Abstract
Heat stress (HS) causes significant economic losses and has become a continual challenge in the dairy industry worldwide. The objective of this study was to evaluate the effects of a dietary supplement on milk performance and immune function in late-lactation cows under HS conditions. The supplement was a fermented Chinese herbal medicines (CHMs) mixture consisting of 18 herbs. Forty lactating Holstein cows (560 ± 51.0 kg of initial BW, 230 ± 10.0 DIM, 16 ± 3.0 kg of milk per day) were randomly assigned into 4 treatment groups (10 cows per group). Each group was fed a dietary supplemented with 0, 25, 50, or 100 g CHMs per cow per day. Cows were housed at high ambient temperature-humidity index (average 74.5) for an experimental period of 42 d during the summer months. Milk yield, composition, immune responses involving blood lymphocyte apoptosis rate, serum biochemical parameters, and genes expression in lymphocytes were evaluated on days 14, 28, and 42, respectively. Results showed that milk yield, milk fat, and protein content were greater (all P < 0.05) for 50 or 100 g/d CHMs compared with the group without CHMs supplements throughout the experimental period. On the other hand, increasing CHMs dose demonstrated a greater lymphocyte or leukocyte count (P < 0.01). By flow cytometry analysis, early or late apoptosis rate of the lymphocytes was decreased (P < 0.05) by CHMs supplements. The immunity-related biochemistry and genes transcript responses involving cytokines (IL-1, IL-2, IL-6, and IL-12), apoptosis (Bak, Mcl-1, Bax, Bcl-2, Bcl-xl, and P53), and immunoglobulins (IgA, IgG, and IgM) were investigated. Compared with the unsupplemented group, the serum IL-2 and IL-6 levels, as well as IL-2 mRNA expression, increased (P < 0.05) for 100 g/d. However, the serum IL-1 level tended to decrease (P = 0.08) with increasing CHMs dose, and IL-1 mRNA expression was down-regulated (P = 0.02) by up to 24% for 100 g/d. Additionally, the serum Bax level decreased (P < 0.01) and Bcl-2 level increased (P = 0.01) for 100 g/d. Bax and Bak mRNA expressions were down-regulated (P < 0.05), and Bcl-2 and Bcl-xl expression were up-regulated (P < 0.05) for 50 or 100 g/d. The mRNA expressions of P53 and Mcl-1 were not affected by CHMs (P > 0.10). Besides, serum IgG levels were greater (P < 0.01) for 50 or 100 g/d, compared with unsupplemented group. In conclusion, CHMs supplements may improve milk performance and immune function in dairy cows under HS conditions.
Keywords: Chinese herbal medicine, lactation cow, milk performance, immune function, heat stress, gene expression
INTRODUCTION
Heat stress (HS) causes significant economic losses in dairy production systems across China and worldwide (Hill et al., 2017). The biological mechanisms mediating HS-induced alteration are not well understood; however, some responses (e.g., immune alteration) to HS have been implicated (Dokladny et al., 2016; McCracken et al., 2015). Several studies have demonstrated that HS impairs immune system in livestock (Lacetera et al., 2006; Carroll et al., 2012). Despite significant advancements, mitigating the incidence of HS continues to be an important challenge in the dairy industry. Often, HS occurs in the late lactation period of dairy cows because of the reduced immune function (Ingvartsen and Moyes, 2013). Thus, it is necessary to improve the immune function of late-lactation cows to alleviate the negative effect of HS on milk production.
Majority of the traditional Chinese herbal medicines (CHMs) have a long history of being used in Asian countries for the maintenance of human health because CHMs have low toxicity and cause fewer side effects (Ma et al., 2013). Evidence has demonstrated that CHMs may influence immune cells and immune-associated cytokine production. In recent years, the interest in CHMs is growing in the prevention and treatment of diseases in livestock including poultry, rabbit, and sheep, due to a broad spectrum of immune regulatory activities of CHMs (Wang et al., 2007; Sun et al., 2016; Xie et al., 2017; Liang et al., 2018). Several studies have demonstrated that CHMs, such as Rhizoma atractylodis and Agastache rugose, have effective effect when used to treat HS syndrome (Song et al., 2009, 2010; Dong et al., 2012). Recently, CHMs fermented by probiotic have been introduced to improve the biological activities of CHMs and exert a greater immune effect on animals (Kim et al., 2017; Kumar et al., 2017). A recent study involving immunomodulatory activity in vitro indicated that fermented CHMs by Rhizopus oligosporus SH effectively enhanced the proliferation of lymphocytes, and promoted mRNA expression of cytokines such as interleukin-6 and interferon-γ (Sun et al., 2017). Until now, little information is available for the effects of fermented CHMs on dairy cows (Hashemzadeh-Cigari et al., 2014). Therefore, the objectives of the present study were to examine the effects of a fermented CHMs mixture offered to late-lactation cows on milk performance and immune responses involving apoptosis characteristics of lymphocytes, biochemical parameters, and gene expressions in peripheral blood under HS conditions.
MATERIALS AND METHODS
All animal procedures were approved by the Animal Care Committee at Agricultural University of Hebei in accordance with the university’s guidelines for animal research.
Preparation of Chinese Herbal medicine fermentation
According to the classical Chinese pharmacopeia (State Pharmacopoeia Commission of the PRC, 2005), a traditional CHMs mixture in this study was prepared from 18 Chinese herbs, including Radix rehmanniae preparata (5.6%, dry weight basis), Fructus crataegi (8.4%), Semen raphani (5.6%), Radix et rhizoma rhei (0.8%), Unguis sus domestica (5.6%), Radix astragali (21.0%), Radix codonopsis (5.6%), Radix angelicae sinensis (5.6%), R. atractylodis (4.2%), Pericarpium citri reticulatae (5.6%), Radix glycyrrhizae (2.0%), Rhizoma chuanxiong (2.0%), Herba cistanches (4.2%), Radix ophiopogonis (4.2%), Radix paeoniae alba (4.2%), Cacumen platycladi (8.4%), Artemisiacapillaris thumb (5.6%), and Fructus gardeniae (1.4%), all of which were purchased from Anguo Jufu Herbal Medicine Co. Ltd. (Hebei, China). Before these herbs were mixed thoroughly and crumbled, the 3 herbs R. rehmanniae preparata, F. crataegi, and S. raphani were heated in marmite at 100–150 °C until the surface of herbs was scorched. Both R. et rhizoma rhei and U. sus domestica were submerged in alcohol for 30 min, heated in marmite at 120 °C, and then cooled down in the air. The minor material, including 4% of corn flour, 0.8% of glucose, and 30% of water, calculated by dry weight of herbal mixture, was added to the mixture and thoroughly mixed, and then 1% of Saccharomyces cerevisiae (2 × 1010 cfu/g) and 0.1% of Bacillus subtilis (6 × 109 cfu/g) were added. The herbal mixture was incubated by aerobic fermentation for 48 h, and further anaerobic fermentation for 1 wk. Fermented product was dried in the air, then added to the ingredients (60% of soybean meal and 40% of corn flour, DM basis), dried, and packed until needed. One kilogram of the final fermented mixture was equivalent to 300 g of the raw herbs. This fermented mixture has been granted a licensed patent as feed additive of dairy cows in China.
Animals, Experimental Design, and Treatments
The present study was conducted at a commercial dairy facility of Hebei province, China, which is characterized as having a warm temperate monsoon climate. Forty lactating primiparous Holstein cows(560 ± 51.0 kg of initial BW (mean ± SD), 230 ± 10.0 DIM, 16 ± 3.0 kg of milk per day at the start of trial)were used in a complete randomized design for 10-d adaptation period and a 42-d experimental period. All cows were randomly divided into 4 groups (10 cows per group) in the same cowshed. Initial BW, DIM, and milk yield were not different among groups at the beginning of the experiment (P = 0.61, P = 0.72, and P = 0.53, respectively). The cows in treated experimental groups were supplemented 25, 50, or 100 g CHMs mixture per cow per day (equal to 0.045, 0.090, or 0.180 g/kg BW per day), and those in the control group were not supplemented CHMs (0 g/d CHMs). The CHMs mixture was supplemented at an equal amount twice daily by top-dress feeding onto the diet for individual cows. The GE and CP content of CHMs supplements was 13.8 MJ/kg and 20.1% (DM basis), respectively, and CHMs supplements had no effect on energy and protein content in the diets in treated groups. All cows were fed a total mixed ration (TMR) twice daily at approximately 0530 and 1630 h after milked. The TMR was based on corn silage as the main forage component and corn grain as the major concentrate component when the concentrate and roughage were mixed in 45:55 DM ratio, and formulated to meet the nutritional requirements according to Chinese feeding standard for lactating dairy cows (China Standard NY/T34, 2004). The ingredients and nutritional composition of TMR are presented in Table 1.
