Abstract
Backgroun
This study aimed to investigate the effects of SIN on ankle fracture and the underlying mechanisms in MG-63 cells.
Mateeial/Methods
qRT-PCR and ELISA assay were used to detect the mRNA and protein levels of cytokines in peripheral blood of children with or without ankle fracture. The expression and activity of antioxidant and detoxifying enzymes were detected by ELISA assay. Pretreated MG-63 cells with/without SIN were stimulated with 1 μg/ml bradykinin (BK). A CCK-8 kit was used to detect the cell viability. The cytokines produced from MG-63 cells were detected by Western blotting and qRT-PCR. Moreover, Western blotting was used to detect the levels of p-p38 and p-NF-κB (p65), and the activation level of the Nrf2 signaling pathway was examined by qRT-PCR and Western blotting.
Results
In this study, we found that compared with the healthy children, the mRNA and protein levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) were significantly upregulated in children with ankle fracture. In addition, the expression and activity of antioxidant and detoxifying enzymes were imbalanced in children with ankle fracture. SIN treatment did not have a cytotoxic effect on MG-63 cells. SIN dose-dependently suppressed BK-induced upregulation of IL-1β, IL-6, TNF-α, p-p38, and p-NF-κB (p65). Furthermore, SIN dramatically inhibited oxidative stress induced by BK via balancing the expression and activity of antioxidant and detoxifying enzymes and inhibited the activation of Nrf2 signaling.
Conclusions
SIN might be a potential agent for the treatment of ankle fracture through reducing inflammatory response and oxidative stress.
MeSH Keywords: Ankle Fractures, Inflammation, Oxidative Stress, Sinomenium
Background
Fractures occur more easily when the skeleton is porous, with weak points at the physes and metaphyses [1], and can result in significant periods of inactivity, costly treatment, and excessive morbidity and mortality [2]. The number of fractures is maximal among children aged 5–11 years [3], and fractures are the cause of 9% of all injuries in children that come to the attention of health services [4]. The incidence of ankle fractures is highest during childhood, adolescence, and early adult life. Some evidence supports the view that the incidence of highly traumatic fractures is higher in individuals with reduced bone mass [5,6].
Improper restoration of bone fracture during treatment can induce skeletal malformations that can cause severe effects on the daily life of children. Children’s imperfect development of the nervous system, low tolerance, and strong reactions after fracture (manifested as anxiety, crying, crying, and kicking) can cause expansion of fracture damage, delay the healing of fractures and other issues, and thus affect the rehabilitation of children. Therefore, high-quality nursing care is especially important for children with fractures, and it is urgent to seek new and effective treatment programs for fracture treatment.
The balance between bone formation by osteoblasts and bone resorption caused by osteoclasts constitutes bone homeostasis [7]. Increased activity of osteoclasts caused by persistent inflammation leads to osteolysis in chronic infections, such as osteomyelitis, and high levels of pro-inflammatory factors in bones and joints can induce pain, cartilage loss, and even joint dysfunction [8]. Nuclear factor kappa B (NF-κB) is a well-known regulator of inflammatory response. Activation of NF-κB in osteoblasts and construction of bone cells effectively inhibits the differentiation and function of osteoblasts [9]. In fact, pharmacological inhibition of NF-κB improves oophorectomy-induced bone loss in mice by increasing bone formation and reducing bone resorption [10].
It has also been reported that oxidative stress may affect osteoporosis and fractures [11]. NF-E2-related factor 2 (Nrf2) is known to be a key redox-sensing transcription factor involved in host defense against oxidative stress and chemical insults [12,13]. Nrf2 is expressed in many cell types, including osteoblasts, osteocytes, and osteoclasts [14]. The normal balance between oxidants and antioxidants seems to be needed to maintain the correct balance between osteoblast and osteoclast activity. Nrf2 functions as a transcription factor in the nucleus to transcriptionally induce its downstream antioxidant and detoxifying enzymes, such as heme oxygenase 1 (HO-1), quinone oxidoreductase 1 (NQO1), and superoxide dismutase (SOD) expression to maintain cell homeostasis and protect against tissue injury [13]. Nrf2 deletion suppresses load-induced bone formation and delays fracture healing [14].
