Abstract
Study objectives were to determine the effects of zinc (Zn) amino acid complex (Availa Zn, Zinpro Corporation, Eden Prairie, MN) on metabolism, biomarkers of leaky gut, and inflammation during and following heat stress (HS) and nutrient restriction. Crossbred gilts (n = 50; 50 ± 2 kg BW) were blocked by initial BW and randomly assigned to one of five treatments: 1) thermoneutral (TN) and ad libitum fed a control diet (TNCtl), 2) TN and pair-fed a control diet (PFCtl), 3) TN and pair-fed a Zn-supplemented diet (PFZn), 4) HS and ad libitum fed a control diet (HSCtl), and 5) HS and ad libitum fed a Zn-supplemented diet (HSZn). The study consisted of 3 experimental periods (P): during P1 (7 d), all pigs were fed their respective diets ad libitum and housed in TN conditions (20.84 ± 0.03 °C, 47.11 ± 0.42% relative humidity). During P2 (7 d), HSCtl and HSZn pigs were exposed to progressive cyclical HS conditions (27 to 30 °C, 41.9 ± 0.5% relative humidity), while TNCtl, PFCtl, and PFZn pigs remained in TN conditions and were fed ad libitum or pair-fed to their respective HSCtl and HSZn counterparts. During P3 (5 d; “recovery phase”), all pigs were housed in TN conditions and fed ad libitum. Pigs exposed to HS had overall increased rectal temperature, skin temperature, and respiration rate (0.33 °C, 3.76 °C, and 27 bpm, respectively; P < 0.01). Relative to TN controls, HS decreased ADFI and ADG (28 and 35%, respectively; P < 0.05), but these variables were unaffected by dietary treatment. Additionally, circulating insulin did not differ between HS and TN pigs (P = 0.41), but was decreased in PF relative to TN pigs (P < 0.01). During recovery, no differences were observed in rectal temperature or respiration rate across treatments, but HSZn pigs had decreased skin temperature relative to TN, PF, and HSCtl pigs (P < 0.01). During P3, no Zn effects were observed in production parameters; however, PF pigs had increased ADFI and ADG relative to TN and HS treatments (P < 0.01). During P3, circulating insulin was increased in pigs that were HS relative to TN and PF pigs (75%, P < 0.05). Interestingly, tumor necrosis factor alpha (TNFα) levels were decreased during P3 (P = 0.04) in Zn relative to Ctl-fed pigs. Circulating lipopolysaccharide-binding protein was not different among periods (P > 0.10). In summary, Zn reduced TNFα (regardless of HS), and the stimulatory effect of HS on insulin secretion is amplified during HS recovery.
Keywords: heat stress, intestinal integrity, pigs, recovery, zinc
INTRODUCTION
Heat stress (HS) severely limits profitable pig production, and although certainly multifactorial, many of the negative consequences of HS on animal health and productivity are mediated by reduced intestinal barrier integrity (Baumgard and Rhoads, 2013). Heat-stressed animals redistribute blood to the periphery in an attempt to increase radiant heat loss, and the gastrointestinal tract vasoconstricts in a coordinated effort to support the altered hierarchy of blood distribution (Hall et al., 1999). Reduced splanchnic blood flow causes ischemic damage and subsequently creates intestinal barrier dysfunction (Lambert, 2009). Furthermore, reduced feed intake also compromises intestinal barrier integrity (Rodriguez et al., 1996; Pearce et al., 2013b; Kvidera et al., 2017) and thus HS causes leaky gut by multiple etiologies. Intestinal infiltrating antigens stimulate a local immune response and, if severe enough, cause systemic endotoxemia that is associated with inflammation and an acute phase protein response (Bouchama and Knochel, 2002; Mani et al., 2012).
Zinc (Zn) plays an important role at maintaining gastrointestinal tract barrier function (Alam et al., 1994), and dietary Zn decreases intestinal permeability in a variety of animal models (Rodriguez et al., 1996; Sturniolo et al., 2001; Lambert et al., 2003). Furthermore, we have demonstrated that supplementing complexed Zn improves intestinal barrier function and villi architecture in chronic and acute heat-stressed pigs and ruminants (Sanz Fernandez et al., 2014; Pearce et al., 2015; Abuajamieh et al., 2016). However, whether Zn can improve “recovery” from HS remains unclear. Therefore, it was of interest to evaluate the effects of supplemental Zn on temporal metabolism and biomarkers of intestinal integrity both during and following HS and/or nutrient restriction. Assessing the recovery period provides an indication of the duration that these 2 insults negatively affect gastrointestinal barrier integrity.
MATERIALS AND METHODS
Animals, Diets, and Experimental Design
Iowa State University Institutional Animal Care and Use committee approved all procedures involving animals (#9-15-8087-S). Fifty crossbred gilts (50 ± 2 kg BW) were utilized in a replicated (25 pigs per replicate) experiment conducted at the Iowa State University Swine Nutrition Farm Research Facility (Ames, IA). On the basis of initial BW, pigs were allotted to 5 blocks and randomly assigned to 1 of 5 environmental dietary treatments: 1) thermoneutral (TN) and ad libitum fed a control diet (TNCtl; n = 10), 2) TN and pair-fed a control diet (PFCtl; n = 9), 3) TN and pair-fed a Zn-supplemented diet (PFZn; n = 10), 4) HS and ad libitum fed a control diet (HSCtl; n = 10), and 5) HS and ad libitum fed a Zn-supplemented diet (HSZn, n = 9). During the experimental period, pigs were allocated to one of two environmentally controlled rooms. Each room had 24 crates (57 × 221 cm) where pigs were housed individually. Each crate was equipped with a stainless steel feeder and a nipple drinker. Water was provided ad libitum during the entire experiment. Two pigs were culled from the study due to health issues (one from the PFCtl and one from the HSZn treatments), and their data were not included in the final analysis.
Two diets were formulated and mixed according to the following specifications: 1) a control (Ctl) diet containing 120 mg/kg of inorganic Zn in the form of Zn sulfate (Sigma-Aldrich, St Louis, MO) and 2) a Zn diet containing 60 mg of inorganic Zn in the form of Zn sulfate and 60 mg/kg of complexed Zn as Zn amino acid complex (Availa Zn; Zinpro Corporation, Eden Prairie, MN). Diets were processed in meal form and mixed at the Iowa State University Swine Nutrition Center Mill. Other than the Zn source, all diets were similar in ingredients and were formulated to meet or exceed the predicted requirements of growing pigs (NRC, 2012) for energy, essential amino acids, protein, minerals, and vitamins (Table 1).
Table 1.
Ingredient composition and chemical and nutritional composition of experimental diets (as-fed basis)
| Item | Control | Zinc |
|---|---|---|
| Ingredient | ||
| Corn, % | 63.11 | 63.11 |
| DDGS1, % | 15.00 | 15.00 |
| Soybean meal, % | 18.00 | 18.00 |
| Soybean oil, % | 1.50 | 1.50 |
| Limestone, % | 1.25 | 1.25 |
| l-Lysine HCl, % | 0.27 | 0.27 |
| l-Threonine, % | 0.01 | 0.01 |
| Quantum Blue (5,000 FTU/g)2, % | 0.005 | 0.005 |
| Vitamin premix3, % | 0.200 | 0.200 |
| Trace mineral premix4, % | 0.150 | 0.150 |
| Availa zinc5 | - | 0.05 |
| Zinc sulfate | 0.05 | 0.025 |
| Copper sulfate | 0.002 | 0.002 |
| Iron sulfate | 0.06 | 0.06 |
| Limestone | 0.03 | 0.01 |
| Manganese | 0.002 | 0.002 |
| Potassium iodate | 0.00003 | 0.00003 |
| Selenium | 0.00005 | 0.00005 |
| NaCl | 0.500 | 0.500 |
| Calculated nutrient levels, % | ||
| ME, kcal/kg | 3,342 | 3,342 |
| NE, kcal/kg | 2,477 | 2,477 |
| CP | 17.97 | 17.97 |
| Ether extract | 4.43 | 4.43 |
| ADF | 5.30 | 5.30 |
| NDF | 12.29 | 12.29 |
| SID6 AA, % | ||
| Lys | 0.86 | 0.86 |
| Met | 0.26 | 0.26 |
| Thr | 0.53 | 0.53 |
| Trp | 0.16 | 0.16 |
| Ca | 0.54 | 0.54 |
| Total P | 0.41 | 0.41 |
| STTD7 P | 0.27 | 0.27 |
1Corn distillers dried grains with solubles.
