Abstract
Background
Patients with acute respiratory distress syndrome (ARDS) who develop acute kidney injury have increased mortality and frequently require renal replacement therapy (RRT). The optimal timing for initiation of RRT after onset of ARDS to improve survival is not known.
Methods
We retrospectively reviewed clinical data on patients admitted to our health system over a 2-year period. Individual charts were carefully reviewed to ascertain that patients met the Berlin criteria for ARDS and to categorize RRT utilization. The Kaplan–Meier analysis was conducted to compare early (£48 hours postintubation) versus late (>48 hours postintubation) initiation of RRT. Associations between RRT initiation and mortality were evaluated using Cox proportional hazards regression.
Results
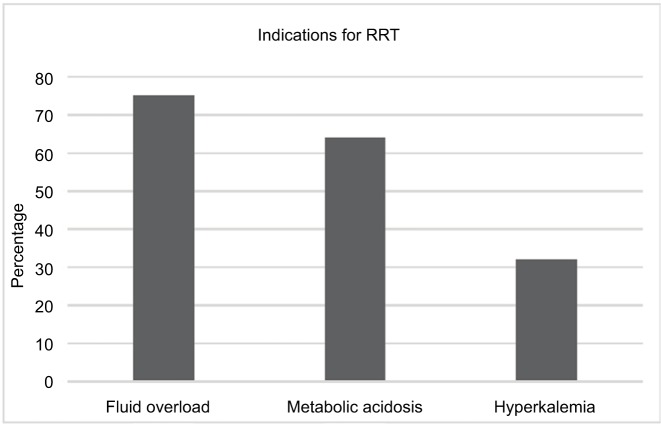
A total of 75 patients were identified with ARDS, 95% of whom received RRT. Mortality of patients who required RRT was 56%. The main indications for RRT initiation were fluid overload (75%), metabolic acidosis (64%), and hyperkalemia (33%). The Kaplan–Meier analysis comparing early initiation of RRT to late initiation of RRT showed no survival benefit. Cox proportional hazard models testing the association between timing of RRT initiation with survival and adjusting for sex, race, ethnicity, and Acute Physiology and Chronic Health Evaluation II score did not reach statistical significance (HR=0.94, 95% CI=0.48–1.86).
Conclusion
Timing of RRT initiation was not associated with a survival benefit. Prospective study in the utilization and outcomes of RRT in ARDS could assist in optimizing its usage in this population.
Keywords: acute respiratory distress syndrome, AKI, dialysis, intensive care, renal
Introduction
Acute respiratory distress syndrome (ARDS), a severe form of acute respiratory failure, is characterized by a disruption of the endothelial barrier of capillaries lining the alveoli with increased permeability, leading to an intense inflammatory insult, alveolar epithelial injury, and influx of a protein-rich edema fluid into the alveoli and causing defective gas exchange, decreased lung compliance and increased work of breathing.1,2 Up to 35% of patients with ARDS develop acute kidney injury (AKI) during the course of stay in the intensive care unit (ICU), with multiple studies over the past 20 years describing a concomitant relationship between acute respiratory failure, including ARDS, and increased mortality in patients who develop AKI.3,4 Extracorporeal renal replacement therapy (RRT) has been used as a supportive treatment in the setting of AKI and has traditionally focused on averting the life-threatening derangements associated with kidney failure, including metabolic acidosis, hyperkalemia, uremia, and/or fluid overload, while allowing time for organ recovery.5
A critical decision in the management of patients with ARDS is when to initiate RRT. There are currently numerous clinical, biochemical, and physiological factors that are considered when deciding to initiate RRT. In routine clinical practice, several factors such as availability of personnel and equipment and placement of appropriate vascular access influence the timing of RRT initiation once patients develop ARDS. The ideal time to initiate RRT in ARDS patients who develop AKI has not been determined. The few studies examining this question have not agreed on what should constitute early versus late in terms of the event that decides early versus late initiation of RRT. Some studies have used admission to the ICU, whereas others have used onset of ARDS.1,6 A recent study prospectively evaluated patients with ARDS of various etiologies for the effect of early initiation of RRT (<12 hours after diagnosis of ARDS versus >48 hours) on mortality.1 Although it did not show a statistically significant decrease in mortality with early initiation of RRT, it did show an improvement in terms of oxygenation and decreased ventilator days. Our objective was to determine the ideal time to initiate RRT in patients with ARDS using time of intubation as the reference event. We hypothesized that early initiation of RRT in patients with ARDS (within 48 hours of intubation) would improve in-hospital mortality when compared with late initiation (>48 hours postintubation).
