Abstract
Background
Let-7c-5p is down-regulated in dental pulp tissues in inflammatory disorders. The microRNA (miR) molecule shows an anti-inflammation potential due to its direct regulation of dentin matrix protein-1 (DMP1), which promotes inflammation changes in dental pulp tissues. In the present study, the effect of let-7c-5p on lipopolysaccharide (LPS)-induced pulpitis was detected and the associated mechanism was explored.
Material/Methods
Dental pulp stem cells (DPSCs) were isolated from rat dental tissues, infected with let-7c-5p lentivirus particles, and subjected to LPS administration to induce inflammation. Then, the effect of let-7c-5p overexpression on LPS-induced impairments on DPSCs were detected and the mechanism was explained by focusing on the DMP1 expression and NF-κB pathway. The role of DMP1 in the anti-inflammation effect of let-7c-5p was assessed by incubating let-7c-5p-expressed DPSCs with DMP1 protein. The results of in vitro assays were verified in LPS-induced rat pulpitis models.
Results
LPS administration increased the production of IL-1β and TNF-α and decreased DPSCs viability by increasing the expression of DMP1 and activating NF-κB pathway. However, the induced expression of let-7c-5p relieved DPSCs from LPS-induced inflammation and suppressed DMP1 as well as NF-κB pathway. The incubation of let-7c-5p-expressed DPSCs with DMP1 protein blocked the effect of let-7c-5p. In in vivo experiments, the injection of let-7c-5p attenuated LPS-induced pulpitis by inhibiting DMP1-mediated NF-κB pathway.
Conclusions
Findings outlined in the current study demonstrated the dental pulp protecting function of let-7c-5p during LPS-induced inflammation, which was exerted by inhibiting the DMP1-mediated NF-κB pathway.
MeSH Keywords: Dental Pulp Diseases, MicroRNAs, Pulpitis
Background
Bacterial infection after the development of caries or dental traumatic lesions will cause pulpitis when the pathogenic microbes penetrates into dentinal tubules and pulpal tissues [1]. The disorder is termed as pulpitis and depends on root canal procedures for alleviation (http://www.aae.org; last accessed November 1, 2011). During the progression of pulpitis, the secretion of pro-inflammatory cytokines induced by bacterial irritants will alter the proliferation, migration, and differentiation behaviors of dental pulp stem cells (DPSCs). The cell type derives from mesenchymal-derived cells characterized by self-renewal and multi-lineage differentiation capability [2], which has been employed as a promising option for the tissue engineering and regenerative medicine. Previous studies have demonstrated that the restoration of DPSC functions in pulpitis can induce a form of tertiary dentin adjacent to injury sites and cell differentiation into odontoblast-like cells [3], showing the central role of DPSCs in maintaining the homeostasis of the dental pulp microenvironment.
With the accumulation of information regarding the microbial infection of dental pulp tissues, microRNAs (miRs) have been recently reported to be a key regulator involved in the response of DPSCs to inflammation [4,5]. MiRs are small (~20–22 nucleotides) noncoding RNA species that regulate diverse gene functions by binding to the 3′ untranslated region (UTR) of targeted mRNA members [6]. Multiple miR members have been reported to be dys-expressed in inflamed human pulps [7], including up-regulated ones that might be involved in the onset of pulpitis and down-regulated ones that might have treatment potential against pulpitis. The expression of miR let-7c-5p is reported to be suppressed in inflamed human dental pulp tissues [7]. Moreover, let-7c-5p contributes to osteogenic differentiation of human stromal mesenchymal stem cells [8], representing the ability of let-7c-5p to store the normal function of DPSCs.
Dentin matrix protein 1 (DMP1) is a non-collagenous protein essential for the mineralization of dentin and bone [9]. Generally, DMP1 is highly expressed in odontoblasts and bone osteocytes, while in osteoblasts and cartilage the expression of DMP1 is suppressed [10–12]. However, during pulpitis, the level of DMP1 increases, suggesting that DMP1 contributes to inflammatory responses in dental pulp tissues [13]. Furthermore, DMP1 is a direct target of let-7c-5p and the interaction between the 2 factors has been verified in normal dental pulp cells [9]. Based on the above information, the hypothesis of the present study was that the suppressed expression of let-7c-5p during pulpitis upregulates the expression of DMP1, thus contributing to the progression of inflammatory responses in dental pulp tissues and impairing the normal biological behavior of DPSCs.
