Abstract
Objectives
This post hoc analysis of data from a randomized, double-blind, placebo-controlled, enriched-enrollment randomized-withdrawal Phase III study evaluated the safety, tolerability, and analgesic efficacy of Oxycodone DETERx extended-release (ER), abuse-deterrent capsules (Xtampza® ER) in subjects with chronic low back pain who were successfully transitioned from immediate-release (IR) oxycodone.
Methods
Continuous outcomes were analyzed using a mixed-model repeated-measures approach; binomial outcomes were analyzed using chi-squared; and time-to-event outcomes using Kaplan–Meier analyses.
Results
A total of 110 subjects previously prescribed IR oxycodone entered the Open-label Titration Phase. Forty-four subjects were randomized to Oxycodone DETERx (n=22) or placebo (n=22) in the 12-week Double-blind Maintenance Phase. Efficacy results in this subgroup showed a statistically significant difference between Oxycodone DETERx and placebo in average pain intensity scores from Randomization Baseline to Week 12 (least squares mean [± standard error], −1.88 [0.70]; P=0.0078). Additional efficacy results indicated that Oxycodone DETERx vs placebo was associated with a statistically significant benefit in durability of effect from Week 2 through Week 12 (P<0.01), numbers of subjects with a ≥30% (n [%] 10 [45.5%] vs 0 [0%]; P=0.0004) and ≥50% (10 [45.5%] vs 0 [0%]; P=0.0004) improvement in pain intensity, longer time-to-exit (P=0.0014), a greater number of subjects who completed the study (14 [63.6%] vs 4 [18.2%]), and less rescue medication use (acetaminophen; mean [SD], 163.5 [337.8] mg) vs 216.2 [377.3] mg). Adverse event profiles were consistent with opioid class effects and results from the original study; Oxycodone DETERx was well tolerated in subjects previously treated with short-acting oxycodone.
Conclusions
Oxycodone DETERx resulted in clinically meaningful and statistically significant efficacy in subjects with chronic low back pain who were previously prescribed IR oxycodone and were successfully switched to ER Oxycodone DETERx.
Keywords: oxycodone, DETERx, Xtampza ER, chronic low back pain, extended-release, immediate-release, opioid
Introduction
The National Academy of Medicine concluded that chronic pain is a significant public health problem affecting up to 100 million Americans.1 There is a large population with severe pain who are unable to achieve adequate pain control using non-pharmacologic and/or non-opioid treatments. For these patients, opioids can be effective for the treatment of their chronic pain; however, clinicians must balance the benefits with the risks, particularly those of misuse, abuse, and addiction.2–6
Consensus guidelines support the use of an immediate-release (IR) formulation for initial therapy of acute and chronic pain severe enough to require an opioid. Starting with an IR formulation offers the safest and most flexible way to determine an effective analgesic dose for a given patient. However, in some patients who require around-the-clock opioid treatment over an extended period of time for moderate-to-severe pain for which other treatment options have failed, extended-release (ER) opioids may be preferred over an IR opioid formulation. Switching these patients to an ER opioid is a clinical practice supported by the Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain – United States 2016, American Pain Society, and the American Academy of Pain Medicine.7–9
There is evidence that IR and ER formulations provide similar tolerability, analgesic efficacy, and improvement in functional outcomes.10 However, ER opioids provide more consistent and prolonged opioid plasma concentrations than IR formulations, which may reduce peak-to-trough fluctuations.11,12 For some patients, this will translate clinically to more consistent pain control and improved coverage throughout the day and night avoiding, for example, sleep disruption from poorly controlled pain. For patients at risk of aberrant drug-related behavior, addiction, and/or diversion, there is an additional, and critically important, consideration when choosing to switch from an IR to an ER formulation; switching patients to an abuse-deterrent formulation (ADF) employs an additional safeguard in that these formulations reduce the ability of those patients to manipulate the ER opioid for abuse.
The US Food and Drug Administration (FDA) has issued a comprehensive action plan that includes expanding the use of ADF opioids as part of a comprehensive strategy to address the ongoing public health crisis of opioid abuse and diversion.13 To date, the FDA has approved 9 ER abuse-deterrent opioid formulations, including Oxycodone DETERx, that mitigate the ability of patients to alter the route of administration and/or manipulate solid, oral dosage formulations to obtain immediate release of the opioid.14–22 Abuse-deterrent IR formulations have been approved; however, none are currently marketed.