2Assume releases 0.08% STTD P.
3Vitamin premix provided the following (per kg diet): 4,900 IU of vitamin A, 560 IU of vitamin D3, 40 IU of vitamin E, 2.4 mg of menadione (to provide vitamin K), 39 μg of vitamin B12, 9 mg of riboflavin, 22 mg of d-pantothenic acid, and 45 mg of niacin.
4Compostion per kilogram of feed: Ca, 129 mg as limestone; Cu, 7 mg as copper sulfate; Fe, 120 mg as iron sulfate; I, 0.28 mg as potassium iodate; Mn, 5 mg manganese sulfate; Zn, 120 mg as zinc sulfate or 50% zinc sulfate + 50% Availa-Zn.
5Availa zinc (Availa Zn; Zinpro Corporation).
6Standardized ileal digestibility.
7Standardized total tract digestibility.
The study consisted of 3 experimental periods (P): P1, P2, and P3. Prior to the start of the study (i.e., before pigs were moved into the environmental rooms), pigs were fed their respective diets for 21 d. During the first 14 d, all animals were group-housed according to their dietary treatment. After this initial feeding phase, pigs were moved to individual crates and allowed 7 d to acclimate to their pens before the initiation of the experimental periods. During P1 (7 d), all pigs were fed their respective diets ad libitum and housed in TN conditions (20.84 ± 0.03 °C; 47.11 ± 0.42% relative humidity [RH]) for collection of baseline body temperature indices and production parameters. During P2 (7 d), HSCtl and HSZn animals were fed ad libitum and exposed to progressive cyclical HS conditions with temperatures ranging from 27 to 30 °C (30.23 ± 0.30 °C, 34.70 ± 0.16% RH from 0800 to 1800 h, and 26.98 ± 0.06 °C, 49.13 ± 0.34% RH from 1800 to 0800 h). During P2, pigs assigned to the TNCtl treatment remained in TN conditions and were fed ad libitum; pigs assigned to the PFCtl and PFZn treatments also remained in TN conditions, but were pair-fed to their HS and dietary counterparts (to evaluate the direct effect of HS while eliminating the confounding effects of dissimilar nutrient intake). Briefly, ADFI during P1 was averaged for each pig and used as a baseline. During P2, the decrease in ADFI in HS pigs was calculated every day as a percentage of ADFI reduction relative to P1. The percentage of ADFI reduction was averaged for all pigs in HS treatments per day of heat exposure and applied individually to the baseline of each pig in the PF treatments. The daily amount of feed provided to the PF treatments was divided into 3 equal portions during P2 (~0800, 1200, and 1800 h) to minimize large metabolic changes associated with gorging. To accomplish pair-feeding, pigs assigned to PF treatments remained 1 calendar day behind TN and HS treatments during the entire experimental period. This technique of pair-feeding has been extensively utilized (Pearce et al., 2013b; Sanz Fernandez et al., 2015a, 2015b). During P3 (recovery period; 5 d), pigs from the HS treatments (HSCtl and HSZn) returned to TN conditions (21.00 ± 0.08 °C, 45.79 ± 0.66% RH) and were fed ad libitum; likewise, pigs in the TNCtl, PFCtl, and PFZn treatments remained in TN conditions and were fed ad libitum.
Throughout the experiment, ambient temperature and humidity were monitored and recorded every 5 min by a data logger (Lascar EL-USB-2-LCD, Erie, PA). One data logger was placed in each quadrant of each room (8 data loggers in total), and data were then condensed by room into hourly averages.
Body Temperature Measurements
During P1, P2, and P3, body temperature indices (rectal temperature [TR], skin temperature [TS], and respiration rate [RR]) were obtained thrice daily (~0700, 1200, and 1800 h). Rectal temperature was measured using an electronic thermometer (SureTemp Plus 590, WelchAllyn, Skaneateles Falls, NY). Skin temperature was measured at the rump using an infrared thermometer (IRT207: The Heat Seeker 8:1 Mid-Range Infrared Thermometer, General Tools, New York, NY) ~12 cm from the skin. Respiration rate was determined by counting flank movements for 15 s and multiplied by 4 to obtain breaths per minute (bpm). All indices recorded were condensed into daily averages.
Production Parameters
Daily feed intake was measured at 0700 h during P1, P2, and P3 as feed disappearance. Body weights were obtained at the beginning and end of P1, and at the end of P2 and P3. Average daily gain and G:F were calculated by period.
Blood Sampling and Analysis
An indwelling jugular catheter was surgically inserted on d 1 of P1 using a percutaneous technique as previously described (Sanz Fernandez et al., 2015a). All pigs received antibiotics (Ceftiofur, Excede, Pfizer Animal Health, New York, NY) and nonsteroidal, anti-inflammatory drugs (Flunixin Meglumine, Banamine-S, Schering-Plough Animal Health Corp., Whitehouse Station, NJ) during the surgery. Blood samples were obtained from the jugular catheter daily at 1200 h into disposable tubes (plasma, K2EDTA tube, BD vacutainers, Franklin Lakes, NJ; serum, culture glass tubes, Fisher Scientific, Waltham, MA). Catheters were flushed afterwards with heparinized saline (100 IU/mL). Plasma and serum samples were harvested by centrifugation at 1,500 × g for 15 min at 4 °C and stored at −20 °C until analysis. Plasma glucose, NEFA, blood urea nitrogen (BUN), insulin, lipopolysaccharide (LPS) binding protein (LBP), and tumor necrosis factor-alpha (TNFα) concentrations were measured using commercially available kits following the manufacturers’ instructions (glucose, Wako Chemicals USA, Inc., Richmond, VA; NEFA, Wako Chemicals USA, Inc.; BUN, Teco Diagnostics, Anaheim, CA; insulin, Mercodia Porcine Insulin ELISA; Mercodia AB, Uppsala, Sweden; LBP, Hycult Biotech, Uden, The Netherlands; TNFα, R&D Systems, Inc., Minneapolis, MN). These procedures were conducted in 96-well microplates and read using a microplate spectrophotometer (Eon, BioTek Instruments, Inc., Winooski, VT). The intra- and interassay coefficients of variation for glucose, NEFA, BUN, insulin, LBP, and TNFα were 4.6 and 13.5%, 5.5 and 14.9%, 4.4 and 5.8%, 9.1 and 7.2%, 3.5 and 15.5%, and 6.2 and 10.9%, respectively.
Statistical Analysis
Data from each experimental period were assessed separately using SAS version 9.4 (SAS Inst. Inc., Cary, NC). Daily measurements (i.e., ADFI, body temperature indices, and blood metabolites) were analyzed by repeated measures using the MIXED procedure of SAS with an autoregressive covariance structure and day of the experiment as the repeated effect. The model included treatment, day, treatment by day interaction, block, and replicate as fixed effects; initial BW was used as a covariate. Production parameters (i.e., BW, ADG, and G:F) were analyzed using the MIXED procedure of SAS with a diagonal covariance structure and initial BW as a covariate. The model included treatment, block, and replicate as fixed effects. Pre-planned contrasts were assessed to evaluate environmental (i.e., TN vs. PF, TN vs. HS, and PF vs. HS) and dietary (i.e., HSCtl vs. HSZn and Ctl vs. Zn-fed pigs) treatment effects. Data are reported as least squares means and a difference between treatments considered significant if P ≤ 0.05 and a tendency for a difference if 0.05 < P ≤ 0.10.
