Abstract
A total of 240 newly weaned pigs (5.25 ± 0.15 kg BW) were used to determine the dietary omega-6-to-omega-3 (ω-6:ω-3) fatty acid ratio that optimized growth performance and immune responses when fed corn and soybean meal (SBM)-based diets with low protein quality. Pigs were randomly assigned to 1 of 5 dietary treatments (n = 6 pens per treatment; day 0 of study): [1] positive control (High; included animal proteins and 5% corn oil), [2] negative control (Low0; corn- and SBM-based and 5% corn oil), or 1 of 3 Low diets with increasing supplementation of fish oil to replace corn oil: [3] 1.25% (Low1.25), [4] 2.5% (Low2.5), [5] 5% (Low5) to achieve 5:1, 3:1, and 1:1 ω-6:ω-3 ratios, respectively. Pigs were fed dietary treatments in 2 phases for 7 and 14 d, respectively, followed by a common phase III diet for 21 d. On day 6 and 20, 12 pigs per treatment were immune sensitized with 0.5 mg ovalbumin (OVA) and 0.5 mg Quil A adjuvant in 1 mL saline. The dermal hypersensitivity response (DHR) was evaluated on day 40 in these same pigs, using intradermal injection of OVA; changes in skin-fold thickness were measured. On day 21, 4 pigs per pen were immune challenged with LPS (30 µg Escherichia coli LPS per kg BW) or saline (n = 12); rectal temperature was monitored over 3 h. During phase I only, ADG, ADFI, and G:F were greater for pigs fed the High diet vs. those fed the Low diet (P < 0.05), and increased with increasing fish oil supplementation up to 2.5% (Low2.5), but decreased for pigs fed the Low5 diet (quadratic; P < 0.05, P = 0.086, and P < 0.05 for ADG, ADFI, and G:F, respectively). On day 21, LPS increased rectal temperature (vs. saline at 1-, 2-, and 3-h post-challenge; P < 0.001); fish oil supplementation reduced rectal temperature 2-h post-challenge in the Low-fed pigs (linear; P < 0.05). On day 22, serum haptoglobin was greatest for pigs fed Low0 and decreased with increasing fish oil supplementation (linear; P < 0.05). Immunization with OVA induced a serum anti-OVA IgG response, which was reduced on day 34 among pigs fed Low diets with increasing fish oil supplementation (linear; P = 0.050). On day 40, and 6 h after intradermal injection of OVA, the DHR was least for pigs fed the Low2.5 diet (P < 0.05). Inclusion of 2.5% fish oil (3:1, ω-6:ω-3) optimized growth performance during the early nursery phase when pigs were most sensitive to diets with low protein quality; the ideal ω-6-to-ω-3 fatty acid ratio may differ when using immune responses as the major outcome.
Keywords: diet protein quality, fish oil, growth performance, immune response, nursery pigs
INTRODUCTION
During the early weaning phase, pigs often experience reduced feed intake and BW gain, and increased vulnerability to disease challenges that are attributed to exposure to food allergens and novel pathogens, maternal antibody depletion, social stress, and an immature gut and immune system (Lallès et al., 2004). High-quality protein sources (e.g., animal-derived products) are often included in nursery diets to combat reduced growth performance during the early weaning phase (Tokach et al., 1994; Whang et al., 2000). These high-quality protein sources contribute to the high cost of nursery diets, and may become less available due to biosafety (e.g., blood products) and sourcing (e.g., whey protein) concerns. Alternatively, soybean meal (SBM) is a poorer quality protein source, with reduced amino acid digestibility (vs. casein; Cervantes-Pahm and Stein, 2010), that induces an inflammatory response in young pigs due to the allergenic properties of the plant proteins (Li et al. 1991; Chen et al. 2011). Soybean meal is also considerably less expensive than animal-based proteins. Using SBM as a major dietary protein source has been shown to reduce pig growth during the early nursery period in comparison to diets containing highly digestible, animal-based proteins; however, a similar BW was achieved by the finisher phase due to compensatory growth (Whang et al., 2000; Skinner et al., 2014). Furthermore, carcass quality including HCW, back fat depth, loin eye depth, and percent lean yield at slaughter was not influenced by early-life nutritional management (Skiba, 2005; Skinner et al., 2014). Using ingredients that contain lower quality proteins may, however, compromise immunity in the nursery phase. For example, during a disease challenge, growth performance appeared more severely impacted for pigs fed low-quality protein sources vs. those fed diets containing animal proteins (Skinner et al., 2014), and the adaptive immune response was weakened (Levesque et al., 2013).
The inclusion of immune-modulating nutrients such as omega-3 (ω-3) fatty acids may be used to moderate inflammation and improve growth performance when pigs are fed diets containing low-quality proteins. Indeed, increased ω-3 intake has been shown to reduce inflammation in both humans (Simopoulos, 2008) and pigs (Duan et al., 2014). Therefore, we hypothesized that feeding nursery diets with SBM as a major protein source will reduce pig growth and increase inflammatory responses during the early nursery period, but inclusion of ω-3 fatty acids would mitigate these responses. The objectives of the current study were to determine the dietary ω-6-to-ω-3 fatty acid ratio that optimized BW gain, feed efficiency, and immune responses when pigs were fed nursery diets containing SBM as the main protein source and supplemented with fish oil.
MATERIALS AND METHODS
The experimental protocol was approved by the University of Guelph Animal Care Committee and followed Canadian Council of Animal Care guidelines (CCAC, 2009). The study was conducted at the Arkell Swine Research Station at the University of Guelph (Guelph, ON, Canada).
Animals and Diets
Two hundred forty Yorkshire pigs (120 castrated males and 120 females) were weaned in 2 blocks at 21 ± 3 d of age (5.25 ± 0.15 kg BW). Weaned pigs were housed in an environmentally controlled (26 °C) nursery room in pens that measured 296 × 107 cm and contained fully slatted, plastic-coated, expanded metal floors. Pigs were housed in the nursery for 3 wk after weaning with 8 pigs (4 castrated males and 4 females) per pen; on day 21, 120 pigs received an immune challenge, and were later sacrificed for tissue sampling [4 pigs per pen (2 castrated males and 2 females)]; the remaining 4 pigs were moved to grower-finisher pens that measured 424 × 197 cm and had partially slatted concrete floors. Pigs were weighed individually and feed disappearance per pen was measured simultaneously.
At weaning, pigs were assigned to 1 of 5 dietary treatments according to a randomized complete block design (n = 6 pens per dietary treatment and 8 pigs per pen; day 0 of study): [1] positive control (High; contained multiple protein sources, including animal proteins and 5% corn oil), [2] negative control (Low0; contained SBM as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) to achieve 5:1, 3:1, and 1:1 ω-6:ω-3 ratios, respectively. Nursery diets were provided as a phase-feeding program; phases I and II were fed for 7 and 14 d, respectively. Thereafter, all remaining pigs were fed a common phase III diet for 21 d (Table 1). The High diet contained highly digestible, purified plant proteins (soybean isolate), animal proteins (i.e., whey, spray-dried blood meal, and blood plasma), and crystalline amino acids. The Low diets included SBM as the main protein source. Wheat was added in all diets to improve pellet quality, and vitamin E was added to all diets to prevent oxidation of the fish and corn oils. Titanium dioxide was included in the phase II diets in order to determine apparent nutrient digestibility. Diets were formulated to approach, or meet, estimated nutrient requirements of pigs (NRC, 2012; Table 1); crude protein (CP) was reduced slightly in the phase I Low diets to minimize digestive disturbances. Both nursery and grower diets were pelleted, and diets and water were provided ad libitum throughout the study.
Table 1.
