Abstract
Effects of α-synuclein deficiency on cellular blood components have not been extensively investigated. This study evaluated ultrastructural changes of leukocytes in α-synuclein knockout (KO) mice using electron microscopy (EM). The following ultrastructural characteristics were quantified in leukocytes: mitochondria, primary granules, specific granules (SG), Golgi apparatus (GA), inclusions, rough-endoplasmic reticulum (RER), smooth-endoplasmic reticulum (SER), and cellular projections (CP). EM showed increased numbers or amounts of SG, inclusions, and SER in KO group (5.3 ± 4.5 in WT vs. 14.1 ± 10.3 in KO, p=0.02; 0.4 ± 0.9 in WT vs. 3.2 ± 2.8 in KO, p=0.007; and 7.7 ± 6.7 in WT vs. 17.7 ± 12.2 in KO, p=0.03, respectively). Although CP number was not significantly different between the two groups (13.4 ± 5.3 in WT vs. 16.3 ± 7.5 in KO, p=0.32), their size and shapes were altered in KO mice. Notably, findings occurred in the setting of significant lymphopenia. α-synuclein deficiency leads to changes in size and shape of secretory particles and increases in SER, SG, and inclusions, indicating a potential role for α-synuclein in vesicular trafficking in leukocytes. Further studies are needed to elucidate functions mediated by α-synuclein.
Keywords: α-synuclein, leukocytes, morphology, ultrastructure
Introduction
α-synuclein is a small protein (14 kDa) that is important to normal brain function and in certain conditions undergoes aberrant protein conformational changes that lead to the pathologic findings seen in Lewy bodies characteristic of Parkinson’s disease [1]. The function of this molecule in the central nervous system still remains to be fully elucidated; but it appears to play important roles in promoting the health of the presynaptic terminal, neurotransmitter release in response to stimuli, and neuronal plasticity [2]. It can also have inhibitory roles, as seen in dopaminergic neurotransmitter release [3, 4]. This protein is co-localized at or near the plasma membrane and cytoplasmic membranous structures, and this localization may mediate its neuronal functional roles.
It has also become evident that this protein is also well expressed in the hematopoietic system, but its functions in this system remain obscure. Reports have indicated that α-synuclein expression is highest in megakaryocytes, platelets, red blood cells (RBC), and erythroid precursors [5, 6]. α-synuclein expression has also been reported to be a specific marker of neoplastic changes in the megakryocytic lineage [7]. Platelets have been shown to have the highest concentration of α-synuclein per milligram of cellular protein in peripheral blood [8], and time-dependent α-synuclein concentration increases in plasma supernatant of stored platelets may be indicative of changes that platelets undergo while in storage [9]. Importantly, αsynuclein in platelets has been shown to inhibit α-granule release in a calcium dependent manner [10]. In addition, α-synuclein deficiency leads to production of small circulating platelets and significant anemia [11].
In leukocytes, the function of α-synuclein appears to be critical since its deficiency leads to severe paucity in the number of mature B and T lymphocytes, lack of immunoglobulin class switching in response to stimuli (with a resulting absence of IgG), and abnormal development of T helper and T regulatory cells [11, 12]. In light of these observations that α-synuclein leads to quantitative and functional impairment of lymphocytes, we proceeded to look at the ultrastructural effects of α-synuclein deficiency on leukocytes by electron microscopy analysis of peripheral blood samples from α-synuclein knockout (KO) mice.
Methods
Mice
α-synuclein−/− mice B6;129X1-Sncatm1Ros1 (stock 003692) (KO), and age- and sex-matched (8–10 week old) α-synuclein+/+ B6;129SF2/J wild type (WT) mice were obtained from Jackson Laboratory (Bar Harbor, ME) [11]. Mice were housed in pathogen-free conditions at room temperature with standard night/light cycle, standard diet and water ad libitum. Animal protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Blood sampling and CBC
Mouse blood samples (200 μL) were collected from the tail vein in heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA) and used for peripheral blood smears, manual blood counts, EM, and automated complete blood counts (CBC). The latter was performed as previously described [11].
