Abstract
There are fundamental differences between humans and the animals we typically use to study the immune system. We have learned much from genetically manipulated and inbred animal models, but instances in which these findings have been successfully translated to human immunity have been rare. Embracing the genetic and environmental diversity of humans can tell us about the fundamental biology of immune cell types and the elasticity of the immune system. Although people are much more immunologically diverse than conventionally housed animal models, tools and technologies are now available that permit high-throughput analysis of human samples, including both blood and tissues, which will give us deep insights into human immunity in health and disease. As we gain a more detailed picture of the human immune system, we can build more sophisticated models to better reflect this complexity, both enabling the discovery of new immunological mechanisms and facilitating translation into the clinic.
Background
Technological and reagent advances have accelerated our ability to perform human immunology research in a rigorous, ethical, and high-throughput manner. The goal of this review is to bring attention to the variety of techniques and options available for studying the human immune system directly and indirectly through judicious use of appropriate models, in vitro assays, and in vivo studies to improve the translatable potential of immunology research. Choosing the right model system for a given immunological question is essential. Much of what we currently know is derived from studies in inbred mice, and while they seem very similar to humans in many basic aspects, it appears likely that the much greater breadth of pathogen exposure experienced by human beings, together with their genetic heterogeneity, will result in many disparities. Intensive efforts over the past 30 years have seen the creation of ‘humanized’ mice with varying degrees of fidelity in accurately modeling human immune responses [1–5]. But the use of these animals presents a daunting problem for translation to humans because of the many incompatibilities between cytokines and cytokine receptors between species.
Although animal models certainly have a place in immunology research, it’s important to recognize their limitations in various aspects of recapitulating human immunity. Some human diseases have no appropriate animal model, and others are hampered by models that incompletely recapitulate key features of a human disease. Striking differences in the T cell responses of inbred mice cohabited with pet store mice have shown clearly that at least some of the differences between inbred mice and humans are due to environmental exposure [6], and even nonhuman primate models (NHP) have failed to predict human immune responses [7, 8]. Mice live one to two years, a miniscule amount of time compared to the average human lifespan. Not only is it virtually impossible to mimic a human lifetime’s worth of antigenic exposure in such a short period of time, but cell turnover is regulated in different ways in different species [9]. Most animal models are based around fixed genetic diversity (e.g., in major histocompatibility loci) and their distributions of immune-relevant receptors and ligands are quite distinct from those of humans (superbly summarized in [10]). If we seek to extend animal model findings to human immunity, it is important to get these genetic and cellular distributions right. Indeed, pre-clinical studies have often been poorly predictive of response in humans [11–17]. The combination of sophisticated animal models that are more relevant for studying human disease and our ability to perform direct ex vivo and in vitro high-throughput assays from human cohort samples suggests a bright future for understanding human immunity. Here, we discuss these new tools and systems that are available to better reflect the complexities of human immunity.
Improved animal models
Owing to logistical and ethical considerations, experimental research in humans has limitations. This is especially the case for the testing of completely novel interventions and for mechanistic immunology research, although the tools that will overcome these challenges are being developed rapidly, as we discuss below. Therefore, animal models still have a place in the translational pipeline due to their ability to overcome these challenges. Myriad models are used for pre-clinical assessments, though generally speaking, the murine and NHP models are best for immunologic studies because of the availability of reagents and tools (Table 1). From small models (mice, guinea pigs, hamsters, zebra fish, and ferrets, among others) to large models (nonhuman primates, pigs, cows, sheep, and more), each model has its own set of advantages and disadvantages, and when choosing them, careful consideration should be made as to how they fit the research question. Here, we focus on the murine and NHP models and the recent advances in, and broad applications to, human translational immunology.
Table 1.
An overview of animal models for translational studies for human immunology
| Model system | Advantages | Disadvantages | Suggested model use | Translatability | Environmental factors |
|---|---|---|---|---|---|
| Conventional inbred, wildtype, and knock-out mice | • Consistency • Widely available • Cost • Easy to handle |
• Poorly represent many human diseases | Fundamental immunology, pre-clinical work in some cases | + | Co-housing and diet may impact microbiota |
| Next-generation mice (humanized through genetic and/or tissue engineering) | • More potential for translation to humans • Many immunologic reagents • Easy to handle |
• More expensive than conventional mice • Partial humanization means murine components can be confounding factors |
Single-organ infections, such as liver cancer, especially metastasis; individual aspects of an immune response, such as Ig or HLA loci | ++ | Similar factors to conventional mice |
| Nonhuman primates (NHP) | • Human-like model • Many immunologic reagents • Limited MHC (certain species) |
• Larger MHC than humans (most species) • Expensive • Ethical concerns |
HIV, tuberculosis, many arthropod-borne viruses | ++/+++ | Typically not co-housed, but length in colony may impact response to perturbations |
| Other animal models | May model particular diseases more accurately than mice or NHP | Reagents limited or non-existent | Non-immunologic disease models, transmission studies (e.g., ferrets for influenza transmission studies recapitulate many features of human infection) | Up to +++ | Many, as typically are not bred for research |
Translatability (from weak (+) to strong (+++)) refers to the relative frequency of successfully identifying an immune phenomenon in the model system that closely mimics the relevant disease or condition in humans
HLA human leukocyte antigen, Ig immunoglobulin, MHC major histocompatibility complex
Murine models
The advantages of mice are universally understood: they are small, tractable, inexpensive, and have many reagents readily available. Their tractability has led to many insights in basic immunology; many of the key insights gained in basic human immunology (such as lymphocyte receptor function, tissue homing, costimulation, and cytokine/chemokine signaling) were first elucidated in murine models. However, the inability of mice to mimic the human immune response means that they can be problematic in studies involving translation to the human system [11, 12, 18–21]. Many diseases that are of human relevance either do not exist or present differently in mice. For example, many viruses that cause disease in humans do not replicate in mice, and when they do, the pathology that results is often different to that observed in humans [22, 23]. Small molecules and other therapeutics can be species-specific and exert effects in humans that are different from those in animal models. Currently, the main tool to bridge this gap is the use of humanized mice.
Three of the most common types of murine models used for pre-clinical research are: genetically engineered mouse models; xenograft models, engrafted with either cell-line-derived (CDX) or patient-derived (PDX) tissue; and humanized models, which incorporate orthotopic implantations or injections and use tissue engineering and/or regenerative medicine approaches [24]. Humanized mice have been used for decades to model human immunity [25–28]. A breakthrough occurred in the early 2000s with IL2Rγnull mice, which after engraftment are considered the most human-like model to date and encompass three main strains of mouse (detailed in [29]). There are a few approaches to engraft human immune cells into mice: using peripheral blood leukocytes (PBL); injection of severe combined immunodeficiency (SCID) reconstituting cells (SRC), also known as CD34+ hematopoietic stem cells (HSC); and the bone marrow/liver/thymus (BLT) model, established by transplantation of fetal liver and thymus and injection of autologous fetal liver HSCs [29]. The method of immune system engraftment [29] is important in relation to the research question being asked; for example, the BLT model would be most appropriate for human immunodeficiency virus (HIV)-related studies because it provides a higher level of engraftment of the human mucosal system [30]. The advantages and limitations of these models have been reviewed exhaustively by others [2, 29, 31–34]; therefore, in this review, we focus on a few recent advances.
