Abstract
Clinical evidence suggests vitamin C (Vit C) may protect against the development of complex regional pain syndrome (CRPS) after fracture and/or surgery. Tibia fracture followed by 4 weeks cast immobilization (fracture/cast) in rats results in nociceptive, vascular, and bone changes resembling clinical CRPS. In the present study, fracture/cast rats were treated with the oxidative stress inhibitors Vit C, N-acetyl cysteine (NAC) or 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) to examine their effects on CRPS-related nociceptive and vascular changes. Administration of these agents significantly reduced fracture/cast-induced cutaneous allodynia by 64–78%, muscle hyperalgesia by 34–40% and hindlimb unweighting by 48–89%. Treatments with Vit C and NAC reduced the oxidative stress markers malondialdehyde in the skin, muscle and sciatic nerve, and lactate in the gastrocnemius muscle of the fracture/cast limb. Furthermore, Vit C treatment inhibited the post fracture up-regulation of SP and CGRP in the sciatic nerve and the increased expression of the pain-related inflammatory mediators, including interleukin 6 (IL-6), and nerve growth factor (NGF) in the skin and interleukin 1β (IL-1β), and IL-6 in the muscle of the post fracture/cast limb. These data suggest that oxidative stress may contribute to the nociceptive features of the rat CRPS model.
Perspective:
Vit C reduced the CRPS-like signs, oxidative stress, and the up regulation of neuropeptide production and inflammatory mediators observed after tibia fracture and casting in rats. Limiting oxidative stress using Vit C or alternative strategies could reduce the risk of developing CRPS after surgery or other forms of trauma.
Keywords: fracture, complex regional pain syndrome, vitamin C, cytokines, oxidative stress, immobilization
Introduction
Complex regional pain syndrome (CRPS) develops after a range of injuries including fractures and soft tissue trauma to the extremities.43 The underlying mechanism of CRPS has not been fully elucidated and there are no specific medications approved for the treatment of CRPS. Oxidative stress results from an imbalance between ROS production and antioxidant defense systems. ROS have been implicated in many degenerative neurological conditions, such as Alzheimer’s disease1 and Parkinson’s disease18 and oxidative stress may also contribute to pain in various diseases, including fibromyalgia,26 diabetes,35, 48 and CRPS.2, 10, 25, 30, 50 Levels of malondialdehyde (MDA, an oxidative stress marker), lactic dehydrogenase and antioxidants in the serum and the saliva of CRPS patients have been shown to be higher compared to healthy controls,10 even though plasma and urine levels of several markers of oxidative stress including MDA, F2-isoprostanes and 8-hydroxy-2’-deoxyguanosine (a modified DNA nucleoside product generated by reactive oxygen species (ROS)) were not found to be elevated in CRPS patients compared to healthy controls.11 Clinical studies suggest that the oral administration of the antioxidant Vit C reduces the incidence of CRPS after trauma and surgery.3, 39, 50, 51 Mechanistically, Vit C may serve to stabilize ROS that could otherwise damage lipid membranes and impair microcirculation,24, 42 nonetheless, the exact mechanism by which Vit C improves CRPS outcomes is still unclear.
ROS may also have pronociceptive effects in animal pain models. Elevated oxidative stress markers have been detected in spinal cord in the capsaicin-induced pain mouse model, in the hind paw muscle of the chronic post ischemic pain (CPIP) CRPS model, and in chronic cast immobilization pain models.7, 20, 28, 29 The free radical scavengers NAC and TEMPOL,7 as well as Vit C,29 reduce mechanical allodynia in the CPIP model.
Distal limb fracture is the most common cause of CRPS8, 36 and we previously established a CRPS model in tibia fracture and casted (fracture/cast) rats and mice.13, 15 The fracture/cast model manifests CRPS-like signs and pathophysiology, including allodynia, unweighting, edema, warmth, facilitated neurogenic inflammation, elevated levels of substance P (SP), calcitonin gene-related peptide (CGRP), and increased expression of pronociceptive inflammatory mediators in the fracture/cast limb. Both SP and CGRP play a critical role in the development of post fracture pain and inflammation and mediate the increased cutaneous expression of pronociceptive inflammatory mediators, including tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), and interleukin 6 (IL-6), and nerve growth factor (NGF) in the fracture/cast limb.13, 15, 21, 22, 33, 34, 44, 46, 47 We therefore tested the hypotheses that; 1) tibia fracture/cast induces oxidative stress in the injured hindlimb contributing to signs of CRPS, 2) that fracture/cast-induced oxidative stress increases SP, CGRP, and inflammatory mediator expression in the injured hindlimb, and 3) that post fracture/cast oxidative stress, CRPS-like signs, and increased SP, CGRP, and inflammatory mediator expression may be reversed by Vit C and other free radical scavenger treatments. The results of the current study demonstrated that Vit C reduced ROS formation, as indicated by the reduction of MDA levels in skin, muscle, and sciatic nerve, inhibited post fracture increases in sciatic SP and CGRP production and the post fracture up-regulation of inflammatory mediators in skin and muscle, and reversed CRPS-like behavioral signs and pathophysiology in the fracture limb. Limiting oxidative stress using Vit C or alternative strategies could potentially inhibit the production of mediators linked to pain and vascular changes in CRPS.
Methods
These experiments were approved by our institute’s Subcommittee on Animal Studies and followed the animal use guidelines of the International Association for the Study of Pain (IASP).49 Adult (9 month old) male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA, USA) were used in all experiments as stated. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were fed and watered ad libitum. During the experimental period the animals were fed Teklad Global 18% Protein Rodent Diet (www.Envigo.com, Madison, WI.) and were kept under standard conditions with a 12 hr light-dark cycle. For all experiments animals were randomly assigned to study groups, and observers were blinded to treatment conditions such as specific drug injections.
Surgery and recovery
Tibia fracture was performed under isoflurane anesthesia as we have previously described.13 The right hindlimb was wrapped in stockinet (2.5 cm wide) and the distal tibia was fractured using pliers with an adjustable stop that had been modified with a 3-point jaw. The hindlimb was wrapped in casting tape so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh. The rats were given subcutaneous saline and buprenorphine (0.03 mg/kg) immediately after the procedure and on the next day after fracture/cast for postoperative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. All rats used in this study had mechanical union at the fracture site after 4 weeks of casting.
