Abstract
Background:
Autophagy is a physiological process that plays an important role in maintaining cellular functions. When aortic valve interstitial cells (AVICs) are stimulated with inflammatory or mechanical stress, one response is elevated pro-osteogenic activity. We hypothesized that autophagy is important in the prevention or regulation of this proosteogenic activity in AVICs.
Materials and methods:
AVICs were isolated. Autophagy activity was examined and its role in AVIC’s pro-osteogenic activity was determined using chemical inhibitors and genetic techniques. The pro-osteogenic biomarker bone morphogenetic protein 2 (BMP-2) and alkaline phosphatase (ALP) were analyzed by immunoblotting and calcium deposition assay.
Results:
Human AVICs from normal aortic valve donors displayed significantly higher autophagic activity than those from calcified aortic valve donors as indicated by lower protein levels of light chain 3-II. Suppression of autophagy by 3-methyladenine, bafilomycin, or knockdown of Atg7 gene induced the expression of BMP-2 and ALP, increased ALP activity, and calcium deposit formation in normal AVICs. Conversely, upregulation of autophagy with rapamycin or overexpression of Atg7 gene decreased the levels of BMP-2 and ALP in diseased AVICs.
Conclusions:
Our data showed that autophagy negatively regulates the pro-osteogenic activity in human AVICs, suggesting that upregulation of autophagy may prevent the progression of calcific aortic valve disease.
Keywords: Aortic valve interstitial cells, Calcification, Autophagy
Introduction
Calcific aortic valve disease (CAVD) is the major heart valve disease but its pathogenesis is not yet fully understood.1 Surgical or transcatheter intervention is the most effective treatment. Many risk factors have been linked to CAVD, such as anatomic, genetic, and clinical abnormalities. Calcification occurs more in patients with bicuspid valve, certain genetic predisposition, diabetes mellitus, hypercholesterolemia, hypertension, male gender, chronic inflammation, ageing, and smoking.2
Autophagy is an active process of “self-eating,” which is responsible for degradation or reutilizing of whole organisms and structures, and supplies cells with another source of nutrients from the recycling of cellular proteins and organelles.3 Therefore, autophagy is crucial for cell survival, differentiation, growth, and response to stress. It is related with cell death, muscular disorder, neurodegenerative diseases, cancer, pathogen infection,4 as well as coronary artery diseases.5 Usually, autophagy occurs constitutively at basal levels in most cells.4 Induction of autophagy often has beneficial effects. Autophagy seems protective in vitro model of calcification of vascular smooth muscle cells, by reducing matrix vesicle release.6 In chondrocytes, autophagy has been shown to protect a mechanical tension‒einduced end-plate chondrocytes calcification.7 Moreover, clinical usage of cholesterol-lowered drugs statins is associated with slowed progression of CAVD and coronary artery atheromatosis in some patients.8 One of the most recent studies has found that atorvastatin alleviated transforming growth factor β1einduced calcification by activating autophagy.9 It is not clear whether autophagy is associated with CAVD. Therefore, we sought to investigate the connection between autophagy and CAVD in an in vitro model of calcification of AVICs.
Materials and methods
Materials
Antibodies against light chain 3-B (LC3B), ATG7, and β-actin were purchased from Cell Signaling, Inc (Beverly, MA). Antibody against bone morphogenetic protein 2 (BMP-2) was purchased from ProSci, Inc (Poway, CA). Antibody against alkaline phosphatase (ALP) was purchased from Abcam (Cambridge, MA). Medium 199 was purchased from Lonza (Walkersville, MD). Laemmli sample buffer for immunoblotting was purchased from Bio-Rad (Hercules, CA). Bafilomycin (BAF) A1 was from Reagents Direct (Encinitas, CA). Rapamycin and 3-methyladenine (3-MA) were purchased from Sigma‒Aldrich Chemical Co (St. Louis, MO).
