Introduction
Colorectal cancer remains the 2nd most common malignancy diagnosed worldwide and accounts for over 100,000 new cases annually in the United States. [1] Current treatment methods typically involve surgical resection with selective use of radiation and chemotherapy depending on disease site and stage. Some of the greatest gains in survival, however, have come from aggressive endoscopic screening. In particular, colonoscopy has reduced the severity of colorectal malignant disease while also providing therapeutic options including resection of early neoplastic disease. [2]
However, several scenarios continue to complicate endoscopic surveillance: identification and assessment of flat neoplasia like sessile polyps and oncologic restaging following neoadjuvant therapy; these can harbor early cancers but remain difficult to identify with standard endoscopic techniques. Additionally, quantitative evaluation of the mucosa for residual rectal cancer after neoadjuvant treatment remains exceedingly difficult using flexible or rigid endoscopic technology. Differentiating a sterile scar from occult residual malignancy is one of the greatest factors limiting adoption of nonoperative strategies for rectal cancer patients. [3]
White light endoscopy (WLE), the gold standard for endoscopic colorectal screening, can only visualize macroscopic morphology and provides no functional assessment of imaged tissue. None of the current optical imaging modalities, such as fluorescence imaging, optical coherence tomography (OCT) and diffuse reflectance spectroscopy (DRS) offer both the high resolution and functional assessments needed to detect small islands of malignant tissue in the setting of early cancer or post-treatment evaluation.
Therefore, a critical need exists for additional endoscopic techniques to detect neoplasia in the colorectal mucosa. One potential solution is an alternative imaging technology, spatial frequency domain imaging (SFDI). SFDI is an emerging label-free, wide field, quantitative optical imaging technique which can provide rapid quantitative absorption and scattering information associated with early neoplastic changes. SFDI has been used for various therapeutic applications such as margin detection in breast and ovarian cancers. [4,5]
The aim of this study was to evaluate the novel application of SFDI in ex vivo human colon tissue specimens and demonstrate the relationship between spectroscopic tissue properties and histology within both normal and neoplastic colon tissue.
Materials and methods
Freshly resected colon samples obtained from patients undergoing surgery at Washington University School of Medicine were imaged immediately after surgery. The study was approved by the Institutional Review Board (IRB) at Washington University, and informed consent was obtained from all patients. Each specimen was opened longitudinally in the operating room, rinsed with sterile water to remove any blood or debris from the mucosal surface, and secured to backing with pins. This exposed the mucosal surface of the organ for imaging. Immediately upon completion of imaging, the samples were placed in formalin and returned to the pathology department for histological processing.
Sinusoidal patterns were projected from a miniaturized projector (RIF6 cube, dimension 2”x2”x1.9”, brightness 50 lumens). The reflected light was recorded by a 12 bit CCD camera (Basler ace, 1296 × 966 pixels, 30 fps, dynamic range 57 dB). For the current study, three wavelengths (460 nm, 530 nm and 630 nm) and two spatial frequencies (0 cm−1 and 1 cm−1) were used. Using a three phase shifting algorithm, the demodulated amplitude image was reconstructed, and the corresponding absorption as well as scattering maps of the tissue samples were reconstructed from pre-computed lookup table. The performance accuracy of the SFDI system was tested with tissue mimicking phantoms of known optical property. The imaging field of view (FOV) was 4 cm x 4 cm, and the average penetration depth at 630 nm was around 3.5 mm. The overall acquisition time for all three wavelengths was around 3–5 seconds.
A total of 9 patients enrolled in the pilot study, and 15 (8 malignant, 7 normal) regions were imaged using the SFDI system. The majority of patients underwent hemicolectomy for cancer and were found to have malignancy on histologic analysis (Table 1).
Table 1.
