Abstract
Regulatory T cells (Tregs) are essential for the maintenance of tolerance and immune homeostasis. In allogeneic hematopoietic stem cell transplantation (aHSCT), transfer of appropriate Treg numbers is a promising therapy for the prevention of graft-versus-host disease (GVHD). We have recently reported a novel approach which induces the marked expansion and selective activation of Tregs in vivo by targeting TNF receptor superfamily 25 (TNFRSF25) and CD25. A potential advance to promote clinical application of Treg cells to ameliorate GVHD and other disorders would be the generation of more potent Treg populations. Here we wanted to determine if very low doses of Tregs generated using the ‘two-pathway’ stimulation protocol via TL1A-Ig fusion protein and low dose IL-2 (targeting TNFRSF25 and CD25, respectively) could be used to regulate pre-clinical GVHD. Analysis of such ‘two-pathway’ expanded Tregs identified higher levels of activation / functional molecules (CD103, ICOS-1, Nrp-1, CD39, CD73, il-10, and tgfb1) vs. unexpanded Tregs. Additionally, in vitro assessment of ‘two-pathway’ stimulated Tregs indicated enhanced suppressor activity. Notably, transplant of extremely low numbers of these Tregs (1:6 expanded Tregs / Tconventional) suppressed GVHD following an MHC-mismatched aHSCT. Overall, these results demonstrate that ‘two-pathway’ stimulated CD4+FoxP3+ Tregs were quantitatively and qualitatively more functionally effective than unexpanded Tregs. In total, the findings in this study support the notion that such ‘two-pathway’ stimulated Tregs may be useful for prevention of GVHD and ultimately promote more widespread application of aHSCT in the clinic.
Keywords: Regulatory T cells (Tregs), Tumor Necrosis Factor Receptor Superfamily 25 (TNFRSF25), Interleukin-2 (IL-2), graft versus host disease (GVHD), hematopoietic stem cell transplantation (HSCT)
Introduction
Allogeneic hematopoietic stem cell transplantation (aHSCT) has been administered as a therapeutic modality for a number of genetic disorders, immune deficiency syndromes as well as hematologic diseases and malignancies. These conditions include reconstitution of the lymphoid system in patients with congenital immunodeficiency diseases (ex. SCID), as a vehicle for gene therapy for individuals with enzyme deficiencies (ex. Gaucher’s Disease), as a tolerance protocol for patients in need of tissue transplants (ex. diabetes, kidney) and as rescue and treatment for patients with sickle cell disease, thalassemias and hematologic cancers (ex. leukemia, lymphoma). A major complication of these transplants is the development of graft versus host disease (GVHD) and several recent reviews have discussed the varied treatment strategies under investigation to ameliorate the initiation, expansion and effector phases of this immune complication post T cell replete aHSCT1.
CD4+ fork head box protein 3 (FoxP3+) regulatory T cells (Tregs) are non-redundant mediators of immune homeostasis and self-tolerance. Several studies have demonstrated that Tregs can be used to treat autoimmune diseases, organ rejection and GVHD following aHSCT2–4. Treg therapy in clinical GVHD was demonstrated to be safe and potentially efficacious5,6. We and others have been examining the application of donor CD4+FoxP3+ Tregs as a prophylactic strategy to prevent development of GVHD4,7–10. Amongst the challenges of such an approach is the production of sufficient numbers of Tregs, which can suppress T conventional (Tconv) cells in T cell replete grafts to facilitate engraftment and provide immediate immune function to recipients9,11,12. Our laboratory recently reported that a marked and rapid expansion of mouse Tregs could be accomplished through concomitant stimulation of CD25 and TNF family receptor superfamily 25 (TNFRSF25)7. The objective of the present investigation was to determine whether very low doses of Tregs generated using our novel ‘two-pathway’ expansion protocol via a fusion protein containing TL1A – the natural ligand of TNFRSF25 – and low dose IL-2 could be used to regulate GVHD. Following sorting to produce high purity CD4+FoxP3+ Tregs, we compared the ability of unexpanded and our ‘two-pathway’ expanded Tregs to prevent GVHD following MHC-mismatched aHSCT. Direct comparison of these Treg populations: a) identified higher levels of activation / differentiation markers as well as functional molecules (specifically: CD103, ICOS-1, Nrp-1, PD-1, KLRG1, CTLA4, CD39, CD73, il-10, tgfb1, granzymes A and B) in the expanded vs unexpanded Tregs, b) found TL1A-Ig+IL-2 expanded Tregs showed enhanced in vitro suppressor activity and notably, c) demonstrated that extremely low numbers of ‘two-pathway’ expanded Tregs (1:6 expanded Tregs / Tconventional) suppressed GVHD post-aHSCT. In total, these findings indicate that the ‘two pathway’ stimulation strategy resulted in quantitatively and qualitatively more effective CD4+FoxP3+ Tregs enabling administration of low cell numbers. These observations support the notion that this expanded Treg population may be useful for prevention of GVHD thereby promoting more widespread application of aHSCT in the clinic.
Materials and Methods
Mice
The FoxP3 reporter mice on a C57BL/6 background (B6-FoxP3RFP) (originally provided by R. Flavell, Yale University, New Haven, CT)13 and B6-CD45.1 (H2d) mice were bred in our facility. Wild-type BALB/c (H2d) mice were purchased from Taconic. Mice were used at 6-12 wk of age and were maintained in pathogen-free conditions at the University of Miami animal facilities. All animal use procedures were approved by the UM IACUC.