Ingredient and nutrient composition of experimental diets (as-fed basis)1
| High | Low0 | Low1.25 | Low2.5 | Low5 | Common | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Phase I | Phase II | Phase I | Phase II | Phase I | Phase I | Phase I | Phase II | Phase I | Phase II | Phase III |
| Ingredient, % | |||||||||||
| Corn | 32.5 | 45.9 | 45.3 | 41.0 | 45.3 | 41.0 | 45.3 | 41.0 | 45.3 | 41.0 | 46.3 |
| Soybean meal, dehulled | 10.0 | 15.0 | 30.0 | 35.0 | 30.0 | 35.0 | 30.0 | 35.0 | 30.0 | 35.0 | 30.0 |
| Soybean isolate2 | 9.3 | 3.0 | – | – | – | – | – | – | – | – | – |
| Wheat | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Whey, dried | 20.0 | 8.00 | – | – | – | – | – | – | – | – | – |
| Corn oil3 | 5.00 | 5.00 | 5.00 | 5.00 | 3.75 | 3.75 | 2.50 | 2.50 | – | – | 5.00 |
| Fish oil4 | – | – | – | – | 1.25 | 1.25 | 2.50 | 2.50 | 5.00 | 5.00 | – |
| Blood plasma5 | 4.50 | 2.00 | – | – | – | – | – | – | – | – | – |
| Blood meal, spray dried | – | 2.00 | – | – | – | – | – | – | – | – | – |
| L-lysine·HCl | – | 0.30 | 0.37 | 0.26 | 0.37 | 0.26 | 0.37 | 0.26 | 0.37 | 0.26 | 0.35 |
| DL-methionine | 0.13 | 0.14 | 0.15 | 0.11 | 0.15 | 0.11 | 0.15 | 0.11 | 0.15 | 0.11 | 0.09 |
| L-tryptophan | – | – | 0.02 | – | 0.02 | – | 0.02 | – | 0.02 | – | – |
| L-threonine | 0.05 | 0.15 | 0.24 | 0.12 | 0.24 | 0.12 | 0.24 | 0.12 | 0.24 | 0.12 | 0.10 |
| Limestone | 0.89 | 1.11 | 1.30 | 1.25 | 1.30 | 1.25 | 1.30 | 1.25 | 1.30 | 1.25 | 1.17 |
| Salt | – | – | 0.50 | 0.40 | 0.50 | 0.40 | 0.50 | 0.40 | 0.50 | 0.40 | 0.40 |
| Monocalcium phosphate | 1.94 | 1.57 | 1.52 | 1.24 | 1.52 | 1.24 | 1.52 | 1.24 | 1.52 | 1.24 | 0.97 |
| Vitamin and mineral mix6 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Vitamin E7 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Titanium oxide | – | 0.20 | – | 0.20 | – | 0.20 | – | 0.20 | – | 0.20 | – |
| Calculated nutrient composition8 | |||||||||||
| ME, Kcal/kg | 3552 | 3536 | 3472 | 3479 | 3472 | 3479 | 3472 | 3479 | 3472 | 3479 | 3496 |
| NE, kcal/kg | 2691 | 2691 | 2611 | 2591 | 2611 | 2591 | 2611 | 2591 | 2611 | 2591 | 2630 |
| Crude protein, % | 23.4 | 20.4 | 20.8 | 22.6 | 20.8 | 22.6 | 20.8 | 22.6 | 20.8 | 22.6 | 20.8 |
| SID Lys, %9 | 1.25 | 1.25 | 1.21 | 1.25 | 1.21 | 1.25 | 1.21 | 1.25 | 1.21 | 1.25 | 1.20 |
| SID Met, % | 0.42 | 0.40 | 0.42 | 0.40 | 0.42 | 0.40 | 0.42 | 0.40 | 0.42 | 0.40 | 0.37 |
| SID Met + Cys, % | 0.79 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.66 |
| SID Thr, % | 0.86 | 0.81 | 0.86 | 0.81 | 0.86 | 0.81 | 0.86 | 0.81 | 0.86 | 0.81 | 0.73 |
| SID Trp, % | 0.29 | 0.23 | 0.24 | 0.25 | 0.24 | 0.25 | 0.24 | 0.25 | 0.24 | 0.25 | 0.22 |
| Calcium, % | 0.84 | 0.79 | 0.84 | 0.79 | 0.84 | 0.79 | 0.84 | 0.79 | 0.84 | 0.79 | 0.70 |
| Total P, % | 0.90 | 0.73 | 0.72 | 0.68 | 0.72 | 0.68 | 0.72 | 0.68 | 0.72 | 0.68 | 0.60 |
| STTD P, %10 | 0.43 | 0.39 | 0.43 | 0.39 | 0.43 | 0.39 | 0.43 | 0.39 | 0.43 | 0.39 | 0.33 |
| Sodium, % | 0.43 | 0.20 | 0.23 | 0.20 | 0.23 | 0.20 | 0.23 | 0.20 | 0.23 | 0.20 | 0.19 |
| Analyzed nutrient composition, % | |||||||||||
| Dry matter | 91.1 | 89.8 | 89.8 | 89.6 | 89.6 | 88.9 | 90.1 | 90.0 | 90.5 | 89.7 | 89.9 |
| Crude protein | 20.8 | 18.7 | 20.7 | 22.1 | 21.1 | 21.9 | 20.7 | 20.2 | 17.7 | 21.8 | 19.1 |
| Calcium | 0.87 | 0.81 | 0.86 | 0.84 | 0.87 | 0.70 | 0.89 | 0.85 | 0.83 | 0.87 | 0.76 |
| Phosphorus | 0.82 | 0.71 | 0.73 | 0.69 | 0.70 | 0.65 | 0.73 | 0.66 | 0.70 | 0.69 | 0.60 |
| Sodium | 0.27 | 0.15 | 0.20 | 0.17 | 0.20 | 0.16 | 0.20 | 0.18 | 0.21 | 0.20 | 0.18 |
| Potassium | 0.94 | 0.81 | 0.97 | 1.08 | 1.00 | 1.10 | 0.95 | 0.95 | 0.90 | 1.01 | 0.91 |
| Magnesium | 0.14 | 0.14 | 0.17 | 0.18 | 0.17 | 0.18 | 0.17 | 0.16 | 0.17 | 0.17 | 0.16 |
1Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk.
2Ardex AF; manufactured by Archer Daniels Midland Company (Decatur, IL).
3Corn oil from Saporito Foods Inc. (Markham, ON, Canada).
4Menhaden fish oil, Grand Valley Fortifiers (Cambridge, ON, Canada).
5AP920; manufactured by APC Nutrition Inc. (Ames, IA).
6Supplied per kg of diet: vitamin A, 12,000 IU as retinyl acetate; vitamin D3, 1,200 IU as cholecalciferol; vitamin E, 48 IU as DL-α-tocopherol acetate; vitamin K, 3 mg as menadione; vitamin B12, 0.03 mg; pantothenic acid, 18 mg; riboflavin, 6 mg; choline, 600 mg; folic acid, 2.4 mg; niacin, 30 mg; thiamine, 18 mg; pyridoxine, 1.8 mg; biotin, 200 µg; Cu, 18 mg as CuSO4·5H2O; Fe, 120 mg as FeSO4; Mn, 24 mg as MnSO4; Zn, 126 mg as ZnO; Se, 0.36 mg as FeSeO3; I, 0.6 mg as KI; DSM Nutritional Products Canada Inc., Ayr, ON, Canada.
7Grand Valley Fortifiers, ON, Canada.
8Calculated on the basis of the NRC (2012) ingredient values.
9Standardized ileal digestible.
10Standardized total tract digestible.
Two and 3 wk after weaning, fresh fecal samples were collected from each pig and combined per pen for determination of apparent total tract digestibility (ATTD) of organic matter (OM); pooled fecal samples were stored frozen until further processing.
