Abstract
Periparturient dairy cows are subjected to altered intracellular reduction–oxidation (redox) balance due to the high metabolic rates and physiological adaptations characteristic of the transition into lactation. Such conditions could alter oxidative stress status. The objective of this study was to investigate the cytoprotective effects of tea polyphenols (TP) in cultured bovine mammary epithelial cells (BMEC) exposed to hydrogen peroxide (H2O2)-induced oxidative stress. To establish oxidative stress, isolated BMEC were exposed to increasing concentrations of H2O2 (0, 100, 200, 400, 600, 800, and 1,000 µM) for 0, 2, 4, 6, 8, 12, and 24 h. Doses of TP (0, 60, 80, and 100 µg/mL) were evaluated by pretreatment of BMEC for 0, 2, 4, 6, 8, 12 and 24 h, followed by an H2O2 (600 µM per culture well) challenge for 6 h. Bovine mammary epithelial cells were preincubated for 30 min with or without 2,4-dinitrochloro-benzene (DNCB), then cultured with or without TP (100 µg/mL) for another 12 h followed by H2O2 (600 µM per culture well) exposure. There were 5 replicate cultures for each treatment in each experiment. Treatment with 600 µM H2O2 per culture well for 6 h induced oxidative damage of BMEC, indicating this system could be used to establish an oxidative stress model. After H2O2 (600 µM per culture well) exposure, a concentration of TP of 100 µg/mL during a 12-h incubation increased cell viability, decreased intracellular reactive oxygen species accumulation, and increased the abundance of nuclear factor-erythroid 2-related factor 2 (NFE2L2). Furthermore, TP upregulated mRNA abundance of genes in the NFE2L2 and mitogen-activated protein kinase (MAPK) pathways of BMEC. The DNCB assay allowed further confirmation that the induction of NFE2L2 and HMOX-1 in response to TP was mediated through the sustained upregulation of the abundance of MAPK3/1 [formerly known as extracellular regulated kinases 1/2] and MAPK11/12/13/14 (formerly known as p38). Overall, results indicate that TP has beneficial effects on BMEC redox balance; it can reduce cellular oxidative stress-related injury and may potentially serve as an antioxidant against oxidative stress in dairy cows.
Keywords: bovine mammary epithelial cells, oxidative stress, tea polyphenols
INTRODUCTION
Oxidative stress (OS) is defined as an imbalance between the formation of oxidative free radicals and the antioxidant defense capacity of cells (Kruk and Duchnik, 2014). Uncontrolled reactive oxygen species (ROS) and reactive nitrogen species production can damage cellular macromolecules, leading to DNA and protein modification and lipid peroxidation [e.g., malondialdehyde (MDA)] (Chang et al., 2008; Chen et al., 2012; Li et al., 2016). Oxidative damage of DNA is considered detrimental, as the replication of damaged DNA can lead to genetic mutations or apoptosis (Wu et al., 2004). These cascades of events create a state of cellular dysfunction and disease (Kruk and Duchnik, 2014).
The transcription factor nuclear factor-erythroid 2-related factor 2 (NFE2L2) regulates multiple antioxidant and detoxifying enzymes and is a key regulator of OS in numerous cell types including bovine mammary epithelial cells (BMEC). In nonruminants, NFE2L2 is primarily regulated by Kelch-like ECH-associated protein 1 (Keap1), a substrate adaptor for a CUL3-containing E3 ubiquitin ligase (Jaramillo and Zhang, 2013). In the absence of OS, NFE2L2 is located in the cytoplasm where it interacts with Keap1 and is rapidly degraded by the ubiquitin–proteasome pathway (Bryan et al., 2013). However, phosphorylation of NFE2L2 under OS leads to its dissociation from Keap1 and subsequent translocation to the nucleus where it binds to the antioxidant response element (ARE) sequence and enhances the transcription of various antioxidant enzymes that can play a key role in BMEC of periparturient dairy cows (Espinosa-Diez et al., 2015).
Phytogenic antioxidants are plant phenolic compounds, and it is estimated that they encompass approximately 8,000 different structures (Działo et al., 2016). Green tea, originally cultivated in China, is considered as one of the most popular beverages in the world (Khan and Mukhtar, 2013). Polyphenols, particularly flavonoids, constitute the primary component of green tea leaves (Khan and Mukhtar, 2013). The antioxidant and ROS-scavenging ability of tea polyphenols (TP), the major hydroxyphenols of green tea leaf extracts, in nonruminants are well known (Ciraj et al., 2001). Besides quenching free radicals, the TP can interrupt lipid peroxidation chain reactions (Takano et al., 2004; Lambert and Elias, 2010).
Phenolic antioxidants can influence ARE-dependent mRNA abundance through the activation of mitogen-activated protein kinase (MAPK) proteins (MAPK3/1, MAPK8/9/10, and MAPK11/12/13/14), probably through the stabilization of NFE2L2 upon its phosphorylation (Yu et al., 1997b). The end result of this cascade of events is the upregulation of phase II enzymes (Yu et al., 1997a). Because of all these benefits of TP and research demonstrating the functionality of the NFE2L2 pathway in BMEC (Jin et al., 2016), the main objective of this study was to determine whether TP have a cytoprotective effect on BMEC during OS and to study the underlying mechanisms whereby TP helps control OS.
MATERIALS AND METHODS
Cell Culture and Treatment
Mammary tissue was obtained from five 4-yr-old healthy lactating multiparous grazing Chinese Holstein cows at a similar stage of lactation (day 200 ± 5 in milk) selected from the Inner Mongolia Academy of Agriculture and Animal Husbandry Sciences. Animals were sacrificed at a local slaughterhouse (Hohhot, China). The midpoint area of left rear side of the udder was clipped and surgically scrubbed. Approximately 150 mg of fresh tissue from each cow was removed and placed in sterilized tubes containing ice-cold Dulbecco’s phosphate buffered saline (Sigma, St Louis, MO) and immediately transported to the laboratory. Animal use was approved by Inner Mongolia Academy of Agriculture and Animal Husbandry Sciences Animal Care and Use Committee. The purification and culture of mammary tissue were described in a previous study from our group (Ma et al., 2018).
To establish OS, isolated BMEC were exposed to increasing concentrations of H2O2 (0, 100, 200, 400, 600, 800, and 1,000 µM) for 0, 2, 4, 6, 8, 12, and 24 h. Doses of TP (0, 60, 80, and 100 µg/mL) were evaluated by pretreatment of BMEC for 0, 2, 4, 6, 8, 12, and 24 h, followed by an H2O2 (600 µM) challenge for 6 h. Bovine mammary epithelial cells were preincubated for 30 min with or without 2,4-dinitrochloro-benzene (DNCB) and then cultured with or without TP (100 µg/mL) for another 12 h followed by H2O2 (600 µM) exposure. There were 5 replicate cultures for each treatment in each experiment.
The basal medium was composed of 95.74 mL DMEM (12400-024, Gibco, Grand Island, NY), 2 mL penicillin streptomycin (Gibco), 0.5 mL 0.5% insulin transferrin solution (Gibco), 100 µL 1 µg/mL hydrocortisone (Gibco), 100 µL 2.5 µg/mL amphotericin B (Gibco), 10 µL 10 ng/mL epidermal growth factor (Gibco), 50 µL 5 µg/mL prolactin (Gibco), and 1.5 mL glutamine (Gibco), which was prepared and kept at 4 °C.
Determination of BMEC Proliferation Rate
The BMEC proliferation rate was performed using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay kit (G3582, Promega, Madison, WI) according to the manufacturer’s instructions. Briefly, 1 × 106/mL BMEC were seeded into 96-well culture plates incubated with the basal medium at 37 °C under 5% CO2 for 24 h. After 24-h incubation, the basal medium was replaced with starvation medium (without fetal bovine serum), and cells were incubated at 37 °C, 5% CO2 for 16 h. After 16-h incubation, the starvation medium was replaced with the treatment medium (basal medium plus different concentration of H2O2 and TP) and incubated at 37 °C under 5% CO2 for 0, 2, 4, 6, 8, 12, and 24 h. Cells were incubated with 20 µL/well of MTS at 37 °C under 5% CO2 for 4 h. Subsequently, 150 µL of dimethyl sulfoxide (Sigma–Aldrich, St. Louis, MO) was added to each well and incubated for 10 min at 37 °C. Last, the absorbance at 490 nm was determined with a microplate reader (Molecular Devices, Sunnyvale, CA).
Detection of Intracellular Antioxidant Enzyme Activity and MDA
To evaluate the potential protective effect of TP against H2O2-induced OS, BMEC were treated as described previously followed by incubation at 37 °C for 6 h with H2O2 (600 µM). The total superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities, the MDA production, 8-hydroxy-2′-deoxyguanosine (8-OHdG), 8-isoprostaglandin (8-iso-PG), and protein carbonyl (PC) content in BMEC were determined using ELISA kits from Nanjing Jiancheng Biotechnology Institute (Nanjing, China) according to the manufacturer’s instructions. In brief, BMEC were washed with PBS and incubated with fresh DMEM (Gibco, Grand Island, NY) at 37 °C for 35 min, and then, 1 × 106 cells were harvested and resuspended in PBS. Absorbance was detected at 560 nm (SOD), 420 nm (GSH-Px), 450 nm (8-OHdG, 8-iso-PG, and PC), and 532 nm (MDA) and was recorded with a microplate reader (Multiskan MS352, Labsystems, Helsinki, Finland).