Antibodies, Reagents, Flow Cytometry and Cell Sorting
Commercial antibodies for use in flow cytometry were purchased from BD Biosciences, Biolegend, or eBioscience. Recombinant mouse IL-2 and α-IL-2 monoclonal antibody, clone JES6-5H4, were purchased from eBioscience, Waltham, MA. IL-2/αIL-2 complex was generated by incubating 1.5μg recombinant mouse IL-2 with 8μg JES6-5H4 (~ 8000 IU/injection) for 15 minutes at room temperature. TL1A-Ig was generated in our laboratory as described previously14.
Single-cell suspensions were prepared from different organs (spleen, peripheral lymph nodes [pLN] and colon). Peripheral blood was collected in heparinized tubes. Peripheral blood mononuclear cells were isolated by standard Ficoll density gradient centrifugation. Next, 106 cells were preblocked with anti-mouse CD16/CD32 and stained with different antibody combinations. Intracellular staining was performed according to standard procedures. The following mAbs to the indicated molecules, the fluorescent labels, and their sources were used in this study: CD4, CD8, CD19, CD25, CD44, CD62L, CD103, KLRG1, CD39, CD73, I-COS, Nrp-1, PD-1, CTLA-4, CCR8, Ly-6C, Ki-67 and Annexin V (Supplementary Materials and Methods, Table S1). Flow cytometric analysis was performed on a BD LSR-Fortessa-HTS instrument (BD Biosciences, San Jose, CA) and the analysis was completed using FlowJo software (FlowJo, LLC, Ashland, OR). Splenic and pLN CD4+FoxP3+ Tregs were sorted using a FACS Aria II cell sorter (BD Biosciences) after enrichment of T cells (surface immunoglobulin depletion of B cells).
RNA isolation, RT-PCR and Quantitative Real-Time PCR
Total RNA was isolated from unexpanded and expanded Tregs using RNAeasy mini kit following the manufacturer’s instructions (QIAGEN). cDNA was retrotranscribed from 1μg of total RNA using qScript cDNA Mastermix (Quanta). Quantitative real-time PCR was (qPCR) performed in triplicate using the ABI PRISM 7300 sequence detection system (Applied Biosystems) with the specific primers for tgfb1, granzyme B, granzyme A, ifng, il-10 and gapdh (Supplementary Materials and Methods, Table S2). The PCR mixture contained 7.5 μl of 2X SYBR Green PCR master mix (Applied Biosystems) in a 15 μl final volume. The specificity of each primer set was monitored by analyzing the dissociation curve. The relative mRNA levels of each gene were calculated using the Livak method with GAPDH as the housekeeping gene.
In Vitro Suppression Assay
CD4+FoxP3− splenocytes (105) were cultured in 96-well plates and activated with 1μg soluble anti-CD3 (clone 2C11) antibody in the presence of APCs (5×104 T cell depleted splenocytes) and titrating numbers of sorted CD4+FoxP3+ Tregs. Cultures were incubated for 72 hours and pulsed with [3H]-Thymidine (0.5 μCi/well; Perkin Elmer) for the last 10 h. Incorporated isotope was measured by liquid scintillation counting (Micro Beta TriLux counter; Perkin Elmer).
HSCT Experiments
For the HSCT in the major MHC-mismatch model (B6→BALB/c), female BALB/c mice (H2d) were ablatively conditioned with 8.5 Gy total body irradiation 1 day before transplantation. BM cells were obtained from femurs, tibias, and vertebrae from sex-matched B6-CD45.1 (H2b; Thy1.2) donor animals. A single-cell suspension of marrow cells was prepared by flushing bones with a 21-gauge needle and the cells were filtered through a 100 μm nylon mesh. Donor marrow cells were depleted of T cells via complement mediated lysis using anti–T-cell–specific antibody HO-13-4 (hybridoma supernatant, mouse anti-Thy1.2 IgM; ATCC, Manassas, VA) generously provided by Dr. Bruce Blazar (University of Minnesota), anti-CD4 mAb (clone 72.4), anti-CD8mAb (clone H02.2), and rabbit complement (Cedarlane Laboratories, Burlington, Ontario, Canada). The marrow cells were incubated at 37°C for 45 minutes, washed twice in RPMI, and resuspended for HSCT. Marrow T cell depletion was routinely >99%. Donor T cells were prepared from spleens or LN obtained from C57BL/6-FoxP3RFP–expanded or unexpanded animals. Donor cells were stained for T cells (anti-CD4, clone RM4-5; anti CD8, clone 53-6-7) and adjusted to 1.0×106 T cells per mouse before mixing with BM. Recipient mice underwent transplantation (day 0) with T cell–depleted (TCD) BM (5.5×106) and 1.0×106 T cells i.v. in a 0.2 mL volume via tail vein injection. GVHD was assessed by monitoring recipients for changes in total body weight, clinical signs, and overall survival. The clinical signs of GVHD were recorded for individual mice. Recipients were scored on a scale from 0 to 2 for 5 clinical parameters15: weight loss, diarrhea, fur texture, posture, and alopecia.