Assessment of Immune Response and Gut Morphology
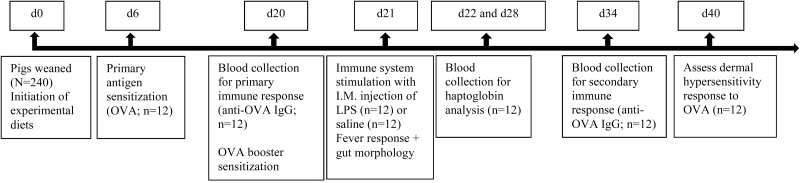
On day 6 and 20 postweaning, 12 pigs per treatment (1 castrated male and 1 female per pen) were randomly selected and immune sensitized with 0.5 mg ovalbumin (OVA) using 0.5 mg Quil A as the adjuvant in 1 mL of saline (Sigma-Aldrich Co., St Louis, MO; Fig. 1) via intramuscular injection. Blood samples were collected on day 20 (primary response) and day 34 (secondary response) postweaning for determination of OVA-specific serum IgG, and on day 22 and 28 for determination of serum haptoglobin (Hp; Fig. 1; time line according to Furesz et al., 1997). Blood samples were collected via orbital-sinus puncture into serum vials (BD Vacutainers, Mississauga, ON, Canada), allowed to clot at room temperature for up to 1 h, and then centrifuged for 20 min at 3,000 × g and 4 °C. The resulting serum was aliquoted into microcentrifuge tubes, and subsequently stored at −20 °C until analysis.
Figure 1.
Time line of procedures for immune responses.
On day 40 postweaning, all OVA-sensitized pigs were intradermally challenged with 200 μg OVA dissolved in 50 μL of saline on the inner thigh of 1 hind leg; 50 μL of saline was also injected as a control at least 5 cm from the OVA test site. Skin thickness measurements (swelling) were determined in triplicate before injection and 6-, 24-, and 48-h post-injection using calipers (Model RH15 9LB, Creative Health Products Inc., Ann Arbor, MI). The percentage change in skin thickness over time relative to the saline-injection measurement was used to determine the dermal hypersensitivity response (DHR).
Serum antibodies and Hp analyses in response to OVA immune sensitization
The serum OVA-specific IgG response was quantified using an indirect ELISA method as described by Begley et al. (2008) that included high-affinity binding 96-well microtiter plates (Corning, Acton, MA); the reference (serum pooled from all pigs on day 34), quality control, and serum samples were tested in triplicate, and the optical density was measured at 405 nm using a Wallac 1420 Victor3 Multilabel counter (Perkin Elmer, Waltham, MA). The optical densities for individual plates were adjusted using a correction factor (CF; Eq. 1). The average intra- and inter-assay variations were 2.7% and 7.6%, respectively.
| (1) |
Serum Hp concentrations were determined by the clinical pathology service of the Animal Health Laboratory, University of Guelph, using a Roche Cobas c501 biochemistry analyzer (Roche Diagnostics, Indianapolis, IN).
Rectal temperature, gut morphology, and apparent ileal digestibility during LPS immune challenge.
On day 21, 120 pigs were administered a single intramuscular injection of either 30 µg Escherichia coli LPS per kg BW (strain 055:B5; Sigma-Aldrich Co., St Louis, MO) or 1 mL of saline (n = 12 pigs per dietary treatment for each LPS and saline injections). Rectal temperature was measured in each pig 1, 2, and 3 h after the LPS immune challenge. After 3 h, pigs were euthanized with 3 mL of Euthasol (Virbac, TX). Immediately thereafter, the entire intestinal tract was excised, and digesta was collected from the terminal ileum (up to 1 m prior to the ileocecal junction) to determine apparent ileal digestibility of CP and OM using the indigestible marker.
Five centimeters of jejunum (1.5 m past the ligament of Trietz) was removed, rinsed with saline, and placed in 10% formalin until further processing. Jejunal tissue segments were prepared for histology analysis according to the procedures of Carleton et al. (1980). Measurements of villi height and crypt depth were collected from the 6 longest villi in each intestinal section using a Leica DMR fluorescence microscope (Leica Microsystems Inc., Wetzlar, Germany) and Openlab Computer Imaging System (Perkin Elmer, Waltham, MA).
Diet Analysis
Samples of feces and digesta were homogenized, freeze-dried, and finely ground prior to nutrient analyses. Diets, feces, and digesta were analyzed in duplicate for DM and ash contents according to AOAC methods (AOAC, 1997). Diets and digesta were analyzed in duplicate for CP (N × 6.25); the N content was determined using a LECO-FP 428 automatic analyzer (Leco Instruments Ltd, Mississauga, ON, Canada; AOAC, 1997). Titanium concentration in feces and ileal digesta (in duplicate) and diets (in quadruplicate) was measured as described by Myers et al. (2004), with minor adaptations (digestion for 24 h at 120 °C in 10 mL tubes and addition of H2O2 after precipitate settled in 100 mL volumetric flasks). Absorbance of standards and samples were measured at 408 nm by spectrophotometry (Power Wave XS KC4; BioTek Instruments Inc., Winooski, VT). Diets were also analyzed for Ca and P contents at Agrifood Laboratories (Guelph, ON, Canada), as described by Heinonen and Lahti (1981) and Themelis et al. (2001), respectively. Diet fatty acid contents were determined at Lipid Analytical Laboratories (Guelph, ON, Canada) according to the method of Bligh and Dyer (1959).
Calculations and Statistical Analysis
The ATTD of OM was calculated 2 and 3 wk after weaning (n = 6; pen as experimental unit) and apparent ileal digestibilities of OM and CP were calculated 3 wk after weaning for pigs that were administered either LPS (n = 12) or saline (n = 12; pig as experimental unit) as described by Columbus et al. (2010). Statistical analyses (for growth performance, ATTD OM, serum Hp, serum OVA-specific IgG, and DHR) were conducted using the MIXED procedure of SAS (Version 9.4; SAS Inst. Inc., Cary, NC) with dietary treatment and time and the interaction between dietary treatment and time as fixed effects, and using the repeated measures option when appropriate. Block and pig (or pen) within dietary treatment were considered random effects. Statistical analysis of rectal temperature, gut morphology, and apparent ileal digestibility of OM and CP were conducted using the MIXED procedure of SAS with dietary treatment, LPS, and the interaction of dietary treatment and LPS immune challenge as the fixed effects, and block and pig within dietary treatment as random effects. For rectal temperature, the interactions among dietary treatment, time after LPS challenge (1, 2, and 3 h), and LPS challenge (+/−) were considered (P > 0.10) but a reduced model was used; the repeated measures option was also included, and initial body temperature (i.e., prior to LPS challenge) was used as a covariate. For analysis of growth performance data and apparent fecal OM digestibility, pen was the experimental unit and initial BW was used as a covariate; for analysis of immune responses and gut morphology, pig was considered the experimental unit. Differences among individual means were assessed using the Tukey–Kramer post hoc test. Linear and quadratic contrasts were constructed to compare the effect of increasing inclusion of fish oil in Low diets. Probability values less than 0.05 were considered significant, whereas 0.05 ≤ P ≤ 0.10 was considered a trend, and P > 0.10 was considered not significant.
RESULTS
The chemical analyses of nursery diets were comparable to calculated values, except for the CP concentration for the phase I diet of Low5, which was approximately 15% lower than expected, likely reflecting a systematic error during CP analysis (Table 1). The fatty acid composition of experimental diets is presented in Table 2 and demonstrates that the ω-6:ω-3 ratio was influenced by altering the inclusion levels of corn and fish oil; eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) made up a significant proportion of total ω-3 fatty acids in the diets containing fish oil.
Table 2.