Table 1.
Ingredients and chemical composition of total mixed ration
| Ingredient | % of DM | Chemical composition | % of DM |
|---|---|---|---|
| Silage | 28.10 | CP | 15.53 |
| Grass hay | 12.73 | Ca | 0.72 |
| Alfalfa hay | 10.63 | P | 0.44 |
| Distillers grain | 10.05 | NDF | 39.62 |
| Cotton seed | 4.45 | ADF | 22.10 |
| Extruded soybean | 1.54 | NEL, MJ/kg | 6.17 |
| Corn | 19.0 | ||
| Soybean meal | 7.90 | ||
| Wheat bran | 2.00 | ||
| NaHCO3 | 0.45 | ||
| NaCl | 0.40 | ||
| Cotton meal | 1.00 | ||
| Calcium phosphate | 0.60 | ||
| Limestone | 0.35 | ||
| Premix1 | 0.80 |
1Formulated to provide (per kg of DM) 500,000 IU of vitamin A; 100,000 IU of vitamin D; 2,100 IU of vitamin E; 4,000 mg of Zn; 30 mg of Se; 50 mg of I; 780 mg of Fe; 68 mg of Co; 1,000 mg of Mn; and 1000 mg of Cu.
Cows were housed in an open sand-bedded cowshed with national ventilation without any coolers, and spent approximately 30 min in the milking parlor where cooling was not provided. The TMR was offered ad libitum to allow approximately 5% of refusals, and water was available ad libitum. Cows were milked twice daily at approximately 0500 and 1600 h, and the milk yield of each milking was automatically recorded (Waikato Milking Systems NZ Ltd., Hamilton, New Zealand) in the milking parlor throughout the experimental period.
Ambient temperature (AT) and relative humidity (RH) in cowshed were measured using KTH-350-I temperature and humidity data-logger (Kimo Industry Co., French) at 30-min intervals over 24 h for a 42-d experimental period to calculate the temperature-humidity index (THI): THI = (1.8 × AT + 32) − [(0.55 − 0.0055 × RH) × (1.8 × AT − 26.8)] (Naderi et al., 2016).
Milk Sampling, Measurement, and Analysis
Milk samples (70 mL) from each cow were collected from 2 consecutive milkings (morning and afternoon, 6:4 by volume) on days 14, 28, and 42 of experiment. Samples were stored at 4 °C with a preservative of potassium dichromate (Sigma-Aldrich, St. Louis, USA) until analysis for milk composition.
Milk samples were measured for milk fat, milk protein, and milk lactose content using MilkoScan FT 120 Milk Analyzer (Foss Electric, Shanghai, China). The 4% fat-corrected milk (4% FCM) was calculated as 0.4 × milk (kg) + 15 × milk fat (kg), as described by Tyrrell and Reid (1965).
Blood Sampling, Measurement, and Analysis
Blood sampling and lymphocyte isolating.
Blood samples (25 mL) were taken from each cow at 0630 h, before the morning feeding on day 0 (before the experiment), days 14, 28, and 42 of the experiment, respectively. Samples were collected into evacuated tubes via the jugular vein. One aliquot (15 mL) was allowed to clot for a minimum of 2 h on ice, and then centrifuged at 3,000 × g for 20 min at 4 °C. The serum was collected and stored at −20 °C until further analysis for serum biochemistry parameters. The second aliquot (10 mL) was immediately transferred into heparinized tubes to obtain whole blood. Part (2 mL) of the whole blood was stored at 4 °C until analysis for total lymphocyte count and leukocyte count, and the rest was separated to isolate peripheral blood lymphocyte (PBLCs) for apoptosis and gene expression measurement.
The isolation of PBLCs was performed using a Peripheral Blood Lymphocyte Isolation Kit (Solarbio Bioscience & Technology Co., Beijing, China) according to the manufacturer’s instructions. Briefly, 8-mL anticoagulated blood was diluted at 1:2 with phosphate buffered saline (PBS) and then transferred into a 50-mL centrifuge tube that contained 16 mL of lymphocytes separating solution. After centrifugation at 500 × g for 30 min, the cell suspension was layered and the buffy coat at interface was collected, washed twice with 10 mL of PBS by centrifuging at 250 × g for 10 min. Then the isolated cells were resuspended in 2 mL of PBS, one aliquot of which was performed immediately for apoptosis measurement, whereas the rest was supplemented with RNAstore (Thermo Fisher, Waltham, MA), and then stored at −80 °C until analysis for gene expression.
PBLCs apoptosis.
Lymphocyte apoptosis was assessed using Fluorescein Isothiocyanate-labeled Annexin V (Annexin V-FITC)/Propidium Iodide (PI) apoptosis kit (MultiSciences Biotech Co., Ltd., Zhejiang, China). The isolated PBLCs were adjusted to 1 × 106 cells/µL in PBS and washed by ice-cold PBS 3 times. After centrifugation, the cells were suspended in 0.5-mL apoptosis positive control solution and then incubated on ice for 30 min. After incubation, cells were centrifuged, washed in ice-cold PBS, and then resuspended in 1 × binding buffer and incubated for 5 min at room temperature in the dark in Annexin V-FITC and PI solutions and 10,000-cell events were acquired using a flow cytometer (Beckman Coulter, California, USA). Results are shown as the percentage of annexin V-FITC and PI negative cells (cell viability), annexin V-FITC positive cells (early apoptosis), PI positive cells (necrosis), annexin V-FITC, and PI positive cells (late apoptosis/necrosis).
Blood biochemistry.
Total leukocyte, lymphocyte, granulocyte, and intermediate cell counts in whole blood were analyzed using Automatic Hematology Analyzer (MEK-6318K, Nihon Kohden corp., Tokyo, Japan). The serum biochemistry analysis, including concentrations of cytokines (interleukin-1 [IL-1], interleukin-2 [IL-2], interleukin-6 [IL-6], and interleukin-12 [IL-12]), lymphocyte apoptosis-associated parameters (Bcl-2 homologous antagonist/killer [Bak], myeloid cell leukemia-1 [Mcl-1], Bcl-2-associated X protein [Bax], B-cell lymphoma/leukemia-2 [Bcl-2], Bcl-extra-large [Bcl-xl], and tumor suppressor protein [P53]), and Immunoglobulins (IgA, IgG, and IgM), was carried out by quantitative sandwich ELISA technique using corresponding commercial ELISA kit (Shanghai Zhuocai Technology Co., Ltd., Shanghai, China) as described in the manufacturer’s protocol.
Gene expression.
The mRNA expressions of 10 genes associated with immune function, including cytokines (IL-1, IL-2, IL-6, and IL-12) and apoptosis-associated genes (Bak, Mcl-1, Bax, Bcl-2, Bcl-xl, and P53), were analyzed in the isolated lymphocytes from whole blood. Total RNA was extracted using a RNA Blood Kit (LS1040, Promega, Shanghai, China). The integrity of total RNA was analyzed by agarose gel electrophoresis, and RNA purity was measured using the NanoDrop 2000 spectrophotometer (Thermo Fisher, Massachusetts, USA). Only samples without signs of degradation and with the absorption ratio of optical density OD260 to OD280 nm between 1.8 and 2.0 were used for further analysis. Approximately 1.0 µg of total RNA was incubated with DNase 1 (Fermantas Inc., Glen Burnie, MD), and reverse transcribed using the iScript Reverse Transcription kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. A 2-step SybrGreen-based quantitative polymerase chain reactions (qPCR) assay was carried out by 7900 HT Fast Real-time PCR System (Applied Biosystems, Foster, CA) to measure mRNA expression levels using GoTaq qPCR Master Mix A 6001 kit (Promega, Madison, USA) according to the manufacturer’s instructions. Briefly, PCR were performed in triplicate by use of 20 µL of reaction mixture, which consisted of 10 µL of 2 × SuperReal PreMix Plus, 0.6 µL of each primer (forward and reverse) at 10 µM, 2 µL of cDNA, 0.4 µL of 50 × ROX Reference Dye, and 6.4 µL of RNase-free ddH2O. Each gene was individually amplified with the same reaction mixture. The qPCR conditions used were as follows: 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min, with a melting curve generated between 60 and 95 °C. The amplification data of each target gene were normalized to GAPDH, and the relative expression was analyzed using the 2−ΔΔCt method (Erskine et al., 2011; Kumar et al., 2017). The primer sequences (Table 2) were designed using Primer Premier 6.0 software (Premier, Canda).