Knock-in (KI) mice have emerged as a powerful tool to engraft whole parts of the human immune system, such as immunoglobulin (Ig) loci [35]. Transgenic human Ig loci were engrafted using bacterial artificial chromosome clones and sequential recombinase-mediated cassette exchange. This model has been utilized to study HIV humoral responses to novel interventions [36, 37] and is likely to be useful in any study in which humoral immune response is key (e.g., Zika or Dengue infection and disease). Advantages include being a controlled system and the maintenance of murine constant regions to avoid incompatibility effects, but this model does not reflect the other genetically diverse aspects of humans or their exposure history, nor does it reflect the immune system as a whole as other leukocyte populations remain murine. Another approach is to knock-in cytokines to enhance other immune responses, as has been achieved, for example, in IL-6 KI mice [5]. One new type of KI mouse (MISTRG) is developed using HSC engraftment on a background with multiple human cytokine knock-ins and demonstrates superior myeloid and natural killer (NK) cell development and hematopoiesis [3]. Human leukocyte antigen (HLA) transgenic mice have shown the ability to present human antigens in vivo in a model using human cytomegalovirus [38]. Engrafting humanized mice with umbilical cord blood is technically straightforward and provides T cells and autologous antigen-presenting cells (APCs) that can present cognate antigen [39]. The de novo transformation of B cells with Epstein-Barr virus (EBV) is observed in this model with tumor masses and tumor microenvironment similar to those observed in humans. Human bone marrow niche-forming cells can also be engrafted in the PDX model by either seeding the cells in vitro or by using a previously implanted scaffold. By using tissue engineering approaches to create a humanized microenvironment in addition to simply engrafting cells, one can study both hematopoiesis and malignancies in a more human-like system [40].
To further these ends, a framework has been proposed to generate a platform that would validate new humanized mice in a standardized manner; this approach merges tissue engineering and regenerative medicine techniques with benchmarks validated against human clinical data with known predictive power [24]. Others have proposed the co-engraftment of human tissues, for example, human HSC with human skin, liver, or lymph nodes to enhance effector and memory responses [41]. These murine models have translational potential for single-organ infections (e.g., hepatitis family viruses and human liver). One drawback to this system is that the model is not completely human, and the remaining murine cells and molecules may confound the interpretation. This could possibly be overcome by co-engraftment with multiple organs or humanization of multiple components, which then would increase the translational potential of this murine system.
NHP models
At first glance, NHP models have several disadvantages compared to mice: they are large, expensive, less tractable, and involve ethical considerations. However, the immune system of NHPs more closely mimics that of humans, thus making them the most translational model system outside of humans themselves. NHPs have other advantages over mice. Some diseases can only be properly modeled in NHPs: for example, human HIV can only be modeled through simian immunodeficiency virus (SIV) and simian/human immunodeficiency virus (SHIV) because HIV cannot infect mice; and infecting mice with the causative agent of human tuberculosis (TB) disease neither causes clinical TB nor recapitulates TB pathology seen in humans, whereas NHP models (particularly the cynomolgus macaque) fully reflect both clinical TB and the disease pathology seen in humans [42, 43]. Although some diseases can be modeled in mice, their immune response may be totally different to that of NHP or humans, and could use immune mediators that may not exist in NHP or humans. Therefore, NHP have great translational value in pre-clinical studies.
NHP as an essential model for HIV have been well characterized with a plethora of experimental manipulations, including consideration of natural or hybrid challenge viruses, choice of NHP species, virus dose, challenge route, and more, all of which should be carefully considered during experimental design [44]. A cynomolgus macaque model of TB has been developed that fully recapitulates human TB, exhibits the full spectrum of clinical disease from latent TB infections to fulminant or septic TB, and has the range and types of pathology seen in humans [42, 43]. Novel frontline Ebola virus vaccines have been developed using the NHP model, because mice develop neither Ebola infection nor disease upon challenge [45, 46]. NHP have also been utilized to model many zoonotic viruses (Flaviviridae, Togaviridae, and others) [22] as well as influenza, although clinical influenza disease in NHP is still slightly different from that in humans [47]. Transplantation tolerance can also be modeled in NHP: a pilot in NHP demonstrated similar tolerance mechanisms to humans [48]. Aging and neurodegenerative diseases have been successfully modeled in NHP, which is a new avenue of interest as these diseases have been shown recently to have immunologic components and potential causes [49]. NHP grow old like humans: aging NHP and human brain transcriptomes are similar; NHP naturally display Alzheimer’s lesions such as amyloid plaques and aggregated hyperphosphorylated tau protein; and they display similar pathology from prion diseases [50]. As most diseases have some genetic component, the need for genetic characterization of NHPs has become apparent [51]. NHP genetics will aid in comparisons between NHP and human genomes, and finding and breeding natural variants will lead to the generation of specific disease models. NHP are outbred, so the impact of genetic background on specific genes or pathways can be measured in this system. Further development of NHP models through genome editing has been pursued [52, 53] but raises serious ethical considerations.
Studying human immunity directly ex vivo and in vitro
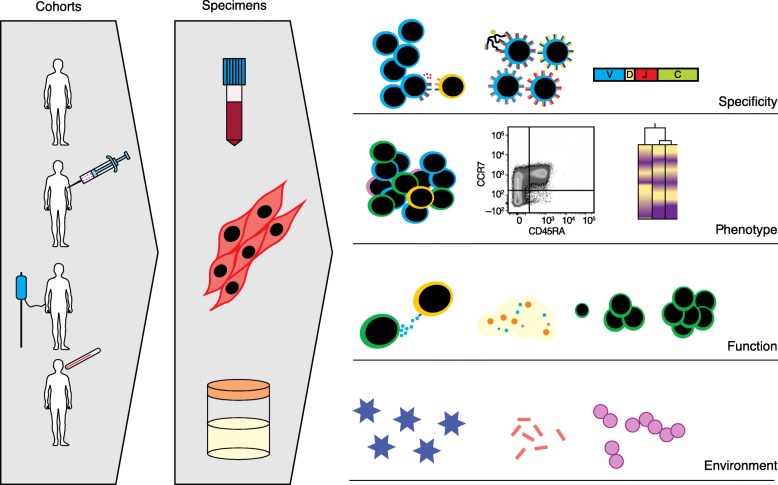
Given that there are many differences between the immune composition and function of humans and those of other animals, recognizing these disparities early on is crucial for translational purposes. One way to circumvent inter-species differences is to study human immune cells directly (Fig. 1). Most work has been (understandably) limited to blood, though discarded tissues and minimally invasive sampling have also been incredibly informative. Assessing tissues directly can be a resource for understanding cell types that do not circulate at high frequency (including resident memory, tissue-specific stroma, and germinal center populations) and in the study of immune infiltration in diseases with tissue- or organ-specific pathologies.
Fig. 1.
The wealth of human data for translational immunology. Consented cohorts of healthy donors and people in immune-perturbed conditions such as during illness, treatment, and immunization can provide insights into human immunity and disease-specific immune responses. Technologies now exist that allow us to study numerous sample types, including blood, tissue biopsies, saliva, urine, and feces, among others. Such samples are usually processed and banked, then run all together to limit batch variation. Depending on the questions to be answered, various assays can be run individually or in combination to gain insights into health or disease processes. These can include immune-cell-specificity assays (restimulation, tetramer staining, or repertoire analysis), broad phenotyping (flow and mass cytometry, RNAseq), functional readouts (cytotoxicity, metabolite detection, proliferation, or differentiation), or environmental contributions (microbiome or virome)
Blood-based immunoassays
Peripheral blood has been used as a surrogate for the human immune system to study pressing immunological systems ranging from cell signaling to clinical trial outcome prediction. Indeed, peripheral blood studies are valuable due to the relative ease of sample acquisition, the low risk to the participant, and the potential for future translational applications in diagnostics and immunotherapeutics. Given that blood is the most frequent sample type used for immunology applications, there are numerous optimized assays for high-throughput analysis (Fig. 1). Peripheral blood has been used to provide information about the fundamental functions of immune cell types in humans [54–58]. Flow and mass cytometry are the tools of choice for establishing immune cell phenotypes and functions directly from ex vivo samples [59–65]. Adaptive immune repertoire analysis [66, 67] has also become prevalent, as discussed in detail below. Transcriptional and epigenetic profiling has revealed fundamental biological information about the regulation of immune genes and their contributions to human variation [68–72]. For example, Qu et al. [69] showed that sex has a strong impact on the regulome of CD4 T cells in healthy adults, and suggest that these differences may play an important modulatory role in autoimmune disease susceptibility. Standardized immunoassays [73–75], as well as more recent higher throughput techniques that have the potential to become standard assays [76–78], are widely available to measure circulating cytokines and other immune markers and metabolites in whole blood, plasma, sera, and culture supernatants for immune monitoring. These technologies can also be combined to provide an in-depth analysis of immune health and even to predict clinical outcome. For example, Lakshmikanth and colleagues [79] recently showed in a combination serum protein and mass cytometry phenotyping study of leukemia patients receiving allogeneic stem cell transplants that they could identify early immune features associated with patient outcome.