Oxycodone DETERx capsules (Xtampza® ER, Collegium Pharmaceutical, Inc., Canton, MA, USA) are an ER, abuse-deterrent, microsphere-in-capsule formulation of oxycodone that have been shown to be effective in both opioid-naïve and opioid-experienced patients with poorly controlled moderate-to-severe chronic low back pain (CLBP).6 Switching patients from IR to ER formulations is common in clinical practice and recommended by consensus guidelines. The transition to an abuse-deterrent long-acting opioid requires that patients be titrated from a short-acting formulation, and clinicians likely need additional information to ensure this is done safely. The primary objective of this post hoc analysis was to characterize the experience of patients switched to Oxycodone DETERx, an ER opioid with abuse-deterrent properties, who were previously prescribed IR oxycodone treatment for CLBP.
Materials and methods
Data for the post hoc analysis were obtained from a Phase III, double-blind, placebo-controlled, parallel-group, enriched-enrollment randomized-withdrawal (EERW), multicenter clinical study that evaluated the safety, tolerability, and analgesic efficacy of Oxycodone DETERx in opioid-naïve and opioid-experienced adults with moderate-to-severe CLBP. All subjects provided written informed consent prior to any study-related procedures or assessments being conducted. The study was approved by the Quorum Internal Review Board (Seattle, WA, USA). The study was registered under ClinicalTrials.gov identifier: NCT01685684. Details on the Materials and Methods for the primary Phase III study have been published previously.6
Study design
The study consisted of a Screening Phase (up to 4 weeks), an Open-label Titration Phase (up to 6 weeks), a 12-week Double-blind Maintenance Phase, and a Follow-up Safety Phase (2 weeks). Eligible opioid-experienced subjects were converted from their current opioid medications to the appropriate starting dose of Oxycodone DETERx based on their equivalent daily dose of morphine sulfate in milligrams.
Subject population
Subjects enrolled into the Phase III study were males and females 18–75 years of age with a clinical diagnosis of moderate-to-severe CLBP (ie, pain intensity score ≥5 to ≤9 on an 11-point pain intensity numerical rating scale [PI-NRS] at screening) for a minimum of 6 months prior to screening.6
The subject population included in this post hoc analysis consisted of a subset of the overall population who had been treated with IR oxycodone as their primary analgesic regimen at Screening Visit 1 and were successfully transitioned to Oxycodone DETERx following a 6-week Open-label Titration Phase. The principal criteria required to achieve a stable dose of study drug during the Open-label Titration Phase in order to be eligible for randomization included as follows: 1) an unchanged dose of Oxycodone DETERx during the last 7 consecutive days prior to randomization, 2) a 24-hour PI-NRS score of ≤4 for ≥6 of the last 7 days prior to the Randomization Visit, 3) a reduction of ≥2 points in the average 24-hour PI-NRS score for ≥6 of the last 7 days prior to Randomization Visit compared with the Screening Phase average pain score, and 4) a maximum of 2,000 mg acetaminophen per day as a rescue medication.
Efficacy and safety/tolerability outcome measures
As this was a post hoc analysis of a subpopulation included in the original Phase III study, all efficacy endpoints were considered exploratory, but were the same as the outcome measures described in the primary manuscript.6 The primary efficacy endpoint was the change in average pain intensity measured by the change in PI-NRS score from Randomization Baseline to Week 12 of the Double-blind Maintenance Phase.
Secondary endpoints included weekly changes in pain intensity score from Randomization Baseline to all weeks, responder analysis (cumulative distribution of subjects with improvement in pain intensity from Screening Baseline to Week 12 and proportions of responders with ≥30% and ≥50% improvement in pain intensity at Week 12), time-to-exit from the study for all causes from Randomization Baseline to Week 12 of the Double-blind Maintenance Phase, and total amount of rescue medication (acetaminophen) used (number of doses and dosage [mg] per day). Patient Global Impression of Change, quality of life (short form 12-question health survey, version 2), and level of physical disability (Roland–Morris Disability Questionnaire) were not included as endpoints in this analysis as there was a small sample size with available data for these endpoints which precluded conducting inferential statistical analysis.
The safety and tolerability of Oxycodone DETERx were evaluated by examining the frequency of treatment-emergent adverse events (TEAEs), clinical laboratory results, vital signs, and physical examination results.