Methods
Subjects and study design
This was a retrospective chart review using a convenience sample of patients aged 18 years or older who were admitted to any ICU (medical, surgical, or trauma) at Banner – University Medical Center Tucson and Banner – University Medical Center South from November 1, 2013, to October 31, 2015, with a diagnosis of ARDS according to the Berlin criteria.7 The exclusion criteria are represented in Table 1. The institutional review board (IRB) of the University of Arizona approved this study. All the information collected were recorded in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects. Additionally, the study used only existing data, documents, or records, and no information prior to November 1, 2013, was collected. Hence, a waiver of authorization for consent was granted by the IRB.
Table 1.
Exclusion criteria
| Patients with ARDS who required use of extracorporal membrane oxygenation during admission |
| Patients with a diagnosis of respiratory failure from CHF |
| Patients with acute exacerbation of COPD or asthma |
| Patients who required RRT prior to intubation |
| Patients with advanced CKD prior to admission (CKD stage 5, estimated glomerular filtration rate <15 mL/min/m2 based on the Modification of Diet in Renal Disease study equation) |
Abbreviations: ARDS, acute respiratory distress syndrome; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disorder; RRT, renal replacement therapy; CKD, chronic kidney disease.
Study procedures
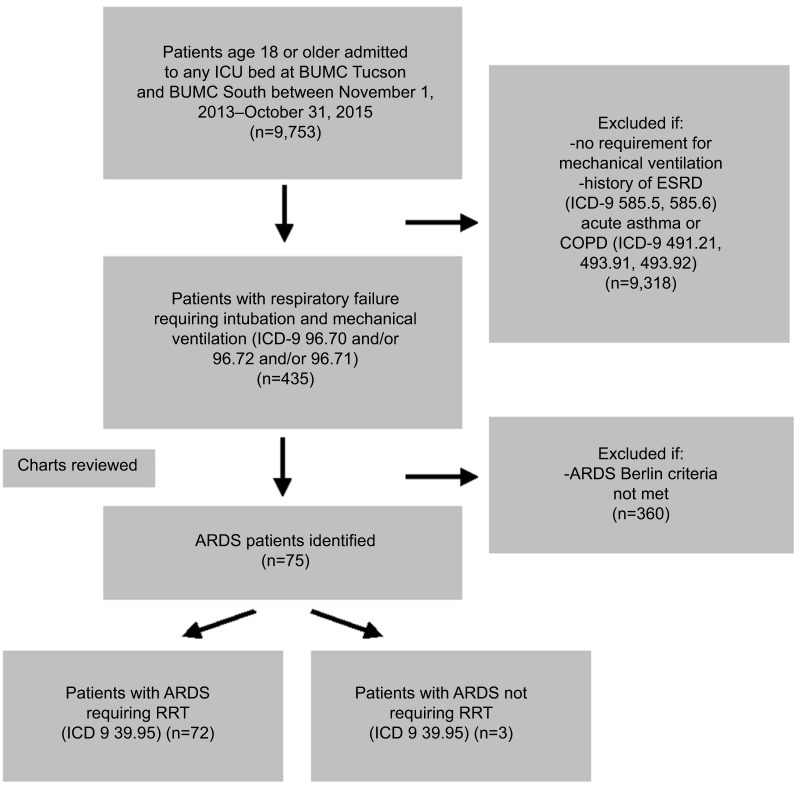
Patients were identified from the electronic health record (EHR) utilizing our Biomedical Informatics Services database that has a collaborative relationship between the University of Health Sciences and Banner–University Medicine. International Classification of Diseases, ninth revision, clinical modification (ICD-9 CM) codes were used to identify specific patients based on our disease-specific algorithm (Figure 1). Patients who required mechanical ventilation (ICD-9 96.70, 96.71, 96.72) and had ARDS based on Berlin criteria were identified. Those who had a history of end-stage renal disease or stage 5 chronic kidney disease (ICD-9 585.5, 585.6) and/or acute exacerbation of asthma or chronic obstructive pulmonary disorder (491.21, 493.91, 493.92) were excluded. The same algorithm was used without ICD-9 585.5 and 585.6 to identify non-RRT patients. Three pulmonary and critical fellows then carefully reviewed individual charts based on prespecified criteria and definitions as mentioned earlier. Patients who met criteria for ARDS were then categorized into those who required RRT and those who did not. Chart review was done to obtain demographic information, past medical history, and other clinical characteristics, such as Acute Physiology and Chronic Health Evaluation II (APACHE) II data, partial pressure of oxygen in arterial blood, and percentage of inspired oxygen at the time of intubation. For each patient, the time of intubation was considered as zero when determining time to initiation of RRT. Patients who were started on RRT within 48 hours postintubation were regarded as early RRT and those who were started on RRT at >48 hours postintubation were regarded as late RRT. A nephrology attending and fellow reviewed all RRT patient charts, and indications were identified retrospectively based on objective data and clinician judgment as documented in the EHR. Acute kidney injury (AKI) was defined based on AKI network classification.