Whole blood and peripheral blood mononuclear cells (PBMCs) can be manipulated in vitro to study responses to a staggering diversity of self and non-self antigens, innate stimuli, and other molecules in health and disease [80, 81]. After in vitro PBMC stimulation with antigens of interest, specific T cells can be identified on the basis of dilution of an intracellular dye, indicating proliferation; cytokine production and capture upon restimulation can be measured with relevant peptides; and target cell killing or antigen specificity can be assayed using peptide:major histocompatibility complex (MHC) tetramers [82, 83]. Similarly, rare B cells can be detected from blood by staining or capturing cells through their specific B cell receptor using labeled or plate-bound antigens, respectively, in flow cytometry and ELISPOT experiments.
In human challenge models (HCMs), healthy people are intentionally infected with a controlled dose of a virus, bacterium, or parasite and then monitored typically through blood sampling (and/or mucosal sampling), sometimes while quarantined, for evidence of immune response and infection progression. These studies are often combined with vaccine efficacy testing or other interventions and post-infection treatment where appropriate. HCMs continue to be used to study human responses to influenza [84, 85] and other infectious diseases, including malaria [86, 87], dengue [88], hookworm [88], and numerous enteric infections such as Salmonella typhi, Vibrio cholerae, and Escherichia coli [89–94]. In a high-dose typhoid challenge model, one group showed that a large pre-infection population of functional, Typhi-responsive CD8 T cells in the blood was a good predictor of progression to typhoid disease. The authors proposed that the additional inflammatory response from a pre-existing pool of Typhi-reactive T cells might be sufficient to promote typhoid fever [89]. Interestingly, only subjects with the highest frequency of reactive CD8 T cells showed delay in disease development, suggesting that CD8 T cells play both a pathogenic and protective role during challenge.
At the same time, these models can tell us much about the immune features that are associated with resistance or susceptibility to infection, as well as the effectiveness of vaccines and treatments. HCMs are valuable because they allow vast quantities of detailed data to be collected from a closely studied cohort in a relatively controlled environment. As the typical population of interest consists of healthy adults, HCMs account for many aspects of human immunity that are absent in animal models, such as genetic variation, pre-existing immunological memory, environmental exposure, and the normal aging of the human immune system.
Repertoire analysis
In recent years, substantial technological advancements and the reduced cost of high-throughput sequencing of T and B cell receptors have led to efforts to identify immune response signatures from sequence. Indeed, several groups have used T cell receptor (TCR) sequence analysis to study fundamental differences between T cell subsets (deeply from the repertoire of a single individual [95] and recently in combination with ATAC-seq (assay for transposase-accessible chromatin sequencing) [95], which allows both the TCR identity and DNA accessibility to be ascertained from individual cells) and the roles of T cells in the context of autoimmunity, cancer, and T cell pathologies [96–98] (Fig. 1). A recent study highlighted the value of TCR repertoire analysis in understanding the response to vaccination. Qi et al. [99] showed, in an elegant twin pair study of older individuals, that immunization with live attenuated varicella zoster virus (VZV) vaccine had numerous effects on the repertoire diversity of VZV-specific CD4 T cells. Overall, they found that diversity increased with immunization (with recruitment from the naïve T cell pool as well) and that although all VZV-specific clones expanded post-vaccination, they did not expand equally. On the basis of these findings, the authors proposed that although broadening the repertoire can have beneficial protective effects, the single immunization strategy employed here may not boost memory responses adequately.
Single cell sequencing [97] is becoming increasingly popular, as obtaining paired alpha and beta chain sequence data from TCRs of interest allows for recombinant expression and because yeast display libraries can be used to probe candidate ligands for TCRs of unknown specificity [100, 101]. Understanding an individual’s immune history and response to immune perturbation from TCR and B cell receptor (BCR) repertoire sequence alone would be transformative, but the incredible diversity of these receptors and the limited overlap between individuals even with the same HLAs and antigen exposure history creates a complicated analysis problem. However, recent advancements in TCR repertoire analysis tools that incorporate V gene usage and local motif searching techniques in the context of similar but non-identical (i.e., ‘convergent’) sequences suggest that, in the future, determining a TCR’s specificity from sequence alone could be possible [102, 103]. Similar strategies are being used for BCR repertoire analysis of similar, non-identical sequences to broaden our understanding of vaccine antigen targets for antibody responses [104, 105].
Modeling immune tissues
Assays that use human tissues as the starting material are more likely to capture the essence of the immune microenvironment. Immune cells can have a relatively low frequency in the overall cellular composition of a tissue, and so studying relevant non-immune cells in concert with immune cells, particularly with pertinent cellular organization, can provide useful insights. We have started to learn a great deal about the tissue-resident immune distribution in human organs from recent studies of organ donors’ tissues [106].
Human PBMCs have also been used to successfully reflect some aspects of tissue-resident and lymph node biology in response to vaccine antigens. Using a system called human modular immune in vitro construct (MIMIC™), purified human T and B cells are combined with in vitro differentiated and antigen-pulsed dendritic cells to elicit antibody responses against vaccine candidates [107–109]. When compared to studies of unmanipulated PBMC cultures, these kinds of model systems show promise for improving vaccine efficacy predictions and for adjusting vaccine candidates prior to clinical trials. But overall, identifying predictive cellular biomarkers in peripheral blood for human vaccine responses and cancer immunotherapies, among many other areas, has been largely unsuccessful. Here, where the microenvironments and spatial organizations are unique, we believe that studying the relevant tissues can provide a clear advantage.
Tissue-based immunity
For vaccine responses, the B cells responsible for forming a neutralizing antibody response are developed inside germinal centers (GCs) within lymphoid organs. Upon antigen arrival into a lymph node, T follicular helper cells (TFH) train GC B cells to form humoral responses. TFH and a variety of other cell types of hematopoietic and non-hematopoietic origin interact and deliver signals to GC B cells to promote survival, proliferation, affinity maturation, class switch recombination, and differentiation into memory B and plasma cells [110–114]. Most of these cellular processes are only briefly, or not at all, detectable in peripheral blood. Gathering information from human lymph nodes after antigen exposure can be problematic depending on node accessibility, size, and the extent of the response, although there have been some studies in which biopsies have been used to study lymph node-based responses [115, 116]. NHP studies have shown that analysis of lymph node fine needle aspirates can better predict neutralizing HIV env vaccine response [117, 118]. Two human studies, one in immunized healthy volunteers [119] and one in multiple sclerosis patients [120], have also shown that it is conceptually possible to study the accessible draining lymph nodes of immunized people. Given that fine needle aspiration is relatively non-invasive and considered a routine medical procedure for biopsy in cancer diagnoses [121], it seems plausible that future human immunization studies will incorporate this sampling strategy.
Similarly, peripheral blood studies have been largely unsuccessful in predicting therapeutic and prognostic indicators for cancer treatment, though this may be possible in some checkpoint blockade-treated cancers such as those treated with anti-PD-1 (anti-programmed death 1) [122]. Nevertheless, no currently approved tests use peripheral immune biomarkers to direct treatment [118, 123]. The tumor microenvironment and the associated immune infiltration has been much more informative in guiding treatment strategies [124–126]. In one study of metastatic melanoma patients treated with anti-CTLA-4 (cytotoxic T lymphocyte-associated protein 4; and later with anti-PD-1), early immune infiltration and activation at the tumor site were significantly correlated with treatment response [124]. The number and type of immune cells infiltrating the tumor site has been shown to have prognostic value [127, 128], warranting further investigation of immune recognition and function at tumor sites.