Histologic Analysis
Briefly, tissues from animals 5 weeks after aHSCT were fixed in 10% formalin and embedded in paraffin. Sections were stained with hematoxylin-eosin (H&E) and images were acquired using the Keyence BZ-X700 microscope. Tissue samples were scored following a modified system described by Kaplan D, et al16. In brief, multiple parameters were used to compare pathology scores between groups in the skin and the colon (3 and 4 parameters, respectively).
Statistical Analysis
All graphing and statistical analysis were performed using GraphPad Prism (San Diego, CA). Values shown in graphs represent the mean of each group±SEM. Survival data were analyzed with the Mantel-Cox log-rank test. Nonparametric unpaired two-tailed t-test was used for comparisons between 2 experimental groups, and multiple variable analysis was performed using ANOVA. A P value less than 0.05 was considered significant.
Results and Discussion
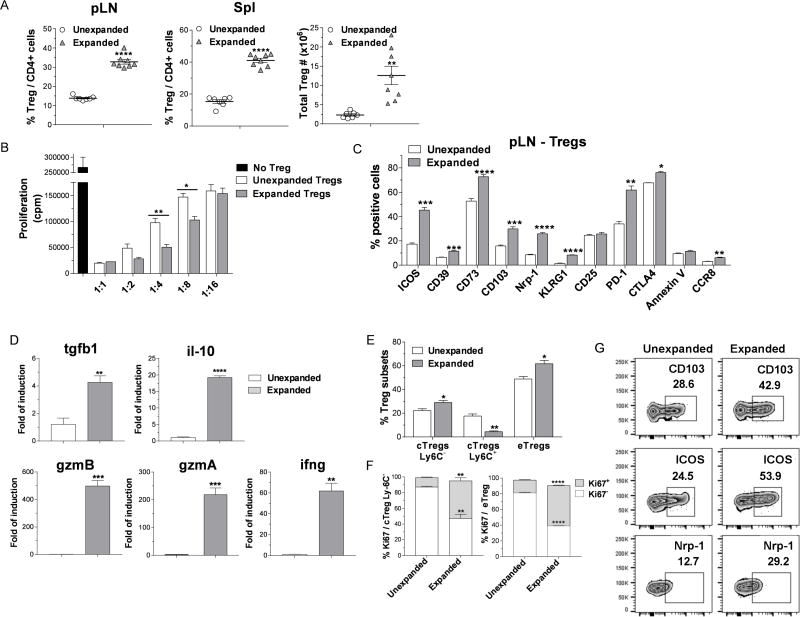
We recently reported that ‘two-pathway’ stimulation of Tregs via TNFRSF25 and CD25 using a TL1A-Ig fusion protein and low dose IL-2 rapidly and markedly enhances the CD4+FoxP3+ Treg compartment while minimally altering Tconv cell populations7. This observation was confirmed by determining the levels of splenic (frequency / numbers) and peripheral lymph node (pLN, frequency) Tregs in C57BL/6-FoxP3RFP (B6-Fir) mice and a second independent strain, BALB/c following this ‘two-pathway’ protocol (Figs. 1A, S1). While in a previous report, we found unexpanded splenic Tregs were more suppressive compared to lymph node Tregs7, to directly compare the functional activity of ‘two-pathway’ expanded Tregs to unexpanded Tregs, an in vitro suppressor assay was performed using these populations isolated from the spleen. On a per cell basis, the expanded Tregs more effectively inhibited proliferation of anti-CD3 mAb stimulated Tconv cells (Fig. 1B). To further analyze and compare expanded and unexpanded Treg populations, activation, differentiation and effector molecules were assessed in pLN (Fig. 1C) and spleen (Fig. S2) using mAbs to defined Treg proteins (Table S1). Significantly increased levels of activation and differentiation molecules, specifically ICOS, CD103, PD-1 and KLRG1 were identified in the expanded Treg population (Fig. 1C). Additionally, the Treg functional suppressive mediators CD39, CD73, Nrp-1 and CTLA-4 were also elevated in this expanded population. Similar results were obtained analyzing splenic Tregs (Fig. S2). The expression of CD127 (IL-7Rα) was low/absent in splenic and lymph node Tregs and Helios expression was not detected in these populations (data not shown). To further explore gene expression of Treg effector cytokines, RNA was obtained from sorted expanded and unexpanded splenic CD4+FoxP3+ Tregs and assessed by qPCR using specific primers (Table S2). Significantly, higher levels of tgfb1, il-10, ifng, gzmA and gzmB (the latter confirming a previous observation7) but not il-35 (i.e. il-12a and Ebi3 - data not shown) were apparent in the ‘two-pathway’ expanded vs. unexpanded Treg cells (Fig. 1D). Tregs can mediate their functional immunosuppressive activity via several mechanisms. These pathways include production of inhibitory cytokines (ex. TGFβ, IL-10), cytolysis (ex. GzmA, B), metabolic disruption (CD39/CD73) and inhibition of DC maturation and function (Nrp-1, CTLA-4)17–19. Notably, this is the first report demonstrating that the ‘two-pathway’ expanded Tregs exhibited elevated levels of molecules which play a role in each of these mechanisms.
