Abstract
Given increasing longevity worldwide, older adults and caregivers are seeking ways to curb cognitive decline especially for those with mild cognitive impairment (MCI, now mild neurocognitive disorder, mNCD, Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-V). This quasi-experimental, within-subjects pilot clinical trial was designed to replicate and extend the study of cognitive benefits for MCI by improving upon our prior interactive Physical and Cognitive Exercise Study (iPACESTM v1.0) by increasing the usability of the neuro-exergame and exploring possible underlying neurobiological mechanisms. Older adults were enrolled in a three-month, in-home trial of a portable neuro-exergame (iPACES™ v2.0) where participants pedaled and steered along a virtual bike path (Memory Lane™). Neuropsychological function was assessed at baseline after component familiarization intervals (e.g., two weeks of exercise-only, game-only, etc.) and after three months of interactive neuro-exergame intervention. Fourteen participants were enrolled in the study and seven completed the final evaluation. Intent-to-treat analyses were conducted with imputed missing data (total n = 14). Significant improvement in executive function (Stroop) was found (d = 0.68, p = 0.02) only. Changes in salivary biomarkers (cortisol and insulin-like growth factor 1; IGF-1) were significantly associated with improved cognition. Further research is needed, but pilot data suggest that a portable in-home neuro-exergame may be an additional, practical tool to fight back against cognitive decline and dementia.
Keywords: exercise, exergame, mild cognitive impairment, neurocognitive disorder, dementia, Alzheimer’s, executive function, IGF-1, cortisol, older adult
1. Introduction
Aging may be accompanied by impactful cognitive changes such as executive function decline, which is often seen in Alzheimer’s disease and related dementias (ADRDs) [1]. There has been growing concern worldwide regarding the increasing prevalence of cognitive decline in our aging population [2]. Dementia (DSM-IV) [3], which is now a major neurocognitive disorder (MND, DSM-V) [4], has been an umbrella term for the general loss of cognitive functions. This often includes impaired performance in domains of intelligence, memory, language, visuo-spatial, and/or executive function. Dementia may also include significant changes in personality or activities of daily living [4]. A mere 15 years ago, 3.8 million older adults (65+) in the United States were diagnosed with dementia [5]. In the United States alone, dementia cases have nearly doubled, reaching more than five million cases [6] and it is projected that worldwide incidence will surpass 115 million by 2050 [7]. Since ADRDs are often marked by declines in executive function that often lead to a loss of independence [8]. Research strives to identify preventative measures to delay the onset of or ameliorate cognitive decline often first presenting as mild cognitive impairment (MCI; DSM-IV [3]). Exercise has shown promising results in slowing the decline in cognition [9]. However, many older adults do not engage in adequate exercise [10]. The present pilot study was concerned with replicating and extending a prior finding than a more engaging neuro-exergame in which physical and mental exercise were intertwined interactively could benefit cognitive performance, specifically executive functioning [11], and also exploring whether changes in biomarkers might correspond with improvements.
1.1. Physical Exercise and Aging
Recent and prior meta-analytic reviews [9,12,13] have found that numerous well-controlled studies have strengthened the claim that physical exercise can positively impact cognitive functioning in later life. Reviews continue to call for more well-controlled clinical trials to incorporate innovations that will reach and engage pre-clinical cases such as MCI where cognitive decline might go undetected yet harbor underlying neuropathology that could potentially be ameliorated with intervention [14,15].
1.2. Physical Exercise and Cognitive Decline (MCI)
A number of meta-analytic reviews have explored the effect of physical exercise on cognition specifically in the MCI population [16,17,18]. Results revealed that physical exercise was positively associated with global cognition. Of all the types of physical exercise interventions, aerobic exercise consistently had a medium effect size on global cognition in the MCI population [17] and an executive function in older adults [19]. Recent work assessing older adult participants also revealed that a physical weakness was associated with an increased amount of amyloid beta in the brain, which is an indicator of Alzheimer’s disease (AD) [20]. Physical exercise and its effect on cognition in the AD population specifically has indicated that physical exercise is among the most impactful of interventions for improving cognition [21].
Despite the preponderance of research supporting physical exercise as a useful intervention in cognitive aging and MCI, most older adults do not get the recommended amount of exercise each week [10]. Research from our own lab chose exergaming as a way to motivate increased exercise compliance and adequate dosing with the intent of maximizing cognitive benefit [22]. We found that older adults pedaling along a virtual reality pathway on a stationary bike (aka “cybercycle”) accrued greater cognitive benefit after three months than those who pedaled a traditional stationary bike. Yet, in the end, motivation and the dose were not the differentiating factors. Instead it appeared that the combination of physical and mental exercise yielded additive and synergistic effects [22]. Recent research has also shown that other technological motivations such as smart watches may encourage exercise [23]. Animal research has also contributed to our understanding by revealing that physical and mental exercise have different neurobiological impacts. Physical exercise leads to neuronal proliferation and mental exercise (aka “environmental enrichment”) leads to neuronal survival [24,25,26]. Similarly, human research has reported that physical and mental exercise are associated with different structural and functional differences in the brain [27]. This research aimed to maximize a cognitive benefit by combining interactive cognitive and physical exercise (herein iPACES™), and tried to identify markers of underlying neurobiological mechanisms.
1.3. Mental Exercise (Cognitive Training) and Cognitive Decline (MCI)
The above finding from our initial cybercycle study, in retrospect, is perhaps not surprising given that there is a considerable growing, albeit controversial, literature (including meta-analyses of controlled trial) indicating the effects of cognitive training interventions [28,29,30,31,32]. Studies that have employed cognitive interventions alone such as computerized tasks, have been suggested to improve cognitive performance in older adults [29,33,34,35]. However, many of these studies did not address cognitively declining samples [36,37] and critiques of the literature question the transfer of apparent cognitive gains [38,39,40,41]. Yet, given larger effect sizes and fewer side effects than medications, the American Academy of Neurology (AAN) [42] and others recommend that health care practitioners working with patients with MCI advocate for physical and mental exercise over and above pharmacologic interventions [21,42,43,44,45].
1.4. Combined Physical and Mental Exercise for MCI (e.g., Combined/Tandem or Interactive/Neuro-Exergaming)
Furthermore, research in humans suggests that neuroplasticity is induced by exercise [46,47], which may prime the substrate prior to or in concert with cognitive exercise and reviews of published studies have found that, when physical exercise is combined with mental exercise, there are added cognitive benefits [47,48,49,50,51,52,53,54,55]. There are many different ways to combine mental and physical exercise, including: 1. sequentially (e.g., cognitive training follows physical activity dis-synchronously often in tandem); 2. simultaneously (e.g., cognitive tasks are presented at the same time while doing physical activity, but they are “disparate,” without interactivity, as in “dual-task” paradigms); and 3. interactively (e.g., physical and cognitive activities are interwoven such that performance in one realm affects the other and vice versa) [11]. Determining which way to combine mental and physical exercise is most effective or under what participant and environmental circumstances requires additional research.
To evaluate the potential to maximize the cognitive benefit of an interactive physical exercise, we compared the impact of different levels of mental challenges in a recently completed randomized clinical trial (RCT, Aerobic and Cognitive Exercise Study [ACES]) for MCI [56]. In the ACES trial, similar outcomes were achieved by six months for participants of either: (1) pedaling and steering along a scenic bike path (exer-tour: low mental challenge); or (2) pedaling and steering through a videogame landscape tagging dragons and coins to score points (exer-score, high mental challenge) [56]. Further comparative analyses had been planned but could not be pursued due to attrition. It was difficult for MCI participants to leave their home and the commercial grade equipment was too large and expensive to distribute in homes. As a result, our lab began developing a portable neuro-exergame for use in the home. The interactive Physical and Cognitive Exercise System (iPACES™) was found to be feasible for older adult use in a single bout study in the lab (v1.0) [57]. This system also yielded promising results in an initial pilot clinical trial of older adult use in the home for three months [11].
It has thus been proposed that both mental and physical exercise interventions together may improve or slow the decline of cognitive abilities in older adults with MCI [58] including sequential/tandem or simultaneous dual-task paradigms [59,60,61,62], yielding improvements in executive function tasks, global-visual memory, processing speed [39,63,64], and improved brain health per neuroimaging [65,66]. Nevertheless, when sequential or in tandem mental and physical exercise interventions were compared to their counterpart interventions alone, they did not produce any greater cognitive improvements than one or the other [52,59,67,68,69,70,71,72,73]. Furthermore, some research has shown that, combined with physical and mental exercise or virtual-reality exergaming interventions, may be the most useful in the early (vs. more severe) stages of cognitive decline [58]. This suggests that there might be a “sweet spot” along the continuum of decline in which MCI might be best suited to extract a benefit from combination interventions. One recent meta-analysis examined four multi-component studies that included MCI samples reporting that “separate” studies (aka sequential/tandem) were more effective than “simultaneous” interventions [74]. The latter compared dual-task vs. interactive aerobic paradigms such as in the cybercycle study [22]. We have hypothesized previously that interactive paradigms are more intuitive, which simulates real-life activities that synergistically activate evolutionarily-adapted networks (e.g., move along a path while seeking a target: destination/food/enemy), vs. forced dual-task paradigms that engage neuronal networks in a competition for resources (e.g., move along a path while being distracted to compute a mathematical problem or memorize an unrelated word list). Theoretical and neurobiological explanations for synergistic effects have been offered [51] and more innovation and research is called for [75] to explore the impact and explanations behind truly interactive physical and cognitive exercises (iPACES) especially for MCI.
