Abstract
Many classes of medications have been evaluated in chronic low back pain (cLBP), however their utilization in the community remains unclear. We examine patterns of prescription medication use among Americans with cLBP in a nationally representative, community-based sample. The Back Pain Survey was administered to a representative sample of US adults aged 20–69 (N = 5103) during the 2009–2010 cycle of the National Health and Nutrition Examination Survey (NHANES). cLBP was defined as self-reported pain in the area between the lower posterior margin of the ribcage and the horizontal gluteal fold on most days for at least 3 months (N = 700). Home-based interviews with pill bottle verification were used to capture commonly prescribed medications for chronic pain. Among the sample of US adults with cLBP aged 20–69, 36.9% took at least one prescription pain medication in the past 30 days. 18.8% used opioids, 9.7% NSAIDs, 8.5% muscle relaxants, 6.9% gabapentin or pregabalin. Non-pain antidepressants and hypnotics were used by 17.8% and 4.7%, respectively. Opioids were used long-term in 76.9% of cases (median 2 years) and were frequently co-administered with antidepressants, benzodiazepines, or hypnotics. 94% of prescription opioids in the cLBP population were used by subjects with less than a college education. Opioids were the most widely used prescription analgesic class in community-based US adults with cLBP and were often co-administered with other CNS-active medications. Opioid use was highly prevalent among less educated Americans with cLBP.
Keywords: low back pain, analgesics, opioids, epidemiology
Introduction
Various medications, including opioids, are widely prescribed for chronic low back pain in the US despite limited supporting evidence, high costs, and often serious health risks [28, 49]. Treatment guidelines for cLBP have been difficult to establish due to heterogeneity of clinical trials and lack of high-quality evidence on long-term medication use [8, 4]. The 2017 American College of Physicians guidelines on pharmacologic management of cLBP highlight small effect sizes from most studied medications and only short-term utility [35]. The 2016 CDC guideline for prescribing opioids for chronic pain stresses the harms of opioids, and recommends pursuing alternative options for managing chronic pain [12].
Previous studies have suggested excessive opioid analgesic prescribing for cLBP in the US. A large claims-based study reported 19% long-term opioid use among patients with low back pain in the Pacific Northwest [9]. A prior National population-based survey from the 1990s reported 12% opioid use among US adults with low back pain, with the highest prevalence of opioid use in the South region [27]. Information on the use of non-opioid pain medications among US adults with cLBP is scarce, and largely comes from convenience-sample surveys. The Gallup-Palmer Annual Report, for example, indicates wide-spread use of NSAIDs and acetaminophen, as well as benzodiazepines [45]. Little is known about National patterns of non-opioid pain medication use among adults with cLBP in the US. Our study aimed to evaluate the use of different classes of prescription medications commonly used in the management of cLBP among Americans with cLBP. Additionally, we described the demographic characteristics of prescription opioid use in the cLBP population, as an aid in directing efforts for opioid use reduction [7].
Materials and Methods
Our primary objective was to identify the patterns of use for medications commonly prescribed for chronic pain among community-based US adults with cLBP, compared to those without cLBP. We analyzed data from The National Health and Nutrition Examination Survey (NHANES), 2009–2010 cycle, in which a comprehensive back pain questionnaire was administered to all adult participants ages 20 to 69 (N=5103) [52]. We identified the cLBP sample from participants who reported current pain in the area between the lower posterior margin of the ribcage and the horizontal gluteal fold at the time of survey with a history of pain lasting almost every day for at least 3 months (N=700) [41]. Prescription medication use information was collected by a trained NHANES interviewer in the participants’ home. The interviewer asked what prescription medications the patient used within the past 30 days, and recorded the medication name into the LexiconPlus® electronic database (a proprietary database of Cerner Multum, Inc.). Medications were matched with generic medication codes and classified according to therapeutic category. The interviewer also asked to see a prescription bottle when available, and recorded duration of use. We used Lexicon Plus® to identify common prescription medications used for cLBP, including analgesics: acetaminophen, NSAIDs, COX2 inhibitors, salicylates (other than aspirin, which was classified as an NSAID), opioid analgesics (including all schedule II and III opioids, combination opioid analgesics, tramadol and all preparations of codeine other than cold and cough medications), gabapentinoids (gabapentin, pregabalin), topical lidocaine, skeletal muscle relaxants, CNS-active medications used for pain: duloxetine, tricyclic antidepressants, benzodiazepines. Additionally, we analyzed the use of CNS-active medications that are not typically used for pain, including selective serotonin reuptake inhibitors (SSRIs), and non-benzodiazepine hypnotics (barbiturates, buspirone, doxepin, zolpidem, 1st generation antihistamines).
We compared pain medication and non-pain CNS-active medication use by category between survey participants with cLBP and without. In the cLBP group (N = 700), we evaluated the median reported duration of use for each therapeutic category, the frequency of drug combinations, and the distribution of common prescription medication use in the population by demographic characteristics. Chi-square tests were used for unadjusted categorical comparisons. Logistic regression was used to produce adjusted odds ratios for binary outcomes, with adjustment for age, race, gender, comorbidities, and education level. In accordance with NHANES methodology, each survey participant represents a specific proportion of the population. Primary sample unit (“sdmvpsu”) and stratum (“sdmvstra”) variables, as well as 2-year interview weights (“wtint2yr”) were used to obtain national estimates for questionnaire variables, and 2-year mobile examination center (MEC) weights (“wtmec2yr”) – for MEC variables. All results were expressed in percentages of the US population. Detailed back pain survey methodology and cLBP sample characteristics have been published elsewhere [52, 41]. A 95% confidence level was set for all tests of significance. All statistical analyses were performed in SAS 9.4 (SAS Institute, Inc., Cary, NC). All research data was obtained from NHANES directly in deidentified form and did not require additional IRB approval. Funding sources: NIH Award Number 5T32DK007784-15. NIH Award Number UL1TR000114.