Figure 1. TL1A-Ig+IL-2 ‘two-pathway’ expanded Tregs exhibit high levels of activation, effector, differentiation and functional molecules together with an increase in their in vitro suppressor activity.
A-G TL1A-Ig (50 μg) was injected i.p. on days 1-4; rmIL-2 (1.5 μg) bound to the a-IL-2 mAb (JES6-5H4; 8ug) was administered on days 4 and 6. Mice were sacrificed on day 7. (A) In vivo treatment with TL1A-Ig + low dose IL-2 induced a strong increase in the overall Treg (CD4+FoxP3+) frequency (%) of total CD4+ cells in pLN (left) and spleen (middle) and total numbers splenic Tregs (right). Data are pooled from 3 independent experiments; unexpanded n=7, expanded n=8. (B) Sorted Tregs from expanded and unexpanded B6-Fir mice were compared for functional activity using an in vitro Treg suppression assay and expanded Tregs demonstrated more effective inhibition on a per cell basis. (C) Expression of activation (i.e. ICOS, CD103), functional (i.e. CD39, CD73, Nrp1, CTLA-4), differentiation (KLRG1) and survival (i.e. PD-1, CCR8, Annexin) molecules in pLN Tregs. (D) Quantitative Real-time PCR (qPCR) analysis of tgfb1, il-10, gzmA, gzmB and ifng mRNA levels (relative to gapdh) of splenic CD4+Foxp3+ Tregs sorted from unexpanded and expanded mice. Significantly higher mRNA levels of tgfb1, il-10, gzmA, gzmB and ifng were observed. Data representative of 2 independent experiments n = 3 mice/group. (E) Treg subset distribution determined by CD62L and Ly-6C staining show an increase in cTregs CD62LhiLy-6C− and eTregs CD62LloLy-6C− in the TL1A-Ig+IL-2 expanded Tregs. (F) cTregs Ly6C− and eTregs expanded Tregs are highly proliferative indicated by Ki67 expression in the pLN. (G) Representative flow cytometry plots assessing expression of CD103, ICOS and Nrp-1 on unexpanded and expanded eTregs show elevated levels of each molecule examined (4 mice/group). Data representative of 3 independent experiments. (A-F) Data are expressed as means ± SEM and were analyzed by a two-tailed unpaired t test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
CCR8 was recently reported to be important in the survival of Treg cells following transplant for their regulation of GVHD20 and was significantly elevated in the TL1A-Ig+IL-2 expanded Treg cells (Fig. 1C, S2B). This chemokine (via CCL1) may also contribute in the regulation of Treg function through its reported association with increase of GzmB and CD3921 - the same pattern observed in the present studies (Figs. 1C, S2B). Additionally, regarding TL1A-Ig+IL-2 expanded Treg survival, we found no evidence of increased apoptotic rates (Annexin V) a finding consistent with their elevated CCR8 levels. PD-1 expression was also significantly elevated in the ‘two-pathway’ expanded Tregs. Interestingly, in the context of in vivo IL-2 treatment, Asano et. al. reported PD-1 is an important homeostatic regulator for Tregs promoting Treg proliferation and survival22.
Ly6C has been used to analyze Treg cell subsets23,24. Three Treg populations have been defined using Ly6C and CD62L expression, i.e. central Tregs (cTregs) consisting of Ly6C-CD62Lhi and Ly6C+CD62Lhi and effector Tregs (eTregs) characterized by the Ly6C− CD62Llo phenotype. Ly6C+ Tregs express poor suppressive ability in vitro and in vivo compared with Ly6C− and the former express lower affinity TCR23,24. Notably, ‘two-pathway’ expanded Tregs contained diminished levels of the Ly6C+CD62Lhi cTregs and increased levels of both Ly6C−CD62Lhi cTregs and Ly6C− CD62Llo eTregs (Fig. 1E). Both of these latter elevated populations exhibited heightened levels of proliferation as assessed by Ki67 expression (Fig. 1F). Representative Treg functional molecules were also elevated in both of these suppressive subsets, eTregs (Fig 1G) and cTregs (data not shown). Tregs have been shown to regulate GVHD following experimental aHSCT4,8,9. It may therefore be noteworthy that the highly suppressive Ly6C− expanded Tregs included a CD62Lhi population. Although Ly6C was not evaluated in an earlier report, those studies proposed that CD62Lhi Tregs were a highly effective population for regulating GVHD25.