Intracellular ROS Detection
A dichlorofluorescein (DCF) staining assay was used to detect intracellular ROS. Briefly, BMEC were washed with PBS and incubated with fresh DMEM containing 10 µM DCF at 37 °C for 35 min, and then, 1 × 106 cells were harvested and resuspended in PBS. The optical density at 450 nm was recorded with a microplate reader (Molecular Devices).
RNA Isolation, cDNA Synthesis, and Quantitative PCR
Total RNA was isolated from 50 mg of cells using the miRNeasy kit (Qiagen, Hilden, Germany) following the manufacturer’s protocols. Samples were treated on-column with DNaseI (Qiagen); quantification was performed using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), and RNA quality was measured using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). All samples had an RNA integrity number factor greater than 7.3. The quantitative PCR was performed as described previously (Osorio et al., 2014a) to determine the relative mRNA abundance of NFE2L2, HMOX-1 (heme oxygenase-1), NQO1 (NAD(P)H:quinone oxidoreductase 1), GCLC (glutamate-cysteine ligase catalytic subunit), GCLM (glutamate-cysteine ligase modifier subunit), MAPK3/1, MAPK8/9/10, MAPK11/12/13/14, MAPK7, and TUBB (tubulin beta; internal control). All reactions were run in triplicate. The primers for NFE2L2 and downstream genes, and MAPK genes used for quantitative real-time PCR (RT-PCR) are listed in Table 1. Reverse transcription of total RNA was performed using the PrimeScript RT Master Mix (Takara Bio, Mountain View, CA). The reverse transcription reaction system (20 µL) contained 14 µL ddH2O, 4 µL PrimeScript RT Master Mix, and 2 µL RNA. Quantitative real-time PCR was carried out in a 7,500c RT-PCR detection system (Bio-Rad, Hercules, CA) with SYBR Premix Ex Tap TM II (Takara Bio) in 20 µL reaction volumes: 7.2 µL ddH2O, 0.4 µL 10 µM forward primer, 0.4 µL 10 µM reverse primer, 10 µL SYBR Premix Ex Tap TM II (Takara Bio), and 2 µL cDNA template.
Table 1.
Primers of downstream genes in the nuclear factor, erythroid 2 like 2 (NFE2L2) signaling pathway evaluated in the present study
| Gene name | Forward primer (5′→3′) | Reverse primer (5′→3′) | Accession number |
|---|---|---|---|
| NFE2L2 | CCAGCACAACACATACCA | TAGCCGAAGAAACCTCATT | 001011678.2 |
| HMOX-1 | GAACGCAACAAGGAGAAC | CTGGAGTCGCTGAACATAG | 001014912.1 |
| NQO1 | CAACAGACCAGCCAATCA | ACCTCCCATCCTTTCCTC | 001034535.1 |
| GCLC | ATTGGGTGGAGAGTGGAA | ACAGCGGGATGAGAAAGT | 001083674.1 |
| GCLM | CCGATGAAAGAGAAGAAATG | CAACAGGAGGTGAAGCAA | 001038143.1 |
| MAPK3/1 | CTGCTCTGCCTTTGTCTG | AGTCCCATCTCGTGTCCT | 001110018 |
| MAPK8/9/10 | GCTCTGGCCCACGAGTGGAGA | GCCCTCGATCACCGTGCAGTT | 001244612.1 |
| MAPK11/12/13/14 | TCTCCCTTACTTCCCCTCT | CAAAGCCAACACCATACC | 001142865.1 |
| MAPK7 | CTGAGGCCCACTGTACCCTA | GGCTGTGAGGAGTGGATGAT | 001099080 |
Western Blot Analysis
To obtain total protein lysates, after treatment, cells were washed twice with 4 °C PBS (0.01M, pH 7.2 to 7.3) and were then lysed using a cell lysis buffer containing 250 µL radio-immunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) standing for 5 min at 4 °C. Total protein was extracted from BMEC using a tissue protein extraction reagent (catalog no. 78510; Thermo Scientific, Waltham, MA) containing inhibitor cocktail (100×, catalog no. 78442; Thermo Scientific). Total protein concentration was determined using the BCA Protein Assay Kit (Sigma, St Louis, MO). Samples were diluted and adjusted to equal amounts of cellular proteins and then mixed with Laemmli’s sample buffer prior to boiling at 100 °C for 5 min. Fifty milligram of total protein per lane was resolved by 10% SDS–PAGE (Bio-Rad) and then transferred from the gel to a polyvinylidene difluoride membrane (0.45 µm, Millipore, Billerica, MA). PageRuler Plus Prestained Protein Ladder (Fermentas, Hanover, MD) was used to confirm protein electrophoresis. Membranes were blocked in Tris-buffered saline (TBST; 50 mM Tris, pH 7.6, 150 mM NaCl, and 0.1% Tween 20), which contains 5% (wt/vol) nonfat dry milk for 2 h at room temperature with gentle agitation. The membranes were then incubated in TBST containing antibodies to NFE2L2 (catalog no. ab137550, Abcam, Cambridge, MA), HMOX-1 (catalog no. ab13248, Abcam), NQO1 (catalog no. ab28947, Abcam), GCLC (catalog no. ab80841, Abcam), and GCLM (catalog no. ab81445, Abcam) with gentle agitation at 4 °C overnight. After incubating with primary antibody, the membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (HRP-labeled Sheep anti-mouse, 1:50,000 and HRP-labeled Sheep anti-rabbit, 1:50,000; catalog no. ab6721; Abcam) in TBST for 1 h at room temperature. The membranes were washed and then incubated with ECL reagent (catalog no. 170–5060; Bio-Rad). Tubulin beta class I (catalog no. ab56676; Abcam) was used as internal control. The images were captured using Chemi DOC MP (Bio-Rad). The intensities of the bands were measured with Image-Pro Plus 6.0 software.
Statistical Analysis
In the preliminary experiments, the effects of time (0, 2, 4, 6, 8, 12, and 24 h) and dose of H2O2 (0, 100, 200, 400, 600, 800, and 1,000 µM) and TP (0, 60, 80, and 100 µg/mL) were analyzed using 1-way ANOVA followed by a Student–Newman–Keuls (SNK) multiple comparison with the MIXED procedure of SAS 9.0 (SAS Institute Inc., Cary, NC). The fixed effects in the model were time and dose of H2O2 and TP. For subsequent experiments evaluating the effect of TP (100 µg/mL) and H2O2 (600 µM), we used an ANOVA followed by SNK multiple comparison using SAS 9.0 (SAS Institute Inc.). The fixed effect was treatment. In all cases, the random effect was BMEC. There were 5 replicate cultures for each treatment in each experiment. All data are expressed as means ± SEM for the indicated number of independent experiments performed. Differences were considered significant at P < 0.05.
RESULTS
Screening for H2O2 Optimum Conditions
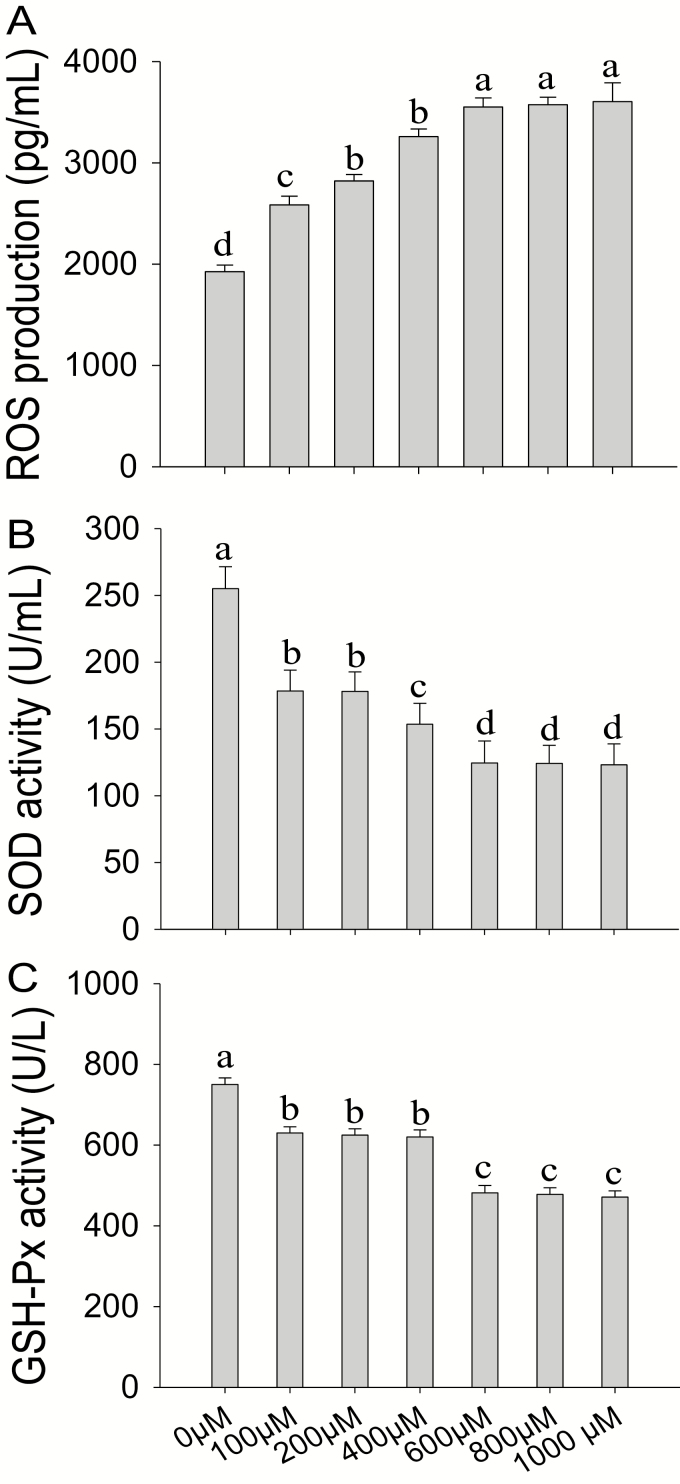
Treatment of BMEC with increasing concentrations of H2O2 (0 to 1,000 µM) for 0 to 24 h elicited a time- and dose-dependent inhibition of cell proliferation rate (Table 2), accompanied with sharp increases of ROS (Fig. 1A) and sharp decreases of SOD (Fig. 1B) and GSH-Px (Fig. 1C). Compared with the control group, the treatment of cells with 600 µM of H2O2 for 6 h decreased cell proliferation to 32% (P < 0.05) and SOD and GSH-PX activities to 50% (P < 0.01) and 36% (P < 0.01), respectively, and increased ROS 1.9-fold (P < 0.01). Thus, 600 µM of H2O2 for 6-h incubation was chosen for subsequent experiments.