Organoid-like culture
Organoids are in vitro representations of an organ or tissue that recapitulate the functional and structural features of the originating organ [129, 130]. Organoid culture has been used to model complex human and murine tissues, including lung, intestine, and brain [130, 131]. The use of the term 'organoid' varies substantially by field; although in many instances they are derived from an originating stem cell population, the consistent features of different organoid systems are relevant tissue patterning and retention of in vivo function. The organoid field has made significant advancements in modeling non-immune organs from mice and humans. Several groups have expanded organoid culture into immune tissues from mice that successfully support humoral responses [132–138]. Ankur Singh and colleagues extended organoid systems to immune tissues in a fully animal-independent way [132, 133]. Using an elegant murine cell-based system, they captured the essence of an immune microenvironment in vitro that permits B cell differentiation, promotes germinal center development, and supports antibody production [132, 133]. Although some facets of organoid culture are currently impractical to translate to a fully human system (dependence on exogenous protein expression from cell lines, re-introduction into living hosts), such methods have great potential to model immune processes. Our group has recently created human immune organoids from primary tonsil tissues that permit in vitro analysis of antigen-specific T and B cell responses. The system we have developed seeks to translate the existing excellent murine organoid models to humans and to allow more mechanistic immune studies to be performed on human tissues.
The organoid field has made substantial progress in modeling the tumor microenvironment and the corresponding tumor-infiltrating lymphocytes. A recent study identified features of treatment success or failure in response to checkpoint blockade using T cell-containing tumor spheroids [139]. These models are promising for providing a better understanding and potentially predicting patient response to checkpoint blockade prior to initiation of treatment in vivo.
In vivo studies
The most physiologically relevant model of human immunity is the study of humans themselves in health and disease. Understanding immune variation among people can also tell us a great deal about how the immune system functions as a holistic unit during steady state and immune perturbations. Experiments dating back to just after the 1918 influenza pandemic indicate that people volunteered for infection challenge studies to improve understanding of disease transmission, immune memory, and the clinical course of infection [140–142]. Current human in vivo studies undergo rigorous ethics review and, for human challenge models in particular, health checkups prior to participation are part of the inclusion/exclusion assessment [143]. In vivo studies can tell us about the fundamental nature of immune cell functions, such as homeostatic proliferation and memory retention, that previously were almost exclusively studied in mice. For example, in a recent 10-year study of yellow fever vaccine recipients, Akondy et al. [144] determined that long-term persisting vaccine-specific CD8 T cells originate from early rapid dividers, subsequently divide less than once a year, and maintain a distinct transcriptional profile [144].
Natural immune variation
There are insights to be gained from understanding human immune variation and so-called ‘experiments of nature’. Large-scale efforts have been undertaken in recent years to quantify genetic and environmental (e.g., pathogen exposure, immunization, chronic infection, microbiome, or maternal health) factors that contribute to observed immune variation among healthy people. The relative contributions seem to vary by cell type and human populations studied, as innate immune responses have been identified as more genetically controlled compared to adaptive responses [145–147]. Understanding immune variation has been a particularly rich area for HIV research too, with progress made in understanding immunological features of resistance against infection despite repeated exposure to the virus, long-term viral control, and non-progression to AIDS even in the absence of anti-retroviral drugs [148, 149].
Primary immunodeficiency patients who present with a constellation of susceptibility to infectious diseases and/or autoimmunity are also a window into the more mechanistic aspects of human immunity. In one recent clinical case, CD70 deficiency was shown to have a detrimental effect on T cell responses to EBV-infected B cells [150]. Izawa et al. [150] showed that disruption of the CD27/CD70 costimulation pathway resulted in defective T cell cytolytic function and proliferation against EBV-infected B cells through a TCR-mediated process. Reconstitution of CD70 expression restored normal functional activity. Individuals with these rare inborn mutations and their subsequent treatment have revealed a great deal about cell signaling in human immune cells and host–pathogen interactions in exquisite detail.
In silico models and bioinformatics
Computational models for translational human immunology are often overlooked but useful tools. Computational power is now robust and sophisticated enough to model the complex processes of human immunity. This power is relatively cheap, easily reproducible, transparent, and high throughput, being able to perform hundreds or even thousands of ‘experiments’ in a single run. There are two main flavors of these tools: in silico models (or mechanistic models of immune processes); and bioinformatics (or data-driven models that link immune processes to clinical outcomes or other endpoints, such as biomarkers and predictive markers) [151].
In silico models incorporate available biological data into systems that can be tractable and high throughput. These biological data can come from small-scale individual studies or large-scale systems immunology projects. In silico models can integrate various types of data, including DNA, RNA, protein, metabolic products, and more. Common approaches to this type of mechanistic modeling include ordinary differential equations (ODE), agent-based models (ABM), and logical models [151]. These models can be deterministic (multiple runs will result in the same conclusion) or stochastic (probabilistic outcomes that may change after repeated runs) [151]. A recent example of the use of these models for translational human immunology is the utilization of molecular dynamics simulations in conjunction with models of affinity maturation to study the development of broadly neutralizing antibody production against HIV [152]. This study and many others from the same laboratory illustrate the power of integrating laboratory experiments, clinical data, computational inference, and mechanistic immune models [153]. Stochastic models have been developed that seek to mimic entire components of the immune system; these models are tunable and include both multi-scale (molecules, up to cells, up to tissues) and multi-compartment (in this case lung, blood, and lymph node) features [154]. These multi-scale, multi-component models have been used successfully to model TB granulomas, feature emergent behavior (i.e., placement of the TB causative agent into the lung model leads to granuloma formation), and are amenable to perturbations that would be impossible biologically (e.g., changes in local drug concentration, or finely tuning cytokine output from particular cells) [155]. In silico models have also been used successfully with cancer applications to model the tumor microenvironment in order to predict optimal anti-angiogenic therapies [156, 157] and to model tumors such as triple-negative breast cancer as a whole [158]. Models that simulate individual cells are currently in development [159]. These can then generate hypotheses that can be tested downstream through mechanistic studies in animal models (although the goal is to test them in human systems).
Bioinformatics relies on a bounty of available public data from a wide variety of sources. This data-driven approach is leveraged to identify biomarkers that can be validated biologically and to generate hypotheses about underlying immune mechanisms; it also serves as a source of carefully curated, publicly available data for comparison purposes [160, 161]. Genomics data, transcriptomics data, and many other -omics data that have been leveraged recently using bioinformatics approaches have been heavily reviewed and only brief examples will be given here. Using genomic data, genome-wide association studies (GWAS) can be performed that link features in DNA to disease traits. For example, a GWAS in patients with multiple sclerosis elucidated over 60% of genetic variation predicting multiple sclerotic onset, and this pattern largely overlapped with other autoimmune disease signatures [162]. Publicly available transcription data have likewise been used to generate predictive signatures for TB [163], systemic sclerosis [164], transplantation allograft survival [165], sepsis [166], anti-TNF (anti-tumor necrosis factor) treatment response in inflammatory bowel disease (IBD) patients [167], and many other conditions. Attention is increasingly turning to the epigenome, due to its role as a bridge between genetic regulation and the environment, and new technologies have recently emerged to probe this system, including ATAC-seq [69] and epigenetic CyTOF (EpiTOF) [168]. Many individual studies that produce these publicly available data are designed to eliminate heterogeneity, but these efforts can fail to exert predictive power and ignore the biological necessity (and relevance) of heterogeneity. One powerful technique that not only deals with but embraces heterogeneity is meta-analysis, which improves the reproducibility of bioinformatic models by integrating data from multiple studies [169]. Recent studies have also identified a bias toward richly annotated genes that should be avoided so that understudied genes, which may play key roles in disease, are not ignored [170]. The continued application and success of these -omics methods will generate more data that can be utilized in these types of models. Predictive markers can be tested in silico to reveal potential biological mechanisms, and in silico models can be used to identify putative predictive markers that can be validated with other approaches. Further development of these models is key to both expand the use of this technique and to even potentially disintermediate animal models.