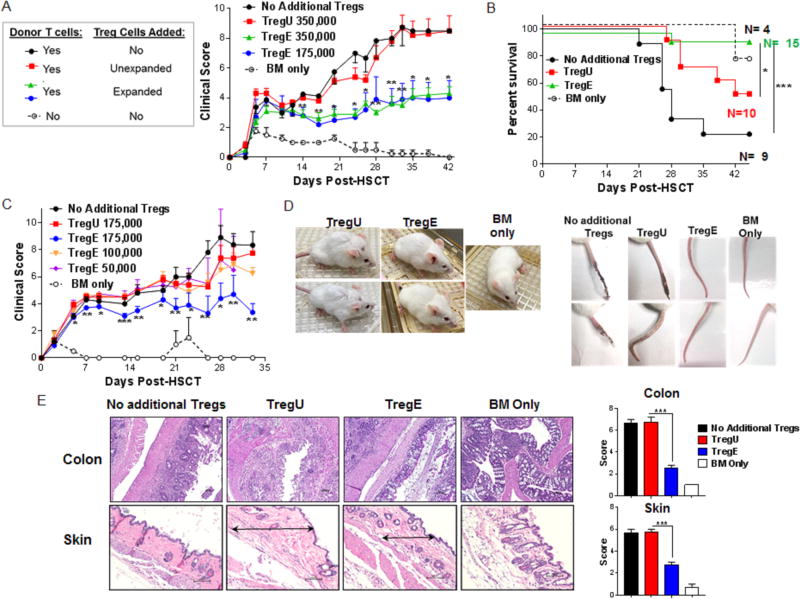
The phenotypic and RNA analyses together with in vitro functional activity implied that potent suppression could be mediated by the ‘two-pathway’ stimulated Tregs in vivo. Therefore, studies were designed to directly compare the ability of this population versus unexpanded Tregs to suppress development of GVHD in a complete MHC-mismatched HSCT model (Fig. S3A). Varying numbers of sorted donor CD4+FoxP3+ Tregs (>99% purity from B6-FoxP3RFP reporter mice Fig. S3B) were combined with B6-wt spleen cells. As anticipated based on published studies, using only 3.5×105 unexpanded Tregs (TregU) together with 1.0 ×106 Tconv cells was not sufficient to prevent GVHD. Notably, transfer of this same number of expanded Tregs (TregE) effectively inhibited GVHD (Fig. 2A). Moreover, as low as 1.75×105 expanded CD4+FoxP3+ cells (ratio: ~1:6 Treg/Tcon) also ameliorated acute clinical GVHD (Fig. 2A, C, D) and promoted survival (Fig. 2B) however, lower numbers of expanded Tregs (0.05 to 1.0×105) were not sufficient to diminish GVHD or prolong survival (Figs. 2C, S3C). Representative photographs of transplanted mice (1 month) and tails (2 months) indicated differences between recipients of TregU and TregE (Fig. 2D). Evaluation of the colon and skin from aHSCT recipients receiving very low Treg numbers was consistent with the clinical scoring (Fig. 2E). Colons from recipients of TregU exhibited mucosal thickening, severe and extensive inflammation and edema with villi distortion. In contrast, colons from TregE showed a mild and patchy inflammation and no distortion of the villi. Skin evaluation in TregU recipients showed general architecture disruption characterized by extensive thickening and collagen deposition (fibrosis) accompanied by decreased hair follicles. In contrast, tissue from TregE recipients showed mild fibrosis with the presence of hair follicles, and minimal dermal thickening (Fig. 2E). It may therefore be noteworthy that increased CD103 levels on ‘two-pathway’ expanded Treg cells were detected (Fig. 1C). This molecule can promote migration of Tregs into tissue sites, including skin and gut and has been associated with enhanced suppressive activity26,27.
Figure 2. Low numbers of sorted TL1A-Ig+IL-2 expanded Tregs ameliorate GVHD and promote survival after MHC-mismatched aHSCT.
Tregs were expanded with TL1A-Ig+IL-2, mice were sacrificed at day 7 and splenic CD4+FoxP3+ Tregs were isolated by FACS. A complete MHC-mismatched aHSCT model (B6→ BALB/c) was utilized and varying numbers of sorted CD4+FoxP3+ unexpanded and expanded Tregs together with B6-wt 1×106 splenic T cells and 5.5×106 TCD B6-CD45.1 BM cells were transplanted. (A) Clinical GVHD scores (0=no disease and 10=severe) and (B) survival curves after receiving BM cells + T cells + unexpanded 350,000 (TregU), expanded 175,000/350,000 (TregE) Tregs or no addition of Tregs. BM only group was used as a negative GVHD control (n=8 mice/group, n=3 mice/BM Only group). Only recipients of “two-pathway” expanded Tregs demonstrated ameliorated GVHD. (C) Clinical GVHD scores of mice receiving unexpanded (TregU)175,000 or expanded (TregE) ≤ 175,000 Tregs corroborated the ability of 175,000 Treg E (however not lower) but not TregU to inhibit GVHD (n=6 mice/group). (D) Representative photographs of recipient mice (4 weeks post-aHSCT) and tails (9 weeks post-aHSCT) of TregU and Treg E 175,000 recipient mice illustrating healthier outcome of TregE transplanted mice. (E) Representative H&E-stained sections of colon and skin from the indicated groups 5 weeks post-aHSCT are shown. Colons from TregE 175,000 recipients showed a mild and patchy inflammation and no distortion of the villi compared with TregU 175,000 (and the no additional Treg) recipient colons which exhibited mucosal thickening, severe and extensive inflammation and edema. In skin of TregE recipients, note minimal dermal thickening (black arrow) with mild cell infiltrate compared with TregU recipients which exhibited a thickened dermis (black arrow) with extensive collagen deposition and moderate infiltration of mononuclear cells. Magnification 100× for colon and 200× for skin. Pathology scores for these tissues are shown on the right. (A-C) Data are expressed as means ± SEM and were analyzed by (A,C) a two-tailed unpaired t test or (B) log-rank test. *p<0.05; **p<0.01; ***p<0.001.