Role of the funding source: National Institutes of Health Award Number 5T32DK007784-15 supported Dr. Shmagel’s training and salary to complete this research. National Institutes of Health Award Number UL1TR000114 provided funding for statistical support of this study.
Results
Demographic characteristics by chronic low back pain status are presented in Table 1. US adults with cLBP compared to those without cLBP were older, more likely to be white, female, and to have a lower socio-economic status. Medical comorbidities and depression were more prevalent among subjects with cLBP. 36.9% of US adults with cLBP aged 20–69 used at least one prescribed pain medication in the past month, 17.8% used non-pain antidepressants, and 4.7% used hypnotics.
Table 1.
Demographic and behavioral characteristics of US adults, ages 20–69, with and without chronic low back pain (N = 5103).
| % with cLBP (SE%) | % w/o cLBP (SE%) | Chi-Sq P-value | Adjusted OR (95% CI) | AOR P-value | |
|---|---|---|---|---|---|
| N=700 | N=4403 | ||||
| Age | |||||
| 20–29 | 15.1 (1.4) | 22.7 (0.9) | <0.0001 | 1 (Ref.) | <0.001 |
| 30–39 | 18.3 (2.2) | 20.7 (0.8) | 1.39 (0.97–1.98) | ||
| 40–49 | 19.3 (2.3) | 23.1 (0.8) | 1.27 (0.86–1.86) | ||
| 50–59 | 27.4 (2.5) | 19.9 (0.9) | 2.03 (1.48–2.78) | ||
| 60–69 | 20.0 (1.5) | 13.6 (0.5) | 2.07 (1.59–2.71) | ||
| Female gender | 55.8 (2.6) | 50.1 (0.7) | 0.036 | 1.28 (1.03–1.58) | 0.027 |
| Race | |||||
| African American | 10.1 (1.4) | 12.1 (0.9) | <0.0001 | 1 (Ref.) | 0.001 |
| Caucasian | 74.9 (3.7) | 64.9 (3.5) | 1.52 (1.14–2.04) | ||
| Hispanic | 11.9 (3.1) | 14.9 (3.0) | 0.98 (0.73–1.32) | ||
| Other | 3.1 (0.8) | 8.1 (1.3) | 0.58 (0.34–0.97) | ||
| Education | |||||
| College and higher | 18.6 (2.6) | 30.2 (1.5) | 0.0001 | 1 (Ref.) | 0.002 |
| GED/AA degree | 60.9 (2.1) | 52.5 (1.4) | 1.99 (1.33–2.98) | ||
| Less than High school | 20.6 (1.2) | 17.3 (0.9) | 2.27 (1.53–3.38) | ||
| Annual HH income $ | |||||
| >100 000 | 17.4 (1.8) | 24.7 (1.3) | 0.0001 | 1 (Ref.) | 0.006 |
| 65–99 000 | 16.8 (2.0) | 18.8 (1.3) | 1.18 (0.75–1.86) | ||
| 45–64 000 | 16.2 (2.0) | 15.8 (1.3) | 1.36 (0.93–1.99) | ||
| 20–44 000 | 29.0 (1.6) | 28.0 (1.1) | 1.42 (1.05–1.93) | ||
| <20 000 | 20.6 (2.9) | 12.6 (1.0) | 2.29 (1.46–3.58) | ||
| PHQ9 score (depression) | |||||
| 1–4 (none) | 56.8 (2.9) | 78.3 (1.2) | <0.0001 | 1 (Ref.) | <0.001 |
| 5–9 (mild) | 21.3 (2.1) | 15.7 (1.1) | 1.86 (1.38–2.52) | ||
| 10–14 (moderate) | 10.0 (1.3) | 4.2 (0.3) | 3.30 (2.46–4.44) | ||
| 15–19 (moderate-severe) | 8.7 (1.7) | 1.5 (0.2) | 8.29 (5.19–13.24) | ||
| 20–27 (severe) | 3.1 (0.8) | 0.4 (0.1) | 10.62 (5.42–20.80) | ||
| Medical comorbidities | |||||
| 0–1 | 57.9 (2.3) | 83.0 (0.7) | <0.0001 | 1 (Ref.) | <0.001 |
| 2–3 | 27.4 (1.4) | 13.9 (0.6) | 2.49 (2.10–2.95) | ||
| >3 | 14.8 (2.2) | 3.1 (0.3) | 6.09 (4.12–9.00) | ||
SE – standard error. cLBP – chronic low back pain. Chi-Sq – Chi-square. AOR – adjusted odds ratio, adjusted for age, race, gender, education. CI – confidence interval. GED – general educational development. AA – associate’s. HH – household. BMI – body mass index. PHQ9 – patient health questionnaire 9.
