Abstract
Vitamin A (VitA) is a micronutrient that is crucial for maintaining vision, promoting growth and development, and protecting epithelium and mucus integrity in the body. VitA is known as an anti-inflammation vitamin because of its critical role in enhancing immune function. VitA is involved in the development of the immune system and plays regulatory roles in cellular immune responses and humoral immune processes. VitA has demonstrated a therapeutic effect in the treatment of various infectious diseases. To better understand the relationship between nutrition and the immune system, the authors review recent literature about VitA in immunity research and briefly introduce the clinical application of VitA in the treatment of several infectious diseases.
Keywords: vitamin A, immunology, infectious disease
1. Introduction
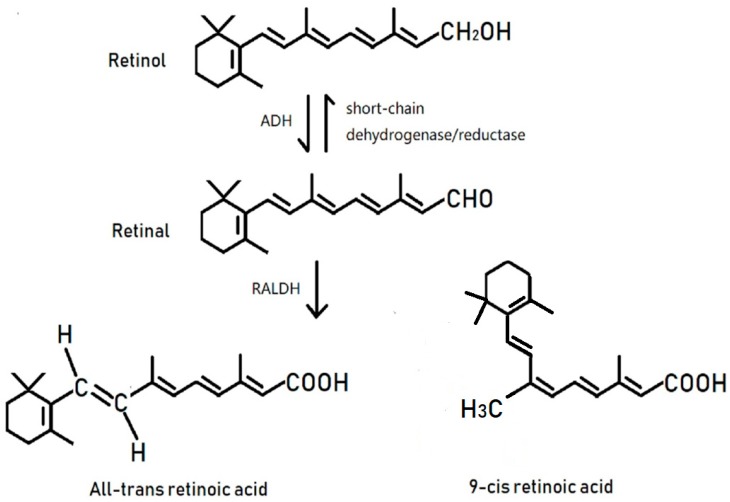
Vitamin A (VitA) is a group of unsaturated monohydric alcohols that contain an alicyclic ring. VitA is insoluble in water but is fat soluble [1]. In 1928, Green and Mellandy reported that VitA could enhance the anti-inflammatory response of organisms and called VitA the “anti-inflammation vitamin” [2]. Later, the anti-inflammatory capacity of VitA was widely studied in the 1980s and 1990s [3,4,5]. VitA exists in the form of retinol, retinal, and retinoic acid (RA), among which RA shows the most biological activity. RA exists in two significant derivatives: 9-cis-RA and all-trans-RA (ATRA) [6] (Figure 1). The primary biological functions of VitA include maintenance of vision, growth, and the integrity of epithelial and mucous tissue [7]. However, the immunoregulatory mechanisms of VitA are not entirely understood. The authors, here, conduct a detailed review on the most recent advances of VitA function in immunology. We briefly introduce the clinical application of VitA in the treatment of several contagious diseases to provide theoretical support for VitA research in immunology and its therapeutic applications.
Figure 1.
Transformation of retinol into bioactive retinoic acid involves a two-step oxidative reaction. To do this, a group of enzymes, divided in three families, will act together to form the final compound retinoic acid (RA). Retinol transforms into retinal under the catalytic action of the alcohol dehydrogenase (ADH) family; this step can also be regulated by the short-chain dehydrogenase/reductase family, which shows a wide affinity for alcohols and aldehydes. The aldehyde dehydrogenase (RALDH) family then catalyzes retinal to form retinoic acid. Both of the oxidation reactions transmit electrons through the electron acceptor NAD or NADP.
2. RA Nuclear Receptors
RA is the ligand of the nuclear retinoic acid receptor (RAR) protein. RAR family has three main members (α(isoforms a1-2), β(isoforms b1-4), and γ), which have additional subtypes produced by the use and splicing of alternating promoters [8]. The nuclear RAR acts as a ligand-activating transcription factor, regulating gene transcription according to cell type and tissue [9]. The ATRA is the highest affinity endogenous ligand of RAR [10]. A member of the second protein family, RA-X receptor (RXR) heterodimers and RAR, give high affinity to binding DNA. The RXR family also contains three members (RXRα, RXRβ, and RXRγ). In addition to targeting RARα, RARβ, and RARγ-like ATRA, 9-cis-RA also activates RXRα, RXRβ, and RXRγ [11]. RAR/RXR heterologous two dimer-bound DNA is known as the retinoic acid reaction element. The consensus retinoic acid reaction element is composed of two direct repeats of PuG (G/T) and TCA that are most often separated by 5 bases [12]. RAR acts as an enhancer, and promotes chromatin opening and changes in the transcriptional activity of RA target genes when occupied by RA/RAR/RXR complexes [13,14]. Binding of RA to RAR leads to release of the corepressor complex and association with coactivator proteins, followed by altered transcription of downstream target genes and, ultimately, changes in cellular function. RA also undergoes further oxidation by the cytochrome P450 (CYP26) family to more polar metabolites. The lipophilic molecule, RA, can act within the same cell in which it is synthesized (autocrine), or can act in a paracrine manner in nearby cells [15,16,17].
3. VitA Is Involved in the Formation of the Epithelial and Mucous Tissues
The epithelium lines all outer surface and most inner surfaces of organisms, and it functions as the “front line” of defense against pathogen invasion. Studies from recent years have shown that VitA plays a crucial role in the morphological formation of the epithelium, epithelial keratinization, stratification, differentiation, and functional maturation of epithelial cells [18]. As a promotor for morphology and a cell differentiation enhancer, VitA is an integral part of the mucus layer of both the respiratory tract and the intestine. Since VitA promotes mucin secretion, it improves the antigen non-specific immunity function of these tissues [18,19]. Research has shown that VitA improves the mechanistic defense of the oral mucosa, increases the integrity of intestinal mucus, and maintains the morphology and amount of urothelium cells [18,19,20].
Even as early as 1925, Wolbach and Howe reported that various epithelia are replaced by stratified squamous keratinizing epithelium when deprived of VitA [21]. It is now clear that under conditions of VitA deficiency (VitAD), epithelial cells shrink, and squamous keratinization may occur in skin, digestive tract, respiratory tract, genitourinary system, cornea, and surrounding soft tissues, leading to symptoms of dry skin, diarrhea, coughing, keratomalacia, corneal opacity, dry eye, and urinary lithiasis [22,23,24,25]. Simultaneously, the resistance of keratinized epithelial tissues to foreign pathogens decreases, and it is no longer able to exert its mechanical barrier function, thus reducing innate immune function and promoting respiratory tract infections, diarrhea, and other diseases in children [26].
4. VitA and Its Impact on the Immune System
Immune organs are organs or tissues that realize immune function, and are places where most immunocompetent cells proliferate, differentiate, mature, aggregate, and respond to immunity. Research has shown that crucial immune organs need constant dietary intake to maintain VitA concentrations, and RA was previously shown both to promote the proliferation and to regulate the apoptosis of thymocytes [27,28,29]. In the thymus, endogenous retinoid synthesis and retinoids similar to glucocorticoids might, indeed, be involved in the regulation of thymic proliferation and selection processes, by being present in the thymus in functionally effective amounts [28]. In mice, VitAD leads to a defect in both T cell-mediated and antibody-dependent immune responses [30,31]. VitAD can also inhibit the normal apoptosis process of bone marrow cells, which leads to an increased number of myeloid cells in the bone marrow, spleen, and peripheral blood, indicating that VitA is involved in the regulation of homeostasis of bone marrow [29]. VitA likely regulates the bone marrow population through binding retinoic acid receptor (RAR) in the bone marrow cell nucleus. This binding alters the expression level of apoptosis genes, such as Bcl-2, Fas, and others. The specific mechanisms by which these apoptosis genes regulate bone marrow homeostasis require further investigation.