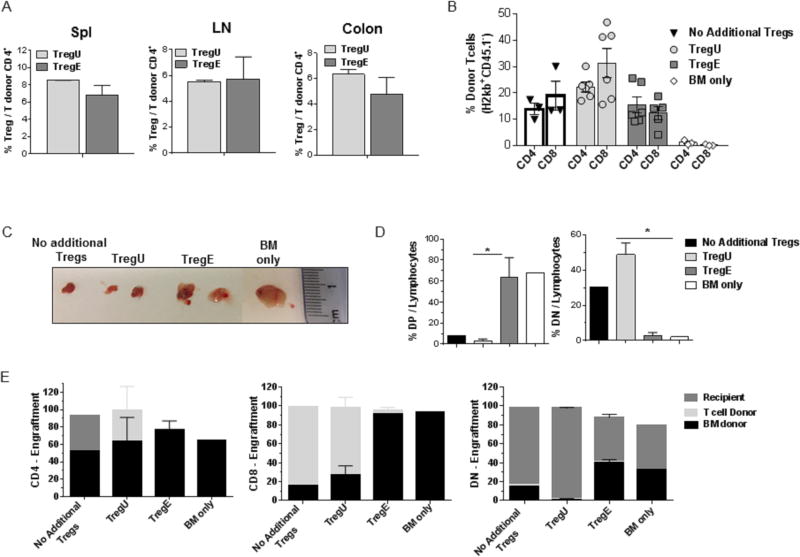
Following HSCT with low numbers of Tregs (1.75-2.0×105), recipients were examined post-transplant. The percentage of CD4+FoxP3+ / CD4+ T cells at Day 2 (data not shown) and 7 in multiple tissues was not different in animals receiving the same number of TregU or TregE cells (Fig. 3A). Assessment of the donor CD4/CD8 ratio in recipients one month post-HSCT demonstrated the anticipated inversion in recipients without additional Treg cells (Fig. 3B). Although not reaching statistical significance, the anticipated CD4/CD8 inversion was observed one month post-HSCT in recipients without additional Tregs or those receiving TregU - but not recipients of TregE (Fig. 3B). These observations support the notion that on a per cell basis, the expanded Tregs are more potent. Recipients of TregU or TregE did not contain an increased frequency of Tregs in the spleen, nodes and colon post-infusion. Such observations may be a result of limited IL-2 availability in these animals and / or trafficking to other compartments. Notably, 5 weeks post-transplant there were differences in the thymic tissue and the origin of thymocytes between recipients of TregU and TregE. Thymic size was greater in recipients of TregE (Figs. 3C) consistent with the clinical score and overall survival of these mice (Figs. 2A-C S3C). Additionally, a normal pattern of thymocyte differentiation was identified in recipients of TregE, i.e. the highest percentage of lymphocytes present were CD4+CD8+ double-positive and the single positive CD4/CD8 (SP) levels were comparable to recipients of bone marrow only (and normal mice, data not shown) (Figs. 3D, S4). Importantly, recipients of TregE contained the highest levels of donor bone marrow derived (CD45.1+H2b+) SP thymocytes, consistent with the finding of a high frequency of donor marrow derived DN thymocyte progenitors (Fig. 3E). Such findings suggest that long-term engraftment may be more effective utilizing administration of ‘two-pathway’ (TL1A-Ig+IL-2) stimulated donor Tregs. Thymic damage is a highly sensitive indicator of GVHD28 and therefore, these results are consistent with those above and support the notion that use of ‘two-pathway’ expanded Tregs is an effective prophylactic GVHD therapy. A recent review discussed that pre-clinical findings with nTregs support the translation of adoptive therapy to prevent clinical GVHD29. Specifically, regarding anti-tumor responses post-aHSCT, it was previously reported that transfer of unexpanded Tregs did not diminish GVL activity30. Importantly, our previous study transplanting spleen cells from TL1A-Ig+IL-2 treated donors (containing ~4×105 Tregs) also found GVL responses were preserved, therefore we anticipate using Treg numbers as low as those reported here will not interfere with effective anti-tumor responses7. Lastly, we speculate that since very low numbers of TNFRSF25 and CD25 stimulated Tregs were found to be effective across a complete MHC-disparate aHSCT, it may be possible to develop an ex-vivo strategy to generate sufficient numbers for therapeutic application14.
Figure 3. Recipients of TL1A-Ig+IL-2 expanded Tregs develop a normal pattern of thymic differentiation and accompanied by donor stem cell derived long term engraftment after MHC-mismatched aHSCT.
A complete MHC-mismatched aHSCT was performed (as in Fig 2) transplanting sorted CD4+FoxP3+ unexpanded and expanded Tregs (175,000-200,000) together with B6-wt 1×106 splenic T cells and 5.5×106 TCD B6-CD45.1 BM cells. Recipient mice were bled / sacrificed at different time points post-aHSCT. (A) Similar Treg levels were found in TregU and TregE recipient spleen (Spl), lymph nodes (LN) and colon 1 week after transplantation. Data are representative of 3 independent experiments. (B) Frequency of CD4+ and CD8+ cells present in the blood of recipients of the indicated groups one month post-aHSCT. Results demonstrated only TregE recipients did not contain an inverted CD4/CD8 ratio (C-E). Five weeks after transplant, the thymus was evaluated. (C) Representative photographs of thymi indicate larger thymic size in the TregE vs TregU recipients. (D) Frequency of CD4+CD8+ double-positive (DP) and CD4−CD8− (DN) thymocytes cells out of total lymphocytes. TregE thymi showed virtually normal levels of DP and DN subsets comparable to recipient to of BM only. Data were analyzed by a two-tailed unpaired t test. *p<0.05. (E) Determination of origin of thymocyte populations, i.e. BM donor, T cell donor or recipient. TregE but not TregU recipients contained levels of BM derived thymocytes comparable to recipients of donor BM only. Data are expressed as means ± SEM.