Transmission electron microscopy
Preparation of cells for electron microscopy was done as previously described [13]. Blood samples from five WT and five KO mice were fixed by immersion in quarter-strength Karnovsky fixative solution for 2 h at room temperature. Specimens were washed and post-fixed for 2 additional hours in an unbuffered 1:1 mixture of 2% osmium tetroxide and 3% potassium ferrocyanide. Specimens were subsequently rinsed with distilled water followed by immersion overnight in an acidified solution of 0.25% uranyl acetate. Specimens were washed a second time in distilled water, dehydrated in ascending concentrations of ethanol, passed through propylene oxide, and embedded in a Poly/Bed 812 embedding media (Polysciences, Warrington, PA). Thin sections were obtained using a RMC MT6000-XL ultramicrotome (Boeckeler Instruments, Inc., Tucson, AZ). Sections were mounted on Gilder square 300 mesh nickel grids (Electron Microscopy Sciences, Hatfield, PA) followed by staining with acidified methanolic uranyl acetate and stable lead staining solution. Sections were subsequently coated on a Denton DV-401 carbon coater (Denton Vacuum LLC, Moorestown, NJ), and analyzed in a JEOL 1200EX electron microscope (JEOL Ltd., Tokyo, Japan). The following morphological and ultrastructural characteristics were recorded from all leukocyte subsets [14–16]: cell diameter, cell perimeter, number of mitochondria, primary granules (PG), specific granules (SG), Golgi apparatus, inclusions, rough endoplasmic reticulum (RER), smooth endoplasmic reticulum (SER), and cellular projections (CP). Separately, peripheral blood smears were obtained and used to derive manual 100-cell differential leukocyte count using light microscopy. Transmission electron microscopy and light microscopy analyses were performed by two pathologists independently.
Cell measurements
Photoshop CC version 19.1.1 (Adobe Systems, San Jose, CA) was used to obtain measurements. Cell diameter and perimeter were obtained by using the select tool that was customized to micrometer units using the scale bar in the EM images.
Statistical analysis
All statistics were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA). Results are presented as mean ± SD. Intergroup data comparisons were performed using Mann Whitney test and unpaired t test. Statistical significance was set at p < 0.05.
Results
Cell differential
Manual 100-cell differential count showed relative lymphopenia (88.4 ± 7.9% in WT vs. 69.2 ± 13.5% in KO, p=0.03) and neutrophilia (5.0 ± 3.6% in WT vs. 21.2 ± 8.0% in KO, p=0.006) in KO mice compared to WT controls (Table 1). These data correlated with automated CBC results and those previously published in this mouse strain [11]. KO mice had a marked absolute lymphopenia (3.8 ± 1.0 × 109/L in WT vs. 2.9 ± 1.0 × 109/L in KO, p=0.029) and a relative percent neutrophilia, the latter likely secondary to the reduced number of lymphocytes since the absolute neutrophil counts were similar between KO and WT mice. Furthermore, KO animals appeared to have a normocytic anemia phenotype since RBC, hemoglobin (Hgb) and hematocrit (Hct) were lower in KO mice compared to WT (9.1 ± 0.8 × 1012/L vs. 10.8 ± 0.8 × 1012/L, p <0.0001; 14.3 ± 0.7 g/dL vs. 16.1 ± 1.2 g/dL, p=0.0005; and 47.8 ± 4.4% vs. 57.8 ± 4.9%, p <0.0001 respectively) in the context of normal mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red cell distribution width (RDW) (Table 1B). There were no statistical differences in monocyte counts between the two mouse groups while basophils and eosinophils could not be detected in the majority of animals from both groups (mean of 0 and SD 0, data not shown).
Table 1.
Cell differentials of peripheral blood samples from KO mice and age-matched WT controls.