RESULTS
Body Temperature Indices, Growth Performance, and Blood Metabolites During P1
No dietary treatment differences were observed in TR, ADG, or blood metabolites during P1 (Supplementary Table S1). However, TS was decreased (1%; P = 0.02) and RR tended to be decreased (5%; P = 0.06) in Zn relative to Ctl-fed pigs (Supplementary Table S1). Moreover, ADFI was decreased (0.16 kg) and G:F tended to be increased (13%) in Zn-fed pigs compared with their Ctl counterparts (P = 0.02 and P = 0.09, respectively; Supplementary Table S1). Additionally, circulating TNFα tended to be decreased (23%) during P1 in Zn-fed pigs compared with controls (P = 0.08; Supplementary Table S1).
Body Temperature Indices
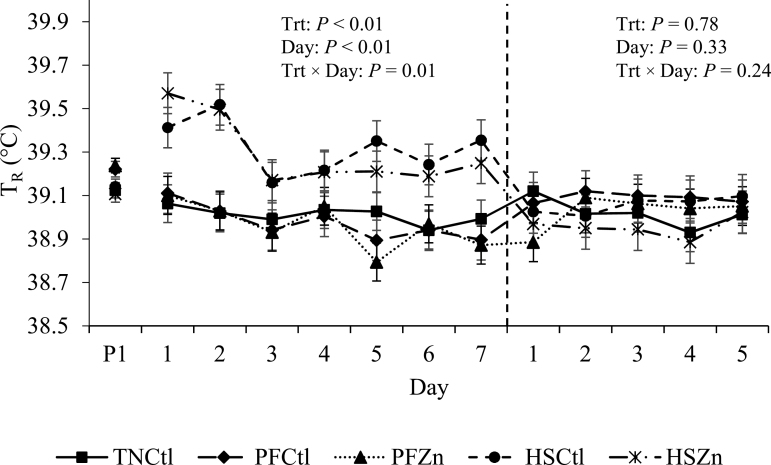
During P2, pigs exposed to HS had an overall increase in TR compared with TN and PF pigs (0.3 °C; P < 0.01; Table 2; Figure 1). Similarly, HS pigs had increased TS relative to TN and PF treatments (~9 and 11%, respectively; P < 0.01; Table 2); however, HSZn pigs tended to have decreased TS compared with HSCtl pigs (0.4 °C; P = 0.06; Table 2). Respiration rate was also increased by ~75% in HS pigs relative to their TN and PF counterparts (27 bpm increase; P < 0.01; Table 2). Interestingly, PF pigs had decreased TS during P2 relative to TN pigs (0.6 °C; P < 0.01; Table 2).
Table 2.
Effects of zinc amino acid complex on body temperature indices in heat-stressed and restricted-fed pigs during P2 and recovery
| Treatment1 | SEM | P | Contrasts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | TNCtl | PFCtl | PFZn | HSCtl | HSZn | Trt2 | Day | Trt × Day3 | TN vs. PF | TN vs. HS | PF vs. HS | HSCtl vs. HSZn | Ctl vs. Zn | |
| Period 2 | ||||||||||||||
| TR4, °C | 39.01b | 38.97b | 38.96b | 39.32a | 39.30a | 0.06 | <0.01 | <0.01 | 0.01 | 0.57 | <0.01 | <0.01 | 0.79 | 0.57 |
| TS5, °C | 36.82b | 36.25c | 36.11c | 40.29a | 39.89a | 0.16 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.06 | 0.11 |
| RR6, bpm | 36.5b | 36.0b | 35.0b | 61.7a | 63.5a | 1.6 | <0.01 | <0.01 | 0.09 | 0.61 | <0.01 | <0.01 | 0.44 | <0.01 |
| Recovery | ||||||||||||||
| TR4, °C | 39.02 | 39.09 | 39.02 | 39.06 | 38.96 | 0.08 | 0.78 | 0.33 | 0.24 | 0.67 | 0.87 | 0.49 | 0.34 | 0.34 |
| TS5, °C | 36.73b | 37.27a | 37.14ab | 36.78b | 36.14c | 0.16 | <0.01 | <0.01 | <0.01 | 0.01 | 0.17 | <0.01 | 0.01 | 0.05 |
| RR6, bpm | 35.0 | 38.4 | 36.5 | 36.1 | 37.7 | 1.3 | 0.35 | <0.01 | <0.01 | 0.12 | 0.24 | 0.68 | 0.36 | 0.60 |
1TNCtl = thermoneutral control; PFCtl = pair-fed control; PFZn = pair-fed zinc; HSCtl = heat-stress control; HSZn = heat-stress zinc.
2Treatment.
3Treatment by day interactions.
4Rectal temperature averaged by day.
5Skin temperature averaged by day.
6Respiration rate averaged by day.
a–cMeans with different superscripts significantly differ (P ≤ 0.05).
Figure 1.
Effects of zinc amino acid complex on rectal temperature (TR) during period 2 and the recovery period. Treatments: TNCtl = thermoneutral ad libitum control diet, PFCtl = pair-fed control diet, PFZn = pair-fed zinc diet, HSCtl = heat stress ad libitum control diet, and HSZn = heat stress ad libitum zinc diet. The dashed line divides period 2 from the recovery period.
During P3 (recovery period), no TR or RR differences between treatments were observed (P > 0.10; Table 2). However, TS was decreased in HSZn compared with TN, PF, and HSCtl pigs (0.8 °C; P < 0.01; Table 2).
Growth Performance
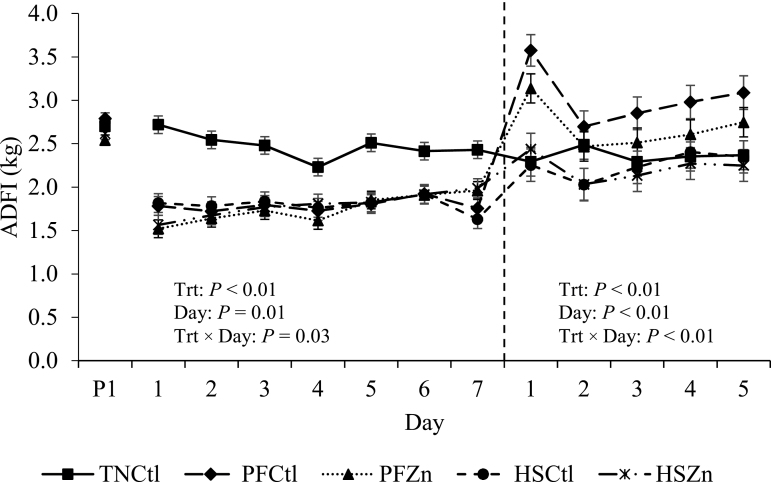
During P2, there was an overall decrease in ADFI in HS pigs compared with TN controls (28%; 0.69 kg/d; P < 0.01; Table 3; Figure 2). Similarly, BW at the end of P2 was decreased in HS treatments relative to TN controls (4.25 kg; P = 0.02; Table 3). Average daily gain was also decreased in HS pigs compared with TN controls (35%; 0.32 kg/d; P < 0.01; Table 3). Feed efficiency did not differ between HS and TN environments (P = 0.45; Table 3) during P2; however, HSZn pigs tended to have increased G:F compared with HSCtl counterparts (27%; P = 0.07; Table 3). By experimental design, PFCtl and PFZn pigs followed the same pattern of reduced ADFI, and their ADG and G:F variables were similar to their HS counterparts (P > 0.10; Table 3).
Table 3.
