Abstract
Background: Bevacizumab-based regimens are used as standard treatments for colorectal cancer. Unfortunately, there are no established predictive markers for bevacizumab response. Methods: Tumor samples from 36 metastatic colorectal cancer patients treated with bevacizumab plus chemotherapy were analyzed by next-generation sequencing of all coding exons of more than 400 genes. Single gene and signaling pathway analyses were performed to correlate genomic data with response. Results: Among the genes most frequently mutated in our cohort, only mutations in PTPRT, a phosphatase involved in JAK/STAT signaling, were associated with response status, with deleterious mutations being enriched in non-responders. Pathway analysis revealed that deleterious mutations in genes of the JAK/STAT pathway, namely in PTPRT and the related gene PTPRD, correlated with resistance. Mutations in RTK/PI3K/RAS, Wnt and TGFβ pathways did not associate with response. Lack of response was observed in all patients with deleterious mutations or copy number loss of PTPRT/PTPRD (n = 10), compared to only 30.8% (n = 8) of patients without such alterations (relative risk, 3.25; 95% CI, 1.83–5.79, p = 0.0003). Similarly, PTPRT/PTPRD deleterious alterations were associated with shorter progression-free survival, an association that was retained in multivariate analysis (HR, 3.33; 95% CI, 1.47–7.54; p = 0.0038). Conclusion: Deleterious alterations in PTPRT/PTPRD are potential biomarkers for bevacizumab resistance.
Keywords: metastatic colorectal cancer, bevacizumab resistance, next-generation sequencing, VEGF, PTPRT/PTPRD mutation and deletion
1. Introduction
Colorectal cancer (CRC) is one of the most common cancer types worldwide [1]. It accounts for 8.5% of all tumor-related mortality, and it is the fourth most common cause of cancer death [1]. Colorectal cancer diagnosed at an early stage is associated with a good prognosis. However, 20–25% of CRC patients initially present with metastases, and approximately half of non-metastatic patients will eventually develop metastatic disease [2]. Unfortunately, metastatic CRC (mCRC) patients have a poor prognosis, and mCRC accounts for the high mortality rates associated with CRC.
Systemic chemotherapy (i.e., fluoropyrimidines, irinotecan, and oxaliplatin) is still the main treatment for mCRC. In the last decades, novel targeted agents combined with chemotherapy or used alone have largely improved therapy outcomes for mCRC patients, and some agents are already established in the clinic. These target agents include the anti-epidermal growth factor receptor (EGFR) antibodies cetuximab and panitumumab, as well as the vascular endothelial growth factor (VEGF) inhibitors bevacizumab, aflibercept, and ramucirumab [3].
Bevacizumab is a monoclonal antibody directed against vascular endothelial growth factor A (VEGF-A). By inhibiting the action of VEGF-A, bevacizumab induces the regression of tumor vasculature and prevents new blood vessels formation, hindering tumor growth [4]. In mCRC, the combination of bevacizumab with chemotherapy (5-fluorouracil/leucovorin/irinotecan or 5-fluorouracil/leucovorin) in first-line therapy has been shown to be associated with increased clinical survival benefit [4,5,6,7]. Therefore, bevacizumab in combination with standard chemotherapy is recommended as a first-line treatment for mCRC patients [8,9]. However, according to clinical practice guidelines, there are currently no predictive biomarkers to determine the use of bevacizumab in mCRC [8,9,10].
Regarding such biomarkers, recent literature indicated that the VEGF-A polymorphisms rs3025039 and rs833061 were associated with survival [11,12]. However, these results could not be confirmed in another study [13]. Sohn et al. [14] reported that a polymorphism in the vascular endothelial growth factor receptor-1 (VEGFR-1), which binds VEGF-A and other ligands, is a predictive biomarker of bevacizumab sensitivity. Furthermore, high levels of VEGF-D, which is not targeted by bevacizumab, have been associated with resistance [15]. In fact, the existence of VEGFs other than VEGF-A and the complexity of angiogenesis signaling could contribute to the challenge to identify biomarkers of response. Further, biomarker analyses have largely neglected genetic alterations in tumor cells that may influence drug sensitivity, and more comprehensive biomarker analyses are needed.
To uncover genetic biomarkers that may predict bevacizumab sensitivity or resistance, we comprehensively analyzed tumor samples of 36 mCRC patients who received bevacizumab therapy. Tumor samples were subjected to high-throughput sequencing of 409 cancer-related genes, and genetic data were correlated with clinical outcome.
2. Results
2.1. Demographic Characterization of Patients
Thirty-six mCRC patients received bevacizumab and chemotherapy combination therapy, with 88.9% (n = 32) of patients receiving first-line and 11.1% (n = 4) of patients receiving second-line treatment (Table 1). Follow-up intervals ranged from weekly to every three months. On average, the follow-up duration was 26.2 months (standard deviation: 18.7 months). Follow-up ended at the time of death or on 1 February 2018. Patients were diagnosed at a median age of 59.5 years (range: 33–87 years, standard deviation 13.3 years), and there were similar proportions of patients with colon cancer (58.3%, n = 21) and rectal cancer (41.7%, n = 15). Tumor staging was performed based on the American Joint Committee on Cancer (AJCC) 2010 guidelines.
Table 1.
Patient characteristics.
| All Patients | Responders | Non-Responders | p Value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total number | 36 (100) | 18 (50) | 18 (50) | |
| Sex | 0.500 | |||
| Male | 21 (58.3) | 12 (67.7) | 9 (50.0) | |
| Female | 15 (41.7) | 6 (33.3) | 9 (50.0) | |
| Age | 0.740 | |||
| <60 | 18 (50.0) | 10 (55.6) | 8 (44.4) | |
| ≥60 | 18 (50.0) | 8 (44.4) | 10 (55.6) | |
| Median (range) | 59.5 (33–87) | 57 (40–84) | 61.5 (33–87) | |
| Histologic grade | 1.000 | |||
| low grade 1 | 33 (91.7) | 16 (88.9) | 17 (94.4) | |
| high grade 2 | 3 (8.3) | 2 (11.1) | 1 (5.6) | |
| Metastatic pattern | 1.000 | |||
| metachronous | 17 (47.2) | 8 (44.4) | 9 (50.0) | |
| synchronous | 19 (52.8) | 10 (55.6) | 9 (50.0) | |
| Primary tumor site | 0.500 | |||
| Colon | 21 (58.3) | 12 (66.7) | 9 (50.0) | |
| Rectum | 15 (41.7) | 6 (33.3) | 9 (50.0) | |
| Metastatic site | ||||
| Liver | 21 (58.3) | 8 (44.4) | 13 (72.2) | |
| Lung | 15 (41,7) | 9 (50.0) | 6 (33.3) | |
| Other | 15 (41.7) | 9 (50.0) | 6 (5.6) | |
| Number of metastatic sites | 1.000 | |||
| 1 | 21 (58.3) | 10 (55.6) | 11 (61.1) | |
| >1 | 15 (41.7) | 8 (44.4) | 7 (38.9) | |
| Treatment regimen | 0.603 | |||
| 1st line | 32 (88.9) | 15 (83.3) | 17 (94.4) | |
| 2nd line | 4 (11.1) | 3 (16.7) | 1 (5.6) | |
| PFS (months) | 0.001 | |||
| Median (range) | 9.8 (3.0–70.9) | 14.0 (7.5–70.9) | 9.2 (3.0–22.5) |
Statistical analysis was performed with the Fisher’s exact test or the Log-rank test, as appropriate. 1 Well-differentiated/moderately-differentiated. 2 Poorly-differentiated. PFS: progression-free survival.