Reference category not displayed for binary variables. All estimates are weighted to represent the national population
The prevalence of prescription medication use among US adults with cLBP and those without cLBP is summarized in Table 2. Current use of virtually all examined prescription pain medications, antidepressants, and hypnotics was more prevalent in the cLBP group than the non-cLBP group (adjusted odds ratios (aOR) ranging from 1.95 (hypnotics) to 6.80 (gabapentinoids)). COX2 inhibitors and topical lidocaine were rarely used in either group. Among prescription pain medications in the cLBP group, opioid analgesics were the most commonly used class, taken within the past 30 days by 18.8% of working-age Americans with cLBP. Other commonly used prescription pain medications in the cLBP population were acetaminophen 9.9% (almost entirely in combination preparations with opioids), NSAIDs 9.7%, muscle relaxants 8.5%, gabapentinoids 6.8%, and benzodiazepines 5.0%. Corresponding values for non-pain CNS-active medications were 12.9% for SSRIs, 5.1% for all other non-pain antidepressants, and 4.7% for hypnotics.
Table 2.
Prescription medication use among US adult participants of the 2009–2010 NHANES back pain survey, ages 20–69 (N = 5103).
| % with cLBP (SE%) | % w/o cLBP (SE%) | Chi-sq P-value | AOR (95% CI) | AOR p-value | |
|---|---|---|---|---|---|
| N=700 | N=4403 | ||||
| Pain-related medications | |||||
| Opioids | 18.8 (1.0) | 4.3 (0.5) | <0.001 | 4.4 (3.44–5.62) | <0.001 |
| Acetaminophen | 9.9 (1.5) | 2.4 (0.3) | <0.001 | 3.87 (2.79–5.36) | <0.001 |
| NSAIDs | 9.7 (1.2) | 4.1 (0.4) | <0.001 | 2.84 (2.00–4.03) | <0.001 |
| Muscle Relaxants | 8.5 (1.1) | 1.3 (0.2) | <0.001 | 5.95 (3.91–9.04) | <0.001 |
| Gabapentinoids | 6.8 (0.9) | 0.7 (0.2) | <0.001 | 6.80 (3.11–14.86) | <0.001 |
| Benzodiazepines | 5.0 (0.9) | 1.2 (0.3) | <0.001 | 3.88 (2.23–6.75) | <0.001 |
| Salicylates | 2.7 (0.5) | 0.7 (0.2) | <0.001 | 3.13 (1.64–5.96) | <0.001 |
| Duloxetine | 2.5 (1.1) | 0.7 (0.1) | 0.008 | 2.65 (1.01–6.92) | 0.047 |
| Tricyclic antidepressants | 2.2 (0.5) | 0.8 (0.2) | <0.001 | 2.41 (1.33–4.36) | 0.004 |
| COX2 Inhibitors | 0.5 (0.3) | 0.3 (0.1) | 0.237 | 1.71 (0.33–8.87) | 0.521 |
| Topical lidocaine | 0.5 (0.4) | 0.1 (0.1) | 0.091 | 4.91 (0.92–26.08) | 0.061 |
| Non-pain Antidepressants | |||||
| SSRI | 12.9 (1.4) | 5.6 (0.7) | <0.001 | 2.50 (1.71–3.65) | <0.001 |
| Other non-pain antidepressants | 5.1 (0.8) | 1.5 (0.2) | <0.001 | 3.00 (1.85–4.88) | <0.001 |
| Hypnotics | 4.7 (1.1) | 2.1 (0.3) | <0.001 | 1.95 (1.20–3.16) | 0.007 |
cLBP – chronic low back pain. SE – standard error. Chi-Sq – Chi-square. AOR – adjusted odds ratio, adjusted for age, race, gender, education. CI – confidence interval. IQR – interquartile range. All estimates are weighted to represent the national population.
The median duration of use for opioid analgesics in the cLBP group was 702 days (interquartile range (IQR) 314–1770 days). 76.9% of current prescription opioid users with cLBP reported taking opioids for 1 year or longer. The median duration of use for NSAIDs was 522 days (IQR 155-1924), for muscle relaxants – 682 days (IQR 258-1688), for gabapentinoids – 534 days (IQR 224-1611), and for benzodiazepines – 947 days (IQR 165-2115). SSRIs were used for a median of 1008 days (IQR 365-1764), and hypnotics for – 820 days (IQR 157-1729). Opioids, acetaminophen, NSAIDs, and muscle relaxants were used significantly longer by subjects with cLBP compared to those without cLBP (Table 3).
Table 3.
Self-reported duration of prescription medication use among US adult participants of the 2009–2010 NHANES back pain survey, ages 20–69 (N = 5103).
| Median number of days (IQR) Subjects with cLBP | Median number of days (IQR) Subjects without cLBP | P-value | |
|---|---|---|---|
| N=700 | N=4403 | ||
| Pain-related medications | |||
| Opioids | 702 (314–1770) | 119 (13–959) | <0.001 |
| Acetaminophen | 690 (324–1756) | 31 (7–352) | <0.001 |
| NSAIDs | 522 (155–1924) | 91 (29–564) | <0.001 |
| Muscle Relaxants | 682 (258–1688) | 142 (15–777) | 0.007 |
| Gabapentinoids | 534 (224–1611) | 495 (173–1046) | 0.64 |
| Benzodiazepines | 947 (165–2115) | 675 (266–2013) | 0.74 |
| Salicylates | 769 (332–1622) | 605 (209–1173) | 0.05 |
| Duloxetine | 522 (221–1139) | 569 (171–959) | 0.76 |
| Tricyclic antidepressants | 1494 (408–2516) | 848 (190–3477) | 0.77 |
| COX2 Inhibitors | 61 (61–162) | 526 (144–1000) | 0.006 |
| Topical lidocaine | 46 (34–59) | 1460 (1460–1460) | <0.001 |
| Non-pain Antidepressants | |||
| SSRI | 1008 (365–1764) | 983 (345–2041) | 0.67 |
| Other non-pain antidepressants | 996 (59–2777) | 658 (276–1567) | 0.92 |
| Hypnotics | 820 (157–1729) | 457 (170–1465) | 0.45 |
cLBP – chronic low back pain. IQR – interquartile range. P values obtained for log-transformed means. All estimates are weighted to represent the national population.
