Abstract
Melanization is a universal defense mechanism of insects against microbial infection. During this response, phenoloxidase (PO) is activated from its precursor by prophenoloxidase activating protease (PAP), the terminal enzyme of a serine protease (SP) cascade. In the tobacco hornworm Manduca sexta, hemolymph protease-14 (HP14) is autoactivated from proHP14 to initiate the protease cascade after host proteins recognize invading pathogens. HP14, HP21, proHP1*, HP6, HP8, PAP1–3, and non-catalytic serine protease homologs (SPH1 and SPH2) constitute a portion of the extracellular SP-SPH system to mediate melanization and other immune responses. Here we report the expression, purification, and functional characterization of M. sexta HP2. The HP2 precursor is synthesized in hemocytes, fat body, integument, nerve and trachea. Its mRNA level is low in fat body of 5th instar larvae before wandering stage; abundance of the protein in hemolymph displays a similar pattern. HP2 exists as an active enzyme in plasma of the wandering larvae and pupae in the absence of an infection. HP14 cleaves proHP2 to yield active HP2. After incubating active HP2 with larval hemolymph, we detected higher levels of PO activity, i.e. an enhancement of proPO activation. HP2 cleaved proPAP2 (but not proPAP3 or proPAP1) to yield active PAP2, responsible for a major increase in IEARpNA hydrolysis. PAP2 activates proPOs in the presence of a cofactor of SPH1 and SPH2. In summary, we have identified a new member of the proPO activation system and reconstituted a pathway of HP14–HP2–PAP2–PO. Since high levels of HP2 mRNA were present in integument and active HP2 in plasma of wandering larvae, HP2 likely plays a role in cuticle melanization during pupation and protects host from microbial infection in a soil environment.
Keywords: insect immunity, hemolymph protein, serine protease cascade, melanization, clip domain, zymogen activation
1. Introduction
Insect immune system is simpler than that in mammals, because only innate mechanisms are involved in the resistance against pathogen invasion (Nappi and Christensen, 2005; Strand, 2008; Jiang et al., 2010; Buchon et al., 2014). Insects mount rapid responses to heal wounds and entrap pathogens via hemolymph coagulation, nodulation, encapsulation and melanization, and destroy the invading microbes by phagocytosis, reactive chemicals, and antimicrobial peptides. They employ serine protease (SP) cascade pathways (Kanost and Jiang, 2015) to coordinate some of these responses including proteolytic activation of phenoloxidase (PO), Spätzle and plasmatocyte spreading peptide (PSP) precursors. PO catalyzes melanization, Spätzle induces antimicrobial peptide synthesis via the Toll pathway, PSP causes hemocyte encapsulation, and stress response peptides modulate innate immunity (Kanost and Jiang, 2015; Schrag et al., 2017). Members of the pathways exist as inactive precursors and, upon wounding or microbial infection, become active by proteolytic cleavage at a specific peptide bond.
Among them, the proPO activation cascade has been studied in several insects (Park et al., 2010; Hillyer, 2010; Kanost and Jiang, 2015; Veillard et al., 2016). POs oxidize monophenols to o-diphenols and then o-quinones that are precursors of melanin. The reactive intermediates (e.g. 5,6-dihydroxyindole) kill pathogens, parasitoids, as well as host cells if not properly regulated (Zhao et al., 2007 and 2011). In the tobacco hornworm Manduca sexta, proPO activation requires a proPO activating protease (PAP) and a high Mr cofactor of non-catalytic serine protease homologs (SPH1 and 2) (Yu et al., 2003; Gupta et al., 2005). PAP1–3 and SPHs are components of the SP-SPH system which also includes HP14, HP21, HP6, HP8 and proHP1* (an active but uncleaved enzyme) (Kanost and Jiang, 2015; Yang et al., 2016; He et al., 2017). HP14 activates proHP21, HP21 activates proPAP2 and 3 for proPO activation (Wang and Jiang, 2006, 2007 and 2017; Gorman et al., 2007; Takahashi et al., 2015) in larval hemolymph. While proHP6 activation remains elusive, HP6 activates proPAP1 and proHP8 (An et al., 2009). PAP1 activates proPO and HP8 generates Spätzle-1 (An et al., 2010) to induce antimicrobial peptide synthesis via the Toll pathway.
Omic research greatly stimulates the understanding of SP-SPH pathways in this biochemical model insect. There are 125 SP/SPH genes in the M. sexta genome, whose protein products do not seem to participate in food digestion (Cao et al., 2015; Kanost et al., 2016). Their expression patterns (e.g. immune inducibility) are available through RNA-seq studies (Zhang et al., 2011; Gunaratna and Jiang, 2013; Cao and Jiang, 2017). More importantly, 36 of the gene products are identified in hemolymph of the 5th instar larvae by proteomic analysis (He et al., 2016), including HP1a, 1b, 2, 5, 6, 8, 9, 14a–21, 22, 25, 26, PAP1–3, SP34, 51, 56, 112, 142, GP57, 59, scolexins A and B, SPH1, 2, 3, 4, 33, and 101. Since the underlined eleven are known to be components of the SP-SPH system, we focus our functional exploration on the other 25 SP-related proteins. In this study, we performed the expression and functional analyses of HP2, one of the first cloned HPs in M. sexta (Jiang et al., 1999). HP2, also known as hemocyte protease-2, is predicted to be a trypsin-like SP with its putative cleavage site located between ADEL and IVGG. Similar as the other HPs, HP2 contains two domains, an amino-terminal regulatory clip domain, a linker region, and a carboxyl-terminal protease domain. HP2 is closely similar to HP21, HP22, HP13, HP18a, HP18b, SP33 and SP144 (Cao et al., 2015), which may severely complicate their activity tests due to potential functional overlaps. The HP2 gene is expressed in both hemocytes and fat body. The mRNA level decreased in hemocytes but increased in fat body after larvae had been injected with bacteria (Jiang et al., 1999). M. sexta proHP2 is present at low levels in plasma and hemocytes of the 5th instar feeding larvae with more processed form inside the cells, as revealed by immunoblot analysis using an HP2 antiserum. Here we report the tissue distribution of HP2 transcripts and changes in protein levels at different life stages. Using purified recombinant proHP2, we have examined its role in proPO activation by identifying its activating protease and protein substrate in the SP pathway. The physiological roles of HP2 are also discussed in this paper.
2. Materials and methods
2.1. Insect rearing, bacterial challenge, hemolymph collection, and tissue dissection
M. sexta eggs were purchased from Carolina Biological supplies and hatched larvae were reared on an artificial diet (Dunn and Drake, 1983). Each day 2, 5th instar larva was injected with a mixture of killed bacteria (1.3×107 Escherichia coli XL1-Blue and 13 μg Micrococcus luteus) and 13 μg insoluble β-1,3-glucan from Alcaligenes faecalis suspended in 20 μl H2O. Hemolymph was collected from cut prolegs of the three larvae 24 h later, centrifuged at 5,000×g for 10 min to harvest induced hemocytes (IH), and supernatants were pooled as induced plasma (IP). Similarly, control plasma (CP) and hemocytes (CH) were collected from three day 3, 5th instar naïve larvae prior to dissection of fat body, integument, midgut, Malpighian tubules, muscle, nerve tissue, salivary glands, and trachea. The control and induced hemocytes and fat body (CH, IH, CF and IF) and the other tissues from the naïve larvae were stored at −80 °C prior to RNA isolation. To measure and compare HP2 mRNA levels at different times, hemocytes, fat body and integument were dissected from insects ranging from 4th instar larvae to day 9, pupae (3 insects per stage) for RNA preparation.
