Abstract
Familial male-limited precocious puberty (FMPP) is an autosomal dominant, male-limited disorder that causes peripheral precocious puberty in boys. Klinefelter syndrome (47, XXY) is the most common chromosomal aberration in males with associated infertility, hypogonadism, and learning disability. We report here a case of Klinefelter syndrome in a patient with FMPP. A 6-year-old boy was referred to our pediatric endocrinology department for accelerated linear growth and premature pubic hair development. He was diagnosed with FMPP based on clinical, laboratory, and genetic sequencing. Increased levels of gonadotropins prompted further investigation, leading to a subsequent diagnosis of Klinefelter syndrome through karyotype analysis. This case illustrates that patients with FMPP and elevated gonadotropins should encourage further investigation by physicians. We recommend the use of karyotype analysis in such patients who are not receiving aromatase inhibitor therapy. We hypothesize that his mutation or pretreatment with aromatase inhibitors may have a protective effect on testosterone production and sperm viability.
Keywords: aromatase inhibitor, familial male-limited precocious puberty, gonadotropins, Klinefelter syndrome, testosterone, testotoxicosis
Familial male-limited precocious puberty (FMPP), also known as testotoxicosis, is a form of GnRH-independent precocious puberty in males inherited in an autosomal dominant pattern. It was first described in 1981 by Schedewie et al. during a study of two, 2-year-old brothers with rapid virilization, increased bone age, and advanced spermatogenesis on testis biopsy [1]. It generally presents between 2 and 4 years of age, with advanced sexual development. Increased testicular volume and accelerated growth rate are commonly observed [2]. Testosterone levels are within adult male ranges with low levels of LH and FSH. Treatment options include androgen receptor antagonists, GnRH agonists, and aromatase inhibitors [3]. We present a case of FMPP in a patient with Klinefelter syndrome.
1. Case Description
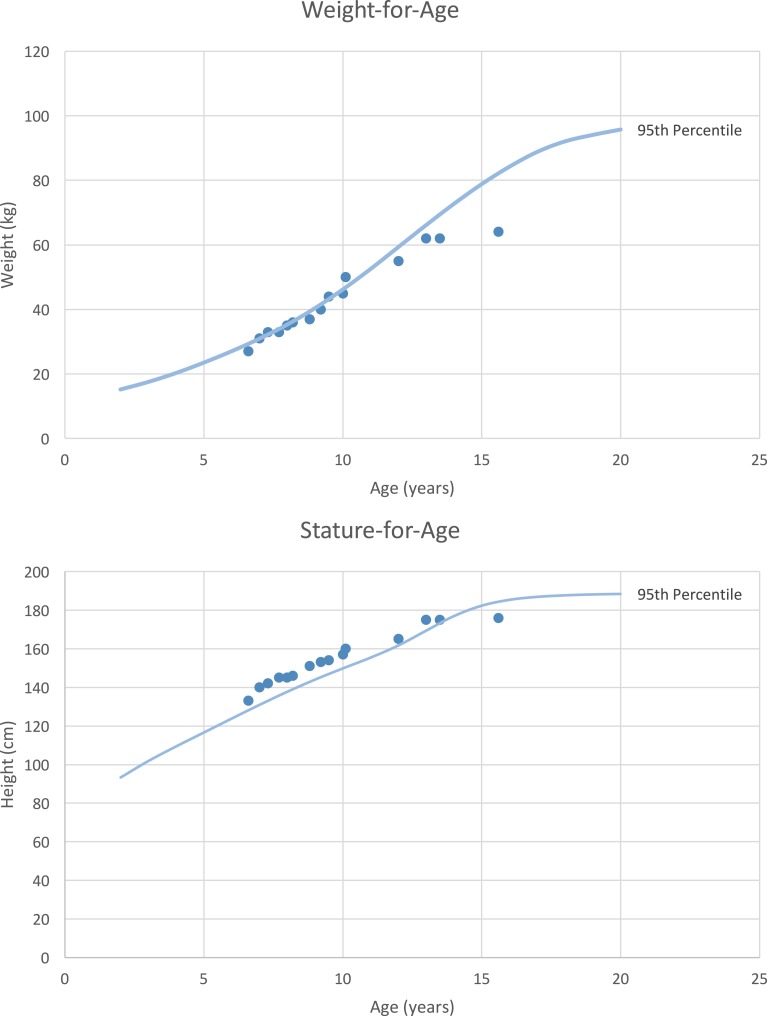
A 6-year, 4-month-old boy was referred to our pediatric endocrinology department by his pediatrician for complaints of pubic hair development and accelerated linear growth. His parents were healthy and there was no family history of precocious puberty. His mother’s and father’s heights were 162.5 cm and 177.8 cm, respectively, giving the patient a midparental height of 176.5 cm. On physical examination, his height was 132.5 cm [+3.4 SD score (SDS)] and weight was 27.9 kg (+1.82 SDS) (Fig. 1). Axillary hair was consistent with Tanner stage 1 and pubic hair with Tanner stage 3. Penile stretch length was 11.5 cm (>+2 SDS) and testicular volume was 2 mL bilaterally. He had no dysmorphic features, gynecomastia, café au lait spots, or abdominal masses. His bone age (BA) was 11 years and 6 months at a chronological age (CA) of 6 years and 2 months (BA/CA: 1.86).