To identify genetic biomarkers associated with bevacizumab response, patients were stratified into responders with partial remissions (50.0%, n = 18) and non-responders (50.0%, n = 18). Among non-responders, 72.2% (n = 13) of patients had stable disease and 27.8% (n = 5) had progressive disease. The disease-control rate was 86.1% (31 of 36) among all subjects. The median progression-free survival (PFS) and response duration were 9.8 months and 7.3 months, respectively. The most common metastatic site was the liver. Regarding the number of metastatic organs, there was no difference between the responder and non-responder group; 58.3% of subjects had one metastatic organ.
2.2. Deleterious Mutations in the Phosphatase Gene PTPRT Are Associated with Bevacizumab Response Status
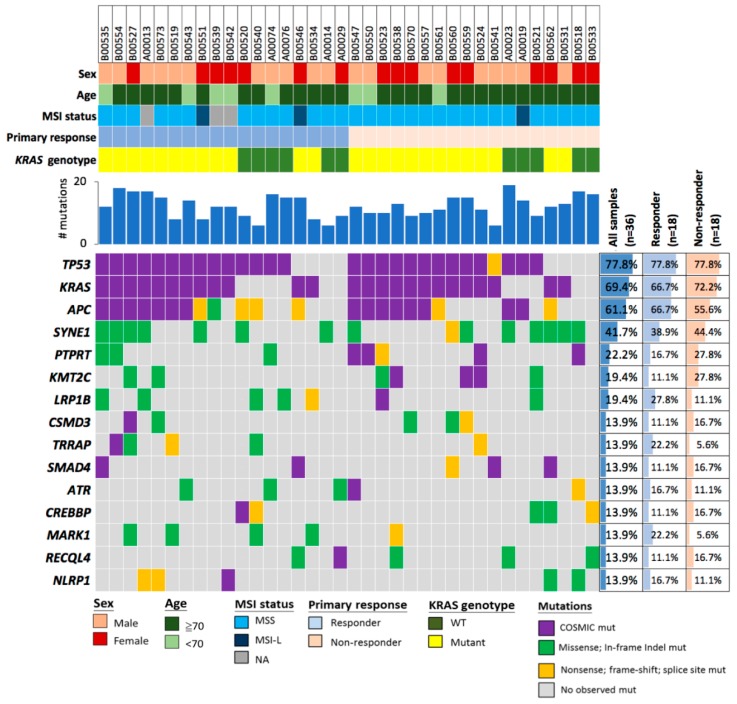
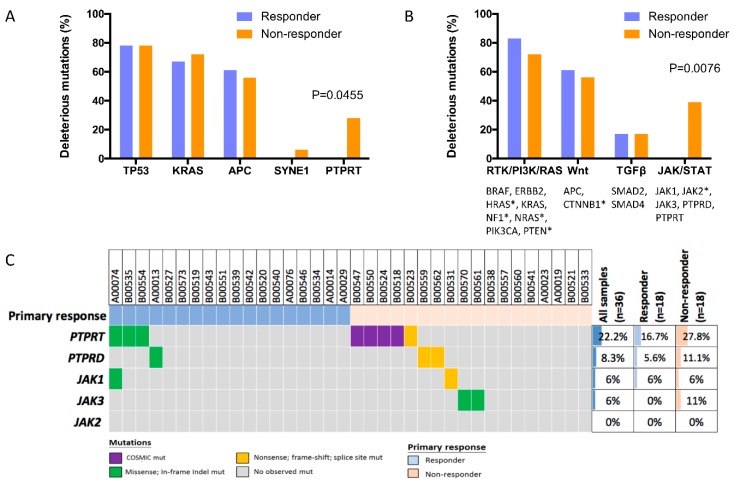
To investigate which genes could be used to discriminate between mCRC patients with or without a response to bevacizumab treatment, we analyzed 409 cancer-related genes (see Supplementary Table S1 for a complete list of genes assayed). A total of 439 mutations (mean 12.2, range 6–19), including 409 base substitutions and 30 small indels, occurring in 185 genes were detected in this cohort. The average number of mutations was similar in tumors of responders (12.3, range 6–18) and non-responders (12.1, range 6–19) (see Supplementary Table S2 for a complete list of all detected sequence variants). The five most frequently mutated genes were TP53, KRAS, APC, SYNE1, and PTPRT, being mutated in 77.8% (n = 28), 69.4% (n = 25), 58.3% (n = 21), 41.7% (n = 15), and 22.2% (n = 8) of patients (Figure 1). We reasoned that previously observed cancer variants with existing COSMIC ID (COSMIC-recurrent mutations), as well as loss-of-function (LoF) mutations in tumor suppressor genes (TSGs), including nonsense, frameshift, and splice-site variants, would be more likely to be deleterious. In line with previous studies on CRC, mutations observed in TP53, KRAS, and APC were mostly such postulated deleterious variants, and had a similar frequency in both responders and non-responders (Figure 2A and Supplementary Table S3). Only one SYNE1 variant was postulated deleterious. However, all five non-responders who harbored mutations of the TSG PTPRT had previously observed cancer variants or truncating variants, whereas this was not the case for any of the three responders with PTPRT variants. These results indicated that postulated deleterious PTPRT variants might discriminate between bevacizumab responders and non-responders, and deleterious PTPRT variants were significantly associated with lack of response (relative risk, 2.39; 95% CI, 1.58–3.61, p = 0.0455, Figure 2A and Supplementary Table S3). In addition to the five most frequently mutated genes, there were two genes with mutations in each 19.4% (n = 7) of patients, namely KMT2C and LRP1B (Figure 1). However, neither the distribution of any variants in those genes, nor the distribution of postulated deleterious variants, were significantly different between responders and non-responders (p > 0.05, Fisher’s exact test).
Figure 1.
Mutations detected in tumor samples of colorectal cancer patients according to bevacizumab response status. Genes for which genetic mutations were detected in at least five study patients are depicted in an oncoprint plot and were ranked according to their observed mutation frequency in the overall study cohort. Patients were grouped according to their bevacizumab response status. Mutations were subdivided into variants with or without existing COSMIC ID. Variants without COSMIC ID were either truncating variants—including nonsense, frameshift, and splice-site variants or missense variants and in-frame indel variants. Additionally, the patient characteristics sex, age, MSI status, and total number of detected mutations are indicated for each patient.