Simultaneous exercise and cognitive interventions have been shown to have positive influences on cognition in older adults with a variety of exercise activities yielding similar results [11,22,75,76,77,78,79,80,81,82]. Meta-analyses comparing combined interventions to mental-only and physical-only interventions indicated that the combined intervention is more effective than the individual components [50,73,74]. Additionally, recent meta-analytic work has shown that overall combined physical and cognitive exercise interventions resulted in significantly improved cognitive performance [47]. In addition, interventions where cognitive and physical activities were occurring simultaneously and were superior to those where these tasks were performed separately [47].
Reported positive physical effects of combined physical and cognitive training have led to an increase in this facet of research such that there are now enough completed trials that they can be analyzed systemically. A couple of published reviews of the literature have explored interactive exergames and their relationship to cognitive functioning in those with neurological disability and it was found that exergaming improved executive functioning with medium-effect sizes [83,84]. Mura and colleagues [83] posited that these benefits may stem from improved decision-making and visuo-spatial perception that create an increased ability to use cognitive resources. Exergaming also had these positive effects when compared to neurologically disabled participants with no intervention [83]. Recent work has also demonstrated that supportive feedback during exergames make them more enjoyable to the user [85], which might increase compliance in these interventions. There is unfortunately a lack of research comparing simultaneous or interactive (exergaming) interventions to sequential interventions, but it is hypothesized that interactive interventions could be most potent due to potentially synergistic effects [22,75,84].
1.5. Exercise, Cognition, and Biomarker Indicators
Insulin-like Growth Factor 1 (IGF-1), Dehydroepiandrosterone sulfate (DHEA-S), and cortisol are three biomarkers that might potentially be associated with the changes in executive functioning that occur after physical and cognitive exercise interventions [73]. IGF-1 levels have been correlated with different levels of cognitive abilities [86]. Recent studies have shown that decreased IGF-1 is associated with decreased cognition [87,88]. IGF-1 has also been shown to increase with exercise [89,90,91]. Low levels of DHEA-S are associated with many conditions such as Alzheimer’s, schizophrenia, and HIV/AIDS, which suggests that DHEA-S has a relationship to cognition [92,93]. Healthy individuals have also displayed this connection with greater levels of DHEA-S corresponding to greater cognitive abilities [94]. Additionally, cortisol has also been linked to cognitive impairments and some neurodegenerative conditions such as Alzheimer’s and other dementias [95,96,97,98,99] and has been responsive to exercise interventions in MCI [100], which makes it another important biomarker to assess in the current study.
It was hypothesized based on prior literature that:
cognition, more specifically executive function, would improve over the course of the three-month neuro-exergame intervention (partial replication and extension of prior findings [11,22])
a component familiarization period would not exceed standard practice effects [pedal-only and game-only practice periods were included to gradually train and prepare cognitively challenged participants for the more complex interactive neuro-exergaming experience (iPACES) and also these periods were anticipated to have a dual benefit of washing out any practice effects from serial cognitive testing such that the learning curve would be similar to that of published normative data] [101].
2. Experimental Section
2.1. Participants
The iPACESTM v2.0 was an IRB-approved quasi-experimental pilot clinical trial (NCT03069391). A within-subjects design was employed such that participants were incrementally exposed to and trained in the independent physical and cognitive components before using the fully interactive iPACES intervention with a dual goal of washing out practice and learning effects from repeated neuropsychological evaluations. Participants were recruited through flyers, newspaper ads, demonstrations at local retirement and community centers, and through the Union College Academy for Lifelong Learning (UCALL) program. Participants sought were age 50+, sufficient visual, auditory, physical functions to participate in testing and exercise, and no known diagnosed neurological condition (e.g., epilepsy, Parkinson’s disease, and Alzheimer’s disease). Additionally, participants had to be co-residing with a partner for safety reasons (buddy system during exercise). All participants were screened with the Impaired Decision-Making Capacity structured interview (IDMC; Veteran’s Health Administration Handbook 2007) [102] and provided informed consent (if applicable, it would have been co-signed by a surrogate or legally-authorized representative per IDMC results).
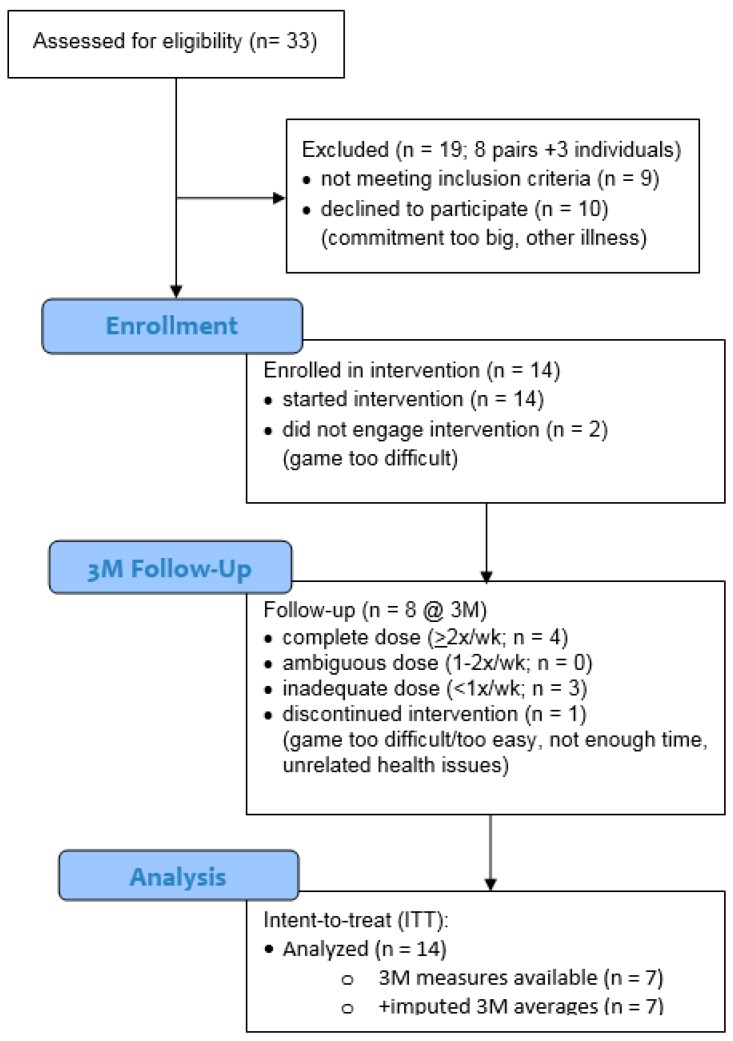
Enrolled (n = 14, 7 pairs) were six females and eight males. The mean age of participants was 82.8 (SD = 3.9), mean level of education was 16.6 years (SD = 2.1), mean body mass index (BMI) was 24.7 (SD = 3.3), average baseline cognitive status was in the MCI range (23.4, SD = 2.8 per the screening test for MCI: Montreal Cognitive Assessment (MoCA) < 26), and all were Caucasian (largely consistent with the catchment area of recruitment in upstate New York). Enrolled participants were co-residing pairs in which each of the partners participated in exercise and assessments. The buddy system was used to limit health risks associated with a typically sedentary older adult exercising alone in their home while also serving to remind one another to complete study activities. Two participants were not able to provide useable data on most measures due to previously existing conditions and were excluded from final analyses. (One participant had macular degeneration, but could play the game and wanted to exercise with their partner and could complete some non-visual tests such as the ADAS. The other participant had more cognitive impairment than apparent at first and could not follow instructions well enough to complete some of the test, but also wanted to continue the exercise with their partner.). Of the 14 participants enrolled, seven completed the final three-month evaluation. Of those seven, four were compliant with the recommended dose of exercising 30 to 45 min/week and 3 to 5 times/week within their ideal heart rate range (averaging a minimum of 2.5 times/week allowing for 1 to 2 weeks of vacation, illness, or equipment breakdown).
2.2. Procedures
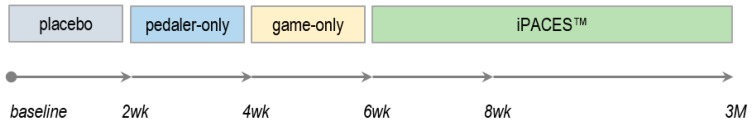
Once a co-residing pair expressed interest in participating in the study, they were screened to determine if they met study criteria and an in-home initial evaluation was scheduled. Two trained research assistants were present for in-home data collection so that testing of each participant could be done simultaneously in separate but adjacent parts of the home (e.g., kitchen and living room). Participants completed a battery of neuropsychological tests that focused on executive function (see measures below). Participants also provided saliva samples through passive-drool collection for the evaluation of biomarkers (per measures below). The initial evaluation also included a series of demographic, exercise history, and mood questionnaires. After each data collection point, the next relevant condition was introduced to participants (e.g., placebo, exercise-only, and game-only). For each condition, participants were asked to participate in the activities for 30 to 45 min on each occasion and 3 to 5 times per week. Evaluations were performed at weeks: 0, 2, 4, 6, 8 weeks, and after three months. An initial placebo period was used to familiarize participants with in-home evaluations (e.g., including completing cognitive tasks on a touchscreen iPad) and, during those two weeks, participants completed a set of on-screen readings on nutrition and exercise by answering a couple of simple multiple choice questions at the conclusion of each session to serve as verification. The second and third two-week windows served to introduce, familiarize, and evaluate each component of the neuro-exergame (physical exercise: pedaler-only and cognitive exercise: game-only) before introducing the interactive use of both in the full intervention (iPACES). The remaining weeks evaluated the full interactive neuro-exergame intervention of iPACES (Figure 1). In all conditions, participants were asked to record their activities in a paper log kept in a binder with study protocol instructions and other information provided at their initial evaluation. Motivation to complete the exercise was measured through a single item Likert scale. Upon study completion, participants were asked to fill out an exit interview questionnaire in which participants described how enjoyable the exergame intervention was and where the exergame intervention could be improved.