Table 2.
Effect of different concentrations and incubation times with hydrogen peroxide (H2O2) on the proliferation rate of bovine mammary epithelial cells (BMEC)1
| H2O2 (µmol/L) | 0 | 100 | 200 | 400 | 600 | 800 | 1,000 | SEM | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | |||||||||
| 0 | 100a2A3 | 96.97aA | 96.20aA | 95.79aA | 85.28abA | 56.93cB | 56.02bB | 5.234 | <0.001 |
| 2 | 100aA | 97.31aA | 97.07aA | 96.57aA | 87.43aA | 58.25cB | 57.09bB | 4.852 | <0.001 |
| 4 | 100aA | 98.13aA | 97.89aA | 97.03aA | 90.99aA | 72.48aB | 69.46aB | 3.323 | <0.001 |
| 6 | 100aA | 95.76aA | 88.34bAB | 86.93bAB | 78.80bcB | 65.37bC | 58.31bC | 3.386 | <0.001 |
| 8 | 100aA | 94.18aA | 88.00bAB | 85.82bAB | 75.27cB | 60.00bcC | 49.46cC | 4.258 | <0.001 |
| 12 | 100aA | 93.55aA | 85.63bAB | 70.09cBC | 59.24dCD | 56.01cD | 38.41dE | 5.001 | <0.001 |
| 24 | 100aAB | 92.43aA | 81.92bAB | 61.13dBC | 48.67eCD | 37.42dCD | 19.81eD | 8.267 | <0.001 |
| SEM | 0 | 2.305 | 2.593 | 1.183 | 2.262 | 2.077 | 1.711 | ||
| P-value | — | 0.5381 | 0.0004 | <0.001 | <0.001 | <0.001 | <0.001 | ||
1BMEC were treated with increasing concentrations of H2O2 (0 to 1,000 µM) for 0 to 24 h. Cell proliferation rate was measured with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). There were 5 replications for each experiment and each well.
2Similar lowercase letters for means in the same column denote lack of significant differences (P > 0.05). Different lowercase letters for means in the same column differ (P < 0.05).
3Similar capital letters for means in the same row denote lack of significant differences (P > 0.05). Different lowercase letters for means in the same row differ (P < 0.05).
Figure 1.
Screening for optimum conditions for in vitro challenge with hydrogen peroxide (H2O2). (A to C) Reactive oxygen species (ROS) production, superoxide dismutase (SOD) activity, and glutathione peroxidase (GSH-Px) activity. Bovine mammary epithelial cells (BMEC) were treated with increasing concentrations of H2O2 (0 to 1,000 µM) for 6 h. ROS production, SOD activity, and GSH-Px activity were measured using commercial kits according to manufacturer’s protocols. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
Screening for Optimum Conditions of TP Incubations
Pretreatment of BMEC with 0 to 120 µg/mL of TP for 0 to 24 h elicited a time- and dose-dependent inhibition of cell proliferation rate (Table 3). Compared with 60 to 100 µg/mL and 12-h incubation, when BMEC were pretreated with 120 µg/mL of TP for 24-h cell proliferation rate was inhibited (P < 0.01). Therefore, 60 to 100 µg/mL and 12-h incubation were considered safe to use for subsequent experiments.
Table 3.
Effect of different concentrations and incubation times with tea polyphenols (TP) on the proliferation rate of bovine mammary epithelial cells (BMEC)1
| Time (h) | |||||||
|---|---|---|---|---|---|---|---|
| TP concentration (µg/mL) | 0 | 6 | 8 | 12 | 24 | SEM | P-value |
| 0 | 100.00a2A3 | 100.00aA | 100.000bA | 100.000bA | 100.000bA | 0 | — |
| 60 | 101.278aD | 103.068aCD | 110.168aBC | 119.384aA | 115.714aAB | 2.834 | 0.0007 |
| 80 | 103.192aB | 106.068aB | 115.048aA | 119.432aA | 117.090aA | 2.458 | 0.0004 |
| 100 | 105.514aB | 107.740aB | 117.264aA | 120.060aA | 119.476aA | 3.046 | 0.0054 |
| 120 | 90.438bA | 92.430aA | 94.106bA | 97.064bA | 95.224bA | 6.102 | 0.9491 |
| SEM | 2.616 | 5.582 | 2.919 | 2.33 | 2.942 | ||
| P-value | 0.0065 | 0.3527 | <0.0001 | <0.0001 | <0.0001 | ||
1BMEC proliferation rate in response to increasing concentrations of TP (0 to 120 µg/mL) without hydrogen peroxide (H2O2). The BMEC were pretreated with 0 to 120 µg/mL of TP for 0 to 24 h prior to analysis of proliferation rate. Proliferation rate was measured with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). There were 5 replications for each experiment and each well.
2Similar lowercase letters for means in the same column denote lack of significant differences (P > 0.05). Different lowercase letters for means in the same column differ (P < 0.05).
3Similar capital letters for means in the same row denote lack of significant differences (P > 0.05). Different lowercase letters for means in the same row differ (P < 0.05).
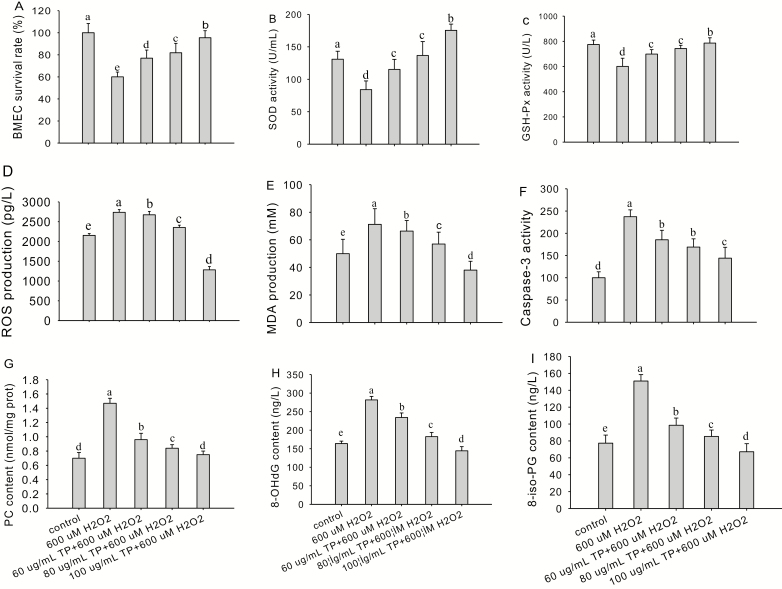
To further determine the optimum concentration of TP to protect the BMEC and assess whether TP has antioxidant effects, we analyzed cell proliferation rate (Fig. 2A), SOD (Fig. 2B) and GSH-Px (Fig. 2C) activities, ROS (Fig. 2D) and MDA (Fig. 2E) production, caspase-3 activity (Fig. 2F), PC (Fig. 2G), 8-OHdG (Fig. 2H), and 8-iso-PG (Fig. 2I) content in oxidative damaged BMEC. The results revealed that compared with the control, the treatment of cells with 600 µM of H2O2 for 6 h sharply decreased the cell proliferation rate (P < 0.01) and SOD and GSH-Px activities (P < 0.01) and strongly enhanced ROS and MDA production (P < 0.01), 8-OHdG, 8-iso-PG, and PC content (P < 0.01), and caspase-3 activity (P < 0.01). However, after pretreatment of cells with 60, 80, and 100 µg/mL of TP for 12 h, followed by H2O2 (600 µM) challenge for 6 h, the cell proliferation rate (P < 0.05) and SOD and GSH-Px activities (P < 0.05) induced by H2O2 increased significantly, whereas ROS and MDA production (P < 0.05); PC, 8-OHdG, and 8-iso-PG content (P < 0.05); and the caspase-3 activity (P < 0.05) induced by H2O2 were decreased markedly.