Fatty acid composition of experimental diets (% as-fed)1
| High | Low0 | Low1.25 | Low2.5 | Low5 | Common | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Phase I | Phase II | Phase I | Phase II | Phase I | Phase II | Phase I | Phase II | Phase I | Phase II | Phase III |
| C14:0 | 0.02 | 0.01 | 0.03 | 0.01 | 0.12 | 0.10 | 0.17 | 0.18 | 0.27 | 0.37 | 0.01 |
| C16:0 | 0.94 | 0.96 | 0.97 | 0.97 | 0.99 | 1.00 | 1.03 | 1.03 | 0.99 | 1.15 | 0.98 |
| C16:1 | 0.02 | 0.01 | 0.04 | 0.01 | 0.16 | 0.13 | 0.24 | 0.25 | 0.36 | 0.50 | 0.01 |
| C18:0 | 0.15 | 0.15 | 0.15 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 | 0.17 | 0.20 | 0.16 |
| C18:1 | 1.77 | 1.84 | 1.72 | 1.78 | 1.43 | 1.51 | 1.32 | 1.29 | 0.83 | 0.86 | 1.86 |
| C18:2n6 | 3.79 | 3.93 | 3.78 | 4.00 | 3.03 | 3.27 | 2.70 | 2.64 | 1.47 | 1.42 | 4.15 |
| C18:3n3 | 0.12 | 0.12 | 0.15 | 0.16 | 0.15 | 0.16 | 0.16 | 0.17 | 0.15 | 0.18 | 0.17 |
| C18:4n3 | 0.00 | 0.00 | 0.01 | 0.00 | 0.04 | 0.04 | 0.06 | 0.07 | 0.10 | 0.14 | 0.00 |
| C20:5n3, EPA2 | 0.01 | 0.00 | 0.04 | 0.01 | 0.19 | 0.16 | 0.28 | 0.29 | 0.44 | 0.61 | 0.00 |
| C22:5n3 | 0.00 | 0.00 | 0.01 | 0.00 | 0.03 | 0.03 | 0.05 | 0.05 | 0.07 | 0.11 | 0.00 |
| C22:6n3, DHA3 | 0.01 | 0.01 | 0.04 | 0.01 | 0.18 | 0.16 | 0.28 | 0.28 | 0.42 | 0.60 | 0.00 |
| Omega-3 | 0.15 | 0.13 | 0.25 | 0.18 | 0.61 | 0.56 | 0.86 | 0.89 | 1.23 | 1.72 | 0.17 |
| EPA + DHA | 0.03 | 0.01 | 0.09 | 0.02 | 0.37 | 0.32 | 0.56 | 0.57 | 0.86 | 1.21 | 0.00 |
| Omega-6 | 3.80 | 3.93 | 3.79 | 4.00 | 3.07 | 3.31 | 2.76 | 2.70 | 1.57 | 1.55 | 4.15 |
| Omega- 6:omega-3 | 26.1 | 30.9 | 15.0 | 22.7 | 5.00 | 5.85 | 3.20 | 3.02 | 1.27 | 0.90 | 24.7 |
1Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk.
2Eicosapentaenoic acid.
3Docosahexaenoic acid.
Growth Performance
The BW of pigs on day 7 and 14 was influenced by dietary treatment (P < 0.05; Table 3). On day 7, BW was lower for pigs fed the Low0 and Low5 diets relative to those fed the High diet (P < 0.05); BW was intermediate for pigs fed Low diets supplemented with 1.25 and 2.5% fish oil. On day 14, BW was lower for pigs fed the Low5 diet than those fed the High diet (P < 0.05) and intermediate for pigs fed Low diets supplemented with 0, 1.25, and 2.5% fish oil. On both day 7 and 14, BW increased with increasing fish oil supplementation up to 2.5% and 1.25%, respectively, for pigs fed the Low diets (quadratic; P < 0.05). During phase I, ADG, ADFI, and G:F were influenced by dietary treatment (P < 0.05) and were lower for pigs fed the Low0 diet relative to those fed the High diet (P < 0.05). Among pigs fed the Low diets, ADG, ADFI, and G:F increased with increasing fish oil supplementation up to 2.5% (quadratic; P < 0.05, P = 0.086, and P < 0.05 for ADG, ADFI, and G:F, respectively); ADG, ADFI, and G:F were not different between pigs fed the Low0 and Low5 diets. In phase II, ADFI was influenced by dietary treatment (P < 0.05) and decreased with increasing fish oil supplementation (linear; P < 0.05). Overall, ADG, ADFI, and G:F (i.e., between day 0 and day 42) were not influenced by dietary treatment. The ATTD of OM was not influenced by the interactive effect of time (week 2 and 3; phase II) and dietary treatment, or the main effect of dietary treatment (Table 3), but was greater in week 3 than in week 2 (84.9 vs. 80.4 ± 0.43%; P < 0.001).
Table 3.
Effect fish oil inclusion in low protein quality diets on growth performance of pigs after weaning
| Dietary treatment1 | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | High | Low0 | Low1.25 | Low2.5 | Low5 | SEM3 | Treatment | Linear | Quadratic |
| BW, kg | |||||||||
| Day 0 | 5.24 | 5.44 | 5.04 | 5.18 | 5.36 | 0.15 | – | – | – |
| Day 7 | 7.19b | 6.86a | 7.04ab | 7.11ab | 6.92a | 0.07 | 0.007 | 0.766 | 0.007 |
| Day 14 | 9.44b | 8.95ab | 9.27ab | 9.18ab | 8.80a | 0.14 | 0.029 | 0.229 | 0.037 |
| Day 21 | 11.83 | 11.76 | 11.89 | 11.84 | 11.46 | 0.33 | 0.616 | 0.252 | 0.346 |
| Day 42 | 25.99 | 24.81 | 25.27 | 25.66 | 24.38 | 0.65 | 0.437 | 0.613 | 0.248 |
| ADG, g | |||||||||
| Phase I, day 0–7 | 107b | 60a | 85ab | 95ab | 68a | 11 | 0.007 | 0.766 | 0.007 |
| Phase II, day 7–21 | 353 | 357 | 350 | 336 | 328 | 29 | 0.475 | 0.124 | 0.776 |
| Phase III, day 21–42 | 654 | 615 | 629 | 651 | 609 | 26 | 0.649 | 0.858 | 0.301 |
| Overall, day 0–42 | 470 | 443 | 454 | 461 | 432 | 16 | 0.469 | 0.594 | 0.275 |
| ADFI, g | |||||||||
| Phase I, day 0–7 | 165b | 129a | 138ab | 145ab | 135a | 19 | 0.040 | 0.773 | 0.086 |
| Phase II, day 7–21 | 467 | 455 | 446 | 429 | 415 | 31 | 0.049 | 0.013 | 0.731 |
| Phase III, day 21–42 | 1005 | 952 | 998 | 1009 | 956 | 32 | 0.573 | 0.910 | 0.175 |
| Overall, day 0–42 | 682 | 646 | 666 | 668 | 635 | 32 | 0.494 | 0.598 | 0.259 |
| G:F | |||||||||
| Phase I, day 0–7 | 0.569b | 0.398a | 0.514ab | 0.564ab | 0.440ab | 0.04 | 0.042 | 0.722 | 0.014 |
| Phase II, day 7–21 | 0.748 | 0.773 | 0.763 | 0.761 | 0.770 | 0.02 | 0.883 | 0.985 | 0.618 |
| Phase III, day 21–42 | 0.661 | 0.650 | 0.641 | 0.659 | 0.644 | 0.02 | 0.783 | 0.916 | 0.764 |
| Overall, day 0–42 | 0.672 | 0.647 | 0.658 | 0.675 | 0.650 | 0.01 | 0.450 | 0.885 | 0.169 |
| ATTD OM, %4 | 83.5 | 81.6 | 83.4 | 82.0 | 82.8 | 0.63 | 0.142 | 0.539 | 0.543 |
1Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk. Each mean represents observations on 6 pens.
