered the CN groups so that detection was also possible at the time of dissection in 4/5 (SP) or 5/5 (LP) groups.
Performance Parameters
The body mass of the animals during (day 7) and at the end of the rearing phase (day 14) showed no significant differences between the individual groups (Table 6). After the adaptation phase to the diet (day 14 to 20) and before experimental infection, the body mass of the SPCN diet animals was higher than the body mass of LPCN- and LPCP diet animals. At the end of the experimental period (day 42), the body mass of the SPCN animals was still significantly higher than the body mass of LPCN animals (P = 0.006). Between SPCN and LPCP animals, there was no difference anymore. The same applied to the increase in body weight during the experimental period. The body weight gain for SPCN animals was significantly higher (P = 0.004) than that for LPCN animals. At the time of dissection, the body weight of SPCN animals was higher than in LPCN animals (P = 0.050), as was the body weight gains between these groups (P = 0.004).
Table 6.
Performance data of broilers depending on experimental infection with C. jejuni using complete diets with different amino acid patterns.
| Item | CN | CP | ||||||
|---|---|---|---|---|---|---|---|---|
| SP diet | LP diet | SP diet | LP diet | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body weight (g) | ||||||||
| Day 7 | 194 | 21.3 | 198 | 21.8 | 197 | 16.5 | 196 | 19.0 |
| Day 14 | 518 | 43.4 | 512 | 49.5 | 513 | 37.8 | 515 | 43.2 |
| Day 21 | 1031a | 95.5 | 996b | 112 | 1006ab | 88.3 | 997b | 109 |
| Day 42 | 3256a | 366 | 3088b | 370 | 3164ab | 385 | 3124ab | 469 |
| Dissection | 3463a | 386 | 3267b | 388 | 3329ab | 412 | 3292b | 498 |
| Carcass weight1 (g) | 2869a | 328 | 2704b | 331 | 2757b | 350 | 2712b | 426 |
| Dressing percentage | 82.9a | 0.93 | 82.8a | 0.96 | 82.8a | 1.08 | 82.4b | 1.35 |
| WFR (g/g) | 1.86a | 0.04 | 1.74b | 0.07 | 1.82ab | 0.05 | 1.77ab | 0.14 |
| FCR (g/g) | 1.63ab | 0.02 | 1.65a | 0.03 | 1.61b | 0.02 | 1.66a | 0.02 |
| CPE (kg/kg DM) | 3.27b | 0.04 | 3.60a | 0.07 | 3.32b | 0.04 | 3.59a | 0.03 |
| AAGL intake | 8.80a | 0.24 | 8.32b | 0.17 | 8.50ab | 0.36 | 8.46ab | 0.25 |
| AACM intake | 13.6a | 0.38 | 11.0b | 0.23 | 13.1a | 0.55 | 11.2b | 0.33 |
CN = without experimental C. jejuni infection; CP = with experimental C. jejuni infection; SP diet = standard protein diet; LP diet = low protein diet; WFR = water-feed ratio in kg water intake/kg diet intake as fed; FCR = feed conversion ratio in kg diet intake as fed/kg body weight gain; CPE = crude protein efficiency in kg body weight gain/kg crude protein intake; AAGL = sum “growth limiting” amino acids like arginine, isoleucine, lysine, methionine, threonine, and valine; AACM = sum of amino acids C. jejuni metabolizable like aspartic acid, glutamic acid, proline, and serine.
1After exsanguation, evisceration and without head and legs, including feathers.
a,bValues within a row with different superscripts differ significantly at P < 0.05.
Finally, the group SPCN showed the significantly highest dressing weight (P < 0.05), and the group LPCP the significant lowest dressing percentage (P < 0.05) at dissection.
DISCUSSION
In the present study, the effects of a diet with reduced crude protein content and a specific amino acid pattern on the number of goblet cells in the ceca, the intestinal release of mucins, the course of an experimental infection of broilers with C. jejuni, and performance were tested. For this purpose, the crude protein content in the diet of broilers was reduced from 212 to 190 g/kg DM. At the same time, crystalline amino acids were added to equalize the concentrations of the amino acids lysine, methionine, threonine, L-isoleucine, valine, and L-arginine to the amounts of the SP diet with a crude protein content of 212 g/kg DM. In this way, the proportion of the amino acids important for the metabolism of C. jejuni (serine, aspartic acid, glutamic acid, and proline; Guccione et al., 2008) was indirectly decreased. These amino acids were therefore available in smaller amounts to the animals.