Patient ID and surgical pathology
| Patient ID | Surgery | Pathology |
|---|---|---|
| 1 | Right hemicolectomy | Intermittently well and poorly differentiated signet ring (T2) |
| 2 | Right hemicolectomy | Moderately to poorly differentiated adenocarcinoma (T3) |
| 3 | Right hemicolectomy | Well-differentiated adenocarcinoma (T3N1) |
| 4 | Left hemicolectomy | Moderately differentiated adenocarcinoma (T3N0) |
| 5 | Right hemicolectomy | No abnormality |
| 6 | Left hemicolectomy | Moderately differentiated adenocarcinoma (T3) |
| 7 | Subtotal colectomy | Well differentiated adenocarcinoma (T2) |
| 8 | Left hemicolectomy | Moderately differentiated adenocarcinoma (T2) |
| 9 | Right hemicolectomy | Adenocarcinoma, moderately differentiated in tubular adenoma (T1) |
T primary tumor, N regional lymph nodes
Depending on the sample size, each SFDI image was subdivided into several non-overlapping regions of interest (ROI) for extracting quantitative spectral as well as spatial features, resulting in a total of 51 nonoverlapping ROIs. Spectral features based on light absorption and scattering included total hemoglobin (tHb), scatter amplitude and scatter spectral slope. Oxygen saturation was not found to be a significant ex vivo feature, mainly due to non-perfusion of the resected specimen. Two additional features related to the spatial heterogeneity of the optical absorption and scattering were extracted from the SFDI images. [10]