Figure 1.
Flow diagram of algorithm used to select patients for chart review.
Note: Any ICU bed includes medical, surgical/trauma, cardiothoracic, or cardiac care.
Abbreviations: ICU, intensive care unit; BUMC, Banner University Medical Center; ESRD, end-stage renal disease; ICD, International Classification of Diseases; COPD, chronic obstructive pulmonary disorder; ARDS, acute respiratory distress syndrome; RRT, renal replacement therapy.
Statistical analysis
Chi-square test was used to compare categorical variables and analysis of variance for continuous data.8 The primary outcome was survival at hospital discharge. Survival curves were constructed using the Kaplan–Meier analysis. We compared patients based on early (≤48 hours) versus late (>48 hours) initiation of RRT. Tests of equality for survival distributions was done using logrank (Mantel–Cox), Breslow (generalized Wilcoxon), and Tarone–Ware tests.9 Cox proportional hazards models were used to estimate HRs and 95% CIs for the association between timing of RRT initiation and mortality in ARDS patients. Potential confounders examined from the medical chart included demographic information, presence of comorbidities, APACHE II score, creatinine, and RRT indication. The software used for analysis were IBM Statistics for Macintosh SPSS, Version 24.0 (IBM Corporation, Armonk, NY, USA) and Stata 14.0 (StataCorp LP, College Station, TX, USA).
Results
Patient demographics
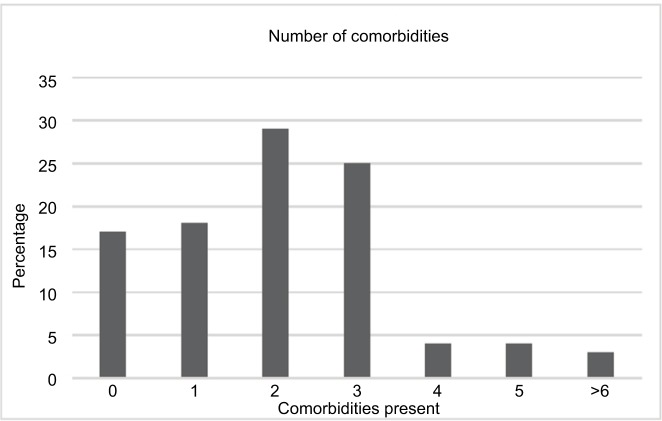
A total of 75 patients were identified using our database algorithm and chart review (Figure 1). In all, 72 (96%) patients underwent RRT during their course of treatment in the ICU. In the RRT group, the patients were 46% female, 79% white, and 38% Latino with a median age of 57 years (interquartile range [IQR] 47–67 years) and a mortality rate of 56% (Table 2). Median values for patients who received RRT were PaO2/FiO2 at intubation of 116 (IQR 68.5–163.6) and APACHE II score at ICU admission of 26 (IQR 20–32). In all, 57 (79%) patients had two or more comorbidities (Figure 2). The most common diagnosis was hypertension, which was present in 50% of patients, with the second most common disease being liver disease (36%) (Table 2).
Table 2.