In the present study, a series of in vitro and in vivo assays were performed to verify this hypothesis. The inflammatory response was induced in DPSCs using lipopolysaccharide (LPS). Then, the effect of let-7c-5p overexpression on the DPSC viability, expression of DMP1, and activity of inflammation-related signaling was assessed. Moreover, the expression of DMP1 was induced in let-7c-5p-overexpressed DPSCs to determine whether the effect of let-7c-5p on inflammation in DPSCs was exerted in a DMP1-inhibition-dependent manner. The data derived from in vitro assays were further verified in rat pulpitis models. Findings outlined in the current study show that let-7c-5p had an anti-inflammation effect in DPSCs by suppressing DMP1 function, which contributes to the amelioration of pulpitis.
Material and Methods
Antibodies and chemicals
Antibody against DMP1 (cat. no. GTX55589) was obtained from GeneTex (USA). Antibodies against IκBα (cat. no. #9242), phosphorylated IκBα (p-IκBα) (cat. no. #2859), IKKβ (cat. no. #8943), NF-κB subunit p65 (cat. no. #8242), and Histone H3 (cat. no. #4499) were purchased from Cell Signaling Technology (USA). Antibody against p-IKKβ (cat. no. ab59195) was purchased from Abcam (USA). Secondary goat anti-rabbit (cat. no. A0208) IgG-HRP antibody was obtained from Beyotime Biotechnology (China). Antibody against β-actin (cat. no. bsm-33139M) was purchased from Bioss (China). Lipopolysaccharides (LPS) (cat. no. L8880) was purchased from Solarbio (China). Rat let-7c-5p agomir was obtained from GenePharma (China). Trizol (cat. no. RP1002), super M-MLV reverse transcriptase (cat. no. RP6502), and 2×Power Taq PCR MasterMix (cat. no. PR1702) were purchased from BioTeke (China). SYBR Green (cat. no. SY1020) was purchased from Solarbio (China). RIPA lysis buffer (cat. no. P0013B), Plasma and Nuclear Protein Extraction Kit (cat. no. P0027), and Protein Concentration Determining Kit using BCA method (cat. no. P0009) were purchased from Beyotime Biotechnology (China). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) (cat. no. M-2128) was obtained from Sigma (USA). Recombinant Mouse DMP-1 Protein (cat. no. 4386-DM-050) was purchased from Sigma (USA). Enzyme-linked Immunosorbent Assay (ELISA) Kits for detection interleukin 1β (IL-1β) (cat. no. EK301B1/2) and tumor necrosis factor α (TNF-α) (cat. no. EK3821/2) were purchased from Multi Sciences (China).
Cell culture
Eight-week-old Sprague Dawley (SD) rats (Huafukang Bioscience Co. Inc., Beijing, China) were killed by i.p. injection of an overdose of pentobarbital sodium and dental tissues were collected. After removing the soft tissues, dentinal cartilage was dissected to expose dental pulp tissues. Then, the dental pulp tissues were cut into pieces and incubated with collagenase type I (3 mg/ml) and neutral protease (3 mg/ml) at 37°C for 20 min. The tissues were then filtered and collected via centrifugation at 309×g for 7 min, repeating these procedures until the tissues were fully digested. Cells were re-suspended and preserved at 37˚C in an atmosphere consisting of 5% CO2 and 95% air. The expression statuses of CD29, CD90, CD146, CD105, CD34, and CD45 were detected with immunofluorescence. All the assays with animals were performed following the Institutional Animal Ethics Committee and Guidelines for the Care and Use of Animals of Jilin University.
Lentiviral vector construction, virus infection, and cell administration
Lentivirus plasmid expression let-7c-5p (5′-UGAGGUAGUA GGUUGUAUGGUU-3′) were constructed and packaged by GenePharma (Shanghai, China). For infection, DPSCs and different lentivirus particles were co-plated into 1 well of 6-well plates at a ratio of 1: 50. Cells stably expressing let-7c-5p were selected with 3 μg/ml ampicillin. The infection efficiency was validated with reverse transcription real time polymerase chain reaction (RT2-PCR). For induction of inflammatory response, cells were incubated with LPS (10 μg/ml) for 24 h. For validation of the role of DMP1 in the effect of let-7c-5p on DPSCs, the let-7c-5p-expressed DPSCs were further incubated with DMP-1 protein (10 ng/ml) for 6 h after LPS administration.
Immunofluorescence
DPSCs were seeded in 14-well chambers and fixed with 4% paraformaldehyde for 15 min before being permeabilized with 0.1% Triton X-100 for 30 min. After being incubated with 10% goat serum for 15 min at room temperature, cells were incubated with the primary antibodies at 4ºC overnight. Following 3 washings using PBS, secondary Cy3-labeled antibody was added and incubated for 1 h at room temperature in the dark. After being washed with PBS, the cells were stained with 4, 6-diamino-2-phenyl indole (DAPI) for 5 min. The images were captured with a fluorescent microscope (BX53, Olympus, Japan) at 400× magnification.
