Abstract
The honey bee is a widely managed crop pollinator that provides the agricultural industry with the sustainability and economic viability needed to satisfy the food and fiber needs of our society. Excessive exposure to apicultural pesticides is one of many factors that has been implicated in the reduced number of managed bee colonies available for crop pollination services. The goal of this study was to assess the impact of exposure to commonly used, beekeeper-applied apicultural acaricides on established biochemical indicators of bee nutrition and immunity, as well as morphological indicators of growth and development. The results described here demonstrate that exposure to tau-fluvalinate and coumaphos has an impact on 1) macronutrient indicators of bee nutrition by reducing protein and carbohydrate levels, 2) a marker of social immunity, by increasing glucose oxidase activity, and 3) morphological indicators of growth and development, by altering body weight, head width, and wing length. While more work is necessary to fully understand the broader implications of these findings, the results suggest that reduced parasite stress due to chemical interventions may be offset by nutritional and immune stress.
Keywords: honey bee, acaricide, nutrition, immunity, growth
The honey bee [Apis mellifera L. (Hymenoptera: Apidae)] plays an important role in satisfying human food and fiber needs. The annual value of pollination services provided by honey bees in the United States exceeds $14 billion (Morse and Calderone 2000), while the global contribution of pollinators to food production is estimated at more than $200 billion (Gallai et al. 2009). Furthermore, an estimated 35% of the food consumed by humans comes from crops that depend on pollinators, and 52 of the 115 leading global food commodities are dependent on honey bee pollination for either fruit or seed set (Klein et al. 2007). Unfortunately, the benefits provided by honey bees in the United States are being threatened by ongoing high rates of mortality among managed colonies (Calderone 2012), which have declined by about 60% between 1947 and 2008 (vanEngelsdorp and Meixner 2010, Ellis et al. 2010). These losses are being driven by a wide range of interrelated factors, at the heart of which is the idea that external stressors such as parasite pressure, pesticide exposure, and poor nutrition reduce immunocompetence and subsequently increase pathogen loads (Goulson et al. 2015, O’Neal et al. 2018).
The ectoparasitic mite Varroa destructor (Parasitiformes: Varroidae), which can produce significant detrimental effects on colony health if left untreated (Bowen-Walker and Gunn 2001, Amdam et al. 2004, Yang and Cox-Foster 2005), is the primary target for beekeeper-applied acaricides, which are among the most common contaminants of the hive environment (Chauzat et al. 2009, Mullin et al. 2010, Li et al. 2015). The two most commonly detected acaricides are the pyrethroid tau-fluvalinate (Apistan) and the organophosphate coumaphos (CheckMite+) (Mullin et al. 2010). As a pyrethroid insecticide, tau-fluvalinate alters the gating kinetics of voltage-gated sodium channels, disrupting the propagation of action potentials in the cholinergic nervous system (Narahashi 1971), while the organophosphate coumaphos prevents the hydrolysis of acetylcholine, causing continual stimulation of the neuron and eventual paralysis of the insect (Fukuto 1990). Both acaricides are lipophilic compounds that are readily absorbed by beeswax (Bogdanov 2006) and have previously been reported to negatively impact bee immunocompetence (Boncristiani et al. 2012, Locke et al. 2012). Consequently, the overall goal of the work presented here is to investigate the effects of tau-fluvalinate and coumaphos on biochemical and morphological indicators of bee nutrition, immunity, and development.
The health of a bee colony can be assessed in a number of different ways, but perhaps the most straightforward approach is to measure the nutritional state of individual bees within the colony. Bee nutrition has long been studied in terms of the major macronutrient profiles, which directly correlate to the dietary requirements of bees (Haydak 1970). Nutrition is also understood to have an important impact on honey bee sensitivity to pesticides (Wahl and Ulm 1983), as well as immunocompetence (Alaux et al. 2010a, Ponton et al. 2013, DeGrandi-Hoffman and Chen 2015), possibly as a result of ion channel regulation of immunity driven by metabolic changes (O’Neal et al. 2017). Colony immunocompetence is determined by both individual- and colony-level immune responses. One common measure of individual immunity is the enzyme phenoloxidase (POX), which is responsible for elements of the cellular immune response such as melanization, wound healing, and sclerotization (Laughton et al. 2011) and has been shown to increase in bees faced with an immune challenge (Chan et al. 2009, Laughton and Siva-Jothy 2011). Colony-level immunity, also known as social immunity, includes behavioral, physiological, and organizational adaptations such as hygienic behavior, necrophoric behavior, nest architecture, the use of propolis in the colony, and glucose oxidase (GOX) production (Traniello et al. 2002, Evans et al. 2006). GOX, which is produced in the hypopharyngeal gland to catalyze hydrogen peroxide production for the sterilization of hive products and honey, is a commonly used indicator of colony-level immunity (Alaux et al. 2010a). Here, we report the effects of tau-fluvalinate and coumaphos exposure on markers of nutrition, immunity, and growth of the honey bee by describing changes in: 1) total proteins; 2) total carbohydrates; 3) total lipids; 4) POX activity; 5) GOX activity; and 6) body weight, head width, and wing length of nurse and forager bees from treated and untreated colonies.