Figure 2.
Mutations in PTPRT/PTPRD, genes of the JAK/STAT pathway, discriminate between bevacizumab responders and non-responders. Postulated deleterious mutations in single genes with high mutation frequencies in our cohort (A) and in multiple genes involved in important colorectal cancer (CRC) signaling pathways (B) were evaluated for bevacizumab responders and non-responders. Genes included in the pathway-based analysis are listed, and genes without any detected mutations in our cohort are indicated by a star. Postulated deleterious variants were variants with existing COSMIC ID or—if the analyzed genes were tumor suppressor genes—truncating variants. In our analysis, genes with detected mutations that were considered as tumor suppressors were APC, PTPRD, PTPRT, SMAD2, SMAD4, SYNE1, and TP53. The proportion of responders and non-responders harboring such variants is depicted. Statistical analysis was performed with the Fisher’s exact test. An oncoprint of genes involved in JAK/STAT signaling is shown (C).
2.3. Deleterious Mutations in PTPRT and PTPRD, Phosphatases of the JAK/STAT Pathway, Predict Bevacizumab Response
To evaluate mutations associated with response more comprehensively and overcome the limitations of analyzing mutations in single genes, genes involved in important CRC cancer signaling pathways were grouped and analyzed together. There was no association of response status with mutations in genes of the RTK/PI3K/RAS pathway (BRAF, ERBB2, HRAS, KRAS, NF1, NRAS, PIK3CA, and PTEN, p = 0.6906), Wnt pathway (APC and CTNNB1, p = 1.0000), and TGFβ pathway (SMAD2 and SMAD4, p = 1.0000) (Figure 2B and Supplementary Table S3).
However, deleterious mutations in TSGs of the JAK/STAT pathway (PTPRD and PTPRT) were significantly associated with bevacizumab resistance, with 38.9% (n = 7) of non-responding patients, but none of the responding patients harboring such mutations. The relative risk for non-response in patients with deleterious mutations in JAK/STAT pathway genes was 2.64 compared to the remaining study patients (95% CI, 1.66–4.20, p = 0.0076). Importantly, non-PTPRT postulated deleterious variants were only detected in the PTPRT-related phosphatase gene PTPRD, occurring in two non-responders (Figure 2C). In contrast, no oncogenic in JAK1-, JAK2- or JAK3-activating mutation that could result in constitutive JAK/STAT activation was detected in both subsets.
2.4. PTPRT Phosphatase Domain Missense Variants and PTPRT/PTPRD Truncating Variants Are Characteristic for Bevacizumab Non-Responders
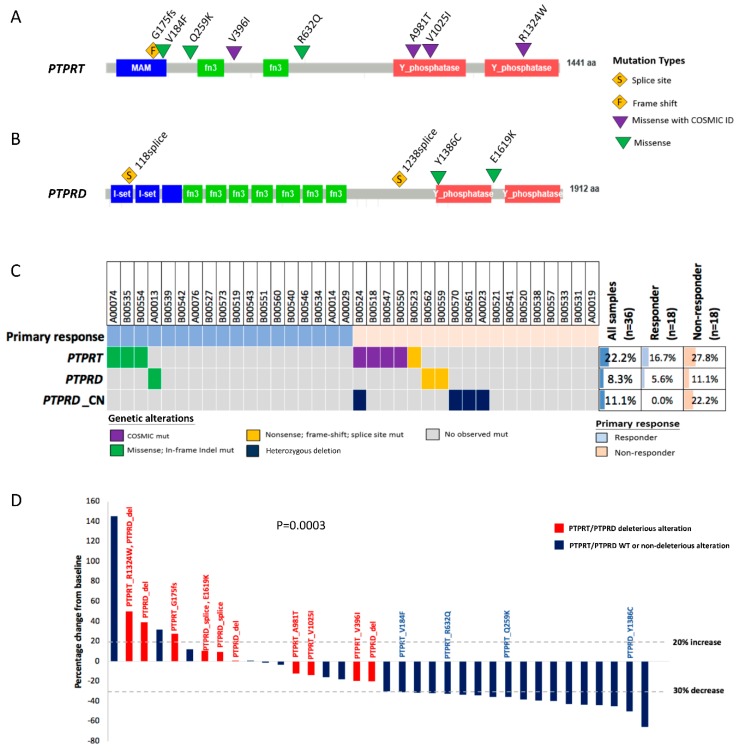
To evaluate the potential functional impact of PTPRT and PTPRD missense variants, we analyzed their protein domain localization. Details regarding all observed PTPRT and PTPRD variants are listed in Table 2. Three of the four PTPRT COSMIC-recurrent mutations observed in non-responders were located in the functionally important protein-tyrosine phosphatase domains (A981T, V1025I and R1324W), whereas the remaining variant (V396I) was located outside of functionally well-defined domains (Figure 3A). Among the three PTPRT missense variants without COSMIC ID observed in responders, only one variant (V184F) was located in a functional domain, the extracellular meprin-A5 antigen-PTP (MAM) domain. Two PTPRD missense variants were observed in each a responder and a non-responder, none of which was listed in COSMIC (Figure 3B). However, the PTPRD variant observed in the non-responding patient (E1619K) co-occurred with a PTPRD splice-site variant. The variant observed in the responding patient (Y1386C) was located in the phosphatase domain.
Table 2.
List of all PTPRT and PTPRD sequence variants and copy number losses detected in our study cohort.
| Sample ID | Response | Gene | cDNA Change | Position/AA Change | COSMIC ID | Protein Domain | Variant Type | Deleterious |
|---|---|---|---|---|---|---|---|---|
| B00524 | PD | PTPRT | c.3970C>T | p.R1324W | COSM577363 | Phosphatase | Missense | Yes |
| B00524 | PD | PTPRD | Heterozygous deletion | Yes | ||||
| B00523 | PD | PTPRT | c.524delG | p.G175fs | Frameshift | Yes | ||
| A00023 | PD | PTPRD | Heterozygous deletion | Yes | ||||
| B00547 | SD | PTPRT | c.2941G>A | p.A981T | COSM1318833 | Phosphatase | Missense | Yes |
| B00550 | SD | PTPRT | c.3073G>A | p.V1025I | COSM3546449 | Phosphatase | Missense | Yes |
| B00559 | SD | PTPRD | c.3715-8T>C | Splice region | Splice region | Splice region | Yes | |
| B00561 | SD | PTPRD | Heterozygous deletion | Yes | ||||
| B00570 | SD | PTPRD | Heterozygous deletion | Yes | ||||
| B00518 | SD | PTPRT | c.1186G>A | p.V396I | COSM1411875 | MAM | Missense | Yes |
| B00562 | SD | PTPRD | c.353-4G>C | Splice region | Splice region | Splice region | Yes | |
| B00562 | SD | PTPRD | c.4855G>A | p.E1619K | Inter-domain | Missense | No | |
| A00074 | PR | PTPRT | c.774_775 delCCinsAA |
p.Q259K | Inter-domain | Missense | No | |
| B00535 | PR | PTPRT | c.1895G>A | p.R632Q | Inter-domain | Missense | No | |
| B00554 | PR | PTPRT | c.550G>T | p.V184F | MAM | Missense | No | |
| A00013 | PR | PTPRD | c.4157A>G | p.Y1386C | Missense | No |
MAM: meprin-A5 antigen-PTP; PR: partial response; SD: stable disease; PD: progressive disease; COSMIC: Catalogue of Somatic Mutations in Cancer.