| A. Manual differential using peripheral blood smears | |||
|---|---|---|---|
| WT | KO | p value | |
| Mean lymph % | 88.4 ± 7.9 | 69.2 ± 13.5 | 0.03 |
| Eosinophil % | 0.6 ± 1.2 | 0.8 ± 0.75 | 0.8 |
| Neutrophil % | 5.0 ± 3.6 | 21.2 ± 8.0 | 0.006 |
| Monocytes % | 8.4 ± 3.8 | 8.8 ± 5.7 | 0.44 |
| Basophil % | 0 | 0 | |
| Band % | 0 | 0 | |
| B. Automated cell blood count | |||
|---|---|---|---|
| WT | KO | p value | |
| White blood cells (× 109/L) | 4.7 ± 1.2 | 4.0 ± 1.4 | 0.210 |
| Neutrophils (%) | 14.7 ± 5.4 | 19.3 ± 5.0 | 0.026 |
| Neutrophils (× 109/L) | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.525 |
| Lymphocytes (%) | 80.0 ± 5.7 | 72.8 ± 4.1 | 0.003 |
| Lymphocytes (× 109/L) | 3.8 ± 1.0 | 2.9 ± 1.0 | 0.029 |
| Monocytes (%) | 5.0 ± 1.2 | 7.3 ± 2.9 | 0.030 |
| Monocytes (× 109/L) | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.325 |
| Erythrocytes (× 1012/L) | 10.8 ± 0.8 | 9.1 ± 0.8 | <0.0001 |
| Hgb (g/dL) | 16.1 ± 1.2 | 14.3 ± 0.7 | 0.0005 |
| Hct (%) | 57.8 ± 4.9 | 47.8 ± 4.4 | <0.0001 |
| MCV (fL) | 53.4 ± 3.0 | 52.5 ± 1.5 | 0.354 |
| MCH (pg) | 15.0 ± 1.2 | 15.7 ± 1.6 | 0.210 |
| MCHC (g/dL) | 28.0 ± 1.9 | 30.2 ± 3.3 | 0.083 |
| RDW (%) | 17.6 ± 1.1 | 17.1 ± 0.6 | 0.234 |
| Platelets (× 109/L) | 624.5 ± 59.3 | 660.9 ± 91.4 | 0.282 |
| Mean Platelet Volume (fL) | 4.1 ± 0.3 | 3.9 ± 0.1 | 0.017 |
Based on 100 cell counts of smears from 5 mice/ group (Mean ± SD)
Based on counts from 11 mice/group (Mean ± SD)
Cell ultrastructure
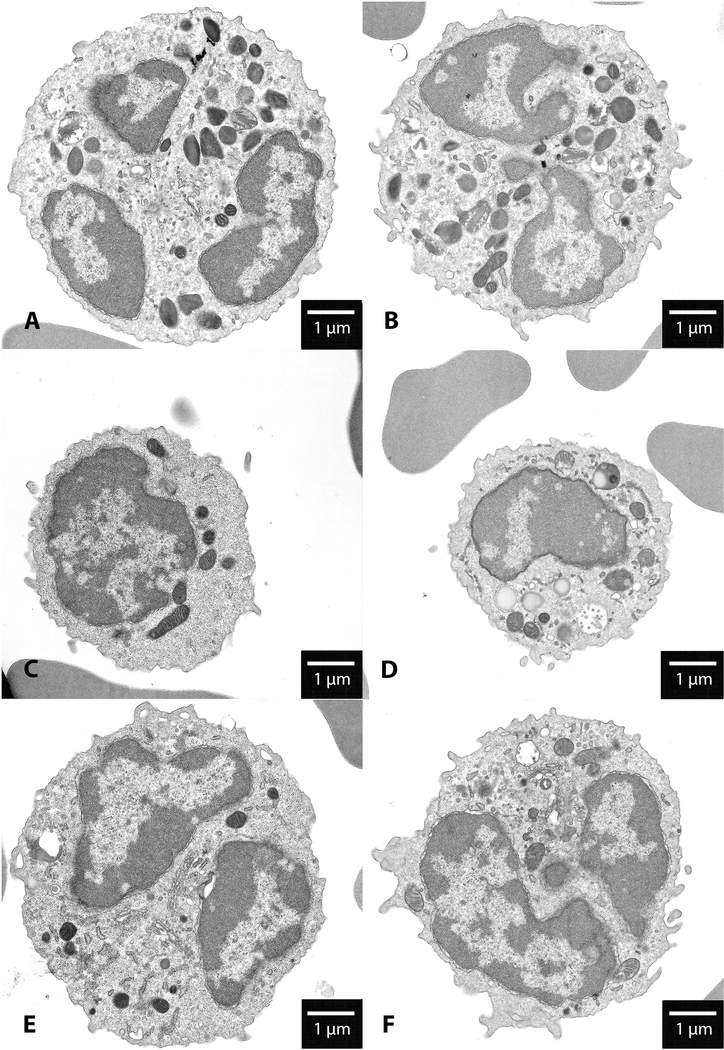
Transmission electron microscopy comparison of the leukocytes of WT and KO mice indicated that the latter had marked increases in the number of SG, inclusions and SER compared to the former (5.3 ± 4.5 in WT vs. 14.1 ± 10.3 in KO, p=0.02; 0.4 ± 0.9 in WT vs. 3.2 ± 2.8 in KO, p=0.007; and 7.7 ± 6.7 in WT vs. 17.7 ± 12.2 in KO, p=0.03, respectively) (Table 2). Analysis of the number of CPs between the two groups showed no significant difference between leukocytes of WT and KO mice (13.4 ± 5.3 in WT vs. 16.3 ± 7.5 in KO, p=0.32). Of note, sizes and shapes of CPs were altered in KO mice (Figure 1). No differences were noted in other organelles between the two groups (data not shown).