Figure 1.
Pilot study design showing component familiarization periods leading to interactive Physical and Cognitive Exercise System (iPACES) intervention.
2.3. Measures
2.3.1. Neuropsychological Evaluation
The targeted outcome of the intervention was executive function, which is consistent with prior literature on this cognitive domain commonly impacted by exercise and exergaming [9,11,19,22,37,49,58,78,79,103,104]. Executive functioning encompasses higher order cognitive processes, akin to the “CEO of the brain,” planning and directing attention and behavior especially in the face of multiple demands requiring set-shifting, response inhibition, and working memory. These are all key to maintaining independence and avoiding institutionalization in later life [105]. For example, an older adult preparing a meal may need to keep track and manage multiple tasks (e.g., something in the oven and on the stovetop) and, if unsuccessful, red flags may be raised (e.g., smoke alarm triggered or worse). Three measures that tap components of executive function (Stroop, Trails, and Flanker) were administered in electronic form on an iPad via the BrainBaseline software version 2.1 [106].
Congruent Correct-Incongruent Incorrect Metric (CCII) [107]. The CCII scaling metric [107] was applied to each of the three executive function measures. The CCII is the percentage of correct congruent responses minus the percentage of the incorrect incongruent responses. This measure is used to gauge the strength of mental processing by quantifying the ability to correctly respond to stimuli when they are relatively easier in contrast to incorrect performance when responding to difficult stimuli. Each of the three executive function measures were scaled to this CCII metric and yielded proportions ranging from −1 to 1 (difference in percentages).
Stroop. The Stroop test has long been used to assess executive function in clinical and research samples with good reliability and validity [105,108,109]. The Stroop task evaluates a controlled, effortful response inhibition. In the present study, an electronic version of the Stroop task was administered to participants at each evaluation. An electronic version was administered through an iPad application: BrainBaseline [106].
Trails. The original black and white [105,110] and the Color Trails [111] have good reliability and validity and have been used for many years to assess processing speed (connecting the “dots”/circled numbers in order as quickly as possible) and executive function (alternating numbers and a second sequence: letters in the black and white version of Trails B or pink/yellow colored numbers in the Color Trails version). The present study used an electronic form of the Trails task by BrainBaseline [106].
Flanker. Additionally, the flanker task was used to further access executive function and stimuli discrimination. The electronic form of the task was used via the BrainBaseline [106] and had participants view five arrows and report which direction the middle arrow is pointing. This task requires dual processing and response inhibition to override the tendency for surrounding arrows to cue a response in a direction different from the actual middle arrow stimuli.
2.3.2. Other Tests Administered
Verbal memory was assessed using the Alzheimer’s Disease Assessment Scale (ADAS) Wordlists for immediate and delayed recall [112,113]. The wordlist task was used to further characterize the sample (e.g., identify participants who might fall into the amnestic subtype of cognitive decline (aMCI) and who might not engage well with the intervention due to a lack of encoding or recall of instructions over time). The ADAS was not hypothesized to change as a result of the iPACES intervention due to memory not consistently being responsive to exercise in prior studies but was included as a manipulation check since the neuro-exergame did have a list-learning task embedded.
The overall cognitive function was assessed with the Montreal Cognitive Assessment (MoCA) and used as a screen for MCI status to characterize the sample. The MoCA is a brief battery of neuropsychological assessments (e.g., clock drawing task, sentence repetition, and letter recognition) that assess various aspects of overall cognitive function. This test has been used frequently over time with high reliably and validity to provide insight of general cognition [114]. The score of MoCA allows for categorization of participant cognitive abilities. Low scores are suggestive of Alzheimer’s type dementia while high scores represent normative cognition and in-between is suggestive of MCI.
Biomarkers: Saliva samples were immediately placed on ice until they could be placed in a −80 °C freezer to prevent degradation. Samples were analyzed for concentrations of cortisol (biochemistry lab protocol), DHEA-S (Salimetrics kit), and IGF-1 (Abcam kit). A bicinchoninic acid (BCA) protein concentration from each sample was used to normalize the protein data.
Additional brief questionnaires regarding affective states and experiences while exercising were administered as part of an exploratory addendum study and are reported elsewhere [115].
2.4. Materials
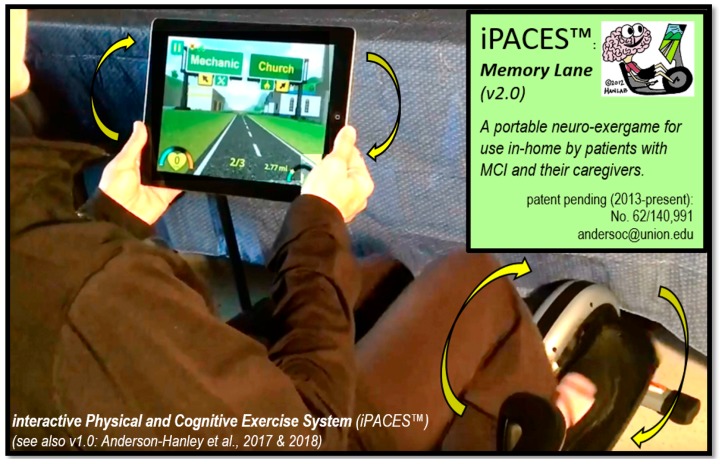
The iPACES neuro-exergame studied in this study (described below) was designed by our lab to target a specific neuropsychological function (in this case: executive function given a primary clinical need of the MCI population per above) and was initially deployed on a touch-screen PC tablet (iPACES v1.0) [11,57]. Version 2 (v2.0) of the game utilized in this study, which is now called Memory Lane, was enhanced through a collaboration between our lab and a software company (1st Playable, Troy, NY, USA) to improve graphics, playability, and other features and was deployed in iOS on an iPad 2 Air. Wireless, Bluetooth-enabled devices were integrated to complete the neuro-exergaming interactive operations (e.g., heart rate monitor ring on finger, cadence meter to track the pedaling motion, and an under-desk elliptical pedaler: Stamina 55-1610 InMotion E1000 Elliptical Trainer). Steering along the virtual bike path of the iPACES Memory Lane neuro-exergame was accomplished by holding the iPad like a steering wheel and tilting left and right accordingly to choose a pathway at each fork in the road (Figure 2).
Figure 2.
Illustration of the use of interactive pedaling and steering of the iPACES™ v2.0 neuro-exergame, Memory Lane.
The iPACES Memory Lane game was designed with the premise of challenging and reinforcing executive functions by simulating the naturalistic task of traveling along a roadway to complete a given list of errands (such as: doctor, pharmacy, grocery, starting with 3 and maxing out at 10 locations) and then returning “home,” traversing (and again having to choose the correct errand locations) in reverse order. The game guides the user down a path that leads the user to a fork in the road. The user must steer (tilt) the iPad “left” or “right” to register their choice of errand location per previously given list. If an error is made, the player is given another chance to complete the list of errands in the correct order. Once all the correct locations are chosen, the player encounters a loop in the path that turns them around to choose, in reverse order, the correct forks in the road to the previously completed errand locations.
The iPACES, as played on the iPad, is held like a steering wheel to give the illusion of riding a bike and maneuvering along a scenic path. The iPACES was intended to be used in its full interactive (physical and cognitive exercise) version, but, for experimental purposes, it can also be enabled such that component parts function separately as in “pedaler-only” (physical exercise only) or “game-only” (mental exercise only) each for use as in component familiarization/training and also potentially as comparative control conditions. When in the interactive mode, the speed of the game is controlled by the speed of participant pedaling (picked up through wireless/Bluetooth cadence meter).
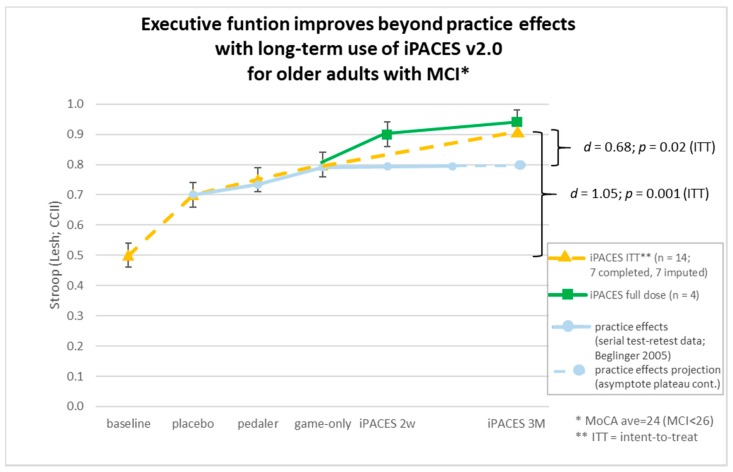
Analyses. The cognitive outcomes were assessed using paired t-tests. Intent-to-treat (ITT) analyses (n = 14) were conducted by imputing mean scores for those participants who did not complete the final three-month evaluation (n = 7). The relationship between changes in cognition and each biomarker was evaluated with Pearson’s correlations (r), which were computed using change scores (post-pre). The significance level was set at p = 0.05. Cohen’s effect sizes (d) were computed to quantify the magnitude of any significant cognitive effect. For comparison, in lieu of a control group, normative test-retest data was culled from Beglinger’s examination of serial Stroop administration (scaling their reported results by way of converting to percent change with tapering of an initial increase to a plateau, which can be seen as follows: 6%, 5%, 8%, 0%, and 0% and plotted alongside results in this study).