Statistical methods
The safety population consisted of all subjects with a prior IR oxycodone regimen who participated in the Open-label Titration Phase. The intent-to-treat (ITT) population and randomized safety population included all subjects previously prescribed IR oxycodone who participated in the Double-blind Maintenance Phase. The ITT population was the primary analysis population for efficacy, and the randomized safety population was the primary analysis population for safety.
For the primary efficacy analysis, the primary analysis methodology for the Week 12 change from Randomization Baseline PI-NRS score used in the primary study analysis was not feasible for this subgroup due to insufficient data for counts in the various reasons for withdrawal6; therefore, a simpler mixed-model repeated-measures analysis was applied to the weekly averages, with fixed effects for treatment, week (categorical), treatment-by-week interaction, and the randomization baseline and random subject intercepts. Least squares (LS) mean differences and P-values were generated for each week, with Week 12 as the primary time point of interest.
The same model used for the primary efficacy analysis was used for the secondary endpoint, durability of effect (ie, treatment group differences over time). The secondary efficacy endpoint of time-to-exit from study was compared between treatment groups using a Kaplan–Meier log-rank test. The difference in proportions of subjects achieving ≥30% and ≥50% improvement from screening pain was tested with a chi-squared approach. Subjects who discontinued treatment or otherwise had missing data were considered non-responders. Responders were those subjects who had a Week 12 pain score ≥30% less than their screening baseline score. The analysis methods for the secondary endpoints were the same as those described in the primary manuscript analyses.6
Use of acetaminophen rescue medication in the Double-blind Maintenance Phase was summarized descriptively by treatment group.
The number of TEAEs and number and percentage of subjects reporting TEAEs, serious TEAEs, treatment-related TEAEs, study drug-associated serious/severe TEAEs, TEAEs leading to study drug discontinuation, and TEAEs leading to death were tabulated.
Results
Subjects
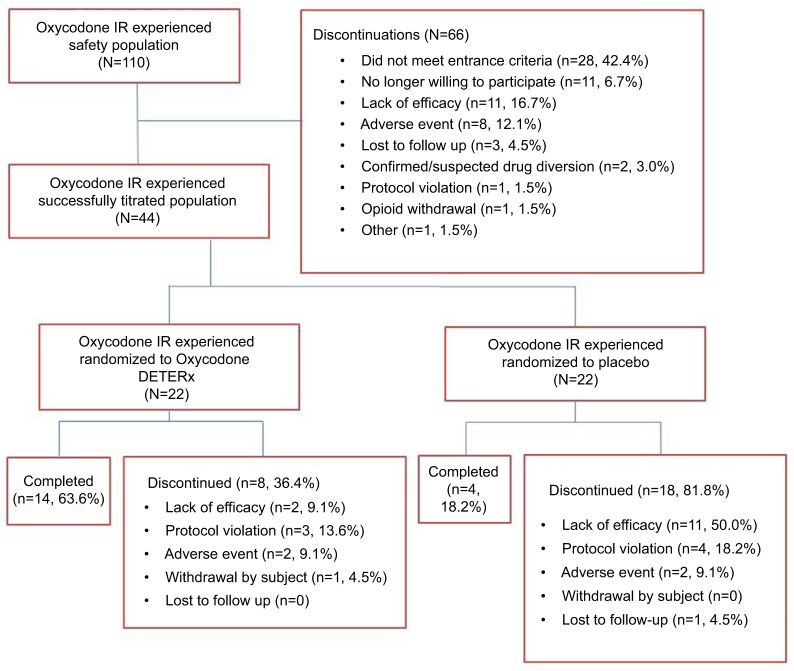
Of the 740 subjects who entered the Open-label Titration Phase, 110 reported previously taking IR oxycodone. Forty-four of the 110 subjects (22 Oxycodone DETERx; 22 placebo) were successfully titrated to a stable dose of Oxycodone DETERx and were randomized to the Double-blind Maintenance Phase (Figure 1). The primary reasons for leaving the study for the 66 subjects who did not enter the Double-blind Treatment Phase were failure to meet entrance criteria (28 [42.4%]), no longer willing to participate (11 [16.7%]), lack of efficacy (11 [16.7%]), adverse event (AE, 8 [12.1%]), lost to follow-up (3 [4.6%]), confirmed/suspected drug diversion (2 [3.0%]), protocol violation (1 [1.5%]), opioid withdrawal (1 [1.5%]), and other (1 [1.5%]; Figure 1). Of those subjects who discontinued during the Double-blind Treatment Phase (8 [36.4%] Oxycodone DETERx and 18 [81.8%] placebo), most did so due to lack of efficacy (2 [9.1%] and 11 [50.0%]), a protocol violation (3 [13.6%] and 4 [18.2%]), or an AE (2 [9.1%] and 2 [9.1%]; Figure 1).