2.2. RNA extraction, cDNA synthesis, and qRT-PCR analysis
The stored tissue samples were treated with TRIZOL reagent (Thermo Fisher Scientific) for RNA extraction. All the total RNA samples (1 μg each) were incubated with 1× iScript Reverse Transcription Supermix (Bio-Rad) in a 10 μl reaction incubated at 25 °C for 5 min and 42 °C for 60 min to synthesize cDNA. The reaction mixtures were heated at 95 °C for 5 min to denature the enzyme. Quantitative real-time PCR was carried out to determine the levels of HP2 and ribosomal protein S3 transcripts using an equal amount of cDNA samples as templates incubated with 1× iTaq™ Universal SYBR Green Supermix (Bio-Rad) and specific primers in 10 μl reactions. The primer pairs were: j1839 (5′-CAGTCATCGAGCTGGAGAAAG) and j1840 (5′-ATTACATCAGATGGAGTAGCGC) for HP2 and j037 (5′-CATGATCCACTCCGGTGACC) and j038 (5′-CGGGAGCATGATTTTGACCTTAA) for rpS3. The thermal cycling conditions were: 95 °C for 2 min, followed by 40 cycles of 95 °C, 10 s and 60 °C, 30 s. Melting curves of all the products were examined to ensure proper shapes and Tm values after reactions were complete on a CFX Connect Real-Time PCR Detection System (Bio-Rad). The mRNA levels were normalized against the internal control of ribosomal protein S3 (set at 1.00) using corresponding Ct values for the same cDNA samples and the relative mRNA levels were calculated as 2−ΔCt, where ΔCt = CtHP2 − CtrpS3.
2.3. Immunoblot analysis of M. sexta HP2 in hemolymph at different life stages
Hemolymph samples were collected from insects at various developmental stages ranging from 4th instar larvae to day 9, pupae. After hemocytes had been removed by centrifugation at 5,000×g for 10 min, the plasma from two or more insects at the same stage were pooled, diluted 10 times in 20 mM Tris-HCl, 50 mM NaCl, pH 7.5, and treated with 1×SDS sample buffer containing dithiothreitol (DTT). The samples (each equivalent to 1.0 μl original hemolymph) were resolved by 10% SDS-PAGE and electro-transferred onto a nitrocellulose membrane for analysis using 1:1000 diluted HP2 antiserum as the primary antibody, 1:1000 diluted goat-anti-rabbit IgG conjugated to alkaline phosphatase (Bio-Rad) as the secondary antibody, and an alkaline phosphatase conjugate substrate kit (Bio-Rad) for color development.
2.4. Plasmid construction and recombinant production of M. sexta proHP2 and proPAP3
The coding region of mature proHP2 was amplified by PCR using forward primer (j1352 5′-GAATTCCTGAAGGAGACGTCTGT, EcoRI site underlined), reverse primer (j589 5′-CTCGAGAGGCCACACGATACTC, XhoI site underlined), and HP2 cDNA in pBluescript as template (Jiang et al., 1999). Similarly, M. sexta proPAP3 was amplified using primers j650 (5′-GAATTCCATGCACGACGCCCAACAAC) and j651 (5′-CAACTCGAGCGGCTTTATGTTAC) from its cDNA (Jiang et al., 2003b). The purified products were ligated with pGEM-T vector (Promega) before transformation and plasmid isolation. Following sequence validation, the EcoRI-XhoI fragments were subcloned into the same sites in pMFH6, which encodes the honeybee melletin signal peptide and a hexahistidine tag at the carboxyl-terminus. The plasmids proHP2/pMFH6 and proPAP3/pMFH6 were used to generate recombinant baculoviruses for producing the zymogens as described before (He et al., 2017). The recombinant proHP2 was isolated from the cell culture medium by cation exchange and Ni-affinity chromatography (Sumathipala and Jiang, 2010). The purified protein was dialyzed against 20 mM Tris-HCl, pH 7.5, 100 mM NaCl at 4 °C and concentrated on Amicon Ultra-30 centrifugal filter devices (Millipore). The protein aliquots were rapidly frozen in liquid nitrogen and stored at −80 °C. Similarly, proPAP3 was isolated, concentrated, and stored for activation tests using HP2 or HP21. The purified proHP2 was analyzed by SDS-PAGE under denaturing or nondenaturing condition, followed by Coomassie blue staining and immunoblotting. The N- and O-linked glycosylation was detected by treating the protein (0.5 μg) with glycoprotein denaturing buffer in 5 μl at 100 °C for 10 min. After 1 μl each of 10 × GlycoBuffer 2 and 10% Nonidet P-40, the treated proHP2 was incubated with PNGase F (0.5 μl, New England BioLabs) in 10 μl for 1 h at 37 °C. Two duplicate samples were treated similarly with 1.0 μl neuraminidase A and 0.5 μl O-glycosidase (New England BioLabs) and with the three enzymes in 10 μl for 2 h at 37 °C. The reaction mixtures (N, O, N+O), as well as untreated control (C), were separated by 7.5% SDS-PAGE under reducing conditions followed by immunoblot analysis.
2.5. Preparation of the proteins for functional assays
M. sexta β-1,3-glucan recognition protein-2 (βGRP2), proHP14 and proPO were purified from larval hemolymph (Jiang et al., 1997 and 2004; Wang and Jiang, 2006). M. sexta microbe binding protein (MBP), peptidoglycan recognition protein-1 (PGRP1), proHP21 and proPAP2 were isolated from the conditioned media of baculovirus-infected insect cells (Wang et al., 2011, Sumathipala and Jiang, 2010; Wang and Jiang, 2007; Ji et al., 2003). Serpin-12 was purified from E. coli cells harboring MsSpn12ΔN/H6pQE60 (Yang et al., 2018).
2.6. Activation of proHP14 and proHP2 using the purified proteins
To test whether or not HP14 activates proHP2 like proHP21 (Wang and Jiang, 2007), the purified proHP2 was incubated with buffer, proHP14, a mixture of E. coli PG, PGRP1 and MBP, or both (i.e. proHP14 and the mixture) for 1 h at 37 °C. The reactions were stopped by SDS-sample buffer treatment with or without DTT, separated by SDS-PAGE, and electro-transferred to a nitrocellulose membrane for immunoblot analysis (Section 2.3).
2.7. Examination of HP2’s role in proPO activation in cell-free hemolymph
Aliquots of cell-free hemolymph (CP or IP) from naïve or immune challenged larvae were incubated with buffer, proHP2, proHP14, a mixture of curdlan and βGRP2, two of the three (i.e. proHP2, proHP14 and the mixture), and all three for 10 min at room temperature. PO activity was measured by adding to each sample well 150 μl of 2.0 mM dopamine in pH 6.5, 50 mM sodium phosphate. Absorbance at 470 nm was monitored on a VersaMax plate reader (Molecular Devices). One unit of PO activity is defined as amount of the enzyme causing an increase of 0.001 absorbance unit per min. The PO activities were plotted in a bar graph as mean ± SD (n = 3). To test effect of serpin-12ΔN on the proHP2 activation by HP14 and the system activation, the mixtures of curdlan, βGRP2, proHP14 were pre-incubated with and without serpin-12ΔN for 1 h at 37 °C, set on ice for 10 min with the serpin, and then incubated with CP or IP for 10 min at room temperature prior to the PO activity assay.