5. VitA Affects Cell Differentiation, Maturity, and Immunological Function in Innate Immunity
Retinoid acid plays crucial roles in the regulation of the differentiation, maturation, and function of cells of the innate immune system. Innate immune cells are comprised of macrophages and neutrophils, which initiate immediate responses to pathogen invasion through phagocytosis and activation of natural killer T cells which perform immunoregulatory functions through cytotoxic activity [32,33]. There is a report that shows that VitA is essential for the proper development and differentiation of colonic CD169+ macrophages [34]. Macrophages mainly include M1 macrophages secreting proinflammatory cytokines and M2 macrophages expressing anti-inflammatory factors. ATRA inhibits inflammatory reactions by inducing monocyte differentiation toward the macrophage lineage while inhibiting the release of an inflammatory factors from macrophages, thus inducing M1 macrophages in the bone marrow to transform into M2 macrophages [35,36]. ATRA acts on RAR in the nucleus of neutrophils, inducing neutrophil differentiation and heterogeneity through activation of the mTOR signaling pathway. This pathway enhances neutrophil extracellular traps and cytotoxicity, allowing for efficient killing of multiple tumor cells [37]. By downregulating the expression level of IFN-γ and upregulating the secretion of IL-5, RA plays a regulatory role in the early differentiation stage of natural killer T cells [32].
Dendritic cells (DCs) are potent and versatile antigen-presenting cells, and they are specialized sentinels of our immune system capable of orchestrating the innate and adaptive immune response [38]. ATRA can regulate the differentiation of DC precursors [39,40,41]. Bone marrow resident pre-DCs have the potential to differentiate into pre-mucosal DCs (pre-μDCs), characterized by the expression of gut-homing receptors. ATRA acts cell-intrinsically in developing gut-tropic pre-μDCs to effect the differentiation and drive the specialization of intestinal CD103+ DCs [42]. Pre-DCs can migrate to the spleen, where they may sense ATRA skewing the differentiation toward CD11b+CD8− DCs instead of CD11b−CD8α+ DCs [40]. The general consensus on the effect of ATRA on DC function is to promote an anti-inflammatory phenotype characteristic of intestinal DCs [43,44]. However, in the presence of IL-15, ATRA was shown to act as an adjuvant in promoting the secretion of the pro-inflammatory cytokines IL-12 and IL-23 by DCs [45], and has unforeseen co-adjuvant properties that induce Th1 immunity to fed antigens. This suggests that under infectious conditions associated with induction of IL-15 and IL-6 in the intestinal mucosa, ATRA will also promote Th17 immunity [46]. These observations caution against the use of VitA and ATRA for the treatment of autoimmunity and inflammatory intestinal disorders associated with high levels of IL-15.
Innate lymphoid cells (ILC) are a subset of lymphocytes different from T and B cells. Located on the surface of intestinal mucosa, ILCs enhance immune response, maintain mucosal integrity, and promote lymphoid organ formation. ILC can be divided into three groups: ILC1, ILC2, and ILC3. ILC3 are characterized by the expression of the transcription factor RORγt and the cytokines IL-22 and IL-17 [47]. In the fetal period, secondary lymphoid organs formation depends on a subset of ILC3 named lymphoid tissue inducer (LTi) cells [48,49]. Fetal ILC3s are controlled by cell-autonomous RA signaling in utero, which pre-sets the immune fitness in adulthood. Embryonic lymphoid organs contain ILC progenitors that differentiate locally into mature LTi cells. Local LTi was controlled by maternal retinoid intake and fetal RA signaling acting in a hematopoietic cell-autonomous manner. RA controlled LTi cell maturation upstream of the transcription factor RORγt [50]. Both IL-22 and IL-17 mediate antibacterial immune responses and prevent bacterial translocation across barriers. Aberrant regulation of ILC3 and, in particular, the expression of IL-17 is a potential driver of chronic gastrointestinal inflammation [51,52]. Animals deficient in VitA display reduced numbers of ILC3 in contrast to mice fed VitA. This reduction in ILC3 has functional consequences for intestinal immunity, as these mice are more susceptible to infection with the bacterial pathogen Citrobacter rodentium than are VitA competent animals [53]. This is primarily due to a lack of ILC3-mediated IL-22 [51,52,53]. RA significantly enhanced IL-22 production by γδ T cells stimulated in vitro with IL-1β or IL-18 and IL-23. In vivo RA shapes early intestinal immune responses by promoting IL-22 synthesis by γδ T cells and ILC [54].
6. Effects of VitA on T Cells
6.1. RA Induces T Cell Migration
T cells originate from pluripotent stem cells in the bone marrow. These T cells migrate to the thymus where they develop into mature T cells and move to targeted peripheral lymphoid tissues. The entire T cell developmental process is based on the interaction of T cell homing receptors with endothelial adhesion molecules [55]. T cell homing is under the regulation of various adhesion molecules that interact with the homing receptor [55,56,57]. Research has shown that under inflammatory conditions, integrin α4β7 and the T cell chemokine receptor, CCR9, are crucial for T cell migration to the intestine [55,58]. After receiving a RA signal, RARα binds to the RA response element in the integrin α4 gene and regulates the expression of α4β7. Simultaneously, the heterodimer of RARα with the RXR binds to the RAR response element in the promoter region of the CCR9 gene, thus playing an additional regulatory role [59,60,61]. In the intestinal lamina propria, RA is an essential regulator for intestinal homing of CD4+ and CD8+ T cells. VitAD caused a reduction in α4β7(+) memory/activated T cells in lymphoid organs, and a lack of T cells from the intestinal lamina propria [56,57]. Based on this, the provision of ATRA during vaccination can augment the ability of T cell-based viral vaccines to promote the gut/mucosal homing of CD8+ T cells, in order to provide increased protection from mucosal viral challenge, and it also resulted in the formation of more vaccine-specific central memory-like CD8+ T cells in systemic sites [62,63]. Further research shows that RA signaling is required for CD8+ T cells survival and expansion in vivo, and the essential requirement is RARα, but not RARβ or RARγ, for CD8+ T cell survival [64,65]. Whole body imaging using a mouse model of rheumatoid arthritis demonstrated that RA signaling is initiated during the development of inflammation. Furthermore, RA signaling is restricted to the site of inflammation both temporally and spatially. Conditional ablation of RA signaling in T cells significantly interferes with CD4+ T cell effector function, migration, and polarity, indicating RA involvement in T cell migration toward the area of inflammation [66].