Effects of zinc amino acid complex on growth performance in heat-stressed and restricted-fed pigs during P2 and recovery
| Treatment1 | SEM | P | Contrasts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | TNCtl | PFCtl | PFZn | HSCtl | HSZn | Trt2 | Day | Trt × Day3 | TN vs. PF | TN vs. HS | PF vs. HS | HSCtl vs. HSZn | Ctl vs. Zn | |
| Period 2 | ||||||||||||||
| ADFI, kg | 2.48a | 1.79b | 1.75b | 1.79b | 1.79b | 0.06 | <0.01 | 0.01 | 0.03 | <0.01 | <0.01 | 0.68 | 0.99 | <0.01 |
| FBW4, kg | 84.4x | 82.9xy | 79.8y | 79.7y | 80.6y | 1.4 | 0.07 | - | - | 0.07 | 0.02 | 0.38 | 0.64 | 0.09 |
| ADG, kg | 0.92a | 0.53b | 0.53b | 0.53b | 0.67b | 0.07 | <0.01 | - | - | <0.01 | <0.01 | 0.31 | 0.18 | 0.27 |
| G:F | 0.37 | 0.30 | 0.30 | 0.30 | 0.38 | 0.03 | 0.14 | - | - | 0.07 | 0.45 | 0.21 | 0.07 | 0.57 |
| Recovery | ||||||||||||||
| ADFI, kg | 2.36bc | 3.04a | 2.69ab | 2.25c | 2.22c | 0.14 | <0.01 | <0.01 | <0.01 | <0.01 | 0.46 | <0.01 | 0.88 | 0.46 |
| FBW4, kg | 89.7x | 89.1xy | 86.1y | 85.1y | 84.9y | 1.7 | 0.08 | - | - | 0.24 | 0.01 | 0.10 | 0.94 | 0.07 |
| ADG, kg | 0.94b | 1.23a | 1.24a | 0.94b | 0.92b | 0.13 | 0.02 | - | - | <0.01 | 0.95 | <0.01 | 0.83 | 0.58 |
| G:F | 0.40 | 0.39 | 0.49 | 0.42 | 0.42 | 0.04 | 0.14 | - | - | 0.26 | 0.61 | 0.48 | 0.88 | 0.07 |
1TNCtl = thermoneutral control; PFCtl = pair-fed control; PFZn = pair-fed zinc; HSCtl = heat-stress control; HSZn = heat-stress zinc.
2Treatment.
3Treatment by day interactions.
4Final body weight.
a–cMeans with different superscripts significantly differ (P ≤ 0.05).
x-yMeans with different superscripts tend to differ (P ≤ 0.10).
Figure 2.
Effects of zinc amino acid complex on ADFI during period 2 and the recovery period. Treatments: TNCtl = thermoneutral ad libitum control diet, PFCtl = pair-fed control diet, PFZn = pair-fed zinc diet, HSCtl = heat stress ad libitum control diet, and HSZn = heat stress ad libitum zinc diet. The dashed line divides period 2 from the recovery period.
During P3 (all treatments housed in TN conditions and fed ad libitum), ADFI was similar between pigs assigned to the HS and TN treatments (P = 0.46; Table 3; Figure 2); however, pigs that had been PF during P2 had increased ADFI compared with TN and HS animals (21 and 28%, respectively; P < 0.01; Table 3; Figure 2). Body weight was not different between TN and PF pigs by the end of the recovery period (P = 0.24; Table 3) but remained decreased in HS pigs compared with their TN counterparts (4.70 kg; P = 0.01; Table 3). Average daily gain did not differ between HS and TN treatments (P = 0.95; Table 3); however, it was increased in pigs that had been PF in P2 relative to TN and HS pigs (32%, 0.30 kg/d; P < 0.01; Figure 2B). No treatment differences were observed for G:F during P3 (P = 0.14; Table 3).
Blood Metabolites
During P2, circulating glucose did not differ among treatments (P = 0.71; Table 4). During P3 (recovery period), blood glucose tended to be increased in HS pigs compared with PF controls (6%; P = 0.10; Table 4). During P3, a tendency for increased glucose levels was observed in the Zn compared with the Ctl-fed pigs (6%, P = 0.07; Table 4).
Table 4.
Effects of zinc amino acid complex on blood metabolites and inflammatory biomarkers in heat-stressed and restricted-fed pigs during P2 and recovery
| Treatment1 | SEM | P | Contrasts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | TNCtl | PFCtl | PFZn | HSCtl | HSZn | Trt2 | Day | Trt × Day3 | TN vs. PF | TN vs. HS | PF vs. HS | HSCtl vs. HSZn | Ctl vs. Zn | |
| Period 2 | ||||||||||||||
| Glucose, mg/dL | 98.1 | 94.8 | 99.0 | 99.6 | 94.4 | 3.3 | 0.71 | <0.01 | 0.65 | 0.77 | 0.79 | 0.98 | 0.31 | 0.79 |
| Insulin:ADFI4 | 0.05xy | 0.03y | 0.03y | 0.06x | 0.06x | 0.01 | 0.07 | 0.58 | 0.13 | 0.08 | 0.52 | <0.01 | 0.94 | 0.87 |
| NEFA, µEq/liter | 84.5 | 83.5 | 110.0 | 72.5 | 95.0 | 10.9 | 0.17 | 0.17 | 0.48 | 0.36 | 0.96 | 0.25 | 0.19 | 0.03 |
| BUN5, mg/dL | 8.46 | 7.13 | 7.34 | 7.09 | 6.97 | 0.58 | 0.41 | 0.72 | 0.31 | 0.09 | 0.07 | 0.73 | 0.90 | 0.44 |
| LBP6, µg/mL | 9.68 | 12.95 | 9.80 | 9.19 | 8.80 | 4.03 | 0.68 | 0.39 | 0.22 | 0.54 | 0.80 | 0.29 | 0.90 | 0.52 |
| Recovery | ||||||||||||||
| Glucose, mg/dL | 94.2xy | 87.8y | 98.4x | 97.9x | 100.2x | 4.0 | 0.09 | 0.60 | 0.09 | 0.79 | 0.31 | 0.10 | 0.67 | 0.07 |
| Insulin: ADFI4 | 0.04bc | 0.03c | 0.04bc | 0.08ab | 0.09a | 0.02 | 0.03 | 0.82 | 0.11 | 0.64 | 0.03 | <0.01 | 0.56 | 0.37 |
| NEFA, µEq/liter | 77.8 | 74.5 | 94.3 | 71.2 | 70.9 | 11.8 | 0.43 | 0.38 | 0.83 | 0.61 | 0.63 | 0.21 | 0.99 | 0.41 |
| BUN5, mg/dL | 8.61 | 8.62 | 8.53 | 7.73 | 8.05 | 0.72 | 0.83 | <0.01 | 0.18 | 0.96 | 0.38 | 0.28 | 0.75 | 0.95 |
| LBP6, µg/mL | 12.72 | 13.73 | 11.59 | 11.38 | 9.99 | 3.08 | 0.84 | <0.01 | 0.04 | 0.98 | 0.50 | 0.43 | 0.69 | 0.42 |
1TNCtl = thermoneutral control; PFCtl = pair-fed control; PFZn = pair-fed zinc; HSCtl = heat-stress control; HSZn = heat-stress zinc.
2Treatment.
3Treatment by day interactions.
4Insulin to average daily feed intake ratio.
6Blood urea nitrogen.
7Lipopolysaccharide binding protein.
a–cMeans with different superscripts significantly differ (P ≤ 0.05).
x-yMeans with different superscripts tend to differ (P ≤ 0.10).