Histology of the Intestine and Mucin Production
The number of goblet cells in the ceca was significantly lower in the groups being fed the LP diet than in the SP diet groups. The number of goblet cells can be regulated relatively quickly. Within 2 d, the differentiation of a goblet cell is completed. After a further 2 to 3 d, the cell undergoes continuous tissue regeneration of the intestinal mucosa (Uni et al., 2003). A short-term adaptation to new external conditions is possible (Uni et al., 2003). The significantly lower number of goblet cells in the LP group was an indication of a lower mucin release in the ceca of those chickens fed the protein-reduced diet.
The daily crude protein intake in the experimental period from day 21 to 42 and the total mucin content in the excreta at day 42 correlated significantly with one another (r2 = 0.73, P < 0.001). These observations are consistent with the interrelationships between the crude protein supply and the release of mucin in the intestine of broilers (Horn et al., 2009; Ravindran et al., 2009).
A correlation between the average daily intake of threonine in the experimental period and the total mucin content in the excrements could be shown in this study (r2 = 0.82, P < 0.001). This correlation was even more marked than the correlation between the crude protein intake and the mucin content of the excrement. At higher amino acid levels in the feed, more amino acids enter the intestinal tract (Lemme et al., 2004) and are available for mucin synthesis. In particular for threonine, a link between the concentration in the diet and the number of goblet cells and the production of mucin is described (Horn et al., 2009). Threonine was artificially added to the LP diet to ensure an optimal supply. Possibly, this counteracted an even clear reduction in the mucin content.
Campylobacter Excretion
Over a period of 4 d after the experimental infection, the prevalence of C. jejuni excretion was slightly lower in animals fed the LP diet infection than those fed the SP diet. Furthermore, a significantly lower shedding of C. jejuni via excreta could be determined.
Hermans et al. (2010) and Robyn et al. (2013) showed the difficulties of influencing the C. jejuni colonization in the ceca of broilers in terms of the protective function of the intestinal mucus layer for the pathogen. While the intestinal mucus layer provides optimal conditions for the growth of C. jejuni (Hugdahl et al., 1988; Van Deun et al., 2008; Hermans et al., 2010), the survival and growth of the pathogen outside this mucus layer is only very limited (Van Deun et al., 2008).
The growth of C. jejuni in the cecal content per se is not possible and a significant reduction in the germ count within 24 h is seen (Van Deun et al., 2008). However, relatively high numbers of C. jejuni (up to 106 CFU/g) were also found in the excreta (Potturi-Venkata et al., 2007).
High levels of C. jejuni counts in excreta were also confirmed in the present study. Although bacterial growth itself does not seem possible, irrespective of the amounts of amino acids present in the cecal content and excreta, the use of these amino acids is still conceivable for maintaining bacterial homeostasis.
A lower prevalence within the flock and lower numbers of bacterial counts in intestinal content are associated with a lower risk of the transfer of microorganisms to the carcass during the slaughtering process (Nauta et al., 2009). A correlation between the Campylobacter spp. prevalence of broilers and the proportion of positive carcasses (Herman et al., 2003) as well as a correlation between bacterial counts in the cecal content and on broiler carcasses after slaughter could be shown (Reich et al., 2008). Also, C. jejuni isolated from cecal content and carcass skin of the same farm or slaughter batch showed corresponding allelic profiles (Chokboonmongkol et al., 2013). Furthermore, C. jejuni is not able to reproduce on the carcasses or the food (Hazeleger et al., 1998). Instead, there is a successive reduction in the number of germs, depending on the storage temperature and storage time (Lee et al., 1998; Georgsson et al., 2006). In the case of a lower contamination of the carcasses in the context of the slaughtering process, a faster reduction of the pathogen occurs on both the carcass and the parts as well as in the further processed products (Alter et al., 2011). Already Black et al. (1988) showed a positive correlation between the number of C. jejuni inoculated and the risk of human Campylobacter infections. Against this background, Rosenquist et al. (2003) used a mathematical model to show that a reduction in the counts of Campylobacter spp. on broiler carcasses by 2 log10 steps can reduce the risk of human campylobacteriosis by a factor of 30.