6.2. RA Is a Control Factor for Regulatory T Cells and Maintains Its Homeostasis
Regulatory T cells (Treg) are a subpopulation of T cells that maintain immune tolerance and regulate the autoimmune response [67,68,69,70]. Foxp3 is a transcription factor that is essential for the differentiation and effector function of Tregs [71,72]. In vivo, ATRA is produced mainly from CD103+ DC in the gut [73]. The cytokine-transforming growth factor-β (TGF-β) converts naïve T cells into Tregs that prevent autoimmunity. However, in the presence of interleukin-6 (IL-6), TGF-β has also been found to promote the differentiation of naïve T lymphocytes into proinflammatory IL-17 cytokine-producing Th17 cells, which promote autoimmunity and inflammation. ATRA, as a key regulator of TGF-β-dependent immune responses, is capable of inhibiting the IL-6-driven induction of proinflammatory Th17 cells and promoting anti-inflammatory Treg cell differentiation [74]. ATRA enhances the expression of Foxp3 in the presence of TGF-β, thus inducing the differentiation of naïve T cells into Tregs and inhibiting the expression of IL-17 [44,71,72,75]. ATRA acts on the nuclear RAR by interacting with TGF-β to activate the ERK1/2 signaling pathway and enhance histone modification of the Foxp3 promotor region and conserved non-coding DNA region. Therefore, ATRA helps maintain Foxp3 gene expression, and regulates Treg differentiation and function [75,76]. Apart from inducing the differentiation of Tregs, ATRA has also been reported to maintain both the stability of Tregs and their immunoregulatory function [45,73,77,78]. In vitro experiments have shown that in pro-inflammatory environments, Tregs are unstable, and can be transformed into other inflammatory cells, such as Th17 cells, by cytokines like IL-6 and IL-21, thus advancing the development of inflammation. Conversely, the addition of ATRA inhibits the transformation of Tregs into Th17 or other Th cells, even in the presence of IL-6, thus maintaining the expression of Foxp3 [73,77]. Local injection of Tregs failed to prevent development in a collagen-induced arthritis model, whereas the injection of ATRA-pretreated Tregs successfully inhibited the development of arthritis [77,78]. ATRA also enhanced the stability and functionality of human natural Treg cells under the inflammatory conditions [79]. ATRA prevented the transformation of Tregs to Th17 cells and other inflammatory cells by inhibiting the expression of IL-6R on the cell surface of peripherally induced Tregs. Therefore, ATRA enhanced IL-2 function, an important immunomodulator, and promoted naïve T cell transformation into natural Tregs while inhibiting the IL-6-induced transformation of naïve T cells into Th17 cells [45,73,78]. Additionally, ATRA also has the ability to induce and promote the development and function of human-induced Treg cells [80].
6.3. RA May Promote the Ongoing Immune Response
Although most evidence shows that, at pharmacological levels, RA inhibits the development of inflammatory cells and induces or expands Tregs, recent work has suggested that RA may also promote T cell activation and T helper cell responses at minimal levels.
As mentioned above, RA is mainly produced by DC from the gut. Some reports show that RA may also be produced at other sites during an ongoing immune response [66,81,82]. We have discussed that RA signaling is initiated during the development of inflammation. Similarly, there is evidence demonstrating that the RA–RARα signaling axis is essential for adaptive CD4+ T cell immunity as RARα-deficient CD4+ T cells were less efficient than wild-type counterparts in polyclonal activation. Also, in RARα-deficient T cells, the phosphorylation of PLCγ and ERK1/2 was reduced, and manifests impaired Ca2+ mobilization and mTOR/AKT activation upon T cell stimulation. Together, RARα may regulate the signaling pathways downstream of T cell receptor engagement [83].
At pharmacological or high doses (10 nM or higher), RA has been proven to inhibit the reaction of Th17 cells and to induce the generation of Tregs [74,84], and high doses of RA can impair the differentiation of human Th17 and Th1 cells in vitro [85]. However, contrary to reports of RA inhibiting Th1 and Th17 responses, some groups reported that RA was beneficial to Th1 and Th17 cell differentiation at low doses. In physiological doses (1 nM), RA promotes Th17 cell differentiation in vitro [86,87]. In addition, under Th1 or Th17 polarization conditions, the RARα-deficient T cells cultured in vitro did not differentiate into Th1 or Th17 cells, supporting the role of RA in the differentiation of Th1 and Th17 cells, and VitAD mice exhibit significant Th1 and Th17 responses in vivo [53,87,88]. All these results have suggested that RA may have a dose differential effect on the differentiation of Th17 cells and Th1 cells [89]. The role of VitA/RA on Tr1 and Tfh cells is unclear, so far, and warrants further study to allow for clarification.
7. Effects of VitA on B Cell Function
7.1. Effects of VitA on Immunoglobulin Production
Antibody production by B cells is central to humoral homeostatic maintenance. Antibodies represent a specific class of immunoglobulins. Animal experiments have demonstrated that the addition of carotenoid-rich foods to rabbit diets can increase their serum levels of IgG, IgM, and IgA, thereby enhancing humoral immunity [90]. Further studies in rat have revealed the association between a paucity of VitA in the diet and increased number of DCs, in addition to the significantly upregulated expression of IL-12, Toll-like receptor 2, and myeloid differentiation factor MyD88 in the intestinal mucosa. When the levels of secretory IgA decrease, rats display a decreased immune function, suggesting that VitA is involved in the synthesis of immunoglobulins, and has an important influence on humoral immunity [91]. A report shows that RA potently synergized with gut-associated lymphoid tissues DC-derived IL-6 or IL-5 to induce IgA secretion [92]. A knockout study demonstrated that the ablation of RARα reduces IgA expression by B cells expressed in vivo and in vitro. This indicates that RA acts on B cells directly through RARα, which affects the synthesis and secretion of IgA [93]. It is also likely that RA affects Tregs first, and then indirectly modulates B cells, since Tregs have an important role in regulating B cell responses [94].
7.2. VitA Regulation of B Cell Activity
Antigen stimulation of immune cells through specific IgE antibodies results in a rapid, specific hypersensitivity response that is involved in most autoimmune conditions [95]. Evidence shows RA has an IgE-repressive activity in vivo. The inhibitory effect of ATRA on IgE mainly downregulates synthesis and secretion of IgE through RARα, and this inhibitory effect depends on IL-10 [96,97,98,99]. Another report shows that exogenous 9-cis-RA in the context of an allergic sensitization profoundly modulates an established humoral IgE response, resulting in reduced specific IgE responses and increased specific IgA responses in mice, indicating that RXR-activating retinoids play a major role in the physiological regulation of IgE due to the endogenous synthesis of 9-cis-RA [95]. These make VitA a very promising therapy for the treatment of IgE-mediated hypersensitivity disease.
Regulatory B cells (Breg) are a class of B cell subsets with immunomodulatory functions that are involved in the maintenance of immune homeostasis, and play an essential regulatory role in various immunopathological processes [100,101]. RA can induce the differentiation of naïve B cells into Bregs, and stimulate Breg synthesis and the secretion of IL-10 through RARα [102,103,104,105]. By secreting IL-10, Bregs have ameliorative effects on experimental colitis, arthritis, and lupus [98,102,103,104,105]. The mechanism by which VitA regulates Bregs activity and how it improves its immunomodulating function is not yet understood. Further research will be required to elucidate this question, and to determine whether the effects of VitA on Bregs are stable.
8. Application of VitA in the Treatment of Infectious Diseases
8.1. Tuberculosis
Tuberculosis, which is a chronic infectious disease caused by the bacterium Mycobacterium tuberculosis, is a global health concern. In recent years, the therapeutic outcomes of drugs traditionally used for tuberculosis treatment have worsened because of the development of drug resistance. Therefore, different treatment strategies are required.
Epidemiological studies have shown that the healthy population has a significantly higher serum level of VitA than tuberculosis patients [106,107,108]. A longitudinal cohort study of tuberculosis showed that VitA deficiency is dose-dependently correlated to the occurrence of tuberculosis [109]. An in vitro study demonstrated that RA inhibits the growth of M. tuberculosis and reduces its survival rate when engulfed by macrophages [110]. For the mechanism of bacteriocidic activity of VitA, Wheelwright et al. found that VitA can induce the expression of NPC2. In NPC2 gene knockout cells, the stimulation of VitA showed no bacteriocidic activity on infected cells. However, the NPC2 gene is commonly known as a regulator of cholesterol transport rather than an immunological regulatory factor. This result can be explained as follows: cholesterol is the nutritional source for tuberculosis bacterial cell walls, whereas NPC2 facilitates the transportation of cholesterol out of lysosomes, therefore depriving tuberculosis bacteria of their nutritional needs. Without the ability of M. tuberculosis to generate protective cell walls, lysozyme can then effectively kill this pathogen [111]. This was demonstrated in a mouse model of tuberculosis in which the addition of ATRA significantly improved the efficacy of traditional anti-tuberculosis drugs [112]. However, more research will be required to elucidate the positive effects ofVitA supplements on the treatment of tuberculosis.