Characteristics of ARDS patient who received ARDS compared with those who did not
| Characteristics | ARDS with RRT (n=72) | ARDS without RRT (n=3) |
|---|---|---|
| Median values with IQR | ||
| Age (years) | 57 (47–67) | 59 (43–75) |
| Height (cm) | 170 (164–176) | 172 (165–179) |
| Weight (kg) | 79 (67–92) | 82 (66–98) |
| PaO2 (mmHg)a | 87 (59–115) | 90.5 (78.5–102.5) |
| FiO2a | 1.0 (0.83–1.17) | 0.7 (0.58–0.83) |
| PaO2/FiO2a | 116 (68.5–163.5) | 116.8 (105.5–128) |
| APACHE II score | 26 (20–32) | 17 (9.1–24.9) |
| Female sex, n (%) | 33 (46) | 1 (33) |
| Race, n (%) | ||
| African American | 4 (6) | 0 |
| White | 57 (79) | 3 (100) |
| Native American | 8 (11) | 0 |
| Asian American | 0 | 0 |
| Other | 3 (4) | 0 |
| Latino ethnicity, n (%) | 27 (38) | 3 (100) |
| Comorbidities, n (%) | ||
| DM (type 1 or 2) | 25 (35) | 0 |
| CHF (any type) | 8 (11) | 0 |
| HIV | 1(1) | 0 |
| Immunocompromised (immunocompromised status from medications) | 10 (14) | 1 (33) |
| CKD (stages 1–4 based on admission history) | 15 (21) | 1 (33) |
| COPD | 6 (8) | 2 (67) |
| Malignancy (active cancer of any kind) | 9 (13) | 0 |
| CAD | 13 (18) | 1 (33) |
| HTN | 36 (50) | 1 (33) |
| Liver disease (any type of chronic liver disease) | 26 (36) | 1 (33) |
Note:
Values represent time of intubation.
Abbreviations: ARDS, acute respiratory distress syndrome; RRT, renal replacement therapy; IQR, interquartile range; PaO2, partial pressure of oxygen in arterial blood; FiO2, percentage of inspired oxygen; PaO2/FiO2, ratio of partial pressure of oxygen in arterial blood to the percentage of inspired oxygen; APACHE II, Acute Physiology and Chronic Health Evaluation II; DM, diabetes mellitus; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disorder; CAD, coronary artery disease; HTN, hypertension.
Figure 2.
Number of patients who had one or more medical comorbidities on admission.
Notes: x-axis represents number of comorbidities present. y-axis represents percentage of patients with each number of comorbidities.
Indications for RRT
The primary modes of RRT were continuous venovenous hemodiafiltration and continuous venovenous hemofiltration. Three different indications for RRT were identified in addition to AKI and oliguria: fluid overload, metabolic acidosis, and hyperkalemia. AKI based on AKI network classification was diagnosed in 97.3% (73/75) patients. In all, 40% of patients requiring RRT had one indication, 49% of patients had two indications, and 11% of patients had three indications for initiating RRT (Figure 3).
Figure 3.
Three indications for RRT.
Note: Patients may have had one or more indications for initiation of RRT.
Abbreviation: RRT, renal replacement therapy.
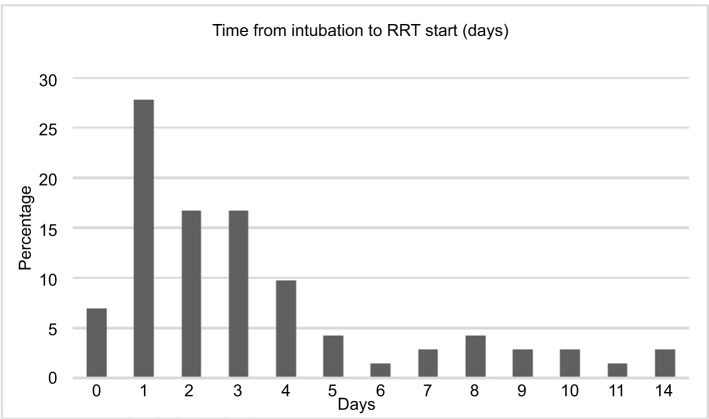
Time to initiation of RRT distribution
The majority of patients (35%) were initiated on RRT within 2 days of intubation. Over the next 48 hours, another 24 (33%) patients were initiated on RRT, with the remaining 23 (32%) of patients being initiated on RRT between days 5 and 15 after intubation (Figure 4).
Figure 4.
Time to initiation of RRT (time 0 at intubation).
Notes: x-axis represents days. y-axis represents number of patients in percentage.
Abbreviation: RRT, renal replacement therapy.