Table 2.
Gene sequences used for PCR amplification
| Gene | GenBank accession no. | Forward primer 5’-3’ | Reverse primer 5ʹ-3ʹ | Size (bp) |
|---|---|---|---|---|
| IL1 | NM_174092.1 | 5ʹ- GCCAGTCATCTCATTGTTGCTA-3ʹ | 5ʹ- TGCCACCATCACCACATTCT-3ʹ | 271 |
| IL2 | NM_174092.2.2 | 5ʹ-ACTCACAGTAACCTCAACTCCT-3ʹ | 5ʹ-AGCAGCAATGACTTCACTTCTT-3ʹ | 144 |
| IL-6 | XM_015468553.1 | 5ʹ-TGCTGGTCTTCTGGAGTATCA-3ʹ | 5ʹ-TTGTGGCTGGAGTGGTTATTAG-3ʹ | 155 |
| IL12 | XM_015472660.1 | 5ʹ-TCTATGAGGACTTGAAGATGTACCA-3ʹ | 5ʹ-CTGAAGGCGTGAAGAAGGATG-3ʹ | 229 |
| Bak | XM_015459725.1 | 5ʹ-TACCGCCATCAGCAGGAAC-3ʹ | 5ʹ-TGGAACTCCGCATCGTAGC-3ʹ | 161 |
| Mcl-1 | NM_001099206.1 | 5ʹ-GCCGCCTGAAGAGATGGAA-3ʹ | 5ʹ-CCGAGCCTGGACTGTTGTT-3ʹ | 155 |
| Bax | XM_015458140.1 | 5ʹ-TTGCT TCAGGG TTTCATCC-3ʹ | 5ʹ-GACACTCGCTCAGCTTCTTG-3ʹ | 112 |
| Bcl-2 | NM_001166486.1 | 5ʹ-TTCTCCTGGCTGTCTCTGAA-3ʹ | 5ʹ-TCTGCTGCTTCTTGAATCTTCT-3ʹ | 139 |
| Bcl-xl | XM_015474117.1 | 5ʹ- TGTGGCTGGTGTGGTTCTG-3ʹ | 5ʹ- GTTCTCCTGGTGGCAATGGT-3ʹ | 133 |
| P53 | XM_010815982.2 | 5ʹ-AGCACTGCCTACCAACAC-3ʹ | 5ʹ-TCGGAACATCTCATAGCG-3ʹ | 109 |
| GAPDH | NM_001034034.2 | 5ʹ-CACTGTCCACGCCATCACT-3ʹ | 5ʹ-GCCTGCTTCACCACCTTCT-3ʹ | 267 |
Statistical Analysis
The data were analyzed as repeated measures using the PROC MIXED procedure of SAS 9.2 (SAS Institute, 2009), and the autoregressive covariance structure was selected on the basis of Akaike’s information criterion. The repeated measures analysis was performed to determine the effects on milk performance and immune function with dose, period, and the interaction on milk performance and immune function with dose, period and the interaction of dose × period as the fixed effects, and cow as random effect. Contrast analysis was used to determine treatment effects. The results are presented as the means and their corresponding standard errors. Significance effects were declared at P ≤ 0.05, and tendencies were declared at 0.05 < P ≤ 0.10.
RESULTS
Milk Performance
The average daily THI, AT, and RH in the cowshed was 74.5 °C, 25.2 °C, and 77.5% during the 42-d experimental period, respectively (Figure 1). Depending on the time of day, the THI ranged from 69.4 to 79.0, and the HS period was 16 h per day, ranging from 0730 to 2330 when THI was over 72.
Figure 1.
Average ambient temperature (AT), relative humidity (RH), and temperature-humidity index (THI) pattern at a different time of day in the cowshed during 42-d experimental period.
The milk production and milk composition are shown in Table 3. The fermented CHMs mixture resulted in an increase in milk yield (P = 0.03) and 4% FCM (P = 0.05) during the 42-d HS period, which peaked at 100 g/d CHMs. Compared with 0 g/d CHMs, the milk yield exhibited an increase (P < 0.05) when cows were offered 50 or 100 g/d CHMs, and an increased tendency (P = 0.09) was also found in the 25 g/d group. The 4% FCM in the 100 g/d group was increased (P = 0.02) compared with the 0 g/d group. Additionally, the milk protein and fat contents were increased (P < 0.01) with greater doses of CHMs. A difference (P = 0.02) between the 100 and the 0 g/d group for milk protein. There was also a difference (P < 0.01) between the 50 or 100 g/d and the 0 g/d group for milk fat. In contrast, no changes were observed for milk lactose content among all groups during the 42-d period, and no dose × period interaction was found for milk yield and composition.
Table 3.
Milk production and composition for dairy cows offered fermented Chinese herbal medicine (CHMs)
| D 14, CHMs dose, g/d | D 28, CHMs dose, g/d | D 42, CHMs dose, g/d | P-value | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | 0 | 25 | 50 | 100 | SEM | 0 | 25 | 50 | 100 | SEM | 0 | 25 | 50 | 100 | SEM | 2D | 3P | 4D×P |
| Milk yield, kg/d | ||||||||||||||||||
| Milk | 16.3 | 16.0 | 16.9 | 16.6 | 0.21 | 15.9 | 16.7 | 17.6 | 18.0 | 0.26 | 15.1 | 16.9 | 16.8 | 17.3 | 0.27 | 0.03 | 0.08 | 0.29 |
| 4% FCM1 | 14.3 | 14.0 | 15.1 | 15.3 | 0.40 | 14.4 | 14.3 | 14.9 | 15.4 | 0.24 | 13.7 | 14.4 | 15.2 | 15.2 | 0.19 | 0.05 | 0.99 | 0.99 |
| Milk composition, % | ||||||||||||||||||
| Protein | 3.46 | 3.46 | 3.55 | 3.63 | 0.04 | 3.43 | 3.42 | 3.60 | 3.66 | 0.03 | 3.44 | 3.49 | 3.60 | 3.69 | 0.04 | 0.05 | 0.72 | 0.98 |
| Fat | 2.89 | 2.80 | 2.86 | 2.82 | 0.06 | 2.90 | 3.09 | 3.37 | 3.49 | 0.05 | 2.84 | 3.12 | 3.38 | 3.34 | 0.08 | <0.01 | <0.01 | 0.24 |
| Lactose | 5.14 | 5.16 | 5.19 | 5.22 | 0.06 | 5.14 | 5.01 | 5.23 | 5.05 | 0.06 | 4.92 | 4.91 | 5.11 | 5.10 | 0.05 | 0.38 | 0.09 | 0.95 |
14% fat-corrected milk.
2D = dose effect: 0, 25, 50, or 100 g CHMs supplementation per cow per day.
3P = period effect: experimental period for 14, 28, and 42 d.
4D × P = interaction between dose and period.
Leukocyte Count, Lymphocyte Count, and Apoptosis
The leukocyte and lymphocyte counts in the peripheral blood are shown in Figure 2. The lymphocyte count was increased (P < 0.01) during the 42-d experiment when cows were fed with greater doses of CHMs. The count in the 50 or 100 g/d group was greater (P < 0.05) than that in the 0 g/d group on days 28 and 42 of the experiment. The granulocyte or intermediate cell count was not affected (P > 0.05) by the increased CHMs doses. Besides, the leukocyte count (sum of lymphocyte, granulocyte, and intermediate) was greater (P < 0.01) with increasing CHMs dose during the entire experimental period.
Figure 2.
Leukocyte and lymphocyte counts in peripheral blood for dairy cows offered fermented Chinese herbal medicine (CHMs). LYM = lymphocyte; MID = intermediate cell; GRA = granulocyte; Leukocyte count is the sum of LYM, GRA, and MID.