Replication-deficient adenoviruses
Adenovirus expressing Atg7, adenoviruses expressing β galactosidase (Ad β-gal), adenoviruses expressing a short hairpin (sh) RNA directed against Atg7 and a control shRNA (AdshCon) were kindly provided by Dr Qiangrong Liang (New York Institute of Technology College of Osteopathic Medicine, Old Westbury, NY). Unless otherwise indicated, AVICs were infected with adenovirus at a multiplicity of infection of 100 plaque-forming unit for 24 h.
Cell isolation and treatment
The normal aortic valve specimens were valves from five male adult patients with cardiomyopathy who were undergoing cardiac transplant, which is relatively “normal” and the valve leaflets were thin, pliable, and normal appearing without any calcification. The stenotic valves were obtained from five male adult patients undergoing aortic valve replacement. All patients gave informed consent for the use of their valves for this study. This study was approved by the COMIRB of the University of Colorado.10 Detailed AVIC isolation and culture protocols have been published elsewhere.11
Immunoblotting
Western blotting was applied to analyze ALP, BMP-2, phosphorylated and total mechanistic target of rapamycin, phosphorylated and total Akt, phosphorylated and total Stat3 with β-actin as a loading control. Details can be seen in previously published materials and methods.11
ALP activity staining
Cells were treated and histochemical staining for ALP activity was performed as previously described.12
Alizarin red S staining
Alizarin red S staining for calcium deposits was performed as previously described.12
Immunofluorescent staining
Cells were cultured in chamber slides to 80% confluence and infected with either AdshAtg7 or AdshCon. After permeabilizing in methanol and fixing in 4% paraformaldehyde, cells were washed with phosphate-buffered saline (PBS) and blocked with 10% donkey serum in 1% bovine serum albumin in PBS for 60 min. Cells were then incubated with anti-LC3A/B antibody (Thermo Fisher Sci., PA1–16931, 1:100 dilution) in PBS Tween (PBST) overnight at 4°C. After washing with PBST, cells were incubated with donkey-anti-rabbit IgG (Invitrogen, A21207, red; 1:200 dilution) in PBST for 60 min. Nuclei were stained with 4,6-diamidino-2-phenylindole. To assess the specificity of the immunostaining, control cells were incubated with nonimmune rabbit IgG. Photography was performed with an Olympus FV1000 FCS/RICS microscope.
Immunohistochemistry staining
Cells were cultured in chamber slides to 80% confluence and infected with either AdshAtg7 or AdshCon. After permeabilizing in methanol and fixing in 4% paraformaldehyde, cells were washed with PBS. Cells were then incubated with anti-ubiquitin antibody (Thermo Fisher Sci., MA1–10035; 1:5000 dilution) and stained using VECTASTAIN Elite ABC Kit (Burlingame, CA) and photographed with an Olympus CH-2 microscope equipped with a camera (S01–0801B) and a software (SSView, Science Supply, Schertz, TX).
Statistical analysis
Data are represented as mean ± standard error of the mean. The t-test is used to compare two groups for statistical difference, and analysis of variance is used to compare more than two groups for statistical difference. P < 0.05 was considered significant.