Materials and Methods
Reagents
Anthrone, l-dopa, and vanillin reagents were purchased from Acros Organics (New Jersey). Bicinchoninic acid, chloroform, copper sulfate, sulfuric acid, Triton X-100, and glucose were purchased from Sigma Aldrich (St. Louis, MO). Chymotrypsin and o-dianisidine were purchased from MP Biomedicals (Solon, OH). Horseradish peroxidase was purchased from Novex Life Technologies (Grand Island, NY). Coumaphos (CheckMite) was purchased from Bayer CropScience (RTP, NC) and tau-fluvalinate (Apistan) was purchased from Zoecon (Charlotte, NC).
Experimental Colonies and Bee Marking
Nine experimental honey bee colonies were established in May at each of the three apiaries maintained by the Department of Entomology at Virginia Tech (a total of 27 colonies) and allowed to reach colony strength by June, approximately 6 wks following establishment. Each of the 27 experimental colonies consisted of a single-story hive constructed using new frames and foundation to limit pesticide pre-exposure; however, given that wax foundation is typically made from recycled commercial beeswax, it is possible that some pesticide contaminants were present. Each hive was also provided with a sister queen to reduce genetic variation among the colonies. Nine colonies were assigned to each of the three treatments (see Experimental Treatments) with the treatments allocated evenly among hives at the three apiaries. In order to reduce variability due to the age of the bees selected for analysis, age-matched adult bees were obtained by removing two random frames of brood from each colony. The frames were caged and housed in an incubator at 34°C with a 50–80% RH for 8 h, during which time adult bees emerged from the brood frames. Groups of approximately 100 bees were marked after emergence using Testors model paint and then smoked with pine needle smoke to eliminate paint odors before the bees were returned to their respective hives. This process was repeated periodically to ensure that marked groups of the appropriate age were available.
Experimental Treatments
Colonies at each apiary received one of three treatments: 1) untreated control (no acaricide), 2) tau-fluvalinate (Apistan, Zoecon), or 3) coumaphos (CheckMite+, Bayer CropScience). For the tau-fluvalinate and coumaphos treatments, colonies were treated with either two tau-fluvalinate-impregnated strips (10.25% active ingredient each) or two coumaphos-impregnated strips (10.00% active ingredient each) for 6 wks according to the manufacturer’s label recommendations. Following the 6-wk treatment period, samples of marked bees from two age groups, nurse and forager bees, were collected. Nurse bees were collected from the brood nest and forager bees from the hive entrance of each colony. Samples consisted of a minimum of 20 bees to ensure that five individuals from each hive were available for protein, carbohydrate, and lipid analysis, five individuals for POX and GOX activity, and 10 individuals for morphometric measurements. Bee samples were frozen in liquid nitrogen and stored at −80°C until analysis. Analysis of total proteins, carbohydrates, lipids, POX activity, and GOX activity was conducted using 45 bees and morphometric measurements were conducted using 90 bees.
Biochemical and Morphological Measurements
Total Protein
The concentration of total proteins in sampled bees was measured according to the method of Smith et al. (Smith et al. 1985), with modifications. Individual bees were homogenized in 1 ml of ice-cold 0.1 M sodium phosphate (pH 7.8) containing 0.3% Triton X-100 using a glass/teflon tissue homogenizer. Homogenates were centrifuged at 10,000 × g for 10 min at 4°C and the supernatants were transferred to clean 1.5 ml microcentrifuge tubes. Ten microliters of each supernatant were added to an individual well of a 96-well microplate containing 10 μl of 0.1 M sodium phosphate (pH 7.8) and 180 μl of bicinchoninic acid with 4% (v/v) copper sulfate. Samples were incubated for 30 min at 37°C and then cooled to room temperature for 5 min. The total protein content in each sample was measured at 560 nm using a Molecular Devices SpectraMax M2 multimode microplate reader (Sunnyvale, CA). The optical densities of the protein samples were compared with those measured for a bovine serum albumin protein standard. The R2 value for the equation was 0.99.
Total Carbohydrates
The concentration of total carbohydrates in sampled bees was measured according to the method of Van Handel and Day (Van Handel and Day 1988), with modifications. Individual bees were homogenized in 1 ml of ice-cold 0.1 M sodium phosphate (pH 7.8) containing 0.3% Triton X-100 using a glass/teflon tissue homogenizer. Homogenates were centrifuged at 10,000 × g for 10 min at 4°C and the supernatants were transferred to clean 1.5 ml microcentrifuge tubes. Twenty microliters of each supernatant were added to a clean 5 ml glass centrifuge tube containing 1.98 ml of anthrone reagent. Samples were incubated at 90°C for 15 min and then cooled at room temperature. Two hundred microliters of each sample were added to an individual well of a 96-well microplate. The total carbohydrate content in each sample was measured at 625 nm using a Molecular Devices SpectraMax M2 multimode microplate reader. The optical densities of the carbohydrate samples were compared with those measured for a glucose carbohydrate standard. The R2 value for the equation was 0.99.