8.2. Acquired Immune Deficiency Syndrome (AIDS)
AIDS patients are known, in general, to be deficient in many vitamins [113]. Since various vitamins have the potential to enhance the immunity of the organism and because AIDS arises from human immunodeficiency virus infection, oxidative stress is thought to have an important effect on the infection process of HIV virus [114,115]. VitA, VitC, and VitE are all-natural antioxidants, and by inhibiting the oxidative stress of the organism, it is postulated that these vitamins can ameliorate the progression of AIDS.
A previous study has shown that HIV infection reduces an organism′s regulation of oxidative stress. However, an external antioxidant, such as VitA, does not have any compensatory effect on regulating the oxidative stress response [116]. Furthermore, although HIV-infected individuals are deficient in many different vitamins, vitamin supplementation showed no clinically important benefits in people living with HIV [117]. Consistently, VitA does not influence the vertical transmission of HIV from mother to child [118]. Therefore, VitA supplementation does not appear to affect HIV per se, but that does not mean that HIV patients or carriers should reject the supplementation of VitA or any other vitamins. HIV lowers the immune function of the body, making the patients susceptible to infectious diseases, including tuberculosis, malaria, herpes, and others [119,120]. As mentioned above, VitA enhances the immunity of organisms, and it has been reported to reduce the incidence of tuberculosis in HIV patients [119]. Moreover, pregnancy and postpartum supplementation with a multivitamin significantly improved hematologic status among HIV-infected women and their children, and reduced the risk of anemia [120]. Antiretroviral therapy is the most effective treatment regimen for HIV; however, antiretroviral therapy alone is not sufficient to improve micronutrient deficiency. Therefore, it is essential to supplement VitA, other vitamins, and micronutrients during HIV treatment [121].
8.3. Infectious Diseases in Children
Infectious diseases in children were once a global threat [122]. Recent research has suggested a close correlation between a deficiency of micronutrients (particularly VitA) and infectious diseases spread through the respiratory and digestive systems in children [26,123,124]. Meanwhile, many infections result in a decrease in systemic VitA levels as a result of infection-induced anorexia and decreased VitA absorption from the intestine [125,126]. VitA may also be lost in substantial amounts in the urine during infection [127]. As mentioned above, VitA plays a crucial role in the establishment and maintenance of the human immune system. More importantly, VitA has demonstrated a therapeutic effect, to some extent, (see Table 1) in diseases transmitted through the respiratory system, such as pneumonia and measles in children, or in contagious digestive diseases in children, such as infantile diarrhea and hand, foot, and mouth disease [128,129,130]. The World Health Organization has suggested that, in less developed countries, a child between 6 months and 5-years-old should be supplemented with high doses of VitA to prevent and cure VitA deficiency-related diseases, and reduce the incidence and mortality rate of these diseases in children [131].
Table 1.
The therapeutic effect of VitA on several infantile infectious diseases.
| Diseases | Role of VitA | Method Setting | Model [Reference] |
|---|---|---|---|
| Measles | Reduce mortality | Meta-analysis | Human [138] |
| Measles | Reduce morbidity and mortality | Systematic review and meta-analysis | Human [129] |
| Measles | Reduce mortality | Meta-analysis | Human [139] |
| Measles | Reduce morbidity | Randomized double-blind controlled trial | Human [140] |
| Acute pneumonia | Promoting the production of specific antibodies | Randomized controlled trial | Mice [141] |
| Acute pneumonia | Relieving clinical symptoms and signs | Meta-analysis | Human [128] |
| Infantile diarrhea | Reduce morbidity and mortality | Systematic review and meta-analysis | Human [129] |
| Infantile diarrhea | Promote the production of IgA in the intestinal tract and enhance the mucosal immune function | Randomized controlled trial | Mice [142] |
| Infantile diarrhea | Reduce morbidity | Randomized double-blind controlled trial | Human [140] |
| Enteric infection | Reduce morbidity and mortality | Randomized controlled trial | Mice [143] |
| Malaria | Reduce morbidity | Randomized double-blind controlled trial | Human [144] |
| Malaria | Reduce morbidity | Randomized controlled trial | Human [145] |
| Malaria | Reduce morbidity | Randomized double-blind controlled trial | Human [146] |
| Hand foot and mouth disease | Promote production of immunoglobulin and enhance antiviral function | Cross-sectional observation and study | Human [130] |
| Mumps | Up-regulation of type 1 interferon and inhibition of viral replication | In vitro controlled experiment | Cells [147] |
The recommended daily intake of VitA for children is 1665 IU [132]. VitA, as retinol, exceeds 20,000 IU/d in short periods, leading to intoxication and, occasionally, death. VitA intoxication is a generalized syndrome, the signs and symptoms of which include desquamative and edematous dermatitis, bone pain and tenderness, edema of the extremities and face, irritability, hepatocellular dysfunction, and hypercalcemia [133,134,135,136]. Furthermore, inflammation affects retinoid metabolism. Serum retinol may be sequestered in tissues, leading to a reduction in serum retinol levels, which implies that assessing VitA status with the use of serum retinol during inflammation may be problematic [137].
9. Summary
As the interdisciplinary approach continues to develop in research, people have been paying increasing attention to the relationship between nutrition and immunity. Furthermore, the influence of micronutrients on the immune function of the organism has been widely studied. VitA has both promoting and regulatory roles in both the innate immune system and adaptive immunity; therefore, it can enhance the organism’s immune function and provide an enhanced defense against multiple infectious diseases. Currently, the VitA’s effect on immune function has been studied at the molecular level, and more research is ongoing about the therapeutic effects of VitA on preventing and curing various infectious diseases. As increasing evidence appears with time, VitA will likely play more critical roles in modern therapeutics.
Acknowledgments
This research was supported in part by The National Natural Science Foundation of China (Grant Nos. 81460411 and 81660450), Guangxi natural science foundation project (No: 2017AD23009, 2017JJD10037 and 2015jjAA40450) and Key Lab development project from Guangxi.
Author Contributions
Z.H. and G.Q. designed the study, and wrote the review. S.G.Z. conceived, designed the review. S.G.Z., Y.L. and D.B. edited and revised review. All authors discussed and approved the final version.