Statistical analysis
To determine the strength of association between each SFDI feature and the histology of the imaged specimen, logistic regression model was used. All statistical tests were performed using MATLAB version 2016. The five spectral and spatial features were used as predictor variables, and the diagnosis from pathology was used as response variable (0 for normal, 1 for cancer). The receiver operating curve (ROC) and the area under the curve (AUC) was used to test the performance accuracy of the logistic classifier.
Results
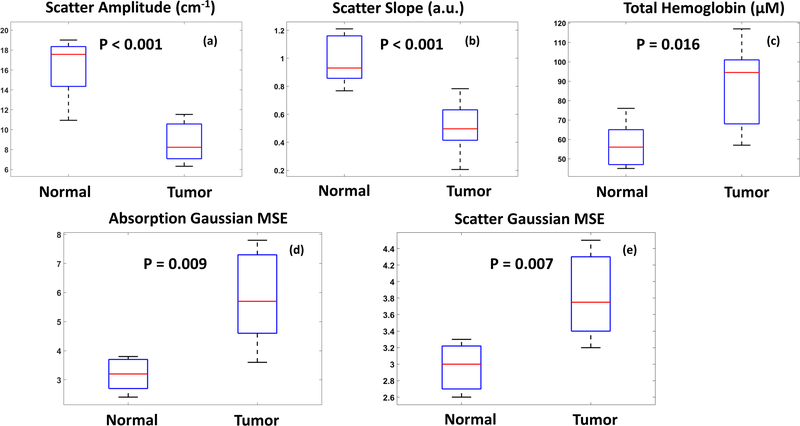
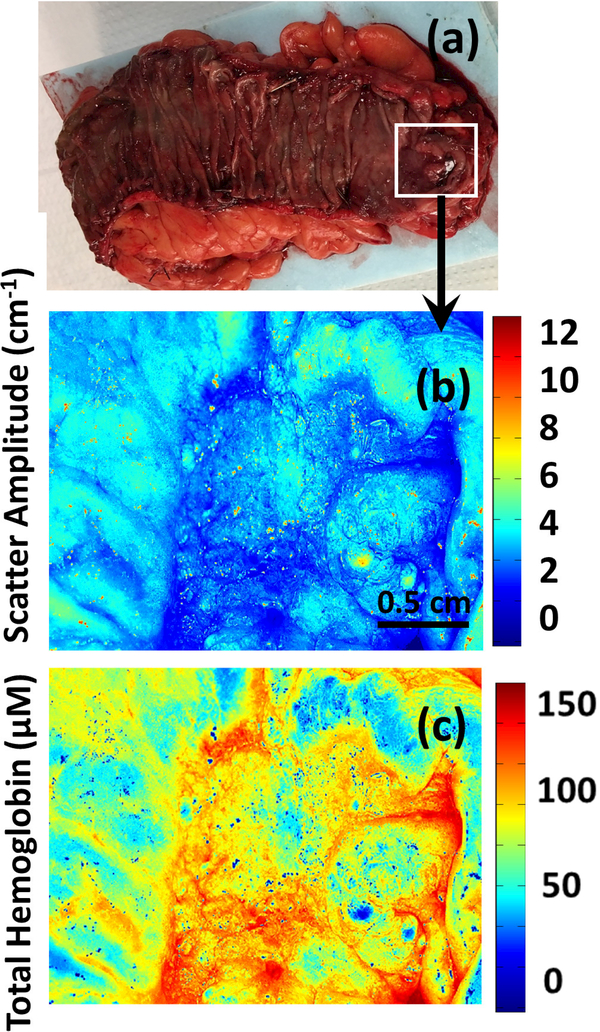
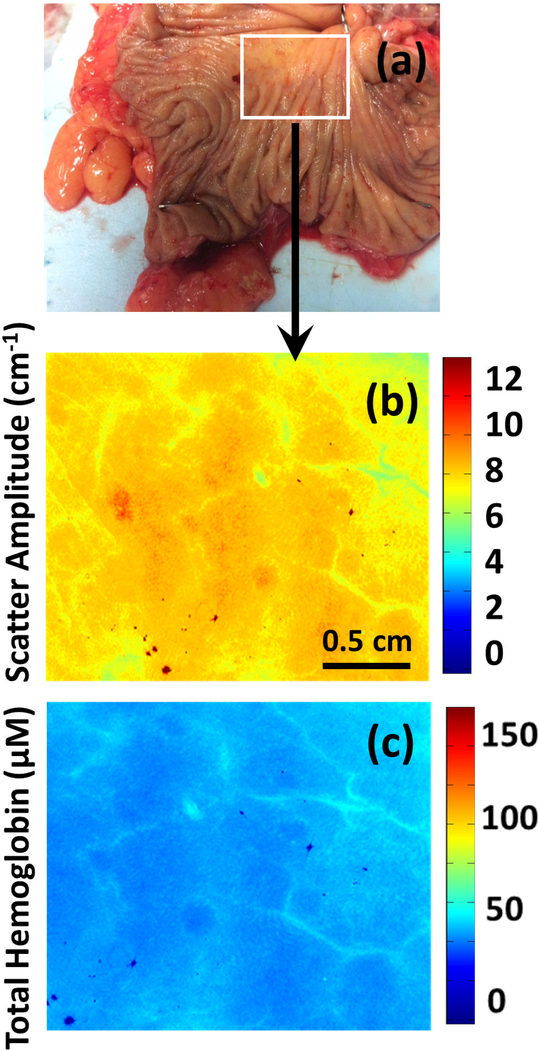
In general, malignant tissue demonstrated several distinct features compared to normal colon when imaged with SDFI. tHb concentrations tended to be elevated in tumors, while light scattering tended to be significantly decreased (Figures 1–4). Additionally, tumor tissue was highly heterogeneous and unorganized with significant intra-tumor variation of both absorption and scattering (Figure 5).
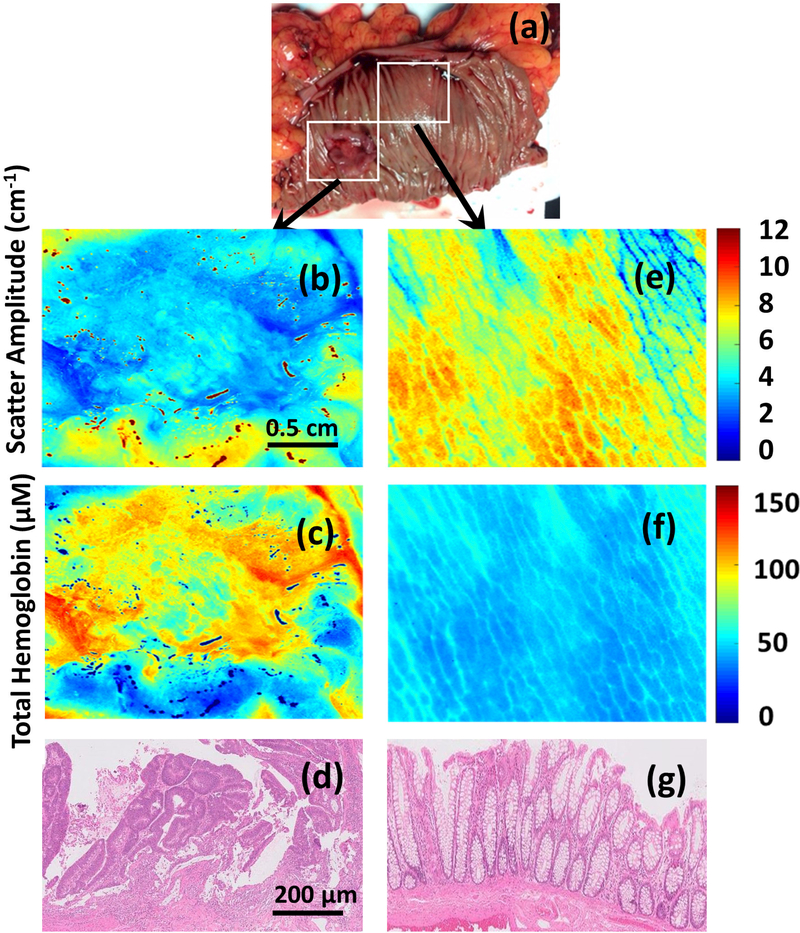
Figure 1.