RT2-PCR
Total RNA in different groups was extracted using Trizol method. cDNA templates were achieved using super M-MLV reverse transcriptase from total RNA. The reaction mixture of real-time PCR contained 10 μl 2×Power Taq PCR MasterMix, 0.3 μl SYBR GREEN, 0.5 μl of each primer (let-7c-5p, forward: 5′-CGTGCGGTGAGGTAGTAGGTT-3′, backward: 5′-GTGCAGGGTCCGAGGTATTC-3′; U6, forward: 5′-GCTTCGG CAGCACATATACT-3′, backward: 5′-GTGCAGGGTCCGAGGTATTC-3′), 1 μl cDNA template, and 7.7 μl ddH2O. Thermal cycling parameters were set as followings: a denaturation step at 94°C for 2 min, followed by 40 cycles of amplification at 94°C for 15 s, 60°C for 15 s and 72°C for 15 s, then the signaling was detected using ExicyclerTM 96 (BIONEER, South Korea). The relative expression levels of the let-7c-5p were calculated using according to the formula of 2−ΔΔct.
MTT assay
Cell viability was detected using MTT assay and DPSCs in different groups were then incubated with 0.5 mg/ml MTT for another 4 h at 37°C. The supernatant was aspirated and 150 μl DMSO was added into each well. The cell viability was represented by the OD570 value detected using a microplate reader (ELX-800, BIOTEK, USA).
ELISA
The production of IL-1β and TNF-α in cell cultures were detected using specific ELISA kits according to the manufacturers’ instructions. Briefly, cells were lysed using ultrasound and total protein was collected via centrifugation at 2500 rpm for 10 min. For ELISA detection, 20 μl protein sample and 50 μl antibody were incubated at room temperature for 2 h. Then, the mixture was reacted with 100 μl TMB at room temperature for 20 min in the dark and the OD values at 450 and 570 nm was determined using a microplate reader (ELX-800, BIOTEK, USA).
Western blotting
Total cellular protein in DPSCs or dental pulp tissues was extracted using 1% PMSF. Protein concentration was determined using BCA method according to the manufacturers’ instructions. We subjected 40 μg protein in 20 μl solution to 5% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred it onto polyvinylidene difluoride (PVDF) membranes. The membranes then were blocked with skimmed milk solution (5%, m/v) for 1 h and primary antibodies against targeted proteins [DMP1 (1: 1000), p-IκBα (1: 1000), IκBα (1: 500), p-IKKβ (1: 1000), IKKβ (1: 1000), NF-κB p65 (1: 1000), β-actin (1: 500), and Histone H3 (1: 1000)] were incubated with the membranes at 4°C overnight. Secondary HRP-conjugated IgG antibodies (1: 5000) were added onto the membranes and incubated for 45 min at 37°C. The blots were developed by incubating membranes with Beyo ECL Plus reagent for 5 min and the relative expression levels of proteins were analyzed using a Gel-Pro-Analyzer (Media Cybernetics, USA).
Rat pulpitis induction and let-7c-5p agomir administration
Six-week-old male SD rats were anesthetized with 50 mg/kg body weight pentobarbital sodium and 2 mm of mandibular left and right incisor crowns were removed by a diamond disc to expose the pulp chamber. After disinfection with 70% ethanol, the coronal pulp chamber was exposed to a sterile paper point infiltrated with LPS (10 mg/ml, 1 ml) for 9 h. Let-7c-5p agomir (50 pM, 10 μl) was injected to the alveolar mucosa of rats. After pulpitis induction, rats were killed by i.p. injection with an overdose of pentobarbital sodium and dental pulp tissues were collected and preserved at −80°C.
Hematoxylin and eosin (H&E) staining
Dental pulp tissues were dehydrated using different concentrations of alcohol and vitrified in dimethylbenzene. Samples of different groups were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). The results were recorded under a microscope (DP73, OLUMPUS, Japan) at 200× magnification.
Immunohistochemistry (IHC)
Dental tissues were dehydrated using different concentrations of alcohol and fixed with dimethylbenzene for 30 min. After incubating in antigen retrieval buffers for 30 min, the sections were incubated with primary antibody for DMP1 (1: 100) at 4°C overnight. Secondary antibodies (1: 200) were added onto the tissues and incubated for 30 min at 37°C. Thereafter, DAB was then added and incubated for 3–10 min before the reaction was stopped. The sections were re-stained with hematoxylin and dehydrated. The results were detected using a microscope (DP73, Olympus, Japan) at 400× magnification.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). One-way ANOVA and post hoc multiple comparisons with Duncan method were performed with a significance level of 0.05 (two-tailed P value). The statistical analyses were performed using GraphPad Prism version 6.0 software (GraphPad Software, Inc., San Diego, CA).