Funding
This research was funded by NIH R01 AR059103, NIH R61 AR073409 and NIH STAR Award.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sommer A. Vitamin a deficiency and clinical disease: An historical overview. J. Nutr. 2008;138:1835–1839. doi: 10.1093/jn/138.10.1835. [DOI] [PubMed] [Google Scholar]
- 2.Mellanby E., Green H.N. Vitamin A as an anti-infective agent. Br. Med. J. 1928;2:691–696. doi: 10.1136/bmj.2.3537.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb E. Recent findings on the importance of vitamin A and its metabolism in man and laboratory animals. Z. Gesamte Inn. Med. 1981;36:897–902. [PubMed] [Google Scholar]
- 4.Semba R.D. Vitamin A, immunity, and infection. Clin. Infect. Dis. 1994;19:489–499. doi: 10.1093/clinids/19.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Muhilal S., Permeisih D., Idjradinata Y.R., Muherdiyantiningsih D., Karyadi D. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: A controlled field trial. Am. J. Clin. Nutr. 1988;48:1271–1276. doi: 10.1093/ajcn/48.5.1271. [DOI] [PubMed] [Google Scholar]
- 6.Pino-Lagos K., Guo Y., Noelle R.J. Retinoic acid: A key player in immunity. Biofactors. 2010;36:430–436. doi: 10.1002/biof.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross S.A., Mccaffery P.J., Drager U.C., De Luca L.M. Retinoids in embryonal development. Physiol. Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 8.Soprano D.R., Qin P., Soprano K.J. Retinoic acid receptors and cancers. J. Nutr. 2002;132:3809S. doi: 10.1093/jn/132.12.3809S. [DOI] [PubMed] [Google Scholar]
- 9.Rochette-Egly C., Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nucl. Recept. Signal. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Repa J.J., Hanson K.K., Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc. Natl. Acad. Sci. USA. 1993;90:7293. doi: 10.1073/pnas.90.15.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940. doi: 10.1096/fasebj.10.9.8801176. [DOI] [PubMed] [Google Scholar]
- 12.Mangelsdorf D., Umesono K., Evans R.M. The Retinoid Receptors. In: Sporn M.B., Roberts A.B., Goodman D.S., editors. The Retinoids: Biology, Chemistry and Medicine. 2nd ed. Raven Press; New York, NY, USA: 1994. pp. 319–350. [Google Scholar]
- 13.Glass C.K., Rosenfeld M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121. [PubMed] [Google Scholar]
- 14.Bastien J., Rochetteegly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Taimi M., Helvig C., Wisniewski J., Ramshaw H., White J., Amad M., Korczak B., Petkovich M. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. Biol. Chem. 2004;279:77–85. doi: 10.1074/jbc.M308337200. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Abed S.S., Beckett B.R., Chiba H., Chithalen J.V., Jones G., Metzger D., Chambon P., Petkovich M. Mouse P450RAIexpression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. Biol. Chem. 1998;273:2409–2415. doi: 10.1074/jbc.273.4.2409. [DOI] [PubMed] [Google Scholar]
- 17.Ribes V., Otto D.M., Dickmann L., Schmidt K., Schuhbaur B., Henderson C., Blomhoff R., Wolf C.R., Tickle C., Dolle P. Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev. Biol. 2007;303:66–81. doi: 10.1016/j.ydbio.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Mccullough F.S., Northropclewes C.A., Thurnham D.I. The effect of vitamin A on epithelial integrity. Proc. Nutr. Soc. 1999;58:289. doi: 10.1017/S0029665199000403. [DOI] [PubMed] [Google Scholar]
- 19.Wang J.L., Swartz-Basile D.A., Rubin D.C., Levin M.S. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J. Nutr. 1997;127:1297–1303. doi: 10.1093/jn/127.7.1297. [DOI] [PubMed] [Google Scholar]
- 20.Amitromach E., Uni Z., Cheled S., Berkovich Z., Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J. Nutr. Biochem. 2009;20:70–77. doi: 10.1016/j.jnutbio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Wolbach S.B., Howe P.R. Tissue changes following deprivation of fat-soluble A vitamin. Nutr. Rev. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson J.R., Dubois R.O. Report of a fatal case of keratomalacia in an infant, with postmortem examination. Am. J. Dis. Child. 1923;26:431–446. doi: 10.1001/archpedi.1923.04120170042006. [DOI] [Google Scholar]
- 23.Blackfan K.D., Wolbach S.B. Vitamin A deficiency in infants: A clinical and pathological study. J. Pediatr. 1933;3:679–706. doi: 10.1016/S0022-3476(33)80022-9. [DOI] [Google Scholar]
- 24.Keenum D.G., Semba R.D., Wirasasmita S., Natadisastra G., Muhilal S., West K.P., Jr., Sommer A. Assessment of vitamin A status by a disk applicator for conjunctival impression cytology. Arch. Ophthalmol. 1990;108:1436–1441. doi: 10.1001/archopht.1990.01070120084034. [DOI] [PubMed] [Google Scholar]
- 25.Natadisastra G., Wittpenn J.R., West K.P., Jr., Muhilal S., Sommer A. Impression cytology for detection of vitamin A deficiency. Arch. Ophthalmol. 1987;105:1224. doi: 10.1001/archopht.1987.01060090082033. [DOI] [PubMed] [Google Scholar]
- 26.Qi Y.J., Niu Q.L., Zhu X.L., Zhao X.Z., Yang W.W., Wang X.J. Relationship between deficiencies in vitamin A and E and occurrence of infectious diseases among children. Eur. Rev. Med. Pharmacol. Sci. 2016;20:5009–5012. [PubMed] [Google Scholar]
- 27.Riabroy N., Tanumihardjo S.A. Oral doses of α-retinyl ester track chylomicron uptake and distribution of vitamin A in a male piglet model for newborn infants. J. Nutr. 2014;144:1188–1195. doi: 10.3945/jn.114.191668. [DOI] [PubMed] [Google Scholar]
- 28.Kiss I., Rühl R., Szegezdi E., Fritzsche B., Toth B., Pongrácz J., Perlmann T., Fésüs L., Szondy Z. Retinoid receptor-activating ligands are produced within the mouse thymus during postnatal development. Eur. J. Immunol. 2008;38:147–155. doi: 10.1002/eji.200737342. [DOI] [PubMed] [Google Scholar]
- 29.Kuwata T., Wang I.M., Tamura T., Ponnamperuma R.M., Levine R., Holmes K.L., Morse H.C., De Luca L.M., Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349. [PubMed] [Google Scholar]
- 30.van Bennekum A.M., Wong Yen Kong L.R., Gijbels M.J., Tielen F.J., Roholl P.J., Brouwer A., Hendriks H.F. Mitogen response of B cells, but not T cells, is impaired in adult vitamin A-deficient rats. J. Nutr. 1991;121:1960–1968. doi: 10.1093/jn/121.12.1960. [DOI] [PubMed] [Google Scholar]
- 31.Darwiche N., Celli G., Sly L., Lancillotti F., De Luca L.M. Retinoid status controls the appearance of reserve cells and keratin expression in mouse cervical epithelium. Cancer Res. 1993;53:2287–2299. [PubMed] [Google Scholar]
- 32.Chang H.K., Hou W.S. Retinoic acid modulates interferon-γ production by hepatic natural killer T cells via phosphatase 2A and the extracellular signal-regulated kinase pathway. J. Interferon Cytokine Res. 2015;35:200–212. doi: 10.1089/jir.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn T.A., Vannella K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiemstra I.H., Beijer M.R., Veninga H., Vrijland K., Borg E.G., Olivier B.J., Mebius R.E., Kraal G., den Haan J.M. The identification and developmental requirements of colonic CD169+ macrophages. Immunology. 2014;142:269. doi: 10.1111/imm.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira W.F., Ribeiro-Gomes F.L., Guillermo L.V., Vellozo N.S., Montalvão F., Dosreis G.A., Lopes M.F. Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T cell responses. J. Leukoc. Biol. 2011;90:1191–1197. doi: 10.1189/jlb.1110608. [DOI] [PubMed] [Google Scholar]