Results
Lower autophagic activity in calcified donors
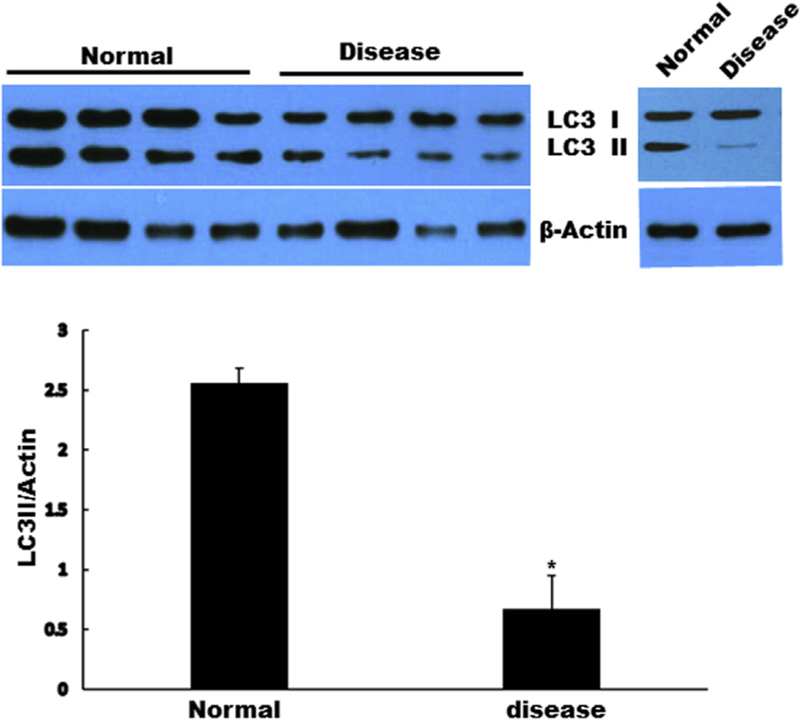
To examine if autophagy is occurring in CAVD, we first investigated the basal level of autophagosome formation in diseased AVICs compared with that in normal AVICs. AVICs from normal donors displayed significantly higher autophagic activity than that from calcified donors as indicated by changes of protein levels of autophagosome marker LC3-II; Fig. 1.
Fig. 1 -.
AVICs from normal aortic valve donors displayed significantly higher autophagic activity than that from calcified aortic valve donors as indicated by changes of the levels of LC3-II. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05 (normal versus diseased). (Color version of figure is available online.)
Suppression of autophagy stimulated osteogenic responses
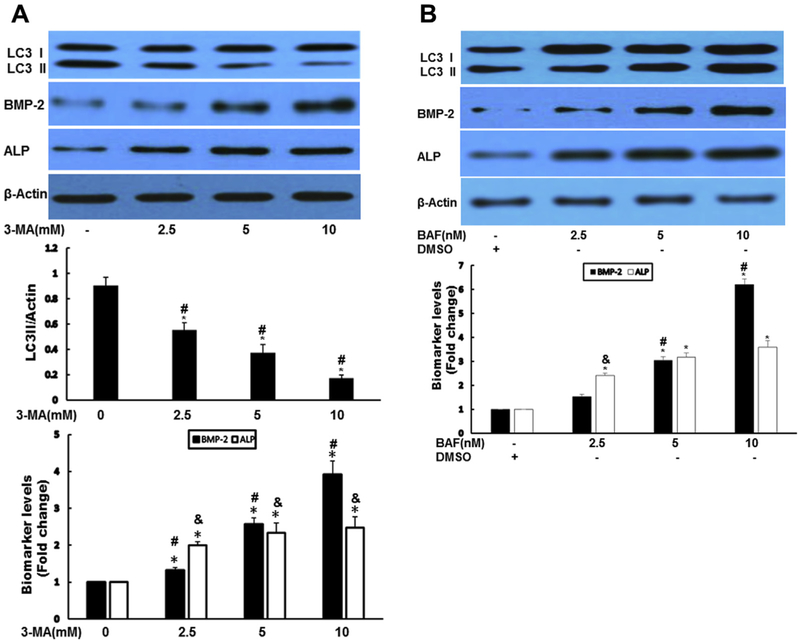
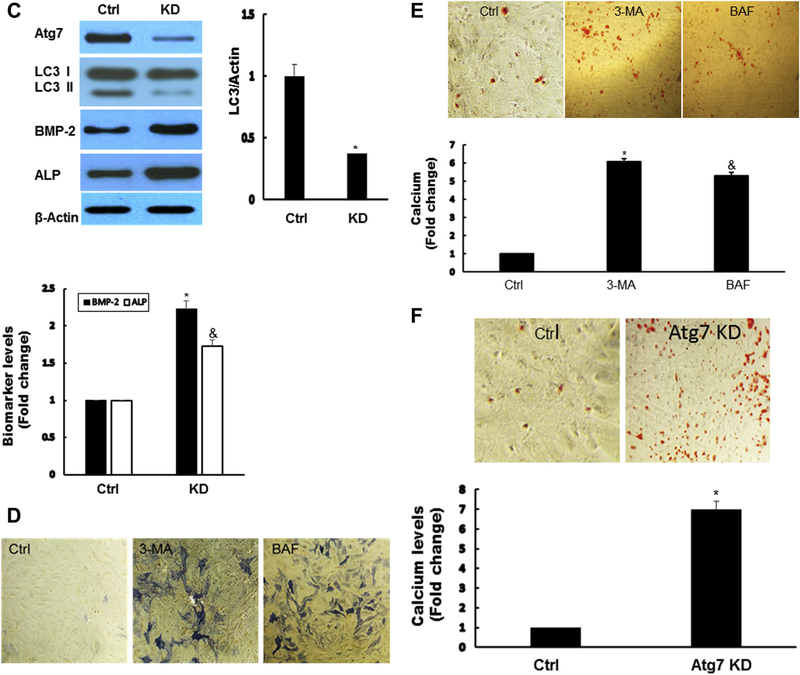
To determine the possible role of autophagy in AVICs, we next used autophagy formation inhibitor 3-MA and auto-phagy degradation inhibitor BAF-A1. Both 3-MA and BAF significantly augmented the expression of BMP-2 and ALP (Fig. 2A and B). To confirm the pharmacologic results of both autophagy inhibitors, short hairpin knockdown of autophagy protein 7 (Atg7) gene was performed in normal human AVICs. Vector encoding a scrambled shRNA was used as a control. Silencing Atg7 resulted in increased expression of BMP-2 and ALP (Fig. 2C). ALP activity staining and calcium staining assays revealed that chemical inhibition of autophagy increased ALP activity (Fig. 2D) and calcium deposit formation (Fig. 2E). Similarly, knockdown Atg7 greatly enhanced calcium deposit formation (Fig. 2F).
Fig. 2 -.