Conclusions
The future of translational human immunology is bright. Continuing to develop more sophisticated models of human immunity will improve our chances of successfully translating findings from fundamental biological studies to the clinic. Humanized mice can be a valuable and potentially translational model, but require further development for reliable and translatable data generation. The publication of negative (and positive) results for benchmarking will aid the development of this model. Developing mice with combined robust human memory B and T cell responses, neutralizing titers, and other immune features remains a major challenge [41]. Pet shop mice that are co-housed with inbred mice represent a novel solution to understanding some of the effects of diverse environmental exposure on immune cell functions [6, 171, 172]. These studies point to the fact that the immune system is finely tuned on the basis of microbial burden and that environment should be considered when attempting to model human immunity in the future. To be broadly useful, these environmentally exposed mice would need to be distributed by a central source. The judicious use of NHP to confirm the translational capabilities of studies before moving to people has clear benefits. That being said, some differences between NHP and humans could hamper translation; for example, MHC-I in NHP, which have multiple A loci and more than ten B loci, is much more complex than in humans [173].
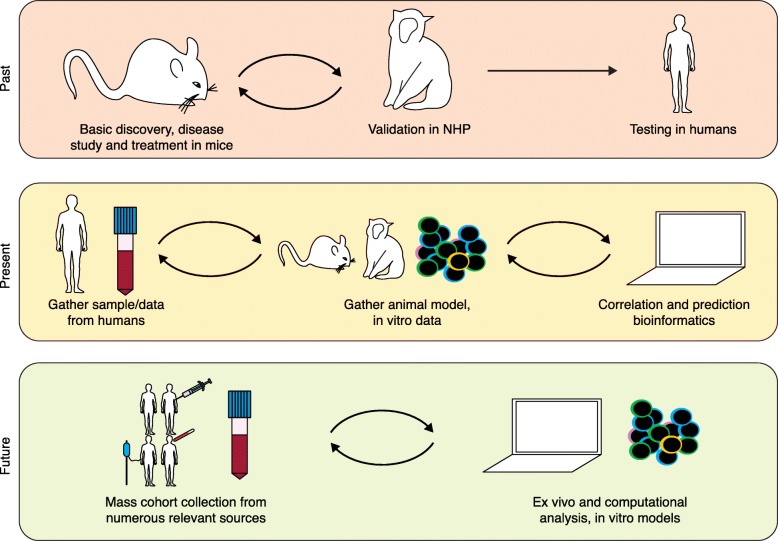
A main goal should be to continue to develop tools to close the animal gap, that is, to develop human-based systems that have the advantages of animal models, including high throughput and the potential for mechanistic, well-controlled studies (Fig. 2). Strong collaborations between scientists, engineers, and clinicians will help to accelerate this process. Along these lines, new approaches such as 'on-a-chip' assays continue to emerge. Microfluidics has been used to both capture immune cells for molecular analysis [174] and to model tissue organization and functions in which immune cells play an important role, including those in the gut, lung, blood vessels, and lymph nodes [175–177]. On-chip-based technologies have been a rich area of study for inflammation research, tumor–immune interactions, and early events of metastasis [178–180]. Nevertheless, there continue to be challenges for studying immune interactions with on-chip methods. There is a delicate balance in creating a model that captures the essence of the immune features to be studied that is, at the same time, not too reductive.
Fig. 2.
The shifting paradigm of translational human models. In the past, animal models were almost exclusively used for pre-clinical analyses, with limited success in translation to humans. NHP often served as a more relevant model for safety testing prior to attempts to test in humans, although on rare occasions this led to unanticipated and devastating effects in human trials. Currently, more strategies are incorporated into translational models, including sampling from people for in vitro assays. The data derived from human ex vivo and in vitro testing is often used to inform animal models and vice versa. As more high throughput data are made publicly available, computational models can contribute to the translational effort as well. In the future, it may be possible to bypass animal models entirely as more information is gathered from a variety of people of diverse health, genetic, and environmental backgrounds. As we gather broad data from human cohorts, our hope is that our predictive abilities and computational models will improve such that we no longer rely on animal models, although they will undoubtedly continue to play at least a supplemental role in translation
We are in an exciting time of human immunology during which high-throughput tools are increasingly accessible to study a wide array of immunological processes in humans. The growing availability of public data sets means that we should be using them more frequently in the hypothesis-generating process when embarking on new studies. At the same time, as a community we should strive to gather data from as diverse a population as possible so as to avoid over-extension from a single or small cohort.
Acknowledgements
We would like to thank Hayley C. Warsinske for helpful discussion.
Funding
The authors are supported by the National Institutes of Health (U19AI057229 to MMD) and the Bill and Melinda Gates Foundation (MMD).
Abbreviations
- ATAC-seq
Assay for transposase-accessible chromatin sequencing
- BCR
B cell receptor
- BLT
Bone marrow/liver/thymus
- EBV
Epstein-Barr virus
- GC
Germinal center
- GWAS
Genome-wide association study
- HCM
Human challenge model
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- HSC
Hematopoietic stem cell
- Ig
Immunoglobulin
- KI
Knock-in
- MHC
Major histocompatibility complex
- NHP
Nonhuman primate
- PBMC
Peripheral blood mononuclear cell
- PD-1
programmed death 1
- PDX
Patient-derived tissue
- TB
Tuberculosis
- TCR
T cell receptor
- TFH
T follicular helper cell
- VZV
Varicella zoster virus
Authors’ contributions
LEW and RMD were major contributors in writing the manuscript. All authors read, edited, and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.2971269. [DOI] [PubMed] [Google Scholar]
- 2.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 3.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herndler-Brandstetter D, Shan L, Yao Y, Stecher C, Plajer V, Lietzenmayer M, et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc Natl Acad Sci U S A. 2017;114:E9626–E9634. doi: 10.1073/pnas.1705301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Borsotti C, Schickel JN, Zhu S, Strowig T, Eynon EE, et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood. 2017;129:959–969. doi: 10.1182/blood-2016-04-709584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attarwala H. TGN1412: from discovery to disaster. J Young Pharm. 2010;2:332–336. doi: 10.4103/0975-1483.66810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 11.von Herrath MG, Nepom GT. Lost in translation: barriers to implementing clinical immunotherapeutics for autoimmunity. J Exp Med. 2005;202:1159–1162. doi: 10.1084/jem.20051224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrand-Rosenberg S. Animal models of tumor immunity, immunotherapy and cancer vaccines. Curr Opin Immunol. 2004;16:143–150. doi: 10.1016/j.coi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010;161:512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallardy M, Hunig T. Primate testing of TGN1412: right target, wrong cell. Br J Pharmacol. 2010;161:509–511. doi: 10.1111/j.1476-5381.2010.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/WNL.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 17.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/S0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 18.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren HS, Tompkins RG, Moldawer LL, Seok J, Xu W, Mindrinos MN, et al. Mice are not men. Proc Natl Acad Sci U S A. 2015;112:E345. doi: 10.1073/pnas.1414857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mak I, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6:114–118. [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin S. Pre-clinical research: make mouse studies work. Nature. 2014;507:423–425. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- 22.Estes JD, Wong SW, Brenchley JM. Nonhuman primate models of human viral infections. Nat Rev Immunol. 2018;18:390–404. doi: 10.1038/s41577-018-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm D, Staeheli P, Hufbauer M, Koerner I, Martinez-Sobrido L, Solorzano A, et al. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci U S A. 2007;104:6806–6811. doi: 10.1073/pnas.0701849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landgraf M, McGovern JA, Friedl P, Hutmacher DW. Rational design of mouse models for cancer research. Trends Biotechnol. 2018;36:242–251. doi: 10.1016/j.tibtech.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 27.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 28.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, et al. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denton PW, Sogaard OS, Tolstrup M. Using animal models to overcome temporal, spatial and combinatorial challenges in HIV persistence research. J Transl Med. 2016;14:44. doi: 10.1186/s12967-016-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramer PC, Chijioke O, Meixlsperger S, Leung CS, Munz C. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol. 2011;89:408–416. doi: 10.1038/icb.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z, Yang YG. Human lymphohematopoietic reconstitution and immune function in immunodeficient mice receiving cotransplantation of human thymic tissue and CD34(+) cells. Cell Mol Immunol. 2012;9:232–236. doi: 10.1038/cmi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo GC, Hasan A, O'Reilly RJ. Use of humanized severe combined immunodeficient mice for human vaccine development. Expert Rev Vaccines. 2009;8:113–120. doi: 10.1586/14760584.8.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee EC, Liang Q, Ali H, Bayliss L, Beasley A, Bloomfield-Gerdes T, et al. Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery. Nat Biotechnol. 2014;32:356–363. doi: 10.1038/nbt.2825. [DOI] [PubMed] [Google Scholar]