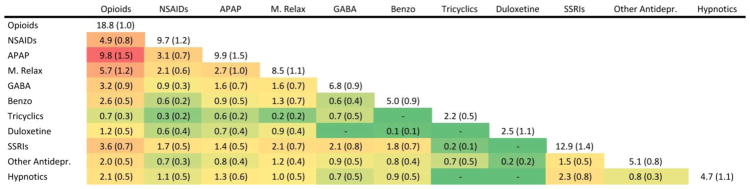
Figure 1 shows the frequency of drug combinations in the cLBP population. Use of more than one pain medication was common and opioid analgesics, in particular, were frequently co-administered with other classes of medications. 9.8% of Americans with cLBP used an opioid with acetaminophen, accounting for over half of the reported opioids and virtually all reported acetaminophen in this population. 5.7% used opioids concomitantly with a muscle relaxant, and 4.9% used an opioid with an NSAID. Other common drug combinations included opioids with SSRIs (3.6%), opioids with gabapentinoids (3.2%), opioids with benzodiazepines (2.6%), and opioids with hypnotics (2.1%).
Figure 1.
Co-administration of pain medications, non-pain antidepressants, and hypnotics in US Adults with cLBP, % (SE%).
cLBP – chronic low back pain. SE – standard error. NSAIDs – non-steroidal anti-inflammatory drugs. APAP – acetaminophen. M. relax muscle relaxants. GABA – gabapentinoids. Benzo benzodiazepines. SSRIs – selective serotonin reuptake inhibitors. Other antidepr. – other non-pain antidepressants. The Heat Map shows the most frequently co-administered medications in red, and the least frequently co-administered medications in green. All estimates are weighted to represent percent of the national population 20–69 years of age. Note that some subjects used more than two classes of medications at the same time.
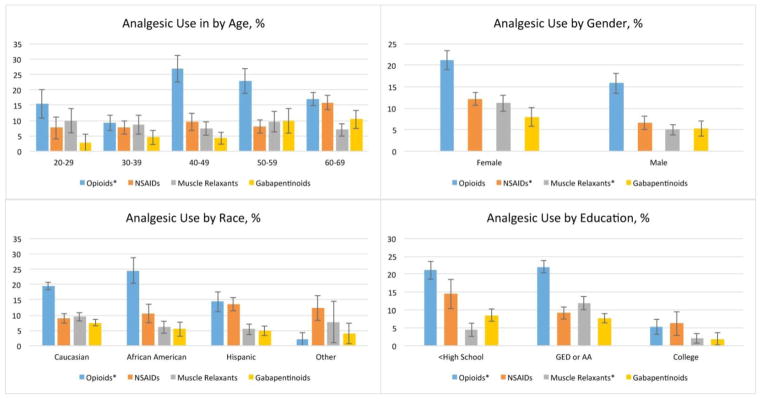
When compared by demographic characteristics, prescription opiate use in the cLBP population was most common among Americans aged 40–49 (27% opioid use), African Americans and Caucasians (24% and 19% opioid use, respectively), and those with less than college education (21% use among subjects with less than high school education and 22% use among those with high school and associates’ degrees, vs 5% use among subjects with a college degree or above), p<0.05 (Figure 2). Although persons with a college education represent 18.6% of the cLBP population, they comprised only 6% of opioid users. 94% of all opioids in the cLBP population were taken by subjects with less than a college degree. Muscle relaxants were most often used by cLBP subjects with high school and associate degrees (12% use, vs 4% use among subjects with less than high school education, and 2% use among subjects with a college degree or above), p = 0.002. Women with cLBP used more NSAIDs and muscle relaxants than men (p = 0.01). In a multivariable model of opioid analgesic use in the cLBP population, which included age, race, gender, education, income, and number of comorbidities, low levels of education remained strongly associated with opioid use: aOR 3.07 (1.12–8.39) for less than high school, and aOR 4.17 (1.73 – 10.03) for high school or associates’ degree, compared with college education. Full models are shown in Table 4. Similar results were observed in an analysis limited to long-term prescription opioid users with cLBP (Appendix 1).
Figure 2.
Demographic distribution of pain medication use in US Adults with cLBP
cLBP – chronic low back pain. NSAIDs – non-steroidal anti-inflammatory drugs. *Chi-square p-value <0.05. The bar charts illustrate the distribution of pain medication use in the cLBP population by age, gender, race, and education level. All estimates are weighted to represent percent of the national population 20–69 years of age. Opioids were most often used by adults in their 40s and 50s. Among races, African Americans were the most likely to use prescription opioids, followed by whites. Americans with cLBP who achieved a college degree or higher, were significantly less likely to use opioid analgesics and muscle relaxants, than less educated Americans. Women with cLBP used more NSAIDs and muscle relaxants than men.
Table 4.