Suppression of autophagy-aggravated osteogenic responses in AVICs. (A) Autophagy inhibitor 3-MA significantly augmented the expression of BMP-2 and ALP. AVICs were treated by 3-MA at indicated time for 24 h. Autophagy indicator LC3-II, BMP-2, and ALP protein levels were analyzed by immunoblotting. Bar graphs indicate densitometry data of LC3-II, as well as BMP-2 and ALP changes. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05; @significant with each other for LC3II; #significant with each other for BMP-2; &significant with each other for ALP. (B) Autophagy inhibitor BAF-A1 significantly increased the expression of BMP-2 and ALP. AVICs were treated by BAF for 24 h, autophagy indicator LC3-II, BMP-2, and ALP protein levels were analyzed by immunoblotting. Bar graphs indicate densitometry data of BMP-2 and ALP changes. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05; #significant with each other for BMP-2; &significant with each other for ALP. (C) Genetically downregulated Atg7 resulted in increased expression of BMP-2 and ALP. AVICS were infected with adshAtg7 (KD) or coshRNA (Ctrl) for 48 h, Atg7, LC3-II, BMP-2, and ALP protein levels were analyzed by immunoblotting. Bar graphs indicate densitometry data of LC3-II, BMP-2, and ALP changes. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05 (KD compared with Ctrl); &P < 0.05 (KD compared with Ctrl). (D) Inhibition of autophagy induces ALP activity in AVICs. AVICs were treated with 3-MA (10 mM) or BAF (5 nM) for 2 wk. ALP activity was assessed by enzyme activity staining in a blinded fashion. (E). Inhibition of autophagy significantly increased mineralization in AVICs. AVICs were treated 3-MA (10 mM) or BAF (5 nM) for 2 wk (conditioning medium). Calcification was assessed by Alizarin Red S staining and quantitation was performed spectrophotometrically in a blinded fashion. *P < 0.05 (3-MA compared with Ctrl); &P < 0.05 (BAF compared with Ctrl). (F) Knockdown of Atg7 greatly increased mineralization in AVICs. AVICS were infected with adshAtg7 or coshRNA (Control, Ctrl) for 2 wk; Calcification was assessed by Alizarin Red S staining and quantitation was performed spectrophotometrically in a blinded fashion. (Color version of figure is available online.)