- 36.Vellozo N.S., Pereiramarques S.T., Cabralpiccin M.P., Filardy A.A., Ribeirogomes F.L., Rigoni T.S., Dosreis G.A., Lopes M.F. All-trans retinoic acid promotes an m1- to m2-phenotype shift and inhibits macrophage-mediated immunity to leishmania major. Front. Immunol. 2017;8:1560. doi: 10.3389/fimmu.2017.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrestha S., Kim S.Y., Yun Y.J., Kim J.K., Lee J.M., Shin M., Song D.K., Hong C.W. Retinoic acid induces hypersegmentation and enhances cytotoxicity of neutrophils against cancer cells. Immunol. Lett. 2017;182:24–29. doi: 10.1016/j.imlet.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Worbs T., Hammerschmidt S.I., Förster R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 39.Beijer M.R., Molenaar R., Goverse G., Mebius R.E., Kraal G., den Haan J.M. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8−CD4− and CD4+ dendritic cells. Eur. J. Immunol. 2013;43:1608–1616. doi: 10.1002/eji.201343325. [DOI] [PubMed] [Google Scholar]
- 40.Klebanoff C.A., Spencer S.P., Torabi-Parizi P., Grainger J.R., Roychoudhuri R., Ji Y., Sukumar M., Muranski P., Scott C.D., Hall J.A., et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J. Exp. Med. 2013;210:1961–1976. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duriancik D.M., Hoag K.A. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8+Gr-1+ memory T lymphocytes in C57BL/6J mice. Cell Immunol. 2010;265:156–163. doi: 10.1016/j.cellimm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng R., Bscheider M., Lahl K., Lee M., Butcher E.C. Generation and transcriptional programming of intestinal dendritic cells: Essential role of retinoic acid. Mucosal. Immunol. 2016;9:183–193. doi: 10.1038/mi.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott C.L., Aumeunier A.M., Mowat A.M. Intestinal CD103+, dendritic cells: Master regulators of tolerance? Trends Immunol. 2011;32:412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 46.DePaolo R.W., Abadie V., Tang F., Fehlner-Peach H., Hall J.A., Wang W., Marietta E.V., Kasarda D.D., Waldmann T.A., Murray J.A., et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 48.Sa V.D.P., Mebius R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 49.Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. An essential function for the nuclear receptor RORgammat in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 50.Sa V.D.P., Ferreira M., Domingues R.G., Ribeiro H., Molenaar R., Moreira-Santos L., Almeida F.F., Ibiza S., Barbosa I., Goverse G., et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., Powrie F. Innate lymphoid cells drive IL-23 dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geremia A., Arancibia-Cárcamo C.V., Fleming M.P., Rust N., Singh B., Mortensen N.J., Travis S.P., Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spencer S.P., Wilhelm C., Yang Q., Hall J.A., Bouladoux N., Boyd A., Nutman T.B., Urban J.F., Jr., Wang J., Ramalingam T.R., et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mielke L.A., Jones S.A., Raverdeau M., Higgs R., Stefanska A., Groom J.R., Misiak A., Dungan L.S., Sutton C.E., Streubel G., et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mora J.R., von Andrian U.H. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Svensson M., Johansson-Lindbom B., Zapata F., Jaensson E., Austenaa L.M., Blomhoff R., Agace W.W. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal. Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosoe N., Miura S., Watanabe C., Tsuzuki Y., Hokari R., Oyama T., Fujiyama Y., Nagata H., Ishii H. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:458–466. doi: 10.1152/ajpgi.00167.2003. [DOI] [PubMed] [Google Scholar]
- 59.Denucci C.C., Pagán A.J., Mitchell J.S., Shimizu Y. Control of α4β7 Integrin Expression and CD4 T Cell Homing by the β1 Integrin Subunit. J. Immunol. 2010;184:2458–2467. doi: 10.4049/jimmunol.0902407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang S.G., Park J., Cho J.Y., Ulrich B., Kim C.H. Complementary roles of retinoic acid and TGF-β1 in coordinated expression of mucosal integrins by T cells. Mucosal. Immunol. 2011;4:66–82. doi: 10.1038/mi.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohoka Y., Yokota A., Takeuchi H., Maeda N., Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex. J. Immunol. 2011;186:733–744. doi: 10.4049/jimmunol.1000913. [DOI] [PubMed] [Google Scholar]
- 62.Tan X., Sande J.L., Pufnock J.S., Blattman J.N., Greenberg P.D. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J. Virol. 2011;85:8316–8327. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allie S.R., Zhang W., Tsai C.Y., Noelle R.J., Usherwood E.J. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. J. Immunol. 2013;190:2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Y., Pino-Lagos K., Ahonen C.A., Bennett K.A., Wang J., Napoli J.L., Blomhoff R., Sockanathan S., Chandraratna R.A., Dmitrovsky E., et al. A retinoic acid--rich tumor microenvironment provides clonal survival cues for tumor-specific CD8+ T cells. Cancer Res. 2012;72:5230–5239. doi: 10.1158/0008-5472.CAN-12-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo Y., Lee Y.C., Brown C., Zhang W., Usherwood E., Noelle R.J. Dissecting the Role of Retinoic Acid Receptor Isoforms in the CD8 Response to Infection. J. Immunol. 2014;192:3336–3344. doi: 10.4049/jimmunol.1301949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pino-Lagos K., Guo Y., Brown C., Alexander M.P., Elgueta R., Bennett K.A., De Vries V., Nowak E., Blomhoff R., Sockanathan S., et al. A retinoic acid–dependent checkpoint in the development of CD4+ T cell–mediated immunity. J. Exp. Med. 2011;208:1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao X.J., Ma J.L., Zhang Y.H., Yu J.N., Cai L., Wang J.H., Zheng S.G. Neutralization of IL-4 and IFN-γ Facilitates inducing TGF-β-induced CD4+Foxp3+ Regulatory Cells. Int. J. Biomed. Sci. 2008;4:52–57. [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng S.G., Gray J.D., Ohtsuka K., Yamagiwa S., Horwitz D.A. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 69.Zheng S.G., Wang J.H., Gray J.D., Soucier H., Horwitz D.A. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: The role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 70.Zheng S.G., Wang J., Wang P., Gray J.D., Horwitz D.A. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 71.Hori S., Nomura T., Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 72.Ma J., Liu Y., Li Y., Gu J., Liu J., Tang J., Wang J., Ryffel B., Shen Y., Brand D., et al. Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J. Leukoc. Biol. 2014;95:275–283. doi: 10.1189/jlb.0513297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z.M., Wang K.P., Ma J., Zheng S.G. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol. Immunol. 2015;12:553–557. doi: 10.1038/cmi.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. Reciprocal th17 and regulatory t cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 75.Schambach F., Schupp M., Lazar M.A., Reiner S.L. Activation of retinoic acid receptor-α favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 76.Lu L., Ma J., Li Z., Lan Q., Chen M., Liu Y., Xia Z., Wang J., Han Y., Shi W., et al. All-Trans Retinoic Acid Promotes TGF-β-Induced Tregs via Histone Modification but Not DNA Demethylation on Foxp3 Gene Locus. PLoS ONE. 2011;6:e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X., Kong N., Wang J., Fan H., Zou H., Horwitz D., Brand D., Liu Z., Zheng S.G. Cutting edge: All-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwok S.K., Park M.K., Cho M.L., Oh H.J., Park E.M., Lee D.G., Lee J., Kim H.Y., Park S.H. Retinoic acid attenuates rheumatoid inflammation in mice. J. Immunol. 2012;189:1062–1067. doi: 10.4049/jimmunol.1102706. [DOI] [PubMed] [Google Scholar]