2.8. Proteolytic activation of proPAP2 and proPAP3 by HP2 and/or HP21
To test if M. sexta HP2 activates proPAP2, we first generated active HP14 using E. coli PG, PGRP1, MBP and proHP14 (Section 2.6) and reacted it with proHP2, proPAP2, or both. The reactions and controls of proHP2, proPAP2, HP14, and a proHP2-proPAP2 mixture were treated with SDS-sample buffer containing DTT, separated by 10% SDS-PAGE, and subjected to immunoblot analysis using diluted PAP2 antiserum (Ji et al., 2003). To study the gel mobility shift of PAP2, proPAP2 and the mixture of HP14, proHP2 and proPAP2 were also treated with SDS sample buffer lacking DTT for SDS-PAGE and immunoblot analysis. Possible activation of proPAP3 by HP2 was analyzed under the same conditions using PAP3 antibody (Jiang et al., 2003b), along with a positive control of HP14, proHP21 and proPAP3. IEARpNa, a synthetic substrate of PAP2 and PAP3, was used to measure their amidase activities. After HP14 had been incubated with proHP2 or 21 and proPAP2 or 3 in 20 mM Tris-HCl, 20 mM NaCl, 1 mM CaCl2, pH 7.5 at 37°C for 1 h, 150 μl aliquots of 100 μM substrate were added to the reaction mixtures. Absorbance at 405 nm was monitored on a plate reader as described in Section 2.7. One unit of IEARase activity is defined as the amount of PAP causing an increase of 0.001 absorbance unit per minute.
3. Results
3.1. Profiling of M. sexta HP2 expression in hemocytes, integument and fat body at different life stages
We first examined immune inducibility of HP2 expression in two major sources of defense proteins in plasma of M. sexta larvae and confirmed the level decrease in hemocytes and increase in fat body (Jiang et al., 1999), but the differences were statistically not significant (Fig. 1A). This generally agree with the RNA-seq data (IF/CF: 0.85, IH/CH: 1.13) and proteomic data (IP/CP: 0.70, p = 0.60) (Gunaratna and Jiang, 2013; He et al., 2016). The mRNA was also abundant in integument, nerve and trachea, comparable or higher than in fat body and hemocytes from day 3, 5th instar larvae. The mRNA levels were much lower in Malpighian tubules and muscles. HP2 expression is negligible in midgut and salivary glands in the same stage.
Fig. 1.
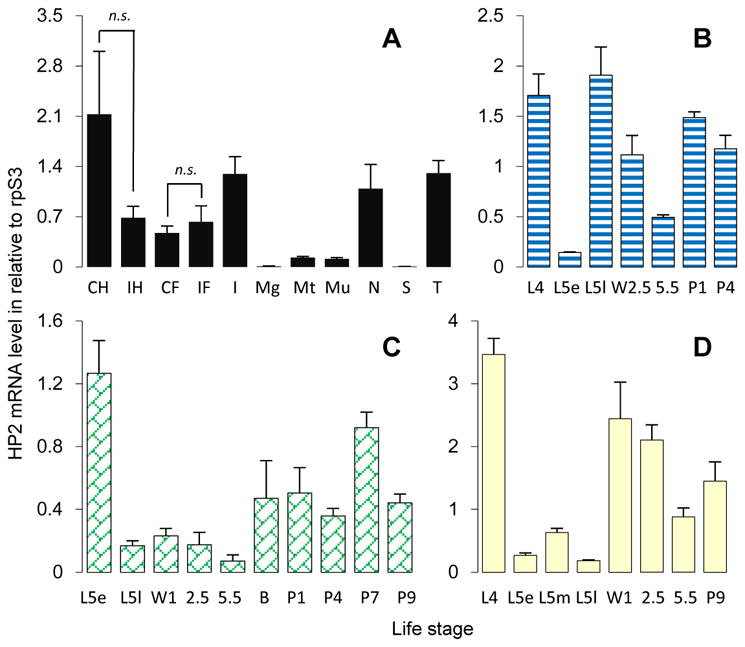
M. sexta HP2 mRNA immune inducibility and expression profiles in various tissues at different life stages. (A) Immune inducibility and tissue distribution. Relative mRNA levels of control (C) and induced (I) hemocytes (H) and fat body (F) were calculated based on Ct values from three biological replicates (3 larvae per sample) and plotted as a bar graph (mean ± SD, n = 3). Pairwise comparisons were performed between C and I using Students’ t-test (*, p < 0.05; n.s. or not significant, p > 0.05). RNA samples of other tissues (I, integument; Mg, midgut; Mt, Malpighian tubules; Mu, muscle; N, nerve tissue; S, salivary gland; T, trachea) from day 3, 5th instar naïve larvae were prepared and analyzed by qPCR under the same conditions. (B–D) Developmental profiles of HP2 transcripts in hemocytes (B), integument (C), and fat body (D). The mRNA levels in these three tissues from larvae and pupae at different stages were determined by the same method. “L4”, 4th instar larvae, day 2; “L5”, 5th instar (“e” for early or day 1; “m” for middle or day 2; “l” for late or day 3–4); “W”, wandering (day 1, 2.5 or 5.5); “B”, bar stage; “P”, pupal (day 1, 4, 7 or 9).
The HP2 expression was further studied in hemocytes, fat body and epithelial cells collected from day 2, 4th instar larvae to day 9, pupae (Fig. 1, B–D). The mRNA was abundant in fat body and hemocytes from 4th instar feeding larvae on day 2. The transcript levels in early 5th instar were less than one-tenth of those in the 4th instar. In comparison, the mRNA level in integument was fairly high when larvae just entered the 5th instar. The low expression ended first in hemocytes (late 5th instar feeding stage), second in fat body (5th instar wandering stage), and last in epithelium (5th instar bar stage). As larvae started to wander, the HP2 mRNA levels in hemocytes decreased substantially and recovered in pupal stage. In fat body, the decrease in HP2 expression occurred at a later time in the wandering stage. Expression of HP2 in integument was moderate in the pupal stage (days 1–9). The expression pattern in fat body is consistent with the RNA-seq data (Fig. S1C). Based on its mRNA levels, we suggest that HP2 is more important in hemolymph of wandering and pupal stages than in 5th instar feeding larvae.
3.2. Detection of M. sexta proHP2 and HP2 in cell-free hemolymph
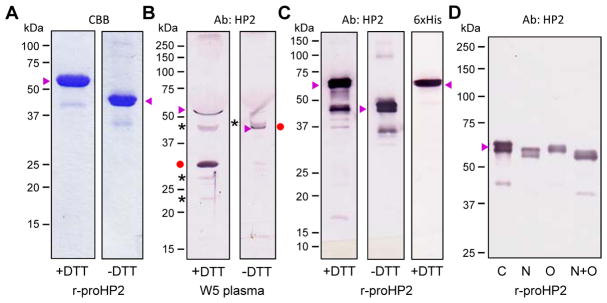
We collected hemolymph samples from M. sexta in different life stages and separated them by 10% SDS-PAGE under reducing conditions for immunoblot analysis using an HP2 antibody. As shown in Fig. 2, proHP2 in the 4th instar larval plasma mainly exists as a zymogen running as a single band at around 60 kDa. The major inconsistency with its theoretical molecular mass of 41.6 kDa is likely caused by N- or O-linked glycosylation at Asn166, T76TSS79, T83STT86, T92, S104, T114S115, T118, S140T141 or T315. Most of these are located in the linker region (V66–L160) between the amino-terminal clip and carboxyl-terminal protease domains. The mucin-type GalNAc O-glycosylation was confirmed using purified proHP2 (Section 3.3). Similar as the mRNA expression data, proHP2 protein was low in 5th instar larvae, especially in the early stage. In the late feeding stage of 5th instar larvae, we detected a 31 kDa immunoreactive band, close to calculated Mr (27.0 kDa) of the catalytic domain of HP2. The band intensity gradually increased, reached the peak on day 5 of wandering stage, remained high in early pupal stage, and reduced to a level close to that in the early wandering stage. Due to the presence of highly abundant storage and transport proteins in the 5th instar larval hemolymph, the proHP2 band was compressed down to about 50 kDa. The HP2 antibody recognized several other bands at around 70, 45, 27 and 22 kDa positions. We suspect that the faint 27 kDa band is the glycosylated light chain (calculated peptide mass: 15.8 kDa) poorly recognized by the antibody raised against proHP2 expressed in E. coli (Jiang et al., 1999). The 31 kDa intense band and the 27 kDa one disappeared under non-reducing conditions (Fig. 3B) and possibly migrated to the 43–45 kDa position recognized by the antibody. The intense band at 43 kDa may represent uncut proHP2. With all the disulfide bonds tethering the polypeptide chains, HP2 and proHP2 particularly are more compact than fully extended proHP2 (about 60 kDa) under reducing conditions. The band shift, in this case, from 27 kDa to 43–45 is a common feature of arthropod clip-domain serine proteases with an interchain disulfide bond (Kanost and Jiang, 2015).