Drugs
The effects of ROS scavengers on fracture/cast-induced nociceptive and vascular abnormalities were tested: Vitamin C (Vit C; Sigma, St. Louis, MO), phenyl N-Acetyl-L-cysteine (NAC; Sigma, St. Louis, MO) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL; Sigma, St. Louis, MO). Based on preliminary data in our lab and previous studies,6, 7, 37, 39 fracture/cast rats were treated with either Vit C (200 mg/kg/day via gavage beginning immediately after fracture and continuing daily for 4 weeks post fracture), NAC (200 mg/kg intraperitoneally once, at 1 hour prior to behavioral testing), or TEMPOL (100 mg/kg intraperitoneally once, at 1 hour prior to behavioral testing). All drugs were dissolved in saline, which was used as the vehicle treatment. All drugs and saline were coded for observer-blind administration. All injection volumes were 1 ml/kg body weight per rat. Behavioral testing (hindpaw allodynia, unweighting, warmth, and edema, and gastrocnemius hyperalgesia) was performed the day after cast removal (4 weeks after fracture). In a preliminary time course experiment, NAC and TEMPOL exerted analgesic effects on nociceptive responses in fracture/cast rats with peak effects at 1 hr after administration, and this time point was used for subsequent experimentation.
Hindpaw nociception
To measure mechanical allodynia an up-down von Frey testing paradigm was used as we have previously described.13 Rats were placed in a clear plastic cylinder (20 cm in diameter) with a wire mesh bottom and allowed to acclimate for 15 minutes. The paw was tested with one of a series of eight von Frey hairs ranging in stiffness from 0.41 g to 15.14 g. The von Frey hair was applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads. The fiber was pushed until it slightly bowed. Hindpaw withdrawal from the fiber was considered a positive response. The initial fiber presentation was 2.1 g and the fibers were presented according to the up-down method of Dixon9 to generate six responses. Data were then processed using the method described by Poree et al.32 to derive withdrawal thresholds suitable for analysis using parametric statistics. Stimuli were presented at an interval of several seconds. Hindpaw mechanical nociceptive thresholds data were analyzed as the difference (R-L) between the fracture/cast side value (right, R) and the contralateral untreated side value (left, L), thus negative values represented fracture hindlimb nociceptive sensitization.
To measure hindpaw unweighting, an incapacitance device (IITC Inc. Life Science, Woodland, CA, USA) was used. The rats were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates such that the entire weight of the rat was supported on the two hindpaws. Weighting on each paw was measured in grams. The duration of each measurement was 6 s, and 10 consecutive measurements were taken at 60-seconds intervals. Eight readings (excluding the highest and lowest ones) were averaged for each paw. Right hindpaw weight-bearing data were analyzed as a percentage between the right hindpaw weighting and half of the sum of the right (R) and left (L) hindpaw weighting values (R/[(R + L)/2] × 100 %), thus any percentage less than 100% represented fracture hindlimb unweighting.
Gastrocnemius mechanical hyperalgesia measurement
Gastrocnemius mechanical hyperalgesia was evaluated by using a Randall-Selitto device (Ugo Basile; Comerio, Varese, Italy) at baseline and 4 weeks after fracture/cast. Briefly, the rats were lightly restrained in a plastic device with an opening allowing easy access to the hind limb, and each leg was successively positioned so that incremental pressure (maximum of 800 g, 16g/kg/sec) could be applied to the mid-gastrocnemius muscle. The pressure required to elicit hind limb withdrawal was determined, and measurements were taken three times at 5 min intervals; the mean value was taken as the hind limb withdrawal threshold.27 Gastrocnemius mechanical hyperalgesia data were analyzed as the difference (R-L) between the fracture/cast side value (right, R) and the contralateral untreated side value (left, L), thus a negative value represents nociceptive sensitization in the fracture limb.
Hindpaw temperature
The room temperature was maintained at 23 °C and humidity ranged between 25 and 45%. The temperature of the hindpaw was measured using a fine wire thermocouple (Omega, Stamford, CT, USA) applied to the paw skin, as previously described.13, 14, 19 The investigator held the thermistor wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw; the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature. Hindpaw temperature data were analyzed as the difference (R-L) between the fracture/cast side value (right, R) and the contralateral untreated side value (left, L), thus an increase in temperature represents warmth in the fracture limb.
Hindpaw thickness
A laser sensor technique was used to determine the dorsal-ventral thickness of the hindpaw, as we have previously described.13 For laser measurements, each rat was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. The paw was gently held flat on the table with a small metal rod applied to the top of the ankle joint. Using optical triangulation, a laser with a distance-measuring sensor was used to determine the distance to the table top and to the top of the hindpaw at a spot on the dorsal skin over the midpoint of the third metatarsal, and the difference was used to calculate the dorsal-ventral paw thickness. The measurement sensor device used in these experiments (4381 Precicura, Limab, Goteborg, Sweden) has 0.01 mm resolution. Hindpaw thickness data were analyzed as the difference (R-L) between the fracture/cast side value (right, R) and the contralateral untreated side value (left, L), thus increased thickness represents edema in the fracture hindlimb.
Tissue collection and processing
Rat ipsilateral hindpaw dorsal skin, gastrocnemius muscle and sciatic nerve were collected under isoflurane anesthesia after behavioral testing and immediately frozen on dry ice. The sciatic nerve collected was that tissue found between the greater trochanter of the femur and the division of the tibia nerve. For measurements of cytokines, NGF, lactate, and MDA levels, these tissues were cut into fine pieces in ice-cold phosphate buffered saline (PBS), pH 7.4, containing protease inhibitors [aprotinin (2 μg/ml), leupeptin (5 μg/ml), pepstatin (0.7 μg/ml), and phenylmethylsulfonyl fluoride (PMSF, 100 μg/ml); Sigma, St. Louis, MO, USA] followed by homogenization using a rotor/stator homogenizer. Homogenates were centrifuged for 5 minutes at 14,000 g, and at 4 °C. Supernatants were transferred to fresh pre-cooled Eppendorf tubes. Triton X-100 (Boehringer Mannheim, Germany) was added at a final concentration 0.01%. The samples were centrifuged again for 5 minutes at 14,000 g at 4 °C. The supernatants were aliquoted and stored at −80 °C. For SP and calcitonin gene-related peptide (CGRP) protein expression in the sciatic nerve, sciatic nerve samples were minced in 1 ml of 3:1 ethanol/0.7 M HCl and homogenized for 20 s. The homogenates were shaken for 2 h at 4 °C and centrifuged at 3000 g for 20 min at 4 °C. The supernatants were frozen and lyophilized. The lyophilized products were stored at −80 °C and reconstituted in PBS containing 0.05% Tween 20 prior to SP and CGRP enzyme immunoassays (EIA).
Measurements of Cytokines, NGF, Lactate, and MDA levels
Measurements of TNFα, IL-1β, IL-6 and NGF levels were carried out in hindpaw skin and gastrocnemius muscle, lactate levels in gastrocnemius muscle, and MDA levels in hindpaw skin, gastrocnemius muscle and sciatic nerve. Tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6) protein levels were determined using EIA kits (R&D Systems, Minneapolis, MN, USA). The nerve growth factor (NGF) concentrations were determined using the NGF Emax® ImmunoAssay System kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The optical density (OD) of the reaction product was read on a microplate reader at 450 nm. The concentrations of TNFα, IL-1β, IL-6, and NGF proteins were calculated from the standard curve for each assay. Positive and negative controls were included in each assay. Each protein concentration was expressed as pg/mg total protein. Total protein contents in all tissue extracts were measured by the Coomassie Blue Protein Assay Kit (Bio-Rad, Hercules, CA).