- 36.Sok D, Briney B, Jardine JG, Kulp DW, Menis S, Pauthner M, et al. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science. 2016;353:1557–1560. doi: 10.1126/science.aah3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verkoczy L, Alt FW, Tian M. Human Ig knockin mice to study the development and regulation of HIV-1 broadly neutralizing antibodies. Immunol Rev. 2017;275:89–107. doi: 10.1111/imr.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas S, Klobuch S, Podlech J, Plachter B, Hoffmann P, Renzaho A, et al. Evaluating human T-cell therapy of cytomegalovirus organ disease in HLA-transgenic mice. PLoS Pathog. 2015;11:e1005049. doi: 10.1371/journal.ppat.1005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zumwalde NA, Gumperz JE. Modeling human antitumor responses in vivo using umbilical cord blood-engrafted mice. Front Immunol. 2018;9:54. doi: 10.3389/fimmu.2018.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abarrategi A, Mian SA, Passaro D, Rouault-Pierre K, Grey W, Bonnet D. Modeling the human bone marrow niche in mice: from host bone marrow engraftment to bioengineering approaches. J Exp Med. 2018;215:729–743. doi: 10.1084/jem.20172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douam F, Ploss A. The use of humanized mice for studies of viral pathogenesis and immunity. Curr Opin Virol. 2018;29:62–71. doi: 10.1016/j.coviro.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scanga C, Flynn JL. Modeling tuberculosis in nonhuman primates. Cold Spring Harb Perspect Med. 2014;4:a018564. doi: 10.1101/cshperspect.a018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunol Rev. 2015;264:60–73. doi: 10.1111/imr.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Prete Gregory Q., Lifson Jeffrey D. Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2017. Nonhuman Primate Models for Studies of AIDS Virus Persistence During Suppressive Combination Antiretroviral Therapy. [DOI] [PubMed] [Google Scholar]
- 45.Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R, et al. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature. 2015;520:688–691. doi: 10.1038/nature14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, et al. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science. 2015;348:439–442. doi: 10.1126/science.aaa4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margine I, Krammer F. Animal models for influenza viruses: implications for universal vaccine development. Pathogens. 2014;3:845–874. doi: 10.3390/pathogens3040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitchens W, Adams A. Nonhuman primate models of transplant tolerance: closer to the holy grail. Curr Opin Organ Transplant. 2016;21:59–65. doi: 10.1097/MOT.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 49.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdier JM, Acquatella I, Lautier C, Devau G, Trouche S, Lasbleiz C, et al. Lessons from the analysis of nonhuman primates for understanding human aging and neurodegenerative diseases. Front Neurosci. 2015;9:64. doi: 10.3389/fnins.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harding JD. Genomic tools for the use of nonhuman primates in translational research. ILAR J. 2017;58:59–68. doi: 10.1093/ilar/ilw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Niu Y, Ji W. Genome editing in nonhuman primates: approach to generating human disease models. J Intern Med. 2016;280:246–251. doi: 10.1111/joim.12469. [DOI] [PubMed] [Google Scholar]
- 53.Sato K, Sasaki E. Genetic engineering in nonhuman primates for human disease modeling. J Hum Genet. 2018;63:125–131. doi: 10.1038/s10038-017-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160:1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alcantara-Hernandez M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, et al. High-dimensional phenotypic mapping of human dendritic cells reveals interindividual variation and tissue specialization. Immunity. 2017;47:1037–1050. doi: 10.1016/j.immuni.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 59.Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y, et al. Standardizing flow cytometry immunophenotyping analysis from the human ImmunoPhenotyping Consortium. Sci Rep. 2016;6:20686. doi: 10.1038/srep20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler's guide to cytometry. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maecker Holden T, McCoy J Philip. A model for harmonizing flow cytometry in clinical trials. Nature Immunology. 2010;11(11):975–978. doi: 10.1038/ni1110-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kleinsteuber K, Corleis B, Rashidi N, Nchinda N, Lisanti A, Cho JL, et al. Standardization and quality control for high-dimensional mass cytometry studies of human samples. Cytometry A. 2016;89:903–913. doi: 10.1002/cyto.a.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicholas KJ, Greenplate AR, Flaherty DK, Matlock BK, Juan JS, Smith RM, et al. Multiparameter analysis of stimulated human peripheral blood mononuclear cells: a comparison of mass and fluorescence cytometry. Cytometry A. 2016;89:271–280. doi: 10.1002/cyto.a.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanner SD, Baranov VI, Ornatsky OI, Bandura DR, George TC. An introduction to mass cytometry: fundamentals and applications. Cancer Immunol Immunother. 2013;62:955–965. doi: 10.1007/s00262-013-1416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaudhary N, Wesemann DR. Analyzing immunoglobulin repertoires. Front Immunol. 2018;9:462. doi: 10.3389/fimmu.2018.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heather JM, Ismail M, Oakes T, Chain B. High-throughput sequencing of the T-cell receptor repertoire: pitfalls and opportunities. Brief Bioinform. 2018;19:554–565. doi: 10.1093/bib/bbw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu K, Zaba LC, Giresi PG, Li R, Longmire M, Kim YH, et al. Individuality and variation of personal regulomes in primary human T cells. Cell Syst. 2015;1:51–61. doi: 10.1016/j.cels.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods. 2009;48:240–248. doi: 10.1016/j.ymeth.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaussabel D, Pascual V, Banchereau J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010;8:84. doi: 10.1186/1741-7007-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendoza LG, McQuary P, Mongan A, Gangadharan R, Brignac S, Eggers M. High-throughput microarray-based enzyme-linked immunosorbent assay (ELISA) BioTechniques. 1999;27:778–780. doi: 10.2144/99274rr01. [DOI] [PubMed] [Google Scholar]
- 75.Chowdhury F, Williams A, Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Porichis F, Hart MG, Griesbeck M, Everett HL, Hassan M, Baxter AE, et al. High-throughput detection of miRNAs and gene-specific mRNA at the single-cell level by flow cytometry. Nat Commun. 2014;5:5641. doi: 10.1038/ncomms6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frei AP, Bava FA, Zunder ER, Hsieh EW, Chen SY, Nolan GP, et al. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat Methods. 2016;13:269–275. doi: 10.1038/nmeth.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen P, Chung MT, McHugh W, Nidetz R, Li Y, Fu J, et al. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano. 2015;9:4173–4181. doi: 10.1021/acsnano.5b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lakshmikanth T, Olin A, Chen Y, Mikes J, Fredlund E, Remberger M, et al. Mass cytometry and topological data analysis reveal immune parameters associated with complications after allogeneic stem cell transplantation. Cell Rep. 2017;20:2238–2250. doi: 10.1016/j.celrep.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Thurm CW, Halsey JF. Measurement of cytokine production using whole blood. Curr Protoc Immunol. 2005;Chapter 7(Unit 7):18B. doi: 10.1002/0471142735.im0718bs66. [DOI] [PubMed] [Google Scholar]