Figure 1.
Patient’s longitudinal growth chart for weight and height compared with the 95th percentile for boys age 2 to 20 years.
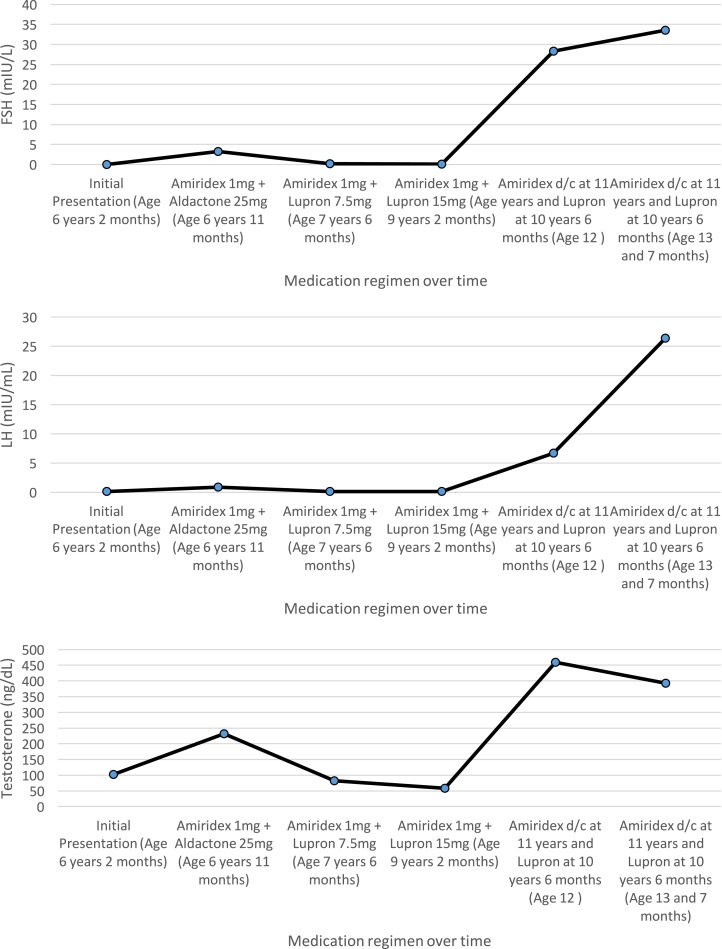
Laboratory data were inconsistent for central precocious puberty (CPP), congenital adrenal hyperplasia, adrenal tumor, or β human chorionic gonadotropin–producing germinoma (Table 1). Remaining differentials included exogenous testosterone exposure and an activating mutation of the LH receptor. However, because none of his family members were using testosterone gel, genetic sequencing of the LH/choriogonadotropin receptor (LHCGR) was performed at Athena Diagnostics. This revealed a nucleotide change of c.A1733G corresponding to an amino acid change of p.Asp578Gly at the transmembrane VI domain, confirming a diagnosis of FMPP. A combination of anastrozole 1 mg and spironolactone 25 mg twice per day by mouth treatment was initiated; however, spironolactone had to be discontinued because of severe stomach discomfort. At the 9-month follow-up, his growth rate accelerated and laboratory tests were repeated, demonstrating that the patient had developed CPP as well. Leuprolide 7.5 mg IM monthly was added to his therapeutic regimen; over the course of approximately 3 years, his dose was gradually increased to 15 mg IM monthly. Lower doses were unable to suppress his gonadotropins to a prepubertal level. His dose was increased to 30 mg IM every 3 months because variable dosing of leuprolide has been shown to achieve adequate hormonal suppression [4]. He discontinued GnRH analog and aromatase inhibitor therapy at chronological age 10 years and 6 months at the request of his parents. Bone age was repeated and was consistent with 12 years and 6 months (BA/CA: 1.18). The patient was lost to follow-up and returned to the clinic at the age of 12. His testicular volume was 8 mL bilaterally and his repeat blood work showed a rebound increase in serum testosterone level to 459 ng/dL, increased LH of 6.7 mIU/mL, and FSH to 28.3 mIU/mL (Fig. 2). The elevated gonadotropin levels suggested potential medication side effect or chromosomal abnormality. A karyotype was obtained and confirmed Klinefelter syndrome (47, XXY), providing an explanation for his elevated gonadotropins. Given that the patient has Klinefelter syndrome, the possibility of needing testosterone replacement therapy in the future was discussed and the family was encouraged to obtain a consultation with urology to discuss a microdissection testicular sperm extraction procedure to preserve fertility. The patient has been followed every 6 months and, despite having persistently elevated gonadotropin levels, he maintains a normal testosterone level 594 ng/dL (reference range, 300 to 950 ng/dL) 4 years after discontinuation of therapy.