Lactate levels in muscle were measured using a lactate determination kit (BioAssay Systems; Hayward, CA). To assess lipid peroxidation, malondialdehyde (MDA) levels in skin, muscle and sciatic nerve were measured by using the Thiobarbituric Acid Reactive Substances (TBARS) Assay Kit (Cayman Chemical; Ann Arbor, MI). MDA was quantified colorimetrically at 535 nm following its controlled reaction with thiobarbituric acid. Standards and samples were assayed at the same time and the standard curve was prepared following the manufacturer’s protocols. Sample MDA concentrations were calculated based on the MDA standard curve and normalized against wet tissue weight.
Enzyme immunoassay procedure for sciatic nerve SP and CGRP levels
The aim of this experiment was to determine whether fracture/cast induced up-regulated SP and calcitonin gene-related peptide (CGRP) protein expression in the sciatic nerve was inhibited by Vit C treatment. After the sciatic nerve supernatants were lyophilized and reconstituted, the sciatic nerve extracts were assayed in duplicate using an EIA kits to determine SP (Assay Designs, Ann Arbor, MI) and CGRP (Cayman Chemical; Ann Arbor, MI) levels following the manufacturer’s protocols.
Study design
To study the effects of Vit C on the development of post fracture/cast nociceptive behavior, vascular signs, and inflammatory responses, rats were randomly assigned to three primary experimental cohorts (n = 10 per cohort); 1) normal Control (nonfracture)+Vehicle (saline), 2) control Fracture (FX)/Cast +Vehicle, 3) FX/Cast+Vit C (ascorbic acid, an anti-oxidant). Fracture/cast rats underwent baseline behavioral testing, then right tibia fracture followed by 4 weeks cast immobilization, then behavioral testing and tissue collection on the day after cast removal. Vit C daily gavage treatment (200 mg/kg/day) began immediately following fracture/cast and lasted for 4 weeks, whereas the Control+Vehicle and FX/Cast+Vehicle groups were given vehicle by gavage daily for the same period. The behavioral measurements included bilateral hindpaw von Frey allodynia, unweighting, warmth, edema, and gastrocnemius muscle hyperalgesia. Following behavioral measurements, hindpaw skin, gastrocnemius muscle, and sciatic nerve tissues were collected and stored for TNFα, IL-1β, IL-6, NGF, SP, CGRP, lactate and MDA assays.
To determine whether ROS contribute to the maintenance of post fracture nociceptive behavior and vascular signs, rats were divided into 4 treatment groups (n = 10 per cohort): 1) normal Control (no fracture)+Vehicle (saline), 2) FX/Cast+Vehicle (saline), 3) FX/Cast+NAC (200 mg/kg, i.p.), and 4) FX/Cast+TEMPOL (100 mg/kg, i.p.). At 4 weeks after fracture the casts were removed and the rats were injected with NAC, TEMPOL, or Vehicle at 1 hr prior to behavioral testing, then tissues were collected for MDA and lactate assays.
Statistical analysis
Statistical analyses were accomplished using a one-way analysis of variance (ANOVA) followed by Neuman-Keuls multiple comparison test to compare among all cohorts. All data are presented as the mean ± standard error of the mean (SEM), and differences were considered significant at a P-value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA, USA).
Results
Vitamin C treatment improved fracture/cast-induced nociceptive and vascular changes in the CRPS model
The nociceptive and vascular effects of tibia fracture followed by cast immobilization (fracture/cast) were examined in fracture/cast rats treated with a 4-week course of Vit C, an antioxidant, started at the time of fracture (Fig. 1). Fracture/cast resulted in hindpaw cutaneous von Frey allodynia with mean von Frey withdrawal threshold 8.5 g lower on the fracture/cast side than the contralateral side (−8.5 ± 0.4 Δg vs control nonfracture rats −0.4 ± 0.3 Δg, P<0.001). Vit C treatment reversed hindpaw allodynia (−8.5 ± 0.4 Δg to −3.3 ± 1.3 Δg, P<0.001). Fracture/cast also reduced ipsilateral hindlimb weight-bearing to 53 ± 1% of the average of total weight bearing in the hindlimbs (P<0.001 compared to control nonfracture rats, Fig. 1B). Vit C treatment improved post fracture weight-bearing from 53 ± 1% to 95 ± 1% (P<0.001), indicating 89% improvement. Fracture/cast also resulted in gastrocnemius muscle hyperalgesia, with the Randall-Selitto withdrawal threshold lower on ipsilateral side than the contralateral side (−410 ± 13 Δg, vs control nonfracture rats 18.6±11.1 Δg, P<0.001). Vit C treatment reversed fracture/cast-induced muscle hyperalgesia (−410 ± 13 Δg to −246 ± 12.5 Δg, P<0.001), indicating 38% improvement. Fracture/cast induced hindpaw warmth, as indicated by the greater difference in skin temperature between ipsilateral and contralateral sides in fracture/cast rats (4.6 ± 0.7 Δ°C) than that in the nonfracture control rats (0.0 ± 0.1 Δ°C, P<0.001, Fig. 1D), but Vit C treatment had no significant effect on hindpaw warmth. Fracture/cast also resulted in edema, as indicated by the greater difference in skin thickness between ipsilateral and contralateral sides in fracture/cast rats (1.5 ± 0.4 Δmm) than that in the control rats (0.0 ± 0.1 Δmm, P<0.01, Fig. 1E), and Vit C treatment had no significant effect on hindpaw edema.
Figure 1. Systemic vitamin C treatment prevented development of nociceptive sensitization after tibia fracture and casting.