- 81.Rivoltini L, Kawakami Y, Sakaguchi K, Southwood S, Sette A, Robbins PF, et al. Induction of tumor-reactive CTL from peripheral blood and tumor-infiltrating lymphocytes of melanoma patients by in vitro stimulation with an immunodominant peptide of the human melanoma antigen MART-1. J Immunol. 1995;154:2257–2265. [PubMed] [Google Scholar]
- 82.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 83.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 84.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167:775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 85.Memoli MJ, Czajkowski L, Reed S, Athota R, Bristol T, Proudfoot K, et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis. 2015;60:693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis. 2014;209(Suppl 2):S40–S45. doi: 10.1093/infdis/jiu063. [DOI] [PubMed] [Google Scholar]
- 87.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 88.Diemert D, Campbell D, Brelsford J, Leasure C, Li G, Peng J, et al. Controlled human hookworm infection: accelerating humanhookworm vaccine development. Open Forum Infect Dis. 2018;5:ofy083. doi: 10.1093/ofid/ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fresnay S, McArthur MA, Magder LS, Darton TC, Jones C, Waddington CS, et al. Importance of Salmonella Typhi-responsive CD8+ T cell immunity in a human typhoid fever challenge model. Front Immunol. 2017;8:208. doi: 10.3389/fimmu.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McArthur MA, Chen WH, Magder L, Levine MM, Sztein MB. Impact of CD4+ T cell responses on clinical outcome following oral administration of wild-type enterotoxigenic Escherichia coli in humans. PLoS Neglect Trop D. 2017;11:e0005291. doi: 10.1371/journal.pntd.0005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Darton TC, Jones C, Blohmke CJ, Waddington CS, Zhou L, Peters A, et al. Using a human challenge model of infection to measure vaccine efficacy: a randomised, controlled trial comparing the typhoid vaccines M01ZH09 with placebo and Ty21a. PLoS Neglect Trop D. 2016;10:e0004926. doi: 10.1371/journal.pntd.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis. 2016;62:1329–1335. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, et al. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med. 2016;14:62. doi: 10.1186/s12967-016-0819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waddington CS, Darton TC, Jones C, Haworth K, Peters A, John T, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis. 2014;58:1230–1240. doi: 10.1093/cid/ciu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satpathy AT, Saligrama N, Buenrostro JD, Wei Y, Wu B, Rubin AJ, et al. Transcript-indexed ATAC-seq for precision immune profiling. Nat Med. 2018;24:580–590. doi: 10.1038/s41591-018-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han A, Newell EW, Glanville J, Fernandez-Becker N, Khosla C, Chien YH, et al. Dietary gluten triggers concomitant activation of CD4+ and CD8+ alphabeta T cells and gammadelta T cells in celiac disease. Proc Natl Acad Sci U S A. 2013;110:13073–13078. doi: 10.1073/pnas.1311861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dziubianau M, Hecht J, Kuchenbecker L, Sattler A, Stervbo U, Rodelsperger C, et al. TCR repertoire analysis by next generation sequencing allows complex differential diagnosis of T cell-related pathology. Am J Transplant. 2013;13:2842–2854. doi: 10.1111/ajt.12431. [DOI] [PubMed] [Google Scholar]
- 99.Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Sci Transl Med. 2016;8:332ra46. doi: 10.1126/scitranslmed.aaf1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gee MH, Han A, Lofgren SM, Beausang JF, Mendoza JL, Birnbaum ME, et al. Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. Cell. 2018;172:549–563. doi: 10.1016/j.cell.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell. 2014;157:1073–1087. doi: 10.1016/j.cell.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Truck J, Ramasamy MN, Galson JD, Rance R, Parkhill J, Lunter G, et al. Identification of antigen-specific B cell receptor sequences using public repertoire analysis. J Immunol. 2015;194:252–261. doi: 10.4049/jimmunol.1401405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galson JD, Truck J, Fowler A, Clutterbuck EA, Munz M, Cerundolo V, et al. Analysis of B cell repertoire dynamics following hepatitis B vaccination in humans, and enrichment of vaccine-specific antibody sequences. EBioMedicine. 2015;2:2070–2079. doi: 10.1016/j.ebiom.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byers AM, Tapia TM, Sassano ER, Wittman V. In vitro antibody response to tetanus in the MIMIC system is a representative measure of vaccine immunogenicity. Biologicals. 2009;37:148–151. doi: 10.1016/j.biologicals.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Dauner A, Agrawal P, Salvatico J, Tapia T, Dhir V, Shaik SF, et al. The in vitro MIMIC(R) platform reflects age-associated changes in immunological responses after influenza vaccination. Vaccine. 2017;35:5487–5494. doi: 10.1016/j.vaccine.2017.03.099. [DOI] [PubMed] [Google Scholar]
- 109.Drake DR, III, Singh I, Nguyen MN, Kachurin A, Wittman V, Parkhill R, et al. In vitro biomimetic model of the human immune system for predictive vaccine assessments. Disruptive Sci Technol. 2012;1:28–40. doi: 10.1089/dst.2012.0006. [DOI] [Google Scholar]
- 110.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 111.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- 113.Qi H, Kastenmuller W, Germain RN. Spatiotemporal basis of innate and adaptive immunity in secondary lymphoid tissue. Annu Rev Cell Dev Biol. 2014;30:141–167. doi: 10.1146/annurev-cellbio-100913-013254. [DOI] [PubMed] [Google Scholar]
- 114.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 115.Shukla GS, Olson WC, Pero SC, Sun YJ, Carman CL, Slingluff CL, Jr, et al. Vaccine-draining lymph nodes of cancer patients for generating anti-cancer antibodies. J Transl Med. 2017;15:180. doi: 10.1186/s12967-017-1283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wendel Ben S., del Alcazar Daniel, He Chenfeng, Del Río-Estrada Perla M., Aiamkitsumrit Benjamas, Ablanedo-Terrazas Yuria, Hernandez Stefany M., Ma Ke-Yue, Betts Michael R., Pulido Laura, Huang Jun, Gimotty Phyllis A., Reyes-Terán Gustavo, Jiang Ning, Su Laura F. The receptor repertoire and functional profile of follicular T cells in HIV-infected lymph nodes. Science Immunology. 2018;3(22):eaan8884. doi: 10.1126/sciimmunol.aan8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Havenar-Daughton C, Carnathan DG. Torrents de la Pena A, Pauthner M, Briney B, Reiss SM, et al. Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing antibody responses to HIV Env Trimer. Cell Rep. 2016;17:2195–2209. doi: 10.1016/j.celrep.2016.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, et al. Elicitation of Robust Tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity. 2017;46:1073–1088. doi: 10.1016/j.immuni.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tatovic D, Young P, Kochba E, Levin Y, Wong FS, Dayan CM. Fine-needle aspiration biopsy of the lymph node: a novel tool for the monitoring of immune responses after skin antigen delivery. J Immunol. 2015;195:386–392. doi: 10.4049/jimmunol.1500364. [DOI] [PubMed] [Google Scholar]
- 120.Jurynczyk M, Walczak A, Jurewicz A, Jesionek-Kupnicka D, Szczepanik M, Selmaj K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol. 2010;68:593–601. doi: 10.1002/ana.22219. [DOI] [PubMed] [Google Scholar]
- 121.Roskell DE, Buley ID. Fine needle aspiration cytology in cancer diagnosis. BMJ. 2004;329:244–245. doi: 10.1136/bmj.329.7460.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, Galon J, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5:44. doi: 10.1186/s40425-017-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 126.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 127.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 128.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 129.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 130.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 131.Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Purwada A, Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat Protoc. 2017;12:168–182. doi: 10.1038/nprot.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. doi: 10.1016/j.biomaterials.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nosenko MA, Drutskaya MS, Moisenovich MM, Nedospasov SA. Bioengineering of artificial lymphoid organs. Acta Nat. 2016;8:10–23. [PMC free article] [PubMed] [Google Scholar]
- 135.Tan JK, Watanabe T. Artificial engineering of secondary lymphoid organs. Adv Immunol. 2010;105:131–157. doi: 10.1016/S0065-2776(10)05005-4. [DOI] [PubMed] [Google Scholar]