Results
Isolation and characterization of DPSCs
DPSCs were successfully generated in the current study: results of immunofluorescence assay showed that the cells were positive for CD90, CD105, CD29, and CD146 and were negative for CD34 and CD45, showing a typical surface antigen characteristic of DPSCs (Supplementary Figure 1) [2].
Let-7c-5p attenuated LPS-induced inflammatory responses in DPSCs
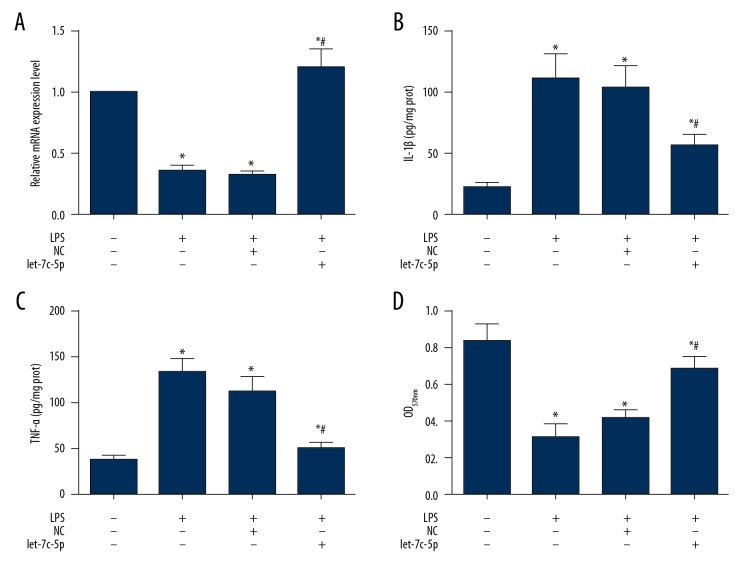
The DPSCs were infected with NC particles or let-7c-5p expression particles (Figure 1A) before incubation with 10 μg/ml LPS for 24 h to induce inflammation. As shown in Figure 1B, incubation of LPS increased the production of pro-inflammation cytokines compared with the Blank group (P<0.05), including IL-1β and TNF-α. However, infection of let-7c-5p lentivirus particles rescued DPSCs from the impairments of LPS administration and production of IL-1β and TNF-α was inhibited by let-7c-5p (Figure 1B).
Figure 1.
Let-7c-5p attenuated LPS-induced inflammation and increased cell viability in DPSCs. DPSCs were infected with NC lentivirus particles and let-7c-5p expression lentivirus particles. At 72 h after infection, the cells were incubated with 10 μg/ml LPS for 24 h to induce inflammatory response. Upon completion of the inflammation induction, the cells were collected and subjected to ELISA for detection of IL-1β and TNF-α production and MTT assay for detection of cell viability. Administration of LPS suppressed the expression of let-7c-5p and infection of let-7c-5p expression vector increased the expression of let-7c-5p (A). The induced expression of let-7c-5p inhibited the production of IL-1β (B) and TNF-α (C) and increased the cell viability of DPSCs due to LPS administration (D). * P<0.05 vs. Blank group. # P<0.05 vs. LPS or LPS+NC group. Each assay was represented by 3 replicates.
Let-7c-5p increased the cell viability in inflammatory DPSCs
The induced inflammatory response suppressed the viability of DPSCs. As detected by MTT assay, DPSCs incubated with LPS had a lower level of OD570 value in comparison to the Blank group (P<0.05) (Figure 1C), but in DPSCs overexpressing let-7c-5p, cell viability was increased to relatively normal level (Figure 1C), indicating the protective effect of let-7c-5p on DPSCs against inflammation.
Let-7c-5p attenuated the LPS-induced inflammatory responses by inhibiting DMP1 expression and NF-κB signaling in DPSCs
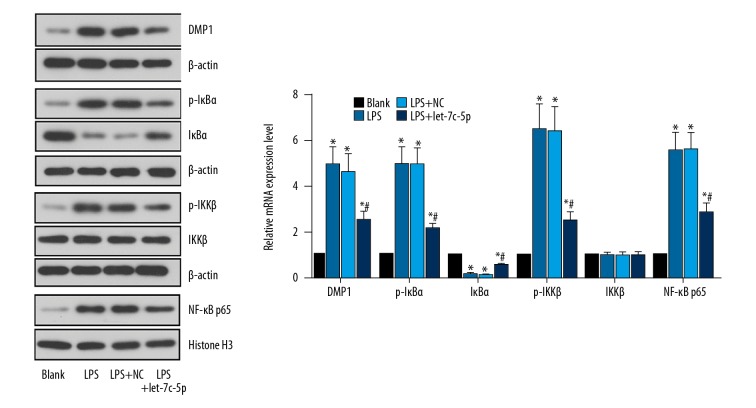
The expression of DMP1 in DPSCs as well as NF-κB signaling was detected to demonstrate the mechanism driving the function of let-7c-5p. It was found that LPS administration increased the expressions of DMP1, p-IκBα, and p-IKKβ, and nuclear translocation of NF-κB p65, but decreased the level of IκBα (Figure 2), showing activation of the NF-κB pathway. The infection of let-7c-5p particles reversed the expression patterns of all indicators, as expected. The results preliminarily indicated that the effect of let-7c-5p in protecting DPSCs was exerted through a-DMP1 and NF-κB-inhibition manner.