Table 2.
Ultrastructural comparisons between leukocytes from WT and KO mice
| WT | KO | p value | |
|---|---|---|---|
| Number of secondary granules | 5.3 ± 4.5 | 14.1 ± 10.3 | 0.02 |
| Number of inclusions | 0.4 ± 0.9 | 3.2 ± 2.8 | 0.007 |
| Number of SER | 7.7 ± 6.7 | 17.7 ± 12.2 | 0.03 |
| Number of cellular projections | 13.4 ± 5.3 | 16.3 ± 7.5 | 0.32 |
| Cell diameter | 5.9 ± 0.8 | 6.8 ± 1.0 | 0.0284 |
| Cell perimeter | 24.4 ± 2.3 | 27.2 ± 4.5 | 0.0465 |
Results are presented as Mean ± SD
Figure 1.
Morphologic changes of leukocytes in α-synuclein knockout mice by transmission electron microscopy. A, C, and E are representative images of eosinophil, monocyte, and neutrophil respectively in WT mice in the context of surrounding erythrocytes. B, D, and F are representative images of eosinophil, monocyte, and neutrophil respectively in KO mice. Scale bars correspond to 1 μm.
Cell dimensions
Cells from KO and WT mice were measured to determine changes in dimensions caused by α-synuclein deficiency. KO mice leukocytes had a >14% larger diameter compared to WT leukocytes (6.8 ± 1.0 vs. 5.9 ± 0.8 respectively, p=0.0284) and 10% larger perimeter (27.2 ± 4.5 vs. 24.4 ± 2.3 respectively, p=0.0465) (Table 2).
Effects of α-synuclein deficiency
To put into context morphological findings, prior results indicating important roles of α-synuclein from our group and recently from others as well are presented in Table 3. Both B and T cells were markedly reduced in KO mice compared to WT and in particular both mature B cells (B220hi) and mature T cells (CD4+ and CD8+) which correlate with the observed absolute lymphopenia. More importantly, lymphocyte reduction occurred in the background of abnormal histological architecture of spleen, thymus and lymph nodes which were also small in size compared to controls. Stimulation of B cells indicated that KO mice were able to mount IgM responses regardless of antigen presented (T-independent or T-dependent) but were unable to class switch upon antigenic challenge with poor to absent IgG responses. In T cells, α-synuclein deficiency led to increases in numbers of double negative and double positive thymocytes which do not appear to reach full maturation. In addition, the small number of T cells that reach maturity do it in an overactive state since they have higher expression of early activation markers (CD49d, CD69). Activation of CD4+ cells, favor a Th1 phenotype since Th2 responses are defective as indicated by minimal IL-4 production and markedly increased IL-2. Similarly, T regulatory cells are also decreased in KO mice. In regard to RBC, we showed that KO mice have anemia at baseline and others since have indicated that this is due to the requirement of α-synuclein expression to allow for enucleation of erythroblasts and to maintain RBC plasma membrane stability. Along the same line, iron deficiency occurs when α-synuclein is absent. Finally, absence of α-synuclein leads to small platelets and to hypercoagulability.
Table 3.
Effects of α-synuclein deficiency in hematopoietic system of KO mice
| B Cells [11] | T Cells [12] | RBC [11, 19, 29] | Platelets [11] |
|---|---|---|---|
| Low absolute lymphocyte count | Low absolute lymphocyte count | Normocytic anemia low Hgb, Hct | Small platelets, low MPV |
| Low B220+IgM+ cells | Low CD3+ T cell absolute count | Decrease in RBC membrane stabilization | |
| Low AA4.1+IgD−, AA4.1+IgD+, AA4.1−IgD+ B cells | Low number of thymocytes/ small thymus | Decrease in erythroblast enucleation | |
| Low B220+AA4.1+, B220+AA4.1−, mature B cells and marginal zone B cells | Low CD4+ and CD8+ single positive splenocytes | Iron deficiency (transferrin) | |
| Normal IgM concentration | High CD4−CD8− thymocytes | ||
| Low IgG concentration | Low CD8+ lymph node T cells | ||
| Abnormal B cell zone architecture in lymphoid organs | Increase in thymic cortex and reduction in medulla | ||
| Low number of lymphoid follicles | High number of CD8+ cells expressing CD69 an CD49d | ||
| Normal IgM response to T-independent antigen | Low IL-4 production upon CD4+ T cell activation | ||
| Absent IgG response to T-independent antigens | High IL-2 production upon CD4+ cell activation | ||
| Normal IgM response to T-dependent antigen | Low IFN-γ production upon CD4+ cell activation | ||
| Absent IgG response to T-dependent antigen | Low Foxp3+ CD4+ cells in thymus |
Low, absent and high refers to statistically significant differences
Discussion
α-synuclein is best characterized with regard to its roles in the central nervous system, which can lead to pathophysiological findings as seen in Parkinson’s disease when structural changes or misfolding of the protein occur. α-synuclein is also essential to late stages of hematopoiesis and especially adaptive immunity as shown by the marked reduction in mature B and T cells in α-synuclein KO mice [11, 12]. Our results complement prior findings from our group indicating that leukocytes in the absence of α-synuclein undergo morphological changes including alterations in cell size, and the ultrastructural distribution and number of intracellular organelles.