Activation of autophagy decreased osteogenic responses
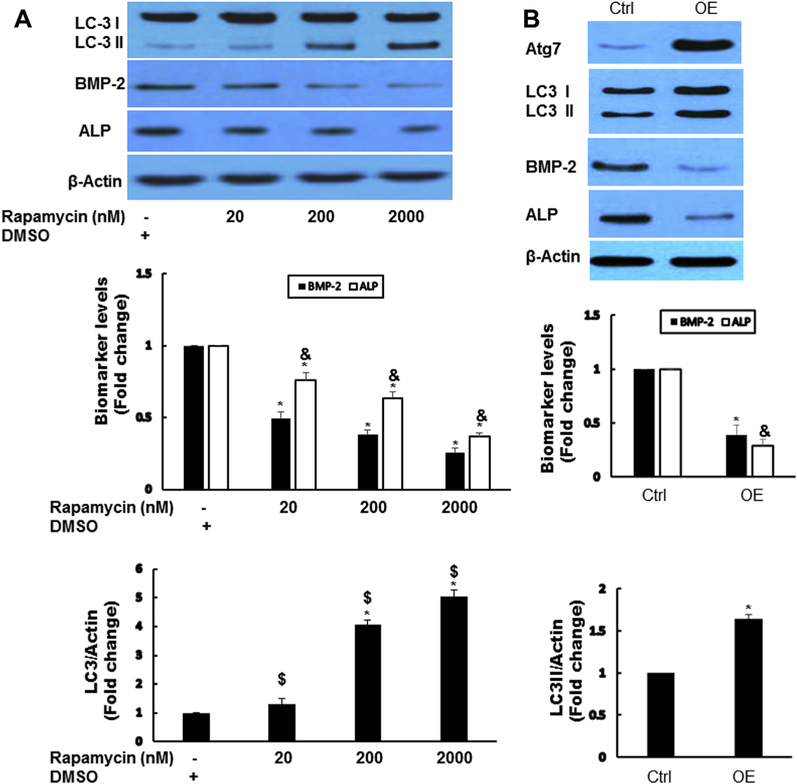
To find further the potential role of autophagy in AVICs, we manipulated autophagy activity using pharmacologic approach. Activation of autophagy by rapamycin (autophagy inducer) dose dependently decreased the levels of BMP-2 and ALP (Fig. 3A). To exclude the potential nonspecific effects of rapamycin, we used adenovial Atg7 (AdAtg7) to increase autophagy in diseased human AVICs. β-galactosidaseeexpressing vector was used as a control. Infection of AVICs with AdAtg7 resulted in enhanced Atg7 protein levels, which led to decreased levels of BMP-2 and ALP (Fig. 3B).
Fig. 3 -.
Activation of autophagy decreased osteogenic responses. (A) Activation of autophagy by rapamycin-reduced osteogenic responses in diseased AVICs. Diseased AVICs were treated by rapamycin for 24 h at indicated dose, autophagy indicator LC3-II, and osteogenic factors ALP and BMP-2 are examined by immunoblotting. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05. #Significant with each other; &significant with each other for ALP; $significant with each other for LC3-II. (B) Overexpression (OE) of Atg7 decreased osteogenic responses in diseased AVICs. Diseased AVICS were infected with adAtg7 or adbgal (Control, Ctrl) for 48 h; Atg7, LC3-II, BMP-2, and ALP protein levels were analyzed by immunoblotting. Bar graphs indicate densitometry data of LC3-II, BMP-2, and ALP changes. Results are expressed as mean ± standard error of the mean; n = 5; *P < 0.05 (OE compared with Ctrl); &P < 0.05 (OE compared with Ctrl for ALP). (Color version of figure is available online.)