Figure 2.
Screening for optimum conditions for in vitro culture with tea polyphenol (TP). (A to I) Cell proliferation rate, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities, reactive oxygen species (ROS), malondialdehyde (MDA) production, caspase-3 activity, protein carbonyl (PC), 8-hydroxy-2′-deoxyguanosine (8-OHdG), and 8-isoprostaglandin (8-iso-PG) content in control and TP-supplemented bovine mammary epithelial cells (BMEC; 0 to 100 µg/mL) challenged with hydrogen peroxide (H2O2; 600 µM). The BMEC were pretreated with TP (0 to 100 µg/mL) for 12 h followed by H2O2 (600 µM) challenge for 6 h. The BMEC proliferation rate was measured by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Enzyme activities and concentrations of metabolites were measured using commercial kits according to manufacturer’s protocols. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
The cell proliferation rate increased to a maximum (95.5%) when the concentration of TP increased to 100 µg/mL, which was significantly greater than that in the H2O2-treated group (60.0%; P < 0.01), 60 µg/mL TP group (77.0%; P < 0.01), and 80 µg/mL TP group (81.8%; P < 0.05). Superoxide dismutase and GSH-Px activities increased to a maximum (175.5 U/mL, 785.9 U/L) when the concentration of TP was 100 µg/mL, which was significantly greater than that of the H2O2-treated group (P < 0.01; 84.1 U/mL, 601.0 U/L), 60 µg/mL TP group (P < 0.01; 115.2 U/mL, 698.7 U/L), and 80 µg/mL TP group (P < 0.01; 136.7 U/mL, 742.5 U/L), respectively.
Content of ROS and MDA decreased to a minimum (1,282.3 pg/mL, 38.0 mM) when the concentration of TP increased to 100 µg/mL, which was significantly less than that of the H2O2-treated group (P < 0.01; 2,736.8 pg/mL, 71.2 mM), 60 µg/mL TP group (P < 0.01; 2,672.9 pg/mL, 66.3 mM), and 80 µg/mL TP group (P < 0.01; 2,353.8 pg/mL, 56.9 mM), respectively. The content of PC, 8-OHdG, and 8-iso-PG decreased to a minimum (0.8 nmol/mg, 144.3 ng/L, and 67.2 ng/L) when the concentration of TP increased to 100 µg/mL, which was significantly less than that of the H2O2-treated group (P < 0.01; 1.5 nmol/mg, 281.6 ng/L, 150.9 ng/L), 60 µg/mL TP group (P < 0.01; 1.0 nmol/mg, 234.2 ng/L, 98.6 ng/L), and 80 µg/mL TP group (P < 0.01; 0.8 nmol/mg, 182.6 ng/L, 85.3 ng/L), respectively.
The activity of caspase-3 decreased to a minimum (144.0) when the concentration of TP increased to 100 µg/mL, which was significantly less than that of the H2O2-treated group (P < 0.01; 237.3), 60 µg/mL TP group (P < 0.05; 185.4), and 80 µg/mL TP group (P < 0.05; 169.9). Thus, 100 µg/mL and 12 h of TP were chosen for subsequent experiments to assess the antioxidant effects of TP in BMEC.
Cytoprotective Effects of TP Against OS in BMEC
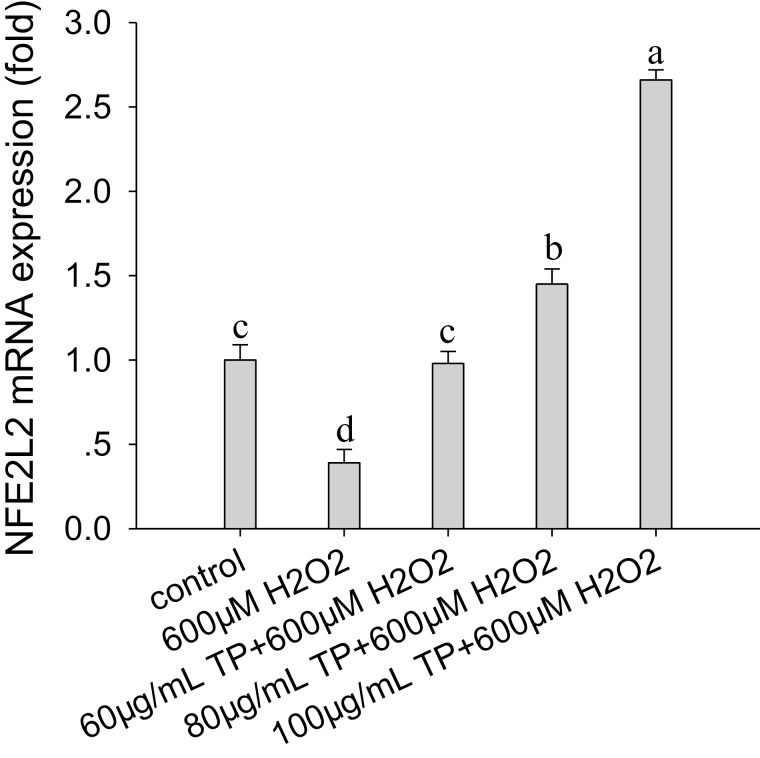
The results indicated that TP significantly upregulated NFE2L2 mRNA abundance in a dose-dependent manner during OS, and NFE2L2 mRNA abundance was greatest under OS when the content of TP was 100 µg/mL. Compared with the control, treatment of cells with 600 µM of H2O2 for 6 h also reduced the mRNA abundance of NFE2L2 by 61% (P < 0.05; Fig. 3).
Figure 3.
Effect of tea polyphenol (TP) on hydrogen peroxide (H2O2)-induced NFE2L2 mRNA abundance in bovine mammary epithelial cells (BMEC). The BMEC were pretreated with TP (0 to 100 µg/mL) for 12 h and then treated with H2O2 (600 µM) for 6 h. Nuclear factor-erythroid 2-related factor 2 (NFE2L2) mRNA abundance was analyzed by quantitative real-time PCR. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
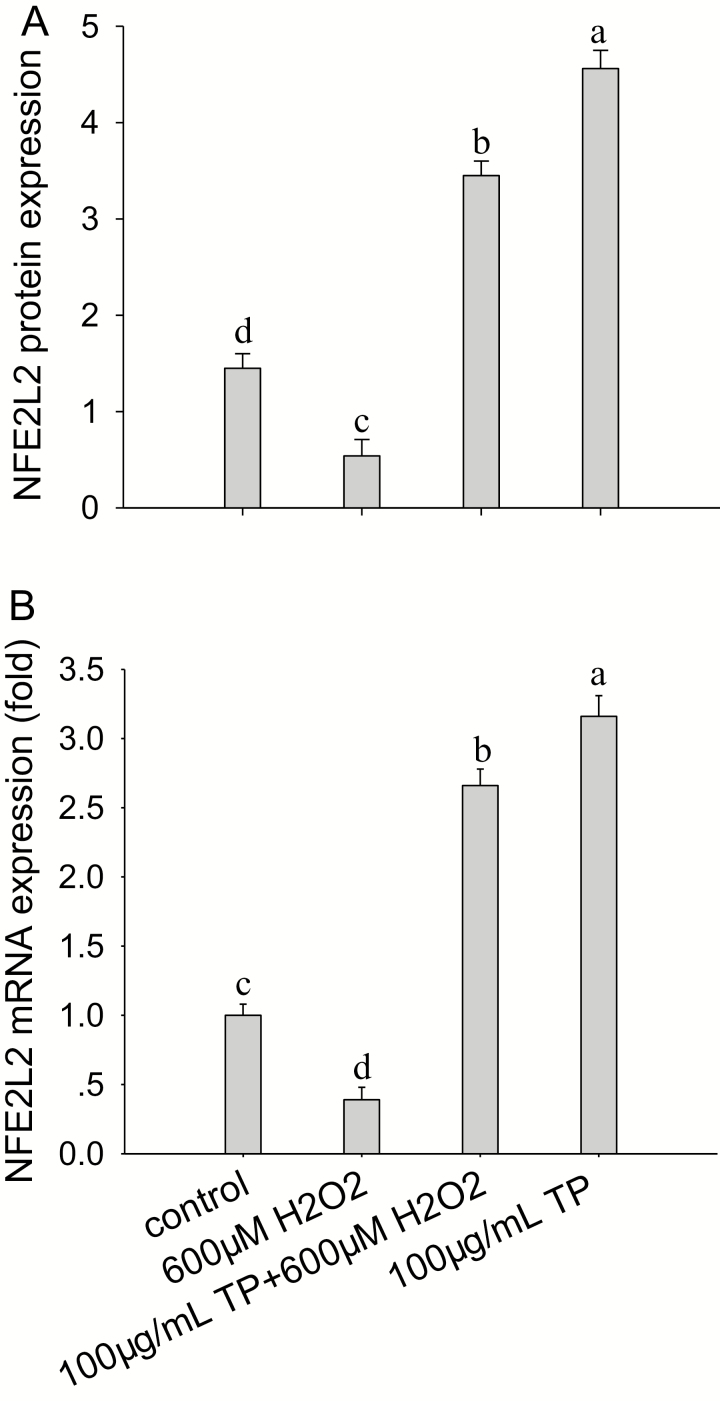
Compared with the control group, after pretreatment of cells with 100 µg/mL of TP for 12 h followed by H2O2 (600 µM) challenge for 6 h, the mRNA abundance of NFE2L2 increased by 2.7-fold (P < 0.01). Without OS, treatment of cells with 100 µg/mL of TP for 12 h led to a 3.2-fold increase in mRNA abundance of NFE2L2 (P < 0.01; Fig. 4A). In addition, after pretreatment of cells with 100 µg/mL of TP for 12 h followed by H2O2 (600 µM) challenge for 6 h, the protein abundance of NFE2L2 was increased compared with the control and H2O2 group (P < 0.05; Fig. 4B). Therefore, TP can increase the protein and mRNA abundance of NFE2L2 in BMEC with or without OS.