Sinomenine (SIN) is an active alkaloid extracted from the plant Sinomenium acutum. Due to its safety and strong anti-inflammatory and immune-regulatory properties, it can effectively treat rheumatoid arthritis and other inflammatory diseases [15]. SIN has significant analgesic, anti-arthritic, anti-inflammatory, and immunosuppressive properties [16]. SIN can attenuate osteoclastogenesis and osteolysis by downregulating TLR4/TRAF6 expression [17]. In addition, SIN can reduce oxidative stress and attenuates renal fibrosis by inhibiting Nrf2-mediated oxidative stress and TGF-β signaling [18]. However, the effect of SIN on ankle fracture has not been previously clarified.
In this study, we investigated the effects of SIN on BK-induced MG-63 cells and explored the underlying molecular mechanism. Our results reveal that SIN inhibits inflammatory response and oxidative stress stimulated by BK, which may be mediated by regulation of the MAPKp38/NF-κB and Nrf2 signaling pathways. Therefore, we show that SIN might be a potential agent for the treatment of ankle fracture.
Material and Methods
Materials
Sinomenine was purchased from Roche (SIN, Roche Pharma, China). BK was obtained from Peptide Institute, Inc. (Osaka, Japan). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), streptomycin, penicillin, glutamine, and sodium pyruvate were obtained from Gibco/Invitrogen, Inc. (Carlsbad, CA, USA). Primary antibodies: anti-TNF-α (1: 1000; cat. no. 3707), anti-IL-1β (1: 1000; cat. no. 12703), anti-IL-6 (1: 1000; cat. no. 12153), anti- phosphorylated-p38 (p-p38) (1: 1000; cat. no. 1170), and β-actin (1: 5000; cat no. 4970) were obtained from Cell Signaling Technology, Inc., (Danvers, MA, USA) and anti-phosphorylated-NF-κB (65) (p-NF-κB) was purchased from Abcam (Cambridge, MA). We obtained anti-Nrf2 from Santa Cruz Biotechnology (USA), anti-HO-1 was purchased from Enzo Life Sciences (USA), and anti-NQO-1 was obtained from Novus biological (USA). Horseradish peroxidase-conjugated secondary antibody was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). IL-1β (cat. no. E-EL-H0149c), IL-6 (cat. no. E-EL-H0102c), and TNF-α (cat. no. E-EL-H0109c) ELISA kits were obtained from Elabscience Biotechnology Co., Ltd. (Wuhan, Hubei, China). Malondialdehyde (MDA) assay kits (cat no. ab118970) and superoxide dismutase (SOD) assay kits (cat no. ab65354) were purchased from Abcam (USA).
Patients
Children at our hospital who had an ankle fracture that required surgical reduction and fixation were included (n=120). Peripheral venous blood was collected from patients and healthy children. All participants provided informed consent and the study approved by our local ethics committee.
Cell culture
Human osteoblastic osteosarcoma cell line MG-63 was grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, USA), 1% penicillin/streptomycin solution, and 1.0 mM sodium pyruvate (Gibco), and incubated in a 37°C 5% CO2 incubator.
ELISA assay
Blood from children with or without ankle fracture was collected and sera were prepared by centrifugation at 3000×g for 15 min. IL-1β, IL-6, and TNF-α concentrations in the sera were determined using ELISA kits according to the relevant manuals.