2 P-values for the main effect of dietary treatment, and for the linear and quadratic contrasts of supplementing Low diets with increasing levels of fish oil.
3Maximum value of standard error of the means.
4Apparent total tract digestibility of organic matter during phase II (week 2 and 3 after weaning; 6 pens per treatment week); interaction between diet and week was not significant; therefore, main effect of diet only is presented; main effect of week P < 0.001 (ATTD OM = 80.4 and 84.9 ± 0.43% on week 1 and week 2 of feeding phase II diets, respectively).
a,bValues with different letters within the same row differ (P < 0.05).
Immune Response to OVA Sensitization
Serum Hp concentrations were greater on day 22 vs. day 28 after weaning (P < 0.001; Fig. 2). Neither dietary treatment (P > 0.10), nor the interaction between dietary treatment and time after weaning (P = 0.112) influenced serum Hp. On day 22, serum Hp concentrations decreased with increasing fish oil supplementation for pigs fed Low diets (linear; P < 0.01) and were not different among pigs fed the High diet or Low diets supplemented with fish oil. On day 28, serum Hp was not influenced by dietary treatment.
Figure 2.
Effect fish oil inclusion in low protein quality diets on serum Hp concentrations of pigs after weaning. Each mean represents observations on 12 pigs. Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk. a,bValues with different letters within the same panel differ (P < 0.05).
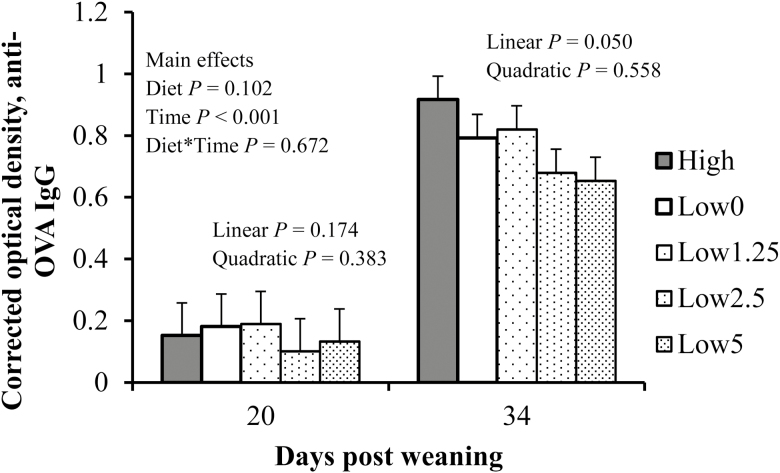
As expected, serum anti-OVA IgG concentration was significantly greater on day 34 (secondary response) than on day 20 (primary response; P < 0.001; Fig. 3). Serum anti-OVA IgG concentration tended to be influenced by the main effect of dietary treatment (P = 0.102); on day 34 and among pigs fed Low diets, anti-OVA IgG tended to be reduced with increasing fish oil supplementation (P = 0.050).
Figure 3.
Effect fish oil inclusion in low protein quality diets on anti-OVA IgG response of pigs after weaning. Each mean represents observations on 12 pigs. Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk.
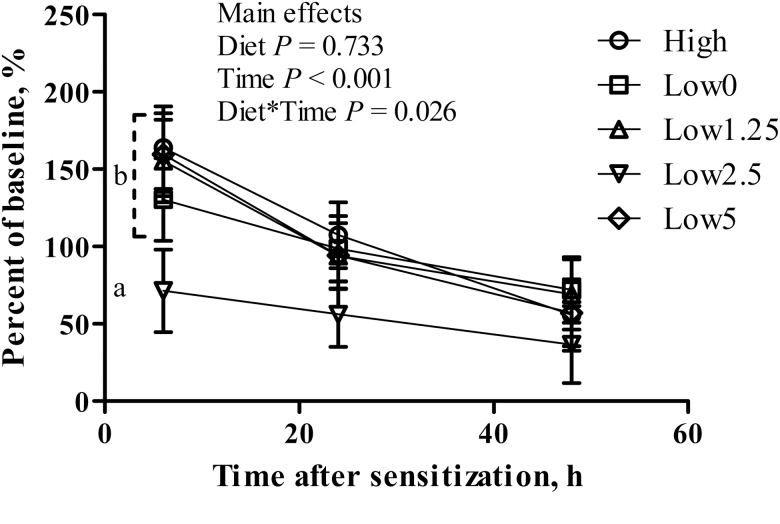
The DHR to OVA challenge was influenced by the interactive effect of dietary treatment and time after intradermal challenge (P < 0.05; Fig. 4); at 6 h after intradermal challenge, pigs fed the Low2.5 diet demonstrated the lowest increase in skin thickness (71 ± 27% of baseline) vs. all other treatment groups (164, 130, 155, and 159 ± 27% of baseline for pigs fed High, Low0, Low1.25, and Low5 diets, respectively; P < 0.05).
Figure 4.
Effect fish oil inclusion in low protein quality diets on DHR of pigs after weaning. Each mean represents observations on 12 pigs. Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk. a,bValues with different letters within the same panel differ (P < 0.05).
Rectal Temperature, Gut Morphology, and Apparent Ileal Digestibility During LPS Immune Challenge
The interaction between dietary treatment and LPS, or dietary treatment and time after LPS challenge did not influence rectal temperature (data not shown). As expected, pigs that received an intramuscular injection of LPS had greater rectal temperatures 1, 2, and 3 h after the LPS immune challenge vs. those that received saline (P < 0.01; Table 4). Rectal temperature was influenced by dietary treatment 2 h after LPS immune challenge (P < 0.05); pigs that received diets with 5% fish oil had lower rectal temperature than those that received the High diet (P < 0.05), and rectal temperature decreased with increasing fish oil supplementation for pigs fed the Low diets (linear; P < 0.05). Before LPS challenge, diet also influenced rectal temperature (P < 0.05); rectal temperature increased between pigs fed Low0 and Low1.25 diets, but decreased with further supplementation of fish oil (quadratic; P < 0.05).
Table 4.
Effect fish oil inclusion in low protein quality diets on rectal temperature of pigs that received LPS or saline on day 21 after weaning
| Dietary treatment1 | LPS challenge2 | P-values3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | High | Low0 | Low1.25 | Low2.5 | Low5 | SEM4 | + | − | SEM5 | Diet | LPS | Linear | Quadratic |
| Hour after challenge | |||||||||||||
| 0 h | 103.0ab | 102.6ab | 103.1b | 102.9ab | 102.5a | 0.2 | 102.9 | 103.0 | 0.1 | 0.027 | 0.034 | 0.240 | 0.010 |
| 1 h | 104.3 | 104.1 | 104.2 | 103.8 | 103.9 | 0.2 | 104.9 | 103.2 | 0.1 | 0.365 | <0.001 | 0.278 | 0.763 |
| 2 h | 104.5b | 104.3ab | 104.1ab | 103.9ab | 103.8a | 0.2 | 105.4 | 102.9 | 0.1 | 0.048 | <0.001 | 0.036 | 0.356 |
| 3 h | 104.2 | 104.0 | 104.2 | 103.7 | 103.8 | 0.2 | 105.3 | 102.7 | 0.1 | 0.262 | <0.001 | 0.315 | 0.706 |
1Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk. Each mean represents observations on 24 pigs for dietary treatment and 60 pigs for LPS challenge (+/−).
2LSmeans for the main effect of LPS challenge.
3 P-values for the main effects of dietary treatment and LPS challenge, and for the linear and quadratic contrasts of supplementing Low diets with increasing levels of fish oil. The interactions between dietary treatment and LPS challenge, or dietary treatment and time after LPS challenge were not significant, therefore are not presented.
4Maximum value of standard error of the means for dietary treatment.