Figure 4.
Effect of tea polyphenol (TP) on hydrogen peroxide (H2O2)-induced nuclear factor-erythroid 2-related factor 2 (NFE2L2) protein and mRNA abundance. (A, B) NFE2L2 protein and mRNA abundance in control bovine mammary epithelial cells (BMEC) or BMEC receiving H2O2 and TP with or without H2O2. The BMEC were pretreated with or without 100 µg/mL of TP for 12 h and then treated with or without 600 µM of H2O2 for 6 h. The NFE2L2 protein and mRNA abundance were analyzed by Western blot and quantitative real-time PCR. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
Effects of TP Treatment on MAPK Signaling Pathways With or Without H2O2
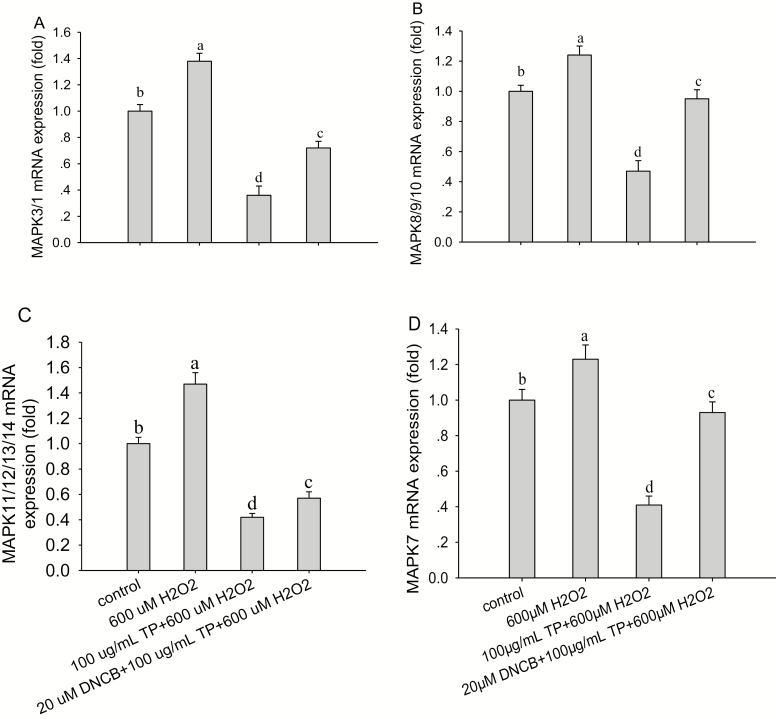
The mRNA abundance of MAPK3/1, MAPK8/9/10, MAPK11/12/13/14, and MAPK7 were all significantly decreased (P < 0.05) after treatment with 600 µM H2O2 for 6 h (Fig. 5) compared with control group. These effects were reversed after the pretreatment of cells with 100 µg/mL of TP for 12 h followed by 600 µM H2O2 for 6 h, causing an increase in mRNA abundance of MAPK3/1 (P < 0.001; Fig. 5A), MAPK8/9/10 (P < 0.01; Fig. 5B), MAPK11/12/13/14 (P < 0.001; Fig. 5C), and MAPK7 (P < 0.01; Fig. 5D) compared with control group. It is noteworthy that the response in mRNA abundance of MAPK3/1, MAPK8/9/10, MAPK11/12/13/14, and MAPK7 was decreased (P < 0.05) after the pretreatment of cells with DNCB (20 µM) for 30 min and then treated with TP (100 µg/mL) for another 12 h, followed by H2O2 (600 µM) exposure for 6 h (P < 0.01) compared with control group. More importantly, the effect of TP on the mRNA abundance of MAPK3/1 and MAPK11/12/13/14 was more pronounced than on abundance of MAPK8/9/10 and MAPK7 (P < 0.01).
Figure 5.
Effect of tea polyphenol (TP) treatment of bovine mammary epithelial cells (BMEC) on mRNA abundance of mitogen-activated protein kinase 3/1 (MAPK3/1), MAPK8/9/10, MAPK11/12/13/14, and MAPK7 with or without hydrogen peroxide (H2O2). (A to D) MAPK3/1, MAPK8/9/10, MAPK11/12/13/14, and MAPK7 mRNA abundance under control and oxidative stress (OS) conditions in BMEC. The BMEC were preincubated with a MAPK-specific inhibitor, 2,4-dinitrochloro-benzene (DNCB), for 30 min and then treated with TP (100 µg/mL) for another 12 h followed by H2O2 (600 µM) exposure for 6 h. The mRNA abundance of MAPK3/1, MAPK8/9/10, MAPK11/12/13/14, and MAPK7 was analyzed by quantitative real-time PCR. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
Effects of TP Treatment on NFE2L2 Signaling Pathways With or Without H2O2
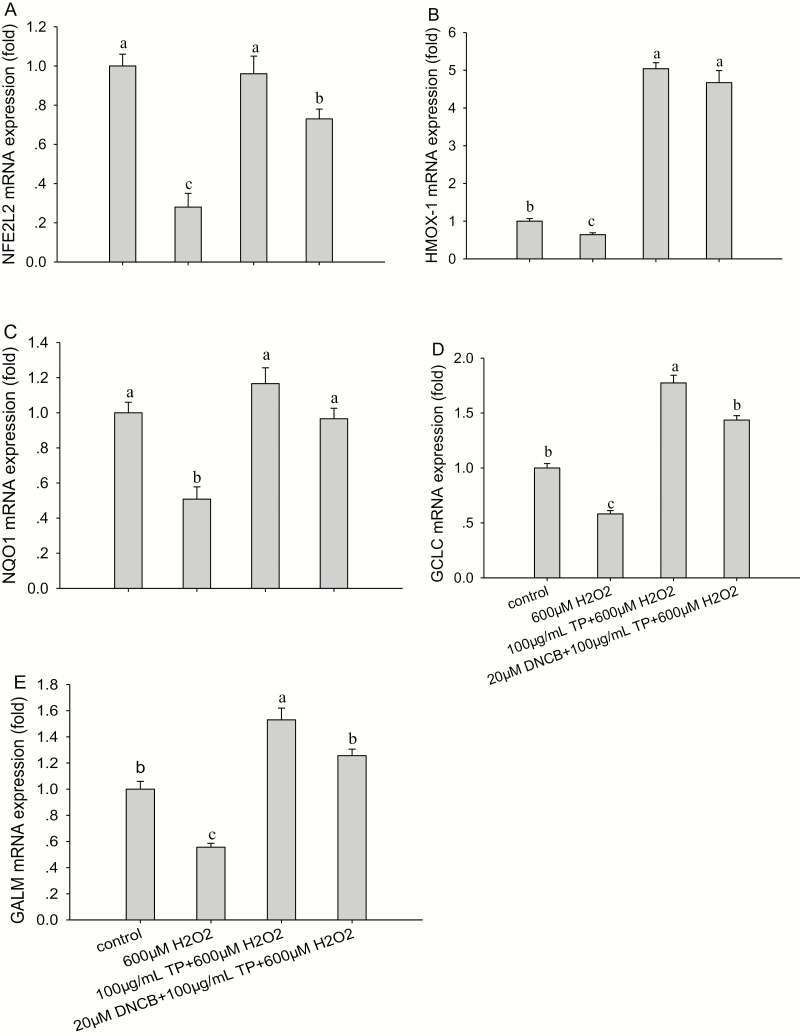
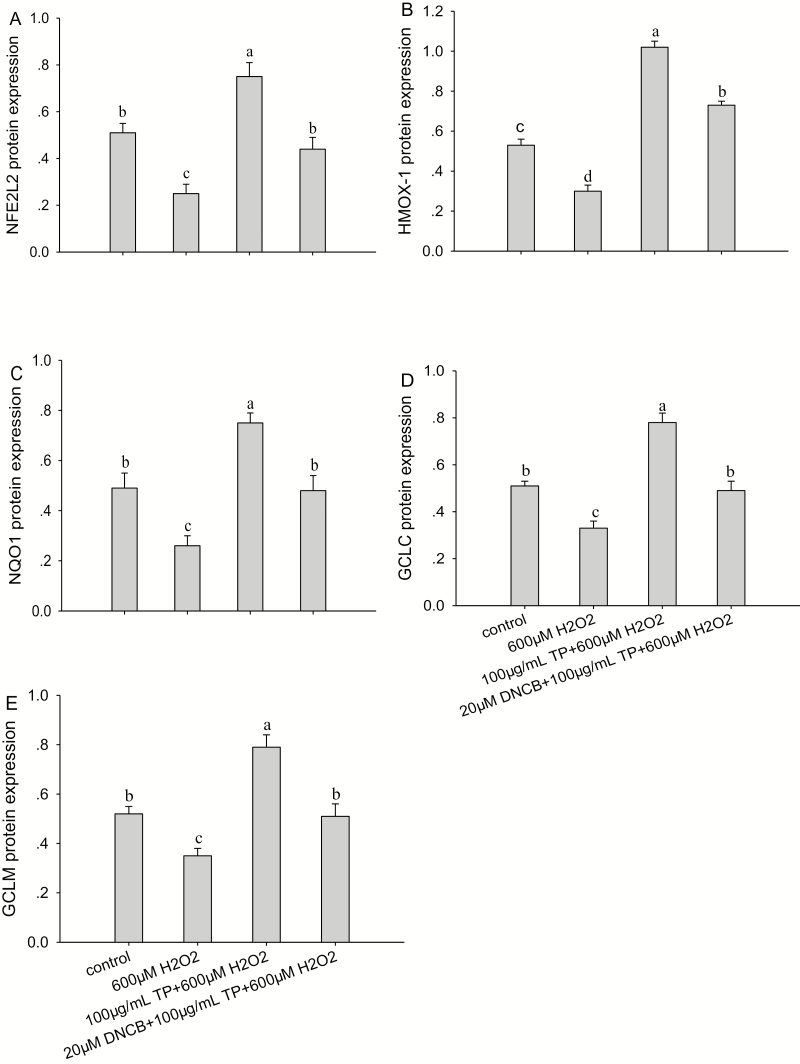
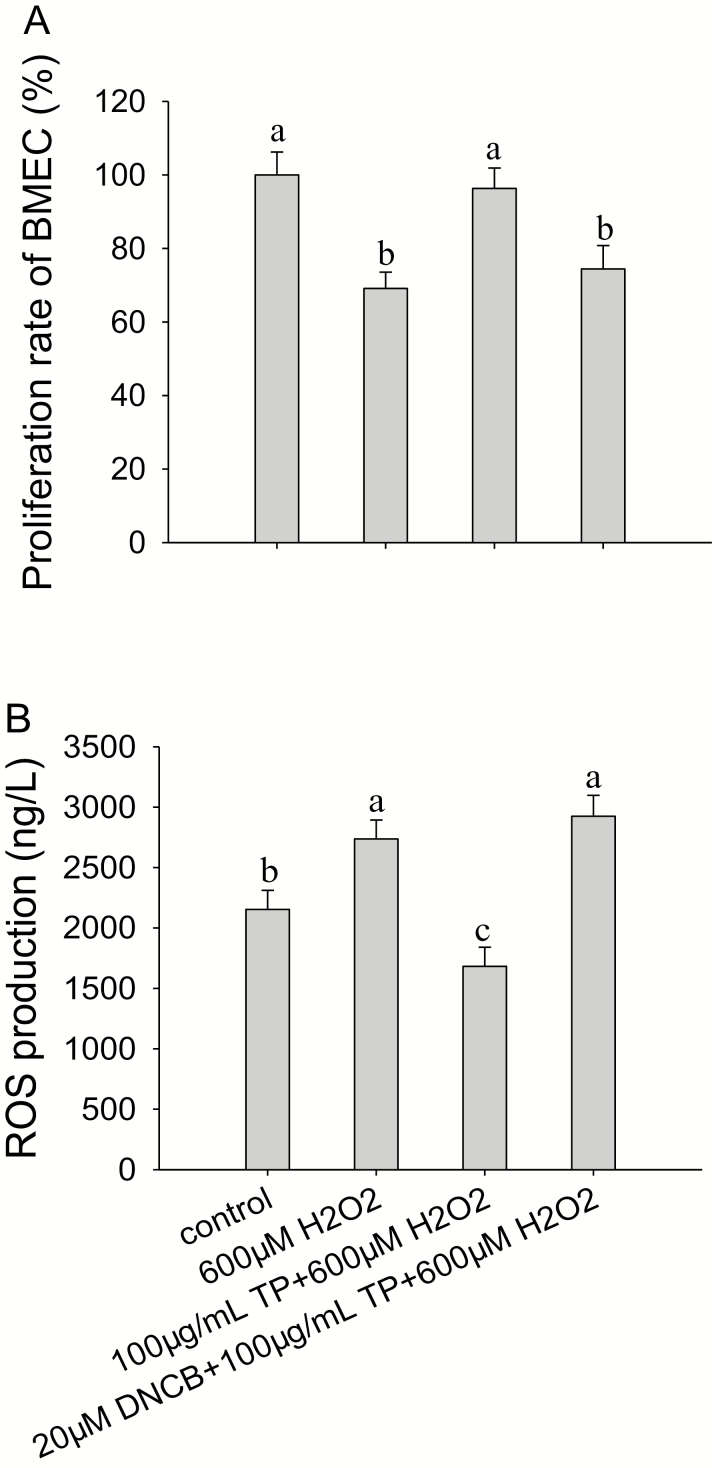