Figure 1.
Subject disposition.
Abbreviations: IR, immediate-release.
Baseline characteristics, including age, body mass index, race, and ethnicity were similar between the Oxycodone DETERx and placebo treatment groups and consistent with the larger Open-label Titration Phase treatment group (Table 1). Mean (SD) PI-NRS scores were similar between the Oxycodone DETERx and placebo treatment groups at both Screening Baseline (6.80 [0.96]) and 6.93 [0.96], respectively) and Randomization Baseline (3.04 [1.13] and 3.08 [0.80], respectively). The stable dose subjects were titrated to during the Open-label Titration Phase (ie, the dose the subject entered the Double-blind Treatment Phase on) or the dose a subject was on at the time of study discontinuation (ie, withdrawal from the Open-label Titration Phase) are available in Table 2.
Table 1.
Demographics and baseline characteristics (safety population)
| Characteristic | Open-label Titration Phase (N=110) | Double-blind Maintenance Phase
|
|
|---|---|---|---|
| Oxycodone DETERx (N=22) |
Placebo (N=22) |
||
| Age (years) | |||
| Mean (SD) | 49.1 (12.3) | 49.4 (14.4) | 50.8 (11.2) |
| Gender, n (%) | |||
| Male | 57 (51.8) | 9 (40.9) | 10 (45.5) |
| Female | 53 (48.2) | 13 (59.1) | 12 (54.5) |
| Race, n (%) | |||
| Caucasian/White | 86 (78.2) | 18 (81.8) | 18 (81.8) |
| Black or African American | 21 (19.1) | 3 (13.6) | 4 (18.2) |
| American Indian or Alaskan Native | 1 (0.9) | 1 (4.5) | 0 |
| Native Hawaiian or other Pacific Islander | 1 (0.9) | 0 | 0 |
| Other | 1 (0.9) | 0 | 0 |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 5 (4.5) | 1 (4.5) | 2 (9.1) |
| Not Hispanic/Latino | 105 (95.5) | 21 (95.5) | 20 (90.9) |
| Height (cm) | |||
| Mean (SD) | 172.3 (10.9) | 169.6 (9.6) | 170.1 (11.2) |
| Weight (kg) | |||
| Mean (SD) | 91.5 (22.5) | 92.9 (22.1) | 88.7 (19.2) |
| BMI (kg/m2) | |||
| Mean (SD) | 30.7 (6.5) | 32.2 (6.9) | 30.6 (5.8) |
Abbreviation: BMI, body mass index.
Table 2.
Stable dose of Oxycodone DETERx at the end of the Open-label Titration Phase
| Stable dose of Oxycodone DETERxa | Subjects successfully titrated to a stable dose (N=44) n (%) |
Subjects unsuccessfully titrated to a stable dose (N=66) n (%) |
Overall (N=110) n (%) |
|---|---|---|---|
| 20 mg/day | 0 | 11 (16.7) | 11 (10.0) |
| 40 mg/day | 7 (15.9) | 10 (15.2) | 17 (15.5) |
| 60 mg/day | 6 (13.6) | 13 (19.7) | 19 (17.3) |
| 80 mg/day | 6 (13.6) | 11 (16.7) | 17 (15.5) |
| 120 mg/day | 10 (22.7) | 6 (9.1) | 16 (14.5) |
| 160 mg/day | 15 (34.1) | 15 (22.7) | 30 (27.3) |
Notes:
The stable dose subjects were titrated to during the Open-label Titration Phase (ie, the dose the subject entered the Double-blind Treatment Phase on) or the dose a subject was on at the time of study discontinuation (ie, withdrawal from the Open-label Titration Phase).
Efficacy
The estimated pain score from Randomization Baseline to Week 12 (change in pain from baseline) was higher for placebo subjects (LS mean [± standard error {SE}]; 2.67 [0.59]) than for Oxycodone DETERx subjects (0.79 [0.38]); this difference (−1.88 [0.70]) was statistically significant (P=0.0078; Figure 2). With respect to durability of effect (ie, subject response over time), the LS mean difference between Oxycodone DETERx and placebo in weekly pain intensity scores was statistically significant starting from Week 2 through Week 12 (P<0.01; Figure 2). Thus, the Oxycodone DETERx treatment group maintained improved pain intensity scores after randomization.