Figure 2.
Let-7c-5p inhibited expression of DMP1 and activation of NF-κB pathway induced by LPS administration in DPSCs. DPSCs were infected with NC lentivirus particles and let-7c-5p expression lentivirus particles. At 72 h after the infection, the cells were incubated with 10 μg/ml LPS for 24 h to induce inflammatory response. The total protein of cells was extracted and the expression of DMP1 and members of NF-κB pathway was detected with Western blotting analysis. The representative images and quantitative analysis results showed that LPS administration increased the expression of DMP1, p-IκBα, p-IKKβ, and the nuclear translocation of NF-κB p65, while decreasing the expression of p-IκBα, showing activation of pro-inflammatory NF-κB signaling. Induced expression of let-7c-5p reversed the expression patterns of the above indicators in DPSCs. * P<0.05 vs. Blank group. # P<0.05 vs. LPS or LPS+NC group. Each assay was represented by 3 replicates.
DMP1 inhibition mediated the inflammation suppressing function of let-7c-5p
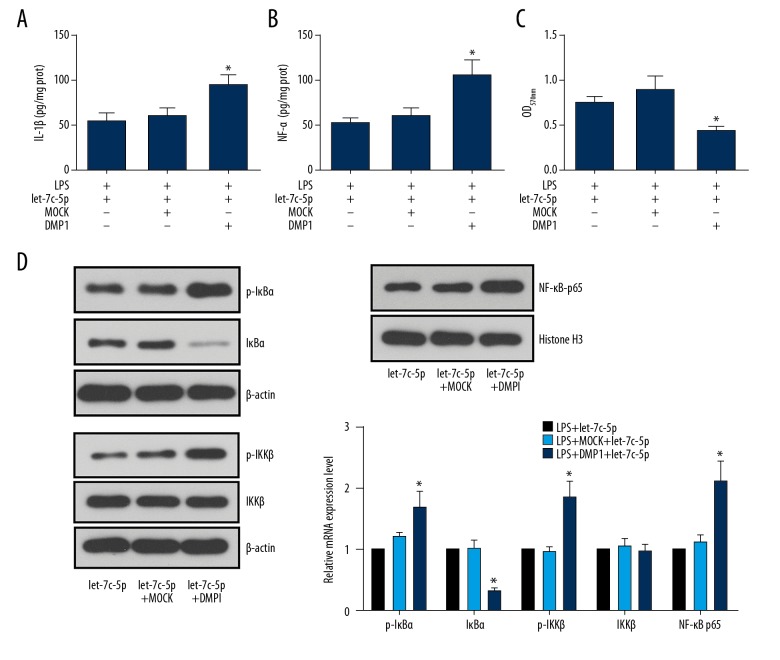
To explicitly explain the role of DMP1 in the inflammation-suppressing effect of let-7c-5p, the let-7c-5p-expressed DPSCs were further treated with DMP1 protein. The results showed that the inhibited production of IL-1β and TNF-α by let-7c-5p was again induced by DMP1 (Figure 3A). DMP1 also impaired the let-7c-5p’s effect on DPSC viability, significantly decreasing the OD570 value when compared with the let-7c-5p group (Figure 3B). Regarding NF-κB signaling, the administration of DMP1 increased the expressions of DMP1, p-IκBα, and p-IKKβ, and nuclear translocation of NF-κB p65 but decreased the level of IκBα even in the presence of let-7c-5p overexpression (Figure 3C).
Figure 3.
let-7c-5p inhibited activation of the NF-κB pathway in a DMP1-inhibition-dependent manner. DPSCs with stable let-7c-5p expression were first incubated with 10 μg/ml LPS for 24 h and then subjected to 10 ng/ml DMP1 protein for 6 h. The production of IL-1β and TNF-α, the cell viability, and the expression of DMP1 and NF-κB pathway members were detected with ELISA, MTT assay, and Western blotting, respectively. Administration of DPSCs with DMP1 protein counteracted the effect of let-7c-5p on LPS-induced impairments in DPSCs, increasing the production of IL-1β (A) and TNF-α (B), decreasing cell viability (C), and activating NF-κB signaling. * P<0.05 vs. let-7c-5p group. Each assay was represented by 3 replicates.