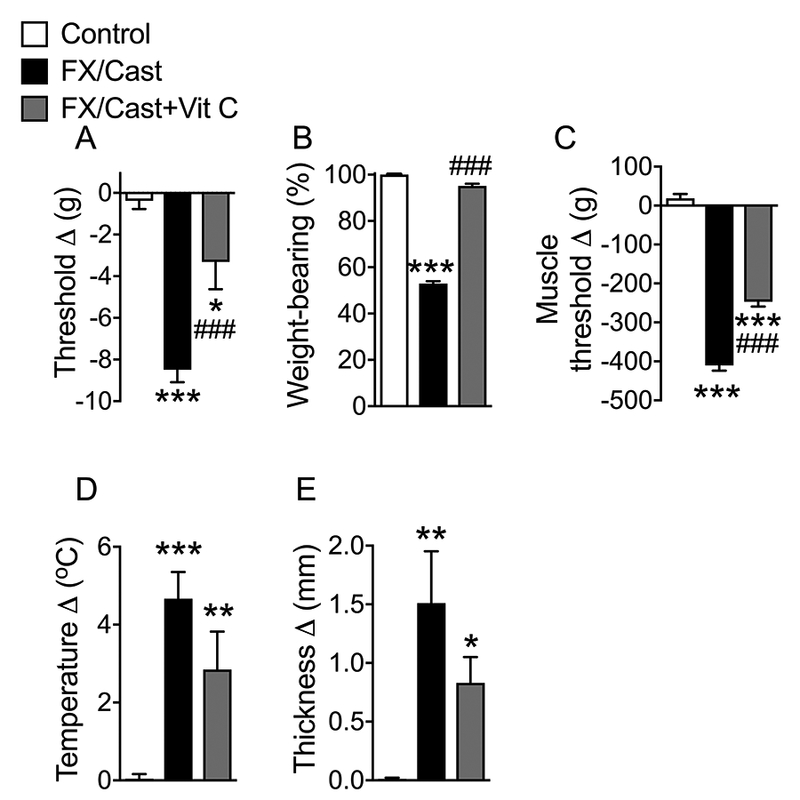
Rats underwent distal tibia fracture with 4 weeks cast immobilization and were treated with either daily saline gavage for 4 weeks (FX/Cast) or Vit C (200 mg/kg daily gavage) for 4 weeks (FX/Cast+Vit C). Additional nonfracture rats were used as Controls. On the day after cast removal behavioral testing was performed. FX/Cast rats developed hindlimb (A) von Frey allodynia, (B) unweighting, (C) gastrocnemius mechanical hyperalgesia, (D) warmth, and (E) edema, and Vit C treatment inhibited the development of the post fracture/cast nociceptive changes, but not warmth and edema. Measurements for (A), (C), (D), and (E) represent the difference between the fracture/cast ipsilateral side (R) and the contralateral paw (L). Thus, negative values (R-L) in graphs (A) and (C) indicate allodynia and hyperalgesia, respectively, whereas positive values (R-L) in Panels (D) and (E) indicate warmth and edema, respectively. The values displayed in panel (B) represent weight-bearing on the fracture/cast hindlimb as a percentage of half of the total bilateral hindlimb weight-bearing, thus any percentage less than 100% represented fracture hindlimb unweighting. Data are expressed as mean values ± SEM and were analyzed by one way ANOVA and post-hoc Newman-Keuls multiple comparison testing (n=10 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 vs. nonfracture Controls treated with vehicle; ###P <0.001 vs. FX/Cast treated with vehicle.
To further assess the impact of free radical generation on fracture/cast-induced nociceptive sensitization and vascular changes, 4-week post fracture/cast rats were injected with either the free radical scavenger NAC or TEMPOL at one hour prior to behavioral testing (Figs. 2A-E). Preliminary studies established these doses to be well tolerated by the animal subjects. Both agents have been used in rat models previously for similar purposes7. Both NAC and TEMPOL significantly reduced fracture/cast-induced hindpaw cutaneous von Frey allodynia (by 59.5% and 78.8%, respectively), hindlimb unweighting (by 47.6% and 52.6%, respectively), and muscle hyperalgesia (by 34.1% and 39.7%, respectively). NAC reduced hindpaw warmth by 75.6%, but TEMPOL did not have a significant effect on warmth. Neither NAC nor TEMPOL had an effect on hindpaw edema.
Figure 2. Systemic NAC or TEMPOL treatment reduced nociceptive sensitization after tibia fracture and casting.
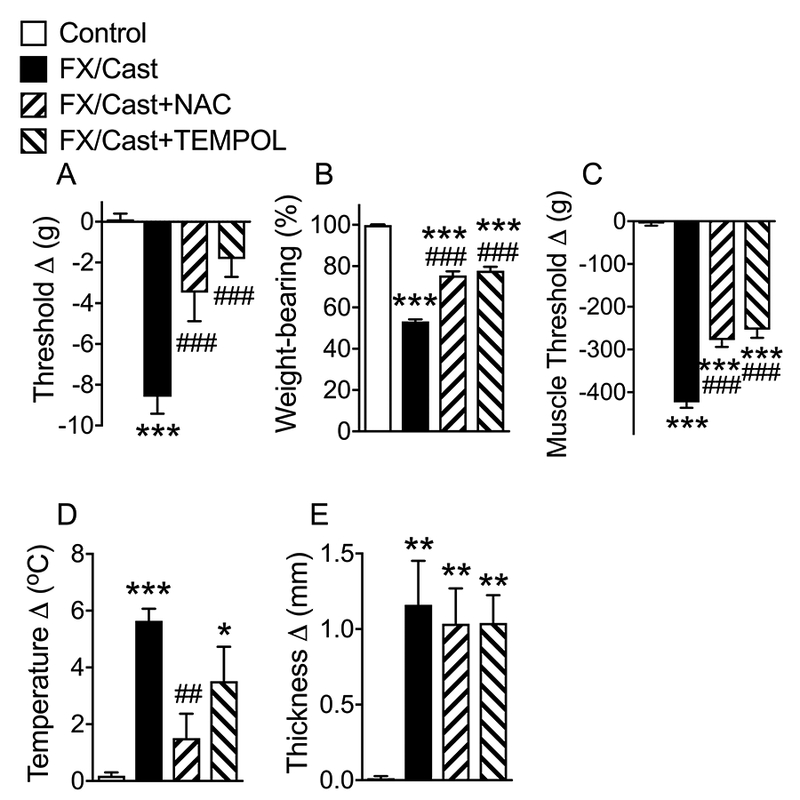
Rats underwent distal tibia fracture with 4 weeks cast immobilization, then the cast was removed and the next day the rats were injected with vehicle or drug and behavioral testing was performed 1 hour later. There were 4 treatment groups; 1) Control (nonfracture), 2) fracture/cast (FX/Cast) rats treated with i.p. saline vehicle, and fracture/cast rats treated with the antioxidants 3) NAC (200 mg/kg, i.p., FX/Cast+NAC), or 4) TEMPOL (100 mg/kg, i.p., FX/Cast+TEMPOL). Intraperitoneal injection of NAC or TEMPOL partially reversed hindpaw (A) von Frey allodynia, (B) unweighting, (C) gastrocnemius mechanical hyperalgesia, and (D) warmth (NAC but not TEMPOL), but had no effect on hindpaw (E) edema. Data are expressed as mean values ± SEM and were analyzed by one way ANOVA and post-hoc Newman-Keuls multiple comparison testing (n=10 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 vs nonfracture Controls treated with vehicle; #P < 0.05 and ###P <0.001 vs. FX/Cast treated with vehicle
Fracture/cast induced increases in skin, muscle, and sciatic nerve MDA levels as well as muscle lactate levels
Because anti-oxidants alleviated fracture/cast-induced nociceptive abnormalities, we hypothesized that fracture/cast resulted in oxidative stress. MDA, a product of lipid peroxidation, is widely used as an indicator of oxidative stress in cells and tissues. Fracture/cast led to an increase in MDA levels in skin (84%, 34 ± 5 to 63 ± 5 nmol/g tissue), muscle (59%, 255 ± 32 to 407 ± 56 nmol/g tissue) and sciatic nerve (71%, 386 ± 59 to 661 ± 44 nmol/g tissue). Post fracture/cast treatments with Vit C or NAC inhibited the fracture/cast-induced increases in MDA levels, although inhibition by NAC treatment in the muscle was not significant (Figs. 3A-C).