5Maximum value of standard error of the means for LPS challenge.
a,bValues with different letters within the same row differ (P < 0.05).
Apparent ileal digestibility of OM and CP and gut morphology were not influenced by the interaction between dietary treatment and LPS. Apparent ileal digestibility of OM and CP was reduced for pigs that were immune challenged with LPS vs. those that received saline (P < 0.05; Table 5). Apparent ileal digestibility of OM was influenced by dietary treatment (P < 0.05), and was lower for pigs that received the Low0 diet vs. those that received the High diet (P < 0.05); intermediate apparent ileal digestibilities of OM were observed for pigs fed Low diets supplemented with fish oil. Jejunal villus height and villus:crypt ratio were reduced, and crypt depth was increased for pigs challenged with LPS vs. those that received saline (P < 0.01; Table 5). Villus height was reduced for pigs fed Low diets that included 0% to 2.5% fish oil but was increased for pigs fed diets containing 5% fish oil (quadratic; P < 0.05); crypt depth decreased with increasing fish oil supplementation for pigs fed the Low diets (linear; P < 0.05).
Table 5.
Effect fish oil inclusion in low protein quality diets on apparent ileal digestibility of organic matter and crude protein and jujunal morphology for pigs that received LPS or saline on day 21 after weaning
| Dietary treatment1 | LPS challenge2 | P-values3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | High | Low0 | Low1.25 | Low2.5 | Low5 | SEM4 | + | − | SEM5 | Diet | LPS | Linear | Quadratic |
| Apparent ileal digestibility, % | |||||||||||||
| Organic matter | 74.8b | 64.0a | 71.5ab | 70.1ab | 70.8ab | 2.5 | 67.8 | 72.7 | 1.5 | 0.039 | 0.021 | 0.158 | 0.162 |
| Crude protein | 60.9 | 60.2 | 67.0 | 64.2 | 60.5 | 4.1 | 54.6 | 70.5 | 2.5 | 0.672 | <0.001 | 0.795 | 0.250 |
| Jejunal morphology, µm | |||||||||||||
| Villus height | 456 | 488 | 456 | 443 | 473 | 16 | 436 | 490 | 9 | 0.248 | <0.001 | 0.630 | 0.023 |
| Crypt depth | 273 | 292 | 285 | 269 | 253 | 16 | 291 | 257 | 11 | 0.255 | 0.005 | 0.014 | 0.878 |
| Villus:crypt ratio | 1.83 | 1.78 | 1.70 | 1.72 | 1.97 | 0.11 | 1.59 | 2.01 | 0.07 | 0.413 | <0.001 | 0.112 | 0.166 |
1Dietary treatments: [1] positive control (High; contained multiple sources of proteins, including animal proteins and 5% corn oil), [2] negative control (Low0; contained soybean meal as the main protein source and 5% corn oil), or 1 of 3 Low diets supplemented with increasing levels of fish oil to replace corn oil: [3] 1.25% fish oil (Low1.25), [4] 2.5% fish oil (Low2.5), [5] 5% fish oil (Low5) fed in 2 phases (7 and 14 d, respectively). All pigs received common phase III diets thereafter for 3 wk. Each mean represents observations on 24 pigs for dietary treatment and 60 pigs for LPS challenge (+/−).
2LSmeans for the main effect of LPS challenge.
3 P-values for the main effects of dietary treatment and LPS challenge, and for the linear and quadratic contrasts of supplementing Low diets with increasing levels of fish oil. The interaction between dietary treatment and LPS was not significant for apparent ileal digestibility of OM and CP or gut morphology, therefore are not presented.
4Maximum value of standard error of the means for the main effect of dietary treatment.
5Maximum value of standard error of the means for the main effect of LPS challenge.
a,bValues with different letters within the same row differ (P < 0.05).
DISCUSSION
To minimize the postweaning growth lag and promote feed intake, newly weaned pigs are often offered diets containing high-quality, animal-derived protein sources that are highly palatable and digestible (Dritz et al., 1996; Sulabo et al., 2010). However, these diets are more expensive than corn- and SBM-based diets. Recent research has suggested that newly weaned pigs can be introduced to simplified or low-quality protein diets without compromising lifetime growth performance, or carcass value at slaughter (Skinner et al., 2014). During a disease challenge, however, the growth performance of weanling pigs fed corn-SBM-based diets was permanently impacted (Skinner et al., 2014), and the adaptive immune response was compromised (Levesque et al., 2013). In this study, including fish oil up to 2.5% in nursery diets containing low-quality proteins quadratically improved growth performance (BW gain, feed intake, and feed efficiency) during the first week after weaning, and tempered the antibody and DHR immune responses to a novel antigen (OVA), while any inclusion level of fish oil in low-quality protein diets moderated the release of the acute-phase protein Hp and improved the apparent ileal digestibility of OM. During LPS immune challenge, which was used to reproduce an acute microbial infection and corresponding inflammatory response, fish oil inclusion into low-quality protein diets lowered body temperature 2 h after the challenge. Notably, the baseline rectal temperature of pigs fed the highest level of fish oil (5%) before LPS immune challenge was also reduced relative to pigs fed lower inclusion levels of fish oil, indicating that basal inflammation 21 d after weaning was also reduced. Therefore, optimizing the ω-6-to-ω-3 fatty acid ratio in nursery diets that contain low-quality protein sources (i.e., SBM) can induce favorable partitioning of dietary nutrients toward BW gain vs. disproportionate inflammation (i.e., improved feed efficiency by reducing maintenance energy expenditure; Gaines et al., 2003; Liu et al., 2003), and may be used to moderate immune responses during various forms of immune system stimulation.
Although many studies have focused on effects of absolute concentrations of ω-3 fatty acids in diets on immune responses, the ratio of ω-6-to-ω-3 fatty acids appears to be more influential for maximizing growth performance (Duan et al., 2014) and modulating the immune response (Broughton et al., 1991). The dietary ratio of ω-6-to-ω-3 fatty acids in the High diet was similar to present Western diets for humans (i.e., 20:1), which has been shown to increase production of pro-inflammatory mediators (Simopoulos, 2008). Conversely, the Low5 diet contained an ω-6-to-ω-3 fatty acid ratio of approximately 1:1; diets containing a reduced ω-6-to-ω-3 ratio are considered immunomodulatory and protective against inflammation (as reviewed by Simopoulos, 2008). In the present study, the ω-6-to-ω-3 ratio of 3:1 maximized growth performance during the first week after weaning for pigs fed low-quality proteins; higher inclusion levels of fish oil (5%; ω-6-to-ω-3 fatty acid ratio of 1:1) in nursery diets negatively impacted feed intake and consequently BW gain during the first week after weaning, likely due to palatability issues or inadequate amino acid intake, but was most effective at reducing body temperature during LPS immune challenge. Therefore, depending on the desired outcome, the optimum ω-6-to-ω-3 fatty acid ratio may differ, which corresponds to previous work (Duan et al., 2014). Moreover, feeding nursery diets with low-quality proteins (SBM) only impacted growth performance during the first week after weaning, when inclusion of fish oil was most efficacious; thereafter, pigs adapted to the low-quality diets, even without the inclusion of fish oil. The carryover effects on immune responses after a relatively short duration of feeding fish oil should be considered in future studies.