Fig. 2.
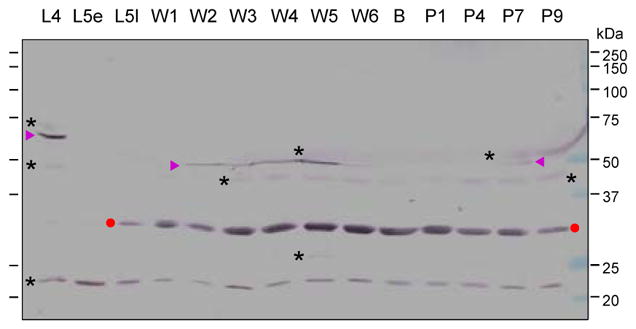
Levels of proHP2 and HP2 in plasma samples from different life stages. The plasma samples (1.0 μl each) were subjected to 10% SDS-PAGE and immunoblot analysis using 1:1000 diluted HP2 antiserum as the primary antibody. “L4”, 4th instar larvae; “L5”, 5th instar (“e” for early or day 1; “l” for late or day 3–4); “W”, wandering (days 1–6); “B”, bar stage; “P”, pupal (days 1, 4, 7 and 9). Sizes and positions of the Mr makers are indicated. ProHP2 (▶); HP2 protease domain (●); unknown protein bands (*) recognized by the antibody.
Fig. 3.
Characterization of proHP2 purified from the conditioned medium of Sf9 cells infected by the recombinant baculovirus and mobility comparison with the natural proHP2 and HP2 in the hemolymph. (A) Following cationic exchange and nickel affinity chromatography, 1.0 μg of the purified proHP2 samples were resolved by 10% SDS-PAGE under reducing and non-reducing conditions and stained with Coomassie brilliant blue (CBB). (B) For mobility comparison, 1.0 μl of plasma from day 5, wandering (W5) larvae was treated with 1×SDS sample buffer in the presence or absence of DTT, separated by 10% SDS-PAGE, and detected by 1:1000 diluted HP2 antiserum and enzyme-linked secondary antibody. The proHP2, HP2 catalytic domain, and uncharacterized protein bands are labeled “▶”, “●”, and “*”, respectively. On the right, the HP2 catalytic domain (●) was attached to the light chain and had migrated slower than proHP2 (▶). (C) The gel-separated proHP2 (50 ng, +/− DTT) was transferred onto a nitrocellulose membrane for immunoblot analysis using 1:1000 diluted HP2 (left, middle) or hexahistidine (right) antiserum as the primary antibody. (D) The purified proHP2 was treated with buffer (C for control), PNGase F (N), neuraminidase A and O-glycosidase (O), or all three enzymes (N+O) prior to 7.5% SDS-PAGE and immunoblot analysis. In panels A–D, sizes and positions of the Mr makers are indicated on the left.
3.3. Recombinant production of M. sexta proHP2 for characterization and functional analysis
To produce the proHP2 for functional assays, we constructed the plasmid proHP2/pMFH6, prepared the bacmid and baculovirus for infecting Sf9 cells, and then captured the proHP2 from 0.7 L of the conditioned medium by cation exchange chromatography. After SDS-PAGE and immunoblot analysis, we pooled the proHP2 fractions, further purified the 6×His tagged protein by nickel affinity chromatography, and obtained 1.2 mg of the zymogen in total. The recombinant proHP2 migrated as a broad band at around 60 kDa on a 10% SDS-PAGE gel (Fig. 3A), similar in size to the compressed protein in plasma (Fig. 3B). Under non-reducing conditions, its mobility became higher due to a less extended structure. As revealed by antibodies against HP2 and 6×His tag, the bands were actually doublets partially resolved by 10% SDS-PAGE (Fig. 3, C and D). The microheterogeneity reflected differences in proHP2 glycosylation because, after deglycosylation with N-glycosidase F, there was an increase in gel mobility and homogeneity of proHP2 (Fig. 3D), consistent with the prediction of N-glycosylation at Asn166. Less increase was found after O-glycosidase treatment, suggesting only a few of the 16 Thr/Ser residues are modified. Treatment with both enzymes caused a greater mobility shift to 54 kDa, still considerably larger than the theoretical Mr (41.6 kDa).
3.4. Cleavage activation of proHP2 by HP14 in vitro
Since HP2 is present as a cleaved protein in hemolymph of the wandering larvae and pupae without an immune challenge, identifying its activating enzyme and substrate(s) should help us understand its physiological role in these stages. We compared the predicted cleavage activation site of proHP2 with other known components of the M. sexta proPO activation system (Fig. 4A) and found proHP2 (ADEL*IVGG) is highly similar to proHP21 (ADDL*IIGG). HP14 activates proHP21 (Wang and Jiang, 2007) and proHP14 by cutting at GTEL*VLGG (Wang and Jiang, 2006). Led by the sequence similarity, we generated HP14 in a mixture of E. coli peptidoglycan, peptidoglycan recognition protein-1 (PGRP1), microbe-binding protein (MBP), and proHP14 (Wang and Jiang, 2017) and detected cleavage of proHP2 added later (Fig. 4B). The sharp band at 32 kDa represented the carboxyl-terminal catalytic domain of recombinant HP2 (calculated Mr: 28.1 kDa), as it was also recognized by the antibody against the 6×His tag (data not shown). The lower broad band at 28 kDa is the amino-terminal light chain (calculated Mr: 16.1 kDa), which is heavily glycosylated. Under non-reducing conditions, the two bands became one at around 55 kDa, much higher than the proHP2 doublet at about 45 kDa. The cleaved but attached HP2 has a more extended structure than proHP2 does. Differences in glycosylation of the natural and recombinant proteins could cause additional difference in gel mobility (Fig. 3B vs. Fig. 4B, no DTT). Because HP2 did not appear after proHP2 had been incubated with proHP14 alone or a mixture of PG, PGRP1 and MBP, HP14 was essential to the proHP2 cleavage activation (Fig. 4).
Fig. 4.