Figure 3. Lipid peroxidation levels increased in skin, muscle and sciatic nerve after fracture/cast and Vit C or NAC treatment reversed this up regulation.
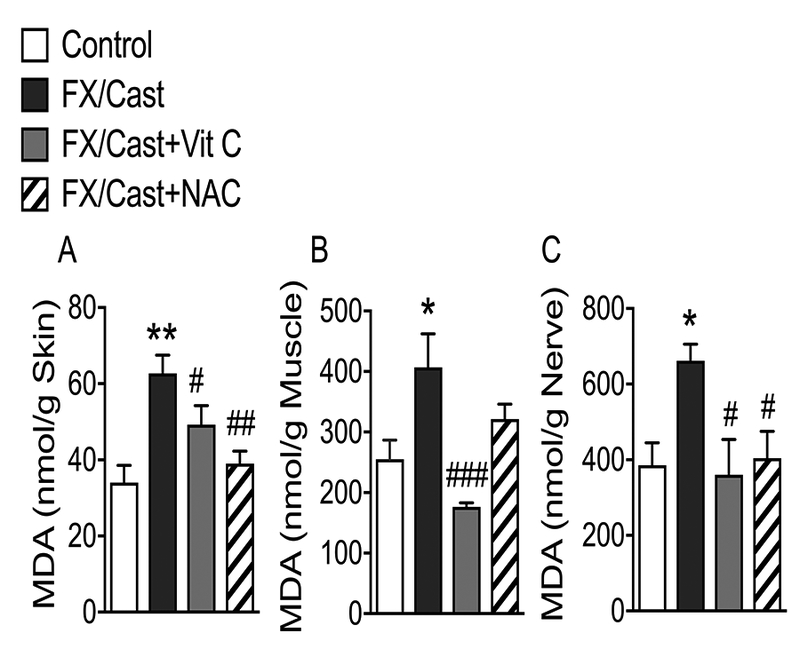
Elevated concentrations of the lipid peroxidation product malondialdehyde (MDA) were detected in the ipsilateral (A) hindpaw skin, (B) gastrocnemius muscle, and (C) sciatic nerve at 4 weeks after tibia fracture/cast. Four weeks treatment with Vit C inhibited the post-fracture/cast increase in MDA levels in skin, muscle and sciatic nerve. Intraperitoneal injection of the antioxidant NAC at 4 weeks post fracture also inhibited the increase in MDA in skin and sciatic nerve. Data are expressed as mean values ± SEM and analyzed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison testing (n=7 per cohort). *P < 0.05, **P < 0.01 and ***P < 0.001 vs. nonfracture Controls treated with vehicle; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. FX/Cast treated with vehicle.
In addition to indicating oxidative stress, lactate has been reported to contribute to muscle pain 7. Therefore, we further tested if fracture and cast immobilization resulted in lactate formation in the muscle in the fracture/cast rat, and whether Vit C or NAC treatment reversed the fracture/cast-induced increase in lactate levels in the muscle. Post fracture/cast lactate levels in the gastrocnemius muscle increased by 100% (from 0.95 ± 0.06 to 1.8 ± 0.08 mmol/kg muscle) and both Vit C and NAC treatments reduced fracture/cast-induced increase in muscle lactate to control levels (Fig. 4).
Figure 4. Muscle lactate levels increased after fracture/cast and Vit C or NAC treatment inhibited this increase.

Muscle lactate levels were increased 100% in the ipsilateral gastrocnemius at 4 weeks after fracture/cast. Both Vit C and NAC treatments reduced the fracture/cast-induced muscle lactate increases by 82 and 73%, respectively. Data are expressed as mean values ± SEM and analyzed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison testing (n=6 per cohort). ***P < 0.001 vs. nonfracture Controls treated with vehicle; #P < 0.05 and ###P < 0.001 vs. FX/Cast treated with vehicle.
Vitamin C treatment reversed fracture/cast induced increases in sciatic nerve SP and CGRP levels
At 4 weeks after fracture and cast immobilization, levels of SP and CGRP in the sciatic nerve were increased by 76% and 27%, respectively (Fig. 5A-B). Treatment with Vit C for 4 weeks significantly reduced fracture/cast-induced SP (63%) and CGRP (99.3%) levels in sciatic nerve, suggesting that oxidative stress contributed to post fracture/cast increases in sciatic neuropeptide content.
Figure 5. Sciatic nerve SP and CGRP levels increased after fracture/cast and Vit C treatment inhibited this increase.
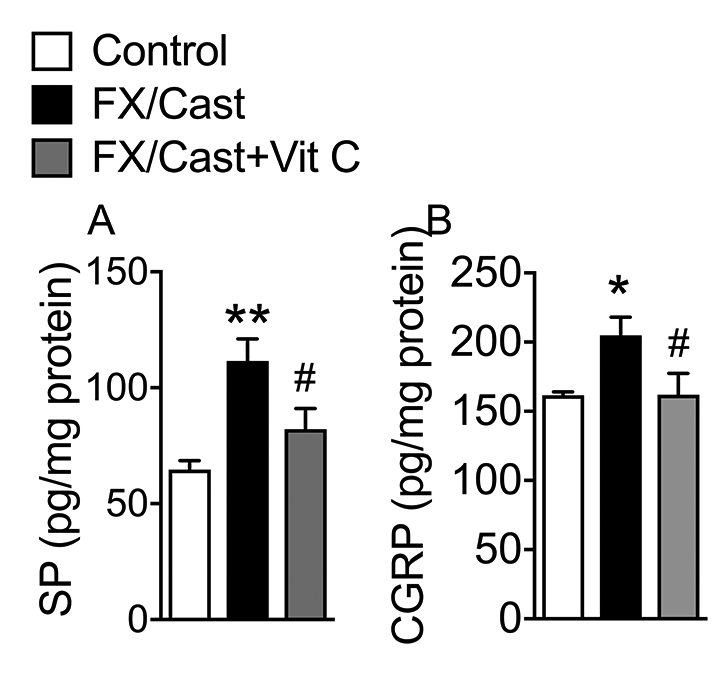
At 4 weeks after fracture/cast both SP (A) as well as CGRP (B) protein levels were increased in the ipsilateral sciatic nerve and a 4 week course of Vit C treatment (200 mg/kg/day p.o.) inhibited this increase. Data are expressed as mean values ± SEM and analyzed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison testing (n=7 per cohort). *P<0.05 and **P < 0.01 vs. nonfracture Controls treated with vehicle; #P < 0.05 vs. FX/Cast treated with vehicle.