Demographic factors associated with pain medication use in US adults with cLBP (n = 700)
| Model 1 Opioids Adjusted OR (95% CI) | Model 2 NSAIDs Adjusted OR (95% CI) | Model 3 Muscle Relaxants Adjusted OR (95% CI) | Model 4 Gabapentinoids Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Age | 1.00 (0.98–1.03) | 1.01 (0.99–1.04) | 0.97 (0.95 – 1.00) | 1.04 (1.00–1.08) |
| Female gender | 1.16 (0.62–2.19) | 1.70 (0.91–3.17) | 2.17 (1.05–4.46)* | 1.56 (0.49–4.97) |
| Race | ||||
| African American | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Caucasian | 0.89 (0.48–1.65) | 1.05 (0.52–2.10) | 1.91 (0.98–3.70) | 1.87 (0.86–4.06) |
| Hispanic | 0.76 (0.33–1.73) | 1.92 (0.91–4.05) | 1.93 (0.58–6.44) | 1.21 (0.43–3.39) |
| Other | 0.09 (0.01–0.70)* | 2.15 (0.66–6.94) | 1.96 (0.09–43.15) | 1.14 (0.17–7.79) |
| Education | ||||
| College and higher | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| GED/AA degree | 4.17 (1.73–10.03)** | 1.13 (0.36–3.57) | 3.83 (1.14–12.91)* | 4.48 (0.63–31.95) |
| Less than High school | 3.07 (1.12–8.39)* | 1.72 (0.35–8.39) | 0.95 (0.23–3.93) | 4.10 (0.55–30.44) |
| Annual HH Income $ | ||||
| >65 000 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 35–64 000 | 1.36 (0.83–2.24) | 2.41 (0.87–6.71) | 6.69 (1.06–42.35)* | 0.70 (0.16–3.01) |
| <35 000 | 1.92 (1.19–3.11)** | 1.92 (0.69–5.36) | 8.03 (1.33–48.49)* | 1.69 (0.57–4.95) |
| Medical comorbidities | ||||
| 0–1 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 2 or more | 3.32 (1.74–6.35)** | 2.06 (1.09–3.90)* | 6.98 (3.09–15.73)** | 1.61 (0.64–4.09) |
cLBP – chronic low back pain. OR – odds ratio. CI – confidence interval. GED – general educational development. AA – associate’s. HH – household.
p<0.05,
p<0.001.
Income expressed in tertiles of the national population. All estimates are weighted to represent the national population.
In summary, American adults with cLBP used multiple classes of analgesics, and were also more likely to use antidepressants and anxiolytics than those without cLBP. All evaluated prescription analgesics were used long-term. Opioid analgesics were the most common prescription analgesics used by community-based Americans with cLBP, and were frequently co-administered with other analgesics, as well as with antidepressants and anxiolytics. Opioid use was disproportionately higher among 40–49 year olds, African Americans, and adults with lower levels of education. After multivariable adjustment, education remained strongly associated with opioid use in the cLBP population.
Discussion
Our study examined the use of various classes of prescription analgesics by community-dwelling US adults with chronic low back pain. We found that opioids were the most commonly used class of prescription pain medications in the cLBP population, and were used long-term. These findings are consistent with previous studies that showed excessive opioid analgesic prescribing for cLBP in the US during the past two decades [9, 27]. After several major professional society recommendations to limit opioid prescribing, the number of prescriptions has declined by 13.1%, but remains 3 times as high as it was in 1999 and 4 times as high as the current prescription rate in Europe [22]. For example, in comparison with a similar population study from Portugal, Americans with cLBP reported 12 times higher prevalence of opioid use than the Portuguese subjects with cLBP [20].
There is no evidence for the benefits of long-term opioids greater than 6 months in chronic non-cancer pain [33]. Even in short-term studies, oral opioids for non-cancer pain may only be marginally more effective than placebo, and not superior to NSAIDs [19, 24, 1, 16]. A systematic review of opioid therapy for chronic pain found good to fair evidence for an increased risk of serious harms, including overdose, opioid abuse, fractures, and myocardial infarction [5]. Moreover, patients may develop opioid-induced hyperalgesia and tolerance as quickly as one month after initiation of opioid therapy [6, 56].
We also found that opioids were frequently used in combination with other CNS-active medications, including non-pain antidepressants and anxiolytics. It can be difficult to separate the beneficial effects of these combinations from potential hazards. For example, in one large study of patients treated with opioids for chronic non-cancer pain, the odds of opioid overdose increased four to seven-fold among subjects with concomitant depression, but treatment of depression with antidepressants was associated with a 20% reduction in overdose risk [47]. In the same study, subjects who used antidepressants together with opioids, but did not have a diagnosis of depression, had an increased risk of opioid overdose. Multiple other studies showed that the use of opioids in patients with mental health conditions or concomitant use with psychiatric medications is associated with increased risk of prolonged opioid use, misuse, and dependence [15, 3, 2].
We also found that opioids were frequently co-administered with other analgesics, most notably acetaminophen. The use of opioid-acetaminophen combinations maybe beneficial in some scenarios, as acetaminophen extends the analgesic effect of opioids and was shown to reduce opioid requirements in short-term studies of post-procedural pain [36, 37]. However, opioid-acetaminophen combination products have become the leading cause of acetaminophen overdose and account for most analgesic-related emergency department visits nationally [55]. Thus, opioid reduction, rather than avoidance of specific drug combinations, may be the most pertinent area for practice improvement.