Supplementary Material
Figure S1. TL1A-Ig+IL-2 ‘two-pathway’ in vivo treatment increases Treg levels in a second strain of mice. TL1A-Ig (50 μg) was injected i.p. on days 1-4; rmIL-2 (1.5 μg) bound to the a-IL-2 mAb (JES-5H4; 8μg) was administered on days 4 and 6. BALB/c mice were sacrificed on day 7. In vivo treatment with TL1A-Ig + low dose IL-2 induced a strong increase in the overall Treg (CD4+FoxP3+) frequency (%) of total CD4+ cells and numbers in pLN (left) and spleen (middle and right) in BALB/c mice. Data are pooled from 2 independent experiments; Unexpanded n=4, Expanded n=4. Data are expressed as means ± SEM and were analyzed by a two-tailed unpaired t test. *p<0.05; **p<0.01.
Figure S2. TL1A-Ig+IL-2 ‘two-pathway’ in vivo expanded Tregs express high levels of activation, effector, differentiation and functional molecules. Tregs were expanded in B6- Fir mice as described above (Fig. S1) and sacrificed on day 7. Expression of activation (i.e. ICOS, CD103), functional (i.e. CD39, CD73, Nrp1, CTLA-4), differentiation (KLRG1) and survival (i.e. PD-1, Annexin) molecules in splenic Tregs. As observed in expanded Tregs from the pLN (Fig. 1B), activation and functional markers were elevated compared with unexpanded Tregs. Data are expressed as means ± SEM and were analyzed by a two-tailed unpaired t test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Figure S3. Survival is prolonged in MHC-mismatched recipients transplanted with low numbers of sorted TL1A-Ig+IL-2 ‘two-pathway’ expanded Tregs. Tregs were expanded with TL1A-Ig+IL-2, mice were sacrifice at day 7 and splenic CD4+FoxP3+ Tregs were sorted. (A) Experimental design of the complete MHC-mismatched aHSCT model used in these studies. (B) Representative dot plots showing the purity (>99%) of unexpanded and expanded CD4+FoxP3+ Tregs after FACS. Titrated numbers were utilized in the aHSCT. (C) Survival curves of groups receiving: BM cells + T cells + unexpanded 175,000 (TregU); expanded 100,000 / 175,000 (TregE) Tregs; or no additional Tregs (n=6 mice/group and n=3 mice/BM only group). Data are expressed as means ± SEM and were analyzed by log-rank test. ns (not significant); *p<0.05.
Figure S4. Recipients transplanted with purified TL1A-Ig+IL-2 expanded Tregs exhibited comparable levels of single positive thymocytes equivalent to BM only recipients. Five weeks after transplant (MHC-mismatched B6➔BALB/c) thymi were counted and stained as described in Methods. There were equivalent levels of CD4 SP and CD8 SP in recipients of TregE and BM only. However, there were abnormal CD4 SP / CD8 SP levels in recipients of TregU or no additional Tregs. Data are expressed as means ± SEM.
Highlights.
Stimulating Tregs in vivo using a ‘two-pathway’ strategy (TNFRSF25 via TL1A-Ig; and CD25 via low dose IL-2) induces differentiation and activation markers characteristic of a distinct phenotype compared to untreated Tregs
‘Two-pathway’ expanded Tregs show increased levels of effector molecules and mediate enhanced in vitro suppressor activity
Low numbers of ‘two-pathway’ expanded Tregs suppressed pre-clinical GVHD
Acknowledgments
The authors thank Dr. Oliver Umland and the Sylvester Comprehensive Cancer Center Flow Cytometry Core for excellent assistance with flow cytometry and cell sorting. This work was supported by funds from the Sylvester Comprehensive Cancer Center and NIH RO1 EY024484-01 (RBL) and funds from the Kalish Family Foundation (KVK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
SC: designed research studies, conducted experiments, analyzed data, wrote the manuscript
DW, BK, HB, COL, CSB and WA: conducted experiments and analyzed data
NHA: analyzed histopathology data
KVK: supervised and supported the studies
RBL: designed research studies, wrote the paper, supervised and supported the work.
Disclosure of Conflicts of Interest
Dr. Levy is a scientific advisory board member of Heat Biologics, Inc. and a consultant for Allergen, Heat Biologics and Pelican Therapeutics. All other authors declared no conflict of interest.