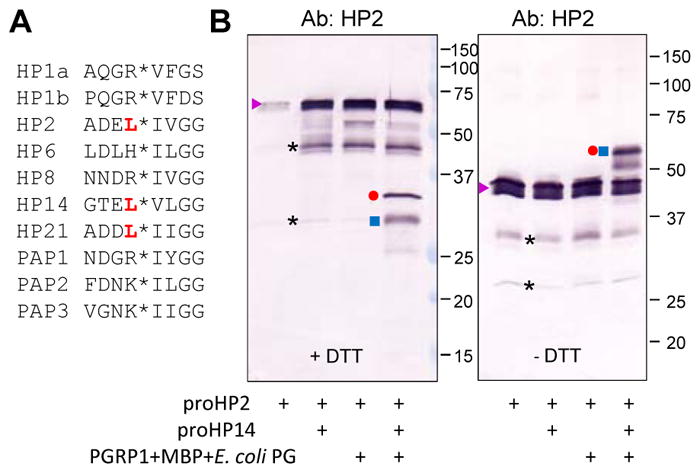
Limited proteolysis of the recombinant proHP2 by auto-activated HP14. (A) Comparison of amino acid sequences near the cleavage activation sites (*) of the known protease components in the M. sexta proPO activation system. The preceding Leu residue in HP2, HP14, and HP21 is red, bold font. (B) Purified proHP2 (200 ng/μl, 1 μl) was reacted with proHP14 (100 ng/μl, 1 μl) only, a mixture of E. coli PG (1 μg/μl, 1 μl), MBP (400 ng/μl, 1 μl), PGRP1 (400 ng/μl, 1 μl) and 15 μl buffer (20 mM Tris-HCl, 1 mM CaCl2, pH 7.5), or both at 37 °C for 2 h. The reaction mixtures were treated with SDS-sample buffer with (left panel) or without (right panel) DTT, separated by 10% SDS-PAGE, and subjected to immunoblot analysis using 1:1000 diluted HP2 antiserum as the primary antibody. The major bands are proHP2 (▶), HP2 catalytic domain (●); HP2 clip domain and linker (■), cleaved yet attached HP2 (●■) under non-reducing conditions (i.e. no DTT), and uncharacterized (*).
3.5. An indirect role of M. sexta HP2 in proPO activation in hemolymph
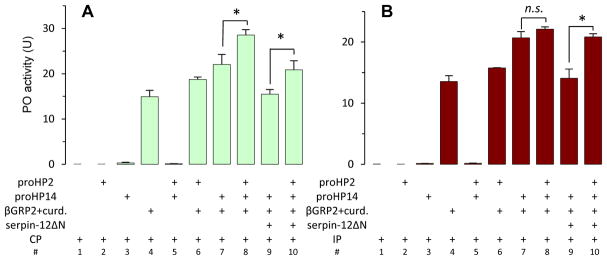
To find out if HP2 is involved in melanization, we first generated HP2 using HP14 produced in the mixture of β-1,3-glucan, β-1,3-glucan recognition protein-2 (βGRP2) and proHP14 (Wang and Jiang, 2006), reacted with purified proPO, but did not detect any cleavage (data not shown). While HP2 is not a direct proPO activator, we reacted the HP2 with cell-free hemolymph from naïve or bacteria-challenged M. sexta, which contains the entire proPO activation system including PGRPs, βGRP(s), MBP, and precursors of HP14, HP21, PAPs, SPHs and POs. Upon HP14 activation, HP21 acts as an activating protease of proPAP2 and proPAP3 (Wang and Jiang, 2007; Gorman et al., 2007). As shown in Fig. 5 (treatments #1–#3, #5 in both panels), the plasma had no basal PO activity and adding proHP2, proHP14, or both had no effect on proPO activation. PO activity increased considerably after curdlan and βGRP2 (#4) were added, indicating the system was intact in CP and IP. Further supplementation of proHP2, proHP14, or both (#6–#8) caused incremental increases of PO activity. We interpret these as results of the auto-activation of endogenous and exogenous proHP14 and the activation of recombinant proHP2 (Fig. 4) and endogenous proHP21 by HP14 (Wang and Jiang, 2007). HP21 activates proPAP2 and 3 (Gorman et al., 2007). The involvement of HP2 in this process was supported by the significant increases in PO activity (#8 vs. #7 in Fig. 5A; #10 vs. #9 in both panels). In the latter case, incubation of HP14 and HP2 with serpin-12ΔN before mixing with CP or IP (Section 2.7) reduced the proPO activation (#9 vs. #7; #10 vs. #8), likely by a direct inhibition of HP14 (Yang et al., 2018).
Fig. 5.
Role of HP2 in the M. sexta proPO activation system. Purified proHP2 (100 ng/μl, 1 μl), proHP14 (100 ng/μl, 0.5 μl), βGRP2 (200 ng/μl, 0.5 μl), curdlan (10 μg/μl, 0.5 μl) and 17 μl of buffer (20 mM Tris-HCl, 1 mM CaCl2, pH 7.5) were incubated at 37 °C for 1.5 h. Serpin-12 (1.0 μg) was then added to the selected samples and left on ice for 10 min. The reaction mixtures (10 μl each) and controls were then incubated with 5 μl of 1:10 diluted plasma from control naïve larvae (CP, panel A) or immune challenged (IP, panel B) at room temperature for 10 min. PO activity was assayed on a plate reader and shown as mean ± SD (n=3) in the bar graphs. Reaction mixtures without all the components were used as controls. *, Student’s t-test p<0.05.
3.6. Effect of HP2 on proPAP2 and proPAP3 activation in vitro
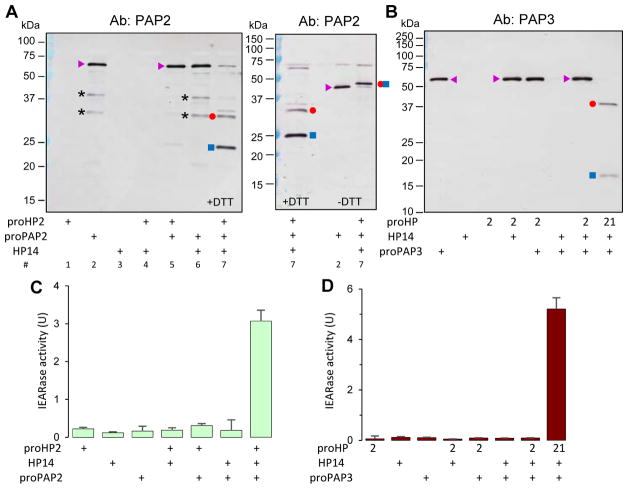
To explain the increases in PO activity (Fig. 5), we analyzed potential substrates of HP2 in hemolymph. HP2 may have a trypsin-like specificity by cutting after a positively charged residue (Jiang et al., 1999). HP1, HP6, HP8 and PAP1–3, known members of the protease system, have an Arg, Lys or His preceding the activation site (Fig. 4A). Therefore, we generated active HP2 in the mixture of E. coli PG, PGRP1, MBP, proHP14 and proHP2, reacted it with proHP1, proHP6, proHP8 and proPAP1 separately, but did not detect any cleavage (data not shown). On the other hand, cleavage of proPAP2 by HP2 yielded 25 and 34 kDa bands (Fig. 6A, left), corresponding to the amino-terminal light chain and carboxyl-terminal heavy chain (Jiang et al., 2003a). The two bands disappeared at these locations and shifted to the 48 kDa position under non-reducing conditions (Fig. 6A, right). In the absence of active HP14 or HP2 (Fig. 6A, treatments #5 and #6), proPAP2 was uncut. We also produced recombinant proPAP3, tested its activation by HP2, but did not find cleavage (Fig. 6B). In the positive control, active HP21 generated by HP14 converted proPAP3 to 37 and 18 kDa bands. Consistent with the proteolytic activation, substantial amounts of IEARpNa were hydrolyzed in the HP14-proHP2-proPAP2 and HP14-proHP21-proPAP3 reactions (Fig. 6, C and D). The amidase activity was a lot lower in the control reactions; unlike PAP2 or PAP3, HP2 and HP21 are poor IEARases. In summary, HP2 activates proPAP2 but not 3, HP21 activates both, and the broader specificity of HP21 suggests a greater role than HP2 during proPO activation. PAP3 activates proPAP3, proSPHs and proPOs (Wang et al., 2014), whereas PAP2’s role in the system seems more limited.
Fig. 6.