Figure 3.
Observed PTPRT/PTPRD genetic alterations and bevacizumab response. Detected variants in the genes PTPRT (A) and PTPRD (B) are depicted according to their protein localization and were classified according to the observed variant type as splice-site variants, frameshift variants, and missense variants with or without COSMIC ID. A PTPRD missense variant (E1619K) and a PTPRD splice variant were observed in the same patient. PTPRT/PTPRD variants and PTPRD copy number loss are depicted in an oncoprint plot, with patients grouped according to bevacizumab response status (C). Maximum tumor lesion changes from baseline and PTPRT/PTPRD genetic alterations are displayed in a waterfall plot (D). The presence or absence of postulated deleterious PTPRT/PTPRD genetic alterations—including mutations and copy number losses—among responders and non-responders was analyzed by the Fisher’s exact test.
2.5. Combining Copy Number Loss and Deleterious Mutations Improves the Predictive Power of PTPRT/PTPRD for Bevacizumab Response
Since deleterious genetic alterations in TSGs are not limited to inactivation by mutations, but also by copy number loss, we analyzed whether such copy number losses in the genes PTPRD and PTPRT occurred in our study cohort (Figure 3C and Table 2). Although no copy number loss was observed for PTPRT, PTPRD heterozygous deletions occurred in four patients, in whom only one gene copy was observed in the tumor tissue. Importantly, PTPRD loss was only detected in bevacizumab non-responders. PTPRD loss co-occurred with a deleterious PTPRT variant (R1324W) in one patient. Otherwise, postulated deleterious PTPRT and PTPRD alterations were mutually exclusive (Figure 3C).
Tumor change from baseline and the genetic alteration status for PTPRT/PTPRD are displayed in a waterfall plot (Figure 3D). In total, 55.6% (n = 10) of bevacizumab non-responders, but none of the responders harbored postulated deleterious PTPRT/PTPRD alterations (p = 0.0003). Therefore, the overall response rate (complete response (CR) + partial response (PR)) among patients with deleterious PTPRT/PTPRD alterations was 0.0% (0/10), compared to 69.2% (18/26) among patients without deleterious PTPRT/PTPRD alterations. The relative risk for the lack of response in patients with deleterious PTPRT/PTPRD alterations was significantly enhanced compared to patients without such alterations (relative risk, 3.25; 95% CI, 1.83–5.79, p = 0.0003).
2.6. PTPRT/PTPRD Alterations and Progression-Free Survival
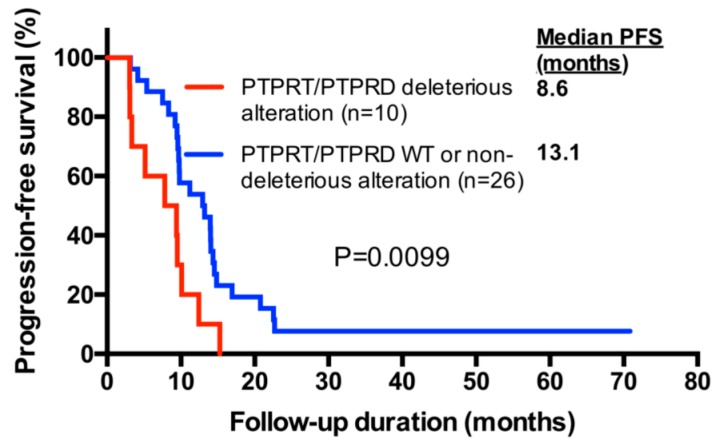
We further evaluated progression-free survival (PFS) depending on the presence of postulated deleterious PTPRT/PTPRD alterations. Progression-free survival was significantly shorter in bevacizumab-treated patients harboring postulated deleterious PTPRT/PTPRD alterations, the median PFS being 8.6 months compared to 13.1 months for patients without deleterious alterations (Hazard Ration (HR), 2.50; 95% CI, 1.40–9.52; p = 0.0099, Log-rank test) (Figure 4). Among the patients without deleterious PTPRT/PTPRD alterations, the eight non-responders had median PFS of 9.4 months, which was similar to the median PFS of 8.6 months in patients harboring such alterations. In contrast, responding patients, all of whom did not harbor deleterious PTPRT/PTPRD alterations, had a median PFS of 14.0 months. The survival in these patients was significantly longer compared to non-responders without deleterious PTPRT/PTPRD alterations (HR, 0.39; 95% CI, 0.09–0.79; p = 0.0197, Log-rank test).
Figure 4.
Progression-free survival (PFS) according to PTPRT/PTPRD alteration status. Progression-free survival upon bevacizumab treatment of all study cohort patients is shown in a Kaplan–Meier plot. Statistical analysis was performed with the Log-rank test.
In addition to PTPRT/PTPRD deleterious alterations, univariate Cox regression identified the primary tumor site as a factor associated with PFS (Table 3). In multivariate analysis, both postulated deleterious PTPRT/PTPRD alterations and rectal tumors were independent factors associated with shorter PFS (HR, 3.33; 95% CI, 1.47–7.54; p = 0.0038 and HR, 2.56; 95% CI, 1.23–5.32; p = 0.0118, respectively). However, although associated with PFS, tumor site was not associated with response (p = 0.4998, Fisher’s exact test).
Table 3.
Uni- and multivariate analysis of progression-free survival.
| Factors | n | HR 1 | 95% CI 1 | p Value 1 | HR 2 | 95% CI 2 | p Value 2 |
|---|---|---|---|---|---|---|---|
| Clinical | |||||||
| Sex (male/female) | 21/15 | 1.34 | 0.66–2.69 | 0.4164 | |||
| Age (≥60/<60) | 18/18 | 1.13 | 0.57–2.25 | 0.7191 | |||
| Histologic Grade (high/low) | 3/33 | 1.61 | 0.48–5.46 | 0.4422 | |||
| Metastatic pattern (meta/syn) | 17/19 | 1.19 | 0.60–2.35 | 0.6144 | |||
| Primary Tumor site (rectum/colon) | 15/21 | 2.11 | 1.04–4.26 | 0.0377 | 2.56 | 1.23–5.32 | 0.0118 |
| Number of metastatic sites (>1/1) | 15/21 | 1.01 | 0.51–2.01 | 0.9757 | |||
| Treatment regimen (2nd line/1st line) | 4/32 | 0.77 | 0.27–2.20 | 0.6274 | |||
| Genetic | |||||||
| PTPRT/PTPRD deleterious alteration (yes/no) | 10/26 | 2.67 | 1.23–5.80 | 0.0130 | 3.33 | 1.47–7.54 | 0.0038 |
HR: hazard ratio. 1 Univariate analysis. 2 Multivariate analysis.