Fracture/cast evoked increases in skin and muscle cytokine and neurotrophin levels were inhibited by vitamin C treatment
Elevated levels of proinflammatory cytokines (TNFα, IL-1β, and IL-6) and the neurotrophin NGF were observed in the hindpaw skin (Fig. 6A-D) and gastrocnemius muscle (Fig. 7A-D) of the injured limb at 4 weeks after fracture/cast. Fracture/cast increased ipsilateral hindpaw skin TNFα, IL-1β, IL-6, and NGF by 216%, 280%, 58%, and 130%, respectively, as compared to nonfracture control skin, and Vit C treatment significantly reversed fracture/cast-induced increase in cutaneous IL-6 and NGF levels (Fig. 6A-D).
Figure 6. Hindpaw skin inflammatory mediator levels increased after fracture/cast and Vit C treatment reversed the increases in IL-6 and NGF.
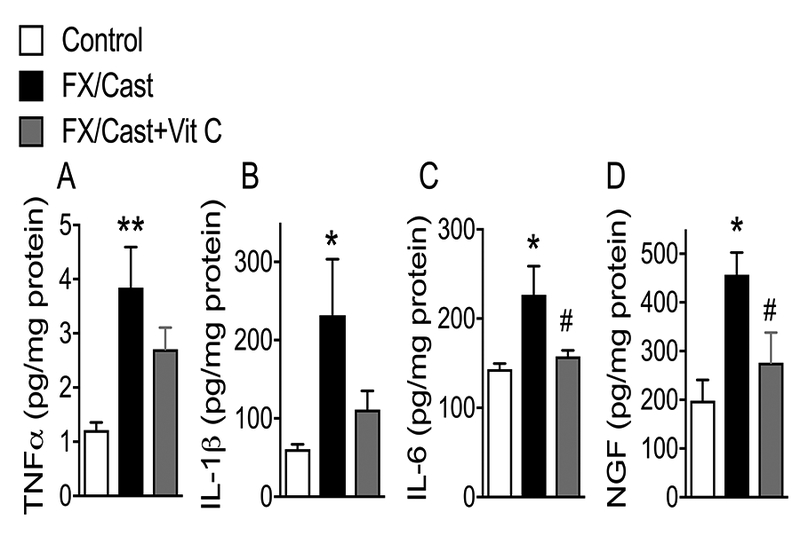
(A) TNFα, (B) IL-1β, (C) IL-6, and (D) nerve growth factor (NGF) levels in hindpaw skin were increased at 4 weeks post fracture/cast and a 4 week course of Vit C treatment (200 mg/kg/day p.o.) inhibited the increases in IL-6 and NGF. Data are expressed as mean values ± SEM and analyzed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison testing (n=6 per cohort). *P<0.05 and **P < 0.01 vs. nonfracture Controls treated with vehicle; #P < 0.05 vs. FX/Cast treated with vehicle.
Figure 7. Muscle inflammatory mediator levels increased after fracture/cast and Vit C treatment reversed the increases in IL-1β and IL-6.
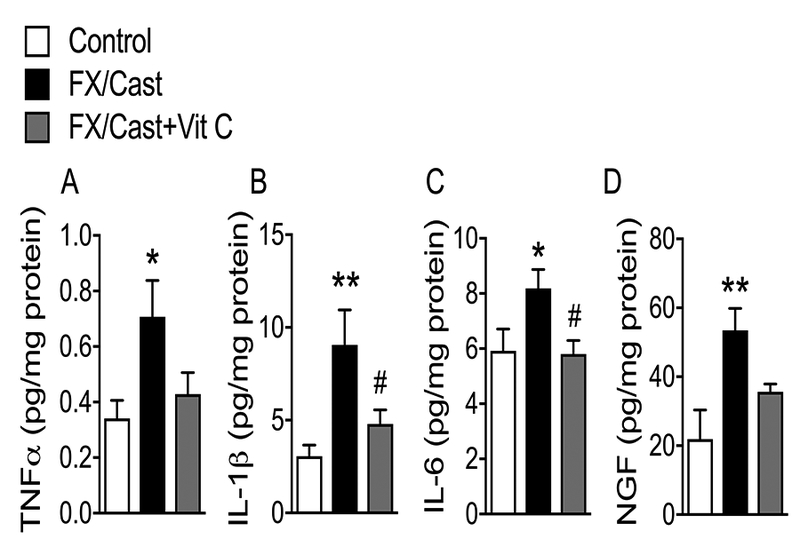
(A) TNFα, (B) IL-1β, (C) IL-6, and (D) nerve growth factor (NGF) levels in the ipsilateral gastrocnemius muscle were increased at 4 weeks post fracture/cast and a 4 week course of Vit C treatment (200 mg/kg/day, p.o.) inhibited this increase. Data are expressed as mean values ± SEM and analyzed using one-way ANOVA followed by post hoc Newman-Keuls multiple comparison testing (n=7 per cohort). *P<0.05 and **P < 0.01 vs. nonfracture Controls treated with vehicle; #P < 0.05 vs. FX/Cast treated with vehicle.
Fracture/cast also significantly increased ipsilateral gastrocnemius muscle TNFα, IL-1β, IL-6, and NGF levels by 107%, 196%, 38%, and 144%, respectively, and Vit C treatment significantly reversed fracture/cast-induced increases in muscle IL-1β, and IL-6 (Fig. 7A-D). Collectively these data demonstrate that post fracture/cast increases in cutaneous and muscle inflammatory mediators were reversed by Vit C treatment, suggesting that oxidative stress may contribute to the enhanced production of these mediators after fracture/cast.