We estimated that Americans with cLBP who have lower levels of education are four times more likely to use prescription opioids than college graduates. This association may in part be explained by known higher incidence and severity of many chronic conditions among less educated adults [10]. However, another possible explanation is overreliance on opioids by prescribers due to lack of resources for safer pain management alternatives. Non-pharmacologic pain management approaches are frequently not covered by health insurance, leaving disadvantaged cLBP patients without access to some of the most widely recommended treatment options [51]. Additionally, patients may not be appropriately informed about low efficacy and high risk of side effects with opioid use [44, 29]. Low levels of education have also been linked to increased drug poisoning and opioid-related mortality [25, 38]. Interestingly, while opioid use was most prevalent among African Americans, race no longer independently predicted opioid use when age, gender, socioeconomic status and comorbidities were taken into account. This finding highlights the relative importance of education level and health status over race when estimating the likelihood of long-term opioid use in individuals with cLBP. When designing patient-centered interventions for prevention and mitigation of opioid misuse in the cLBP population, understanding patients’ race and education level, as well as socioeconomic status, may aid in selecting the most effective tactics. Patient education strategies that include assessment of patients’ health literacy and post-intervention knowledge have been effective in changing pain perception, ensuring correct pain medication dosing, and promoting safe storage and disposal of prescription opioids [40, 11, 30]. As African Americans are more likely to have a lower quality of life due to pain [39], and less likely to receive care for chronic pain [21], interventions aimed at improving coping strategies and healthcare access may specifically benefit African Americans. Community involvement and church-based self-management programs have been effective in improving health outcomes and wellbeing among African Americans [26, 50]. Several strategies have also been developed to target healthcare providers’ racial bias when caring for African Americans with pain, including perspective-taking [14], and shared decision-making [13, 46]. Expansion of Medicaid coverage to include care coordination and peer support for disadvantaged opioid users has also been proposed in some states [31, 53].
While opioids are overused, other pain medications are possibly underutilized by Americans with cLBP. Celecoxib, for example, which was rarely used in our study, was found superior to tramadol in two randomized controlled trials in cLBP [34]. Addressing cardiovascular safety concerns, a recent trial in the osteoarthritis population showed a low risk of cardiovascular events with long-term celecoxib and NSAID use [32]. While gastrointestinal and renal side effects remain a concern in elderly patients with comorbid conditions [17, 47], Celecoxib has been associated with fewer GI side effects compared with non-selective NSAIDs, in both short term and long term use [32, 18]. It should be noted, however, that due to its high cost celecoxib may not be available to low-income groups in the US. Duloxetine and tricyclic antidepressants may also be modestly effective in chronic low back pain, while simultaneously addressing depressive symptoms [23, 49, 42]. SSRIs, which were much more often used in the cLBP population, have not shown benefit for pain management [43]. Using antidepressants with pain-relieving properties instead of SSRIs maybe a potential area of practice improvement, and appears to be a cost-effective strategy [54].
Our study’s strengths include a large nationally-representative sample, and the use of participant-reported medication information, which allow to make community-level population estimates, relatively unbiased by healthcare-seeking behavior. However, this study has several limitations. NHANES is a cross-sectional survey, with many self-report variables that may be subject to measurement error and recall bias. Temporal relationships and causality cannot be established from this study design, and, as with any observational study, residual confounding is likely present. Data collection took place during 2009–2010, and was limited to non-institutionalized subjects between 20 and 69 years of age, thus results may not be extrapolated on other populations and other time periods. We were unable to evaluate dosage and frequency of use for prescription medications. It is also likely that the use of over the counter medications, such as NSAIDs, acetaminophen, and some hypnotics is underreported in this study. While our study does not directly infer that the studied medications were used to treat back pain, we saw markedly elevated odds of every analgesic class use in American adults with cLBP compared with the non-cLBP population.
In conclusion, the patterns of analgesic use among US adults with cLBP highlight long-term, and likely excessive use of opioids, possible underutilization of other classes of analgesics, and demographic disparities in the use of different classes of analgesics. As clinical guidelines continue to emphasize opioid-related harms and advocate for cautious prescribing, evidence-based education for patients and providers, and expanded access to non-opioid cLBP treatments are urgently needed.
Perspective.
As prescription opioid use is an issue of national concern, we examined pain-related prescription medication use in community-dwelling among US adults with cLBP. Opioids were the most common prescription pain medication, typically used long-term, in combination with other CNS-active agents, and disproportionately among subjects with less than a college education.
Highlights.
Opioids were the most common prescription pain medications among US adults with cLBP.
Opioids were typically used long-term, and combined with other CNS-active agents.
Low level of education was strongly associated with opioid use in cLBP population.
Acknowledgments
Authors do not have any conflict of interest relevant to this manuscript. All research data was obtained from NHANES directly in deidentified form and did not require additional IRB approval.
Funding sources: NIH Award Number 5T32DK007784-15. NIH Award Number UL1TR000114.
The authors thank Dr. Erin Krebs for providing critical review of this manuscript.
Footnotes
Disclosures:
Dr. Shmagel was funded by a National Institutes of Health T32 grant for this research, Award Number 5T32DK007784-15. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicts of interest to disclose.
Dr. Ensrud is a VA employee. This material is the result of work supported with resources and the use of facilities at the Minneapolis VA. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Anna Shmagel, Assistant Professor of Medicine in the Division of Rheumatic and Autoimmune Diseases at the University of Minnesota.
Dr. Linh Ngo, Clinical fellow in the Division of Rheumatic and Autoimmune Diseases at the University of Minnesota.
Dr. Kristine Ensrud, Professor of Medicine and Epidemiology & Community Health at the University of Minnesota, and a Core Investigator at the Minneapolis VA Center for Chronic Disease Outcomes Research.
Dr. Robert Foley, Associate Professor of Medicine at the University of Minnesota, in the Division of Renal Diseases and Hypertension.