LC3 is the better marker of autophagy
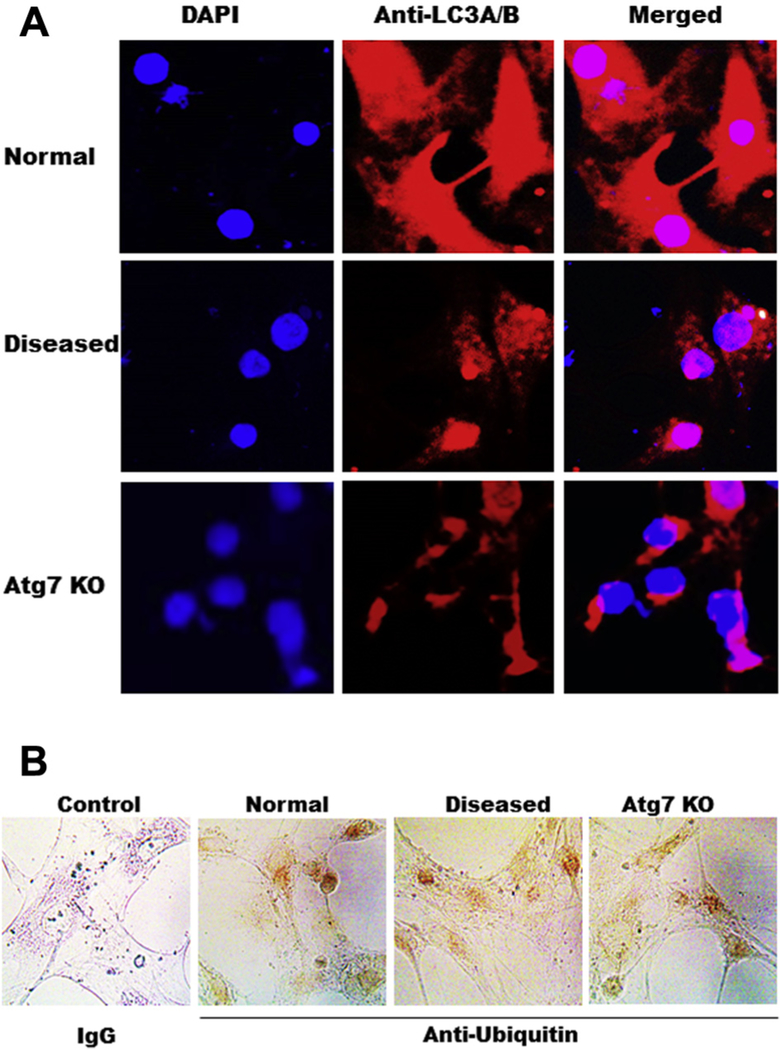
To further determine if LC-3 is a better marker of autophagy in AVICs, we compared LC3 with ubiquitin by immunofluorescent and immunohistochemistry staining. Weak ubiquitin staining is observed in AVICs but there is no difference among normal, diseased, and Atg7 knockdown, whereas LC3 is higher in normal AVIC and lower in disease AVIC as well as in AVIC of Atg7 knockdown (Fig. 4A and B).
Fig. 4 -.
LC3 is a better marker for autophagy than ubiquitin. (A) Immunofluorescent staining of LC3 in AVICs of normal, diseased, and Atg7 knockdown, 20×; (B) Immunohistochemical staining of ubiquitin in AVICs of normal, diseased, and Atg7 knockdown. Ubiquitin is stained as brown, 40×. (Color version of figure is available online.)
Discussion
In this study, we showed that AVICs from normal donors displayed significantly higher autophagic activity than that from calcified donors. Inhibition of autophagy by 3-MA, BAF, or silencing Atg7 gene induced the expression of BMP-2 and ALP, increased ALP activity and calcium deposit formation. On the other hand, induction of autophagy with rapamycin or knock-in of Atg7 gene decreased the levels of BMP-2 and ALP. It seems that autophagy prevents the osteogenic responses in human AVICs.
Autophagy is a conserved and highly controlled lysosomal pathway that breaks down cytosolic macromolecules, membranes, and organelles that maintain cellular homeostasis.13 During aging, accumulation of mis-folded proteins,14 lipid oxidation by-products,15 and damaged macromolecules have been observed and diminished autophagy is associated with those dysfunctional changes.16 This may be due to a general failure of lysosomal enzymes, leading to an acumination of damaged organelles and slow removal of autophagosomes in the aging tissues.17 Interestingly, CAVD is an age-related progressive disorder. The prevalence of mild CAVD (aortic sclerosis) is about 25% of all adults aged >65 y,18 and the epidemiologic evidence supports the association between aging and CAVD. Therefore, it is conceivable there may be a correlation between CAVD and autophagy.