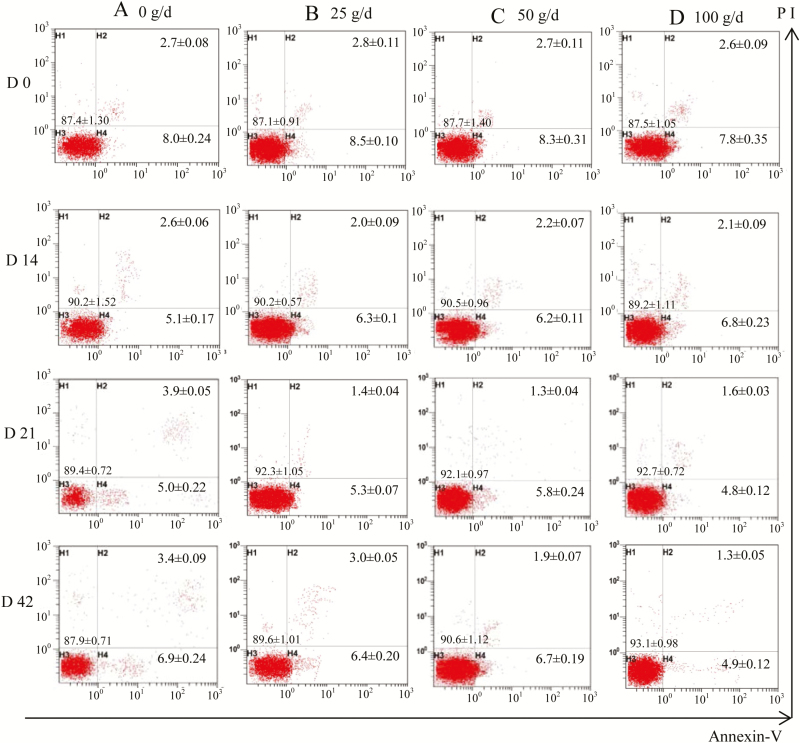
The effect of CHMs on lymphocyte apoptosis was evaluated and the results are shown in Figure 3. The percentage of early apoptosis (Annexin V+ PI−) cells was lowered (P = 0.02) by raising the CHMs dose throughout the experimental period. Particularly on day 42, the early apoptosis cells decreased (P < 0.05) in the 100 g/d group compared with the 0, 25, or 50 g/d group, and the early apoptosis rate decreased by 8%, 4%, and 29% in the 25, 50, and 100 g/d group, respectively, compared with the 0 g/d group. Similarly to the results of early apoptosis, the percentage of late apoptosis/necrotic (Annexin V+ PI+) cells was also decreased (P < 0.01) by CHMs supplements. An interaction between dose and period was observed because the apoptosis rate was not different (P > 0.10) among the 25, 50, and 100 g/d groups on days 14 and 28, whereas the apoptosis rate in the 100 or 50 g/d group was lowered (P < 0.01) than that in the 25 g/d group on days 42.
Figure 3.
Lymphocyte apoptosis assessed by flow cytometry for dairy cows offered fermented Chinese herbal medicine (CHMs). The values mentioned in each quadrant represent the percentage of cells. The cell populations discriminated in individual quadrant are viable cells (PI and Annexin-V negative cells) in the lower left quadrant, early apoptotic cells (Annexin-V positive and PI negative cells) in the lower right quadrant, late apoptotic/necrotic cells (PI and Annexin-V positive cells) in the upper right quadrant. (A), (B), (C), and (D) represent lymphocyte apoptosis rate on day 0 (before experiment), days 14, 28, and 42 of the experiment in 0, 25, 50, and 100 g/d CHMs groups.
Biochemistry Parameters
The serum concentrations of cytokines (IL-1, IL-2, Il-6, and IL-12), apoptosis-associated parameters (Bak, Mcl-1, Bax, Bcl-2, Bcl-xl, and P53), and immunoglobulins (IgA, IgG, and IgM) are listed in Table 4. The concentration levels of IL-2 and IL-6 were increased (P < 0.05) by CHMs supplements, peaked in the 100 g/d group. However, there was no difference (P > 0.10) between the 0 and 25 g/d groups during the entire experimental period. On the contrary, the IL-1 level tended to decrease (P = 0.08) with a greater CHMs dose, and the IL-12 level was not affected (P = 0.48) by CHMs supplements. For the apoptosis-related parameters, the CHMs supplement of 100 g/d increased Bcl-2 (P = 0.01) level and decreased Bax level (P < 0.01) throughout the experimental period, and the Bax level demonstrated a declined tendency (P = 0.06) with the supplement of 50 g/d, compared with the 0 g/d supplement. The Bax and Bcl-2 levels in cows feeding 25 g/d CHMs had no difference (P > 0.05) comparing with those feeding 0-g/d CHMs. Other apoptosis-related parameters (Bak, Mcl-1, Bcl-xl, and P53) were not affected (P > 0.10) by increasing doses of CHMs. For the immunoglobulins, the IgG level was greater (P < 0.01) for the 50 or 100 g/d group when compared with the 0 or 25 g/d group during the 42-d period. Additionally, no interaction (P > 0.01) between CHMs dose and experimental period was found for all biochemistry parameters.
Table 4.
Serum biochemistry parameters associated immune function for dairy cows offered fermented Chinese herbal medicine (CHMs)
| IL-1 | IL-2 | IL-6 | IL-12 | IgA | IgG | IgM | Bak | Mcl-1 | Bax | Bcl-2 | Bcl-xl | P53 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | (pg/mL) | (pg/mL) | (pg/mL) | (pg/mL) | (µg/mL) | (µg/mL) | (µg/mL) | (ng/mL) | (pg/mL) | (ng/mL) | (ng/mL) | (ng/mL) | (pg/mL) | |
| D 0 CHMs dose, g/d |
0 | 66.6 | 853.4 | 151.9 | 546.7 | 318.1 | 278.7 | 126.6 | 10.27 | 1181.3 | 4.46 | 81.7 | 10.04 | 536.3 |
| 25 | 69.3 | 846.3 | 162.0 | 556.4 | 309.5 | 285.1 | 133.6 | 10.91 | 1199.5 | 4.44 | 75.5 | 9.83 | 546.2 | |
| 50 | 74.6 | 840.1 | 145.5 | 540.9 | 311.9 | 280.6 | 132.0 | 10.70 | 1152.1 | 4.53 | 85.5 | 10.90 | 568.1 | |
| 100 | 63.8 | 839.31 | 156.5 | 557.4 | 315.5 | 282.6 | 130.0 | 10.68 | 1188.2 | 4.38 | 76.4 | 10.75 | 529.6 | |
| SEM | 2.76 | 6.61 | 4.56 | 13.56 | 4.42 | 7.94 | 4.20 | 0.42 | 65.77 | 0.19 | 2.86 | 0.47 | 31.57 | |
| D 14 CHMs dose, g/d |
0 | 67.6 | 906.3 | 152.6 | 526.7 | 311.0 | 283.6 | 126.3 | 12.22 | 1344.1 | 4.81 | 73.4 | 9.95 | 534.1 |
| 25 | 53.6 | 882.9 | 137.5 | 521.6 | 311.5 | 285.0 | 131.6 | 13.29 | 1190.5 | 4.83 | 80.2 | 10.29 | 535.2 | |
| 50 | 52.1 | 914.7 | 140.0 | 536.3 | 323.2 | 293.5 | 124.4 | 13.32 | 1346.3 | 4.87 | 80.1 | 9.13 | 613.2 | |
| 100 | 52.0 | 953.0 | 160.7 | 529.5 | 317.0 | 292.9 | 124.1 | 11.58 | 1062.9 | 3.54 | 86.1 | 11.58 | 563.7 | |
| SEM | 4.07 | 8.15 | 5.19 | 17.79 | 5.06 | 7.54 | 7.02 | 0.45 | 68.03 | 0.21 | 2.43 | 0.36 | 28.79 | |
| D 28 CHMs dose, g/d |
0 | 58.7 | 950.5 | 150.1 | 535.9 | 302.2 | 278.0 | 127.7 | 10.49 | 1387.0 | 4.95 | 73.2 | 10.57 | 523.2 |
| 25 | 49.5 | 985.7 | 138.6 | 541.3 | 301.7 | 295.4 | 135.0 | 11.85 | 1423.1 | 3.97 | 79.5 | 10.29 | 467.0 | |
| 50 | 48.9 | 1066.0 | 139.4 | 537.0 | 325.3 | 318.6 | 128.4 | 10.87 | 1398.3 | 3.00 | 78.4 | 11.36 | 515.3 | |
| 100 | 53.9 | 1014.9 | 151.0 | 542.9 | 310.1 | 317.7 | 118.1 | 10.23 | 1123.3 | 2.90 | 94.8 | 10.99 | 611.0 | |
| SEM | 2.92 | 18.50 | 4.15 | 11.33 | 7.26 | 7.75 | 2.45 | 0.50 | 59.94 | 0.22 | 2.11 | 0.39 | 36.72 | |
| D 42 CHMs dose, g/d |
0 | 65.7 | 1016.4 | 146.2 | 496.0 | 305.4 | 259.9 | 119.0 | 10.56 | 1272.3 | 4.52 | 77.6 | 9.17 | 558.0 |
| 25 | 47.6 | 1035.5 | 142.3 | 521.0 | 303.6 | 269.7 | 124.3 | 10.24 | 1301.8 | 3.70 | 85.7 | 10.98 | 357.9 | |
| 50 | 47.6 | 1145.3 | 150.1 | 550.0 | 311.6 | 323.8 | 132.6 | 9.79 | 1612.6 | 3.32 | 93.1 | 12.24 | 458.2 | |
| 100 | 41.1 | 1149.8 | 197.5 | 562.7 | 311.7 | 338.1 | 138.1 | 8.96 | 1467.1 | 2.76 | 106.3 | 12.22 | 320.3 | |
| SEM | 3.42 | 14.96 | 5.09 | 12.24 | 9.74 | 8.96 | 5.72 | 0.24 | 38.15 | 0.25 | 2.28 | 0.46 | 23.78 | |
| P-value | 1D | 0.08 | 0.02 | 0.01 | 0.48 | 0.71 | 0.01 | 0.84 | 0.17 | 0.31 | 0.03 | 0.05 | 0.25 | 0.29 |
| 2P | 0.04 | <0.01 | 0.21 | 0.75 | 0.89 | 0.36 | 0.95 | <0.01 | 0.05 | <0.01 | <0.01 | 0.38 | 0.03 | |
| 3D×P | 0.60 | 0.08 | 0.21 | 0.98 | 0.98 | 0.46 | 0.94 | 0.96 | 0.43 | 0.10 | 0.06 | 0.45 | 0.44 |
1D = dose effect: 0, 25, 50, or 100 g CHMs supplement per cow per day.