The results indicated that the mRNA (Fig. 6) and protein abundance (Fig. 7) of NFE2L2, HMOX-1, NQO1, GCLC, and GCLM and proliferation rate of BMEC (Fig. 8A) were all significantly decreased (P < 0.05) after treatment with 600 µM H2O2 for 6 h compared with control group. Although ROS production (Fig. 8B) was significantly increased (P < 0.05) after treatment with 600 µM H2O2 for 6 h compared with control group. These effects were reversed after the pretreatment of cells with 100 µg/mL of TP for 12 h followed by 600 µM H2O2 for 6 h, causing an increase in the mRNA and protein abundance of NFE2L2 (P < 0.05; Figs. 6A and 7A), HMOX-1 (P < 0.01; Figs. 6B and 7B), NQO1 (P < 0.01; Figs. 6C and 7C), GCLC (P < 0.01; Figs. 6D and 7D), and GCLM (P < 0.01; Figs. 6E and 7E) and causing a decrease in ROS production (P < 0.01) compared with the control group, respectively.
Figure 6.
Effect of tea polyphenol (TP) treatment on mRNA abundance of nuclear factor-erythroid 2-related factor 2 (NFE2L2) signaling pathway proteins in bovine mammary epithelial cells (BMEC) under control and oxidative stress (OS) conditions. (A to E) mRNA abundance of NFE2L2, heme oxygenase-1 (HMOX-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) in BMEC under control and OS conditions. The BMEC were preincubated with a MAPK-specific inhibitor, 2,4-dinitrochloro-benzene (DNCB), for 30 min and then treated with or without TP (100 µg/mL) for another 12 h followed by hydrogen peroxide (H2O2; 600 µM) exposure for 6 h. The mRNA abundance of NFE2L2, HMOX-1, NQO1, GCLC, and GCLM was measured by quantitative real-time PCR. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
Figure 7.
Effect of tea polyphenol (TP) treatment on protein abundance of nuclear factor-erythroid 2-related factor 2 (NFE2L2) signaling pathway proteins in bovine mammary epithelial cells (BMEC) under control and oxidative stress (OS) conditions. (A to E) Protein abundance of NFE2L2, heme oxygenase-1 (HMOX-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase modifier subunit (GCLM) in BMEC under control and OS. The BMEC were preincubated with a MAPK-specific inhibitor, 2,4-dinitrochloro-benzene (DNCB), for 30 min and then treated with or without TP (100 µg/mL) for another 12 h, followed by hydrogen peroxide (H2O2; 600 µM) exposure for 6 h. The protein abundance of NFE2L2, HMOX-1, NQO1, GCLC, and GCLM was measured by Western blot. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
Figure 8.
Effect of tea polyphenol (TP) treatment on proliferation rate of bovine mammary epithelial cells (BMEC) and reactive oxygen species (ROS) production in BMEC under control and oxidative stress (OS) conditions. (A, B) Proliferation rate of BMEC and ROS production in BMEC under control and OS conditions. The BMEC were preincubated with or without a MAPK-specific pathway inhibitor, 2,4-dinitrochloro-benzene (DNCB), for 30 min and then treated with or without TP (100 µg/mL) for another 12 h followed by hydrogen peroxide (H2O2; 600 µM) exposure for 6 h. Cell proliferation rate was measured by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), and ROS production was measured by a commercial kit following the manufacturer’s instructions. Means with different superscript letters differ (P < 0.05). There were 5 replications for each experiment and each well.