Let-7c-5p attenuated the LPS-induced pulpitis by inhibiting DMP1/NF-κB signaling in SD rats
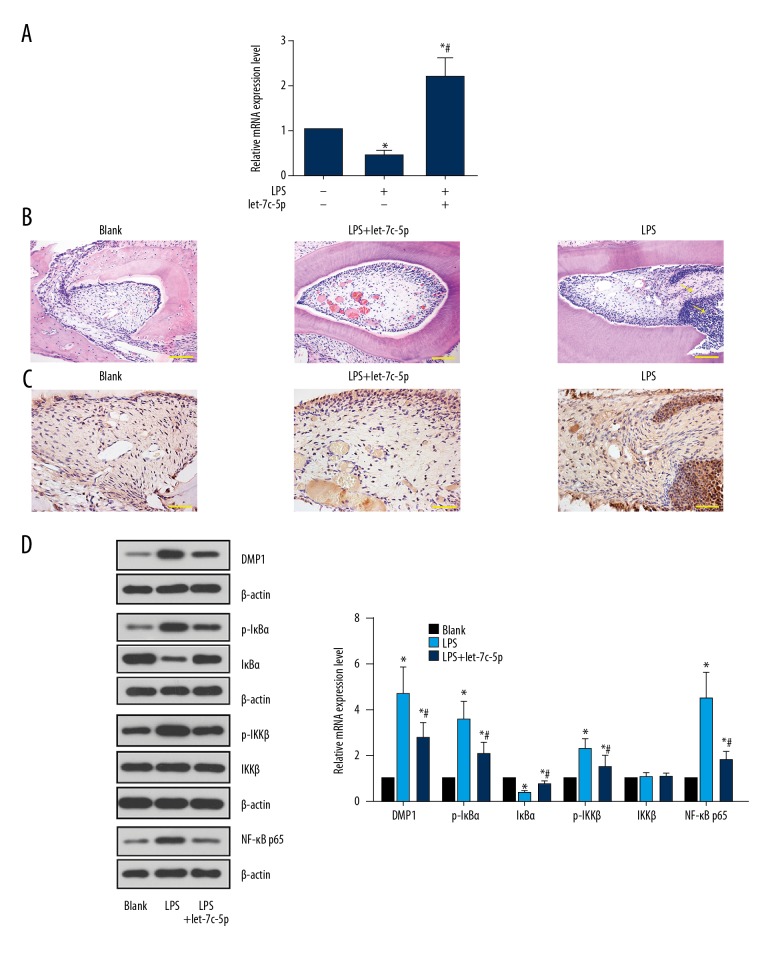
Rats were first injected with let-7c-5p agomir (Figure 4A) and subjected to pulpitis induction by LPS. The results of H&E staining showed a higher level of neutrophils infiltration in the LPS group when compared with the Blank group (Figure 4B), showing the induction of inflammation in dental pulp tissues of rats. Similar to the results of in vitro assays, the expression and distribution of DMP1 (stained dark brown in IHC) was expanded in dental pulp tissues by the LPS administration (Figure 4C, 4D), which led to the activation of NF-κB signaling (Figure 4D). In rats pre-injected with let-7c-5p agomir, the effect of LPS was diminished; the lower level of neutrophil infiltration was accompanied by the suppressed activity of DMP1/NF-κB signaling (Figure 4D), further confirming the DPSC-protecting effect of let-7c-5p during pulpitis.
Figure 4.
let-7c-5p ameliorated LPS-induced pulpitis by suppressing DMP1-mediated NF-κB signaling in vivo. SD rats were first injected with NC or let-7c-5p agomir and then subjected to pulpitis induction using LPS. At 9 h after LPS administration, rats were sacrificed and the dental tissues were collected for H&E staining, immunochemistry, RT2-PCR, and Western blot analysis. LPS decreased the level of let-7c-5p in dental tissues and injection of let-7c-5p agomir restored the level (A). As shown by H&E staining, LPS induced neutrophil infiltration in dental tissues and let-7c-5p agomir inhibited the LPS-induced neutrophils infiltration (B). LPS also increased the expression and distribution of DMP1 (C, D), which further induced the activation of NF-κB signaling (D). However, increased expression of let-7c-5p by agomir injection effectively inhibited the LPS-induced activation of DMP1/NF-κB signaling transduction. * P<0.05 vs. Blank group. # P<0.05 vs. LPS group. Arrows indicate neutrophil infiltration detected by H&E staining. Scale bar for H&E staining, 100 μm. Scale bar for immunochemistry, 50 μm. Each assay was represented by 6 replicates.