References
- 1.Berthelot J-M, Darrieutort-Lafitte C, Le Goff B, Maugars Y. Strong opioids for noncancer pain due to musculoskeletal diseases: Not more effective than acetaminophen or NSAIDs. Joint Bone Spine. 2015;82:397–401. doi: 10.1016/j.jbspin.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–70. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chelminski PR, Ives TJ, Felix KM, Prakken SD, Miller TM, Perhac JS, Malone RM, Bryant ME, DeWalt DA, Pignone MP. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity. BMC Health Serv Res. 2005;5:3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, Casey D, Cross JT, Shekelle P, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Physicians, American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–91. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–86. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 6.Chu LF, D’Arcy N, Brady C, Zamora AK, Young CA, Kim JE, Clemenson AM, Angst MS, Clark JD. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153:1583–92. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–8. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. REPORT OF THE NIH TASK FORCE ON RESEARCH STANDARDS FOR CHRONIC LOW BACK PAIN. J Pain. 2014;15:569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyo RA, Smith DHM, Johnson ES, Donovan M, Tillotson CJ, Yang X, Petrik AF, Dobscha SK. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med. 2011;24:717–27. doi: 10.3122/jabfm.2011.06.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Cesare M, Khang Y-H, Asaria P, Blakely T, Cowan MJ, Farzadfar F, Guerrero R, Ikeda N, Kyobutungi C, Msyamboza KP, Oum S, Lynch JW, Marmot MG, Ezzati M. Inequalities in non-communicable diseases and effective responses. The Lancet. 2013;381:585–97. doi: 10.1016/S0140-6736(12)61851-0. [DOI] [PubMed] [Google Scholar]
- 11.Donovan HS, Ward SE, Song M-K, Heidrich SM, Gunnarsdottir S, Phillips CM. An Update on the Representational Approach to Patient Education. Journal of Nursing Scholarship. 2007;39:259–65. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315:1624–45. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drwecki BB. Education to identify and combat racial bias in pain treatment. AMA J Ethics. 2015;17:221–8. doi: 10.1001/journalofethics.2015.17.3.medu1-1503. [DOI] [PubMed] [Google Scholar]
- 14.Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain. 2011;152:1001–6. doi: 10.1016/j.pain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Edlund MJ, Martin BC, Fan M-Y, Braden JB, Devries A, Sullivan MD. An analysis of heavy utilizers of opioids for chronic noncancer pain in the TROUP study. J Pain Symptom Manage. 2010;40:279–89. doi: 10.1016/j.jpainsymman.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eken C, Serinken M, Elicabuk H, Uyanik E, Erdal M. Intravenous paracetamol versus dexketoprofen versus morphine in acute mechanical low back pain in the emergency department: a randomised double-blind controlled trial. Emerg Med J. 2014;31:177–81. doi: 10.1136/emermed-2012-201670. [DOI] [PubMed] [Google Scholar]
- 17.Enthoven WTM, Roelofs PDDM, Deyo RA, van Tulder MW, Koes BW. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst Rev. 2016;2:CD012087. doi: 10.1002/14651858.CD012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frampton JE, Keating GM. Celecoxib: a review of its use in the management of arthritis and acute pain. Drugs. 2007;67:2433–72. doi: 10.2165/00003495-200767160-00008. [DOI] [PubMed] [Google Scholar]
- 19.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174:1589–94. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouveia N, Rodrigues A, Ramiro S, Eusébio M, Machado PM, Canhão H, Branco JC. The Use of Analgesic and Other Pain-Relief Drugs to Manage Chronic Low Back Pain: Results from a National Survey. Pain Pract. 2016 doi: 10.1111/papr.12455. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 21.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kaloukalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The Unequal Burden of Pain: Confronting Racial and Ethnic Disparities in Pain. Pain Med. 2003;4:277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 22.Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalita J, Kohat AK, Misra UK, Bhoi SK. An open labeled randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. Journal of the Neurological Sciences. 2014;342:127–32. doi: 10.1016/j.jns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain. 2007;130:66–75. doi: 10.1016/j.pain.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of Increased Opioid-Related Mortality in the United States and Canada, 1990–2013: A Systematic Review. Am J Public Health. 2014;104:e32–42. doi: 10.2105/AJPH.2014.301966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehrer HM, Dubois SK, Brown SA, Steinhardt MA. Resilience-based Diabetes Self-management Education: Perspectives From African American Participants, Community Leaders, and Healthcare Providers. Diabetes Educ. 2017;43:367–77. doi: 10.1177/0145721717714894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine. 2004;29:884–890. doi: 10.1097/00007632-200404150-00012. discussion 891. [DOI] [PubMed] [Google Scholar]
- 28.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–64. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy DM, Cameron KA, King JP, Mullen RJ, Bailey SC, Jacobson KL, Di Francesco L, Davis TC, Parker RM, Wolf MS. Patient Recall of Health Care Provider Counseling for Opioid-Acetaminophen Prescriptions. Pain Med. 2014;15:1750–6. doi: 10.1111/pme.12499. [DOI] [PubMed] [Google Scholar]
- 30.McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addictive Behaviors. 2013;38:2230–5. doi: 10.1016/j.addbeh.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minnesota State Targeted Response to the Opioid Crisis [Internet] [cited 2018 Jan 10]. Available from. https://mn.gov/dhs/assets/mn-opioid-str-project-narrative-april-2017_tcm1053-289624.pdf.