Total Lipids
The concentration of total lipids in sampled bees was measured according to the method of Van Handel and Day (Van Handel and Day 1988), with modifications. Individual bees were homogenized in 1 ml of ice-cold 0.1 M sodium phosphate (pH 7.8) containing 0.3% Triton X-100 using a glass/teflon tissue homogenizer. Homogenates were centrifuged at 10,000 × g for 10 min at 4°C and the supernatants were transferred to clean 1.5 ml microcentrifuge tubes. Twenty microliters of each supernatant were added to a clean 5 ml glass centrifuge tube containing 200 μl of chloroform and 200 μl of sulfuric acid. The lipid samples were incubated at 90°C for 10 min followed by the addition of vanillin. Samples were then cooled at room temperature. Two hundred microliters of each sample were added to an individual well of a 96-well microplate. The total lipid content in each sample was measured at 625 nm using a Molecular Devices SpectraMax M2 multimode microplate reader. The optical densities of the lipid samples were compared with those measured for a vegetable oil standard. The R2 value for the equation was 0.99.
POX Activity
POX activity in sampled bees was measured according to the method of Laughton and Siva-Jothy (Laughton and Siva-Jothy 2011), with modifications. Using 1 μl capillary tubes, 2 μl hemolymph were collected from the fourth abdominal tergite of each individual honey bee and diluted in ice-cold 0.1 M sodium phosphate (pH 7.8) containing 0.3% Triton X-100. Nine microliters of diluted hemolymph were added to the individual well of a 96-well microplate containing 20 μl 0.1 M sodium phosphate (pH 7.8) and 135 μl deionized H2O. Five microliters of chymotrypsin were added to the wells. Samples were incubated for 5 min at 37°C followed by the addition of 20 μl l-dopa. POX activity was measured at 490 nm for 60 min at 15 s intervals on a Molecular Devices SpectraMax M2 multimode microplate reader. Activity was recorded as the change in optical density over time (ΔmOD) and standardized using the total protein concentration for each hemolymph sample. The total protein concentration was determined as described above using a bovine serum albumin standard.
GOX Activity
GOX activity in sampled bees was measured according to the method of Alaux et al. (2010b), with modifications. Heads were dissected from individual bees and homogenized in 1 ml of ice-cold 0.1 M sodium phosphate (pH 7.8) containing 0.3% Triton X-100 using a glass/teflon tissue homogenizer. Homogenates were centrifuged at 10,000 × g for 10 min at 4°C and the supernatants were transferred to clean 1.5 ml microcentrifuge tubes. Fifty microliters of each supernatant were added to an individual well of a 96-well microplate containing 0.5 M potassium phosphate (pH 7.0), 0.1 M glucose, and 2.5 U of horseradish peroxidase. Samples were incubated for 10 min at 37°C followed by the addition of 3 mM O-dianisidine. GOX activity was measured at 430 nm for 90 min at 15 s intervals on a Molecular Devices SpectraMax M2 multimode microplate reader. Activity was recorded as the change in optical density over time (ΔmOD) and standardized using the total protein concentration for each sample. The total protein concentration was determined as described above using a bovine serum albumin standard.
Head Width, Wing Length, and Body Mass