The proliferation rate of BMEC was not affected by 100 µg/mL TP with 600 µM H2O2 compared with control group. In addition, after the pretreatment of cells with DNCB (20 µM) for 30 min followed by treatment with TP (100 µg/mL) for another 12 h and H2O2 (600 µM) exposure for 6 h, the mRNA abundance of NFE2L2 (P < 0.05), mRNA and protein abundance of HMOX-1 (P < 0.01), and proliferation rate of BMEC (P < 0.05) all decreased and ROS production increased compared with the control group. In contrast, compared with control group, mRNA and protein abundance of NQO1, GCLM, and GCLC were not affected by 20 µM DNCB when incubating with 100 µg/mL TP during OS.
DISCUSSION
Reactive oxygen species are normally produced via aerobic metabolism in the mitochondrial electron transport chain (Decoursey and Ligeti, 2005). However, large amounts of ROS production that exceed antioxidant capabilities by the host can cause OS and consequent inflammatory reactions leading to disease (Wiegman et al., 2015). In the bovine mammary gland, the sudden increase in metabolic activity, cellular proliferation, and physiological adaptations after calving, all expose mammary tissue to a considerable amount of ROS (Wiegman et al., 2015). For example, excessive lipid peroxidation and a deficiency of antioxidants within mammary gland result in OS during lactation (Castillo et al., 2006; Nathan and Cunningham-Bussel, 2013).
The search for natural antioxidants that can scavenge free radicals and protect cells from oxidative damage has been activated for a number of years. For instance, TP, a natural chemical compound found in tea, possessed antioxidant capacity in a number of in vitro and in vivo studies (Frei and Higdon, 2003; Lambert and Elias, 2010). Therefore, an important aim of the present studied was to uncover a potential role of TP in ameliorating OS in BMEC. We further explored the possible molecular mechanisms underlying the antioxidant effects of TP against oxidative damage in H2O2-treated BMEC.
In vitro oxidative damage models induced by H2O2 have been widely used (Jiao et al., 2003). Hydrogen peroxide is one important type of ROS with well-defined physical and chemical properties (Tkachev et al., 2011). The present study revealed that incubation of BMEC with 600 µM H2O2 for 6 h caused substantial damage resulting in altered cell morphology and decreased cell viability. This is consistent with previous studies in BMEC (Jin et al., 2016). In addition, the marked increase in caspase-3 activity indicated that H2O2 treatment decreased BMEC proliferation rate but increased apoptosis.
A greater supply of antioxidants in the diet could protect dairy cattle against OS, especially during the transition period (Loor et al., 2013; Osorio et al., 2014b). A number of previous studies have indicated that supplementation of vitamin E (Politis, 2012), Se (Gong and Xiao, 2016), and conjugated linoleic acid (Hanschke et al., 2016) plays a positive role in protecting against OS while maintaining immune function during negative energy balance. In addition to the above nutrients, recent studies revealed that caffeic acid (Kilani-Jaziri et al., 2017) and resveratrol (Jin et al., 2016) can protect mammary epithelial cells from OS. In the present study, we first provide the evidence of the potential benefit for the utilization of TP as antioxidants. The data revealed that the dose-dependent cytoprotective effect of TP against H2O2-induced OS in BMEC occurred at least in part through the induction of multiple antioxidant response genes and the reduction of 8-OHdG, 8-iso-PG, and PC content, as well as caspase-3 activity.
To date, cytoprotective effects of TP/epigallocatechin gallate (EGCG) in bovine or BMEC have not been reported. The antioxidant effect of EGCG on human umbilical vein endothelial cells is related to an increase in the amount of redox-regulated antioxidant proteins that can scavenge free radicals (Yang et al., 2015). Therefore, the upregulation of several major antioxidant enzymes involved in cytoprotection against OS (Zhai et al., 2013) indicated that TP could help restore cellular redox state in BMEC.
Through its interaction with ARE, the transcription factor NFE2L2 plays a central role in the regulation of abundance of several antioxidant/detoxifying enzymes and protects cells against cytotoxicity caused by OS. In several cellular models, there is evidence that TP could activate NFE2L2 by disrupting the NFE2L2/Keap1 interaction (Yang et al., 2015). Therefore, the positive effect of TP on mRNA abundance of NFE2L2 and the protein abundance and mRNA abundance of HMOX-1, NQO1, GCLC, and GCLM under H2O2 treatment confirmed the previous data.
Moreover, a number of cellular kinases, including MAPK, may regulate NFE2L2. MAPK are implicated in the upregulation of antioxidant/detoxificant enzymatic activities (Kim and Novak, 2007), which together with NFE2L2 induction constitutes an important pathway to protect cells against oxidative damage (Masella et al., 2005). The activation of MAPK and PI3k signaling pathway initiated by antioxidants results in the activation of the NFE2L2 signaling pathway and upregulates the abundance of HMOX-1 (Lee et al., 2013). One of the possible mechanisms by which TP prevents H2O2-induced BMEC injury might be the activation of the NFE2L2/HMOX-1 pathway. For example, pretreating endothelial cells with EGCG exerted significant cytoprotective effects against H2O2, suggesting that the induction of HMOX-1 is an important component of the protective effects against OS (Wu et al., 2006; Wung et al., 2006). The present results indicated that TP can decrease intercellular ROS production through a similar mechanism, underscoring the role of HMOX-1 activation in the prevention of ROS-induced cellular injury.
The findings that TP increased the mRNA abundance of MAPK3/1, MAPK8/9/10, MAPK11/ 12/13/14, and MAPK7 underscore its role in BMEC survival against OS and confirm the previous data in non-ruminant cells (Ou et al., 2010; Pullikotil et al., 2012; Wang et al., 2016). In this study, the findings that DNCB abolished the protective effects of TP by inhibiting the abundance of MAPK3/1 and MAPK11/12/13/14 in the MAPK pathway, decreasing the proliferation rate, and increasing intracellular ROS by downregulating NFE2L2/HMOX-1 demonstrate that MAPK3/1 and MAPK11/12/13/14 pathways participate in the nuclear transport of NFE2L2. As such, abundance of HMOX-1 is induced by NFE2L2 and probably plays an important role in mediating the protective effect of TP as shown in other studies (Takano et al., 2004). Therefore, the present data in BMEC indicate that the activation of MAPK3/1 and MAPK11/12/13/14 might be required for the protective effects of TP.
There are few in vivo data studying the role of TP or other plant extracts on OS in transition cows (e.g., Winkler et al., 2015). The fact that grape pomace/marc (high in polyphenols) fed to late-lactation cows induced changes in ruminal bacterial and archeal communities along with a reduction in methane production (Moate et al., 2014) indicates that these products may need to be protected from rumen metabolism to enhance delivery of TP to tissues of the cow. Data from a study with transition cows fed green tea and curcuma extract (rich in TP) revealed some positive effects in terms of alleviation of hepatic inflammation postpartum (Winkler et al., 2015). If, in fact, systemic inflammation could be alleviated by long-term supplementation of TP during transition, it could enhance energy utilization for milk production (Winkler et al., 2015).
CONCLUSIONS
Treatment with TP protects BMEC against H2O2-induced OS by scavenging ROS formation and stimulating the NFE2L2–ARE self-defense mechanism. This molecule regulates the abundance of NFE2L2 and HMOX-1, along with increasing the abundance of MAPK3/1 and MAPK11/12/13/14 in the MAPK pathway. These beneficial effects of TP provide us with information about its potential application as a therapeutic antioxidant in ruminants. Further in vivo studies with periparturient cows are warranted.
Footnotes
This research was supported partly by grants from China National Natural Science Foundation (Nos. 31601975 and 31460616), Youth Foundation of Inner Mongolia Academy of Agriculture & Animal Husbandry Science (No. 2015QNJJM07), and the China Agriculture Research System (No. CARS-36). Y.M. received a scholarship from China Scholarship Council (CSC; Beijing, China) to train at the University of Illinois, Urbana–Champaign.