2P = period effect: experimental period for 14, 28, and 42 d.
3D × P = interaction between dose and period.
Gene Expression
The effects of CHMs supplements on the immunity-related genes in blood lymphocyte were evaluated by measuring the transcriptional expression of a panel of genes associated with cytokine production (IL-1, IL-2, Il-6, and IL-12) and lymphocyte apoptosis (Bak, Mcl-1, Bax, Bcl-2, Bcl-xl, and P53). The results are shown in Figure 4. Among the tested genes, the IL-1 expression was affected (P = 0.05) by CHMs dose during the 42-d period. It was decreased by 11% (P = 0.02) and 24% (P < 0.01) on days 28 and 42 in the 100 g/d group, respectively, compared with the 0 g/d group. In contrast, the IL-2 expression in the 100 g/d group was greater than the 0, 25, or 50 g/d groups (P < 0.05) and a dose × period interaction was found (P = 0.01). The IL-2 demonstrated the greatest expression in the 100 g/d group on days 28 and greater expression was observed in the 50 or 100 g/d group than that in the 0 or 25 g/d group on days 42.
Figure 4.
Immunity-related genes expression for dairy cows offered fermented Chinese herbal medicine (CHMs). In each period subset, values with different lowercase letters differ significantly (P < 0.05) among 4 groups for 0, 25, 50, and 100 g/d supplements of CHMs.
For apoptosis-related genes, CHMs demonstrated effects on the mRNA expression of Bax (P = 0.04) and Bak genes (P = 0.05) during the entire experimental period. There was a decrease of Bax expression (P < 0.01) in the 50 g/d group, compared with the 0 g/d group. At the end of the experiment (on days 42), all the supplemented groups (25, 50, and 100 g/d) demonstrated the decrease of Bax expression (all P < 0.05) by 27%, 33%, and 24%, respectively, compared with the 0 g/d group. For Bak gene, the mRNA expression in the 50 or 100 g/d group was lower (P < 0.05) than that in the 0 or 25 g/d group. On the contrary, CHMs dose increased the expression of Bcl-2 and Bcl-xl genes during the 42-d experiment (P < 0.05). An interaction (P < 0.05) between dose and period was observed for Bcl-2 expression, exhibiting the expression in the 25 g/d group peaked among all groups (P < 0.05) from the 0 or 50 g/d group on days 28, whereas the expression was greatest in the 100 g/d group on days 42, increasing by 50%, 19%, and 46%, compared with the 0, 25, and 50 g/d groups, respectively. Also, the Bcl-xl expression in the 25, 50, or 100 g/d group was greater (P < 0.05) than that in the 0 g/d group during the 42-d experiment. Additionally, there was no difference (P > 0.10) among all groups for the mRNA expression of P53 and Mcl-1 genes during the entire experimental period.
DISCUSSION
HS reduces animal productivity worldwide. Despite significant efforts in management strategies, ventilation design of cowshed and modification of cooling facilities, HS remains an incredibly costly issue for the dairy industry (West, 2003; Shwartz et al., 2009). In the present study, Holstein cows were exposed to an environmental heat load above the thermal comfort zone with an average temperature of 25.2 °C and THI over 72 for 16 h each day. Early study suggested that Holstein cows, due to its genetic and physiological property, are sensitive to HS conditions when the ambient THI is over 72 and AT is higher than 25 °C (Rhoads et al., 2009). The purpose of this work was to investigate the influence of supplements with fermented CHMs mixture on milk performance and immune function in dairy cows to mitigate HS. Our recent data suggest that the CHMs supplements influenced milk yield and milk quality in late-lactation cows, as well as immunity-related parameters, such as transcriptional expression of genes, serum biochemistry, and lymphocyte apoptosis. When the CHMs supplements ranged from 25 to 100 g/d per cow, the milk yield increased compared with the group without CHMs supplements; however, the effect for the addition of 50 g/d on milk yield was similar to that for 100 g/d throughout the experimental period. This is in agreement with Hu et al. (2015), who showed that the milk yield increased by 20%, 24%, and 17%, when heat-stressed cows were fed fermented CHMs mixture for 50, 100, and 200 g/d per cow, respectively. Previous studies in pigs and prawns have also showed that CHMs supplements in diet significantly enhanced growth performance of pigs and prawns under high temperature stress (Windisch et al., 2008; Liu et al., 2010; Song et al., 2010; Dong et al., 2012). However, some inconsistent observations were reported, suggesting dietary CHMs supplements had no effect on animal performance (Botsoglou et al., 2002; Hwang et al., 2017). These different responses to CHMs in different studies might be due to herbal species and their complexity of bioactive components in herbs. In our prescription, R. astragali, as one of the major components, is a popular health-promoting herb in oriental medicines for thousands of years (Fu et al., 2014). Astragali polysaccharides are the main ingredient of R. astragali and have been used as immune enhancers and display antibacterial, antiviral, and antiparasitic activities (Zhang et al., 2014; Adesso et al., 2018). Also, the 2 herbs of F. crataegi and C. platycladi are another important components in this prescription. Previous studies suggested that flavonoids extracted from the 2 herbs are likely responsible for the wide health benefits, such as immunoregulatory, anti-oxidative, and anti-inflammatory activities (Lu et al., 2006; Wen et al., 2017). Despite the favorable bioactivities of the single herbs, the synergic effect may occur if 2 or more herbal medicines act on different pharmacological targets, and the coordinate effect has been estimated to raise by 50 to 2,000 folds compared with equivalent concentrations of single herb (Burns et al., 2010; Jantan et al., 2015). In recent years, the preparation and development of herbal formula from numerous conventional or complementary and alternative herbs are gaining interest because it is difficult to attain a single herb with high target selectivity and potency. In our experiment, the milk fat and protein contents for cows fed 50 or 100 g/d CHMs also increased when compared with unsupplemented CHMs, but no significant difference was found between 50 and 100 g/d CHMs. These results suggest that the CHMs supplement of 50 g/d per cow was likely financially advantageous for milk performance in heat-stressed cows during a 42-d period.