Figure 2.
Weekly pain intensity scores for patients with previous IR oxycodone use.
Note: *P<0.01.
Abbreviation: IR, immediate-release.
During the Double-blind Maintenance Phase, a statistically significantly greater number of subjects in the Oxycodone DETERx vs placebo treatment group had ≥30% improvement (10 [45.5%] vs 0 [0.0%]; P=0.0004) or ≥50% improvement (10 [45.5%] vs 0 [0.0%]; P=0.0004) in pain intensity (Table 3). In addition, subjects in the Oxycodone DETERx treatment group remained in the study for a statistically significantly longer period than those in the placebo group (Kaplan–Meier time-to-exit survival analysis: P=0.0014; Table 3), and fewer subjects in the Oxycodone DETERx treatment group than in the placebo group exited the study due to all causes (4 [18.2%] vs 14 [63.6%]; Table 3).
Table 3.
Summary of secondary endpoints from Randomization Baseline to Week 12 (intent-to-treat population)
| Secondary endpoint | Statistic | Oxycodone DETERx (N=22) | Placebo (N=22) | P-value |
|---|---|---|---|---|
| Responder analysisa | ||||
| >30%b | n (%) | 10 (45.5) | 0 (0.0) | 0.0004 |
| >50%b | n (%) | 10 (45.5) | 0 (0.0) | 0.0004 |
| Completers | n (%) | 14 (63.6) | 4 (18.2) | 0.0014c |
| Rescue medication | ||||
| Tablets per day | Mean (SD) | 0.16 (0.35) | 0.30 (0.59) | NAd |
| Dosage (mg) per day | Mean (SD) | 163.45 (337.83) | 216.20 (377.26) | NAd |
Notes:
Subjects who discontinued treatment or otherwise had missing data were considered non-responders. Responders were those subjects who had a Week 12 pain score ≥30% less than their screening baseline score.
Subjects with ≥30 or≥50% improvement.
P-value based on Kaplan–Meier time-to-exit analysis.
Rescue medication was reported using descriptive statistics.
Abbreviation: NA, not applicable.
Compared with subjects in the Oxycodone DETERx treatment group, subjects in the placebo group used a numerically greater number of tablets (0.30 [0.59] vs 0.16 [0.35]) and dosage per day (mg; 216.2 [377.3] vs 163.5 [377.8]) of rescue medication (Table 3).
There were no clinically meaningful differences between the treatment groups with respect to clinical laboratory results, vital signs, and physical examination results.
Safety
Overall, 70 (63.6%) subjects experienced at least 1 TEAE during the Open-label Titration Phase, and 29 (65.9%; 16 [72.7%] Oxycodone DETERx, and 13 [59.1%] placebo) subjects experienced at least 1 TEAE during the Double-blind Maintenance Phase (Table 4). The most frequently reported TEAEs during the Open-label Titration Phase were headache (18 subjects [16.4%]), constipation (16 [14.5%]), and nausea (13 [11.8%]). Similarly, the most frequently reported TEAEs in the Oxycodone DETERx treatment group during the Double-blind Maintenance Phase were constipation (4 subjects [18.2%] vs 5 subjects [22.7%] placebo), headache (4 [18.2%] vs 3 [13.6%] placebo), nausea (4 [18.2%] vs 1 [4.5%] placebo), and sedation (2 [9.1%] vs 1 [4.5%] placebo). Three subjects (of 110) in the Open-label Titration Phase experienced a TEAE of withdrawal syndrome; all 3 subjects withdrew from the study: one withdrew due to this withdrawal event, 1 was listed as a titration failure (ie, failed to meet entrance criteria), and 1 withdrew due to an AE other than withdrawal syndrome.
Table 4.