Discussion
The etiology of pain and disability in CRPS remain enigmatic despite years of study in animals and humans. In our previous studies, tibia fracture followed by cast immobilization for 4 weeks in rats initiated hindpaw cutaneous allodynia, postural unweighting, exaggerated neurogenic inflammation, edema, and warmth, with increased neuropeptide and inflammatory mediator levels, and nociceptive sensitization lasting for at least 16 weeks.45 These signs and pathophysiologic changes closely resembled CRPS in humans.4
In the present study, our data demonstrate that oxidative stress plays an important role in the development of post fracture CRPS-like features. Fracture/cast resulted in elevated oxidative stress (as indicated by MDA levels) in hindpaw skin, sciatic nerve, and gastrocnemius muscle as well as allodynia in the hindpaw skin and hyperalgesia over the gastrocnemius muscle. The systemic treatment with the antioxidant Vit C significantly reduced the fracture/cast-induced oxidative stress and nociceptive abnormalities although we have not isolated the contributions of oxidative stress in each tissue individually to nociceptive abnormalities. Consistent with our findings, oxidative stress has been suggested to contribute to CRPS development in humans.30, 31 While the mechanism of fracture/cast-induced oxidative stress is not clear in the fracture/cast CRPS model, short-term nociceptive changes observed after immobilization alone observed by Ohmichi et. al.28 were sensitive to the administration of anti-oxidants although evidence of oxidative stress was not collected in those experiments. Results from our group using immobilization alone suggest that nociceptive and inflammatory changes are less robust and shorter lived.13 Similar findings were reported using the chronic post-ischemia pain (CPIP) rat model7 where a tourniquet (a tight fitting O-ring) was placed on one hindlimb of an anesthetized rat just proximal to the ankle joint for 3 h, and removed to allow reperfusion. In the CPIP model, microvascular dysfunction after ischemia-reperfusion injury is initiated by oxygen free radicals when oxidases that accumulate in ischemic tissue reduce molecular oxygen arriving on reperfusion.17 Furthermore in the CPIP model, hindlimb cutaneous tactile allodynia was associated with microvascular dysfunction in muscle where lactate levels were increased.20 Therefore, deep tissue microvascular dysfunction may partially contribute to allodynia measured in more superficial tissue layers in laboratory animals and humans after limb injury.7 In addition to microvascular dysfunction, we observed a post fracture/cast increase in muscle lactate levels that may support muscle hyperalgesia in fracture/cast rats. An increase of skin lactate has been reported in CRPS patients, although venous lactate was unaltered, suggesting enhanced anaerobic glycolysis, probably as a result of local chronic tissue hypoxia.5 Thus, we speculate that the rise of lactate in peripheral tissues also contributes to fracture/cast-induced hyperalgesia. The reduction in pH resulting from lactic acid accumulation may contribute to pain because acidosis causes excitation of nociceptors leading to mechanical hyperalgesia and spontaneous pain.40
Consistent with the hypothesis that the generation of free radicals may be partly responsible for fracture/cast-induced allodynia, Vit C and two free radical scavengers, NAC and TEMPOL, were able to reduce signs of mechanical allodynia in this fracture/cast model. Both 4 weeks of treatment with Vit C and the administration of NAC for 1 hour before tissue analysis reduced MDA levels in skin, muscle and sciatic nerve in the fracture/cast hindlimb and lactate levels in the gastrocnemius muscle. These observations suggest that free radicals or peroxidation products directly contribute to hyperalgesia, in addition to lactic acid. We speculate that accumulation of free radicals in earlier stage may initiate a lipid oxidation chain reaction that may persist over the 4 weeks of fracture/cast recovery and thus contribute to the maintenance of hyperalgesia. If true, early treatment with antioxidants may prevent or reduce subsequent CRPS development.3, 50
In addition to the possibility that muscular microcirculatory abnormalities support the allodynia and hyperalgesia observable in the fracture/cast model, we previously reported that SP and CGRP receptor activation contributes to nociceptive sensitization and the generation of pronociceptive inflammatory mediators in the fracture/cast model.13, 15, 21, 22, 33, 34, 44, 46, 47 Hypothesizing that limiting oxidative stress might impact this pathway, we examined effects of Vit C on neuropeptide levels in the fracture/cast limb. Interestingly, Vit C anti-oxidant treatment throughout the 4 weeks post fracture/cast period reduced post fracture lipid peroxidation (a marker of ROS generation) in skin, muscle, and sciatic nerve, partially reversed nociceptive sensitization, and inhibited the up-regulation of sciatic nerve SP and CGRP contents. These data support the premise that post fracture/cast ROS generation induced CRPS-like signs through the neuropeptide signaling pathway. Furthermore, there is a ROS-dependent component to neurogenic inflammatory responses mediated by SP and CGRP,41 and SP neurokinin 1 (NK1) receptor activation reportedly induced ROS generation in epithelial cells.12 Using cultured dorsal root ganglia (DRG) neurons, Linley et al23 reported that SP increased release of reactive oxygen species from the mitochondrial electron transport chain, suggesting that augmented neuropeptide expression could provide a positive feedback loop amplifying ROS release.
Since innate inflammatory responses in the injured limb are regulated by exaggerated neuropeptide signaling after fracture/cast,15, 38 we measured inflammatory mediators in the skin and gastrocnemius muscle at 4 weeks post fracture/cast. Levels of inflammatory mediators were elevated in skin and muscle (Figures 6 and 7), modeling the increased cytokine levels observed in the affected skin of CRPS patients,4, 16 and Vit C reduced cytokine and NGF levels in the skin and muscle. Previously we observed increased ipsilateral epidermal keratinocyte expression of TNFα, IL-1β, IL-6, and NGF after fracture/cast and demonstrated that each of inflammatory mediators contributes to nociceptive sensitization in this CRPS model.21, 22, 33, 34, 47
Collectively, our data support the hypothesis that limb trauma/immobilization-induced oxidative stress supports inflammation by activating the SP and CGRP signaling pathways leading to the pain-related symptoms in CRPS. Using our well-validated model of CRPS4 we have demonstrated that therapies directed at reducing the oxidative state of the tissue or the production of free radicals maybe clinically effective. There are, however, issues left unresolved. For example, we do not know if the oxidative changes contribute throughout the course of the disease or only during the acute phase of CRPS when the inflammatory features are more striking. On a more basic level, we do not understand the etiology of the microvascular dysregulation ostensibly responsible for oxidative stress after trauma. Controlling that process may prevent CRPS-related pain at an even more basic level. Relatedly, the mechanisms by which oxidative stress impacts the functioning of peripheral nerves may help us to understand not only CRPS and trauma-related pain, but other disease states in which peripheral nerves are subjected to oxidative stress, low pH and elevated levels of free radicals such as myocardial ischemia and peripheral vascular disease.
Conclusions
Fracture/cast and immobilization of the hindlimb induces oxidative stress in several tissues, including skin, muscle, and sciatic nerve. Free radicals may play a role in the mechanism responsible for nociceptive sensitization in these circumstances. Therefore, early treatment with an antioxidant such as Vit C may prevent pain sensitization and other features of CRPS after limb trauma.