Several published studies have demonstrated the effectiveness of CHMs in boosting immune system of livestock (Mao et al., 2005; Kim et al., 2008; Manzanilla et al., 2009; Liu et al., 2017). Particularly, the effect of CHMs on immune cells and cytokine secretion is garnering increasing attention. In our study, the leukocyte or lymphocyte counts in peripheral blood were significantly increased when the fermented CHMs were supplemented to late-lactation cows for 50 or 100 g/d per cow under high THI conditions where lowered numbers of leukocytes was reported by early studies (Zecchini et al., 2003; Zhang et al., 2014). The increase in leukocyte and lymphocyte counts observed in this study might lead to an improvement of immunomodulatory action including cellular and humoral immune. Similar results were obtained by Lien et al. (2007), who reported that traditional CHMs Bazhen increased blood leukocyte count in piglets and promoted antibody IgG production. It might be explained that the increased level of cytokine (e.g., IL-6) stimulated B cell proliferation and differentiation leading to enhance humoral immune function (Ma et al., 2013). Similarly, the significant increase of IgG level induced by CHMs was also found in an early study of pigs (Yan et al., 2012) and in our current study on dairy cows, suggesting that when the CHMs supplement level was 50 and 100 g/d per cow, the IgG level increased by 25% and 30%, respectively, at the end of experiment (on day 42) compared with unsupplemented group. On the other hand, cytokine, a soluble extracellular protein or glycoprotein, is mainly produced from leukocytes (often lymphocyte) and possesses multiple targets and multiple functions such as modulating the development, differentiation, activation and function of immune cells, and engaging in innate and adaptive host defenses against inflammation. Recent studies have indicated that some CHMs influenced immune responses through modulation of mRNA transcript and protein expression of cytokines such as IL-2, IL-4, IL-6, IL-10, and IL-12 (Spelman et al., 2006; Ma et al., 2013; Okoye et al., 2016). Results of our study suggest that the CHMs supplements increased the concentration of both type-1 cytokine IL-2 and type-2 cytokine IL-6 in serum for late-lactation cows. Particularly for IL-2, the serum protein level as well as mRNA expression level in peripheral lymphocytes was significantly increased on days 42 of the experiment when cows were fed 50 or 100 g/d CHMs, compared with un-supplemented group. Generally, type-1 cytokines predominantly defend against intracellular pathogens and inflammatory diseases (Lucey et al., 1996). Our data are in agreement with previous results by Hsu et al. (2015), Burns et al. (2010), and Sun et al. (2016), who reported that CHMs or formulas stimulated IL-2 and IL-6 production. On the contrary, the mRNA expression in peripheral lymphocyte and protein levels of IL-1 in serum in this study were inhibited by CHMs, which agrees with previous literature from Trivedi et al. (2017) when they reported a reduction for IL-1β expression in mouse splenocytes exposed to herbal formula in vitro. This inhibitory response of CHMs on proinflammatory cytokine IL-1 might play an important role in mediating immunity-related diseases as a complementary and alternate medicine.
We also investigated the effects of CHMs on lymphocyte apoptosis involving apoptosis and the mRNA expression of apoptosis-associated genes. The lymphocyte apoptosis, a process programmed by cell genome, plays an important role in maintaining normal immune function. However, excess apoptosis can lead to many chronic infectious diseases, which has been widely discussed (Erskine et al., 2011; Verçosa et al., 2012). Some external stimuli, such as heat shock, cold stimulation, or γ-irradiation, may trigger cell apoptosis and contribute to the failure of cellular immunity (Moulin et al., 2006; Takahashi et al., 2012; Chen and Chen, 2017). Analysis of our data in this case indicates that CHMs may regulate the HS-induced apoptosis, suggesting that early- or late-stage apoptosis rate of peripheral lymphocytes reduced when increasing doses of CHMs were supplemented to dairy cows exposed to HS condition. Moreover, the expression responses of a panel of apoptosis-associated genes were investigated in this study, showing the transcript expression of both Bcl-2 and Bcl-xl in lymphocytes for CHMs-fed cows was up-regulated by up to 50% and 64%, respectively, relative to the unfed CHMs cows, whereas the expressions of both Bax and Bak genes were down-regulated by up to 33% and 19%, respectively. Early studies indicated that Bcl-2 family, including both anti-apoptotic members (e.g., Bcl-2, Bcl-xl, and Mcl-1) and pro-apoptotic members (e.g., Bax and Bak), is key regulators of the progression of apoptosis implicated in a number of diseases (Huang, 2000). Among these Bcl-2 family members, Bcl-2 (antiapoptotic member) and Bax (proapoptotic member) are considered to be critical for the maintenance of both dynamic balance and integrity of many tissues, which has been demonstrated in the present study that up-regulated Bcl-2 and down-regulated Bax were observed in CHMs-fed cows for gene expression as well as serum concentration. This finding is also consistent with results from Mei et al. (2017), who reported that fermented herbal formula Shuan-Tong-Ling composed of R. astragali, R. paeoniae alba, F. crataegi, and so on, up-regulated the mRNA expression of Bcl-2 and down-regulated the mRNA expression of Bax in brain cells of rats. A recent study involving pigs also evaluated that Bcl-2 and Bax are important regulators of cell apoptosis induced by heat shock (Fan et al., 2017). Although the mechanism that Bcl-2 family regulates cell apoptosis is not now fully understood, it has been suggested that the regulation of apoptosis is mainly linked to mitochondrial dysfunction (Xu et al., 2013; Wang et al., 2017). A previous study (Rogério et al., 2006) has demonstrated that Bcl-2 is located on the outer membrane of mitochondria, whereas Bax is considered to be either on the mitochondrial outer membrane or in the cytosol. Moreover, protein–protein interactions associated with Bcl-2 family play an important role in regulating apoptotic pathway. During the progression of cell apoptosis, Bax-activated translocates to the mitochondria or cytosol and stimulates signaling pathway of apoptosis such as apoptosome formation and caspase activation. Conversely, Bcl-2 inhibits Bax-induced apoptosis by complex mechanisms involving heterodimerization process, membrane stabilization, and channel inhibition. The cell fate depends mainly on the intracellular balance between Bcl-2 and Bax. The ratio of Bcl-2 to Bax would play an important role in apoptosis induction, and increased ratio would promote cell viability while reduced ratio would induce cell apoptosis. Findings from our study exhibit that under HS conditions, the ratio of above 2 genes expression in lymphocytes increased when cows were supplemented greater dose of CHMs, suggesting that the CHMs supplements would protect peripheral lymphocytes against apoptosis upon heat stimulation via some regulating pathway. Additionally, P53, in response to a wide spectrum of stress signals via mitochondrial pathway, can bind to antiapoptotic Bcl-xl protein and directly induce the production of pro-apoptotic Bax protein, resulting in increasing membrane permeabilization of mitochondria and cell apoptosis. However, there was no significant effect in gene expression as well as serum concentration of P53 when CHMs were fed to dairy cows in each time point from days 14 to 56 in our present experiment. Although this inconsistency in P53 response could not be well explained due to complex signaling pathway of the progression of apoptosis, among apoptosis-mediated genes and related proteins in our study, both Bcl-2 and Bax seem to play a predominant role in the molecular mechanism of herbal mixture–suppressing apoptosis. In combination with the cytokines response discussed above, we assume that the alterations of gene and protein levels of cytokines may be an important contributor to the apoptosis biological responses implicated in apoptosis-associated protein and gene expression as well as apoptotic rate. It was worth noting that the effect of CHMs supplements on blood biochemistry and molecular parameters may depend on not only supplement dose, but also supplement duration. A tendency was also observed that the positive response to CHMs supplements was more obvious with prolonging experimental time, for example, a stronger response occurred at the end period of the experiment (days 42) compared with the first period (days 14) when dose of CHMs supplements to cows was equivalent. This can be explained by the physiological response in cows feeding CHMs over 5 wk. The first period of experiment might be an initiating stage on CHMs stimuli, suggesting little effect on milk performance and blood biochemistry; however, with durative supplements of CHMs, the relationships between milk performance and some biochemistry parameters showed the tendency of allometry. Based on these findings, CHM would indeed exert a more beneficial consequence in alleviating HS of dairy cows. However, in vivo study is further needed to determine the molecular pathway for response to herbal mixture to regulate heat stimulation.
CONCLUSIONS
Our data suggest that the fermented CHMs supplements promoted milk production and immune function in late-lactation cows exposed to HS condition. The CHMs supplements of 50 or 100 g/d per cow might be advantageous for both milk yield and milk quality. On the other hand, the alterations implicated in lymphocyte apoptosis, gene expression, and serum biochemistry by CHMs may afford protection against HS in dairy cows; however, the response to CHMs is dependent on CHMs dose and supplement duration. Our present results would contribute to further exploring the molecular pathways of HS regulation in dairy cows via CHMs supplements.
Conflict of interest statement. None declared.
Acknowledgments
The authors thank the funding supported by Dairy Cow Industrial Research Program of Hebei Province (number HBCT2018120202), and from Science and Technology Research and Development Program of Chengde City (number 201701A150). We also acknowledge the funding from Double First-Class Construction Project of Hebei Agricultural University.