In regard to leukocytes, deficiency of this protein leads to maturation arrest of lymphocytes and this is likely at the core of the abnormal architecture seen in lymphoid organs. We observed that leukocytes from KO mice were larger in size compared to WT. This is an observation that has been reported in microglia from α-synuclein KO mice, which are significantly larger and have a greater number of cytoplasmic vacuoles [17]. This larger microglial size is associated with decreased phagocytic activity, elevated cellular prostaglandin levels, and accumulation of multiple microglial enzymes needed for responses to signaling events [18]. Since α-synuclein is closely linked to cellular membranes, including granules, these prior results and our current data suggest its absence may lead to accumulation of material that under normal physiologic conditions is secreted to the extracellular space resulting in larger cells. Likewise, its close plasma membrane and nuclear membrane association hint at one of its functions in RBC since KO mice erythroblasts fail to enucleate leading to impaired RBC maturation and decreased membrane stability [19]. Taken together, these results seem to indicate that of α-synuclein may be important to proper cellular function.
The hallmark of Parkinson’s disease and Lewy body dementia is the formation of cytoplasmic inclusions called Lewy bodies, which are mainly aggregates of misfolded α-synuclein protein, and their accumulation in the neuronal endoplasmic reticulum results in activation of the unfolded protein response [20]. This response helps to resolve protein misfolding or trigger apoptosis in cases of chronic stress [21]. Our results suggest that lack of α-synuclein leads to a significant increase in cytoplasmic inclusions in leukocytes. Furthermore, leukocytes from the KO group had an increase in SER and SG. α-synuclein could potentially modulate vesicle/SG trafficking in leukocytes, and the observed inclusions may represent SG that failed to be secreted. Future research in this area will help elucidate these potential mechanisms.
Since α-synuclein is essential to normal physiological intracellular trafficking, the arrest of lymphocyte development in α-synuclein KO mice may be caused by a lack of receptor delivery to the cell surface. This is similar to the case seen in the synapse, where absence of αsynuclein leads to dysregulated vesicle trafficking [22]. This α-synuclein function may be mediated by its close relationship with membranes and ability to closely associate with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes while promoting their assembly [23]. In T cells, SNARE complex activity is essential to TCR localization to the immunological synapse [24, 25], and SNARE-mediated membrane fusion is required for granule exocytosis and release from cytotoxic T cells and NK cells [26]. Additionally, in T cells α-synuclein-deficiency can lead to enhanced localization of TCR to immunological synapses on developing thymocytes [27], resulting in higher affinity interaction and enhanced negative selection of cells during maturation. Similarly, in the B cell lineage, another SNARE protein member, SNAP-23, is essential in plasma cells for antibody production and secretion [28]; and through protein interactions with such SNAREs α-synuclein may also participate in SNARE-related membrane fusion in B cells and be essential to B cell biology.
In conclusion, our findings suggest that absence of α-synuclein is associated with ultrastructural changes in leukocytes, including alterations in cell size and the shape of secretory particles, and increases in amount or number of SER, SG, and inclusions. These morphological findings correlate with quantitative and qualitative defects seen in lymphocytes of KO mice. Further studies are needed to elucidate physiological mechanisms mediated by α-synuclein, and their effects on different leukocyte populations.