The reduction in bacterial counts in the excreta 2 d after experimental infection at day 23 determined in this study was 1.9 log10 steps. Therefore, if the initial infection in a flock is close to the slaughter date, the effect can be promising, finally through a lower exposure of the consumer to C. jejuni through the consumption of poultry meat products.
Performance
The increase in body weight during the experimental period (day 21 to 42) was significantly higher (P < 0.001) than the target of the breeding company Aviagen in all groups of the trial. This indicates a supply covering demand even with lower crude protein contents of the ingested diet. In the case of an experimental C. jejuni infection, there was no difference in performance.
Limitations
Keeping broilers in subgroups of 15 animals does not correspond to the conditions in practice in livestock production. Here, tens of thousands of animals are kept together in 2 flock. This fact must be taken into account in particular when interpreting the spreading modus of the experimental C. jejuni infection, since a higher animal contact is to be expected under the experimental conditions. On the other hand, as far as the 3R principle is concerned, the greatest possible reduction in animal numbers is to be achieved.
Scientific and Clinical Implications
A lower crude protein content and a specific amino acid composition of the complete diet reduces the mucin release in the digestive tract of broilers. This, in combination with a lower concentration of free amino acids in the ingesta, may lead to lower substrate availability for C. jejuni in the intestinal contents and the excreta. Therefore, these conditions may potentially reduce the survival of C. jejuni in these substrates.
Additional research is needed to further optimize the amino acid composition in the diet of broilers.
Conclusion
A positive influence of lowering the crude protein content in the diet on reducing the spread and the shedding of a C. jejuni infection in broilers was observed in this study (Patent No 17,187,659.2–1106). In combination with other approaches this optimization could ultimately help to develop an efficient strategy for reducing C. jejuni prevalence in broiler flocks and thus reduce the potential exposure of consumer to C. jejuni through contaminated poultry meat products. A delayed spreading of C. jejuni infection among flocks is of special interest shortly before slaughter. This could lead to a reduction in the number of C. jejuni bacteria in the digestive tract and thus to a reduced risk of a transfer to the carcasses.
ACKNOWLEDGMENTS
We would like to thank Martin Beyerbach for his kind support regarding all questions concerning the statistics (planning, evaluation, etc.) and Frances Sherwood-Brock for proof reading the manuscript to ensure correct English.
Conflict of Interest
Dr. Ariane Helmbrecht is an employee of Evonik Nutrition & Care GmbH.
REFERENCES
- Abd El-Wahab A., Visscher C. F., Wolken S., Reperant J. M., Beineke A., Beyerbach M., Kamphues J.. 2012. Foot-pad dermatitis and experimentally induced coccidiosis in young turkeys fed a diet without anticoccidia. Poult. Sci. 91:627–635. [DOI] [PubMed] [Google Scholar]
- Adedokun S. A., Adeola O., Parsons C. M., Lilburn M. S., Applegate T. J.. 2011. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult. Sci. 90:1737–1748. [DOI] [PubMed] [Google Scholar]
- Allen A. 1981. Structure and function of gastrointestinal mucus. Physiol. Gastrointest. Tract 1:617–639. [Google Scholar]
- Alter T., Weber R. M., Hamedy A., Glünder G.. 2011. Carry-over of thermophilic Campylobacter spp. between sequential and adjacent poultry flocks. Vet. Microbiol. 147:90–95. [DOI] [PubMed] [Google Scholar]
- Awad W. A., Dublecz F., Hess C., Dublecz K., Khayal B., Aschenbach J. R., Hess M.. 2016. Campylobacter jejuni colonization promotes the translocation of Escherichia coli to extra-intestinal organs and disturbs the short-chain fatty acids profiles in the chicken gut. Poult. Sci. 95:2259–2265. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Han Y.. 1994. Ideal amino acid profile for chicks during the first three weeks posthatching. Poult. Sci. 73:1441–1447. [DOI] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J.. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472–479. [DOI] [PubMed] [Google Scholar]