Activation of proPAP2 but not proPAP3 by M. sexta HP2. The purified recombinant proPAP2 (A, C) or proPAP3 (B & D) (1 μl, 50 ng/μl) was mixed with HP2 reaction mixtures (see legend to Fig. 4) and incubated at 37 °C for 1.5 h. Half of the reactions were used for SDS-PAGE and immunoblot analysis using PAP2 (A) or PAP3 (B) antiserum under reducing (A, left panel and lane 1 of right panel and B) or non-reducing conditions (A, right panel, lanes 2 and 3). The other half of the reaction mixtures were used for amidase activity assay (C and D). In the enzyme assay, 150 μl of 100 μM IEARpNa in 20 mM Tris-HCl, 1 mM CaCl2, pH 7.5 was incubated with the mixtures at room temperature and changes of absorbance at 405 nm were monitored in the kinetic mode. One unit of IEARase activity was defined as change in A405 of 0.001 per min. For the proPAP3 assay, proHP21 was also used as substitute for proHP2 in the reactions as a positive control (B, last lane; D, last bar). The major bands are proPAP2/3 (▶), PAP2/3 catalytic domain (●); PAP2/3 dual clip domain and linker (■), cleaved yet attached PAP2 (●■) under non-reducing conditions (i.e. no DTT), and uncharacterized (*).
4. Discussion
In the last three decades, SPs, their non-catalytic homologs, and their inhibitors in the serpin superfamily have been investigated to understand their roles as mediators and regulators in insect embryonic development and innate immunity (Kanost and Jiang, 2015; Veillard et al., 2016; Meekins et al., 2017). Much progress has been made through genetic and biochemical studies. As more insect genomes, transcriptomes, and proteomes become available, we are now at a position to study the entire gene families, even though functional studies of individual members are still challenging due to system complexity. There are at least 193, 257 and 337 SP and SPH genes in the genomes of M. sexta, Drosophila melanogaster and Anopheles gambaie, respectively (Cao et al., 2015, 2017 and unpublished data). Two third of them in each species likely participate in processes unrelated to food digestion. In this study, we elucidated the function of M. sexta HP2 in the proPO activation system by taking a biochemical approach. HP2 is one of the first four HPs cloned (Jiang et al., 1999), and one of the 36 SP-related proteins identified in the larval hemolymph of M. sexta (He et al., 2016).
4.1. Physiological role of M. sexta HP2 in protection against microbial infection of the host
The HP2 mRNA levels in different tissues and stages indicate complex regulation at the transcription level (Fig. 1). Hemocytes and fat body synthesize and secrete proHP2 into plasma and their low expression in early 5th instar larvae is consistent with the low HP2 level in the feeding stage (Fig. 2). In contrast, the high mRNA level in epithelial cells and trachea at the same time may be responsible for defense against microbes on external surface of insects (Brey et al., 1993).
HP2 was cloned from feeding larvae but its function seems to associate with innate immunity of the wandering larvae and pupae (Fig. 2). The infection-independent expression in the later stages and proteolytic activation of proHP2 are probably required by insects to endure harsher conditions. Compared with heathy leafs and young stems the feeding larvae consume, wandering larvae and pupae face soil microbes at much higher density and diversity. Melanin deposition and cuticle scelotization during pupation provide insects a tough physiochemical barrier against pathogenic invaders in the vulnerable stages.
Functional elucidation of a gene product can take years to accomplish. Using partially purified proHP2 to test its possible involvement in proPO activation in larval plasma was a disaster lasting for many years. In fact, even after knowing how to produce active HP2 using purified proHP14, proHP2, along with E. coli peptidoglycan, PGRP1 and MBP (Fig. 4) or with β-1,3-glucan and βGRP2 (Fig. 5), we still experienced problems to show its role in melanization in the background of proHP21, proPAP2 and proPAP3 in control and induced plasma. Having an overlap with HP2’s function, HP21 yielded a considerable amount of PO (Fig. 5 A and B, #4), making it difficult to discern the enhancement caused by HP2. The background PO activity was a result of PAP2 and PAP3 activated by HP21 (Wang and Jiang, 2007; Gorman et al., 2007). Since HP2 activates proPAP2 only (Fig. 6), its contribution to the PO activity increase was less pronounced. After the baseline proPO activation was reduced by serpin-12, it became straightforward to reveal the HP14-HP2-PAP2-PO pathway.
Readily available plasma from the lepidopteran larvae provides a good opportunity for us to fine-tune the experimental conditions in functional tests, which is nearly impossible in Drosophila or mosquito. With hundreds of SP-related proteins, functional overlapping or pathway redundancy is expected to be a norm rather than exception, which quickly diminishes the power of tools for genetic research. For instance, in a genetic screen to identify clip-domain SPs functioning in Drosophila immunity, only one of the twenty-four mutants had a significant phenotype (Nam et al., 2012). As such, knowledge and antibodies acquired over the years from biochemical studies of M. sexta HPs are valuable for future research on other SPs or SPHs identified in the larval plasma.
4.2. Evidence for the new pathway from the M. sexta transcriptome data
Because the pathway of HP14-HP2-PAP2-PO is elucidated by in vitro assays using purified proteins, we further examined the expression patterns of its members (i.e. HP14, HP2, HP21, PAP2, PAP3 genes) in the 52 libraries (Fig. S1) to test if they generally support the model. M. sexta proPO genes, expressed in oenocytoids (Jiang et al., 1997), were excluded since no hemocyte sample was sequenced in the transcriptome analysis associated with the genome research. On the other hand, we included HP14b in the expression study because of its high similarity to HP14(a) (Cao et al., 2015) but soon realized HP14b expression was negligible in all the libraries except for early embryo (i.e. 0–3 h eggs) (Fig. S1B). In comparison, HP14a had a broad profile of expression in various tissues and stages (Fig. S1A). In fat body, its mRNA was detected from late 4th instar larvae to late adults, covering the entire periods of HP2 and HP21 expression.
While the mRNA levels of HP21 were about 5-fold higher than those of HP14, their profiles closely resemble (Fig. S1, A vs. D), as shown in the cluster analysis (Cao et al., 2015). The mRNA levels of HP2 in the 5th instar feeding stage were much lower than in wandering larvae, pupae, and adults, consistent with the detection of active HP2 in wandering larvae and pupae (Fig. 2). Even in the later stages, the role of HP2 seems secondary as compared to HP21, whose mRNA levels were several fold higher than HP2’s (Fig. S1, C vs. D). Another difference is that the HP2 expression in muscle of the feeding larvae was much higher than HP21, but we do not yet know the function of proHP2 made by muscle. We further noticed that the profiles of HP2 and PAP2 are vastly similar (Fig. S1, C vs. E) (Cao et al., 2015), which supports the HP2-PAP2 link revealed in the biochemical tests (Fig. 6). In comparison with PAP2, PAP3 expression changes abruptly (Fig. S1F).