- 79.Lu L., Lan Q., Li Z., Zhou X., Gu J., Li Q., Wang J., Chen M., Liu Y., Shen Y., et al. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl. Acad. Sci. USA. 2014;111:3432–3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu L., Zhou X.H., Wang J.L., Zheng S.G. Correction: Characterization of Protective Human CD4+CD25+ FOXP3+ Regulatory T Cells Generated with IL-2, TGF-β and Retinoic Acid. PLoS ONE. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammerschmidt S.I., Ahrendt M., Bode U., Wahl B., Kremmer E., Forster R., Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molenaar R., Greuter M., van der Marel A.P., Roozendaal R., Martin S.F., Edele F., Huehn J., Forster R., O’Toole T., Jansen W., et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. Immunology. 2009;183:6395–6402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 83.Hall J.A., Cannons J.L., Grainger J.R., Dos Santos L.M., Hand T.W., Naik S., Wohlfert E.A., Chou D.B., Oldenhove G., Robinson M., et al. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao S., Jin H., Korn T., Liu S.M., Oukka M., Lim B., Kuchroo V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. Immunology. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rampal R., Awasthi A., Ahuja V. Retinoic acid-primed human dendritic cells inhibit Th9 cells and induce Th1/Th17 cell differentiation. J. Leukoc. Biol. 2016;100:111–120. doi: 10.1189/jlb.3VMA1015-476R. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi H., Kanno T., Nakayamada S., Hirahara K., Sciume G., Muljo S.A., Kuchen S., Casellas R., Wei L., Kanno Y., et al. TGF-[beta] and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat. Immunol. 2012;13:587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang C., Kang S.G., HogenEsch H., Love P.E., Kim C.H. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J. Immunol. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cha H.R., Chang S.Y., Chang J.H., Kim J.O., Yang J.Y., Kim C.H., Kweon M.N. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. Immunology. 2010;184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- 89.Raverdeau M., Mills K.H. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014;192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 90.Ghodratizadeh S., Kanbak G., Beyramzadeh M., Dikmen Z.G., Memarzadeh S., Habibian R. Effect of carotenoid β-cryptoxanthin on cellular and humoral immune response in rabbit. Vet. Res. Commun. 2014;38:59–62. doi: 10.1007/s11259-013-9584-8. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y., Yuan Y., Tao Y., Wang W. Effects of vitamin A deficiency on mucosal immunity and response to intestinal infection in rats. Nutrition. 2011;27:227–232. doi: 10.1016/j.nut.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 92.Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 93.Pantazi E., Marks E., Stolarczyk E., Lycke N., Noelle R.J., Elgueta R. Cutting Edge: Retinoic Acid Signaling in B Cells Is Essential for Oral Immunization and Microflora Composition. J. Immunol. 2015;195:1368–1371. doi: 10.4049/jimmunol.1500989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu A., Liu Y., Chen W., Wang J., Xue Y., Huang F., Rong L., Lin J., Liu D., Yan M., et al. TGF-β-induced regulatory T cells directly suppress B cell responses through a non-cytotoxic mechanism. J. Immunol. 2016;196:3631–3641. doi: 10.4049/jimmunol.1501740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heine G., Hollstein T., Treptow S., Radbruch A., Worm M. 9-cis retinoic acid modulates the type I allergic immune response. J. Allergy. Clin. Immunol. 2018;141:650–658. doi: 10.1016/j.jaci.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 96.Seo G.Y., Lee J.M., Jang Y.S., Kang S.G., Yoon S.I., Ko H.J., Lee G.S., Park S.R., Nagler C.R., Kim P.H. Mechanism underlying the suppressor activity of retinoic acid on IL4-induced IgE synthesis and its physiological implication. Cell Immunol. 2017;322:49–55. doi: 10.1016/j.cellimm.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dilillo D.J., Matsushita T., Tedder T.F. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 98.Yanaba K., Bouaziz J.D., Haas K.M., Poe J.C., Fujimoto M., Tedder T.F. A regulatory B cell subset with a unique CD1dhi CD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 99.Yanaba K., Bouaziz J.D., Matsushita T., Tsubata T., Tedder T.F. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 cells) Requires Antigen Receptor Diversity and TLR Signals. J. Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf S.D., Dittel B.N., Hardardottir F., Janeway C.A., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 102.Di Caro V., Phillips B., Engman C., Harnaha J., Trucco M., Giannoukakis N. Retinoic acid-producing, ex-vivo-generated human tolerogenic dendritic cells induce the proliferation of immunosuppressive B lymphocytes. Clin. Exp. Immunol. 2013;174:302–317. doi: 10.1111/cei.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwata Y., Matsushita T., Horikawa M., Dilillo D.J., Yanaba K., Venturi G.M., Szabolcs P.M., Bernstein S.H., Magro C.M., Williams A.D., et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mauri C., Gray D., Mushtaq N., Londei M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blair P.A., Noreña L.Y., Flores-Borja F., Rawlings D.J., Isenberg D.A., Ehrenstein M.R., Mauri C. CD19+ CD24hi CD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 106.Ramachandran G., Santha T., Garg R., Baskaran D., Iliayas S.A., Venkatesan P., Fathima R., Narayanan P.R. Vitamin A levels in sputum-positive pulmonary tuberculosis patients in comparison with household contacts and healthy ‘normals’. Int. J. Tuberc. Lung Dis. 2004;8:1130–1133. [PubMed] [Google Scholar]
- 107.Mugusi F.M., Rusizoka O., Habib N., Fawzi W. Vitamin A status of patients presenting with pulmonary tuberculosis and asymptomatic HIV-infected individuals, Dar es Salaam, Tanzania. Int. J. Tuberc. Lung Dis. 2003;7:804–807. [PubMed] [Google Scholar]
- 108.Qrafli M., El Kari K., Aguenaou H., Bourkadi J.E., Sadki K., El Mzibri M. Low plasma vitamin A concentration is associated with tuberculosis in Moroccan population: A preliminary case control study. BMC Res. Notes. 2017;10:421. doi: 10.1186/s13104-017-2737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aibana O., Franke M.F., Huang C.C., Galea J.T., Calderon R., Zhang Z., Becerra M.C., Smith E.R., Ronnenberg A.G., Contreras C., et al. Impact of Vitamin A and Carotenoids on the Risk of Tuberculosis Progression. Clin. Infect. Dis. 2017;65:900–909. doi: 10.1093/cid/cix476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anand P.K., Kaul D., Sharma M. Synergistic action of vitamin D and retinoic acid restricts invasion of macrophages by pathogenic mycobacteria. J. Microbiol. Immunol. Infect. 2008;41:17–25. [PubMed] [Google Scholar]
- 111.Wheelwright M., Kim E.W., Inkeles M.S., De Leon A., Pellegrini M., Krutzik S.R., Liu P.T. All-trans retinoic acid triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J. Immunol. 2014;192:2280–2290. doi: 10.4049/jimmunol.1301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mourik B.C., Leenen P.J., de Knegt G.J., Huizinga R., van der Eerden B.C., Wang J., Krois C.R., Napoli J.L., Bakker-Woudenberg I.A., de Steenwinkel J.E. Immunotherapy added to antibiotic treatment reduces relapse of disease in a mouse model of tuberculosis. Am. J. Respir. Cell Mol. Biol. 2017;56:233–241. doi: 10.1165/rcmb.2016-0185OC. [DOI] [PubMed] [Google Scholar]
- 113.Drain P.K., Kupka R., Mugusi F., Fawzi W.W. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am. J. Clin. Nutr. 2007;85:333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 114.Chin J. The AIDS Pandemic: The collision of epidemiology with political correctness. Drugs Alcohol. Today. 2007;7:46–47. doi: 10.1108/17459265200700016. [DOI] [Google Scholar]