- Chokboonmongkol C., Patchanee P., Gölz G., Zessin K. H., Alter T.. 2013. Prevalence, quantitative load, and antimicrobial resistance of Campylobacter spp. from broiler ceca and broiler skin samples in Thailand. Poult. Sci. 92:462–467. [DOI] [PubMed] [Google Scholar]
- Devleesschauwer B., Bouwknegt M., Mangen M.-J. J., Havelaar A. H.. 2017. Health and economic burden of Campylobacter. Pages 27–40 in Campylobacter. Klein G., ed. Academic Press, London, UK. [Google Scholar]
- EFSA. 2013. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2011. EFSA J. 11:3129 ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps S. V., Harvey R. B., Hume M. E., Phillips T. D., Anderson R. C., Nisbet D. J.. 2013. Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 10:6292–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgsson F., Thornorkelsson A. E., Geirsdottir M., Reiersen J., Stern N. J.. 2006. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 23:677–683. [DOI] [PubMed] [Google Scholar]
- GfE. 1999. Recommendations for the Energy and Nutrient Supply of Laying Hens and Broilers. DLG-Verlag, Frankfurt am Main, Germany. [Google Scholar]
- Gracia M. I., Millán C., Sánchez J., Guyard-Nicodème M., Mayot J., Carre Y., Csorbai A., Chemaly M., Medel P.. 2016. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: Part B. Poult. Sci. 95:886–892. [DOI] [PubMed] [Google Scholar]
- Guccione E., Leon-Kempis M. D., Pearson B. M., Hitchin E., Mulholland F., van Diemen P. M., Stevens M. P., Kelly D. J.. 2008. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 69:77–93. [DOI] [PubMed] [Google Scholar]
- Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Le Berre B., Sánchez J., Vesseur P., Martín Á., Medel P., Chemaly M.. 2016. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period1. Poult. Sci. 95:298–305. [DOI] [PubMed] [Google Scholar]
- Hazeleger W. C., Wouters J. A., Rombouts F. M., Abee T.. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64:3917–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerchove D., Rollier I., De Zutter L.. 2003. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 131:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Martel A., Van Deun K., Verlinden M., Van Immerseel F., Garmyn A., Messens W., Heyndrickx M., Haesebrouck F., Pasmans F.. 2010. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult. Sci. 89:1144–1155. [DOI] [PubMed] [Google Scholar]
- Horn N. L., Donkin S. S., Applegate T. J., Adeola O.. 2009. Intestinal mucin dynamics: Response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 88:1906–1914. [DOI] [PubMed] [Google Scholar]
- Hugdahl M. B., Beery J. T., Doyle M. P.. 1988. Chemotactic Behavior of Campylobacter jejuni. Infect. Immun. 56:1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen W., Reich F., Klein G.. 2014. Large-scale feasibility of organic acids as a permanent preharvest intervention in drinking water of broilers and their effect on foodborne Campylobacter spp. before processing. J. Appl. Microbiol. 116:1676–1687. [DOI] [PubMed] [Google Scholar]
- Khattak F., Paschalis V., Green M., Houdijk J. G. M., Soultanas P., Mahdavi J.. 2018. TYPLEX® Chelate, a novel feed additive, inhibits Campylobacter jejuni biofilm formation and cecal colonization in broiler chickens. Poult. Sci. 97:1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Khan W. I.. 2013. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens 2:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurekci C., Al Jassim R., Hassan E., Bishop-Hurley S. L., Padmanabha J., McSweeney C. S.. 2014. Effects of feeding plant-derived agents on the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 93:2337–2346. [DOI] [PubMed] [Google Scholar]
- Lee A., Smith S. C., Coloe P. J.. 1998. Survival and growth of Campylobacter jejuni after artificial inoculation onto chicken skin as a function of temperature and packaging conditions. J. Food Prot. 61:1609–1614. [DOI] [PubMed] [Google Scholar]