Survival analysis for timing of RRT initiation
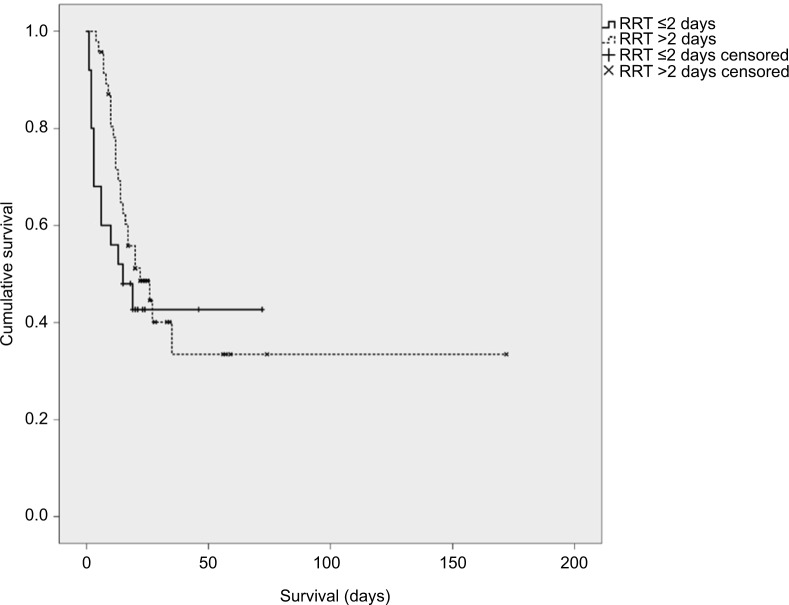
The Kaplan–Meier analysis was conducted to compare early initiation of RRT (within 48 hours of intubation for ARDS) to late initiation of RRT (>48 hours post intubation for ARDS), but no survival benefit was identified (Figure 5). Cox proportional hazard models testing the association between timing of RRT initiation with survival and adjusting for sex, race, ethnicity, and APACHE II score did not reach statistical significance (HR=0.94, 95% CI=0.48–1.86).
Figure 5.
Kaplan–Meier survival analysis for timing of RRT initiation comparing patients who had initiated RRT £2 days (48 hours) after intubation versus >2 days after intubation.
Abbreviation: RRT, renal replacement therapy.
Discussion
This retrospective observational study comparing early versus late initiation of RRT in critically ill patients with ARDS suggests that there is no difference in survival between these two groups. To the best of our knowledge, this is the first study to address the question of whether the time from intubation and mechanical ventilation to initiation of RRT would influence survival in the patients with ARDS. In contrast to previous studies, we intentionally decided to take time of intubation as the reference point for calculating the time to RRT initiation because of the inherent challenges in determining the actual timing of onset of ARDS. Although a patient might have been developing ARDS based on reviewing the chart for 12–24 hours prior to the intubation, the objective data (ie, recent chest X-ray, arterial blood gas, positive end-expiratory pressure) most often were not there to make a definitive assessment. Using time of intubation gave those needed data despite its other imperfections. Furthermore, we reasoned that the time of intubation represents the closest approximation of the worst patient deterioration during the course of ARDS. Our results do not confirm some of the previous findings suggesting that early RRT was associated with favorable outcomes in critically ill patients.1,10,11 One of the reasons for this discrepancy could be because the initiation of RRT in those studies occurred within a few hours of admission to the ICU. Interestingly, our survival analysis showed a trend toward a survival benefit for patients with RRT initiation after >48 hours but was not statistically significant.