LITERATURE CITED
- Bryan H. K., Olayanju A., Goldring C. E., and Park B. K.. 2013. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 85:705–717. doi: 10.1016/j.bcp.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Castillo C., Hernández J., Valverde I., Pereira V., Sotillo J., Alonso M. L., and Benedito J. L.. 2006. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 80:133–139. doi: 10.1016/j.rvsc.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Chang D., Wang F., Zhao Y. S., and Pan H. Z.. 2008. Evaluation of oxidative stress in colorectal cancer patients. Biomed. Environ. Sci. 21:286–289. doi: 10.1016/S0895-3988(08)60043-4 [DOI] [PubMed] [Google Scholar]
- Chen L., Hu J. Y., and Wang S. Q.. 2012. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 67:1013–1024. doi: 10.1016/j.jaad.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Ciraj A. M., Sulaim J., Mamatha B., Gopalkrishna B. K., and Shivananda P. G.. 2001. Antibacterial activity of black tea (Camelia sinensis) extract against salmonella serotypes causing enteric fever. Indian J. Med. Sci. 55:376–381. [PubMed] [Google Scholar]
- Decoursey T. E., and Ligeti E.. 2005. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 62:2173–2193. doi: 10.1007/s00018-005-5177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., and Kulma A.. 2016. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 17:160. doi: 10.3390/ijms17020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., and Lamas S.. 2015. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 6:183–197. doi: 10.1016/j.redox.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., and Higdon J. V.. 2003. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 133:3275S–3284S. doi: 10.1093/jn/133.10.3275S [DOI] [PubMed] [Google Scholar]
- Gong J., and Xiao M.. 2016. Selenium and antioxidant status in dairy cows at different stages of lactation. Biol. Trace Elem. Res. 171:89–93. doi: 10.1007/s12011-015-0513-2 [DOI] [PubMed] [Google Scholar]
- Hanschke N., Kankofer M., Ruda L., Höltershinken M., Meyer U., Frank J., Dänicke S., and Rehage J.. 2016. The effect of conjugated linoleic acid supplements on oxidative and antioxidative status of dairy cows. J. Dairy Sci. 99:8090–8102. doi: 10.3168/jds.2015-10685 [DOI] [PubMed] [Google Scholar]
- Jaramillo M. C., and Zhang D. D.. 2013. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 27:2179–2191. doi: 10.1101/gad.225680.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H. L., Ye P., and Zhao B. L.. 2003. Protective effects of green tea polyphenols on human HepG2 cells against oxidative damage of fenofibrate. Free Radic. Biol. Med. 35:1121–1128. [DOI] [PubMed] [Google Scholar]
- Jin X., Wang K., Liu H., Hu F., Zhao F., and Liu J.. 2016. Protection of bovine mammary epithelial cells from hydrogen peroxide-induced oxidative cell damage by resveratrol. Oxid. Med. Cell. Longev. 2016:2572175. doi: 10.1155/2016/2572175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., and Mukhtar H.. 2013. Tea and health: Studies in humans. Curr. Pharm. Des. 19:6141–6147. doi: 10.2174/1381612811319340008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilani-Jaziri S., Mokdad-Bzeouich I., Krifa M., Nasr N., Ghedira K., and Chekir-Ghedira L.. 2017. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure-activity relationship study. Drug Chem. Toxicol. 40:416–424. doi: 10.1080/01480545.2016.1252919 [DOI] [PubMed] [Google Scholar]
- Kim S. K., and Novak R. F.. 2007. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol. Ther. 113:88–120. doi: 10.1016/j.pharmthera.2006.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk J., and Duchnik E.. 2014. Oxidative stress and skin diseases: Possible role of physical activity. Asian Pac. J. Cancer Prev. 15:561–568. doi: 10.7314/APJCP.2014.15.2.561 [DOI] [PubMed] [Google Scholar]
- Lambert J. D., and Elias R. J.. 2010. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 501:65–72. doi: 10.1016/j.abb.2010.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Bae S., and Yoon Y.. 2013. The anti-adipogenic effects of (−)epigallocatechin gallate are dependent on the WNT/β-catenin pathway. J. Nutr. Biochem. 24:1232–1240. doi: 10.1016/j.jnutbio.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Li H., Song F., Duan L. R., Sheng J. J., Xie Y. H., Yang Q., Chen Y., Dong Q. Q., Zhang B. L., and Wang S. W.. 2016. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/akt pathway. Sci. Rep. 6:23693. doi: 10.1038/srep23693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor J. J., Bionaz M., and Drackley J. K.. 2013. Systems physiology in dairy cattle: Nutritional genomics and beyond. Annu. Rev. Anim. Biosci. 1:365–392. doi: 10.1146/annurev-animal-031412-103728 [DOI] [PubMed] [Google Scholar]
- Ma Y. F., Wu Z. H., Gao M., and Loor J. J.. 2018. Nuclear factor erythroid 2-related factor 2 antioxidant response element pathways protect bovine mammary epithelial cells against H2O2-induced oxidative damage in vitro. J. Dairy Sci. 101:5329–5344. doi: 10.3168/jds.2017-14128 [DOI] [PubMed] [Google Scholar]
- Masella R., Di Benedetto R., Varì R., Filesi C., and Giovannini C.. 2005. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 16:577–586. doi: 10.1016/j.jnutbio.2005.05.013 [DOI] [PubMed] [Google Scholar]
- Moate P. J., Williams S. R., Torok V. A., Hannah M. C., Ribaux B. E., Tavendale M. H., Eckard R. J., Jacobs J. L., Auldist M. J., and Wales W. J.. 2014. Grape marc reduces methane emissions when fed to dairy cows. J. Dairy Sci. 97:5073–5087. doi: 10.3168/jds.2013-7588 [DOI] [PubMed] [Google Scholar]
- Nathan C., and Cunningham-Bussel A.. 2013. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13:349–361. doi: 10.1038/nri3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio J. S., Ji P., Drackley J. K., Luchini D., and Loor J. J.. 2014a. Smartamine M and metasmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways. J. Dairy Sci. 97:7451–7464. doi: 10.3168/jds.2014-8680 [DOI] [PubMed] [Google Scholar]
- Osorio J. S., Trevisi E., Ji P., Drackley J. K., Luchini D., Bertoni G., and Loor J. J.. 2014b. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 97:7437–7450. doi: 10.3168/jds.2013-7679 [DOI] [PubMed] [Google Scholar]
- Ou H. C., Song T. Y., Yeh Y. C., Huang C. Y., Yang S. F., Chiu T. H., Tsai K. L., Chen K. L., Wu Y. J., Tsai C. S.,. et al. 2010. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J. Appl. Physiol. (1985) 108:1745–1756. doi: 10.1152/japplphysiol.00879.2009 [DOI] [PubMed] [Google Scholar]
- Politis I. 2012. Reevaluation of vitamin E supplementation of dairy cows: Bioavailability, animal health and milk quality. Animal 6:1427–1434. doi: 10.1017/S1751731112000225 [DOI] [PubMed] [Google Scholar]
- Pullikotil P., Chen H., Muniyappa R., Greenberg C. C., Yang S., Reiter C. E., Lee J. W., Chung J. H., and Quon M. J.. 2012. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K., Nakaima K., Nitta M., Shibata F., and Nakagawa H.. 2004. Inhibitory effect of (−)-epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. J. Agric. Food Chem. 52:4571–4576. doi: 10.1021/jf0355194 [DOI] [PubMed] [Google Scholar]
- Tkachev V. O., Menshchikova E. B., and Zenkov N. K.. 2011. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc) 76:407–422. doi: 10.1134/S0006297911040031 [DOI] [PubMed] [Google Scholar]
- Wang H., Li D., Hu Z., Zhao S., Zheng Z., and Li W.. 2016. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol. Cells 39:508–513. doi: 10.14348/molcells.2016.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegman C. H., Michaeloudes C., Haji G., Narang P., Clarke C. J., Russell K. E., Bao W., Pavlidis S., Barnes P. J., Kanerva J.,. et al. ; COPDMAP. 2015. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 136:769–780. doi: 10.1016/j.jaci.2015.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A., Gessner D. K., Koch C., Romberg F. J., Dusel G., Herzog E., Most E., and Eder K.. 2015. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch. Anim. Nutr. 69:425–441. doi: 10.1080/1745039X.2015.1093873 [DOI] [PubMed] [Google Scholar]
- Wu L. L., Chiou C. C., Chang P. Y., and Wu J. T.. 2004. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 339:1–9. doi: 10.1016/j.cccn.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Wu C. C., Hsieh C. W., Lai P. H., Lin J. B., Liu Y. C., and Wung B. S.. 2006. Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol. Appl. Pharmacol. 214:244–252. doi: 10.1016/j.taap.2005.12.013 [DOI] [PubMed] [Google Scholar]
- Wung B. S., Hsu M. C., Wu C. C., and Hsieh C. W.. 2006. Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol. Res. 53:113–122. doi: 10.1016/j.phrs.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Yang G. Z., Wang Z. J., Bai F., Qin X. J., Cao J., Lv J. Y., and Zhang M. S.. 2015. Epigallocatechin-3-gallate protects HUVECs from PM2.5-induced oxidative stress injury by activating critical antioxidant pathways. Molecules 20:6626–6639. doi: 10.3390/molecules20046626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Jiao J. J., Duh J. L., Gudehithlu K., Tan T. H., and Kong A. N.. 1997a. Activation of mitogen-activated protein kinases by green tea polyphenols: Potential signaling pathways in the regulation of antioxidant-responsive element-mediated phase II enzyme gene expression. Carcinogenesis 18:451–456. [DOI] [PubMed] [Google Scholar]
- Yu R., Tan T. H., and Kong A. N.. 1997b. Butylated hydroxyanisole and its metabolite tert-butylhydroquinone differentially regulate mitogen-activated protein kinases. The role of oxidative stress in the activation of mitogen-activated protein kinases by phenolic antioxidants. J. Biol. Chem. 272:28962–28970. doi: 10.1074/jbc.272.46.28962 [DOI] [PubMed] [Google Scholar]
- Zhai X., Lin M., Zhang F., Hu Y., Xu X., Li Y., Liu K., Ma X., Tian X., and Yao J.. 2013. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 57:249–259. doi: 10.1002/mnfr.201200536 [DOI] [PubMed] [Google Scholar]