3. Results
Of the 12 participants that were able to provide useable evaluation data, 11 were compliant through the end of the component familiarization period (week 6) and seven were retained through the final three-month evaluation (see Figure 3 CONSORT flow diagram for further details showing participant progress through the trial). No adverse events occurred at any point during the three-month study.
Figure 3.
CONSORT Flow Diagram: enrollment and progress of participants through the trial.
3.1. Cognitive Results
Participants’ average cognitive performance across timepoints and conditions is presented in Table 1 including the subvariables that were used to compute the CCII metric for each executive function variable (see above). The three CCII metrics of executive function (Stroop, Trails, and Flanker) were the focus of a priori hypotheses even though only Stroop and Flanker could be analyzed due to too many out-of-range scores on Trails (likely due to multiple restarts from older adult participants whose dexterity or lack of familiarity inflates touch screen sensitivities during required continuous contact while drawing with a finger to connect dots).
Table 1.
Cognitive and biomarker data. Note: bold indicates p < .05 (ITT: n = 7, and ITT imputed: n = 14) compared both with baseline and end of component familiarization (end of game-only).
| 0 | 2w | 4w | 6w | 8w | 3M | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| baseline (n = 14) | placebo (n = 12) | pedaler (n = 11) | game (n = 11) | iPACES (n = 11, 7) | |||||||||
| ave | SD | ave | SD | ave | SD | ave | SD | ave | SD | ave | SD | ||
| Stroop | Incongruent % correct | 0.61 | 0.31 | 0.77 | 0.33 | 0.78 | 0.28 | 0.86 | 0.13 | 0.79 | 0.24 | 0.93 | 0.05 |
| Congruent % correct | 0.88 | 0.13 | 0.94 | 0.10 | 0.97 | 0.06 | 0.94 | 0.07 | 0.96 | 0.05 | 0.98 | 0.03 | |
| Incongruent ave time (s) | 1.45 | 0.22 | 1.38 | 0.17 | 1.42 | 0.22 | 1.41 | 0.21 | 1.35 | 0.16 | 1.33 | 0.16 | |
| Congruent ave time (s) | 1.37 | 0.18 | 1.22 | 0.25 | 1.13 | 0.14 | 1.22 | 0.17 | 1.23 | 0.19 | 1.10 | 0.07 | |
| Total duration (s) | 269.0 | 104.3 | 186.8 | 63.7 | 149.9 | 68.3 | 107.3 | 54.9 | 100.6 | 44.6 | 70.7 | 5.7 | |
| CCII | 0.48 | 0.41 | 0.71 | 0.39 | 0.75 | 0.33 | 0.80 | 0.16 | 0.75 | 0.28 | 0.91 | 0.07 | |
| Trails | restarts | 4.77 | 6.56 | 2.00 | 1.95 | 1.64 | 2.25 | 1.36 | 1.12 | 0.73 | 1.19 | 1.43 | 1.90 |
| A % error | 0.42 | 0.58 | 0.20 | 0.24 | 0.15 | 0.23 | 0.11 | 0.09 | 0.06 | 0.09 | 0.13 | 0.16 | |
| B % error | 0.29 | 0.27 | 0.18 | 0.17 | 0.16 | 0.16 | 0.10 | 0.10 | 0.10 | 0.09 | 0.28 | 0.15 | |
| B duration (s) | 212.6 | 175.5 | 160.9 | 151.6 | 145.8 | 81.1 | 124.5 | 69.2 | 125.0 | 81.0 | 163.1 | 55.4 | |
| CCII | 0.28 | 0.73 | 0.64 | 0.31 | 0.69 | 0.30 | 0.79 | 0.16 | 0.84 | 0.15 | 0.59 | 0.28 | |
| Flanker | Incongruent % correct | 0.81 | 0.25 | 0.91 | 0.14 | 0.90 | 0.17 | 0.93 | 0.07 | 0.92 | 0.12 | 0.76 | 0.36 |
| Congruent % correct | 0.91 | 0.10 | 0.89 | 0.14 | 0.97 | 0.03 | 0.95 | 0.08 | 0.95 | 0.08 | 0.98 | 0.02 | |
| Incongruent ave time (s) | 0.78 | 0.21 | 0.65 | 0.08 | 0.71 | 0.12 | 0.66 | 0.12 | 0.64 | 0.06 | 0.77 | 0.22 | |
| Congruent ave time (s) | 0.74 | 0.25 | 0.63 | 0.08 | 0.64 | 0.07 | 0.61 | 0.12 | 0.61 | 0.07 | 0.67 | 0.12 | |
| Total duration (s) | 244.9 | 47.7 | 207.9 | 94.2 | 152.7 | 63.4 | 97.8 | 64.5 | 97.4 | 57.9 | 71.9 | 16.7 | |
| CCII | 0.72 | 0.28 | 0.79 | 0.27 | 0.87 | 0.19 | 0.87 | 0.13 | 0.87 | 0.20 | 0.74 | 0.36 | |
| ADAS | Word List (sum trials correct) | 18.64 | 4.58 | 19.29 | 5.68 | 20.33 | 4.33 | 19.92 | 3.96 | 21.00 | 4.63 | 21.13 | 4.67 |
| Word List (delay correct) | 5.08 | 2.66 | 5.85 | 2.44 | 5.58 | 2.23 | 6.42 | 2.02 | 5.75 | 2.83 | 5.63 | 2.13 | |
| Biomarkers | cortisol | 3.55 | 3.36 | 5.08 | 5.37 | 5.52 | 5.23 | 2.58 | 3.38 | 3.06 | 4.00 | 17.61 | 7.17 |
| DHEA-S | 8733 | 5923 | 7729 | 8620 | 7301 | 9153 | 6508 | 6085 | 4999 | 3547 | 6072 | 6503 | |
| IGF-1 | 3.16 | 2.73 | 3.43 | 3.66 | 1.82 | 1.01 | 2.64 | 1.91 | 1.83 | 1.37 | 2.56 | 2.46 | |
Notes: bold indicates p ≤ 0.05 (ITT: n = 7, and ITT imputed n = 14) compared both with baseline and end of component familiarization (end of game-only).
A significant improvement was found in ITT analyses of executive function (Stroop CCII) from baseline to the three-month iPACES neuro-exergame intervention [t(13) = −4.34, p < 0.001, d = 1.05, Figure 4]. A significant improvement was also observed with a moderate effect size [t(13) = −3.53, p = 0.004, d = 0.68] when comparing as “baseline” the more stringent end of the component familiarization periods (week 6), which was also the start of full interactive iPACES (after which any practice effects were washed out as affirmed by the plotting of the grey line in Figure 4, which shows the plateaued asymptote of Beglinger’s normative test-retest data [101]. See Gray and colleagues for further discussion [116]). Also, plotted for anecdotal visual inspection were the results of those participants that completed the full recommended dose of iPACES (n = 4). No significant effect was observed for Flanker.
Figure 4.
Changes in cognition over three-month iPACES neuro-exergame intervention exceed practice effects.
3.2. Biomarker Results
Given the significant improvement in executive function (Stroop) noted above, the correlations between the changes on the three biomarkers (cortisol, DHEA-S, and IGF-1) and the change in Stroop were examined. Greater improvement in Stroop performance was significantly related to a decrease in cortisol (r = −0.24, p = 0.04) and IGF-1 (r = −0.28; p = 0.04).
4. Discussion
Co-residing pairs of older adults (n = 14) were enrolled in a three-month in-home pilot study to examine the cognitive and biomarker outcomes of pedaling and steering an enhanced iPad-based neuro-exergame: the interactive Physical and Cognitive Exercise System (iPACES v2.0). Seven participants (six with MCI) completed the final three-month evaluation and intent-to-treat analyses of all 14 enrollees revealed a significant improvement in executive function (Stroop, but not Flanker) that exceeded practice effects. This was affirmed by evaluating change from the initial baseline as well as from the end of familiarization after washing out serial-testing practice effects and also by comparing with normative test-retest data. The quasi-experimental within-subjects design gradually and sequentially familiarized participants with each of the component conditions (e.g., game-only and exercise-only) before introducing the fully interactive neuro-exergame (iPACES). The effect sizes observed ranged from medium to large even when including those with less than the full recommended dose. As hypothesized, the improvement in executive function was found to significantly correlate with a change in cortisol and IGF-1 (but not DHEA-S). The individuals, which experienced the smallest biomarker changes had the greatest improvement on the Stroop, while individuals with the greatest change in biomarkers had the least Stroop improvement. Contrary to expectations, cortisol significantly increased after the three-month exergame intervention. Although unanticipated, the finding of increased cortisol levels in elderly individuals post intervention has been seen in previous research [117,118].
There are a number of limitations to this pilot study. In particular, the small sample size yields narrow variability of participant limits generalizability, constricts exploration of factors that might affect outcomes, and diminishes power to detect significant effects. Furthermore, the lack of a control group for comparison makes interpretation challenging. However, these results are preliminary given the small sample size and pilot. Due to the quasi-experimental nature of the study, there are a few observations that can be cautiously made to guide further research. First, this pilot study partially replicates and extends our prior pilot of iPACES v1.0 (Anderson-Hanley et al. in press), which confirms that an in-home neuro-exergame intervention for MCI is feasible and potentially effective warranting further study. This is also consistent with other published research on in-home exercise interventions, which are not without their challenges but demonstrate that it is increasingly feasible to incorporate innovated technology into a patient’s home environment [119]. Second, despite a small sample and even with challenges in accruing a full dose of the iPACES intervention, a significant and sizeable effect on one of three measures of executive function was found and the results of this enhanced v2.0 (d = 0.68) seem even stronger than the initial pilot of iPACES v1.0 (d = 0.39 [11]). Lastly, biomarkers available in readily-obtained saliva samples seem to provide a fruitful avenue for exploring possible underlying mechanisms to explain any cognitive benefits of neuro-exergaming interventions, which is seen with the significant relationship found between cortisol and executive function in other cross-sectional and intervention studies [95,96,97,98,99].