3. Discussion
In mCRC, bevacizumab has been shown to prolong survival and increase response rates when used in first-line treatment with irinotecan-based regimens [4,5,6,7]. However, although not all patients benefit from bevacizumab, there are no clinically established predictive biomarkers to guide patient selection, according to clinical practice guidelines [8,9] and the European Medicines Agency (EMA) report [10]. Our study is the first to identify the predictive utility of PTPRD/PTPRT genetic variants in mCRC patients receiving bevacizumab-based chemotherapy. In our study cohort, the absence of PTPRD/PTPRT deleterious genetic variants conferred a statistically significant and clinically meaningful increase in response rate and survival benefit.
Our results showed that deleterious mutations in PTPRT, but not mutations in other genes frequently mutated in our cohort, such as TP53, KRAS, APC, and SYNE1, were associated with lack of bevacizumab response (relative risk, 2.39; 95% CI, 1.58–3.61, p = 0.0455). Pathway-based analysis revealed that additional consideration of mutations in the PTPRT-related gene PTPRD involved in JAK/STAT signaling further improved the prediction of bevacizumab response (in total, 38.9% (n = 7) of non-responders harbored deleterious PTPRT/PTPRD mutations, whereas such mutations were not detected in any responders (relative risk of non-response in patients with deleterious PTPRT/PTPRD mutations, 2.64; 95% CI, 1.66–4.20, p = 0.0076). In contrast, no associations of deleterious mutations in genes of the RTK/RAS/PI3K pathway, the Wnt pathway, and the TGFβ pathway with response were found.
Both PTPRD and PTPRT are protein tyrosine phosphatases (PTPs). Protein tyrosine phosphatases can act as tumor suppressors which interfere with signaling in malignant cells by counteracting tyrosine kinases [16,17,18]. Among PTPs, PTPRD and PTPRT belong to the receptor protein tyrosine phosphatases (PTPRs). Their functional relevance is demonstrated by the fact that ectopic PTPRD expression induces apoptosis in cancer cells and inhibits their oncogenic, metastatic properties [19,20], and PTPRT knockout mice are highly sensitive to carcinogen-induced colon cancer [21].
Among PTPRs, PTPRT exhibits the highest mutation prevalence in malignancies. PTPRT mutations were identified in various cancer types and are particularly common in CRC [22,23,24]. In our study, we found PTPRT to be mutated in approximately 25% of patients. A previous study found a prevalence of 26% for mutations in PTPRT and other phosphatases, with a high proportion of PTPRT mutations [22]. We postulate five of eight PTPRT mutations detected in our cohort to be deleterious, one variant being a truncating variant and the other four being cancer-recurrent missense mutations. Three of those cancer-recurrent missense mutations were detected in the phosphatase domains, and such mutations have been demonstrated to be associated with reduced phosphatase activity [22].
PTPRD has also been found to be mutated in CRC and in various other cancer types [19,25,26] and is frequently inactivated by copy number loss in a broad range of cancer types [27,28,29]. Similar to TCGA data [30], copy number loss of PTPRD was more frequent than that of PTPRT in our cohort, in which no PTPRT loss was observed. Copy number loss of PTPRD was only detected in bevacizumab non-responders. When combining PTPRT/PTPRD copy number loss and deleterious mutations, 55.6% of bevacizumab non-responders, but none of the responders harbored deleterious genetic PTPRT/PTPRD alterations ((relative risk, 3.25; 95% CI, 1.83–5.79, p = 0.0003). Interestingly, deleterious PTPRT and PTPRD alterations were mutually exclusive in the vast majority of our study patients. This result indicates that the two phosphatases may not be functionally redundant, since the loss of one phosphatase cannot be compensated by the other phosphatase.
The finding that inactivation of PTPRT/PTPRD is associated with bevacizumab resistance may be explained by their role in signal transduction. PTPRT/PTPRD dephosphorylate various signaling molecules, stabilize cell-cell adhesions and inhibit tumor migration [21,31,32,33]. One of their target molecules is the signal transducer and activator of transcription factor 3 (STAT3) [34,35]. STAT3 is induced in tumor cells under hypoxic conditions, which triggers tumor cell production of hypoxia inducible factor 1 alpha (HIF-1α) and VEGF; VEGF then acts on endothelial cells to promote cell proliferation and angiogenesis [36]. Additionally, VEGF can stimulate tumor cells in an autocrine manner, and bevacizumab resistance has been associated with upregulation of VEGF and its receptors VEGFR1 and VEGFR2 by tumor cells [37,38]. Therefore, PTPRT/PTPRD deleterious alterations may promote bevacizumab resistance by the upregulation of components of the VEGF pathway.
Most biomarker studies have focused on components of the VEGF pathway to predict bevacizumab outcome. However, results have been mostly disappointing. Baseline VEGF-A levels were not associated with bevacizumab response [39,40]. Contradictory results regarding the predictive value of response and/or survival were published for VEGFR1 rs9582036 [13,41] and VEGFA rs833061 [11,13,14,42], although ethnic differences between cohorts may explain part of the observed differences. A comparison of response rates of our study and the abovementioned studies with statistically significant findings demonstrate that in the present study, there is a high difference in response rates between biomarker-positive versus -negative patients (PTPRT/PTPRD deleterious alterations vs no deleterious alterations; 0% vs. 69.2%, VEGFR1 rs9582036 CC vs. AA; 36% vs. 56% [41]; VEGFA rs833061 CC vs. TT/TC: 20% vs. 56% [42] and VEGFA rs833061 all genotypes other than T/T vs. T/T; 51% vs. 76% [14]). However, it was suggested that angiogenesis might be too complex for single germline SNPs to serve as reliable predictors of bevacizumab benefit [13], and a combination of biomarkers might improve patient stratification. In this regard, in a very small cohort (n = 14) for which a biomarker combination approach was used for VEGF-A/VEGFR1/VEGFR2 mRNA expression, 0% of responders, but 71% of non-responders had a low signature expression [43]. Our approach includes a combination of genetic alterations in two genes, and in the future, it may be interesting to evaluate effects of combinations with other biomarkers.