Cell viability analysis
MG-63 cells were cultured in 96-well plates and incubated with SIN (0.25, 0.5, or 1 mM) for 24 h. Then, a CCK-8 kit (Vazyme, China) was used to measure cell viability according to the manufacturer’s instructions.
qRT-PCR
MG-63 cells (5×104) were plated in 6-well plates and incubated with SIN (0.25, 0.5 or 1 mM) for 2 h, and then incubated in the presence or absence of BK (1 μg/ml) for 24 h. Total RNA from MG-63 cells was extracted from Trizol reagent (Invitrogen, USA) following the manufacturer’s protocol. We reversely transcribed 1 μg of RNA into cDNA using the HiScript Reverse Transcriptase Kit (Vazyme, China) according to the manufacturer’s protocol. Quantitative real-time PCR was performed in an ABI 7500 Real-time PCR instrument (Applied Biosystems, Carlsbad, CA, USA) with SYBR Premix Ex Taq (TaKaRa). The PCR primer sequences were:
Nrf2-F, 5′-CAGGTGATGCTGACAGAGGA-3′,
Nrf2-R, 5′-TCTCTGCAGGGGCAGTA-3′;
HO-1-F, 5′-ATGTGGCCCTGGAGGAGGAGA-3′;
HO-1-R, 5′-CGCTGCATGGCTGGTGTGTAG-3′;
NQO1-F, 5′-GGATTGGACCGAGCTGGAA-3′;
NQO1-R, 5′-AATTGCAGTGAAGATGAAGGCAAC-3′;
TNF-α-F, 5′-CATCTTCTCAAAATTCGAGTGAC-3′;
TNF-α-R, 5′-TGGGAGTAGACAAGGTACAACCC-3′,
IL-1β-F, 5′-TGGAAAAGCGGTTTGTCTTC-3′;
IL-1β-R 5′-TACCAGTTGGGGAACTCTGC-3′;
IL-6-F, 5′-GCTGGTGACAACCACGGCCT-3′;
IL-6-R, 5′-AGCCTCGACTTGTGAAGTGGT-3′;
GAPDH-F, 5′-GGCCTTCCGTGTTCCTAC-3′;
GAPDH-R, 5′-TGTCATCATATCTGGCAGGTT-3′.
GAPDH was used as a control and the 2−ΔΔCq method [19] was used to analyze the data.
Measurement of MDA and antioxidant enzyme activities
Blood from children with or without ankle fracture were collected and sera were prepared by centrifugation at 3000 g for 15 min. MG-63 cells (5×104) were plated into 6-well plates and were incubated with SIN (0.25, 0.5, or 1 mM) for 2 h, and then incubated in the presence or absence of BK (1 μg/ml) for 24 h. After treatment, MG-63 cells were lysed and the supernatants were collected after centrifugation for 10 min at 1600 g at 4°C. Commercial colorimetric detection kits were used to assay the level of MDA and enzymatic activities of SOD and CAT in children with or without ankle fracture and MG-63 cells in accordance with the manufacturer’s instructions. The enzyme activity in cells is presented as units/mg of protein [20].
Western blot analysis
MG-63 cells (5×104) were plated in 6-well plates and were incubated with SIN (0.25, 0.5, or 1 mM) for 2 h, and then incubated in the presence or absence of BK (1 μg/ml) for 24 h. Total proteins from the MG-63 cells were extracted using lysis buffer. Protein concentrations were detected using the bicinchoninic acid (BCA) protein assay (Thermo Scientific, USA). Equal amounts (25 μg) of the protein samples were separated using 12% SDS-PAGE and then transferred onto PVDF membranes (Millipore, USA). Subsequently, the membranes were blocked with 5% skim milk for 2 h at 37°C, and then incubated with primary antibodies – anti-TNF-α, anti-IL-1β, anti-IL-6, anti-p-p38, anti-NF-kB (p65), anti-Nrf2, anti-HO-1, anti-NQO-1, and anti-β-actin – at 4°C overnight. After incubation with horseradish peroxidase-conjugated secondary antibody, the blots were visualized using chemiluminescent ECL reagent (Millipore, MA, USA) and we quantified the protein bands by densitometry (QuantityOne 4.5.0 software; Bio-Rad, Inc., USA).