The preliminary pilot findings in this study are consistent with prior research, which has found cognitive benefits of exercise [9] and exergaming [11,56,84]. Chuang and colleagues [120] similarly found an executive function benefit following an exergaming intervention (dance-dance-revolution: DDR). Yet, this pilot adds to the smaller set of literature addressing MCI specifically and extends to prior work on neuro-exergaming by reaching the oft-isolated MCI population with a widely-applicable, safely-seated intervention for in-home use and with an effect size that seems to exceed prior reports [11,56]. There is scant literature on in-home interventions, but notably this echoes Chew and colleagues [120] who reported some benefits to patients enrolled in tandem intervention (physical and cognitive exercises separately). Yet, this study also addresses caregivers directly (some with insidious MCI and/or at least known heavy caregiver burden [121] involving a caregiver in the exercise intervention as directly as participants themselves).
This study had several weaknesses that point to the next steps in the research. The first was a lack of a control group and, while various comparisons were made within subjects (via initial baseline and after washing out practice effects) and also with published normative test-retest data, a matched control group in a larger trial would be more ideal for future research. The small sample does limit statistical power, which means that, while it is encouraging that a significant effect could be detected despite the small sample, a larger sample might shed light on smaller effects that are, perhaps, not detected (e.g., possibly via Flanker) and would also afford greater diversity allowing for more nuanced consideration of generalizability and analytic integration of covariates (such as age, education, sex, etc.). For example, research has shown that benefits of exercise may vary by sex [122,123] and a larger sample could clarify whether there are any inadvertent effects such as ruling out possible gender bias in the choice or applicability of errand locations. Additionally, most participants reported enjoying the study. However, it was often reported that the iPad game became redundant and participants suggested incorporating more challenging and dynamic game features.
The move from paper in the first iPACES pilot to electronic cognitive testing in this second iPACES pilot study revealed some challenges for seniors using the touch screen. For example, high restart and error rates were seen in the Trails task (seven of the 14 participants had >10 restarts on the Trails task at one or more evaluations throughout the study). Additionally, the switch from paper to electronic proved difficult in deriving comparable scores and metrics from a breadth of computer-captured data. The high rate of attrition is not uncommon in intensive exercise interventions [124] and it may have been exacerbated in this study given that these unpaid senior volunteers were either very busy or also dealing with other health and familial complications. Furthermore, it may have been exacerbated in that recruiting pairs was useful for safety afforded via the in-home buddy system but may have also inflated attrition since two were lost when one partner was not able to continue. Last, compliance with the full dose of exercise was challenging for our participants to achieve with four of the seven study completers reaching the recommended target dose of three to five times per week. Participant feedback suggests that those who volunteer for an unpaid study of this sort tend to also be busy with many other commitments, but it may be necessary to further fine-tune the game’s challenge to the ability of the participant. Some participants were only capable of finishing a limited challenge (e.g., maintaining three to four errand locations yet achieving a sense of accomplishment vs. frustration) while others needed a bigger challenge (e.g., maxing out quickly at 10 errand locations and perhaps needing varied scenarios/story-boards to maintain interest such as pedaling along roadways of a state or country to recall and tag a given list of tourist attractions).
Future research might also aim to replicate and extend research on related multimodal interventions [125], which incorporated a nutritional component along with physical and cognitive exercise (in tandem in that study). It makes sense that, if exercise interventions (both physical and mental) are to impact cognitive and brain health via cardiovascular benefits and neuroplasticity, nutritional support for building or repairing neurons could magnify the impact of an intervention. Specifically, an expanding list of nootropics, derived from plant nutraceuticals (e.g., Gingko Biloba, Bacopa Monnieri, Huperzine A, Choline, Phosphatidylserine, Vinpocetine, Rhodiola Rosea, Methylcobalamin) and other potential cognitive enhancers [126] are finding their way into mainstream use among the general population with varying degrees of scientific support on neuronal and brain health [124]. Thus, examining the combined effects of neuro-exergaming with nootropics may be supra-additive in terms of cognitive effects and warrants further study.
Alternative forms of exercise that incorporate interactive physical and cognitive components have been increasingly explored such as dance [127,128,129] and might be contrasted in a future RCT with neuro-exergaming even though care would need to be taken regarding additional variables such as intensity of exercise, aerobic achievement, and influences of socialization. Naturalistic outdoor cycling could be compared with virtual reality cycling and a preliminary pilot in our lab of this type of comparison suggests it is feasible [130], but it is also complex to tease out the impact of multidimensional interactivity, the intensity of cognitive challenges, and the impact of daylight, nature, or green-scapes on outcomes [131]. Examining the role of various forms of mental engagement during exercise could also be fruitful. For instance, this pilot of iPACES asserts a certain level of complexity of the mental challenge (memorizing a list of errand locations and reversing them while pedaling “home”). However, it may also be possible that cognitive performance and brain health is also improved due to less effortful and more relaxing or meditative modes of exercise, which is seen in achieving a flow state while engaging a challenging exercise or in Tai Chi [132].
Additional biomarkers might also be useful to examine in future research such as the brain-derived neurotrophic factor (BDNF), which has been found to predict a cognitive benefit of a dual-task paradigm for those with MCI [133].
5. Conclusions
In conclusion, the results of this pilot study indicate that a portable, iPad-based neuro-exergame is feasible for MCI and caregiver co-residing pairs to use in the home and it appears to have a sizeable effect on executive function, which warrants further research. It is anticipated that there will continue to be calls for additional research and funding for RCTs to evaluate these types of innovations for addressing the encroaching dementia epidemic [75,134]. It is hoped that these interventions could potentially ameliorate the cognitive decline of increasing numbers of those with MCI [75] including reaching them at home where they often become secluded. Perhaps with enough evidence, the scientific community might arrive at “prescribable video games” as a non-pharmacological intervention to address cognitive decline [135] or, as in this pilot study of iPACES (v2), an impactful multimodal neuro-exergame intervention.
6. Patents
iPACES™ patent pending (US15087351).
Acknowledgments
Lindsey Barrett, Hannah Christian, Carolyn Doty, and Anvit Kalra-Lall for their help in data collection. Earlier versions of these results were presented at the annual meetings of the Cognitive Neuroscience Society (March 2018, Boston) and Experimental Biology (April 2018, San Diego).
Author Contributions
Conceptualization, C.A.-H. Methodology, C.A.-H. Software, E.S., E.M. and K.S. Validation, C.A.-H. and A.F.K. Formal Analysis, K.W. and C.A.-H. Investigation, K.W., J.S. and A.S. Resources, A.F.K. and B.D.C. Data Curation, K.W., J.S. and A.S. Writing—Original Draft Preparation, K.W. Writing—Review & Editing, K.W., J.S., C.A.-H., P.J.A., B.D.C., A.F.K., A.S. and K.S. Visualization, C.A.-H. Supervision, C.A.-H. Project Administration, C.A.-H. Funding Acquisition, C.A.-H.
Funding
This research was funded by the NIH/NIA STTR: R41AG053120 and faculty and student grants from Union College. ClinicalTrials.gov Identifier: NCT03069391
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kirova A.M., Bays R.B., Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biol. Med. Res. Int. 2015;2015 doi: 10.1155/2015/748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M., Wimo A., Guerchet M., Ali G., Wu Y., Prina M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International; London, UK: 2015. [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Volume 4. American Psychiatric Publishing; Arlington, VA, USA: 2010. [DOI] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Volume 5. American Psychiatric Publishing; Arlington, VA, USA: 2013. [DOI] [Google Scholar]
- 5.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B., Steffens D.C. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. [(accessed on 26 July 2018)]; Available online: https://www.alz.org/aaic/overview.asp.
- 7.Alzheimer’s Disease International: World Alzheimer Report 2009. [(accessed on 27 August 2018)]; Available online: https://www.alz.co.uk/research/GlobalImpactDementia2013.pdf.