(a-g) Photograph, scatter amplitude map and total hemoglobin (HbT) map of a moderately differentiated adenocarcinoma (Images b-d) and corresponding normal tissue (images e-g).
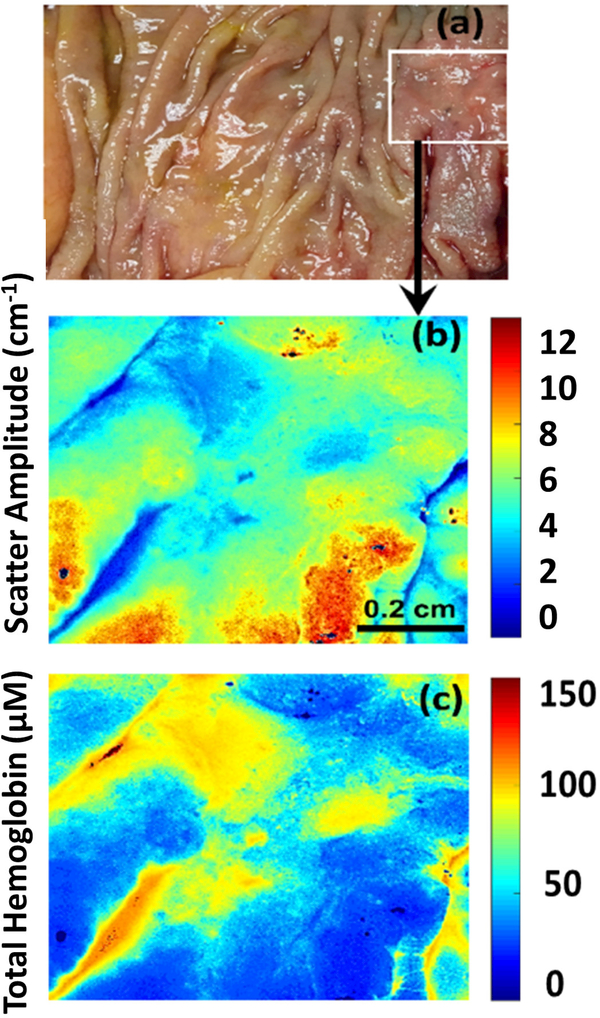
Figure 4.
(a-c) Photograph, scatter amplitude map and total hemoglobin (HbT) map of moderately differentiated adenocarcinoma in tubular adenoma (T1).
Figure 5.
Boxplot of the spectral and spatial features.
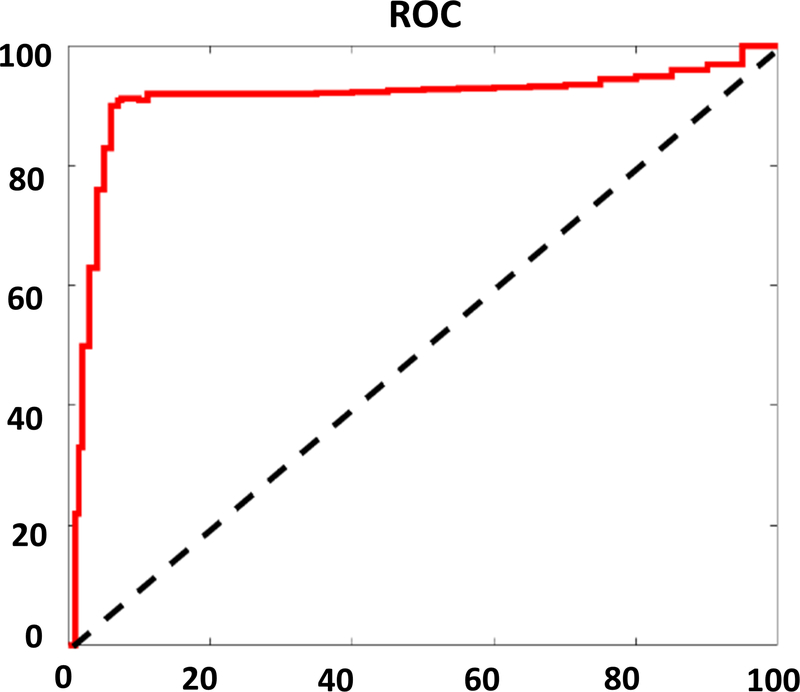
All the ROIs were separated into two groups based on underlying histology classification. 31 images (17 tumor, 14 normal) were used to develop the regression model using the 5 aforementioned classifiers, and the remaining 20 images (11 tumor, 9 normal) were used for model calibration testing. The ROC demonstrated an optimum sensitivity of 90.91%, specificity of 88.89% and accuracy of 90% (Figure 6). The mean AUC of the regression model was 0.902.