Morphometric measurements of the sampled bees were conducted according to the method of Wilson-Rich et al. (2008). The total body weight (wet weight) of individual bees was measured to the nearest milligram using a Mettler AE 100 analytical balance (Mettler, Toledo). The head width (mm) and forewing length (mm) of individual bees were measured using a Dinolite Pro AM413T/AD413T.
Statistical Analysis
All calculations and statistical analyses were carried out using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Caste differences in total proteins, carbohydrates, lipids, POX, GOX activities, and morphometrics for each acaricide treatment were statistically compared to untreated controls using a two-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test (Zar 2007). All statistical tests were carried out at a significance level (α) of 0.05.
Results
Total Proteins
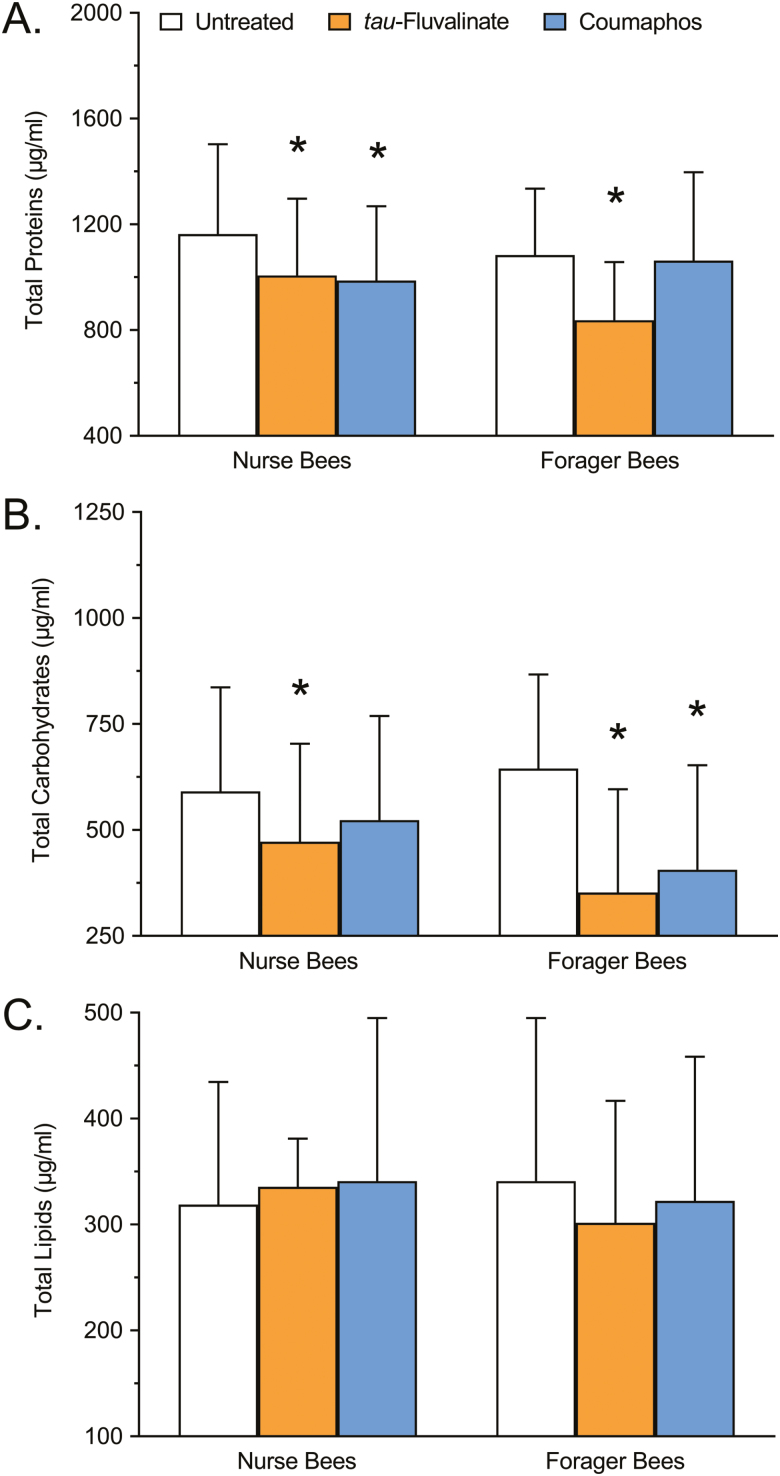
The total protein concentration of nurse and forager honey bees treated with tau-fluvalinate or coumaphos is shown in Fig. 1A. No significant interaction due to caste was detected (F = 2.677; df = 1, 264; P = 0.1030), but a significant interaction due to treatment was observed (F = 10.89; df = 2, 264; P < 0.0001). The total protein concentration of nurse bees was significantly lowered following exposure to tau-fluvalinate (13.50%; P = 0.0206), as well as coumaphos (15.13%; P = 0.0083), relative to untreated controls. The total protein concentration of forager bees was also significantly lowered following exposure to tau-fluvalinate (22.76%; P < 0.0001), but was not significantly altered following exposure to coumaphos (2.03%; P = 0.9137), relative to untreated controls.
Fig. 1.
Nutritional analysis showing (A) total protein, (B) total carbohydrate, and (C) total lipid content of nurse and forager honey bees following exposure to tau-fluvalinate (Apistan, 10.25% a.i.) or coumaphos (CheckMite+, 10.00% a.i.), compared with an untreated control. Bars represent mean protein level (µg/ml) ± SD (n = 45). Asterisks denote that the means are significantly different from the respective untreated control according to a two-way ANOVA and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
Total Carbohydrates
The total carbohydrate concentration of nurse and forager honey bees treated with tau-fluvalinate or coumaphos is shown in Fig. 1B. A significant interaction due to both caste (F = 4.341; df = 1, 261; P = 0.0382) and treatment (F = 17.62; df = 2, 261; P < 0.0001) was observed. The total carbohydrate concentration of nurse bees was significantly lowered following exposure to tau-fluvalinate (20.10%; P = 0.0399), but was not significantly altered following exposure to coumaphos (11.46%; P = 0.3124), relative to untreated controls. The total carbohydrate concentration of forager bees, however, was significantly lowered following exposure to tau-fluvalinate (45.36%; P < 0.0001), as well as coumaphos (37.02%; P < 0.0001), relative to untreated controls.
Total Lipids
The total lipid concentration of nurse and forager honey bees treated with tau-fluvalinate or coumaphos is shown in Fig. 1C. No significant interaction due to either caste (F = 0.4409; df = 1, 264; P = 0.5073) or treatment (F = 0.2832; df = 2, 264; P = 0.7536) was detected. The total lipid concentration of nurse bees was not significantly altered following exposure to either tau-fluvalinate (5.27%; P = 0.7508) or coumaphos (6.97%; P = 0.6128), nor was the total lipid concentration of forager bees significantly altered following exposure to either tau-fluvalinate (11.53%; P = 0.2389) or coumaphos (5.49%; P = 0.7022), relative to untreated controls.