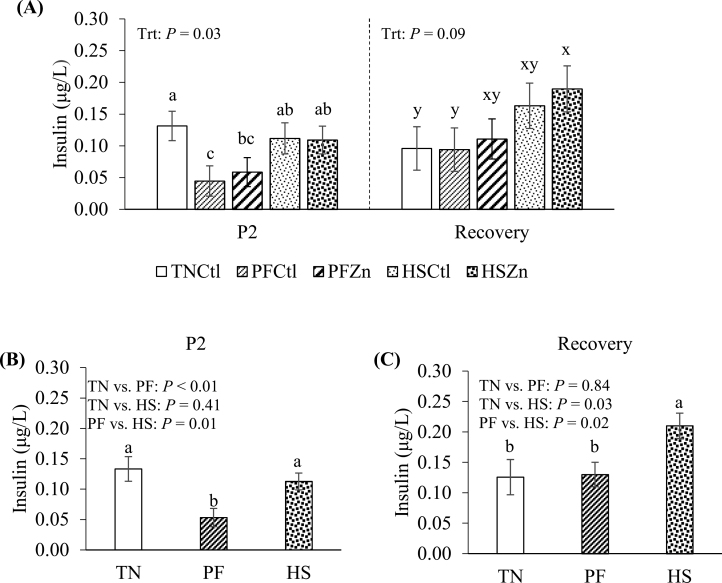
During P2, despite marked differences in ADFI, insulin levels did not differ between HS and TN treatments (P = 0.41; Figure 3A and B); however, insulin was decreased in the PF relative to TN and HS pigs (58 and 50%, respectively; P < 0.01; Figure 3B). No differences in the insulin:ADFI were observed between TN and HS pigs during P2 (P = 0.52; Table 4); however, the insulin:ADFI was increased in HS pigs compared with their PF counterparts (100%; P < 0.01; Table 4). No differences in insulin levels or the insulin:ADFI were detected between Ctl and Zn-fed pigs during P2 (P > 0.10; Table 4). During P3 (recovery period), circulating insulin was increased in pigs that were assigned to HS during P2 relative to TN and PF pigs (75%, P < 0.05; Figure 3A–C). Similarly, the insulin:ADFI was increased in HS pigs relative to TN and PF controls (113 and 143%, respectively; P < 0.05; Table 4). When comparing Ctl vs. Zn-fed pigs, no differences in insulin levels or the insulin:ADFI were detected during P3 (P > 0.10; Table 4).
Figure 3.
Effects of zinc amino acid complex on (A) circulating levels of insulin during period 2 and the recovery period. Treatments: TNCtl = thermoneutral ad libitum control diet, PFCtl = pair-fed control diet, PFZn = pair-fed zinc diet, HSCtl = heat stress ad libitum control diet, and HSZn = heat stress ad libitum zinc diet. The dashed line divides period 2 from recovery period. Circulating insulin levels during (B) period 2 and (C) the recovery period when comparing between thermoneutral (TN), pair-fed (PF), and heat stress (HS) treatments. a–cValues with differing superscripts denote differences (P ≤ 0.05) between treatments. x–yValues with different superscripts denote tendencies (P ≤ 0.10) between treatments.
During P2 and despite marked differences in ADFI, NEFA levels were similar across treatments (P = 0.17; Table 4). However, when comparing Ctl vs. Zn-fed pigs, NEFA levels were increased in Zn relative to Ctl-fed pigs (28%; P = 0.03; Table 4). During P3, circulating NEFA was similar across treatments (P = 0.43; Table 4) and no differences in NEFA levels were observed between Ctl and Zn-fed pigs (P = 0.41; Table 4). During P2, there were no overall treatment differences detected in BUN (P = 0.41; Table 4); however, there was a tendency for decreased BUN levels in HS and PF pigs relative to TN controls (P = 0.07 and P = 0.09, respectively; Table 4). No differences in BUN were detected between Ctl and Zn-fed pigs during P2 (P = 0.44; Table 4). During P3, BUN levels were similar across treatments (P = 0.83; Table 4) and did not differ between Ctl and Zn-fed pigs (P = 0.95; Table 4). There were no treatment differences in circulating LBP during P2 or P3 (P = 0.68 and P = 0.84, respectively; Table 4). Likewise, LBP levels did not differ between Ctl and Zn-fed pigs during P2 or P3 (P = 0.52 and P = 0.42, respectively).
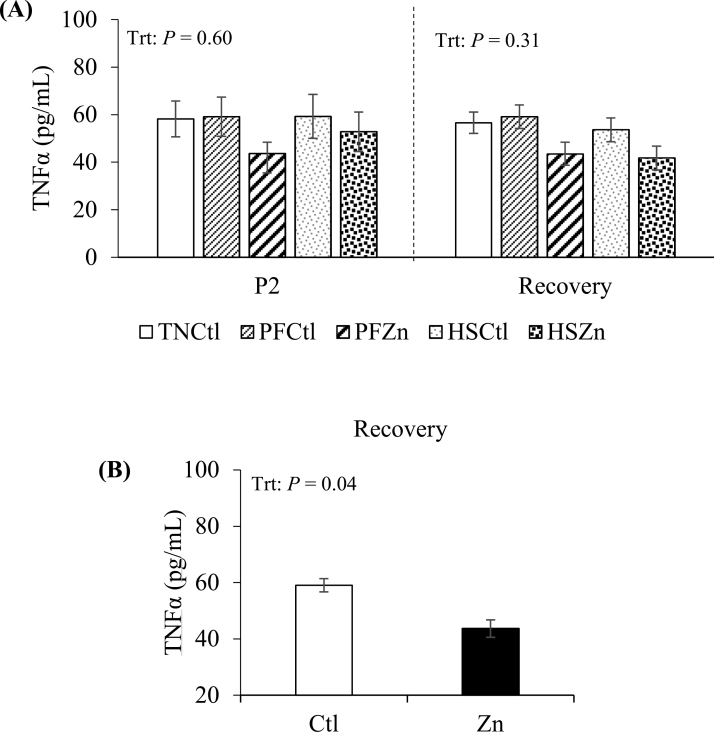
During P2, TNFα levels were similar across treatments (P = 0.60; Figure 4A), and no differences were detected between Ctl and Zn-fed pigs (P = 0.15). No overall treatment effect was observed in circulating TNFα during P3 (P = 0.31; Figure 4A); however, TNFα levels were decreased in Zn relative to Ctl-fed pigs (25%; P = 0.04; Figure 4B).
Figure 4.
Effects of zinc amino acid complex on circulating levels of (A) tumor necrosis factor alpha during period 2 and the recovery period. Treatments: TNCtl = thermoneutral ad libitum control diet, PFCtl = pair-fed control diet, PFZn = pair-fed zinc diet, HSCtl = heat stress ad libitum control diet, and HSZn = heat stress ad libitum zinc diet. The dashed line divides period 2 from recovery period. Circulating TNFα levels during recovery period (B) when comparing between dietary control (Ctl) and zinc (Zn) treatments.
DISCUSSION
HS negatively impacts a variety of animal agriculture parameters including growth, body composition, milk yield, and reproduction (St-Pierre et al., 2003; Baumgard and Rhoads, 2013). The detrimental effects of HS on animal productivity may be mediated in large part by its effects on intestinal health and barrier function (Baumgard and Rhoads, 2013). During hyperthermia, blood is redistributed away from the splanchnic bed to the periphery in an attempt to maximize radiant heat dissipation (Lambert, 2004). This survival strategy results in increased reactive oxygen species production, membrane damage, and disruption of tight junction complexes in the intestinal epithelium (Hall et al., 2001; Lambert, 2009). Additionally, hypophagia (a conserved response to HS; Baumgard and Rhoads, 2013) causes intestinal hyperpermeability in multiple species (Ferraris and Carey, 2000; Pearce et al., 2013b; Zhang et al., 2013). In both situations, the loss of intestinal barrier function to luminal content (e.g., bacterial endo- and exotoxins, food-borne antigens, etc.) results in LPS appearance in portal and systemic circulation (Pearce et al., 2013b). Endotoxin stimulates an immune response associated with increased circulating inflammatory biomarkers (Bouchama et al., 1991, 1993; Hall et al., 2001; Lambert, 2004). Additionally, LPS (a catabolic molecule) inexplicably increases circulating insulin (an anabolic hormone) in a variety of animal models (Baumgard et al., 2016). Consequently, HS-induced endotoxemia and inflammation alter metabolism and nutrient partitioning, and this partially explains why HS decreases productivity (Baumgard and Rhoads, 2013). Furthermore, while the negative effects of HS have been intensively studied during the heat load, little is known regarding the biology of “recovery” following HS exposure. Therefore, studying HS recovery provides insight into the length of time that HS and/or nutrient restriction adversely affect intestinal integrity. Identifying effective dietary strategies aimed at alleviating the adverse effects of HS on intestinal health and/or accelerating the recovery following HS exposure could have enormous impact on animal health and agriculture economics.