In the current study, pigs that received nursery diets with fish oil supplemented between 1.25% and 5% (ω-6-to-ω-3 fatty acid ratio between 5:1 and 1:1) had reduced concentrations of the Hp serum acute-phase protein compared to those fed 0% fish oil. This is consistent with other reports in pigs (Bazinet et al., 2004), ewes (Stryker et al., 2013), and humans (Ernst et al., 1991) fed diets with reduced ω-6-to-ω-3 fatty acid ratios. During immune system stimulation, cytokines are released into circulation by inflammatory cells such as neutrophil granulocytes and macrophages, which induce the liver to produce acute-phase proteins such as Hp. Decreasing the dietary ω-6-to-ω-3 fatty acid ratio may reduce the production of arachidonic acid-derived eicosanoids (e.g., prostaglandins E2 and leukotriene β4; Dinarello, 1998; Thies et al., 1999; Liu et al., 2003), and consequently reduce the production of pro-inflammatory cytokines. Previous studies have demonstrated that pigs receiving diets supplemented with 5% to 7% fish oil had lower concentrations of plasma cytokines (i.e., interleukin-1β, tumor necrosis factor-α, and interferon-γ) vs. pigs that received diets supplemented with 6% to 7% corn oil (Carroll et al., 2003; Gaines et al., 2003; Liu et al., 2003). Although inflammatory cytokines were not measured in the current study, it is likely that the blunted acute-phase and body temperature responses observed for pigs that received diets supplemented with fish oil were due to reduced inflammatory cytokine production.
With respect to acquired immunity, OVA-specific IgG was detected in all pigs that were immune sensitized; greater responses were observed on day 34 than on day 20, which indicated that the immunization protocol was efficacious. Similar protocols have been used effectively in pigs, sheep, and cows (Bazinet et al., 2004; Crawley et al., 2005; Stryker et al., 2013). In the current study, anti-OVA IgG during the secondary response tended to be reduced for pigs fed diets containing low-quality proteins with decreasing ω-6-to-ω-3 fatty acid ratios (linear). This is in agreement with a previous study that found the antibody-mediated immune responses were reduced in mice fed diets high in fish oil (Harbige, 2001). Furthermore, in the current study, supplementing Low diets with 2.5% fish oil (i.e., ω-6-to-ω-3 fatty acid ratio of 3:1) attenuated the DHR, which likely involved both antibodies and granulocytes. Therefore, reducing the ω-6-to-ω-3 fatty acid ratio to 3:1 seems to provide growth benefits, while still attenuating inflammation to some degree. It is important to note that some innate inflammation is important because it helps to drive the immune response, while excessive inflammation contributes to the pathology of many diseases (especially acute microbial infection), is damaging to tissues, and triggers sickness behavior that reduces appetite and growth (e.g., Liu et al., 2003).
In the current study, immune system stimulation 21 d after weaning with a single intramuscular injection of LPS severely impacted jejunal morphology within 3-h post-challenge, but dietary supplementation with fish oil did not provide a protective effect, contrary to previous research (Liu et al., 2012). However, among all pigs (saline + LPS) fed the Low diets, jejunal villus height was increased (at 5% inclusion level of fish oil) and crypt depth decreased with increasing supplementation of fish oil. Increased crypt depth indicates increased cell production (Lauronen et al., 1998; Mei and Xu, 2005) and typically occurs in response to villus atrophy during periods of recovery or regeneration (e.g., during postweaning transition in young pigs; Smith et al., 1984; Pluske et al., 1997). Therefore, increasing the inclusion level of fish oil during the first 3 wk after weaning may protect the microstructure of the jejunum when the newly weaned pig is exposed to foreign immune-reactive plant proteins and perturbations in feed intake during the early weaning phase. Furthermore, the inclusion of fish oil to low protein quality diets improved the apparent ileal digestibility of OM, which may be a result of the superior absorptive surface of the jejunum. It is also possible that diet composition influenced the diversity of the gut microbiome, which may impact apparent nutrient digestibility, although previous research in our laboratory demonstrated limited diet-induced effects on the gut microbiome (Hooda et al., 2016).
In conclusion, nursery diets containing low-quality proteins from SBM appear to influence growth performance only during the early phase of the postweaning period, after which pigs are able to adapt to a corn- and SBM-based diet. When fed diets with low protein quality, fish oil quadratically improved feed efficiency during the first week after weaning, attenuated acute-phase protein production, and reduced the IgG response and DHR to antigen challenge as well as body temperature after an acute microbial infection. Therefore, reducing the ω-6-to-ω-3 fatty acid ratio is a potential nutritional method to improve feed efficiency and moderate immune reactions after weaning and when pigs are vulnerable to acute microbial infection. The benefit of immune-modulatory nutrients, such as ω-3 fatty acids, during common and multifactorial disease challenges that occur on commercial farms should be examined in future studies. Moreover, sources of ω-3 fatty acids other than fish oil that are both economical and environmentally sustainable (e.g., cultured algae meal) must be investigated.
ACKNOWLEDGMENTS
Financial support was provided by the Ontario Ministry of Agriculture, Food and Rural Affairs (Guelph, ON, Canada); Ontario Pork (Guelph, ON, Canada); Canadian Agriculture and Agri-food Swine Research Cluster (Swine Innovation Porc; Ottawa, ON, Canada).
LITERATURE CITED
- AOAC 1997. Official methods of analysis. 16th ed. Assoc. Off. Anal. Chem, Washington, DC. [Google Scholar]
- Bazinet R. P., H. Douglas E. G. McMillan B. N. Wilkie, and Cunnane S. C.. 2004. Dietary 18:3omega3 influences immune function and the tissue fatty acid response to antigens and adjuvant. Immunol. Lett. 95:85–90. doi: 10.1016/j.imlet.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Begley N., Bucklet F., Burnside E. B., Schaeffer L., Pierce K., and Mallard B. A.. 2008. Immune responses of Holstein and Norwegian Red Holstein calves on Canadian dairy farms. J. Dairy Sci. 92:518–525. doi: 10.3168/jds.2008-1300 [DOI] [PubMed] [Google Scholar]
- Bligh E. G. and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. doi: 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- Broughton K. S., J. Whelan I. Hardardottir, and Kinsella J. E.. 1991. Effect of increasing the dietary (n-3) to (n-6) polyunsaturated fatty acid ratio on murine liver and peritoneal cell fatty acids and eicosanoid formation. J. Nutr. 121:155–164. doi: 10.1093/jn/121.2.155 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care 2009. Guidelines on the care and use of farm animals in research, teaching and testing. CCAC, Ottawa, ON, Canada. [Google Scholar]
- Carleton H. M., Drury R. A. B., and Wallington E. A.. 1980. Carleton’s histological techniques. 5th ed. E. A., Wallington, editor. Oxford-New York-Toronto: Oxford University Press. [Google Scholar]
- Carroll J. A., A. M. Gaines J. D. Spencer G. L. Allee H. G. Kattesh M. P. Roberts, and Zannelli M. E.. 2003. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs. I. Effects on the immune axis when fed diets containing spray-dried plasma. Domest. Anim. Endocrinol. 24:341–351. doi:10.1016/S0739-7240(03)00017-1 [DOI] [PubMed] [Google Scholar]