POX Activity
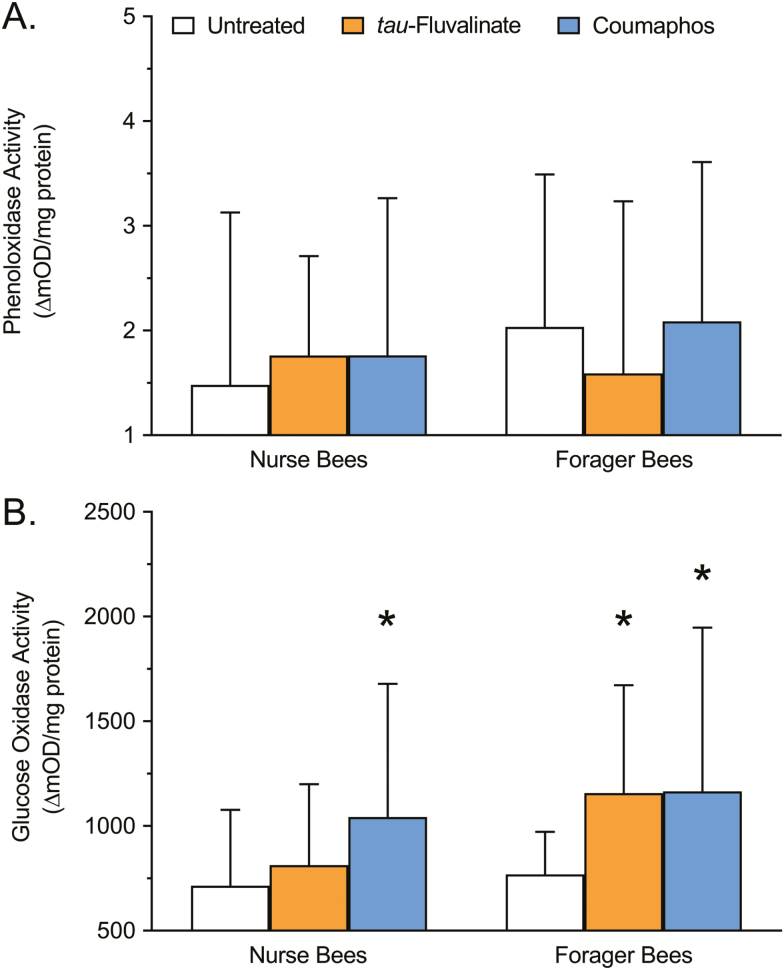
POX activity of nurse and forager honey bees treated with tau-fluvalinate or coumaphos is shown in Fig. 2A. No significant interaction due to either caste (F = 1.724; df = 1, 261; P = 0.1904) or treatment (F = 0.6670; df = 2, 261; P = 0.5141) was detected. POX activity of nurse bees was not significantly altered following exposure to either tau-fluvalinate (18.92%; P = 0.5683) or coumaphos (19.05%; P = 0.5640), nor was POX activity of forager bees significantly altered following exposure to either tau-fluvalinate (21.82%; P = 0.2743) or coumaphos (2.56%; P = 0.9802), relative to untreated controls.
Fig. 2.
Analysis of immune responsiveness showing (A) total POX and (B) total GOX activity of nurse and forager honey bees following exposure to tau-fluvalinate (Apistan, 10.25% a.i.) or coumaphos (CheckMite+, 10.00% a.i.), compared with an untreated control. Bars represent mean activity level (ΔmOD/mg protein) ± SD (n = 45). Asterisks denote that the means are significantly different from the respective untreated control according to a two-way ANOVA and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
GOX Activity
GOX activity of nurse and forager honey bees treated with tau-fluvalinate or coumaphos is shown in Fig. 2B. A significant interaction due to both caste (F = 7.586; df = 1, 262; P = 0.0063) and treatment (F = 11.50; df = 2, 262; P < 0.0001) was observed. GOX activity of nurse bees was not significantly altered following exposure to tau-fluvalinate (13.71%; P = 0.5698), but was significantly higher following exposure to coumaphos (45.94%; P = 0.5683), relative to untreated controls. GOX activity of forager bees was significantly higher following exposure to tau-fluvalinate (50.51%; P = 0.0010), as well as coumaphos (51.68%; P = 0.0006), relative to untreated controls.