- Lemme A., Ravindran V., Bryden W.. 2004. Ileal digestibility of amino acids in feed ingredients for broilers. Worlds Poult. Sci. J. 60:423–438. [Google Scholar]
- Lien K. A., Sauer W. C., Fenton M.. 1997. Mucin output in ileal digesta of pigs fed a protein-free diet. Z. Ernährungswiss 36:182–190. [DOI] [PubMed] [Google Scholar]
- Mack S., Bercovici D., De Groote G., Leclercq B., Lippens M., Pack M., Schutte J. B., Van Cauwenberghe S.. 1999. Ideal amino acid profile and dietary lysine specification for broiler chickens of 20 to 40 days of age. Br. Poult. Sci. 40:257–265. [DOI] [PubMed] [Google Scholar]
- Metcalf J. H., Donoghue A. M., Venkitanarayanan K., Reyes-Herrera I., Aguiar V. F., Blore P. J., Donoghue D. J.. 2011. Water administration of the medium-chain fatty acid caprylic acid produced variable efficacy against enteric Campylobacter colonization in broilers. Poult. Sci. 90:494–497. [DOI] [PubMed] [Google Scholar]
- Naumann C., Bassler R.. 2012. Methoden der landwirtschaftlichen Forschungs-und Untersuchungsanstalt, Biochemische Untersuchung von Futtermitteln. Methodenbuch III (einschließlich der achten Ergänzungen) VDLUFA, Darmstadt, Germany. [Google Scholar]
- Nauta M., Hill A., Rosenquist H., Brynestad S., Fetsch A., van der Logt P., Fazil A., Christensen B., Katsma E., Borck B., Havelaar A.. 2009. A comparison of risk assessments on Campylobacter in broiler meat. Int. J. Food Microbiol. 129:107–123. [DOI] [PubMed] [Google Scholar]
- Parsons C. M. 1984. Influence of caecectomy and source of dietary fibre or starch on excretion of endogenous amino acids by laying hens. Br. J. Nutr. 51:541–548. [DOI] [PubMed] [Google Scholar]
- Potturi-Venkata L. P., Backert S., Lastovica A. J., Vieira S. L., Norton R. A., Miller R. S., Pierce S., Oyarzabal O. A.. 2007. Evaluation of different plate media for direct cultivation of Campylobacter species from live broilers. Poult. Sci. 86:1304–1311. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Bryden W. L.. 1999. Amino acid availability in poultry - In vitro and in vivo measurements. Aust. J. Agric. Res. 50:889–908. [Google Scholar]
- Ravindran V., Morel P. C., Rutherfurd S. M., Thomas D. V.. 2009. Endogenous flow of amino acids in the avian ileum as influenced by increasing dietary peptide concentrations. Br. J. Nutr. 101:822–828. [DOI] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Haunhorst E., Klein G.. 2008. The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. Int. J. Food Microbiol. 127:116–120. [DOI] [PubMed] [Google Scholar]
- Robyn J., Rasschaert G., Hermans D., Pasmans F., Heyndrickx M.. 2013. Is allicin able to reduce Campylobacter jejuni colonization in broilers when added to drinking water? Poult. Sci. 92:1408–1418. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Nielsen N. L., Sommer H. M., Nørrung B., Christensen B. B.. 2003. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 83:87–103. [DOI] [PubMed] [Google Scholar]
- Schneitz C., Hakkinen M.. 2016. The efficacy of a commercial competitive exclusion product on Campylobacter colonization in broiler chickens in a 5-week pilot-scale study. Poult. Sci. 95:1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedat J. 2015. Aktuelle Statistik meldepflichtiger Infektionskrankheiten, Deutschland. Epidemiologisches Bull. 27:1–4. [Google Scholar]
- Slaoui M., Fiette L.. 2011. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods. Mol. Biol. 691:69–82. [DOI] [PubMed] [Google Scholar]
- Uni Z., Smirnov A., Sklan D.. 2003. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 82:320–327. [DOI] [PubMed] [Google Scholar]
- Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., Van den Broeck W., Van Immerseel F., Haesebrouck F.. 2008. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 130:285–297. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Grant A. J., Hurd D., Harrison M., Guccione E. J., Kelly D. J., Maskell D. J.. 2009. Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology 155:80–94. [DOI] [PubMed] [Google Scholar]