TEAEs during the Open-label Titration and Double-blind Maintenance Phases
| Open-label Titration Phase (N=110) n (%)
|
Double-blind Maintenance Phase
|
||
|---|---|---|---|
| Oxycodone DETERx (N=22) n (%) | Placebo (N=22) n (%) | ||
| Number of AEs | 182 | 38 | 23 |
| Subjects with TEAEs | 70 (63.6) | 16 (72.7) | 13 (59.1) |
| Subjects with serious TEAEs | 3 (2.7) | 0 | 0 |
| Subjects with treatment-related TEAEs | 54 (49.1) | 8 (36.4) | 7 (31.8) |
| Subjects with study drug-associated serious/severe TEAEs | 1 (0.9) | 0 | 1 (4.5) |
| Subjects with TEAEs leading to study drug termination | 9 (8.2) | 2 (9.1) | 2 (9.1) |
| Subjects with TEAEs leading to death | 0 | 0 | 0 |
| TEAEs occurring in ≥5% of subjects | |||
| Headache | 18 (16.4) | 4 (18.2) | 3 (13.6) |
| Constipation | 16 (14.5) | 4 (18.2) | 5 (22.7) |
| Nausea | 13 (11.8) | 4 (18.2) | 1 (4.5) |
| Sedation | 5 (4.5) | 2 (9.1) | 1 (4.5) |
| Fatigue | 4 (3.6) | 1 (4.5) | 2 (9.1) |
| Somnolence | 4 (3.6) | 1 (4.5) | 2 (9.1) |
Notes: TEAEs were considered treatment-related or study drug-associated if the relationship to study drug was possible, probable, definite, or missing.
Abbreviations: AE, adverse event; TEAE, treatment-emergent adverse event.
Discussion
In this post hoc analysis of a Phase III, double-blind, placebo-controlled, EERW, clinical study, a minority of subjects (40% [44 of 110]; 22 were randomized to Oxycodone DETERx and 22 to placebo) with CLBP previously prescribed IR oxycodone were able to successfully convert to Oxycodone DETERx, an ER ADF of oxycodone. The percentage of patients who were successfully converted in the overall study was 53%6; in general, successful titration across published EERW opioid studies ranges from 53% to 78%,23 regardless of prior opioid experience. It is not unexpected that the titration failure rate would be higher in patients previously prescribed IR oxycodone – an inclusion criterion requires patients to have inadequately controlled pain (PI-NRS score of 5–9) at screening. Therefore, it could be assumed that some of the patients included in this post hoc analysis were simply oxycodone non-responders (ie, the inclusion criterion effectively enriched the population assessed in this analysis for oxycodone non-responders). Additionally, achieving “efficacy” in this study is arguably harder than in clinical practice due to stringent predefined criteria (eg, low pain intensity scores, a minimum of a 2-point reduction on the PI-NRS scale).
Compared with placebo, treatment with Oxycodone DETERx in this group was associated with greater pain control, greater clinically meaningful improvement in pain,24 and less rescue medication use. TEAEs with Oxycodone DETERx were consistent with those common for the opioid class. The efficacy and safety findings of this post hoc analysis support those of the primary analysis (N=740); transitioning subjects with poorly controlled pain (baseline PI-NRS scores were 6.8±0.96) from IR oxycodone to Oxycodone DETERx was as successful as converting a broader population (ie, subjects in the Phase III study) with poorly controlled pain to Oxycodone DETERx, regardless of prior treatment regimen or opioid experience (opioid-naïve or opioid-experienced).6
Clinicians may choose to switch a patient from an IR to an ER opioid formulation to provide more continuous and consistent pain control. The process of completing an IR to ER switch is neither always uneventful nor simple, even in cases where the patient is treated with the same opioid molecule for both the IR and ER medications. IR formulations are intended to provide patients rapid, acute pain relief. Effective IR products must have rapid oral bioavailability and typically release about 80% of the active pharmaceutical ingredient in the first 30 minutes after dosing. Unsuccessful attempts may occur because some patients prefer their IR formulation as it affords them the ability to quickly treat episodic flares of pain on an as-needed basis.25 Other patients, especially those who have had prolonged exposure to IR formulations, may associate multiple sensations that result from the rapid onset of an IR opioid with efficacy, and consequently report decreased efficacy in the absence of these sensations. Therefore, it is common practice to provide patients with pre-switch and ongoing education and counseling during the switching process.
While it can be argued that the attention and support bestowed upon subjects in clinical studies exceeds that which patients in clinical care usually encounter, specifically helping patients to prepare for and develop a changed relationship with their pain, pain relief, and ability to function when pain is managed with an ER rather than an IR opioid, is not typical clinical study support, including in this study. In practice, it is more likely to be an ongoing dialogue between the patient and pain psychologists and clinicians sensitive to this issue. The absence of this dialogue in a study setting is likely to contribute to study discontinuation, even when the same dosage of the same molecule is being provided in either case.