4.3. Possible reasons for the difference between HP2 and HP21 in proPAP2 and 3 activation
Why does HP2 only activate proPAP2 but HP21 also activate proPAP3? HP2, 13, 18a, 18b, 21, 22, SP33, and 144 form a clade of single clip-domain SPs whereas M. sexta PAP2, PAP3, and six other dual clip-domain SPs (HP12, 15, 23, 24, 26, GP33) form another in the phylogenetic tree (Cao et al., 2015). In other words, the overall structural similarity of HP2 and HP21, as well as the protein substrates proPAP2 and 3, is high. In particular, the proteolytic activation site of PAP2 (FDNK*ILGG) is nearly identical to that of PAP3 (VGNK*IIGG). A variation in the S3 specificity pocket of HP2 (for D at PAP2’s P3 site) and HP21 (for D/G at P3) may account for the differential activation of proPAP2 and 3. Alternatively, exosite interactions of clip domains could also lead to differential associations of HP2-proPAP2 and HP2-proPAP3 (Jiang and Kanost, 2000). To explore the latter hypothesis, we compared the charge status of HP2, HP21, PAP2, PAP3 (Table 1), and nine other clip-domain SPs in the same clades (data not shown). Overall, the four proteases are slightly acidic (pI: 6.04–6.60). The single clip-domain HP2 has a basic catalytic domain (pI: 8.36), whereas HP21’s is slightly acidic (6.47). For the dual clip-domain SPs, clip-1 of HP26 is acidic (6.05) whereas those of the other seven are basic (8.23–8.67). Contrarily, clip-2s of PAP3 and HP23 are basic (8.78 and 7.51) but those of the other six are acidic (4.25–4.49). We do not know if any of these differences is responsible for HP2’s activation of proPAP2 only. Swapping clip-2, linker, and catalytic domain of PAP3 with their counterparts in PAP2 may allow us to locate a region responsible for the differential protease-substrate interaction. If clip-2 of PAP3 is involved in the process, a more focused study may reveal molecular interaction at the subdomain level. The structures of PAP2’s clip-1 and clip-2 (Huang et al., 2007) will provide guidance for site-directed mutagenesis and biochemical experiments. In the past, mutation in the clip domain of Drosophila Snake yielded indirect but useful evidence for its functional importance (Tian and LeMosy, 2008).
Table 1.
Calculated isoelectric points (i.e. pI values) of the clip and protease domains in M. sexta HP2, HP21, PAP2 and PAP3 *
| protein | clip-1 | clip-2 | linker | catalytic | entire |
|---|---|---|---|---|---|
| HP2 | 8.63 (−) | n.a. | 4.83 (+) | 8.36 (−) | 6.60 (0) |
| HP21 | 8.65 (−) | n.a. | 5.11 (+) | 6.47 (+) | 6.04 (+) |
| PAP2 | 8.62 (−) | 4.49 (+) | 4.09 (+) | 8.48 (−) | 6.05 (+) |
| PAP3 | 8.23 (−) | 8.78 (−) | 8.61 (−) | 5.60 (+) | 6.49 (+) |
Net charges at pH 7.0 are indicated in parenthesis as “+”, “−”, and “0” when pI values are <6.5, >7.5, and 6.5–7.5, respectively.
4.4. Concluding remarks
After nearly three decades of research, a framework of the SP-SPH system has been established in the hemolymph of M. sexta larvae using biochemical methods, which includes the newly discovered HP14–HP2–PAP2–PO pathway. Transcriptional regulation of the pathway members, particularly HP2, is complex and maybe closely related to the defense role of melanization in wandering larvae and pupae. The HP2 specific cleavage of proPAP2 but not proPAP3 is remarkable, and exploration of its mechanism may lead to function elucidation of clip domains. These regulatory units are widely distributed in arthropod SPs and SPHs, but little is certain about their roles in molecular recognition. Overlap of the HP2 and HP21 functions has posed a fierce challenge in this study. This cautions us the possibility that a single gene knockout may not yield any phenotype, although its protein does have a function. Given the system complexity, redundancy of a pathway or its member(s) can be severe. On the other hand, the omic data, baculovirus system, biochemical assays, and hemolymph availability have provided unique opportunities to reveal the entire system.
Supplementary Material
Highlights.
ProHP2 mRNA is relatively abundant in hemocytes, fat body, integument, nerve, and trachea
ProHP2 protein is low in hemolymph of 5th instar larvae but becomes active and abundant during wandering stage
ProHP2 is activated by HP14 in vitro
Active HP2 cleaves proPAP2 but not proPAP3. PAP2 then produces active PO.
Acknowledgments
This work was supported by National Institutes of Health Grants GM58634 and AI112662 (to HJ). This article was approved for publication by the Director of the Oklahoma Agricultural Experiment Station and supported in part under project OKLO2450.
Abbreviations
- CP, IP, CH, IH, CF and IF
control and induced plasma (i.e. cell-free hemolymph), hemocytes, and fat body from naïve or immune challenged larvae
- DTT
dithiothreitol
- βGRP
β-1,3-glucan recognition protein
- HP
hemolymph (serine) protease
- MBP
microbe binding protein
- PO and proPO
phenoloxidase and its precursor
- PAP
proPO activating protease
- PG and PGRP
peptidoglycan and its recognition protein
- PSP
plasmatocyte spreading peptide
- SP and SPH
serine protease and non-catalytic serine protease homolog
References
- An C, Ishibashi J, Ragan EJ, Jiang H, Kanost MR. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J Biol Chem. 2009;284:19716–19726. doi: 10.1074/jbc.M109.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spätzle in innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey PT, Lee WJ, Yamakawa M, Koizumi Y, Perrot S, François M, Ashida M. Role of the integument in insect immunity: epicuticular abrasion and induction of cecropin synthesis in cuticular epithelial cells. Proc Natl Acad Sci USA. 1993;90:6275–6279. doi: 10.1073/pnas.90.13.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster – from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Gulati M, Jiang H. Serine protease-related proteins in the malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol. 2017;88:48–62. doi: 10.1016/j.ibmb.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, He Y, Hu Y, Zhang X, Wang Y, Zou Z, Chen Y, Blissard GW, Kanost MR, Jiang H. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect Biochem Mol Biol. 2015;62:51–63. doi: 10.1016/j.ibmb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jiang H. An analysis of 67 RNA-seq datasets from various tissues at different stages of a model insect, Manduca sexta. BMC Genomics. 2017;18:796. doi: 10.1186/s12864-017-4147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PE, Drake DR. Fate of bacteria injected into naïve and immunized larvae of the tobacco hornworm Manduca sexta. J Invertebr Pathol. 1983;41:77–85. [Google Scholar]
- Gorman MJ, Wang Y, Jiang H, Kanost MR. Manduca sexta hemolymph proteinase 21 activates prophenoloxidase-activating proteinase 3 in an insect innate immune response proteinase cascade. J Biol Chem. 2007;282:11742–11749. doi: 10.1074/jbc.M611243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratna R, Jiang H. A comprehensive analysis of Manduca sexta immunotranscriptome. Dev Com Immunol. 2013;39:388–398. doi: 10.1016/j.dci.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase proPO) activation requires proPO-activating proteinase and serine proteinase homologs simultaneously. Insect Biochem Mol Biol. 2005;35:241–248. doi: 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Cao X, Zhang S, Rogers J, Hartson S, Jiang H. Changes in the plasma proteome of Manduca sexta larvae in relation to the transcriptome variations after an immune challenge: evidence for high molecular weight immune complex formation. Mol Cell Proteomics. 2016;15:1176–1187. doi: 10.1074/mcp.M115.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang Y, Yang F, Jiang H. Manduca sexta hemolymph protease-1, activated by an unconventional non-proteolytic mechanism, mediates immune responses. Insect Biochem Mol Biol. 2017;84:23–31. doi: 10.1016/j.ibmb.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer JF. Mosquito immunity. Adv Exp Med Biol. 2010;708:218–38. doi: 10.1007/978-1-4419-8059-5_12. [DOI] [PubMed] [Google Scholar]