In agreement with limited response to bevacizumab, patients with PTPRT/PTPRD deleterious alterations also had an inferior PFS (8.6 months versus 13.1 months, HR, 2.50; 95% CI, 1.40–9.52, p = 0.0099). In multivariate analysis, PTPRT/PTPRD deleterious alterations retained their statistical significance as a predictor of short PFS (HR, 3.33; 95% CI, 1.47–7.54; p = 0.0038). The other factor associated with short PFS were rectal tumors, although rectal tumors were not associated with the lack of response. The association of rectal tumors with inferior PFS was surprising, considering that PFS in rectal tumor patients treated with bevacizumab and chemotherapy has been reported to be longer than for most other tumor sites [44]. Regarding tumor sites, it has further been reported that splenic flexure tumors have a high proportion of PTPRD mutations [45]. Although it is possible that patients with specific tumor locations are more likely to harbor PTPRD mutations and could be more likely to be bevacizumab resistant, our cohort only included two patients with splenic flexure tumors, and was therefore not suitable to confirm those results. However, we observed no PTPRD mutation in any of those two patients, although one patient harbored a heterozygous PTPRD loss.
The strength of our study is a comprehensive genetic analysis of patient samples without preselecting potential biomarkers. The potential of the identified marker genes to interfere with angiogenesis supports their biological validity. However, the retrospective nature and the small cohort size represent major limitations. Therefore, our results need to be confirmed in larger prospective studies.
4. Materials and Methods
4.1. Patients
For this study, tumor samples from 36 mCRC patients treated at the Oncology Department of the Chang Gung Memorial Hospital, Tao-Yuan, Taiwan from 2011 to 2015 were analyzed. Study eligibility criteria were the histologically confirmed diagnosis of colorectal adenocarcinoma, the presence of metachronous or synchronous metastases, measurable tumor lesions to evaluate therapeutic responses, ECOG performance status 0–1 and no previous bevacizumab-based therapy. The study received approval from the Chang Gung Memorial Foundation Institutional Review Board (IRB 102-2850A3). All patients provided written informed consent before tumor samples were obtained. Patient characteristics are listed in Table 1.
4.2. Treatment Regimens
In the first-line therapy settings, patients with mCRC received bevacizumab plus chemotherapy with FOLFIRI (i.e., irinotecan and infusional 5-fluorouracil with leucovorin) [46]. In the second-line settings, patients with mCRC who had progressed after oxaliplatin-based chemotherapy received bevacizumab plus chemotherapy with FOLFIRI [46]. The detailed chemotherapy and bevacizumab schedules are described as follows: bevacizumab (5 mg per kg of bodyweight) infused initially over 30–90 min on day 1, thereafter with 60–90 min infusion of irinotecan (180 mg per m2 of body-surface area); 120 min infusion of folic acid (400 mg per m2 of body-surface area) with an intravenous bolus of 5-fluorouracil (400 mg per m2 of body-surface area), and then a continuous 46 h infusion of 5-fluorouracil (2400 mg per m2 of body-surface area) [47,48]. Response to therapy was evaluated by CT scans using Response Evaluation Criteria in Solid Tumors (RECIST) criteria 1.1. [49].
4.3. Tumor Sequencing and Analysis of Genetic Alterations
DNA extraction from formalin-fixed paraffin-embedded (FFPE) tumor samples was performed with the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). For DNA quantification, the Qubit™ dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA) was used. DNA amplification and sequencing were performed with the Ion AmpliSeq Comprehensive Cancer Panel (Life Technologies, Carlsbad, CA, USA) and an Ion Proton sequencer with an Ion P1 chip (Life Technologies), respectively. The mean sequencing depth was >800×. For variant analysis, the human genome sequence hg19, the Torrent Suite Server version 5.0 and the Torrent Suite Variant Caller plug-in, version 5.0 were used. Variants with a frequency of ≥5%were included in the further analysis. In addition to single nucleotide variants and small insertions and deletions, copy number variants were analyzed by ONCOCNV (https://github.com/BoevaLab/ONCOCNV) [50].
Mutations were postulated to be deleterious if they were cancer-recurrent variants and therefore had a corresponding COSMIC ID. Additionally, mutations in TSGs were considered as deleterious if they were frameshift, nonsense or splice-site mutations. For the localization of mutations in PTPRT/PTPRD functional domains and their display in protein graphs, tools from cbioportal were used [51,52].
4.4. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (v. 6.0; GraphPad Inc, San Diego, CA, USA) and SPSS (v. 20.0.0; IBM, New York, NY, USA). Categorical outcomes were compared using the Fisher’s exact test, whereas PFS was analyzed by the Log-rank test or Cox regression, as indicated. For multivariate Cox regression, all factors with a p-value < 0.25 in univariate Cox regression were included.
5. Conclusions
The present study demonstrated the clinical utility of performing comprehensive NGS profiling in mCRC patients to identify genetic markers of bevacizumab response. Through integrative analysis of cancer genetic and clinicopathological data, deleterious alterations in PTPRT/PTPRD were associated with bevacizumab resistance, as indicated by a poor response rate and shorter survival. This is the first study, to our knowledge, reporting that PTPRT and PTPRD deleterious alterations predict bevacizumab sensitivity in mCRC. However, the potential of PTPRT/PTPRD alterations as predictive markers for bevacizumab therapy in mCRC warrants further confirmation by pre-clinical and clinical studies.
Acknowledgments
Some of the data included in the present manuscript were shared at the ESMO Asia 2016 Congress. Annals of Oncology (2016) 27 (suppl_9): ix9-ix18. 10.1093/annonc/mdw574. This work was supported by ACT Genomics, Co. Ltd., and by grants from the Chang Gung Memorial Hospital, Taiwan (CORPG3F0062) and (CMRPG3C1841) to J.F. You and H.C. Hsu.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6694/10/9/314/s1, Table S1: list of genes assayed, Table S2: complete list of all sequence variants detected in the study cohort, Table S3: association of postulated deleterious mutations in frequently mutated genes and key signaling pathway genes with bevacizumab response status. (statistical analysis was performed with the Fisher’s exact test).
Author Contributions
Conceptualization, H.-C.H., T.K.T. and J.-F.Y.; Data curation, H.-C.H., C.-Y.Y., W.-S.T., H.-Y.H., J.C.-H.H., T.-S.Y. and J.-F.Y.; Formal analysis, H.-C.H., N.L., S.-J.C., Y.-J.L., R.-S.J. and T.K.T.; Funding acquisition, H.-C.H. and J.-F.Y.; Investigation, H.-C.H., C.-Y.Y., W.-S.T., H.-Y.H., J.C.-H.H., T.-S.Y. and J.-F.Y.; Methodology, S.-J.C., Y.-J.L., R.-S.J. and T.K.T.; Project administration, H.-C.H., T.K.T. and J.-F.Y.; Resources, H.-C.H., S.-J.C., Y.-J.L., R.-S.J., C.-Y.Y., W.-S.T., H.-Y.H., J.C.-H.H., T.-S.Y. and J.-F.Y.; Software, N.L., S.-J.C., Y.-J.L., R.-S.J. and T.K.T.; Supervision, T.K.T. and J.-F.Y.; Validation, H.-C.H., N.L., T.K.T. and J.-F.Y.; Visualization, H.-C.H., N.L., T.K.T. and J.-F.Y.; Writing-original draft, H.-C.H.; Writing-review and editing, N.L. and T.K.T.; T.K.T. and J.-F.Y. contributed equally as corresponding authors.