Head-to-head studies comparing IR vs ER opioids are limited and focus on initiating opioid therapy rather than converting patients from IR to ER.10 Future studies are needed to establish treatment benefits of IR vs ER opioids.
It is well documented that both IR and ER formulations are abused,26–28 and that abuse can often involve product manipulation to alter both the route (eg, oral, injected) and mode (eg, solid, crushed, chewed) of administration to obtain more drug in less time.29 In the last quarter of 2015, the population-adjusted rate of intentional abuse of IR opioids was 4.6 times higher than ER opioids, and the rate of drug diversion was 6.1 times higher.30 An additional benefit of ER opioids is that many are formulated to be abuse deterrent.30 By limiting prescribing of opioids for chronic pain to IR formulations, patients and providers may be missing out on the clinical advantages of ER opioids, mentioned previously, including prescribing products that may be abused because they have no abuse-deterrent properties.
The limitations of this study include that it is a post hoc analysis based on data gathered electronically from a large, prospective, randomized, controlled, Phase III clinical study; the sample size of this cohort of subjects was small, with only 22 subjects in each of the active and placebo treatment groups, which limited the ability to detect differences using inferential analyses between the 2 treatment groups for some of the secondary endpoint measures. Additionally, the clinical study design was an EERW design of a Phase III study that adhered to FDA requirements for a chronic pain indication, which may limit the generalizability of the results. It should be noted that, while analyses in the IR subpopulation generally followed the pre-specified outcomes and analyses of the main protocol statistical analysis plan and the analysis population was selected without first examining the resulting outcomes, these were not pre-planned analyses and were chosen and executed after database lock and unblinding. Therefore, the analyses could be subject to the biases of the authors, which may have biased the study in the direction of positive results. The specific IR oxycodone dose at screening was not captured in a consistent way, however, the dose range allowed at entry was >10 to ≤240 mg morphine sulfate equivalents per day for opioid-experienced subjects.
Conclusion
Data herein support that some patients on an IR oxycodone regimen who continue to have uncontrolled pain (PI-NRS score of 5–9) can be switched to a dose of Oxycodone DETERx to effectively and safely control their pain. The formulation characteristics of Oxycodone DETERx allow for continued release of oxycodone over a 12-hour period in an ADF that retains its ER properties despite physical manipulation (eg, crushing or chewing).31 Clinical judgment of health care providers is required to identify the appropriate patient and clinical scenario most suitable for such transitions. The successful completion of an IR to ER switch is challenging and requires time and patience on the part of both the clinician and the patient. A better understanding of the dosing and non-pharmacological strategies to facilitate a safer transition from IR to ER opioids may make this switch safer and more beneficial to patients in some clinical situations.
Acknowledgments
Editorial support for writing this manuscript was provided by Rho, Inc. (Joseph Watson) and was funded by Collegium Pharmaceutical, Inc.
Footnotes
Disclosure
DSM, EAK, MLO, and SDP were employees of Collegium Pharmaceutical at the time of this study. JM has served as the Chair in the Data Safety Monitoring Board for Novartis. He served in the advisory board and received grants and personal fees from Pfizer. He also received personal fees as a consultant from Biogen, Diaachi Sankyo, and Quark and received grants from Depomed. He is also a consultant for Aptinyx, Grunenthal, Plamasurgical, Quartet, Sanofi, and Trevenaand. JM also served at the advisory board for Collegium, Chromocell, Endo, and Purdue. JM also has other engagements with Allergan, Nektar and Teva. The authors report no other conflicts of interest in this work
References
- 1.Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse Pain Management and Prescription Opioid-Related Harms: Exploring the State of the Evidence Washington DC: The National Academic Press; 2016 [Google Scholar]
- 2.Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine. 2014;39(7):556–563. doi: 10.1097/BRS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 3.Hale ME, Ahdieh H, Ma T, Rauck R, Oxymorphone ER Study Group 1 Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: a 12-week, randomized, double-blind, placebo-controlled study. J Pain. 2007;8(2):175–184. doi: 10.1016/j.jpain.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine. 1998;23(23):2591–2600. doi: 10.1097/00007632-199812010-00014. [DOI] [PubMed] [Google Scholar]
- 5.Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;8:CD004959. doi: 10.1002/14651858.CD004959.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz N, Kopecky EA, O’Connor M, Brown RH, Fleming AB. A phase 3, multicenter, randomized, double-blind, placebo-controlled, safety, tolerability, and efficacy study of Xtampza ER in patients with moderate-to-severe chronic low back pain. Pain. 2015;156(12):2458–2467. doi: 10.1097/j.pain.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 7.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 8.ScienceDirect Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. [Accessed August 07, 2018];J pain. 2019 10(2):113–130.e22. doi: 10.1016/j.jpain.2008.10.008. Available from: https://www.sciencedirect.com/science/article/pii/S1526590008008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federation of State Medical Boards of the United States Model policy on the use of opioid analgesics in the treatment of chronic pain. [Accessed August 07, 2018]. Available from: http://www.painpolicy.wisc.edu/sites/default/files/sites/www.painpolicy.wisc.edu/files/pain_policy_july2013.pdf.