Statistical analysis
Data are expressed as mean ±SD. Statistical analysis was performed using SPSS 17.0 software (IBM, USA). One-way analysis of variance (ANOVA) and Dunnett’s t test were used to assess the significance between groups. p<0.05 was considered statistically significant.
Results
Ankle fracture causes a dramatic increase in the levels of inflammatory bio-markers
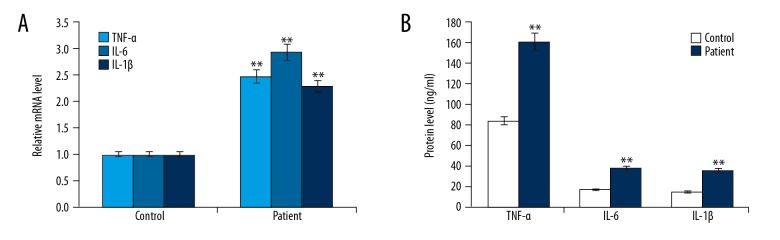
qRT-PCR and ELISA assay results showed that the mRNA and protein levels of TNF-α, IL-6, and IL-1β were significantly higher in children with ankle fracture than in healthy children (Figure 1).
Figure 1.
TNF-α, IL-1β, and IL-6 expression levels in children with or without fractured ankles. (A) The mRNA levels of TNF-α, IL-1β, and IL-6 in children with or without fractured ankle were detected by qRT-PCR; (B) The protein levels of TNF-α, IL-1β, and IL-6 in children with or without fractured ankles were detected by ELISA assay. Controls: healthy children; Patients: children with fractured ankle. ** p<0.01 vs. Control.
Ankle fracture causes a dramatic increase in oxidative stress
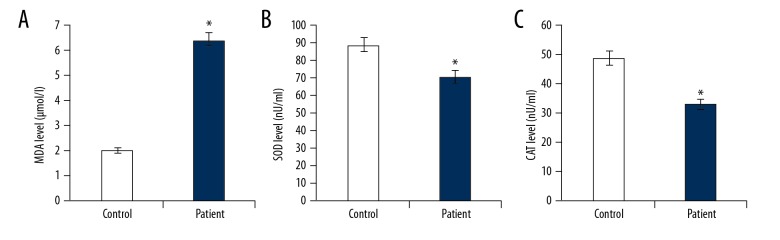
Compared with the healthy children, the MDA level was significantly increased in children with ankle fracture. In addition, the SOD and CAT activities were significantly reduced in children with ankle fracture (Figure 2). These findings indicate the enhanced oxidative stress in ankle fracture patients.
Figure 2.
MDA, SOD, and CAT level in children with or without fractured ankle. The levels of MDA, SOD, and CAT in children with or without fractured ankles were detected in the present study. Controls: healthy children; Patients: children with fractured ankles. * p<0.01 vs. Control.
SIN has no cytotoxicity on MG-63 cells
As shown in Figure 3, the effect of SIN on MG-63 cell viability was determined by CCK-8 assay. The results showed that the cell viability was not markedly affected by SIN at concentrations of 0.25, 0.5, or 1 mM.
Figure 3.
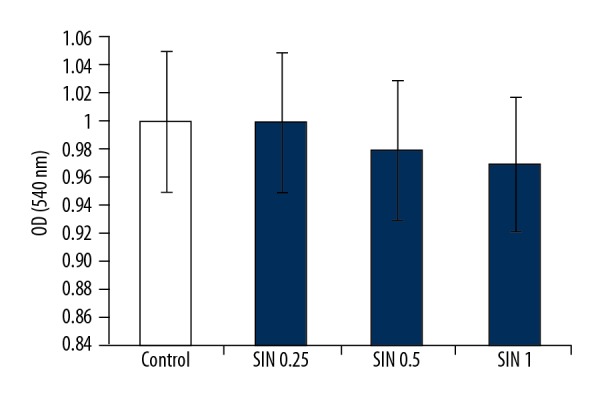
SIN shows no cytotoxicity on MG-63 cells. Cell viability of MG-63 cells was measured by CCK-8 assay. Controls: cells without any treatment; SIN 0.25, SIN 0.5, SIN 1: MG-63 cells were treated with 0.25, 0.5, or 1 mM for 24 h.