Body Weight, Head Width, and Wing Length
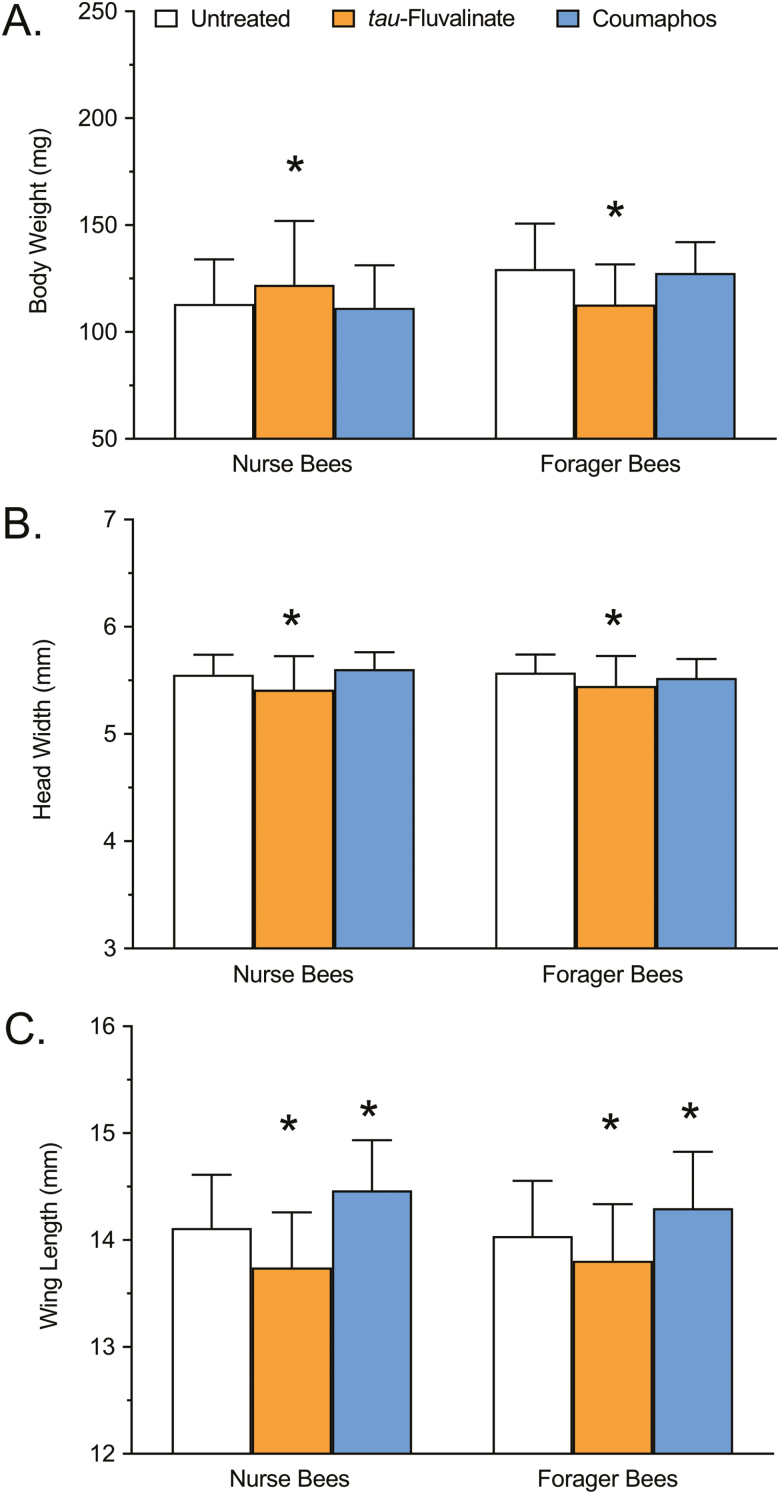
The results of the morphometric measurements of nurse and forager honey bees treated with tau-fluvalinate or coumaphos are shown in Fig. 3A–C. For body weight, a significant interaction due to caste was observed (F = 17.99; df = 1, 534; P < 0.0001), but no significant interaction due to treatment was detected (F = 1.512; df = 2, 534; P = 0.2214). Relative to untreated controls, the body weight of nurse bees was significantly higher following exposure to tau-fluvalinate (7.77%; P = 0.0110), but was unchanged following exposure to coumaphos (1.68%; P = 0. 7798) (Fig. 3A). Likewise, the body weight of forager bees was significantly lower following exposure to tau-fluvalinate (12.83%; P < 0.0001), but was unchanged following exposure to coumaphos (1.39%; P = 0.7932) (Fig. 3A).
Fig. 3.
Morphometric analysis showing (A) body weight, (B) head width, and (C) wing length of nurse and forager honey bees exposed to tau-fluvalinate (Apistan, 10.25% a.i.) or coumaphos (CheckMite+, 10.00% a.i.), compared with an untreated control. Bars represent mean body weight (mg), head width (mm), or wing length (mm) ± SD (n = 90). Asterisks denote that the means are significantly different from the respective untreated control according to a two-way ANOVA and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
For head width, no significant interaction due to caste was detected (F = 0.2259; df = 1, 534; P = 0.6347), but a significant interaction due to treatment was observed (F = 21.49; df = 2, 534; P < 0.0001). Relative to untreated controls, the head width of nurse bees was significantly decreased following exposure to tau-fluvalinate (2.52%; P < 0.0001), but was unchanged following exposure to coumaphos (0.94%; P = 0. 2015) (Fig. 3B). Likewise, the head width of forager bees was significantly decreased following exposure to tau-fluvalinate (2.24%; P = 0.0003), but was unchanged following exposure to coumaphos (0.92%; P = 0.2129) (Fig. 3B).
For wing length, no significant interaction due to caste was detected (F = 2.693; df = 1, 534; P = 0.1013), but a significant interaction due to treatment was observed (F = 57.16; df = 2, 534; P < 0.0001). Relative to untreated controls, wing length was significantly decreased in nurse bees following exposure to tau-fluvalinate (2.62%; P < 0.0001), but was significantly increased in nurse bees following exposure to coumaphos (2.48%; P < 0.0001). Likewise, wing length was significantly decreased in nurse bees following exposure to tau-fluvalinate (1.64%; P = 0.0048), but was significantly increased following exposure to coumaphos (1.85%; P = 0.0014) (Fig. 3C).