Acknowledgements
This study was supported by grant funding from the Office of Diversity and Inclusion of the University Hospitals Cleveland Medical Center and by the Dr. Harry Taylor Scholar Award also from UHCMC (both awarded to RWM). Laboratory facilities were supported by NIH R01 AI034343 grant (awarded to CVH). The authors are grateful for the assistance provided by Dr. Hisashi Fujioka from Case Western Reserve University School of Medicine Electron Microscopy Core Facility. The authors would also like to acknowledge technological and logistical assistance provided by Nancy Nagy,
Footnotes
Conflict of Interest: The authors report no conflicts of interest with the submission of this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–90. [DOI] [PubMed] [Google Scholar]
- [3].Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. [DOI] [PubMed] [Google Scholar]
- [4].Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79:1044–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, et al. Expression of alphasynuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoietic lineage. Biochem Biophys Res Commun. 2007;358:104–10. [DOI] [PubMed] [Google Scholar]
- [6].Hashimoto M, Yoshimoto M, Sisk A, Hsu LJ, Sundsmo M, Kittel A, et al. NACP, a synaptic protein involved in Alzheimer’s disease, is differentially regulated during megakaryocyte differentiation. Biochem Biophys Res Commun. 1997;237:611–6. [DOI] [PubMed] [Google Scholar]
- [7].Maitta RW, Wolgast LR, Wang Q, Zhang H, Bhattacharyya P, Gong JZ, et al. Alpha- and beta-synucleins are new diagnostic tools for acute erythroid leukemia and acute megakaryoblastic leukemia. Am J Hematol. 2011;86:230–4. [DOI] [PubMed] [Google Scholar]
- [8].Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5:55–9. [DOI] [PubMed] [Google Scholar]
- [9].Stefaniuk CM, Hong H, Harding CV, Maitta RW. alpha-synuclein concentration increases over time in plasma supernatant of single donor platelets. Eur J Haematol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park SM, Jung HY, Kim HO, Rhim H, Paik SR, Chung KC, et al. Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood. 2002;100:2506–14. [DOI] [PubMed] [Google Scholar]
- [11].Xiao W, Shameli A, Harding CV, Meyerson HJ, Maitta RW. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology. 2014;219:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shameli A, Xiao W, Zheng Y, Shyu S, Sumodi J, Meyerson HJ, et al. A critical role for alpha-synuclein in development and function of T lymphocytes. Immunobiology. 2016;221:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hong H, Xiao W, Maitta RW. Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS One. 2014;9:e85465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol. 2014;5:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 2008;295:C1354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ghadially F Ultrastructual pathology of the cell and matrix: A text and atlas of physiological and pathological alterations in the fine structure of cellular and extracellular components. Third ed. London, UK: Butterworths; 1988. [Google Scholar]
- [17].Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Austin SA, Rojanathammanee L, Golovko MY, Murphy EJ, Combs CK. Lack of alphasynuclein modulates microglial phenotype in vitro. Neurochem Res. 2011;36:994–1004. [DOI] [PubMed] [Google Scholar]
- [19].Araki K, Sugawara K, Hayakawa EH, Ubukawa K, Kobayashi I, Wakui H, et al. The localization of alpha-synuclein in the process of differentiation of human erythroid cells. Int J Hematol. 2018;108:130–8. [DOI] [PubMed] [Google Scholar]
- [20].Mercado G, Castillo V, Soto P, Sidhu A. ER stress and Parkinson’s disease: Pathological inputs that converge into the secretory pathway. Brain Res. 2016;1648:626–32. [DOI] [PubMed] [Google Scholar]
- [21].Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scott D, Roy S. alpha-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012;32:10129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 2004;20:577–88. [DOI] [PubMed] [Google Scholar]
- [25].Pattu V, Qu B, Schwarz EC, Strauss B, Weins L, Bhat SS, et al. SNARE protein expression and localization in human cytotoxic T lymphocytes. Eur J Immunol. 2012;42:470–5. [DOI] [PubMed] [Google Scholar]
- [26].Chiang SC, Theorell J, Entesarian M, Meeths M, Mastafa M, Al-Herz W, et al. Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood. 2013;121:1345–56. [DOI] [PubMed] [Google Scholar]
- [27].Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–48. [DOI] [PubMed] [Google Scholar]
- [28].Reales E, Mora-Lopez F, Rivas V, Garcia-Poley A, Brieva JA, Campos-Caro A. Identification of soluble N-ethylmaleimide-sensitive factor attachment protein receptor exocytotic machinery in human plasma cells: SNAP-23 is essential for antibody secretion. J Immunol. 2005;175:6686–93. [DOI] [PubMed] [Google Scholar]
- [29].Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson’s disease. Free Radic Biol Med. 2016;97:292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]