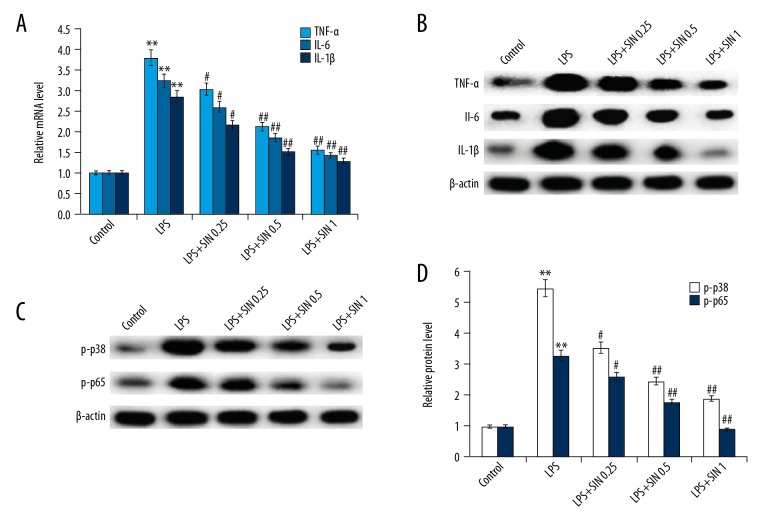
SIN pretreatment remarkably alleviated BK-induced inflammation in MG-63 cells
As shown in Figure 4, the mRNA and protein levels of TNF-α, IL-1β, and IL-6 were markedly increased in MG-63 cells stimulated by BK compared with the control group. Pretreatment with SIN attenuated the expression of BK-induced inflammatory mediators in a dose-dependent manner. To investigate the mechanism by which SIN inhibits inflammatory responses, we investigated the effect of SIN on MAPKp38/NF-κB signaling. The results demonstrated that compared with the control group, the protein levels of p-p38 and p-NF-κB (p65) were significantly increased in BK-induced MG-63 cells. Remarkably, administration of SIN significantly decreased p-p38 and p-NF-κB protein expression when compared with the BK group. Taken together, these data suggested that SIN inhibited inflammation may be mediated by MAPKp38/NF-κB signaling.
Figure 4.
Effect of SIN on inflammatory response. After treatment with 0.25, 0.5, or 1 mM for 24 h, mRNA (A) and protein (B) levels of TNF-α, IL-1β, and IL-6 were detected by qRT-PCR and Western blot, respectively; Protein levels of p-p38 and p-p65 were detected using Western blotting (C) and the data were analyzed (D). Controls: cells without any treatment; BK: 1 μg/ml BK treatment group; BK + SIN 0.25: 1 μg/ml BK + 0.25 nM SIN treatment group; BK + SIN 0.5: 1 μg/ml BK + 0.5 nM SIN treatment group; BK + SIN 1: 1 μg/ml BK + 1 nM SIN treatment group;** p<0.01 vs. Control; # p<0.05 vs. BK; ## p<0.01 vs. BK.