Discussion
There are numerous stressors that negatively impact honey bee health and immunocompetence, including exposure to pesticides (O’Neal et al. 2018), as well as the presence of the ectoparasitic mite V. destructor. By directly feeding on bees throughout their life cycle, mites reduce the overall health and immune responsiveness of the insect, in addition to facilitating the spread of pathogens and causing previously covert infections to become devastating outbreaks (Genersch and Aubert 2010, Le Conte et al. 2010, Nazzi et al. 2012, Ryabov et al. 2014). At this time, however, the most effective strategy for controlling mite populations is the use of chemical interventions. An extensive survey of managed bee colonies in North America detected a wide range of agricultural and apicultural pesticides contaminating the hive environment, among the most common of which were the beekeeper-applied acaricides tau-fluvalinate (Apistan) and coumaphos (CheckMite+) (Mullin et al. 2010). Although tau-fluvalinate and coumaphos have been found to be less efficacious in recent years as a result of increasing metabolic and target-site resistance in Varroa populations (Pettis 2004), their high prevalence is likely due to a combination of their continued use by beekeepers and their lipophilic nature, which allows them to persist in beeswax (Bogdanov 2006).
The results of this study show that tau-fluvalinate and coumaphos exposure had an impact on indicators of bee nutrition, evident in reduced protein levels and carbohydrate levels, on social immunity, evident through increased GOX activity, and on growth and development, evident through altered body weight, head width, and wing length. Low macronutrient concentrations have been associated with decreased colony population growth (Zheng et al. 2014); reduced worker lifespan (Knox et al. 1971); and impairment of energy-intensive tasks such as flight, thermoregulation, and comb building (Brodschneider and Crailsheim 2010). Not surprisingly, nutritionally deficient bees also display signs of impaired growth and development when assessing general morphometric indicators. These results, in particular as they relate to tau-fluvalinate, stand in contrast to previously reported studies showing that tau-fluvalinate did not have an effect on body weight or protein and carbohydrate levels in treated bees (Feazel-Orr et al. 2016). The differences in the observed results are possibly due to genetic and/or age-related variation, as the previous study did not establish colonies using sister queens, nor were the sampled bees age-matched. The observed increases in GOX activity suggest that exposure to these acaricides is potentially inducing a social immune response. Interestingly, GOX levels previously have been shown to decrease in the presence of a neonicotinoid pesticide (Alaux et al. 2010b), which could be due to either the different modes of action, or differences in experimental design.
In the effort to understand the factors that influence honey bee health, it is generally understood that interactions between pesticide exposure, mite stress, limits to nutrition, and immune challenges are all factors that contribute to colony stress and can decrease overall colony health. Beekeepers are faced with an often difficult choice between utilizing chemical interventions to treat their hives for mites or risking colony loss due to the stress caused by overwhelming mite populations. The results of this study suggest that the use of tau-fluvalinate and coumaphos, while reducing stress due to mite feeding, may increase nutritional stress and decrease the effectiveness of select social immune responses, though more work is needed to evaluate the long-term impact of these changes.
Author Competing Interests
The authors declare no competing financial interests.
Acknowledgments
This study was partially funded by a grant from the Virginia Department of Agriculture and Consumer Services (MOA 2013-001; registration fees paid by pesticide companies).
Author Contributions
A.M.R., R.D.F., C.C.B., and T.D.A. contributed in conceptualization for this manuscript. A.M.R. participated in investigation and S.T.O., C.C.B., and T.D.A. contributed in formal analysis of manuscript. A.M.R. and S.T.O. contributed in original draft preparation. A.M.R., S.T.O., R.D.F., C.C.B., and T.D.A. contributed in review and editing.
References Cited
- Alaux C., Ducloz F., Crauser D., and Le Conte Y.. 2010a. Diet effects on honeybee immunocompetence. Biol. Lett. 6: 562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaux C., Brunet J. L., Dussaubat C., Mondet F., Tchamitchan S., Cousin M., Brillard J., Baldy A., Belzunces L. P., and Le Conte Y.. 2010b. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 12: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam G. V., Hartfelder K., Norberg K., Hagen A., and Omholt S. W.. 2004. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering?J. Econ. Entomol. 97: 741–747. [DOI] [PubMed] [Google Scholar]
- Bogdanov S. 2006. Contaminants of bee products. Apidologie 37: 1–18. [Google Scholar]
- Boncristiani H., Underwood R., Schwarz R., Evans J. D., Pettis J., and vanEngelsdorp D.. 2012. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 58: 613–620. [DOI] [PubMed] [Google Scholar]
- Bowen-Walker P. L., and Gunn A.. 2001. The effect of the ectoparasitic mite, varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomol. Experiment. Appl. 101: 207–217. [Google Scholar]
- Brodschneider R., and Crailsheim K.. 2010. Nutrition and health in honey bees. Apidologie 41: 278–294. [Google Scholar]
- Calderone N. W. 2012. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992-2009. Plos One. 7: e37235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, Q. W., A. P. Melathopoulos, S. F. Pernal, and L. J. Foster. 2009. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genomics 10: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauzat M. P., Carpentier P., Martel A. C., Bougeard S., Cougoule N., Porta P., Lachaize J., Madec F., Aubert M., and Faucon J. P.. 2009. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ. Entomol. 38: 514–523. [DOI] [PubMed] [Google Scholar]