- 8.Fichman H.C., Oliveira R.M., Fernandes C.S. Neuropsychological and neurobiological markers of the preclinical stage of alzheimer’s disease. Psychol. Neurosci. 2011;4:245–253. doi: 10.3922/j.psns.2011.2.010. [DOI] [Google Scholar]
- 9.Kramer A.F., Colcombe S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study—Revisited. Perspect. Psychol. Sci. 2018;13:213–217. doi: 10.1177/1745691617707316. [DOI] [PubMed] [Google Scholar]
- 10.Chodzko-Zajko W.J., Proctor D.N., Singh M.A., Minson C.T., Nigg C.R., Salem G.J., Skinner J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 11.Anderson-Hanley C., Stark J., Wall K.M., Van Brakle M., Michel M., Maloney M., Barcelos N., Striegnitz K., Cohen B.D., Kramer A.F. The interactive Physical and Cognitive Exercise System (iPACES™): Effects of a 3-month in-home pilot clinical trial for mild cognitive impairment and caregivers. Clin. Int. Aging. 2018 doi: 10.2147/CIA.S160756. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colcombe S., Kramer A. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 13.Rathore A., Lom B. The effects of chronic and acute physical activity on working memory performance in healthy participants: A systematic review with meta-analysis of randomized controlled trials. Syst. Rev. 2017;6:1–16. doi: 10.1186/s13643-017-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui M.Y., Lin Y., Sheng J.Y., Zhang X., Cui R.J. Exercise Intervention Associated with Cognitive Improvement in Alzheimer’s Disease. Neural Plast. 2018;2018 doi: 10.1155/2018/9234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistridis P., Krumm S., Monsch A.U., Berres M., Taylor K.I. The 12 years preceding mild cognitive impairment due to Alzheimer’s disease: The temporal emergence of cognitive decline. J. Alzheimer’s Dis. 2015;48:1095–1107. doi: 10.3233/JAD-150137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G., Xia R., Zhou W., Tao J., Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016;50:1443–1450. doi: 10.1136/bjsports-2015-095699. [DOI] [PubMed] [Google Scholar]
- 17.Song D., Yu D.S.F., Li P.W.C., Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018;79:155–164. doi: 10.1016/j.ijnurstu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Hess N.C.L., Dieberg G., Mcfarlane J.R., Smart N.A. The effect of exercise intervention on cognitive performance in persons at risk of, or with, dementia: A systematic review and meta-analysis. Health Aging Res. 2014;3:1–10. doi: 10.12715/har.2014.3.3. [DOI] [Google Scholar]
- 19.Ludyga S., Gerber M., Brand S., Holsboer-Trachsler E., Pühse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology. 2016;53:1611–1626. doi: 10.1111/psyp.12736. [DOI] [PubMed] [Google Scholar]
- 20.Yoon J.H., Minzenberg M.J., Ursu S., Walter R., Wendelken C., Ragland J.D., Carter C.S. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: Relationship with impaired cognition, behavioral disorganization, and global function. Am. J. Psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang J.-H., Xu Y., Lin L., Jia R.-X., Zhang H.-B., Hang L. Comparison of multiple interventions for older adults with Alzheimer disease or mild cognitive impairment: A PRISMA-compliant network meta-analysis. Medicine (Baltimore) 2018;97:e10744. doi: 10.1097/MD.0000000000010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson-Hanley C., Arciero P.J., Brickman A.M., Nimon J.P., Okuma N., Westen S.C., Merz M.E., Pence B.D., Woods J.A., Kramer A.F., et al. Exergaming and older adult cognition: A cluster randomized clinical trial. Am. J. Prev. Med. 2012;42:109–119. doi: 10.1016/j.amepre.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Pope Z., Zeng N., Zhang R., Lee H.Y., Gao Z. Effectiveness of Combined Smartwatch and Social Media Intervention on Breast Cancer Survivor Outcomes: Randomized Trial. Med. Sci. Sports Exer. 2018;50:137. doi: 10.1249/01.mss.0000535536.13395.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Praag H., Kempermann G., Gage F.H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 25.Van Praag H., Shubert T., Zhao C., Gage F. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Praag H. Neurogenesis and exercise: past and future directions. Neuromol. Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 27.Suo C., Singh M.F., Gates N., Wen W., Sachdev P., Brodaty H., Baune B.T. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry. 2016;21:1633–1642. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappa K., Walsh S., Snyder P. Immediate and delayed effects of cognitive interventions in healthy elderly: A review of current literature and future directions. Alzheimer’s Dement. 2009;5:50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Anguera J.A., Boccanfuso J., Rintoul J.L., Al-Hashimi O., Faraji F., Janowich J., Kong E., Larraburo Y., Rolle C., Johnston E., et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K.Z.H., Lindenberger U., Freund A.M., Baltes P.B. WALKING WHILE MEMORIZING: Age-Related Differences in Compensatory Behavior. Psychol. Sci. 2001;12:230–238. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- 31.Toril P., Reales J.M., Ballesteros S. Video game training enhances cognition of older adults: A meta-analytic study. Psychol. Aging. 2014;29:706. doi: 10.1037/a0037507. [DOI] [PubMed] [Google Scholar]
- 32.Wang C., Yu J., Wang H., Tan C., Meng X., Tan L. Non-pharmacological interventions for patients with mild cognitive impairment: A meta-analysis of randomized controlled trials of cognition-based and exercise interventions. J. Alzheimer’s Dis. 2014;42:663–678. doi: 10.3233/JAD-140660. [DOI] [PubMed] [Google Scholar]
- 33.Ballesteros S., Prieto A., Mayas J., Toril P., Pita C., Ponce de León L., Reales J.M., Waterworth J. Brain training with non-action video games enhances aspects of cognition in older adults: A randomized controlled trial. Front. Aging Neurosci. 2014;6:277. doi: 10.3389/fnagi.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbett A., Owen A., Hampshire A., Grahn J., Stenton R., Dajani S., Burns A., Howard R., Williams N., Williams G., et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2015;16:990–997. doi: 10.1016/j.jamda.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Mowszowski L., Lampit A., Walton C., Naismith S. Strategy-Based Cognitive Training for Improving Executive Functions in Older Adults: A Systematic Review. Neuropsychol. Rev. 2016;26:252–270. doi: 10.1007/s11065-016-9329-x. [DOI] [PubMed] [Google Scholar]
- 36.Bahar-Fuchs A., Clare L., Woods B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer’s or vascular type: A review. Alzheimer’s Res. Ther. 2013;5:35. doi: 10.1186/alzrt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karr J.E., Areshenkoff C.N., Rast P., Garcia-Barrera M.A. An empirical comparison of the therapeutic benefits of physical exercise and cognitive training on the executive functions of older adults: A meta-analysis of controlled trials. Neuropsychology. 2014;28:829. doi: 10.1037/neu0000101. [DOI] [PubMed] [Google Scholar]
- 38.Martin M., Clare L., Altgassen A.M., Cameron M.H., Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Libr. 2011 doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Simons D.J., Boot W.R., Charness N., Gathercole S.E., Chabris C.F., Hambrick D.Z., Stine-Morrow E.A. Do “Brain Training” Programs Work? Psychol. Sci. Public Interest Suppl. 2016;17:103–186. doi: 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- 40.Reijnders J., van Heugten C., van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res. Rew. 2013;12:263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Zokaei N., MacKellar C., Čepukaitytė G., Patai E.Z., Nobre A.C. Cognitive training in the elderly: Bottlenecks and new avenues. J. Cogn. Neurosci. 2017;29:1473–1482. doi: 10.1162/jocn_a_01080. [DOI] [PubMed] [Google Scholar]
- 42.AAN Summary of Practice Guidelines for Clinicians, Practice Guideline Update: Mild Cognitive Impairment. [(accessed on 1 July 2018)]; Available online: https://www.aan.com/Guidelines/Home/GetGuidelineContent/882.
- 43.Eshkoor S.A., Hamid T.A., Mun C.Y., Ng C.K. Mild cognitive impairment and its management in older people. Clin. Int. Aging. 2015;10:687–693. doi: 10.2147/CIA.S73922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagamatsu L.S., Flicker L., Kramer A.F., Voss M.W., Erickson K.I., Hsu C.L., Liu-Ambrose T. Exercise is medicine, for the body and the brain. Br. J. Sports Med. 2014:943–944. doi: 10.1136/bjsports-2013-093224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Studenski S., Carlson M.C., Fillit H., Greenough W.T., Kramer A., Rebok G.W. From bedside to bench: does mental and physical activity promote cognitive vitality in late life? Sci. SAGE KE. 2006;2006:pe21. doi: 10.1126/sageke.2006.10.pe21. [DOI] [PubMed] [Google Scholar]
- 46.Foster P.P., Rosenblatt K.P., Kuljiš R.O. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front. Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gheysen F., Poppe L., DeSmet A., Swinnen S., Cardon G., De Bourdeaudhuij I., Chastin S., Fias W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018;15:63. doi: 10.1186/s12966-018-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constans A., Pin-barre C., Temprado J.J., Decherchi P., Laurin J. Influence of aerobic training and combinations of interventions on cognition and neuroplasticity after stroke. Front. Aging Neurosci. 2016;8:164. doi: 10.3389/fnagi.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diamond A. Effects of Physical Exercise on Executive Functions: Going beyond Simply Moving to Moving with Thought. Ann. Sport Med. Res. 2015;2:1011. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X., Yin S., Lang M., He R., Li J. The more the better? A meta-analysis on effects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016;31:67–79. doi: 10.1016/j.arr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Geda Y.E., Silber T.C., Roberts R.O., Knopman D.S., Christianson T.J., Pankratz V.S., Boeve B.F., Tangalos E.G., Petersen R.C. Computer activities, physical exercise, aging, and mild cognitive impairment: A population-based study. Mayo Clin. Proc. 2012;87:437–442. doi: 10.1016/j.mayocp.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah T., Verdile G., Sohrabi H., Campbell A., Putland E., Cheetham C., Dhaliwal S., Weinborn M., Maruff P., Darby D., et al. A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. Transl. Psychiatry. 2014;4:e487. doi: 10.1038/tp.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karssemeijer E.E., Aaronson J.J., Bossers W.W., Smits T.T., Rikkert M.M., Kessels R.R. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017 doi: 10.1016/j.arr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Styliadis C., Kartsidis P., Paraskevopoulos E., Ioannides A.A., Bamidis P.D. Neuroplastic effects of combined computerized physical and cognitive training in elderly individuals at risk for dementia: An eLORETA controlled study on resting states. Neural Plast. 2015;2015 doi: 10.1155/2015/172192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards M.K., Loprinzi P.D. Experimental Effects of Acute Exercise and Meditation on Parameters of Cognitive Function. J. Clin. Med. 2018;7:125. doi: 10.3390/jcm7060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson-Hanley C., Barcelos N.M., Zimmerman E.A., Gillen R.W., Dunnam M., Cohen B.D., Yerokhin V., Miller K.E., Hayes D.J., Arciero P.J., et al. The Aerobic and Cognitive Exercise Study (ACES) for community-dwelling older adults with or at-risk for mild cognitive impairment (MCI): Neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson-Hanley C., Maloney M., Barcelos N., Striegnitz K., Kramer A. Neuropsychological Benefits of Neuro-Exergaming for Older Adults: A Pilot Study of an Interactive Physical and Cognitive Exercise System (iPACES) J. Aging Phys. Act. 2017;25:73–83. doi: 10.1123/japa.2015-0261. [DOI] [PubMed] [Google Scholar]