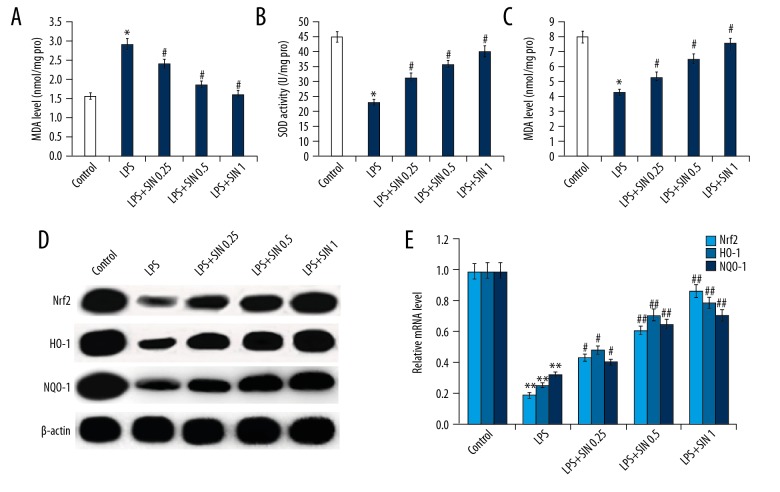
SIN pretreatment remarkably alleviated BK-induced oxidative stress in MG-63 cells
As shown in Figure 5A, the levels of MDA significantly increased in MG-63 cells stimulated with BK compared to the control group. Pretreatment with SIN at a concentration of 0.25, 0.5, or 1 mM significantly decreased the level of MDA. Furthermore, antioxidant enzymes, such as SOD and CAT was significantly decreased in MG-63 cells exposed to BK compared with the control group. However, the decreased activity of SOD and CAT induced by BK was dose-dependently attenuated when MG-63 cells were pretreated with SIN at a concentration of 0.25, 0.5, or 1 mM (Figure 5B, 5C). These findings suggest that SIN protected MG-63 cells against BK-induced oxidative stress.
Figure 5.
Effect of SIN on oxidative stress. After treatment with 0.25, 0.5, or 1 mM for 24 h, the level of MDA (A) and activity of SOD (B) and CAT (C) was detected; Protein (D) and mRNA (E) levels of Nrf2, HO-1, and NQO-1 were detected using Western blotting and qRT-PCR, respectively. Controls: cells without any treatment; BK: 1 μg/ml BK treatment group; BK + SIN 0.25: 1 μg/ml BK + 0.25 nM SIN treatment group; BK + SIN 0.5: 1 μg/ml BK + 0.5 nM SIN treatment group; BK + SIN 1: 1 μg/ml BK + 1 nM SIN treatment group; * p<0.05 vs. Control; ** p<0.01 vs. Control; # p<0.05 vs. BK; ## p<0.01 vs. BK.
SIN pretreatment increased the expression of Nrf2-related antioxidant genes in MG-63 cells
The Nrf2 antioxidant system is thought to be a good candidate molecule for regulating intracellular redox state. To investigate the mechanism by which SIN inhibits oxidative stress, we investigated the effect of SIN on Nrf2 antioxidant system. As shown in Figure 5D and 5E, compared with the control group, Nrf2, HO-1, and NQO-1 mRNA and protein expression were significantly decreased in MG-63 cells exposed to BK. Pretreatment with SIN significantly increased Nrf2, HO-1, and NQO-1 protein and mRNA expression, respectively, in a dose-dependent manner when compared with the BK group.
Discussion
Our results suggest that pretreatment with SIN, a natural alkaloid extracted from the climbing plant S. acutum and extensively used in the clinical treatment of multiple inflammatory disorders, downregulated inflammatory mediators, including TNF-α, IL-1β, and IL-6, inhibited the MAPKp38/NF-κB pathway, and upregulated the levels of Nrf2, HO-1, and NQO-1 in BK-induced MG-63 cells. Evidence collected in this study support that SIN could be a novel agent for treating ankle fractures.
The balance between the activity of osteoblasts and osteoclasts is involved in bone formation, and the healing of fractures requires large quantity of osteoblasts [21]. There is compelling evidence indicating that inflammation directly affects bone formation in trauma-induced fractures. Our study shows that inflammatory factors in children with ankle fractures are significantly higher than those in healthy children. It has been reported that BK stimulates bone resorption in mice [22]. A previous study has reported that SIN reduces osteoclasts activity and bone resorption both in vivo and in vitro via inhibition of inflammation and immune responses [23]. The results from the present study indicate that SIN significantly decreases the levels of inflammatory cytokines, including TNF-α, IL-1β, and IL-6 in MG-63 cells induced by BK and did not have cytotoxic effects on MG-63 cells.