Discussion
The role of miR in regulating inflammatory response is complex in that a single miR member can have diverse targets. Regarding the interaction between miRs and inflammation in dental pulp tissues, different studies have identified hundreds of dys-expressed miR molecules, of which let-7c-5p has been proposed as an anti-inflammation factor during the onset of pulpitis [7,14]. In the present study, the possible role of let-7c-5p in protecting dental pulp tissues against inflammation was further investigated and the results showed that miR could attenuate LPS-induced dental pulp cell inflammation both in vitro and in vivo. Moreover, our results also preliminarily explained the mechanism driving the anti-inflammation effect of let-7c-5p: the miR blocked NF-κB signaling activation through a DMP1-inhibition manner.
The conserved let-7 miR was first identified in Caenorhabditis elegans as a switch inducing cells to exit the cell cycle when C. elegans reaches adulthood [15–17]. Similar to that in C. elegans, the expression of let-7 in human is generally detectable after tissue differentiation [18]. More recently, the members of the let-7 family have been shown to play a suppressing role in the progression of cancers by directly inhibiting the function of oncogenes such as Ras, HMGA2, and c-Myc [19–22]. As a typical isoforms of the let-7 family, let-7c-5p is reported to determine the intrinsic chemo-resistance of renal cell carcinoma [23] and acts as a suppresser of breast cancer [24]. Moreover, let-7c-5p can also protect brain tissues against ischemia injuries by inhibiting the activation of microglia [25]. The dys-expression of let-7c-5p in inflammatory dental pulp tissues was also previously reported [7,14], indicating the possible role of the molecule in antagonizing the inflammation during pulpitis. Our study provides preliminary evidence for the hypothesis that the administration of LPS inhibits the expression of let-7c-5p both in vivo and in vitro, and the induced expression of let-7c-5p attenuates impairment of dental pulp cells due to LPS administration. The overexpression of let-7c-5p suppressed the production of pro-inflammatory cytokines, which led to the restored viability of DPSCs and weaker neutrophil infiltration in dental tissues. At the molecular level, the overexpression of let-7c-5p also inhibited the LPS-induced activation of NF-κB signaling by inhibiting the phosphorylation of IκBα and IKKβ and increasing the expression of total IκBα, hence suppressing the nuclear translocation of NF-κB p65. The results confirmed the anti-inflammation function of let-7c-5p.
To further explain the mechanism driving the effect of let-7c-5p on dental pulp inflammation, we focused on the function of DMP1. It was previously reported that DMP1 is a direct target of let-7c-5p [9] and DMP1 is induced during pulpitis [13]. The molecule is also involved in the regulation of mineralization of bone and dentin [9]. The deficiency of DMP1 causes hypomineralization and dental deformity in model animals [26]. However, the over-production of DMP1 is associated with augmented inflammatory response in dental pulp tissues [13], suggesting the possibility that the factor participates in the development of inflammatory responses in dental pulp tissues. Given the interaction between let-7c-5p and DMP1, it is reasonable to hypothesize that the anti-inflammation function of let-7c-5p depends on the inhibition of DMP1 function. Therefore, let-7c-5p-overexpressed DPSCs were subjected to DMP1 protein incubation. Higher DMP1 levels counteracted the anti-inflammation effect of let-7c-5p, increasing the production of pro-inflammation cytokines, and decreasing DPSC viability. Moreover, we found that the administration of DMP1 activated NF-κB signaling, partially explaining the pathway through which DMP1 participates in the inflammatory responses in dental pulp tissues. These results help explain the mechanism through which let-7c-5p inhibits the NF-κB pathway, but further research is needed to clearly define the interaction between DMP1 and the NF-κB pathway.
Conclusions
let-7c-5p was shown to protect dental pulp cells against LPS-induced inflammation and the effect was exerted by inhibiting DMP1-mediated NF-κB pathway activation. However, a full elucidation of the role of DMP1 in the inflammation of dental pulp tissues is needed to elucidate the downstream pathway transducing the anti-inflammation effect of let-7c-5p and to identify novel therapeutic targets for treating dental inflammatory disorders.