Zinc is essential for normal barrier function as it improves intestinal integrity during different disease states including malnutrition (Rodriguez et al., 1996), ethanol-induced intestinal damage (Lambert et al., 2003), chronic inflammatory bowel diseases (Sturniolo et al., 2001), and infectious diarrhea (Alam et al., 1994). Furthermore, our group has reported beneficial effects of complexed Zn supplementation during a thermal load as it decreased circulating endotoxin levels and improved intestinal epithelial resistance, intestinal architecture, and the acute phase response in heat-stressed pigs and ruminants (Sanz Fernandez et al., 2014; Pearce et al., 2015; Abuajamieh et al., 2016). Therefore, we hypothesized supplemental Zn would reduce the temporal pattern of inflammatory biomarkers and subsequent altered metabolism in a HS and feed restriction porcine model and would improve the recovery response after both insults.
In the current study, pigs in the HS environment experienced marked hyperthermia as demonstrated by increased TR, TS, and RR (0.3 °C, 3.3 °C, and 26 bpm, respectively) relative to TN pigs. Conversely, decreased TS (0.6 °C) was observed in PF pigs during P2 relative to their TN counterparts, indicating decreased heat increment of feeding as reported previously by our group (Pearce et al., 2013a; Seibert et al., 2018). During the recovery period, all body temperature indices returned to baseline values, and no TR or RR differences were observed across treatments. However, despite being in similar environmental conditions, HSZn pigs had a slight decrease in TS (0.8 °C) relative to TN, PF, and HSCtl pigs. Understanding why HSZn pigs had decreased TS during recovery and whether this is biologically meaningful and industry relevant is of interest.
As expected, HS exposure markedly decreased ADFI (28%) and ADG (35%) relative to TN ad libitum fed pigs. By experimental design, PF treatments mirrored this pattern of reduced ADFI during P2. The magnitude and pattern of nutrient intake reduction were comparable to our previous studies (Johnson et al., 2015; Sanz Fernandez et al., 2015b). The negative effects of HS on voluntary ADFI have been well-documented (as reviewed by Nyachoti et al., 2004), and it is a highly conserved response that is partially responsible for decreased growth performance observed in heat-stressed animals (Baumgard and Rhoads, 2013). During the recovery period (P3), ADFI and ADG in pigs exposed to HS returned to TN levels; however, pigs previously assigned the PF treatment had increased ADFI and ADG (21 and 32%, respectively) above TN levels. Reasons why appetite was blunted in HS relative to PF pigs during recovery are not entirely clear. Our group previously observed that ADFI in pigs exposed to 3 d of HS gradually increased during the recovery period but remained less than the TN controls for the remainder of the experiment (Abuajamieh et al., 2015). Additionally, Johnson et al. (2016) observed that pigs exposed to a 3-h heat load had a progressively increased feeding behavior relative to that of the TN pigs during a 3-h recovery period. Mechanisms underlying compensatory growth are difficult to understand as factors influencing an animal’s ability to recover may be associated with the nature, severity, and/or duration of nutrient restriction (Wilson and Osbourn, 1960; Lovatto et al., 2006). Additionally, compensatory growth can also reflect changes in metabolism as circulating levels of insulin, thyroid, and growth hormones play a key role in the initiation of this response (Prince et al., 1983; Hornick et al., 2000). Insulin has been hypothesized to be an important regulator of feed intake and energy balance (Rezek, 1976; Woods et al., 2006; Perry and Wang, 2012); furthermore, exogenous insulin administration reduces feed consumption in different rodent models (Schwartz et al., 1992; Woods et al., 1996; Air et al., 2002). Interestingly, in the current study, pigs previously exposed to HS during P2 had increased circulating insulin relative to TN and PF pigs during the recovery period (as described below), and this may have contributed to the blunted appetite response. Regardless, the absence of full recovery in nutrient intake and growth in HS pigs is of practical interest and suggests the negative consequences of HS have long-lasting effects on animal productivity and farm profitability.
Animals exposed to HS have altered postabsorptive carbohydrate, lipid, and protein metabolism independent of nutrient intake. This energetic phenotype is primarily characterized by increased basal and stimulated circulating insulin levels and blunted adipose tissue mobilization (Baumgard and Rhoads, 2013). In the current study, no overall treatment differences in blood glucose, NEFA, or BUN were observed during P2 or recovery. Despite marked reductions in ADFI during P2, circulating insulin from HS pigs was similar to TN controls, but it was decreased in PF pigs compared with their TN counterparts. The aforementioned insulin response corroborates similar observations in heat-stressed rodents, pigs, and ruminants (Torlinska et al., 1987; O’Brien et al., 2010; Wheelock et al., 2010, Pearce et al., 2013a; Sanz Fernandez et al., 2015b). Reasons why insulin homeostasis is altered during HS remain unclear, but likely involve insulin’s role in activating the cellular stress response (Li et al., 2006) and facilitating immune system fuel uptake (as reviewed by Baumgard et al., 2016). In fact, previous reports indicate diabetic rodents and humans are more susceptible to heat-related illness and death (Schuman et al., 1972; Semenza et al., 1999; Niu et al., 2003). Interestingly, during the recovery period, PF pigs returned to euinsulinemia while pigs previously exposed to HS had a large increase in circulating insulin relative to both TN and PF pigs (~75%). This is surprising because insulin secretion is very sensitive to changes in nutrient intake, yet ADFI in pigs that were exposed to HS remained below that of PF pigs during the recovery period. Reasons why circulating insulin levels were increased during recovery in pigs that were exposed to HS are not clear but likely suggest the stimulatory effect of LPS on insulin secretion is amplified during HS recovery. This also likely explains why the carcass from heat-stressed pigs have more adipose tissue than energetically expected (Close et al., 1971; Verstegen et al., 1978; Collin et al., 2001).
Previous studies suggest the pathophysiological responses to a thermal load might not be due to the direct effects of heat exposure, but the result of a systemic and uncontrolled inflammatory response following thermal injury (Leon, 2007; Heled et al., 2013). The increased inflammatory response after the cessation of HS is thought to stem from increased LPS leakage into circulation, which in turn elicits an immune response and stimulates production of proinflammatory cytokines (Lambert, 2009). Accordingly, Johnson et al. (2016) observed increased circulating and tissue cytokine levels in pigs after a 3-h recovery period following an acute heat load. Additionally, our group has recently reported a progressive increase in circulating LBP during recovery from an intense HS in pigs (Abuajamieh et al., 2015), suggesting the inflammatory and acute phase response is constrained or blunted during the heat load, but is expressed during recovery and remains robust for at least 1 wk following an intense heat load. Contrarily, in the current study no differences were observed in circulating LBP among environments during P2 or recovery. Although not clear, inconsistencies in this response may be explained by differences in the intensity and length of the heat load utilized between experiments.