Acknowledgments
Disclosures: This study was supported by National Institutes of Health grants NS072168 and NS094438, and Department of Veterans Affairs, Rehabilitation Research and Development Merit grant RX001475. The authors do not have financial or other relationships that might lead to conflict of interest.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
Authors’ Contributions: TG performed the behavioral studies. TW carried out the immunoassays and biochemical analyses, performed the statistical analyses and drafted the manuscript. TH helped to design the study. WK conceived of and coordinated the study, participated in its design and helped to draft the manuscript. JC helped to design the study and to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Balazs L, Leon M. Evidence of an oxidative challenge in the Alzheimer’s brain. Neurochemical research. 19:1131–1137, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Baykal T, Seferoglu B, Karsan O, Kiziltunc A, Senel K. Antioxidant profile in patients with complex regional pain syndrome type I. International journal of rheumatic diseases. 17:156–158, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot and ankle surgery : official journal of the European Society of Foot and Ankle Surgeons. 15:179–182, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Ibrahim A, Schlereth T, Kingery WS. The rodent tibia fracture model: a critical review and comparison with the complex regional pain syndrome literature. J Pain. In Press, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birklein F, Weber M, Neundorfer B. Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology. 55:1213–1215, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chung JM. The role of reactive oxygen species (ROS) in persistent pain. Molecular interventions. 4:248–250, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 112:94–105, 2004 [DOI] [PubMed] [Google Scholar]
- 8.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 129:12–20, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ. Staircase bioassay: the up-and-down method. Neuroscience and biobehavioral reviews. 15:47–50, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg E, Shtahl S, Geller R, Reznick AZ, Sharf O, Ravbinovich M, Erenreich A, Nagler RM. Serum and salivary oxidative analysis in Complex Regional Pain Syndrome. Pain. 138:226–232, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Fischer SG, Perez RS, Nouta J, Zuurmond WW, Scheffer PG. Oxidative stress in Complex Regional Pain Syndrome (CRPS): no systemically elevated levels of malondialdehyde, F2-isoprostanes and 8OHdG in a selected sample of patients. International journal of molecular sciences. 14:7784–7794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free radical biology & medicine. 43:581–589, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 108:95–107, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 121:158–167, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Molecular pain. 8:85, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. Journal of pain and symptom management. 27:101–103, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Inauen W, Suzuki M, Granger DN. Mechanisms of cellular injury: potential sources of oxygen free radicals in ischemia/reperfusion. Microcirculation, endothelium, and lymphatics. 5:143–155, 1989 [PubMed] [Google Scholar]
- 18.Jenner P Oxidative damage in neurodegenerative disease. Lancet. 344:796–798, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 104:75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Laferriere A, Millecamps M, Xanthos DN, Xiao WH, Siau C, de Mos M, Sachot C, Ragavendran JV, Huygen FJ, Bennett GJ, Coderre TJ. Cutaneous tactile allodynia associated with microvascular dysfunction in muscle. Molecular pain. 4:49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 151:843–852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 144:303–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C, Gamper N. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 109:E1578–1586, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda T, Tanaka H, Yuasa H, Forrest R, Matsuda H, Hanumadass M, Reyes H. The effects of high-dose vitamin C therapy on postburn lipid peroxidation. The Journal of burn care & rehabilitation. 14:624–629, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Meena S, Sharma P, Gangary SK, Chowdhury B. Role of vitamin C in prevention of complex regional pain syndrome after distal radius fractures: a meta-analysis. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 25:637–641, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets? Expert opinion on therapeutic targets. 17:1081–1089, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Meotti FC, Campos R, da Silva K, Paszcuk AF, Costa R, Calixto JB. Inflammatory muscle pain is dependent on the activation of kinin B(1) and B(2) receptors and intracellular kinase pathways. British journal of pharmacology. 166:1127–1139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohmichi Y, Sato J, Ohmichi M, Sakurai H, Yoshimoto T, Morimoto A, Hashimoto T, Eguchi K, Nishihara M, Arai YC, Ohishi H, Asamoto K, Ushida T, Nakano T, Kumazawa T. Two-week cast immobilization induced chronic widespread hyperalgesia in rats. European journal of pain. 16:338–348, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Park JM, Kim CK, Lee HC, Jung H, Choi KU, Hong SW, Lim DG, Baek WY, Kwak KH. Antiallodynic effects of vitamin C and vitamin E in chronic post-ischemia pain rat model. Korean journal of anesthesiology. 65:442–448, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez RS, Zollinger PE, Dijkstra PU, Thomassen-Hilgersom IL, Zuurmond WW, Rosenbrand KC, Geertzen JH, force CIt. Evidence based guidelines for complex regional pain syndrome type 1. BMC neurology. 10:20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez RS, Zuurmond WW, Bezemer PD, Kuik DJ, van Loenen AC, de Lange JJ, Zuidhof AJ. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 102:297–307, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesthesia and analgesia. 87:941–948, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 137:507–519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 138:47–60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. International journal of endocrinology. 2014:674987, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 103:199–207, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 138:514–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi X, Wang L, Li X, Sahbaie P, Kingery WS, Clark JD. Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1beta production in keratinocytes. Anesthesia and analgesia. 113:175–183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery--systematic review and meta-analysis. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 52:62–66, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Sieweke N, Birklein F, Riedl B, Neundorfer B, Handwerker HO. Patterns of hyperalgesia in complex regional pain syndrome. Pain. 80:171–177, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Starr A, Graepel R, Keeble J, Schmidhuber S, Clark N, Grant A, Shah AM, Brain SD. A reactive oxygen species-mediated component in neurogenic vasodilatation. Cardiovascular research. 78:139–147, 2008 [DOI] [PubMed] [Google Scholar]
- 42.van der Laan L, Kapitein PJ, Oyen WJ, Verhofstad AA, Hendriks T, Goris RJ. A novel animal model to evaluate oxygen derived free radical damage in soft tissue. Free radical research. 26:363–372, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Wasner G, Backonja MM, Baron R. Traumatic neuralgias: complex regional pain syndromes (reflex sympathetic dystrophy and causalgia): clinical characteristics, pathophysiological mechanisms and therapy. Neurologic clinics. 16:851–868, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. Journal of neuroinflammation. 9:181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. Journal of neuroinflammation. 13:14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 144:278–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain. 13:253–262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao X, Li XL, Liu X, Wang C, Zhou DS, Ma Q, Zhou WH, Hu ZY. Antinociceptive effects of fisetin against diabetic neuropathic pain in mice: Engagement of antioxidant mechanisms and spinal GABAA receptors. Pharmacological research. 102:286–297, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann M Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 16:109–110, 1983 [DOI] [PubMed] [Google Scholar]
- 50.Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. The Journal of bone and joint surgery. American volume. 89:1424–1431, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 354:2025–2028, 1999 [DOI] [PubMed] [Google Scholar]