A key autophagic pathway regulator is LC3, which controls the growth of autophagic membranes and the formation of autophagosomes.19,20 Two forms of LC3 is present, LC3-I and II. LC3 is processed from cytoplasm LC3-I to the membrane-bound form LC-II via conjugation with phosphatidylethanolamine in the presence of the E1-like enzyme Atg7 and the E2-like enzyme Atg3.21 Thus, LC3-II has been used as an indicator of autophagy, and the examination of LC3 levels can monitor autophagy and autophagy-related processes.22 We found that LC-II level is highly lower in diseased AVICs than that in normal AVICs.
Functional inhibition or activation of autophagy will allow us to find the connection between autophagy and human diseases, and add some new insight into the clinical development of autophagy-targeting drugs for the abnormalities. Here we found that suppression of autophagy by both the autophagy formation inhibitor 3-MA and degradation inhibitor BAF greatly induced the osteogenic responses, ALP activity, and calcium deposition in human AVICs. As expected, genetic knockdown of Atg7 significantly enhanced the osteogenic responses and calcium deposit formation in human AVICs. Actually, disrupted autophagy occurs in various diseases and animal models, which results in cell dysfunction and pathogenesis. For instance, kidneys from Beclin 1 knock-out mice reveal a profibrotic phenotype with augmented collagen deposition.23 Conversely, some auto-phagy induction is protective. Our results showed that upregulation of autophagy with autophagy inducer rapamycin or overexpression of autophagy gene Atg7 highly decreased the osteogenic responses. Recent studies have shown that induction of autophagy improved phosphate-induced calcium deposition in aortic ring explants and smooth muscle cells.6 In fact, basal autophagy is involved in maintaining chondrocyte survival and in regulating bone formation.24 Defected auto-phagy has been found in the articular cartilage in old mice compared with that from young mice.25
There is some discrepancy between this study and Somers’s study.26 They histologically evaluated ubiquitin as an auto-phagy marker on the whole tissue of calcified aortic valves. Compared with normal valve tissues, they found that ubiquitin was expressed more in diseased tissues. But they did not further investigate where ubiquitin was located. Actually, in another similar tissue histology study using aortic and mitral valves with atherosclerotic involvement, ubiquitin was found positive only in monocyte-derived foam cells.27 The cause of this discrepancy may be because Somer’s study was based on whole tissues while the findings in this study are on AVICs. Furthermore, there are drawbacks of using immunohisto-chemistry for ubiquitin as a marker of autophagy. For instance, ubiquitinated depositions can also come from faults in auto-phagic activities or from protein structural changes.28 And high ubiquitin expression is not always consistent with high auto-phagic activities. For example, increased accumulation of cytosolic ubiquitin aggregates is present in Atg7-deficient mice even though they have impaired autophagy.29 In this study, we demonstrated that weak ubiquitin staining is seen in AVICs but there is no difference among normal, diseased, and Atg7 knockdown, whereas LC3 is higher in normal AVIC and lower in disease AVIC as well as in AVIC of Atg7 knockdown, which is in agreement with our immunoblotting results. Hence, LC3 is more reliable that ubiquitin as an autophagy marker.
The primary limitation of the study is that only AVICs from male donor were used. Therefore, potential sex difference in the calcified versus noncalcified valves was not investigated.
In conclusion, our study indicated that autophagy negatively regulates osteogenic responses in human AVICs, which suggested that autophagy induction may have a therapeutic implication for initiation and development of CAVD.
Acknowledgment
This study was funded in part by the grant from the National Institutes of Health, United States (RO1 HL106582) and by the grant from the Natural Science Foundation of Guangdong Province (2015A030313297).