- DeGrandi-Hoffman G., and Chen Y.. 2015. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 10: 170–176. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D., and Meixner M. D.. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 103 (Suppl 1): S80–S95. [DOI] [PubMed] [Google Scholar]
- Ellis J. D., Evans J. D., and Pettis J.. 2010. Colony losses, managed colony population decline, and colony collapse disorder in the United States. J. Apicult. Res. 49: 134–136. [Google Scholar]
- Evans J. D., Aronstein K., Chen Y. P., Hetru C., Imler J. L., Jiang H., Kanost M., Thompson G. J., Zou Z., and Hultmark D.. 2006. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 15: 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feazel-Orr H. K., Catalfamo K. M., Brewster C. C., Fell R. D., Anderson T. D., and Traver B. E.. 2016. Effects of pesticide treatments on nutrient levels in worker honey bees (Apis mellifera). Insects 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto T. R. 1990. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 87: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallai N., Salles J. M., Settele J., and Vaissiere B. E.. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68: 810–821. [Google Scholar]
- Genersch E., and Aubert M.. 2010. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 41: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D., Nicholls E., Botías C., and Rotheray E. L.. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347: 1255957. [DOI] [PubMed] [Google Scholar]
- Haydak M. H. 1970. Honey bee nutrition. Annu. Rev. Entomol. 15: 143–156. [Google Scholar]
- Klein A. M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., and Tscharntke T.. 2007. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. A., Shimanuki H., and Herbert E. W.. 1971. Diet and the longevity of adult honey bees. J. Econ. Entomol. 64: 1415–1416. [Google Scholar]
- Laughton A. M., and Siva-Jothy M. T.. 2011. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie 42: 140–149. [Google Scholar]
- Laughton A. M., Boots M., and Siva-Jothy M. T.. 2011. The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J. Insect Physiol. 57: 1023–1032. [DOI] [PubMed] [Google Scholar]
- Le Conte Y., Ellis M., and Ritter W.. 2010. Varroa mites and honey bee health: can varroa explain part of the colony losses?Apidologie 41: 353–363. [Google Scholar]
- Li Y., Kelley R. A., Anderson T. D., and Lydy M. J.. 2015. Development and comparison of two multi-residue methods for the analysis of select pesticides in honey bees, pollen, and wax by gas chromatography-quadrupole mass spectrometry. Talanta 140: 81–87. [DOI] [PubMed] [Google Scholar]
- Locke B., Forsgren E., Fries I., and de Miranda J. R.. 2012. Acaricide treatment affects viral dynamics in varroa destructor-infested honey bee colonies via both host physiology and mite control. Appl. Environ. Microbiol. 78: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse R. A., and Calderone N. W.. 2000. The value of honey bees as pollinators of U.S. crops in 2000. Bee Culture Pollin. 2000: 1–15. [Google Scholar]
- Mullin C. A., Frazier M., Frazier J. L., Ashcraft S., Simonds R., Vanengelsdorp D., and Pettis J. S.. 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. Plos One. 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. 1971. Mode of action of pyrethroids. Bull. World Health Organ. 44: 337–345. [PMC free article] [PubMed] [Google Scholar]
- Nazzi F., Brown S. P., Annoscia D., Del Piccolo F., Di Prisco G., Varricchio P., Della Vedova G., Cattonaro F., Caprio E., and Pennacchio F.. 2012. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. Plos Pathog. 8: e1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S. T., Swale D. R., and Anderson T. D.. 2017. ATP-sensitive inwardly rectifying potassium channel regulation of viral infections in honey bees. Sci. Rep. 7: 8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S. T., Anderson T. D., and Wu-Smart J. Y.. 2018. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 26: 57–62. [DOI] [PubMed] [Google Scholar]
- Pettis J. S. 2004. A scientific note on varroa destructor resistance to coumaphos in the United States. Apidologie 35: 91–92. [Google Scholar]
- Ponton F., Wilson K., Holmes A. J., Cotter S. C., Raubenheimer D., and Simpson S. J.. 2013. Integrating nutrition and immunology: a new frontier. J. Insect Physiol. 59: 130–137. [DOI] [PubMed] [Google Scholar]
- Ryabov E. V., Wood G. R., Fannon J. M., Moore J. D., Bull J. C., Chandler D., Mead A., Burroughs N., and Evans D. J.. 2014. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after varroa destructor-mediated, or in vitro, transmission. Plos Pathog. 10: e1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., and Klenk D. C.. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- Traniello J. F. A., Rosengaus R. B., and Savoie K.. 2002. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl. Acad. Sci. USA. 99: 6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E., and Day J. F.. 1988. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control Assoc. 4: 549–550. [PubMed] [Google Scholar]
- Wahl O., and Ulm K.. 1983. Influence of pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica. Oecologia. 59: 106–128. [DOI] [PubMed] [Google Scholar]
- Wilson-Rich N., Dres S. T., and Starks P. T.. 2008. The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 54: 1392–1399. [DOI] [PubMed] [Google Scholar]
- Yang X., and Cox-Foster D. L.. 2005. Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA. 102: 7470–7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. H. 2007. Biostatistical Analysis 5th ed Pearson, Upper Saddle River, NJ. [Google Scholar]
- Zheng B., Wu Z., and Xu B.. 2014. The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. J. Insect Sci. 14: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]