NF-κB is one of the most important inflammatory signaling molecules and is responsible for the transcription of cytokines and chemokines. A previous study reported that NF-κB activation is essential in osteoclastogenesis, and SIN regulates immune response and inhibits inflammation by inhibiting NF-κB activation [24]. It has been reported that SIN inhibits the activation of NF-κB and related gene expression in BK-induced osteoclastogenesis [25]. Therefore, we detected the NF-κB signaling to explore the potential mechanisms underlying SIN suppression of inflammatory response in BK-induced MG-63 cells. Our results showed that SIN repressed NF-κB activity in BK-induced MG-63 cells. In addition, lower levels of p38 phosphorylation participate in osteoclast formation [26] and SIN had been shown to down-regulate the phosphorylation of MAPKp38 (but not JNK) in BK-induced osteoclastogenesis [25]. We thus hypothesized that SIN protects MG-63 cells against BK-induced inflammation, also involving regulation of MAPKp38 signaling. Our results showed that SIN significantly inhibited p38 activation as manifested by the strikingly lower levels of phosphorylated p38. Thus, the suppressive effect of SIN on BK-induced inflammation may be mediated partly by the suppression of activated MAPKp38/NF-κB.
Numerous studies have found that SIN has antioxidant activity in different cells [27,28]. The Nrf2 signaling pathway is the major defense against inflammatory responses and oxidative stress. SIN has been reported to activate the Nrf2 pathway in the kidneys [29]. Therefore, we assessed the effects of SIN on the Nrf2 system in MG-63 cells. The results showed that after treatment with SIN, the expression levels of Nrf2 system-associated genes in MG-63 cells, including Nrf2, HO-1 and NQO-1, were concentration-dependently upregulated.
In summary, our investigation indicates that SIN protects against BK-induced inflammation and oxidative stress in MG-63 cells and inhibits MAPKp38/NF-κB and Nrf2 pathways. SIN might be a potential drug for treatment of ankle fracture.
Conclusions
SIN exerts an anti-inflammatory and anti-oxidative stress effect on MG-63 cells in vitro. SIN may be a promising and effective agent for the treatment of ankle fracture.
Footnotes
Conflicts of interests
None.
Source of support: Departmental sources
References
- 1.Clark EM. The epidemiology of fractures in otherwise healthy children. Curr Osteoporos Rep. 2014;12:272–78. doi: 10.1007/s11914-014-0227-y. [DOI] [PubMed] [Google Scholar]
- 2.Pasco JA, Lane SE, Brennan-Olsen SL, et al. The epidemiology of incident fracture from cradle to senescence. Calcif Tissue Int. 2015;97:568–76. doi: 10.1007/s00223-015-0053-y. [DOI] [PubMed] [Google Scholar]
- 3.Mansoor K, Shahnawaz S, Ahmad A, et al. Epidemiology of childhood fractures in the city of Karachi. J Ayub Med Coll Abbottabad. 2015;27:608–12. [PubMed] [Google Scholar]
- 4.Spady DW, Saunders DL, Schopflocher DP, Svenson LW. Patterns of injury in children: A population-based approach. Pediatrics. 2004;113:522–29. doi: 10.1542/peds.113.3.522. [DOI] [PubMed] [Google Scholar]
- 5.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–88. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 6.Sanders KM, Pasco JA, Ugoni AM, et al. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: The Geelong Osteoporosis Study. J Bone Miner Res. 1998;13:1337–42. doi: 10.1359/jbmr.1998.13.8.1337. [DOI] [PubMed] [Google Scholar]
- 7.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–36. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 8.Lukens JR, Gross JM, Calabrese C, et al. Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci USA. 2014;111:1066–71. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Li A, Strait K, et al. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–55. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 10.Alles N, Soysa NS, Hayashi J, et al. Suppression of NF-kappaB increases bone formation and ameliorates osteopenia in ovariectomized mice. Endocrinology. 2010;151:4626–34. doi: 10.1210/en.2010-0399. [DOI] [PubMed] [Google Scholar]