- 136.Grikscheit TC, Sala FG, Ogilvie J, Bower KA, Ochoa ER, Alsberg E, et al. Tissue-engineered spleen protects against overwhelming pneumococcal sepsis in a rodent model. J Surg Res. 2008;149:214–218. doi: 10.1016/j.jss.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 137.Okamoto N, Chihara R, Shimizu C, Nishimoto S, Watanabe T. Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. J Clin Invest. 2007;117:997–1007. doi: 10.1172/JCI30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nojima T, Haniuda K, Moutai T, Matsudaira M, Mizokawa S, Shiratori I, et al. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 139.Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018;8:196–215. doi: 10.1158/2159-8290.CD-17-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wahl H, White G, Lyall H. Some experiments on the transmission of influenza. J Infect Dis. 1919;25:419–426. doi: 10.1093/infdis/25.5.419. [DOI] [Google Scholar]
- 141.Yamanouchi T, Sakakami K, Iwashima S. The infecting agent in influenza: an experimental research. Lancet. 1919;193:971. doi: 10.1016/S0140-6736(01)30275-1. [DOI] [Google Scholar]
- 142.Oxford JS, Oxford JR. Clinical, scientific and ethnographic studies of influenza in quarantine. Expert Rev Vaccines. 2012;11:929–937. doi: 10.1586/erv.12.77. [DOI] [PubMed] [Google Scholar]
- 143.Bambery B, Selgelid M, Weijer C, Savulescu J, Pollard AJ. Ethical criteria for human challenge studies in infectious diseases. Public Health Ethics. 2016;9:92–103. doi: 10.1093/phe/phv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552:362–367. doi: 10.1038/nature24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19:302–314. doi: 10.1038/s41590-018-0049-7. [DOI] [PubMed] [Google Scholar]
- 146.Piasecka B, Duffy D, Urrutia A, Quach H, Patin E, Posseme C, et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc Natl Acad Sci U S A. 2018;115:E488–E497. doi: 10.1073/pnas.1714765115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis. 2009;200:1714–1723. doi: 10.1086/646609. [DOI] [PubMed] [Google Scholar]
- 149.Stranford SA, Skurnick J, Louria D, Osmond D, Chang SY, Sninsky J, et al. Lack of infection in HIV-exposed individuals is associated with a strong CD8(+) cell noncytotoxic anti-HIV response. Proc Natl Acad Sci U S A. 1999;96:1030–1035. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Izawa K, Martin E, Soudais C, Bruneau J, Boutboul D, Rodriguez R, et al. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med. 2017;214:73–89. doi: 10.1084/jem.20160784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vodovotz Y, Xia A, Read EL, Bassaganya-Riera J, Hafler DA, Sontag E, et al. Solving immunology? Trends Immunol. 2017;38:116–127. doi: 10.1016/j.it.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ovchinnikov V, Louveau JE, Barton JP, Karplus M, Chakraborty AK. Role of framework mutations and antibody flexibility in the evolution of broadly neutralizing antibodies. elife. 2018;7. 10.7554/eLife.33038. [DOI] [PMC free article] [PubMed]
- 153.Chakraborty AK. A perspective on the role of computational models in immunology. Annu Rev Immunol. 2017;35:403–439. doi: 10.1146/annurev-immunol-041015-055325. [DOI] [PubMed] [Google Scholar]
- 154.Kirschner DE, Hunt CA, Marino S, Fallahi-Sichani M, Linderman JJ. Tuneable resolution as a systems biology approach for multi-scale, multi-compartment computational models. Wiley Interdiscip Rev Syst Biol Med. 2014;6:289–309. doi: 10.1002/wsbm.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Linderman JJ, Kirschner DE. In silico models of M. tuberculosis infection provide a route to new therapies. Drug Discov Today Dis Model. 2015;15:37–41. doi: 10.1016/j.ddmod.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Norton KA, Popel AS. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci Rep. 2016;6:36992. doi: 10.1038/srep36992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chu LH, Ganta VC, Choi MH, Chen G, Finley SD, Annex BH, et al. A multiscale computational model predicts distribution of anti-angiogenic isoform VEGF165b in peripheral arterial disease in human and mouse. Sci Rep. 2016;6:37030. doi: 10.1038/srep37030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Norton KA, Wallace T, Pandey NB, Popel AS. An agent-based model of triple-negative breast cancer: the interplay between chemokine receptor CCR5 expression, cancer stem cells, and hypoxia. BMC Syst Biol. 2017;11:68. doi: 10.1186/s12918-017-0445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carrera J, Covert MW. Why build whole-cell models? Trends Cell Biol. 2015;25:719–722. doi: 10.1016/j.tcb.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, et al. The Human Cell Atlas. elife. 2017;6. 10.7554/eLife.27041. [DOI] [PMC free article] [PubMed]
- 161.Shugay M, Bagaev DV, Zvyagin IV, Vroomans RM, Crawford JC, Dolton G, et al. VDJdb: a curated database of T-cell receptor sequences with known antigen specificity. Nucleic Acids Res. 2018;46:D419–D427. doi: 10.1093/nar/gkx760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med. 2016;4:213–224. doi: 10.1016/S2213-2600(16)00048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lofgren S, Hinchcliff M, Carns M, Wood T, Aren K, Arroyo E, et al. Integrated, multicohort analysis of systemic sclerosis identifies robust transcriptional signature of disease severity. JCI Insight. 2016;1:e89073. doi: 10.1172/jci.insight.89073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Azad TD, Donato M, Heylen L, Liu AB, Shen-Orr SS, Sweeney TE, et al. Inflammatory macrophage-associated 3-gene signature predicts subclinical allograft injury and graft survival. JCI Insight. 2018;3. 10.1172/jci.insight.95659. [DOI] [PMC free article] [PubMed]
- 166.Sweeney TE, Perumal TM, Henao R, Nichols M, Howrylak JA, Choi AM, et al. A community approach to mortality prediction in sepsis via gene expression analysis. Nat Commun. 2018;9:694. doi: 10.1038/s41467-018-03078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gaujoux R, Starosvetsky E, Maimon N, Vallania F, Bar-Yoseph H, Pressman S, et al. Cell-centred meta-analysis reveals baseline predictors of anti-TNFalpha non-response in biopsy and blood of patients with IBD. Gut. 2018. 10.1136/gutjnl-2017-315494. [DOI] [PMC free article] [PubMed]
- 168.Cheung P, Vallania F, Warsinske HC, Donato M, Schaffert S, Chang SE, et al. Single-cell chromatin modification profiling reveals increased epigenetic variations with aging. Cell. 2018;173:1385–1397. doi: 10.1016/j.cell.2018.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sweeney TE, Haynes WA, Vallania F, Ioannidis JP, Khatri P. Methods to increase reproducibility in differential gene expression via meta-analysis. Nucleic Acids Res. 2017;45:e1. doi: 10.1093/nar/gkw797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haynes WA, Tomczak A, Khatri P. Gene annotation bias impedes biomedical research. Sci Rep. 2018;8:1362. doi: 10.1038/s41598-018-19333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abolins S, King EC, Lazarou L, Weldon L, Hughes L, Drescher P, et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat Commun. 2017;8:14811. doi: 10.1038/ncomms14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Viney M, Lazarou L, Abolins S. The laboratory mouse and wild immunology. Parasite Immunol. 2015;37:267–273. doi: 10.1111/pim.12150. [DOI] [PubMed] [Google Scholar]
- 173.Maness NJ. The importance of understanding MHC-I diversity in nonhuman primate models of human infectious diseases. Toxicol Pathol. 2017;45:157–160. doi: 10.1177/0192623316672072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kotz KT, Xiao W, Miller-Graziano C, Qian WJ, Russom A, Warner EA, et al. Clinical microfluidics for neutrophil genomics and proteomics. Nat Med. 2010;16:1042–1047. doi: 10.1038/nm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 176.Moura Rosa P, Gopalakrishnan N, Ibrahim H, Haug M, Halaas O. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip. 2016;16:3728–3740. doi: 10.1039/C6LC00702C. [DOI] [PubMed] [Google Scholar]
- 177.Rigat-Brugarolas LG, Elizalde-Torrent A, Bernabeu M, De Niz M, Martin-Jaular L, Fernandez-Becerra C, et al. A functional microengineered model of the human splenon-on-a-chip. Lab Chip. 2014;14:1715–1724. doi: 10.1039/C3LC51449H. [DOI] [PubMed] [Google Scholar]
- 178.Boussommier-Calleja A, Li R, Chen MB, Wong SC, Kamm RD. Microfluidics: a new tool for modeling cancer-immune interactions. Trends Cancer. 2016;2:6–19. doi: 10.1016/j.trecan.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Irimia D, Wang X. Inflammation-on-a-Chip: probing the immune system ex vivo. Trends Biotechnol. 2018;36:923–937. doi: 10.1016/j.tibtech.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]