- 58.Bamidis P.D., Vivas A.B., Styliadis C., Frantzidis C., Klados M., Schlee W., Siountasa A., Papageorgioud S.G. A review of physical and cognitive interventions in aging. Neurosci. Biobehav. Rev. 2014;44:206–220. doi: 10.1016/j.neubiorev.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Van Het Reve E., De Bruin E.D. Strength-balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: A multicenter randomized-controlled trial. BMC Geriatr. 2014;14:1–15. doi: 10.1186/1471-2318-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaefer S., Schumacher V. The interplay between cognitive and motor functioning in healthy older adults: Findings from dual-task studies and suggestions for intervention. Gerontology. 2011;57:239–246. doi: 10.1159/000322197. [DOI] [PubMed] [Google Scholar]
- 61.Law L.L., Barnett F., Yau M.K., Gray M.A. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Res. Rev. 2014;15:61–75. doi: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Lauenroth A., Ioannidis A., Teichmann B. Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 2016;16:141. doi: 10.1186/s12877-016-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noack H., Lövdén M., Schmiedek F. On the validity and generality of transfer effects in cognitive training research. Psychol. Res. 2014;78:773–789. doi: 10.1007/s00426-014-0564-6. [DOI] [PubMed] [Google Scholar]
- 64.Ratner E., Atkinson D. Why cognitive training and brain games will not prevent or forestall dementia. J. Am. Geriatr. Soc. 2015;63:2612–2614. doi: 10.1111/jgs.1_13825. [DOI] [PubMed] [Google Scholar]
- 65.Maffei L., Picano E., Andreassi M.G., Angelucci A., Baldacci F., Baroncelli L., Begenisic T., Bellinvia P.F., Berardi N., Biagi L., et al. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017;7:39471. doi: 10.1038/srep39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voss M.W., Weng T.B., Burzynska A.Z., Wong C.N., Cooke G.E., Clark R., Fanning J., Awick E., Gothe N.P., Olson E.A., et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage. 2016;131:113–125. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabre C., Chamari K., Mucci P. Improvement of Cognitive Function by Mental and / or Individualized Aerobic Training in Healthy Elderly Subjects. Int. J. Sports Med. 2002;33:415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 68.Oswald W.D., Gunzelmann T., Rupprecht R., Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: The SimA study in a 5-year perspective. Eur. J. Ageing. 2006;3:179–192. doi: 10.1007/s10433-006-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 2013;5:8. doi: 10.3389/fnagi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linde K., Alfermann D. Single versus combined cognitive and physical activity effects on fluid cognitive abilities of healthy older adults: A 4-month randomized controlled trial with follow-up. J. Aging Phys. Act. 2014;22:302–313. doi: 10.1123/JAPA.2012-0149. [DOI] [PubMed] [Google Scholar]
- 71.Rahe J., Petrelli A., Kaesberg S., Fink G.R., Kessler J., Kalbe E. Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clin. Int. Aging. 2015;10:297. doi: 10.2147/CIA.S74071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desjardins-Crépeau L., Berryman N., Fraser S.A., Vu T.T.M., Kergoat M.J., Li K.Z., Bosquet L., Bherer L. Effects of combined physical and cognitive training on fitness and neuropsychological outcomes in healthy older adults. Clin. Interv. Aging. 2016;11:1287–1299. doi: 10.2147/CIA.S115711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rahe J., Becker J., Fink G.R., Kessler J., Kukolja J., Rahn A., Rosen J.B., Szabados F., Wirth B., Kalbe E. Cognitive training with and without additional physical activity in healthy older adults: cognitive effects, neurobiological mechanisms, and prediction of training success. Front. Aging Neurosci. 2015;7:187. doi: 10.3389/fnagi.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruderer-Hofstetter M., Rausch-Osthoff A.K., Meichtry A., Münzer T., Niedermann K. Effective multicomponent interventions in comparison to active control and no interventions on physical capacity, cognitive function and instrumental activities of daily living in elderly people with and without mild impaired cognition: A systematic review and network meta-analysis. Ageing Res. Rev. 2018;45:1–14. doi: 10.1016/j.arr.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Ballesteros S., Voelcker-Rehage C., Bherer L. Editorial: Cognitive and Brain Plasticity Induced by Physical Exercise, Cognitive Training, Video Games, and Combined Interventions. Front. Hum. Neurosci. 2018;12:169. doi: 10.3389/fnhum.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiyamizu M., Morioka S., Shomoto K., Shimada T. Effects of dual task balancetraining on dual task performance in elderly people: A randomized controlled trial. Clin. Rehabil. 2012;26:58–67. doi: 10.1177/0269215510394222. [DOI] [PubMed] [Google Scholar]
- 77.Theill N., Schumacher V., Adelsberger R., Martin M., Jäncke L. Effects of simultaneously performed cognitive and physical training in older adults. BMC Neurosci. 2013;14:103. doi: 10.1186/1471-2202-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayama H., Okamoto K., Nishiguchi S., Yamada M., Kuroda T., Aoyama T. Effect of a Kinect-based exercise game on improving executive cognitive performance in community-dwelling elderly: Case control study. J. Med. Internet Res. 2014;16:e61. doi: 10.2196/jmir.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barcelos N., Shah N., Cohen K., Hogan M.J., Mulkerrin E., Arciero P.J., Cohen B.D., Kramer A.F., Anderson-Hanley C. Aerobic and cognitive exercise (ACE) pilot study for older adults: Executive function improves with cognitive challenge while exergaming. J. Int. Neuropsychol. Soc. 2015;21:768–779. doi: 10.1017/S1355617715001083. [DOI] [PubMed] [Google Scholar]
- 80.Nishiguchi S., Yamada M., Tanigawa T., Sekiyama K., Kawagoe T., Suzuki M., Yoshikawa S., Abe N., Otsuka Y., Nakai R., et al. A 12-Week Physical and Cognitive Exercise Program Can Improve Cognitive Function and Neural Efficiency in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015;63:1355–1363. doi: 10.1111/jgs.13481. [DOI] [PubMed] [Google Scholar]
- 81.Leon J., Urena A., Bolanos M.J., Bilbao A., Ona A. A combination of physical and cognitive exercise improves reaction time in persons 61–84 years old. J. Aging Phys. Activ. 2015;23:72–77. doi: 10.1123/JAPA.2012-0313. [DOI] [PubMed] [Google Scholar]
- 82.Yokoyama H., Okazaki K., Imai D., Yamashina Y., Takeda R., Naghavi N., Ota A., Hirasawa Y., Miyagawa T. The effect of cognitive-motor dual-task training on cognitive function and plasma amyloid β peptide 42/40 ratio in healthy elderly persons: a randomized controlled trial. BMC Geriatr. 2015;15:60. doi: 10.1186/s12877-015-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mura G., Carta M.G., Sancassiani F., Machado S., Prosperini L. Active exergames to improve cognitive functioning in neurological disabilities: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017 doi: 10.23736/S1973-9087.17.04680-9. [DOI] [PubMed] [Google Scholar]
- 84.Stanmore E.S., Brendon V.D., de Bruin E.D., Firth J. The effect of active video games on cognitive functioning in clinical and non-clinical populations: A meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2017;78:34–43. doi: 10.1016/j.neubiorev.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Kim J., Timmerman C.E. Effects of Supportive Feedback Messages on Exergame Experiences. Mass Commun. Soc. 2018;30:29–40. doi: 10.1027/1864-1105/a000175. [DOI] [Google Scholar]
- 86.Sonntag W.E., Ramsey M., Carter C.S. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res. Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Dik M.G., Pluijm S.M.F., Jonker C., Deeg D.J.H., Lomecky M.Z., Lips P. Erratum: Insulin-like growth factor I (IGF-I) and cognitive decline in older persons Neurobiol. Aging. 2004;25:271. doi: 10.1016/S0197-4580(02)00136-7. [DOI] [PubMed] [Google Scholar]
- 88.Al-Delaimy W.K., Von Muhlen D., Barrett-Connor E. Insulinlike growth factor-1, insulinlike growth factor binding protein-1, and cognitive function in older men and women. J. Am. Geriatr. Soc. 2009;57:1441–1446. doi: 10.1111/j.1532-5415.2009.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vega S.R., Knicker A., Hollmann W., Bloch W., Strüder H.K. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab. Res. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- 90.Bellar D., Glickman E.L., Juvancic-Heltzel J., Gunstad J. Serum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience. 2011;178:133–137. doi: 10.1016/j.neuroscience.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 91.Voss M.W., Prakash R.S., Erickson K.I., Basak C., Chaddock L., Kim J.S., Alves H., Heo S., Szabo A.N., White S.M., et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ray L., Khemka V.K., Behera P., Bandyopadhyay K., Pal S., Pal K., Basu D., Chakrabarti S. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in Alzheimer’s disease and vascular dementia. Aging Dis. 2013;4:57. [PMC free article] [PubMed] [Google Scholar]
- 93.Maggio M., De Vita F., Fisichella A., Colizzi E., Provenzano S., Lauretani F., Valenti G. DHEA and cognitive function in the elderly. J. Steriod. Biochem. Mol. Biol. 2015;145:281–292. doi: 10.1016/j.jsbmb.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Davis S.R., Shah S.M., McKenzie D.P., Kulkarni J., Davison S.L., Bell R.J. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J. Clin. Endocrinol. Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 95.Lupien S., Nair N., Briére S., Maheu F., Tu M., Lemay Μ., Meaney M. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia in later life. Rev. Neurosci. 1999;10:117–140. doi: 10.1515/REVNEURO.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- 96.Lupien S.J., Leon M.D., Santi S.D., Convit A., Tarshish C., Nair N.P., Meaney M.J. Cortisol, human aging, hippocampal atrophy, and memory deficits. Neuroscientist. 1998;4:389–390. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 97.Csernansky J.G., Dong H., Fagan A.M., Wang L., Xiong C., Holtzman D.M., Morris J.C. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am. J. Psychiatry. 2006;163:2164–2169. doi: 10.1176/ajp.2006.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lara J., Cooper R., Nissan J., Ginty A.T., Khaw K.T., Deary I.J., Lord J.M., Kuh D., Mathers J.C. A proposed panel of biomarkers of healthy ageing. BMC Med. 2015;13:222. doi: 10.1186/s12916-015-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lara V.P., Caramelli P., Teixeira A.L., Barbosa M.T., Carmona K.C., Carvalho M.G., Fernandes A.P., Gomes K.B. High cortisol levels are associated with cognitive impairment no-dementia (CIND) and dementia. Clin. Chim. Acta. 2013;423:18–22. doi: 10.1016/j.cca.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Tortosa-Martínez J., Clow A., Caus-Pertegaz N., González-Caballero G., Abellán-Miralles I., Saenz M.J. Exercise increases the dynamics of diurnal cortisol secretion and executive function in people with amnestic mild cognitive impairment. J. Aging Phys. Act. 2015;23:550–558. doi: 10.1123/japa.2014-0006. [DOI] [PubMed] [Google Scholar]
- 101.Beglinger L.J., Gaydos B., Tangphao-Daniels O., Duff K., Kareken D.A., Crawford J., Fastenau P.S., Siemers E.R. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch. Clin. Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Veterans Health Administration Handbook 2007. [(accessed on 1 July 2018)]; Available online: https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2966.