Early initiation of hemofiltration within 12 hours of ICU admission can help volume management and pulmonary edema better than if initiated after 48 hours.1 In addition to the benefits of maintaining fluid balance and correcting electrolyte abnormalities, initiation of RRT also allows for the administration of adequate nutrition, medications, and blood products, while avoiding further fluid accumulation. However, at the same time, early initiation of RRT also has its disadvantages. Early aggressive fluid removal may be associated with worsening of hemodynamic instability in the presence of capillary leak, an important cause of intravascular volume loss in patients with sepsis.2,12 This may in turn impact the oxygenation of tissues resulting from hypoperfusion of organs, and adequate organ perfusion is an important component of management of critically ill patients.2 There are clinical studies showing that early hemofiltration results in worse outcomes and prolongation of organ support.13,14 Another important issue with early initiation of RRT in critically ill patients is the removal of antibiotics. We are still unaware of the effect of RRT on these lifesaving drugs. There could be under dosing of antibiotics that in turn could result in lack of benefits to some of these patients.15
In our study, the most common indication for initiation of RRT was fluid overload (75%). Similar trends have been noted in other RRT clinical trials. In a renal trial that was not specific for ARDS, 43% of the patients were started on RRT due to organ edema.16 This high percentage of fluid overload noted at the time of initiation of RRT may be due to the liberal fluid strategy we use in our ICU patients with hemodynamic instability as part of the early goal-directed therapy. The administration of excess fluids beyond what is recommended for the initial management of patients, especially in the presence of AKI, capillary leak, and myocardial dysfunction, will expand the extracellular fluid compartment. This in turn can result in impaired tissue perfusion and progressive organ dysfunction.17 While massive fluid resuscitation in the context of underlying disorders including sepsis could be detrimental, clinical studies have come up with conflicting reports. The effects of fluid overload and outcomes were investigated by the ARDS network trial. In the ARDS network fluids and catheters treatment trial, liberal versus conservative fluid strategy in lung injury patients did not change mortality, although the conservative arm had less ventilator days.18 Similarly, studies showing the use of diuretics to maintain fluid balance have not found substantial benefit in patients with ARDS.19
The mortality rate in our study (56%) was similar to findings reported in other studies involving critically ill patients who underwent RRT. In the study performed on patients admitted to a Canadian tertiary ICU, the mean APACHE II score was 28 and the overall hospital mortality was close to 60%.20 Similarly, in the Acute Renal Failure Trial Network trial, the mean APACHE II score was 26 and the 60-day mortality was 52%.21 Mortality data on ARDS patients also give a similar picture. A prospective, multicenter, cohort study looking at ARDS recognition and treatment reported unadjusted mortality based on mild, moderate, and severe ARDS at 35%, 40%, and 46%, respectively.22 Our RRT patient group had a mean PaO2/FiO2 of 133.5, which would categorize as moderate ARDS. However, adjusting for the APACHE II score of 27.6, the predicted mortality rate in moderate ARDS patients can be in the range of 50%–60%, which is similar to our observed mortality rate.23
Another key finding in our study is that the utilization of RRT in critically ill patients with AKI is up to 96%. RRT is utilized in 8%–10% of critically ill patients and has grown by >10%/year in the last 10 years.24 The AKI-epidemiologic prospective investigation study found that continuous renal replacement therapy (CRRT) is the predominant form of RRT utilized in critically ill patients. It is the initial RRT modality used in >75% of critically ill patients.25 This high utilization of CRRT also represents a significant escalation in the complexity and cost. There is need for circuit anticoagulation, patient immobility, and intensive nursing requirements. A high utilization of CRRT despite a significant amount of supportive evidence is mainly due to lack of effective alternate options. There are only limited treatment options available at this point for renal replacement in patients who are hemodynamically unstable. CRRT has been advocated as a means of mitigating blood pressure lability that may occur with conventional intermittent hemodialysis. At the same time, use of intermittent forms of dialysis like sustained low efficiency dialysis (SLED) has come up as an alternative to CRRT in hemodynamically unstable patients. Data have shown that there is no survival benefit for CRRT over SLED and the ultrafiltration and solute removal are comparable.26,27 Although some studies show that the patients treated with CRRT have a higher likelihood of renal recovery and improved renal outcomes over the long term, this has not been proven in a randomized trial.28
Despite some of the interesting findings, inferences from our study are limited for various reasons. Consequent to the relatively low number of subjects and retrospective design of the study, we were unable to calculate effect sizes for the outcome of interest, which is mortality. Although we defined patients into early and late based on relation to intubation, the actual initiation of CRRT will be determined by several factors, including clinical status, volume status, decision of the treating physician, and technical delays in the initiation of RRT. This may make the definition of early versus late imprecise. These limitations of the retrospective study emphasize the importance of hypothesis testing using randomized controlled trial (RCT) designs. Interestingly, there are only a few RCT done in this field, and a definite conclusion could not be obtained from these studies.