- 115.Schreck R., Rieber P., Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Makinde O., Rotimi K., Ikumawoyi V., Adeyemo T., Olayemi S. Effect of vitamin A and vitamin C supplementation on oxidative stress in HIV and HIV-TB co-infection at Lagos University Teaching Hospital (LUTH) Nigeria. Afr. Health Sci. 2017;17:308–314. doi: 10.4314/ahs.v17i2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Visser M.E., Durao S., Sinclair D., Irlam J.H., Siegfried N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst. Rev. 2017;5:CD003650. doi: 10.1002/14651858.CD003650.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiysonge C.S., Ndze V.N., Kongnyuy E.J., Shey M.S. Vitamin A supplements for reducing mother-to-child HIV transmission. Cochrane Database Syst. Rev. 2017;9:CD003648. doi: 10.1002/14651858.CD003648.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campa A., Baum M.K., Bussmann H., Martinez S.S., Farahani M., van Widenfelt E., Moyo S., Makhema J., Essex M., Marlink R. The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana. Nutr. Diet. Suppl. 2017;2017:37–45. doi: 10.2147/NDS.S123545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fawzi W.W., Msamanga G.I., Kupka R., Spiegelman D., Villamor E., Mugusi F., Wei R., Hunter D. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am. J. Clin. Nutr. 2007;85:1335–1343. doi: 10.1093/ajcn/85.5.1335. [DOI] [PubMed] [Google Scholar]
- 121.Shivakoti R., Christian P., Yang W.T., Gupte N., Mwelase N., Kanyama C., Pillay S., Samaneka W., Santos B., Poongulali S., et al. Prevalence and risk factors of micronutrient deficiencies pre- and post-antiretroviral therapy (ART) among a diverse multicountry cohort of HIV-infected adults. Clin. Nutr. 2016;35:183–189. doi: 10.1016/j.clnu.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rytter M.J., Kolte L., Briend A., Friis H., Christensen V.B. The Immune System in Children with Malnutrition—A Systematic Review. PLoS ONE. 2014;9:e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen K., Zhang X., Li T.Y., Chen L., Qu P., Liu Y.X. Co-assessment of iron, vitamin A and growth status to investigate anemia in preschool children in suburb Chongqing, China. World J. Pediatr. 2009;5:275–281. doi: 10.1007/s12519-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 124.Semba R.D., de Pee S., Sun K., Campbell A.A., Bloem M.W., Raju V.K. Low intake of vitamin A-rich foods among children, aged 12–35 months, in India: Association with malnutrition, anemia, and missed child survival interventions. Nutrition. 2010;26:958–962. doi: 10.1016/j.nut.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 125.Sivakumar B., Reddy V. Absorption of labelled vitamin A in children during infection. Br. J. Nutr. 1972;27:299–304. doi: 10.1079/BJN19720094. [DOI] [PubMed] [Google Scholar]
- 126.Sivakumar B., Reddy V. Absorption of vitamin A in children with ascariasis. J. Trop. Med. Hyg. 1975;78:114–115. [PubMed] [Google Scholar]
- 127.Stephensen C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 128.Nan H.U., Qu-Bei L.I., Zou S.Y. Effect of vitamin A as an adjuvant therapy for pneumonia in children: A meta analysis. Chin. J. Contemp. Pediatr. 2018;20:146–153. doi: 10.7499/j.issn.1008-8830.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mayo-Wilson E., Imdad A., Herzer K., Yakoob M.Y., Bhutta Z.A. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen S., Yang Y., Yan X., Chen J., Yu H., Wang W. Influence of vitamin A status on the antiviral immunity of children with hand, foot and mouth disease. Clin. Nutr. 2012;31:543–548. doi: 10.1016/j.clnu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 131.Fisker A.B., Bale C., Rodrigues A., Balde I., Fernandes M., Jørgensen M.J., Danneskiold-Samsøe N., Hornshøj L., Rasmussen J., Christensen E.D., et al. High-dose Vitamin A with Vaccination after 6 Months of Age: A Randomized Trial. Pediatrics. 2014;134:739–748. doi: 10.1542/peds.2014-0550. [DOI] [PubMed] [Google Scholar]
- 132.Beare-Rogers J.L. Recommended Nutrient Intakes for Canadians. Dept. Natl. Health Welf. 1983:48–50. doi: 10.1016/S0315-5463(84)72296-6. [DOI] [Google Scholar]
- 133.Oliver T.K. Chronic vitamin A intoxication. Am. J. Dis. Child. 1953;95:57–68. doi: 10.1001/archpedi.1958.02060050059010. [DOI] [PubMed] [Google Scholar]
- 134.Bush M.E., Dahms B.B. Fatal hypervitaminosis A in a neonate. Arch. Pathol. Lab Med. 1984;108:838–842. [PubMed] [Google Scholar]
- 135.James M.B., Leonard J.C., Fraser J.J., Jr., Stuemky J.H. Hypervitaminosis A: A case report. Pediatrics. 1982;69:112–115. [PubMed] [Google Scholar]
- 136.Lippe B., Hensen L., Mendoza G., Finerman M., Welch M. Chronic vitamin A intoxication: A multisystem disease that could reach epidemic proportions. Am. J. Dis. Child. 1981;135:634–636. doi: 10.1001/archpedi.1981.02130310040014. [DOI] [PubMed] [Google Scholar]
- 137.Rubin L.P., Ross A.C., Stephensen C.B., Bohn T., Tanumihardjo S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017;8:197–212. doi: 10.3945/an.116.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang H.M., Mao M., Wan C. Vitamin A for treating measles in children. Cochrane Database Syst. Rev. 2005;5:85–86. doi: 10.1002/14651858.CD001479.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sudfeld C.R., Marie N.A., Halsey N.A. Effectiveness of measles vaccination and vitamin A treatment. Int. J. Epidemiol. 2010;39:i48–i55. doi: 10.1093/ije/dyq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bhandari N., Bhan M.K., Sazawal S. Impact of massive dose of vitamin A given to preschool children with acute diarrhoea on subsequent respiratory and diarrhoeal morbidity. BMJ. 1994;309:1404–1407. doi: 10.1136/bmj.309.6966.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cui D., Moldoveanu Z., Stephensen C.B. High-level dietary vitamin A enhances T-helper type 2 cytokine production and secretory immunoglobulin A response to influenza A virus infection in BALB/c mice. J. Nutr. 2000;130:1132–1139. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- 142.Nikawa T., Odahara K., Koizumi H., Kido Y., Teshima S., Rokutan K., Kishi K. Vitamin A prevents the decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J. Nutr. 1999;129:934–941. doi: 10.1093/jn/129.5.934. [DOI] [PubMed] [Google Scholar]
- 143.McDaniel K.L., Restori K.H., Dodds J.W., Kennett M.J., Ross A.C., Cantorna M.T. Vitamin A-deficient hosts become nonsymptomatic reservoirs of escherichia coli-like enteric infections. Infect. Immun. 2015;83:2984–2991. doi: 10.1128/IAI.00201-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Elom M.O., Okafor F.C., Eyo J.E. Vitamin A supplementation of malaria-infected pregnant women and infant birth weight outcomes a case study of Ebonyi State, Nigeria. Gastro. 2014;2:109. [Google Scholar]
- 145.Owusu-Agyei S., Newton S., Mahama E., Febir L.G., Ali M., Adjei K., Tchum K., Alhassan L., Moleah T., Tanumihardjo S.A. Impact of vitamin A with zinc supplementation on malaria morbidity in Ghana. Nutr. J. 2013;12:131. doi: 10.1186/1475-2891-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zeba A.N., Sorgho H., Rouamba N., Zongo I., Rouamba J., Guiguemdé R.T., Hamer D.H., Mokhtar N., Ouedraogo J.B. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: A randomized double blind trial. Nutr. J. 2008;7:1–7. doi: 10.1186/1475-2891-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Soye K.J., Trottier C., Di Lenardo T.Z., Restori K.H., Reichman L., Miller W.H., Jr., Ward B.J. In vitro inhibition of mumps virus by retinoids. Virol. J. 2013;10:337. doi: 10.1186/1743-422X-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]