- 103.Eggenberger P., Wolf M., Schumann M., de Bruin E. Exergame and Balance Training Modulate Prefrontal Brain Activity during Walking and Enhance Executive Function in Older Adults. Front. Aging Neurosci. 2016;8:66. doi: 10.3389/fnagi.2016.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ogawa E., You T., Leveille S. Potential benefits of exergaming for cognition and dual-task function in older adults: A systematic review. J. Aging Phys. Act. 2016;24:332–336. doi: 10.1123/japa.2014-0267. [DOI] [PubMed] [Google Scholar]
- 105.Strauss E., Sherman E.M., Spreen O. A compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York, NY, USA: 2006. [Google Scholar]
- 106.Lee K., Baniqued P., Cosman J., Mullen S., McAuley E., Severson J., Kramer A.F. Examining cognitive function across the lifespan using a mobile application. Comput. Hum. Behav. 2012;28:1934–1946. doi: 10.1016/j.chb.2012.05.013. [DOI] [Google Scholar]
- 107.Lesh T.A., Westphal A.J., Niendam T.A., Yoon J.H., Minzenberg M.J., Ragland J.D., Carter C.S. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. NeuroImage Clin. 2013;2:590–599. doi: 10.1016/j.nicl.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van der Elst W., Van Boxtel M., Van Breukelen G., Jolles J. The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 2006;13:62–79. doi: 10.1177/1073191105283427. [DOI] [PubMed] [Google Scholar]
- 109.Scarpina F., Tagini S. The stroop color and word test. Front. Psychol. 2017;8:557. doi: 10.3389/fpsyg.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wecker N.S., Kramer J.H., Wisniewski A., Delis D.C., Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14:409. doi: 10.1037/0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 111.D’Elia L.G., Satz P., Uchiyama C.L., White T. Color Trails Test (CTT) Psychological Assessment Resources; Odessa, FL, USA: 1996. [Google Scholar]
- 112.Harrison J. Measuring cognitive change in Alzheimer’s disease clinical drug trials. J. Nutr. Health Aging. 2007;11:327–329. [PubMed] [Google Scholar]
- 113.Podhorna J., Krahnke T., Shear M., Harrison J.E. Alzheimer’s Disease Assessment Scale-Cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: Change over time and the effect of enrichment strategies. Alzheimer’s Res. Ther. 2016;8:8. doi: 10.1186/s13195-016-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 115.Schillaci A. Bachelor’s Thesis. Union College; Schenectady, NY, USA: June 2017. Neuropsychological Effects of the Interactive Physical and Cognitive Exercise System (iPACES™) for Older Adults: Executive Function and Mood. [Google Scholar]
- 116.Gray W.D. Plateaus and asymptotes: Spurious and real limits in human performance. Curr. Dir. Psychol. Sci. 2017;26:59–67. doi: 10.1177/0963721416672904. [DOI] [Google Scholar]
- 117.Guazzo E.P., Kirkpatrick P.J., Goodyer I.M., Shiers H.M., Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J. Clin. Endocrinol. Metabol. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- 118.Mura G., Cossu G., Migliaccio G.M., Atzori C., Nardi A.E., Machado S., Carta M.G. Quality of life, cortisol blood levels and exercise in older adults: Results of a randomized controlled trial. Clin. Pract. Epidemiol. Ment. Health. 2014;10:67–72. doi: 10.2174/1745017901410010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chew J., Chong M.S., Fong Y.L., Tay L. Outcomes of a multimodal cognitive and physical rehabilitation program for persons with mild dementia and their caregivers: A goal-oriented approach. Clin. Int. Aging. 2015;10:1687. doi: 10.2147/CIA.S93914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chuang L.Y., Hung H.Y., Huang C.J., Chang Y.K., Hung T.M. A 3-month intervention of Dance Dance Revolution improves interference control in elderly females: A preliminary investigation. Exp. Brain Res. 2015;233:1181–1188. doi: 10.1007/s00221-015-4196-x. [DOI] [PubMed] [Google Scholar]
- 121.Dunkin J., Anderson-Hanley C. Dementia caregiver burden: A review of the literature and guidelines for assessment and intervention. Neurology. 1998;51:S53–S60. doi: 10.1212/WNL.51.1_Suppl_1.S53. [DOI] [PubMed] [Google Scholar]
- 122.Loprinzi P.D., Frith E. The Role of Sex in Memory Function: Considerations and Recommendations in the Context of Exercise. J. Clin. Med. 2018;7 doi: 10.3390/jcm7060132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barha C., Davis J., Falck R., Nagamatsu L., Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocr. 2017;46:71–85. doi: 10.1016/j.yfrne.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Osho O., Owoeye O., Armijo-Olivo S. Adherence and Attrition in Fall Prevention Exercise Programs for Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2018;26:304–326. doi: 10.1123/japa.2016-0326. [DOI] [PubMed] [Google Scholar]
- 125.Köbe T., Witte A.V., Schnelle A., Lesemann A., Fabian S., Tesky V.A., Pantel J., Flöel A. Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. Neuroimage. 2016;131:226–238. doi: 10.1016/j.neuroimage.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 126.Tricco A.C., Soobiah C., Berliner S., Ho J.M., Ng C.H., Ashoor H.M., Chen M.H., Hemmelgarn B., Straus S.E. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: A systematic review and meta-analysis. Can. Med. Assoc. J. 2013;185:1393–1401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kosmat H., Vranic A. The efficacy of a dance intervention as cognitive training for the old-old. J. Aging Phys. Act. 2017;25:32–40. doi: 10.1123/japa.2015-0264. [DOI] [PubMed] [Google Scholar]
- 128.Merom D., Grunseit A., Eramudugolla R., Jefferis B., Mcneill J., Anstey K.J. Cognitive benefits of social dancing and walking in old age: The dancing mind randomized controlled trial. Front. Aging Neurosci. 2016;8:26. doi: 10.3389/fnagi.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Burzynska A.Z., Jiao Y., Knecht A.M., Fanning J., Awick E.A., Chen T., Gothe N., Voss M.W., McAuley E., Kramer A.F. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front. Aging Neurosci. 2017;9:59. doi: 10.3389/fnagi.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cohen J., Rudolph E., Anderson-Hanley C. Aerobic and cognitive exercise over time: Virtual versus outdoor cycling. Presented at the annual meeting of the Society of Behavioral Medicine, Philadelphia, PA. Ann. Behav. Med. 2013;47:S282. [Google Scholar]
- 131.Nelson L., Tabet N. Slowing the progression of Alzheimer’s disease; what works? Ageing Res. Rev. 2015;23:193–209. doi: 10.1016/j.arr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Wayne P.M., Walsh J.N., Taylor-Piliae R.E., Wells R.E., Papp K.V., Donovan N.J., Yeh G.Y. Effect of Tai Chi on cognitive performance in older adults: Systematic review and meta-Analysis. J. Am. Geriatr. Soc. 2014;62:25–39. doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Ito K., Shimokata H., Washimi Y., Endo H., Kato T. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8:e61483. doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith G.E. Healthy cognitive aging and dementia prevention. Am. Psychol. 2016;71:268. doi: 10.1037/a0040250. [DOI] [PubMed] [Google Scholar]
- 135.Abbasi J. Adam Gazzaley, MD, PhD: Developing Prescribable Video Games. JAMA. 2018;320:16–18. doi: 10.1001/jama.2018.4985. [DOI] [PubMed] [Google Scholar]