LITERATURE CITED
- Adesso S., Russo R., Quaroni A., Autore G., and Marzocco S.. 2018. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-kB activation and Nrf2 response. Int. J. Mol. Sci. 19:800. doi:10.3390/ijms19030800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsoglou N. A., Florou-Paneri P., Christaki E., Fletouris D. J., and Spais A. B.. 2002. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br. Poult. Sci. 43:223–230. doi: 10.1080/00071660120121436 [DOI] [PubMed] [Google Scholar]
- Burns J. J., Zhao L., Taylor E. W., and Spelman K.. 2010. The influence of traditional herbal formulas on cytokine activity. Toxicology 278:140–159. doi: 10.1016/j.tox.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Carroll J. A., Burdick N. C., Chase C. C. Jr, Coleman S. W., and Spiers D. E.. 2012. Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to a provocative immune challenge. Domest. Anim. Endocrinol. 43:146–153. doi: 10.1016/j.domaniend.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Chen L., and Chen H.. 2017. Effect of mahuang gancao ganjiang decoction on fusion and fission of mitochondria and apoptosis of lymphocytes in mice under cold stress. Evid. Based Complemet. Alternat. Med. 2017:5132963. doi:10.1155/2017/5132963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K., Zuhl M. N., and Moseley P. L.. 2016. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. (1985). 120:692–701. doi: 10.1152/japplphysiol.00536.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Zhong Y., Liu F., Yang K., Yu J., and Xu J.. 2012. Regulating effects and mechanisms of Chinese medicine decoction on growth and gut hormone expression in heat stressed pigs. Livest. Sci. 143:77–84. doi:10.1016/j.livsci.2011.08.015 [Google Scholar]
- Erskine R. J., Corl C. M., Gandy J. C., and Sordillo L. M.. 2011. Effect of infection with bovine leukosis virus on lymphocyte proliferation and apoptosis in dairy cattle. Am. J. Vet. Res. 72:1059–1064. doi: 10.2460/ajvr.72.8.1059 [DOI] [PubMed] [Google Scholar]
- Fan X., Xi H., Zhang Z., Liang Y., Li Q., and He J.. 2017. Germ cell apoptosis and expression of bcl-2 and bax in porcine testis under normal and heat stress conditions. Acta Histochem. 119:198–204. doi: 10.1016/j.acthis.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Fu J., Wang Z., Huang L., Zheng S., Wang D., Chen S., Zhang H., and Yang S.. 2014. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 28:1275–1283. doi: 10.1002/ptr.5188 [DOI] [PubMed] [Google Scholar]
- Hashemzadeh-Cigari F., Khorvash M., Ghorbani G. R., Kadivar M., Riasi A., and Zebeli Q.. 2014. Effects of supplementation with a phytobiotics-rich herbal mixture on performance, udder health, and metabolic status of Holstein cows with various levels of milk somatic cell counts. J. Dairy Sci. 97:7487–7497. doi: 10.3168/jds.2014-7989 [DOI] [PubMed] [Google Scholar]
- Hill D. L., and Wall E.. 2017. Weather influences feed intake and feed efficiency in a temperate climate. J. Dairy Sci. 100:1–18. doi:10.3168/jds.2016-11047 [DOI] [PubMed] [Google Scholar]
- Hsu Y. J., Hsu J. H., and Lin K. C.. 2015. Yam storage protein dioscorins modulate cytokine gene expression in BALB/c and C57BL/6 lymphocytes. Food Agric. Immunol. 26:909–923. doi:10.1080/09540105.2015.1048787 [Google Scholar]
- Hu Y. L., Ye S. L., and Luo J. J.. 2015. Effect of Chinese herbal medicine on milk production, anitioxidant capacity and immunity of dairy cows. Acta Pratac. Sin. 24:132–140. [Google Scholar]
- Huang Z. 2000. Bcl-2 family proteins as targets for anticancer drug design. Oncogene 19:6627–6631. doi: 10.1038/sj.onc.1204087 [DOI] [PubMed] [Google Scholar]
- Hwang J. W., Cheong S. H., Kim Y. S., Lee J. W., You B. I., Moon S. H., Jeon B. T., and Park P. J.. 2017. Effects of dietary supplementation of oriental herbal medicine residue and methyl sulfonyl methane on the growth performance and meat quality of ducks. Anim. Prod. Sci. 57:948–957. doi:10.1071/AN15134 [Google Scholar]
- Ingvartsen K. L., and Moyes K.. 2013. Nutrition, immune function and health of dairy cattle. Animal Supple 1:112–122. doi:10.1017/S175173111200170X [DOI] [PubMed] [Google Scholar]
- Jantan I., Ahmad W., and Bukhari S. N.. 2015. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 6:655. doi: 10.3389/fpls.2015.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W., Fan M. Z., and Applegate T. J.. 2008. Nonruminant nutrition symposium on natural phytobiotics for health of young animals and poultry: mechanisms and application. J. Anim. Sci. 86 (14 Suppl):E138–E139. doi: 10.2527/jas.2007-0769 [DOI] [PubMed] [Google Scholar]
- Kim D. G., Lee M. R., Yoo J. M., Park K. I., and Ma J. Y.. 2017. Fermented herbal formula KIOM-MA-128 protects against acute colitis induced by dextran sodium sulfate in mice. BMC Complement. Altern. Med. 17:354. doi: 10.1186/s12906-017-1855-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Bass B. E., Bandrick M., Loving C. L., Brockmeier S. L., Looft T., Trachsel J., Madson D. M., Thomas M., Casey T. A.,. et al. 2017. Fermentation products as feed additives mitigate some ill-effects of heat stress in pigs. J. Anim. Sci. 95:279–290. doi: 10.2527/jas.2016.0662 [DOI] [PubMed] [Google Scholar]
- Lacetera N., Bernabucci U., Scalia D., Basiricò L., Morera P., and Nardone A.. 2006. Heat stress elicits different responses in peripheral blood mononuclear cells from brown Swiss and Holstein cows. J. Dairy Sci. 89:4606–4612. doi: 10.3168/jds.S0022-0302(06)72510-3 [DOI] [PubMed] [Google Scholar]
- Liang X., Jin J., Bi X., Kamruzzaman M., Kudo T., and Sano H.. 2018. Effects of Chinese herbal medicine and cold exposure on plasma glucose, leucine and energy metabolism in sheep. J. Anim. Physiol. Anim. Nutr. 102:e534–e541. doi:10.1111/jpn.12792 [DOI] [PubMed] [Google Scholar]
- Lien T. F Y. M. Horng, and Wu C. P.. 2007. Feasibility of replacing antibiotic feed promoters with the Chinese traditional herbal medicine Bazhen in weaned piglets. Livest. Prod. Sci. 107:97–102. doi:10.1016/j.livsci.2006.09.008 [Google Scholar]
- Liu B., Xie J., Ge X., Xu P., Wang A., He Y., Zhou Q., Pan L., and Chen R.. 2010. Effects of anthraquinone extract from Rheum officinale bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol. 29:49–57. doi: 10.1016/j.fsi.2010.02.018 [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Yin R. Q., Liang S. S., Duan Y. L., Yao J. H., Duan Y. L., and Yang X. J.. 2017. Effect of dietary Lycium barbarum polysaccharide on growth performance and immune function of broilers. J. Appl. Poult. Res. 26:200–208. doi:10.3382/japr/pfw063 [Google Scholar]
- Lu Y. H., Liu Z. Y., Wang Z. T., and Wei D. Z.. 2006. Quality evaluation of Platycladus orientalis (L.) Franco through simultaneous determination of four bioactive flavonoids by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 41:1186–1190. doi: 10.1016/j.jpba.2006.02.054 [DOI] [PubMed] [Google Scholar]
- Lucey D. R., Clerici M., and Shearer G. M.. 1996. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 9:532–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. D., Deng Y. R., Tian Z., and Lian Z. X.. 2013. Traditional Chinese medicine and immune regulation. Clin. Rev. Allergy Immunol. 44:229–241. doi: 10.1007/s12016-012-8332-0 [DOI] [PubMed] [Google Scholar]
- Manzanilla E. G., Pérez J. F., Martín M., Blandón J. C., Baucells F., Kamel C., and Gasa J.. 2009. Dietary protein modifies effect of plant extracts in the intestinal ecosystem of the pig at weaning. J. Anim. Sci. 87:2029–2037. doi: 10.2527/jas.2008-1210 [DOI] [PubMed] [Google Scholar]
- Mao X. F., Piao X. S., Lai C. H., Li D. F., Xing J. J., and Shi B. L.. 2005. Effects of beta-glucan obtained from the Chinese herb Astragalus membranaceus and lipopolysaccharide challenge on performance, immunological, adrenal, and somatotropic responses of weanling pigs. J. Anim. Sci. 83:2775–2782. doi: 10.2527/2005.83122775x [DOI] [PubMed] [Google Scholar]