Funding
This research was funded by Chang Gung Memorial Hospital at Linkou, Taiwan (CORPG3F0062) and (CMRPG3C1841).
Conflicts of Interest
Tan Kien Thiam, Nina Lapke, Shu-Jen Chen, Yen-Jung Lu, and Ren-Shiang Jhou are employees of ACT Genomics, Co. Ltd.
References
- 1.International Agency for Research on Cancer WHO GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. [(accessed on 8 September 2016)];2012 World Fact Sheet. (Last Update 2012) Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 2.National Cancer Institute SEER Cancer Statistics Factsheets: Colon and Rectum Cancer. [(accessed on 23 August 2016)];2016 (Last Update 2016) Available online: http://seer.cancer.gov/ statfacts/html/colorect.html.
- 3.Kasi P.M., Hubbard J.M., Grothey A. Selection of biologics for patients with metastatic colorectal cancer: The role of predictive markers. Expert Rev. Gastroenterol. Hepatol. 2015;9:273–276. doi: 10.1586/17474124.2015.1001743. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Tebbutt N.C., Wilson K., Gebski V.J., Cummins M.M., Zannino D., Van Hazel G.A., Robinson B., Broad A., Ganju V., Ackland S.P., et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 6.Kabbinavar F., Irl C., Zurlo A., Hurwitz H. Bevacizumab improves the overall and progression-free survival of patients with metastatic colorectal cancer treated with 5-fluorouracil-based regimens irrespective of baseline risk. Oncology. 2008;75:215–223. doi: 10.1159/000163850. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D., Lang I., Marcuello E., Lorusso V., Ocvirk J., Shin D.B., Jonker D., Osborne S., Andre N., Waterkamp D., et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E., Cervantes A., Nordlinger B., Arnold D., ESMO Guidelines Working Group Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(Suppl. 3):iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network Colon Cancer. Version 2. [(accessed on 11 June 2015)];2015 Available online: www.nccn.org.
- 10.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) European Public Assessment Reports. Avastin: EPAR—Product Information. [(accessed on 11 June 2015)]; Available online: http://www. ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf.
- 11.Loupakis F., Ruzzo A., Salvatore L., Cremolini C., Masi G., Frumento P., Schirripa M., Catalano V., Galluccio N., Canestrari E., et al. Retrospective exploratory analysis of VEGF polymorphisms in the prediction of benefit from first-line FOLFIRI plus bevacizumab in metastatic colorectal cancer. BMC Cancer. 2011;11:247. doi: 10.1186/1471-2407-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibertin-Blanc C., Mancini J., Fabre A., Lagarde A., Del Grande J., Levy N., Seitz J.-F., Olschwang S., Dahan L. Vascular Endothelial Growth Factor A c.*237C>T polymorphism is associated with bevacizumab efficacy and related hypertension in metastatic colorectal cancer. Dig. Liver Dis. 2015;47:331–337. doi: 10.1016/j.dld.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F., Cremolini C., Yang D., Salvatore L., Zhang W., Wakatsuki T., Bohanes P., Schirripa M., Benhaim L., Lonardi S., et al. Prospective validation of candidate SNPs of VEGF/VEGFR pathway in metastatic colorectal cancer patients treated with first-line FOLFIRI plus bevacizumab. PLoS ONE. 2013;8:e66774. doi: 10.1371/journal.pone.0066774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn B.S., Park S.J., Kim J.E., Kim K.P., Hong Y.S., Suh C., Kim Y.S., Kim S.Y., Im S.-A., Kim S.Y., et al. Single-nucleotide polymorphisms in the vascular endothelial growth factor pathway and outcomes of patients treated with first-line cytotoxic chemotherapy combined with bevacizumab for advanced colorectal cancer. Oncology. 2014;87:280–292. doi: 10.1159/000365593. [DOI] [PubMed] [Google Scholar]
- 15.AWeickhardt J.A., Williams D., Lee C., Simes J., Murone C., Cummins M., Asadi K., Price T.J., Mariadason J., Tebbutt N.C., et al. Vascular endothelial growth factors (VEGF) and VEGF receptor expression as predictive biomarkers for benefit with bevacizumab in metastatic colorectal cancer (mCRC): Analysis of the phase III MAX study. J. Clin. Oncol. 2011;29(Suppl. 15):3531. doi: 10.1200/jco.2011.29.15_suppl.3531. [DOI] [Google Scholar]
- 16.Guan K.L., Haun R.S., Watson S.J., Geahlen R.L., Dixon J.E. Cloning and expression of a protein-tyrosine-phosphatase. Proc. Natl. Acad. Sci. USA. 1990;87:1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czernilofsky A.P., Levinson A.D., Varmus H.E., Bishop J.M., Tischer E., Goodman H.M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980;287:198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- 18.Charbonneau H., Tonks N.K., Kumar S., Diltz C.D., Harrylock M., Cool D.E., Krebs E.G., Fischer E.H., Walsh K.A. Human placenta protein-tyrosine-phosphatase: Amino acid sequence and relationship to a family of receptor-like proteins. Proc. Natl. Acad. Sci. USA. 1989;86:5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veeriah S., Brennan C., Meng S., Singh B., Fagin J.A., Solit D.B., Paty P.B., Rohle D., Vivanco I., Chmielecki J., et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc. Natl. Acad. Sci. USA. 2009;106:9435–9440. doi: 10.1073/pnas.0900571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuwanita I., Barnes D., Monterey M.D., O’Reilly S., Andrechek E.R. Increased metastasis with loss of E2F2 in Myc-driven tumors. Oncotarget. 2015;6:38210–38224. doi: 10.18632/oncotarget.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y., Zhang X., Guda K., Lawrence E., Sun Q., Watanabe T., Iwakura Y., Asano M., Wei L., Yang Z., et al. Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T. Proc. Natl. Acad. Sci. USA. 2010;107:2592–2597. doi: 10.1073/pnas.0914884107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Shen D., Parsons D.W., Bardelli A., Sager J., Szabo S., Ptak J., Silliman N., Peters B.A., Van der Heijden M.S., et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 23.Stransky N., Egloff A.M., Tward A.D., Kostic A.D., Cibulskis K., Sivachenko A., Kryukov G.V., Lawrence M.S., Sougnez C., McKenna A., et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker H., Yoshida K., Blagitko-Dorfs N., Claus R., Pantic M., Abdelkarim M., Niemöller C., Greil C., Hackanson B., Shiraishi Y., et al. Tracing the development of acute myeloid leukemia in CBL syndrome. Blood. 2014;123:1883–1886. doi: 10.1182/blood-2013-10-533844. [DOI] [PubMed] [Google Scholar]