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
REFERENCES
- 1.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630‒634. [DOI] [PubMed] [Google Scholar]
- 2.Cowell SJ, Newby DE, Boon NA, Elder AT. Calcific aortic stenosis: same old story? Age Ageing. 2004;33:538‒544. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463‒477. [DOI] [PubMed] [Google Scholar]
- 4.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990‒995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryter SW, Lee SJ, Smith A, Choi AM. Autophagy in vascular disease. Proc Am Thorac Soc. 2010;7:40‒47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai XY, Zhao MM, Cai Y, et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83:1042‒1051. [DOI] [PubMed] [Google Scholar]
- 7.Xu HG, Yu YF, Zheng Q, et al. Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone. 2014;66:232‒239. [DOI] [PubMed] [Google Scholar]
- 8.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316‒3326. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Cui W, Liu B, et al. Atorvastatin protects vascular smooth muscle cells from TGF-β1-stimulated calcification by inducing autophagy via suppression of the β-catenin pathway. Cell Physiol Biochem. 2014;33:129‒141. [DOI] [PubMed] [Google Scholar]
- 10.Yao Q, Song R, Ao L, et al. Over-expression of neurotrophin 3 in human aortic valves affected by calcific disease induces the osteogenic responses via the Trk-Akt pathway. Biochim Biophys Acta. 2015;1852:1940‒1949. [DOI] [PubMed] [Google Scholar]
- 11.Meng X, Ao L, Song Y, et al. Expression of functional toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:C29‒C35. [DOI] [PubMed] [Google Scholar]
- 12.Deng XS, Meng X, Song R, Fullerton D, Jaggers J. Rapamycin decreases the osteogenic response in aortic valve interstitial cells through the Stat3 pathway. Ann Thorac Surg. 2016;102:1229‒1238, 13. [DOI] [PubMed] [Google Scholar]
- 13.Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol. 2008;266:207‒247. [DOI] [PubMed] [Google Scholar]
- 14.Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol. 2014;24:161‒170. [DOI] [PubMed] [Google Scholar]
- 15.Porta EA. Pigments in aging: an overview. Ann N Y Acad Sci. 2002;959:57‒65. [DOI] [PubMed] [Google Scholar]
- 16.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842‒846. [DOI] [PubMed] [Google Scholar]
- 17.Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611‒619. [DOI] [PubMed] [Google Scholar]
- 18.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly [comment]. N Engl J Med. 1999;341:142‒147. [DOI] [PubMed] [Google Scholar]
- 19.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165‒178. [DOI] [PubMed] [Google Scholar]
- 20.Noda NN, Ohsumi Y, Inagaki F. Atg8-family interacting motif crucial for autophagy. Inagaki FEBS Lett. 2010;584:1379‒1385. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488‒492. [DOI] [PubMed] [Google Scholar]
- 22.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008;445:77‒88. [DOI] [PubMed] [Google Scholar]
- 23.Kim SI, Na HJ, Ding Y, Wang Z, Lee SJ, Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J Biol Chem. 2012;287:11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivas V, Bohensky J, Zahm AM, Shapiro IM. Autophagy in mineralizing tissues: microenvironmental perspectives. Cell Cycle. 2009;8:391‒393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caramés B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568‒1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somers P, Knaapen M, Kockx M, van Cauwelaert P, Bortier H, Mistiaen W. Histological evaluation of autophagic cell death in calcified aortic valve stenosis. J Heart Valve Dis. 2006;15:43‒47. [PubMed] [Google Scholar]
- 27.Yamada T, Satoh S, Sueyoshi S, et al. Ubiquitin-positive foam cells are identified in the aortic and mitral valves with atherosclerotic involvement. J Atheroscler Thromb. 2009;16:472‒479. [DOI] [PubMed] [Google Scholar]
- 28.Mistiaen W, Knaapen M. Evaluation of cell death markers in severe calcified aortic valves (Chapter 18) in autophagy in disease and clinical applications, Part C. Methods Enzymol. 2009;453:365‒378. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu M, Waguri S, Ueno T, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425‒434. [DOI] [PMC free article] [PubMed] [Google Scholar]