- Chen C. C., Lee T. T., Bin Hsu C., Huang C. W., and Yu B.. 2011. Associations of allergenic soybean proteins with piglet skin allergic reaction and application of polyclonal antibodies. Anim. Prod. Sci. 51:1008–1014. doi:10.1071/AN11142 [Google Scholar]
- Cervantes-Pahm S. K. and Stein H. H.. 2010. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J. Anim. Sci. 88:2674–2683. doi: 10.2527/jas.2009-2677 [DOI] [PubMed] [Google Scholar]
- Columbus D., S. J. Niven C. L. Zhu, and de Lange C. F.. 2010. Phosphorus utilization in starter pigs fed high-moisture corn-based liquid diets steeped with phytase. J. Anim. Sci. 88:3964–3976. doi: 10.2527/jas.2010-3011 [DOI] [PubMed] [Google Scholar]
- Crawley A. M., B. Mallard, and Wilkie B. N.. 2005. Genetic selection for high and low immune response in pigs: effects on immunoglobulin isotype expression. Vet. Immunol. Immunopathol. 108:71–76. doi: 10.1016/j.vetimm.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 856:1–11. doi:10.1111/j.1749-6632.1998.tb08307.x [DOI] [PubMed] [Google Scholar]
- Dritz S. S., K. Q. Owen J. L. Nelssen R. D. Goodband, and Tokach M. D.. 1996. Influence of weaning age and nursery diet complexity on growth performance and carcass characteristics and composition of high-health status pigs from weaning to 109 kilograms. J. Anim. Sci. 74:2975–2984. doi:10.2527/1996.74122975x [DOI] [PubMed] [Google Scholar]
- Duan Y., F. Li L. Li J. Fan X. Sun, and Yin Y.. 2014. N-6:n-3 PUFA ratio is involved in regulating lipid metabolism and inflammation in pigs. Br. J. Nutr. 111:445–451. doi: 10.1017/S0007114513002584 [DOI] [PubMed] [Google Scholar]
- Ernst E., T. Saradeth, and Achhammer G.. 1991. N-3 fatty acids and acute-phase proteins. Eur. J. Clin. Invest. 21:77–82. [DOI] [PubMed] [Google Scholar]
- Furesz S. E., B. A. Mallard J. T. Bossé S. Rosendal B. N. Wilkie, and MacInnes J. I.. 1997. Antibody- and cell-mediated immune responses of Actinobacillus pleuropneumoniae-infected and bacterin-vaccinated pigs. Infect. Immun. 65:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines A. M., J. A. Carroll G. F. Yi G. L. Allee, and Zannelli M. E.. 2003. Effect of menhaden fish oil supplementation and lipopolysaccharide exposure on nursery pigs. II. Effects on the immune axis when fed simple or complex diets containing no spray-dried plasma. Domest. Anim. Endocrinol. 24:353–365. doi: 10.1016/S0739-7240(03)00016-X [DOI] [PubMed] [Google Scholar]
- Harbige L. S. and Fisher B. A.. 2001. Dietary fatty acid modulation of mucosally-induced tolerogenic immune responses. Proc. Nutr. Soc. 60:449–456. doi: 10.1079/PNS2001123 [DOI] [PubMed] [Google Scholar]
- Heinonen J. K. and Lahti R. J.. 1981. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphate. Anal. Biochem. 113:313–317. [DOI] [PubMed] [Google Scholar]
- Hooda S., Levesque C. L., Lepp D., Yu H., Gong J., and de Lange C. F. M.. 2016. Ileal mucosa and digesta associated microbiota of starter pigs and changes linked to time post weaning and dietary interventions. J. Anim. Sci. 94: 344–348. doi: 10.2527/jas2015-9749 [DOI] [Google Scholar]
- Lallès J. P., Boudry G., Favier C., LeFloc’h N., Luron I., Montagne L., Oswald I. P., Pié S., Piel C., and Sève B.. 2004. Gut function and dysfunction in young pigs: physiology. Anim. Res. 53: 301–316. doi: 10.1051/animres:2004018 [DOI] [Google Scholar]
- Lauronen J., M. P. Pakarinen P. Kuusanmäki E. Savilahti P. Vento T. Paavonen, and Halttunen J.. 1998. Intestinal adaptation after massive proximal small-bowel resection in the pig. Scand. J. Gastroenterol. 33:152–158. doi: 10.1080/00365529850166879 [DOI] [PubMed] [Google Scholar]
- Levesque C. L., Miller E., Zhu J., de Lange K.. 2013. Simple assessment of piglet robustness in relation to nursery diet quality and feeding antibiotics. J. Anim. Sci. 91(Suppl. 2):093 (Abstr.) [Google Scholar]
- Li D. F., J. L. Nelssen P. G. Reddy F. Blecha R. Klemm, and Goodband R. D.. 1991. Interrelationship between hypersensitivity to soybean proteins and growth performance in early-weaned pigs. J. Anim. Sci. 69:4062–4069. [DOI] [PubMed] [Google Scholar]
- Liu Y., F. Chen J. Odle X. Lin S. K. Jacobi H. Zhu Z. Wu, and Hou Y.. 2012. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 142:2017–2024. doi: 10.3945/jn.112.164947 [DOI] [PubMed] [Google Scholar]
- Liu Y. L., D. F. Li L. M. Gong G. F. Yi A. M. Gaines, and Carroll J. A.. 2003. Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after an escherichia coli lipopolysaccharide challenge. J. Anim. Sci. 81:2758–2765. doi: 10.2527/2003.81112758x [DOI] [PubMed] [Google Scholar]
- Mei J. and Xu R. J.. 2005. Transient changes of transforming growth factor-beta expression in the small intestine of the pig in association with weaning. Br. J. Nutr. 93:37–45. doi: 10.1079/BJN20041302 [DOI] [PubMed] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. 2004. Technical note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–783. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- NRC.. 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Simopoulos A. P. 2008. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 233:674–688. doi: 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- Skiba G. 2005. Physiological aspects of compensatory growth in pigs. J. Anim. Feed Sci. 14:191–203. doi: 10.22358/jafs/70362/2005 [DOI] [Google Scholar]
- Skinner L. D., C. L. Levesque D. Wey M. Rudar J. Zhu S. Hooda, and de Lange C. F.. 2014. Impact of nursery feeding program on subsequent growth performance, carcass quality, meat quality, and physical and chemical body composition of growing-finishing pigs. J. Anim. Sci. 92:1044–1054. doi: 10.2527/jas.2013-6743 [DOI] [PubMed] [Google Scholar]
- Smith M. W., J. Y. Patterson, and Peacock M. A.. 1984. A comprehensive description of brush border membrane development applying to enterocytes taken from a wide variety of mammalian species. Comp. Biochem. Physiol. A. Comp. Physiol. 77:655–662. [DOI] [PubMed] [Google Scholar]
- Stryker J. A., R. Fisher Q. You M. M. Or-Rashid H. J. Boermans M. Quinton B. W. McBride, and Karrow N. A.. 2013. Effects of dietary fish meal and soybean meal on the ovine innate and acquired immune response during pregnancy and lactation. Animal 7:151–159. doi: 10.1017/S175173111200136X [DOI] [PubMed] [Google Scholar]
- Sulabo R. C., M. D. Tokach J. M. Derouchey S. S. Dritz R. D. Goodband, and Nelssen J. L.. 2010. Influence of feed flavors and nursery diet complexity on preweaning and nursery pig performance. J. Anim. Sci. 88:3918–3926. doi: 10.2527/jas.2009-2724 [DOI] [PubMed] [Google Scholar]
- Themelis D. G., Tzanavaras P. D., Trellopoulos A. V., and Sofoniou M. C.. 2001. Direct and selective flow-injection method for the simultaneous spectrophotometric determination of calcium and magnesium in red and white wines using online dilution based on zone sampling. J. Agric. Food Chem. 49:5152–5155. doi: 10.1021/jf0107353 [DOI] [PubMed] [Google Scholar]
- Thies F., L. D. Peterson J. R. Powell G. Nebe-von-Caron T. L. Hurst K. R. Matthews E. A. Newsholme, and Calder P. C.. 1999. Manipulation of the type of fat consumed by growing pigs affects plasma and mononuclear cell fatty acid compositions and lymphocyte and phagocyte functions. J. Anim. Sci. 77:137–147. doi:10.2527/1999.771137x [DOI] [PubMed] [Google Scholar]
- Tokach M. D., Goodband R. D., and Nelssen J. L.. 1994. Recent developments in nutrition for the early-weaned pig. Comp. Cont. Ed. Pract. Vet. 16:407–419. [Google Scholar]
- Whang K. Y., F. K. McKeith S. W. Kim, and Easter R. A.. 2000. Effect of starter feeding program on growth performance and gains of body components from weaning to market weight in swine. J. Anim. Sci. 78:2885–2895. doi: 10.2527/2000.78112885x [DOI] [PubMed] [Google Scholar]