- McCracken V. L., Xie G., Deaver S. E., Baumgard L. H., Rhoads R. P., and Rhoads M. L.. 2015. Short communication: hepatic progesterone-metabolizing enzymes cytochrome P450 2C and 3A in lactating cows during thermoneutral and heat stress conditions. J. Dairy Sci. 98:3152–3157. doi: 10.3168/jds.2014-8826 [DOI] [PubMed] [Google Scholar]
- Mei Z. G., Tan L. J., Wang J. F., Li X. L., Huang W. F., and Zhou H. J.. 2017. Fermented Chinese formula shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 12:425–432. doi: 10.4103/1673-5374.202946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China. 2004. Feeding standard of dairy cattle. NY/T 34-2004. China Agriculture Press, Beijing. [Google Scholar]
- Moulin M., and Arrigo A. P.. 2006. Long lasting heat shock stimulation of trail-induced apoptosis in transformed T lymphocytes. Exp. Cell Res. 312:1765–1784. doi: 10.1016/j.yexcr.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Naderi N., Ghorbani G. R., Sadeghi-Sefidmazgi A., Nasrollahi S. M., and Beauchemin K. A.. 2016. Shredded beet pulp substituted for corn silage in diets fed to dairy cows under ambient heat stress: feed intake, total-tract digestibility, plasma metabolites, and milk production. j. Dairy Sci. 99:8847–8857. doi: 10.3168/jds.2016-11029 [DOI] [PubMed] [Google Scholar]
- Okoye F. B., Odimegwu D. C., Nworu C. S., Agbo M. O., Esimone C. O., Osadebe P. O., and Proksch P.. 2016. Modulation of intracellular expression of ifnγ and IL-2 in culture of splenic T lymphocytes by some flavonoid glycosides of Alchornea floribunda. Pharm. Biol. 54:1873–1880. doi: 10.3109/13880209.2015.1133659 [DOI] [PubMed] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating holstein cows: I. production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi: 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Rogério F., Jordão H. Jr, Vieira A. S., Maria C. C., Santos de Rezende A. C., Pereira G. A., and Langone F.. 2006. Bax and bcl-2 expression and TUNEL labeling in lumbar enlargement of neonatal rats after sciatic axotomy and melatonin treatment. Brain Res. 1112:80–90. doi: 10.1016/j.brainres.2006.07.021 [DOI] [PubMed] [Google Scholar]
- SAS Institute. 2009. SAS/STAT 9.2 User’s Guide, Second Edition. SAS Inst. Inc., Cary, NC. [Google Scholar]
- Shwartz G., Rhoads M. L., VanBaale M. J., Rhoads R. P., and Baumgard L. H.. 2009. Effects of a supplemental yeast culture on heat-stressed lactating holstein cows. J. Dairy Sci. 92:935–942. doi: 10.3168/jds.2008-1496 [DOI] [PubMed] [Google Scholar]
- Song X. Z., Xu J. Q., Liu F. H., and Wang T.. 2009. Chinese medicine granule affects the absorption and transport of glucose in porcine small intestinal brush border membrane vesicles under heat stress. Asian-Aust. J. Anim. Sci. 26: 246–253. [Google Scholar]
- Song X. Z., Xu J. Q., and Wang T.. 2010. Traditional Chinese medicine decoction enhances growth performance and intestinal glucose absorption in heat stressed pigs by up-regulating the expressions of SGLT1 and GLUT2 mRNA. Livest. Sci. 128:75–81. doi: 10.1016/ j.livsci.2009.11.002 [Google Scholar]
- Spelman K., Burns J., Nichols D., Winters N., Ottersberg S., and Tenborg M.. 2006. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern. Med. Rev. 11:128–150. [PubMed] [Google Scholar]
- State Pharmacopoeia Commission of the PRC 2005. Pharmacopoeia of the People’s Republic of China. People’s Med. Publ. House, Beijing, China. [Google Scholar]
- Sun H., Ni X., Song X., Wen B., Zhou Y., Zou F., Yang M., Peng Z., Zhu H., Zeng Y.,. et al. 2016. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 100:8105–8120. doi: 10.1007/s00253-016-7619-0 [DOI] [PubMed] [Google Scholar]
- Sun H., Ni X., Zeng D., Zou F., Yang M., Peng Z., Zhou Y., Zeng Y., Zhu H., Wang H.,. et al. 2017. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res. Vet. Sci. 110:22–28. doi: 10.1016/j.rvsc.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Takahashi A., Torigoe T., Tamura Y., Kanaseki T., Tsukahara T., Sasaki Y., Kameshima H., Tsuruma T., Hirata K., Tokino T.,. et al. 2012. Heat shock enhances the expression of cytotoxic granule proteins and augments the activities of tumor-associated antigen-specific cytotoxic T lymphocytes. Cell Stress Chaperones 17:757–763. doi: 10.1007/s12192-012-0348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi M. K., Mondal S. C., Gangwar M., and Jana S.. 2017. Effect of a novel ashwagandha-based herbomineral formulation on pro-inflammatory cytokines expression in mouse splenocyte cells: a potential immunomodulator. Pharmacogn. Mag. 13 (Suppl 1):S90–S94. doi: 10.4103/0973-1296.197709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell H. F., and Reid J. T.. 1965. Prediction of the energy value of cow’s milk. J. Dairy Sci. 48:1215–1223. doi: 10.3168/jds.S0022-0302(65)88430-2 [DOI] [PubMed] [Google Scholar]
- Verçosa B. L., Melo M. N., Puerto H. L., Mendonça I. L., and Vasconcelos A. C.. 2012. Apoptosis, inflammatory response and parasite load in skin of Leishmania (leishmania) chagasi naturally infected dogs: a histomorphometric analysis. Vet. Parasitol. 189:162–170. doi: 10.1016/j.vetpar.2012.04.035 [DOI] [PubMed] [Google Scholar]
- Wang H., Tao L., Ni T., Gu H., Jin F., Dai X., Feng J., Ding Y., Xiao W., Guo S.,. et al. 2017. Anticancer efficacy of the ethyl acetate extract from the traditional chinese medicine herb Celastrus orbiculatus against human gastric cancer. J. Ethnopharmacol. 205:147–157. doi: 10.1016/j.jep.2017.04.030 [DOI] [PubMed] [Google Scholar]
- Wang J., and Zhou H.. 2007. Comparison of the effects of Chinese herbs, probiotics and prebiotics with those of antibiotics in diets on the performance of meat ducks. J. Anim. Feed Sci. 16:96–103. doi:10.22358/jafs/66730/2007 [Google Scholar]
- Wen L., Guo R., You L., Abbasi A. M., Li T., Fu X., and Liu R. H.. 2017. Major triterpenoids in Chinese hawthorn “Crataegus pinnatifida” and their effects on cell proliferation and apoptosis induction in MDA-MB-231 cancer cells. Food Chem. Toxicol. 100:149–160. doi: 10.1016/j.fct.2016.12.032 [DOI] [PubMed] [Google Scholar]
- West J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., and Kroismayr A.. 2008. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 86(14 Suppl):E140–E148. doi: 10.2527/jas.2007-0459 [DOI] [PubMed] [Google Scholar]
- Xie Z., Zhang J., Ma S., Huang X., and Huang Y.. 2017. Effect of Chinese herbal medicine treatment on plasma lipid profile and hepatic lipid metabolism in hetian broiler. Poult. Sci. 96:1918–1924. doi: 10.3382/ps/pew456 [DOI] [PubMed] [Google Scholar]
- Xu X. F., Zhang T. L., Jin S., Wang R., Xiao X., Zhang W. D., Wang P. Y., and Wang X. J.. 2013. Ardipusilloside I induces apoptosis by regulating bcl-2 family proteins in human mucoepidermoid carcinoma mc3 cells. BMC Complement. Altern. Med. 13:322. doi: 10.1186/1472-6882-13-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Wang K., Chen L., He Y. M., and Tang Z. X.. 2012. Effects of feeding an herbal preparation to sows on immunological performance of offspring. J. Anim. Sci. 90:3778–3782. doi: 10.2527/jas.2011-4946 [DOI] [PubMed] [Google Scholar]
- Zecchini M., Barbieri S., Boureima K., and Crimella C.. 2003. Heat stress parameters in Azawak cattle (Bos indicus): four seasons of data collection. Ital. J. Anim. Sci. 2:142–144. doi:10.4081/ijas.2003.s1.142 [Google Scholar]
- Zhang C. L., Ren H. J., Liu M. M., Li X. G., Sun D. L., Li N., and Ming L.. 2014. Modulation of intestinal epithelial cell proliferation, migration, and differentiation in vitro by Astragalus polysaccharides. Plos One 9:e106674. doi: 10.1371/journal.pone.0106674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. J., Weng X. G., Wang J. F., Zhou D., Zhang W., Zhai C. C., Hou Y. X., and Zhu Y. H.. 2014. Effects of temperature-humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows. J. Anim. Sci. 92:3026–3034. doi: 10.2527/jas.2013-6932 [DOI] [PubMed] [Google Scholar]