Interestingly, despite no differences observed during HS exposure, dietary Zn supplementation reduced TNFα levels during the recovery period by 25% in Zn compared to Ctl-fed pigs. Additionally, circulating TNFα tended to be decreased during P1 (Supplementary Table S1) and was numerically decreased during P2 in Zn relative to Ctl-fed animals. Reasons for decreased circulating TNFα during Zn supplementation are not well-understood but suggest the importance of Zn’s role in modulating the immune and inflammatory responses (as reviewed by Haase and Rink, 2007, 2014). A likely explanation based upon the literature and our previous experiments is that Zn decreased intestinal permeability and thus reduced passage of luminal antigens across the intestinal barrier, consequently reducing the extent of immune activation. A second possible mechanism by which Zn ameliorates the inflammatory response is by regulating the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) pathway (Prasad, 2008) as previous in vitro studies indicated that Zn inhibited NF-κB activation (Jarosz et al., 2017). The NF-κB signaling pathway plays a key role in the expression of proinflammatory cytokines that regulate the immune response (TNFα, IL-1, IL-6, and IL-8), as well as in different cellular processes including proliferation and apoptosis (as reviewed by Hoesel and Schmid, 2013). Additionally, Hu et al. (2013a, 2013b) and Liu et al. (2014) observed decreased mRNA expression of TNFα, IL-6, IL-8, and interferon-γ along different sections of the gastrointestinal tract with increasing concentrations of dietary Zn (inorganic Zn) in weaning pigs. Decreased circulating TNFα levels observed in this study indicate that feeding a more bioavailable dietary Zn may be able to ameliorate the inflammatory response and enhance the progression of recovery in pigs that were exposed to HS or were nutrient-restricted.
In conclusion, chronic HS exposure increased all body temperature indices and caused a marked and constant reduction in ADFI and ADG. Additionally, our data suggest HS has long-lasting effects on phenotypic and metabolic responses, and thus on animal health and productivity. Herein, we also demonstrated that feeding a Zn amino acid complex might be beneficial at reducing biomarkers of inflammation during and following stress. Overall, Zn amino acid supplementation may be a plausible dietary strategy to ameliorate some of the negative effects of HS on animal health.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
Footnotes
1Results described herein were supported by the Zinpro Corporation and the Agricultural and Food Research Initiative Competitive Grant no. 2011-67003-30007 from the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Abuajamieh M., Kvidera S. K., Horst E. A., Mayorga E. J., Seibert J. T., Johnson J. S., Ross J. W., Al-Qaisi M. A., Gorden P. J., DeFrain J., Rhoads R. P., and Baumgard L. H.. 2016. The effects of zinc amino acid complex on biomarkers of gut integrity and metabolism in heat-stressed steers. J. Dairy Sci. 99 (E-Suppl. 1):1175. doi: 10.2527/jam2016-1175 [DOI] [PubMed] [Google Scholar]
- Abuajamieh M., Laughlin E., Lei S., Stoakes S., Mayorga E. J., Seibert J. T., Nolan E. A., Sanz Fernandez M. V., Ross J. W., Selsby J., Rhoads R. P., and Baumgard L. H.. 2015. The effects of recovery time from heat stress on circulating bioenergetics variables and biomarkers of leaky gut. FASEB J. 29:LB365 (Abstr.). doi: 10.1096/fasebj.29.1_supplement.lb365 [DOI] [Google Scholar]
- Air E. L., Benoit S. C., Blake Smith K. A., Clegg D. J., and Woods S. C.. 2002. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol. Biochem. Behav. 72:423–429. [DOI] [PubMed] [Google Scholar]
- Alam A. N., Sarker S. A., Wahed M. A., Khatun M., and Rahaman M. M.. 1994. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut 35:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgard L. H., Hausman G. J., and Sanz Fernandez M. V.. 2016. Insulin: pancreatic secretion and adipocyte regulation. Domest. Anim. Endocrinol. 54:76–84. doi: 10.1016/j.domaniend.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Bouchama A., and Knochel J. P.. 2002. Heat stroke. N. Engl. J. Med. 346:1978–1988. doi: 10.1056/NEJMra011089 [DOI] [PubMed] [Google Scholar]
- Bouchama A., Parhar R. S., el-Yazigi A., Sheth K., and al-Sedairy S.. 1991. Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J. Appl. Physiol. (1985). 70:2640–2644. doi: 10.1152/jappl.1991.70.6.2640 [DOI] [PubMed] [Google Scholar]
- Bouchama A., al-Sedairy S., Siddiqui S., Shail E., and Rezeig M.. 1993. Elevated pyrogenic cytokines in heatstroke. Chest 104:1498–1502. [DOI] [PubMed] [Google Scholar]
- Close W. H., Mount L. E., and Start I. B.. 1971. The influence of environmental temperature and plane of nutrition on heat losses from groups of growing pigs. Anim. Prod. 13:285–294. [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. 2001. Effect of high temperature on feeding behavior and heat production in group-housed young pigs. Br. J. Nutr. 86:63–70. doi: 10.1079/BJN2001356 [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., and Carey H. V.. 2000. Intestinal transport during fasting and malnutrition. Annu. Rev. Nutr. 20:195–219. doi: 10.1146/annurev.nutr.20.1.195 [DOI] [PubMed] [Google Scholar]
- Haase H., and Rink L.. 2007. Signal transduction in monocytes: the role of zinc ions. Biometals 20:579–585. doi: 10.1007/s10534-006-9029-8 [DOI] [PubMed] [Google Scholar]
- Haase H., and Rink L.. 2014. Zinc signals and immune function. Biofactors 40:27–40. doi: 10.1002/biof.1114 [DOI] [PubMed] [Google Scholar]
- Hall D. M., Baumgardner K. R., Oberley T. D., and Gisolfi C. V.. 1999. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am. J. Physiol. 276:G1195–G1203. [DOI] [PubMed] [Google Scholar]
- Hall D. M., Buettner G. R., Oberley L. W., Xu L., Matthes R. D., and Gisolfi C. V.. 2001. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am. J. Physiol. Heart Circ. Physiol. 280:H509–H521. doi: 10.1152/ajpheart.2001.280.2.H509 [DOI] [PubMed] [Google Scholar]
- Heled Y., Fleischmann C., and Epstein Y.. 2013. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 24:85–96. doi: 10.1515/jbcpp-2012-0040 [DOI] [PubMed] [Google Scholar]
- Hoesel B., and Schmid J. A.. 2013. The complexity of NF-κb signaling in inflammation and cancer. Mol. Cancer 12:86. doi: 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick J. L., Van Eenaeme C., Gérard O., Dufrasne I., and Istasse L.. 2000. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 19:121–132. [DOI] [PubMed] [Google Scholar]
- Hu C., Song J., Li Y., Luan Z., and Zhu K.. 2013a. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 110:681–688. doi: 10.1017/S0007114512005508 [DOI] [PubMed] [Google Scholar]
- Hu C. H., Xiao K., Song J., and Luan Z. S.. 2013b. Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Anim. Feed Sci. Technol. 181:65–71. doi: 10.1016/j.anifeedsci.2013.02.003 [DOI] [Google Scholar]
- Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., and Librowski T.. 2017. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25:11–24. doi: 10.1007/s10787-017-0309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. S., Sanz Fernandez M. V., Patience J. F., Ross J. W., Gabler N. K., Lucy M. C., Safranski T. J., Rhoads R. P., and Baumgard L. H.. 2015. Effects of in utero heat stress on postnatal body composition in pigs: II. Finishing phase. J. Anim. Sci. 93:82–92. doi: 10.2527/jas2014-8355 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Sapkota A., and Lay D. C. Jr. 2016. Rapid cooling after acute hyperthermia alters intestinal morphology and increases the systemic inflammatory response in pigs. J. Appl. Physiol. (1985). 120:1249–1259. doi: 10.1152/japplphysiol.00685.2015 [DOI] [PubMed] [Google Scholar]