References
- 1.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017;377:2167–79. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, et al. Administration of CD4+CD25highCD127-regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817–20. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–36. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 5.Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–6. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf D, Barreras H, Bader CS, et al. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:757–66. doi: 10.1016/j.bbmt.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 10.Nishikii H, Kim BS, Yokoyama Y, et al. DR3 signaling modulates the function of Foxp3+ regulatory T cells and the severity of acute graft-versus-host disease. Blood. 2016;128:2846–58. doi: 10.1182/blood-2016-06-723783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shatry A, Chirinos J, Gorin MA, Jones M, Levy RB. Targeting Treg cells in situ: emerging expansion strategies for (CD4(+)CD25(+)) regulatory T cells. Biol Blood Marrow Transplant. 2009;15:1239–43. doi: 10.1016/j.bbmt.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–31. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SQ, Tsai MS, Schreiber TH, Wolf D, Deyev VV, Podack ER. Cloning, expression, and functional characterization of TL1A-Ig. J Immunol. 2013;190:1540–50. doi: 10.4049/jimmunol.1201908. [DOI] [PubMed] [Google Scholar]
- 15.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–9. [PubMed] [Google Scholar]
- 16.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–75. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 17.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 19.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Coghill JM, Fowler KA, West ML, et al. CC chemokine receptor 8 potentiates donor Treg survival and is critical for the prevention of murine graft-versus-host disease. Blood. 2013;122:825–36. doi: 10.1182/blood-2012-06-435735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barsheshet Y, Wildbaum G, Levy E, et al. CCR8(+)FOXp3(+) Treg cells as master drivers of immune regulation. Proc Natl Acad Sci U S A. 2017;114:6086–91. doi: 10.1073/pnas.1621280114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asano T, Meguri Y, Yoshioka T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129:2186–97. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delpoux A, Yakonowsky P, Durand A, et al. TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J Immunol. 2014;193:5914–23. doi: 10.4049/jimmunol.1400477. [DOI] [PubMed] [Google Scholar]
- 24.Toomer KH, Yuan X, Yang J, Dee MJ, Yu A, Malek TR. Developmental Progression and Interrelationship of Central and Effector Regulatory T Cell Subsets. J Immunol. 2016;196:3665–76. doi: 10.4049/jimmunol.1500595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 26.Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci U S A. 2010;107:5919–24. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–55. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 28.Seemayer TA, Lapp WS, Bolande RP. Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia. Am J Pathol. 1977;88:119–34. [PMC free article] [PubMed] [Google Scholar]
- 29.Heinrichs J, Bastian D, Veerapathran A, Anasetti C, Betts B, Yu XZ. Regulatory T-Cell Therapy for Graft-versus-host Disease. J Immunol Res Ther. 2016;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. TL1A-Ig+IL-2 ‘two-pathway’ in vivo treatment increases Treg levels in a second strain of mice. TL1A-Ig (50 μg) was injected i.p. on days 1-4; rmIL-2 (1.5 μg) bound to the a-IL-2 mAb (JES-5H4; 8μg) was administered on days 4 and 6. BALB/c mice were sacrificed on day 7. In vivo treatment with TL1A-Ig + low dose IL-2 induced a strong increase in the overall Treg (CD4+FoxP3+) frequency (%) of total CD4+ cells and numbers in pLN (left) and spleen (middle and right) in BALB/c mice. Data are pooled from 2 independent experiments; Unexpanded n=4, Expanded n=4. Data are expressed as means ± SEM and were analyzed by a two-tailed unpaired t test. *p<0.05; **p<0.01.
Figure S2. TL1A-Ig+IL-2 ‘two-pathway’ in vivo expanded Tregs express high levels of activation, effector, differentiation and functional molecules. Tregs were expanded in B6- Fir mice as described above (Fig. S1) and sacrificed on day 7. Expression of activation (i.e. ICOS, CD103), functional (i.e. CD39, CD73, Nrp1, CTLA-4), differentiation (KLRG1) and survival (i.e. PD-1, Annexin) molecules in splenic Tregs. As observed in expanded Tregs from the pLN (Fig. 1B), activation and functional markers were elevated compared with unexpanded Tregs. Data are expressed as means ± SEM and were analyzed by a two-tailed unpaired t test. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Figure S3. Survival is prolonged in MHC-mismatched recipients transplanted with low numbers of sorted TL1A-Ig+IL-2 ‘two-pathway’ expanded Tregs. Tregs were expanded with TL1A-Ig+IL-2, mice were sacrifice at day 7 and splenic CD4+FoxP3+ Tregs were sorted. (A) Experimental design of the complete MHC-mismatched aHSCT model used in these studies. (B) Representative dot plots showing the purity (>99%) of unexpanded and expanded CD4+FoxP3+ Tregs after FACS. Titrated numbers were utilized in the aHSCT. (C) Survival curves of groups receiving: BM cells + T cells + unexpanded 175,000 (TregU); expanded 100,000 / 175,000 (TregE) Tregs; or no additional Tregs (n=6 mice/group and n=3 mice/BM only group). Data are expressed as means ± SEM and were analyzed by log-rank test. ns (not significant); *p<0.05.
Figure S4. Recipients transplanted with purified TL1A-Ig+IL-2 expanded Tregs exhibited comparable levels of single positive thymocytes equivalent to BM only recipients. Five weeks after transplant (MHC-mismatched B6➔BALB/c) thymi were counted and stained as described in Methods. There were equivalent levels of CD4 SP and CD8 SP in recipients of TregE and BM only. However, there were abnormal CD4 SP / CD8 SP levels in recipients of TregU or no additional Tregs. Data are expressed as means ± SEM.