- 11.Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. 2007;8:519–25. doi: 10.2174/138920007780866852. [DOI] [PubMed] [Google Scholar]
- 12.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 13.Sajadimajd S, Khazaei M. Oxidative stress and cancer: The role of Nrf2. Curr Cancer Drug Targets. 2017 doi: 10.2174/1568009617666171002144228. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Sun YX, Xu AH, Yang Y, Li J. Role of Nrf2 in bone metabolism. J Biomed Sci. 2015;22:101. doi: 10.1186/s12929-015-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol. 2012;50:1053–61. doi: 10.3109/13880209.2012.656847. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–76. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 17.He L, Duan H, Li X, et al. Sinomenine down-regulates TLR4/TRAF6 expression and attenuates lipopolysaccharide-induced osteoclastogenesis and osteolysis. Eur J Pharmacol. 2016;779:66–79. doi: 10.1016/j.ejphar.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFbeta signaling. Toxicol Appl Pharmacol. 2016;304:1–8. doi: 10.1016/j.taap.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625–34. doi: 10.1016/j.cellsig.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Kang J, Hu K, et al. Angiotensin-(1–7) inhibits inflammation and oxidative stress to relieve lung injury induced by chronic intermittent hypoxia in rats. Braz J Med Biol Res. 2016;49:e5431. doi: 10.1590/1414-431X20165431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z, Selvamurugan N, Warshaw J, Partridge NC. Pulsed electromagnetic fields inhibit human osteoclast formation and gene expression via osteoblasts. Bone. 2018;106:194–203. doi: 10.1016/j.bone.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Tominari T, Ichimaru R, Yoshinouchi S, et al. Effects of O-methylated (−)-epigallocatechin gallate (EGCG) on BK-induced osteoclastogenesis, bone resorption, and alveolar bone loss in mice. FEBS Openbio. 2017;7:1972–81. doi: 10.1002/2211-5463.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, He L, Hu Y, et al. Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS One. 2013;8:e74274. doi: 10.1371/journal.pone.0074274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Li J, Yu K, et al. Sinomenine inhibits maturation of monocyte-derived dendritic cells through blocking activation of NF-kappa B. Int Immunopharmacol. 2007;7:637–45. doi: 10.1016/j.intimp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 25.He L, Duan H, Li X, et al. Sinomenine down-regulates TLR4/TRAF6 expression and attenuates lipopolysaccharide-induced osteoclastogenesis and osteolysis. Eur J Pharmacol. 2016;779:66–79. doi: 10.1016/j.ejphar.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Fan H, Shu Q, Guan X, et al. Sinomenine protects PC12 neuronal cells against H2O2-induced cytotoxicity and oxidative stress via a ROS-dependent up-regulation of endogenous antioxidant system. Cell Mol Neurobiol. 2017;37:1387–98. doi: 10.1007/s10571-017-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Jiao Y, Wang Z, et al. Sinomenine hydrochloride inhibits human glioblastoma cell growth through reactive oxygen species generation and autophagy-lysosome pathway activation: An in vitro and in vivo study. Int J Mol Sci. 2017;18(9) doi: 10.3390/ijms18091945. pii: E1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin T, Du R, Huang F, et al. Sinomenine activation of Nrf2 signaling prevents hyperactive inflammation and kidney injury in a mouse model of obstructive nephropathy. Free Radic Biol Med. 2016;92:90–99. doi: 10.1016/j.freeradbiomed.2016.01.011. [DOI] [PubMed] [Google Scholar]