Table 1.
Patient’s Laboratory Values on Admission
| FSH, mIU/mL | <0.1 |
| LH, mIU/mL | 0.1 |
| Testosterone, ng/dL | 103 |
| Dehydroepiandrosterone sulfate, μg/dL | 18 |
| β-human chorionic gonadotropin, mIU/mL | <2 |
| Adrenocorticotropin hormone, pg/mL | 16 |
| Cortisol, μg/dL | 8.2 |
| 17-hydroxyprogesterone, ng/dL | <40 |
| 11-deoxycorticosterone, ng/dL | 36 |
Figure 2.
Longitudinal serum concentrations of changes of FSH, LH, and testosterone are shown according to varying treatment modalities and age.
2. Discussion
FMPP results from an activating mutation of the LH receptor expressed in testicular Leydig cells [5]. The constitutively active receptor results in GnRH-independent production of testosterone and subsequent precocious puberty. In 1993, Shenker et al. suggested that FMPP might be due to a mutated G protein–coupled receptor, which is activated without an agonist. They discovered that a single base change from adenine to guanine resulted in an amino acid change of aspartate to glycine [6]. This missense mutation, present in our patient, is found on transmembrane VI domain at position 578, and accounts for 56% of all mutations found in FMPP [7]. As a result of this activating mutation, patients commonly present with signs of puberty by the age of 4 years. Associated increases in testosterone and decreased levels of LH are also demonstrated [3].
No treatment guidelines have been established for FMPP. Evidence of effective treatment modalities has been weak, primarily because of the limited number of reported cases, small sample sizes, and short-term outcomes. Historically, FMPP has been treated with ketoconazole, an androgen receptor antagonist [8]. Treatment of patients with ketoconazole has resulted in decreased testosterone and growth velocity, but has shown limited efficacy in attaining normal adult height [9, 10]. More recently, a combination of an aromatase inhibitor and antiandrogen has been used in the treatment of FMPP. A review of cases reported by Özcabı et al. demonstrated that combination therapy with anastrozole and bicalutamide was effective in lowering testosterone synthesis and reducing virilization [11].
Treatment of FMPP is often complicated by early onset of CPP. Long-term exposure to sex steroids can induce maturation of the hypothalamic center, leading to central precocious puberty requiring GnRH analog therapy [12]. Because estrogen acts as the primary feedback inhibitor of gonadotropin release, decreased levels from aromatase inhibitor therapy can lead to testicular enlargement and elevated gonadotropin levels [13]. However, our patient demonstrated elevated gonadotropin levels 2 years after discontinuation of anastrozole. This led us to consider the possibility of testicular damage as a long-term side effect of anastrozole vs the possibility of Klinefelter syndrome.
Additionally, a testosterone receptor antagonist was not used in our treatment regimen during a period in which the patient’s bone age advanced only 1 year over a duration of 4 years. This suggests that estrogen, not testosterone, is indeed the main hormone that promotes bone fusion [14]. This raises the question of whether a testosterone antagonist as an adjunctive therapy is needed to maximize the final height in these patients.