- 25.Sjoblom T., Jones S., Wood L.D., Parsons D.W., Lin J., Barber T.D., Mandelker D., Leary R.J., Ptak J., Silliman N., et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 26.Weir B.A., Woo M.S., Getz G., Perner S., Ding L., Beroukhim R., Lin W.M., Province M.A., Kraja A., Johnson L.A., et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singchat W., Hitakomate E., Rerkarmnuaychoke B., Suntronpong A., Fu B., Bodhisuwan W., Peyachoknagul S., Yang F., Koontongkaew S., Srikulnath K. Genomic Alteration in Head and Neck Squamous Cell Carcinoma (HNSCC) Cell Lines Inferred from Karyotyping, Molecular Cytogenetics, and Array Comparative Genomic Hybridization. PLoS ONE. 2016;11:e0160901. doi: 10.1371/journal.pone.0160901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beothe T., Zubakov D., Kovacs G. Homozygous losses detected by array comparative genomic hybridization in multiplex urothelial carcinomas of the bladder. Cancer Genet. 2015;208:434–440. doi: 10.1016/j.cancergen.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Acun T., Demir K., Oztas E., Arango D., Yakicier M.C. PTPRD is homozygously deleted and epigenetically downregulated in human hepatocellular carcinomas. OMICS. 2015;19:220–229. doi: 10.1089/omi.2015.0010. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funato K., Yamazumi Y., Oda T., Akiyama T. Tyrosine phosphatase PTPRD suppresses colon cancer cell migration in coordination with CD44. Exp. Ther. Med. 2011;2:457–463. doi: 10.3892/etm.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becka S., Zhang P., Craig S.E., Lodowski D.T., Wang Z., Brady-Kalnay S.M. Characterization of the adhesive properties of the type IIb subfamily receptor protein tyrosine phosphatases. Cell Commun. Adhes. 2010;17:34–47. doi: 10.3109/15419061.2010.487957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besco J.A., Hooft van Huijsduijnen R., Frostholm A., Rotter A. Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT) Brain Res. 2006;1116:50–57. doi: 10.1016/j.brainres.2006.07.122. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz B., Fabius A.W., Wu W.H., Pedraza A., Brennan C.W., Schultz N., Pitter K.L., Bromberg J.F., Huse J.T., Holland E.C., et al. Loss of the tyrosine phosphatase PTPRD leads to aberrant STAT3 activation and promotes gliomagenesis. Proc. Natl. Acad. Sci. USA. 2014;111:8149–8154. doi: 10.1073/pnas.1401952111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Guo A., Yu J., Possemato A., Chen Y., Zheng W., Polakiewicz R.D., Kinzler K.W., Vogelstein B., Velculescu V.E., et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc. Natl. Acad. Sci. USA. 2007;104:4060–4064. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao P., Niu N., Wei T., Tozawa H., Chen X., Zhang C., Zhang J., Wada Y., Kapron C.M., Liu J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. 2017;8:69139–69161. doi: 10.18632/oncotarget.19932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesange P., Poindessous V., Sabbah M., Escargueil A.E., de Gramont A., Larsen A.K. Intrinsic bevacizumab resistance is associated with prolonged activation of autocrine VEGF signaling and hypoxia tolerance in colorectal cancer cells and can be overcome by nintedanib, a small molecule angiokinase inhibitor. Oncotarget. 2014;5:4709–4721. doi: 10.18632/oncotarget.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Videira P.A., Piteira A.R., Cabral M.G., Martins C., Correia M., Severino P., Gouveia H., Carrascal M., Almeida J.F., Trindade H., et al. Effects of bevacizumab on autocrine VEGF stimulation in bladder cancer cell lines. Urol. Int. 2011;86:95–101. doi: 10.1159/000321905. [DOI] [PubMed] [Google Scholar]
- 39.Marisi G., Scarpi E., Passardi A., Nanni O., Ragazzini A., Valgiusti M., Gardini A.C., Neri L.M., Frassineti G.L., Amadori D., et al. Circulating VEGF and eNOS variations as predictors of outcome in metastatic colorectal cancer patients receiving bevacizumab. Sci. Rep. 2017;7:1293. doi: 10.1038/s41598-017-01420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegde P.S., Jubb A.M., Chen D., Li N.F., Meng Y.G., Bernaards C., Elliott R., Scherer S.J., Chen D.S. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clin. Cancer Res. 2013;19:929–937. doi: 10.1158/1078-0432.CCR-12-2535. [DOI] [PubMed] [Google Scholar]
- 41.Hansen T.F., Christensen R., Andersen R.F., Garm Spindler K.L., Johnsson A., Jakobsen A. The predictive value of single nucleotide polymorphisms in the VEGF system to the efficacy of first-line treatment with bevacizumab plus chemotherapy in patients with metastatic colorectal cancer: Results from the Nordic ACT trial. Int. J. Colorectal Dis. 2012;27:715–720. doi: 10.1007/s00384-011-1382-6. [DOI] [PubMed] [Google Scholar]
- 42.Cui W., Li F., Yuan Q., Chen G., Chen C., Yu B. Role of VEGFA gene polymorphisms in colorectal cancer patients who treated with bevacizumab. Oncotarget. 2017;8:105472–105478. doi: 10.18632/oncotarget.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S.D., McCrudden C.M., Meng C., Lin Y., Kwok H.F. The significance of combining VEGFA, FLT1, and KDR expressions in colon cancer patient prognosis and predicting response to bevacizumab. OncoTargets Ther. 2015;8:835–843. doi: 10.2147/OTT.S80518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boisen M.K., Johansen J.S., Dehlendorff C., Larsen J.S., Osterlind K., Hansen J., Nielsen S.E., Pfeiffer P., Tarpgaard L.S., Holländer N.H., et al. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann. Oncol. 2013;24:2554–2559. doi: 10.1093/annonc/mdt253. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira D.M., Laudanna C., Migliozzi S., Zoppoli P., Santamaria G., Grillone K., Elia L., Mignogna C., Biamonte F., Sacco R., et al. Identification of different mutational profiles in cancers arising in specific colon segments by next generation sequencing. Oncotarget. 2018;9:23960–23974. doi: 10.18632/oncotarget.25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs C.S., Marshall J., Mitchell E., Wierzbicki R., Ganju V., Jeffery M., Schulz J., Richards D., Soufi-Mahjoubi R., Wang B., et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C Study. J. Clin. Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 47.Petrelli F., Borgonovo K., Cabiddu M., Ghilardi M., Lonati V., Maspero F., Sauta M.G., Beretta G.D., Barni S. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: A pooled analysis of 29 published trials. Clin. Colorectal Cancer. 2013;12:145–151. doi: 10.1016/j.clcc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Heinemann V., Von Weikersthal L.F., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S.E., Heintges T., Lerchenmüller C., Kahl C., Seipelt G., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 49.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Boeva V., Popova T., Lienard M., Toffoli S., Kamal M., Le Tourneau C., Gentien D., Servant N., Gestraud P., Rio Frio T., et al. Multi-factor data normalization enables the detection of copy number aberrations in amplicon sequencing data. Bioinformatics. 2014;30:3443–3450. doi: 10.1093/bioinformatics/btu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobson A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobson A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.