- Huang R, Lu Z, Dai H, Velde DV, Prakash O, Jiang H. The solution structure of the clip domains from Manduca sexta prophenoloxidase activating proteinase-2. Biochemistry. 2007;46:11431–11439. doi: 10.1021/bi7010724. [DOI] [PubMed] [Google Scholar]
- Ji C, Wang Y, Ross J, Jiang H. Expression and in vitro activation of Manduca sexta prophenoloxidase-activating proteinase-2 precursor from baculovirus-infected insect cells. Protein Express Purif. 2003;29:235–243. doi: 10.1016/s1046-5928(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30:95–105. doi: 10.1016/s0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Ma C, Lu Z, Kanost MR. β-1,3-glucan recognition protein-2 from Manduca sexta: an acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem Mol Biol. 2004;34:89–100. doi: 10.1016/j.ibmb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Kanost MR. Four serine proteinases expressed in Manduca sexta hemocytes. Insect Mol Biol. 1999;8:39–53. doi: 10.1046/j.1365-2583.1999.810039.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Ma C, Kanost MR. Subunit composition of prophenoloxidase from Manduca sexta: molecular cloning of subunit proPO-p1. Insect Biochem Mol Biol. 1997;27:835–850. doi: 10.1016/s0965-1748(97)00066-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Kanost MR. Prophenoloxidase-activating proteinase-2 (PAP-2) from hemolymph of Manduca sexta: a bacteria-inducible serine proteinase containing two clip domains. J Biol Chem. 2003a;278:3552–3561. doi: 10.1074/jbc.M205743200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Zhu Y, Kanost MR. Prophenoloxidase-activating proteinase-3 from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol. 2003b;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Arrese EL, Cao X, Chen YR, Chellapilla S, Goldsmith MR, Grosse-Wilde E, Heckel DG, Herndon N, Jiang H, Papanicolaou A, Qu J, Soulages JL, Vogel H, Walters J, Waterhouse RM, Ahn SJ, Almeida FC, An C, Aqrawi P, Bretschneider A, Bryant WB, Bucks S, Chao H, Chevignon G, Christen JM, Clarke DF, Dittmer NT, Ferguson LC, Garavelou S, Gordon KH, Gunaratna RT, Han Y, Hauser F, He Y, Heidel-Fischer H, Hirsh A, Hu Y, Jiang H, Kalra D, Klinner C, Konig C, Kovar C, Kroll AR, Kuwar SS, Lee SL, Lehman R, Li K, Li Z, Liang H, Lovelace S, Lu Z, Mansfield JH, McCulloch KJ, Mathew T, Morton B, Muzny DM, Neunemann D, Ongeri F, Pauchet Y, Pu LL, Pyrousis I, Rao XJ, Redding A, Roesel C, Sanchez-Gracia A, Schaack S, Shukla A, Tetreau G, Wang Y, Xiong GH, Traut W, Walsh TK, Worley KC, Wu D, Wu W, Wu YQ, Zhang X, Zou Z, Zucker H, Briscoe AD, Burmester T, Clem RJ, Feyereisen R, Grimmelikhuijzen CJ, Hamodrakas SJ, Hansson BS, Huguet E, Jermiin LS, Lan Q, Lehman HK, Lorenzen M, Merzendorfer H, Michalopoulos I, Morton DB, Muthukrishnan S, Oakeshott JG, Palmer W, Park Y, Passarelli AL, Rozas J, Schwartz LM, Smith W, Southgate A, Vilcinskas A, Vogt R, Wang P, Werren J, Yu XQ, Zhou JJ, Brown SJ, Scherer SE, Richards S, Blissard GW. Multifaceted biological insights from a draft genome sequence of the tobacco hornworm moth, Manduca sexta. Insect Biochem Mol Biol. 2016;76:118–147. doi: 10.1016/j.ibmb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr Opin Insect Sci. 2015;11:47–55. doi: 10.1016/j.cois.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins DA, Kanost MR, Michel K. Serpins in arthropod biology. Semin Cell Dev Biol. 2017;62:105–109. doi: 10.1016/j.semcdb.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Jang IH, You H, Lee KA, Lee WJ. Genetic evidence of a redox-dependent systemic wound response via Hayan protease-phenoloxidase system in Drosophila. EMBO J. 2012;31:1253–1265. doi: 10.1038/emboj.2011.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35:443–459. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Park JW, Kim CH, Rui J, Park KH, Ryu KH, Chai JH, Hwang HO, Kurokawa K, Ha NC, Söderhäll I, Söderhäll K, Lee BL. Beetle immunity. Adv Exp Med Biol. 2010;708:163–180. doi: 10.1007/978-1-4419-8059-5_9. [DOI] [PubMed] [Google Scholar]
- Schrag LG, Herrera AI, Cao X, Prakash O, Jiang H. Structure and function of stress responsive peptides in insects. In: Srivastava VP, editor. Peptide-based Drug Discovery: Challenges and New Therapeutics. Royal Society of Chemistry; London, UK: 2017. pp. 438–451. [Google Scholar]
- Strand MR. The insect cellular immune response. Insect Sci. 2008;15:1–14. [Google Scholar]
- Sumathipala N, Jiang H. Involvement of Manduca sexta peptidoglycan recognition protein-1 in the recognition of bacteria and activation of prophenoloxidase system. Insect Biochem Mol Biol. 2010;40:485–495. doi: 10.1016/j.ibmb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Garcia BL, Kanost MR. Initiating protease with modular domains interacts with β-1,3-glucan recognition protein to trigger innate immune response in insects. Proc Natl Acad Sci USA. 2015;112:13856–13861. doi: 10.1073/pnas.1517236112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, LeMosy EK. Mutagenesis of the cysteine-rich clip domain in the Drosophila patterning protease, Snake. Arch Biochem Biophys. 2008;475:169–174. doi: 10.1016/j.abb.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillard F, Troxler L, Reichhart JM. Drosophila melanogaster clip-domain serine proteases: structure, function and regulation. Biochimie. 2016;122:255–269. doi: 10.1016/j.biochi.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Interaction of β-1,3-glucan with its recognition protein activates hemolymph proteinase 14, an initiation enzyme of the prophenoloxidase activation system in Manduca sexta. J Biol Chem. 2006;281:9271–9278. doi: 10.1074/jbc.M513797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: Snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase- activating proteinase-2 precursor. Insect Biochem Mol Biol. 2007;37:1015–1025. doi: 10.1016/j.ibmb.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Prophenoloxidase activation and antimicrobial peptide expression induced by the recombinant microbe binding protein of Manduca sexta. Insect Biochem Mol Biol. 2017;83:35–43. doi: 10.1016/j.ibmb.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu Z, Jiang H. Manduca sexta proprophenoloxidase activating proteinase-3 (PAP3) stimulates melanization by activating proPAP3, proSPHs, and proPOs. Insect Biochem Mol Biol. 2014;50:82–91. doi: 10.1016/j.ibmb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sumathipala N, Rayaprolu S, Jiang H. Recognition of microbial molecular patterns and stimulation of prophenoloxidase activation by a β-1,3-glucanase-related protein in Manduca sexta larval plasma. Insect Biochem Mol Biol. 2011;41:322–331. doi: 10.1016/j.ibmb.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang Y, He Y, Jiang H. In search of a function of Manduca sexta hemolymph protease-1 in the innate immune system. Insect Biochem Mol Biol. 2016;76:1–10. doi: 10.1016/j.ibmb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang Y, Sumathipala N, Cao X, Kanost MR, Jiang H. Manduca sexta serpin-12 controls the prophenoloxidase activation system in larval hemolymph. Insect Biochem Mol Biol. 2018;99:27–36. doi: 10.1016/j.ibmb.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XQ, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;33:197–208. doi: 10.1016/s0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Zhang S, Gunaratna R, Zhang X, Najar F, Wang Y, Roe B, Jiang H. Pyrosequencing-based expression profiling and identification of differentially regulated genes from Manduca sexta, a lepidopteran model insect. Insect Biochem Mol Biol. 2011;41:733–746. doi: 10.1016/j.ibmb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Li J, Wang Y, Jiang H. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem Mol Biol. 2007;37:952–959. doi: 10.1016/j.ibmb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Lu Z, Strand MR, Jiang H. Antiviral, anti-parasitic, and cytotoxic effects of 5,6-dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response. Insect Biochem Mol Biol. 2011;41:645–652. doi: 10.1016/j.ibmb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.