- 10.Klepstad P, Kaasa S, Jystad A, Hval B, Borchgrevink PC. Immediate- or sustained-release morphine for dose finding during start of morphine to cancer patients: a randomized, double-blind trial. Pain. 2003;101(1-2):193–198. doi: 10.1016/s0304-3959(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 11.Argoff CE, Silvershein DI. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84(7):602–612. doi: 10.1016/S0025-6196(11)60749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine PG, Mahajan G, Mcpherson ML. Long-acting opioids and short-acting opioids: appropriate use in chronic pain management. Pain Med. 2009;10(Suppl 2):S79–S88. doi: 10.1111/j.1526-4637.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 13.Califf RM, Woodcock J, Ostroff S. A Proactive Response to Prescription Opioid Abuse. N Engl J Med. 2016;374(15):1480–1485. doi: 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- 14.OxyContin® Purdue Pharma, LP, Stamford, CT. 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022272s034lbl.pdf.
- 15.Embeda®. Pfizer Inc; New York, NY: Dec, 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022321s022lbl.pdf. [Google Scholar]
- 16.Hysingla ER™. Purdue Pharma, LP, Stamford, CT. 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206627s004lbl.pdf.
- 17.MorphaBond ER™ Inspirion Delivery Sciences, LLC, Basking Ridge, NJ. 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/206544s002s005lbl.pdf.
- 18.Xtampza® ER. Collegium Pharmaceutical, Inc; Canton, MA: 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208090s003lbl.pdf. [Google Scholar]
- 19.Troxyca® ER. Pfizer Inc; New York NY: 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207621s004lbl.pdf. [Google Scholar]
- 20.Arymo™ ER. Egalet US Inc; Wayne, PA: 2017. [AccessedNovember 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208603s000lbl.pdf. [Google Scholar]
- 21.Vantrela™ ER. Teva Pharmaceuticals USA, Inc; North Wales PA: 2017. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207975s000lbl.pdf. [Google Scholar]
- 22.Targiniq™ ER. Purdue Pharma LP; Stamford CT: 2016. [Accessed November 3, 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205777s004lbl.pdf. [Google Scholar]
- 23.Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res. 2018;11:923–934. doi: 10.2147/JPR.S160255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Miaskowski C, Dodd M, West C, et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22(9):1713–1720. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 26.Webster L. Update on abuse-resistant and abuse-deterrent approaches to opioid formulations. Pain Med. 2009;10(Suppl 2):S124–S133. doi: 10.1111/j.1526-4637.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 27.Iwanicki JL, Severtson SG, Mcdaniel H, et al. Abuse and Diversion of Immediate Release Opioid Analgesics as Compared to Extended Release Formulations in the United States. PLoS One. 2016;11(12):e0167499. doi: 10.1371/journal.pone.0167499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicero TJ, Ellis MS, Kasper ZA. Relative preferences in the abuse of immediate-release versus extended-release opioids in a sample of treatment-seeking opioid abusers. Pharmacoepidemiol Drug Saf. 2017;26(1):56–62. doi: 10.1002/pds.4078. [DOI] [PubMed] [Google Scholar]
- 29.Moorman-Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412–418. [PMC free article] [PubMed] [Google Scholar]
- 30.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res. 2017;10:383–387. doi: 10.2147/JPR.S129553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopecky EA, Fleming AB, Noonan PK, et al. Impact of physical manipulation on in vitro and in vivo release profiles of oxycodone DETERx®: an extended-release, abuse-deterrent formulation. J Opioid Manag. 2014;10(4):233–246. doi: 10.5055/jom.2014.0211. [DOI] [PubMed] [Google Scholar]