Figure 6.
Receiver operating curve using all five features
Discussion
The quantitative images of tumors captured by SFDI in this pilot study demonstrate drastically different characteristics from normal mucosa. Elevated tHb concentrations among tumors, potentially due to malignant angiogenesis, was uniformly noted along with decreased scatter. In fact, these findings correlate well with histologic findings in these tumors as well; increased microvascular proliferation was found among multiple specimens on pathologic review.
Additionally, we found drastically altered mucosal morphology among the neoplastic images in comparison to normal tissue. In contrast to the regular, homogenous patterns noted among the benign images, the neoplasia showed no particular pattern or regularity. This too was corroborated on histological exam; the normal structure of the colonic mucosa and submucosa was disrupted by invasive carcinoma.
There were several limitations of this pilot study. The number of samples was limited, all imaged tumors were greater than 1cm in size, and there were no pre-cancerous lesions such as polyps included in the current data set. Additionally, the absorption parameters such as tHb and absorption heterogeneity can be affected by surface ulceration, hemorrhage or reduced blood supply after surgery. Thus, these factors may perform differently in in vivo imaging settings. Furthermore, only visible wavelengths were used in the current study, where blood hemoglobin is the dominant chromophore. Alternative wavelengths, such as near infrared, may yield additional information about other chromophores such as lipid, collagen and water content. These may also be important prognosticators of neoplastic changes related to tumor growth and metabolism.
Conclusions
Our results demonstrate both the feasibility of functional imaging based on light absorption and the high discriminatory capability of SFDI parameters in differentiating normal from neoplastic tissue. Further exploratory studies of the SFDI system for screening or diagnostic colonoscopy, targeted biopsy, or post treatment evaluation of rectal or colonic neoplasia must be performed, and the device must be tested in vivo to evaluate the clinical applicability of these promising results.
Figure 2.
(a-c) Photograph, scatter amplitude map and total hemoglobin (HbT) map of a moderately differentiated adenocarcinoma (T3).
Figure 3.
(a-c) Photograph, scatter amplitude map and total hemoglobin (HbT) map of a colon sample with no histopathologic abnormalities.
Acknowledgements:
This work was supported in part by National Institutes of Health R01CA151570 (QZ) and T32 CA009621 (WC).
Footnotes
The research protocol described herein was approved by Institutional Review Board (IRB# 201707066).
Conflict of Interest: The authors declare that they have no conflict of interest.
References:
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Pan J, Xin L, Ma YF, Hu LH, Li ZS. (2016) Colonoscopy Reduces Colorectal Cancer Incidence and Mortality in Patients With Non-Malignant Findings: A Meta-Analysis. The American journal of gastroenterology;111(3):355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rex DK, Ahnen DJ, Baron JA, et al. (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. The American journal of gastroenterology. 107(9):13151329; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughney AM, Krishnaswamy V, Rizzo EJ, et al. (2013); Spectral discrimination of breast pathologies in situ using spatial frequency domain imaging. Breast Cancer Research :BCR 15(4):R61. doi: 10.1186/bcr3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandy S, Hagemann I S, Powell M A, Siegel C, and Zhu Q (2018) Quantitative multispectral ex vivo optical evaluation of human ovarian tissue using spatial frequency domain imaging, Biomed. Opt. Express 9 (5), 2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]