Conclusion
Our study did not find a survival benefit in regard to timing of RRT initiation in our ARDS patient population. A total of 96% of patients received RRT during their hospital stay, with fluid overload being the most frequent indication for RRT initiation. In all, 35% of patients were initiated on RRT within 48 hours after intubation and 68% of patients were initiated within 96 hours from intubation. Timing of RRT initiation could be a good area for future prospective study to definitively answer the question of its benefit in this population.
Acknowledgments
The authors acknowledge the support of Vern Pilling; Natt Bhupinder, MD; Hem Desai, MD; Mark Borgstrom; and Lindsay Kohler, PhD. This study was presented as a poster at the American Thoracic Society International Conference, Washington DC, USA, 2017. It was also published as an abstract in American Journal of Respiratory and Critical Care Medicine (2017;195:A7138).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Han F, Sun R, Ni Y, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. Am J Med Sci. 2015;349(3):199–205. doi: 10.1097/MAJ.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 2.Garzia F, Todor R, Scalea T. Continuous arteriovenous hemofiltration countercurrent dialysis (CAVH-D) in acute respiratory failure (ARDS) J Trauma. 1991;31(9):1277–84. doi: 10.1097/00005373-199109000-00013. discussion 84–85. [DOI] [PubMed] [Google Scholar]
- 3.Darmon M, Clec’h C, Adrie C, et al. Acute respiratory distress syndrome and risk of AKI among critically Ill patients. Clin J Am Soc Nephrol. 2014;9(8):1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley EJ. Updates in the management of acute lung injury: a focus on the overlap between AKI and ARDS. Adv Chronic Kidney Dis. 2013;20(1):14–20. doi: 10.1053/j.ackd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Wierstra BT, Kadri S, Alomar S, et al. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20(1):122. doi: 10.1186/s13054-016-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15(1):R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4(5):91. doi: 10.21037/atm.2016.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarone RE, Ware J. On distribution-free tests for equality of survival distributions. Biometrica. 1977;64(1):156–160. [Google Scholar]
- 10.DiCarlo JV, Alexander SR, Agarwal R, Schiffman JD. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol. 2003;25(10):801–805. doi: 10.1097/00043426-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Hong J, Zeng Q, et al. Improvement of oxygenation in severe acute respiratory distress syndrome with high-volume continuous veno-venous hemofiltration. Glob Pediatr Health. 2016;3 doi: 10.1177/2333794X16645699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, Agostoni P. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol. 2001;38:963–968. doi: 10.1016/s0735-1097(01)01479-6. [DOI] [PubMed] [Google Scholar]
- 13.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37(3):803–810. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 14.Hoste EA, Vanholder RC, Lameire NH, et al. No early respiratory benefit with CVVHDF in patients with acute renal failure and acute lung injury. Nephrol Dial Transplant. 2002;17(12):2153–2158. doi: 10.1093/ndt/17.12.2153. [DOI] [PubMed] [Google Scholar]
- 15.Reetze-Bonorden P, Böhler J, Keller E. Drug dosage in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet. 1993;24(5):362–379. doi: 10.2165/00003088-199324050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 17.Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17:109. doi: 10.1186/s12882-016-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 19.Roch A, Guervilly C, Papazian L. Fluid management in acute lung injury and ARDS. Ann Intensive Care. 2011;1:16. doi: 10.1186/2110-5820-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlEnezi F, Alhazzani W, Ma J, et al. Continuous venovenous hemofiltration versus continuous venovenous hemodiafiltration in critically ill patients: a retrospective cohort study from a Canadian tertiary centre. Can Respir J. 2014;21(3):176–180. doi: 10.1155/2014/965479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 24.Rewa OG, Villeneuve PM, Lachance P, et al. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med. 2017;43(6):750–763. doi: 10.1007/s00134-016-4579-x. [DOI] [PubMed] [Google Scholar]
- 25.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 26.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24(2):512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 27.Misset B, Timsit JF, Chevret S, Renaud B, Tamion F, Carlet J. A randomized cross-over comparison of the hemodynamic response to intermittent hemodialysis and continuous hemofiltration in ICU patients with acute renal failure. Intensive Care Med. 1996;22(8):742–746. doi: 10.1007/BF01709515. [DOI] [PubMed] [Google Scholar]
- 28.Ronco C. Renal replacement therapy for acute kidney injury: let’s follow the evidence. Int J Artif Organs. 2007;30(2):89–94. doi: 10.1177/039139880703000202. [DOI] [PubMed] [Google Scholar]