- 32.Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM. PRECISION Trial Investigators. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N Engl J Med. 2016 [Google Scholar]
- 33.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM, Chou R. The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet] Chichester, UK: John Wiley & Sons, Ltd; 2010. Long-term opioid management for chronic noncancer pain. [cited 2017 Mar 8]. Available from: http://doi.wiley.com/10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell JB, Ekman EF, Spalding WM, Bhadra P, McCabe D, Berger MF. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789–802. doi: 10.1177/147323000903700615. [DOI] [PubMed] [Google Scholar]
- 35.Qaseem A, Wilt TJ, McLean RM, Forciea MA for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain. A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine [Internet] 2017 doi: 10.7326/M16-2367. [cited 2017 Mar 6]. Available from: http://annals.org/article.aspx?doi=10.7326/M16-2367. [DOI] [PubMed]
- 36.Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. Journal of Clinical Pharmacy and Therapeutics. 2001;26:257–64. doi: 10.1046/j.1365-2710.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 37.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–13. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 38.Richardson R, Charters T, King N, Harper S. Trends in Educational Inequalities in Drug Poisoning Mortality: United States, 1994–2010. Am J Public Health. 2015;105:1859–65. doi: 10.2105/AJPH.2015.302697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley JL, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002;100:291–8. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 40.Rimer BK, Kedziera P, Levy MH. The Role of Patient Education in Cancer Pain Control. The Hospice Journal. 1992;8:171–91. doi: 10.1080/0742-969x.1992.11882724. [DOI] [PubMed] [Google Scholar]
- 41.Shmagel A, Foley R, Ibrahim H. Epidemiology of Chronic Low Back Pain in US Adults: Data From the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res (Hoboken) 2016;68:1688–94. doi: 10.1002/acr.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, Detke MJ, Iyengar S, Atkinson JH, Backonja M. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine. 2010;35:E578–585. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 43.Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine. 2003;28:2540–5. doi: 10.1097/01.BRS.0000092372.73527.BA. [DOI] [PubMed] [Google Scholar]
- 44.Stein C, Reinecke H, Sorgatz H. Opioid use in chronic noncancer pain: guidelines revisited. Curr Opin Anaesthesiol. 2010;23:598–601. doi: 10.1097/ACO.0b013e32833c57a8. [DOI] [PubMed] [Google Scholar]
- 45.The Gallup-Palmer College Annual Report [Internet] [cited 2018 Jan 8]. Available from: http://www.palmer.edu/alumni/research-publications/gallup-report/
- 46.Torke AM, Corbie-Smith GM, Branch WT. African American Patients’ Perspectives on Medical Decision Making. Arch Intern Med. 2004;164:525–30. doi: 10.1001/archinte.164.5.525. [DOI] [PubMed] [Google Scholar]
- 47.Turner BJ, Liang Y. Drug Overdose in a Retrospective Cohort with Non-Cancer Pain Treated with Opioids, Antidepressants, and/or Sedative-Hypnotics: Interactions with Mental Health Disorders. J Gen Intern Med. 2015;30:1081–96. doi: 10.1007/s11606-015-3199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: A systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26:285–91. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Vogt MT, Kwoh CK, Cope DK, Osial TA, Culyba M, Starz TW. Analgesic usage for low back pain: impact on health care costs and service use. Spine. 2005;30:1075–81. doi: 10.1097/01.brs.0000160843.77091.07. [DOI] [PubMed] [Google Scholar]
- 50.Walls CT, Zarit SH. Informal Support From Black Churches and the Well-Being of Elderly Blacks. Gerontologist. 1991;31:490–5. doi: 10.1093/geront/31.4.490. [DOI] [PubMed] [Google Scholar]
- 51.Weeks J. Influential U.S. Medical Organizations Call for Insurance Coverage of Non-Pharmacologic Approaches to Pain. J Altern Complement Med. 2016;22:947–9. doi: 10.1089/acm.2016.29016.jjw. [DOI] [PubMed] [Google Scholar]
- 52.Weisman MH, Witter JP, Reveille JD. The prevalence of inflammatory back pain: population-based estimates from the US National Health and Nutrition Examination Survey, 2009–10. Ann Rheum Dis. 2013;72:369–73. doi: 10.1136/annrheumdis-2012-201403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitewashed AA Opioid Crisis 11-15-17_EMBARGOED_FINAL.pdf [Internet] [cited 2018 Jan 10]. Available from: https://www.thechicagourbanleague.org/cms/lib/IL07000264/Centricity/Domain/1/Whitewashed%20AA%20Opioid%20Crisis%2011-15-17_EMBARGOED_%20FINAL.pdf.
- 54.Wielage RC, Bansal M, Andrews JS, Wohlreich MM, Klein RW, Happich M. The cost-effectiveness of duloxetine in chronic low back pain: a US private payer perspective. Value Health. 2013;16:334–44. doi: 10.1016/j.jval.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Willy M, Kelly JP, Nourjah P, Kaufman DW, Budnitz DS, Staffa J. Emergency department visits attributed to selected analgesics, United States, 2004–2005. Pharmacoepidem Drug Safe. 2009;18:188–95. doi: 10.1002/pds.1691. [DOI] [PubMed] [Google Scholar]
- 56.Wm H, TjLCT Opioid-induced hyperalgesia in community-dwelling adults with chronic pain, Opioid-Induced Hyperalgesia in Community-Dwelling Adults with Chronic Pain. Pain. 2015;156:1145–52. doi: 10.1097/j.pain.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]