3. Conclusion
This case report highlights the importance of using karyotype analysis in FMPP patients with elevated gonadotropins not receiving aromatase inhibitor therapy. Our findings demonstrate that our patient was able to maintain normal testosterone levels for 4 years after discontinuation of therapy. The exact mechanism is unclear whether this could be due to an activating mutation in the LH receptor, the use of long-term aromatase inhibitors, or a combination of both. Further investigation on whether aromatase inhibitors have a protective effect is needed in patients with Klinefelter syndrome. We suggest that aromatase inhibitors may potentially have a protective effect on the testes in prolonging testosterone production and protecting sperm viability.
Although numerous cases of FMPP have been documented in literature, this is a rare disorder with an estimated prevalence of <1/1,000,000. Klinefelter syndrome, on the other hand, is one of the most common genetic disorders, with an estimated frequency of 1 in 500 to 1 in 1000 males. Klinefelter syndrome associated with FMPP has not been reported until now, and the chance of having both is estimated at 1 in one-half of a billion to 1 in a billion.
Acknowledgments
Informed consent was obtained from the participant and the institution approved the investigation.
Disclosure Summary: The authors have nothing to disclose.
Glossary
- Abbreviations: BA
bone age
- CA
chronological age
- CPP
central precocious puberty
- FMPP
familial male-limited precocious puberty
- SDS
SD score
References and Notes
- 1. Schedewie HK, Reiter EO, Beitins IZ, Seyed S, Wooten VD, Jimenez JF, Aiman EJ, DeVane GW, Redman JF, Elders MJ. Testicular Leydig cell hyperplasia as a cause of familial sexual precocity. J Clin Endocrinol Metab. 1981;52(2):271–278. [DOI] [PubMed] [Google Scholar]
- 2. Aziz AA, Jafri SM, Haque NU. Testotoxicosis: gonadotrophin-independent male sexual precocity. Postgrad Med J. 1992;68(797):225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leschek EW, Flor AC, Bryant JC, Jones JV, Barnes KM, Cutler GB Jr. Effect of antiandrogen, aromatase inhibitor, and gonadotropin-releasing hormone analog on adult height in familial male precocious puberty. J Pediatr. 2017;190:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen D, Janfaza M, Klein KO. Importance of leuprolide acetate variable dosing for precocious puberty: a range of acceptable suppression. J Pediatr Endocrinol Metab. 2009;22(7):629–634. [DOI] [PubMed] [Google Scholar]
- 5. Kawate N, Kletter GB, Wilson BE, Netzloff ML, Menon KMJ. Identification of constitutively activating mutation of the luteinising hormone receptor in a family with male limited gonadotrophin independent precocious puberty (testotoxicosis). J Med Genet. 1995;32(7):553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shenker A, Laue L, Kosugi S, Merendino JJ Jr, Minegishi T, Cutler GB Jr. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365(6447):652–654. [DOI] [PubMed] [Google Scholar]
- 7. Laue L, Chan WY, Hsueh AJ, Kudo M, Hsu SY, Wu SM, Blomberg L, Cutler GB Jr. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci USA. 1995;92(6):1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holland FJ, Kirsch SE, Selby R. Gonadotropin-independent precocious puberty (“testotoxicosis”): influence of maturational status on response to ketoconazole. J Clin Endocrinol Metab. 1987;64(2):328–333. [DOI] [PubMed] [Google Scholar]
- 9. Bertelloni S, Baroncelli GI, Lala R, Cappa M, Matarazzo P, De Sanctis C, Saggese G. Long-term outcome of male-limited gonadotropin-independent precocious puberty. Horm Res. 1997;48(5):235–239. [DOI] [PubMed] [Google Scholar]
- 10. Almeida MQ, Brito VN, Lins TS, Guerra-Junior G, de Castro M, Antonini SR, Arnhold IJ, Mendonca BB, Latronico AC. Long-term treatment of familial male-limited precocious puberty (testotoxicosis) with cyproterone acetate or ketoconazole. Clin Endocrinol (Oxf). 2008;69(1):93–98. [DOI] [PubMed] [Google Scholar]
- 11. Özcabı B, Tahmiscioğlu Bucak F, Ceylaner S, Özcan R, Büyükünal C, Ercan O, Tüysüz B, Evliyaoğlu O. Testotoxicosis: report of two cases, one with a novel mutation in LHCGR gene. J Clin Res Pediatr Endocrinol. 2015;7(3):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update. 2001;7(3):292–302. [DOI] [PubMed] [Google Scholar]
- 13. Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF Jr. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85(9):3027–3035. [DOI] [PubMed] [Google Scholar]
- 14. Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev. 2017;97(1):135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]