Supplementary Figure
Identification of DPSCs with immunofluorescence. DPSCs were isolated from dental tissues of SD rats and the molecular surface antigen markers in the cells were analyzed with immunofluorescence. The results showed that cells were positive for CD90, CD105, CD29, and CD146 while negative for CD34 and CD45. Magnification, 400×.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Hahn C-L, Liewehr FR. Innate immune responses of the dental pulp to caries. J Endodont. 2007;33(6):643–51. doi: 10.1016/j.joen.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.He X, Jiang W, Luo Z, et al. IFN-γ regulates human dental pulp stem cells behavior via NF-κB and MAPK signaling. Sci Rep-UK. 2017;7:40681. doi: 10.1038/srep40681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper PR, Takahashi Y, Graham LW, et al. Inflammation – regeneration interplay in the dentine – pulp complex. J Dent. 2010;38(3):687–97. doi: 10.1016/j.jdent.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Elsevier; 2008. pp. 131–40. [DOI] [PubMed] [Google Scholar]
- 5.Sugatani T, Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem. 2009;284(7):4667–78. doi: 10.1074/jbc.M805777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mott JL, Mohr AM. Overview of MicroRNA biology. Semin Liver Dis. 2015;35(1):3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong S, Zhang S, Bair E, et al. Differential expression of microRNAs in normal and inflamed human pulps. J Endodont. 2012;38(6):746–52. doi: 10.1016/j.joen.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Nishino J, Kim I, Chada K, et al. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16 Ink4a and p19 Arf expression. Cell. 2008;135(2):227–39. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue J, Wu B, Gao J, et al. DMP1 is a target of let-7 in dental pulp cells. Int J Mol Med. 2012;30(2):295–301. doi: 10.3892/ijmm.2012.982. [DOI] [PubMed] [Google Scholar]
- 10.George A, Sabsay B, Simonian PA, et al. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268(17):12624–30. [PubMed] [Google Scholar]
- 11.Weng Y, Liu Y, Du H, et al. Glycosylation of DMP1 is essential for chondrogenesis of condylar cartilage. J Dent Res. 2017;96(13):1535–45. doi: 10.1177/0022034517717485. [DOI] [PubMed] [Google Scholar]
- 12.Pan M, Weng Y, Sun Y. Overexpression of Dentin matrix protein 1 in Nestin(+) cells causes bone loss in mouse long bone. Biochem Biophys Res Commun. 2017;490(2):356–63. doi: 10.1016/j.bbrc.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 13.Abd-Elmeguid A, Donald CY, Kline LW, et al. Dentin matrix protein-1 activates dental pulp fibroblasts. J Endodont. 2012;38(1):75–80. doi: 10.1016/j.joen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Sehic A, Tulek A, Khuu C, et al. Regulatory roles of microRNAs in human dental tissues. Gene. 2017;596:9–18. doi: 10.1016/j.gene.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Abbott AL. Uncovering new functions for microRNAs in Caenorhabditis elegans. Curr Biol. 2011;21(17):R668–71. doi: 10.1016/j.cub.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson SM, Lin S-Y, Slack FJ. The time of appearance of the C. elegans let-7 microRNA is transcriptionally controlled utilizing a temporal regulatory element in its promoter. Dev Biol. 2003;259(2):364–79. doi: 10.1016/s0012-1606(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 17.Abbott AL, Alvarez-Saavedra E, Miska EA, et al. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9(3):403–14. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin H, Lv S, Yang J, et al. Use of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cells. PLoS One. 2011;6(7):e21307. doi: 10.1371/journal.pone.0021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boominathan L. The guardians of the genome (p53, TA-p73, and TA-p63) are regulators of tumor suppressor miRNAs network. Cancer Metastasis Rev. 2010;29(4):613–39. doi: 10.1007/s10555-010-9257-9. [DOI] [PubMed] [Google Scholar]
- 20.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–30. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Tang Y, Cui H, et al. Let-7/miR-98 regulate Fas and Fas-mediated apoptosis. Genes Immun. 2011;12(2):149–54. doi: 10.1038/gene.2010.53. [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Mo R, Ma J, et al. let-7b and let-7c are determinants of intrinsic chemoresistance in renal cell carcinoma. World J Surg Oncol. 2015;13(1):175. doi: 10.1186/s12957-015-0596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Mao X, Wang Y, et al. Let-7c-5p inhibits cell proliferation and induces cell apoptosis by targeting ERCC6 in breast cancer. Oncol Rep. 2017;38(3):1851–56. doi: 10.3892/or.2017.5839. [DOI] [PubMed] [Google Scholar]
- 25.Ni J, Wang X, Chen S, et al. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav Immun. 2015;49:75–85. doi: 10.1016/j.bbi.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Hirst KL, Ibaraki-O’Connor K, Young MF, et al. Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J Dent Res. 1997;76(3):754–60. doi: 10.1177/00220345970760030701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of DPSCs with immunofluorescence. DPSCs were isolated from dental tissues of SD rats and the molecular surface antigen markers in the cells were analyzed with immunofluorescence. The results showed that cells were positive for CD90, CD105, CD29, and CD146 while negative for CD34 and CD45. Magnification, 400×.