- Kvidera S. K., Dickson M. J., Abuajamieh M., Snider D. B., Sanz Fernandez M. V., Johnson J. S., Keating A. F., Gorden P. J., Green H. B., Schoenberg K. M., and Baumgard L. H.. 2017. Intentionally induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 100:4113–4127. doi: 10.3168/jds.2016-12349 [DOI] [PubMed] [Google Scholar]
- Lambert G. P. 2004. Role of gastrointestinal permeability in exertional heatstroke. Exerc. Sport Sci. Rev. 32:185–190. [DOI] [PubMed] [Google Scholar]
- Lambert G. P. 2009. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 87(14 Suppl):E101–E108. doi: 10.2527/jas.2008-1339 [DOI] [PubMed] [Google Scholar]
- Lambert J. C., Zhou Z., Wang L., Song Z., McClain C. J., and Kang Y. J.. 2003. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J. Pharmacol. Exp. Ther. 305:880–886. doi: 10.1124/jpet.102.047852 [DOI] [PubMed] [Google Scholar]
- Leon L. R. 2007. Heat stroke and cytokines. Prog. Brain Res. 162:481–524. doi: 10.1016/S0079-6123(06)62024-4 [DOI] [PubMed] [Google Scholar]
- Li G., Ali I. S., and Currie R. W.. 2006. Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am. J. Physiol. Heart Circ. Physiol. 291:H1709–H1721. doi: 10.1152/ajpheart.00201.2006 [DOI] [PubMed] [Google Scholar]
- Liu P., Pieper R., Rieger J., Vahjen W., Davin R., Plendl J., Meyer W., and Zentek J.. 2014. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS ONE 9:e91091. doi: 10.1371/journal.pone.0091091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatto P. A., Sauvant D., Noblet J., Dubois S., and van Milgen J.. 2006. Effects of feed restriction and subsequent refeeding on energy utilization in growing pigs. J. Anim. Sci. 84:3329–3336. doi: 10.2527/jas.2006-048 [DOI] [PubMed] [Google Scholar]
- Mani V., Weber T. E., Baumgard L. H., and Gabler N. K.. 2012. Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90:1452–1465. doi: 10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- Niu C. S., Lin M. T., Liu I. M., and Cheng J. T.. 2003. Role of striatal glutamate in heatstroke-induced damage in streptozotocin-induced diabetic rats. Neurosci. Lett. 348:77–80. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed National Academies Press, Washington, DC. [Google Scholar]
- Nyachoti C. M., Zijlstra R. T., de Lange C. F. M., and Patience J. F.. 2004. Voluntary feed intake in growing-finishing pigs: a review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 84:549–566. doi: 10.4141/A04-001 [DOI] [Google Scholar]
- O’Brien M. D., Rhoads R. P., Sanders S. R., Duff G. C., and Baumgard L. H.. 2010. Metabolic adaptations to heat stress in growing cattle. Domest. Anim. Endocrinol. 38:86–94. doi: 10.1016/j.domaniend.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. 2013a. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Mani V., Weber T. E., Rhoads R. P., Patience J. F., Baumgard L. H., and Gabler N. K.. 2013b. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 91:5183–5193. doi: 10.2527/jas.2013-6759 [DOI] [PubMed] [Google Scholar]
- Pearce S. C., Sanz Fernandez M. V., Torrison J., Wilson M. E., Baumgard L. H., and Gabler N. K.. 2015. Dietary organic zinc attenuates heat stress-induced changes in pig intestinal integrity and metabolism. J. Anim. Sci. 93:4702–4713. doi: 10.2527/jas.2015-9018 [DOI] [PubMed] [Google Scholar]
- Perry B., and Wang Y.. 2012. Appetite regulation and weight control: the role of gut hormones. Nutr. Diabetes 2:e26. doi: 10.1038/nutd.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S. 2008. Zinc in human health: effect of zinc on immune cells. Mol. Med. 14:353–357. doi: 10.2119/2008-00033.Prasad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince T. J., Jungst S. B., and Kuhlers D. L.. 1983. Compensatory responses to short-term feed restriction during the growing period in swine. J. Anim. Sci. 56:846–852. [DOI] [PubMed] [Google Scholar]
- Rezek M. 1976. The role of insulin in the glucostatic control of food intake. Can. J. Physiol. Pharmacol. 54:650–665. [DOI] [PubMed] [Google Scholar]
- Rodriguez P., Darmon N., Chappuis P., Candalh C., Blaton M. A., Bouchaud C., and Heyman M.. 1996. Intestinal paracellular permeability during malnutrition in guinea pigs: effect of high dietary zinc. Gut 39:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Isom S. C., Rhoads R. P., and Baumgard L. H.. 2015a. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Pearce S. C., Gabler N. K., Patience J. F., Wilson M. E., Socha M. T., Torrison J. L., Rhoads R. P., and Baumgard L. H.. 2014. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 8:43–50. doi: 10.1017/S1751731113001961 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. 2015b. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3:e12478. doi: 10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman S. H. 1972. Patterns of urban heat-wave deaths and implications for prevention: data from New York and St. Louis during July, 1966. Environ. Res. 5:59–75. [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Figlewicz D. P., Baskin D. G., Woods S. C., and Porte D. Jr. 1992. Insulin in the brain: a hormonal regulator of energy balance. Endocr. Rev. 13:387–414. doi: 10.1210/edrv-13-3-387 [DOI] [PubMed] [Google Scholar]
- Seibert J. T., Abuajamieh M., Sanz Fernandez M. V., Johnson J. S., Kvidera S. K., Horst E. A., Mayorga E. J., Lei S., Patience J. F., Ross J. W., Rhoads R. P., Johnson R. C., Lonergan S. M., Perfield J. W. II, and Baumgard L. H.. 2018. Effects of heat stress and insulin sensitizers on pig adipose tissue. J. Anim. Sci. 96:510–520. doi: 10.1093/jas/skx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J. C., McCullough J. E., Flanders W. D., McGeehin M. A., and Lumpkin J. R.. 1999. Excess hospital admissions during the July 1995 heat wave in Chicago. Am. J. Prev. Med. 16:269–277. [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86(E. Suppl):E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Sturniolo G. C., Di Leo V., Ferronato A., D’Odorico A., and D’Incà R.. 2001. Zinc supplementation tightens “leaky gut” in crohn’s disease. Inflamm. Bowel Dis. 7:94–98. [DOI] [PubMed] [Google Scholar]
- Torlinska T., Banach R., Paluszak J., and Gryczka-Dziadecka A.. 1987. Hyperthermia effect on lipolytic processes in rat blood and adipose tissue. Acta Physiol. Pol. 38:361–366. [PubMed] [Google Scholar]
- Verstegen M. W. A., Brascamp E. W., and Van Der Hel W.. 1978. Growing and fattening of pigs in relation to temperature of housing and feeding level. Can. J. Anim. Sci. 58:1–13. [Google Scholar]
- Wheelock J. B., Rhoads R. P., Vanbaale M. J., Sanders S. R., and Baumgard L. H.. 2010. Effects of heat stress on energetic metabolism in lactating holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Wilson P. N., and Osbourn D. F.. 1960. Compensatory growth after undernutrition in mammals and birds. Biol. Rev. Camb. Philos. Soc. 35:324–363. [DOI] [PubMed] [Google Scholar]
- Woods S. C., Chavez M., Park C. R., Riedy C., Kaiyala K., Richardson R. D., Figlewicz D. P., Schwartz M. W., Porte D. Jr, and Seeley R. J.. 1996. The evaluation of insulin as a metabolic signal influencing behavior via the brain. Neurosci. Biobehav. Rev. 20:139–144. [DOI] [PubMed] [Google Scholar]
- Woods S. C., Lutz T. A., Geary N., and Langhans W.. 2006. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361:1219–1235. doi: 10.1098/rstb.2006.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. j. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.