Abstract
Vaccine design efforts against the human immunodeficiency virus (HIV) have been greatly stimulated by the observation that many infected patients eventually develop highly potent, broadly neutralizing antibodies (bnAbs). Importantly, these bnAbs have evolved to recognize not only the two protein components of the viral envelope protein (Env), but also the numerous glycans that form a protective barrier on the Env protein. Because Env is vastly over-glycosylated compared to host glycoproteins, the glycans have become targets for the antibody response. Therefore, considerable efforts have been made in developing and validating biophysical methods to elucidate the complex structure of the Env spike glycoprotein with its combination of glycan and protein epitopes. We illustrate here how the application of robust biophysical methods have transformed our understanding of the structure and function of the HIV Env spike and stimulated innovation in vaccine design strategies that takes into account the essential glycan components.
Keywords: viral glycoprotein, broadly neutralizing antibody, glycosylation, glycan composition, EM structure, X-ray structure
Introduction
Determining high resolution structures of viral attachment and fusion glycoproteins has emerged a critical step in developing immunization strategies where traditional vaccine approaches have failed (16). Currently, there is no effective vaccine against the type-1 human immunodeficiency virus (HIV-1). To aid in that goal, a sustained effort was mounted over many years to decipher the three-dimensional structure of the heavily glycosylated HIV Envelope spike (Env) (118, 120, 142). Env consists of a trimer of gp120-gp41 heterodimers and this viral component is the sole target for neutralizing antibodies (87). Env is resposible for virion attachment and fusion with target cells by first binding to host cell surface proteins: CD4 is the primary receptor and chemokine receptors, such as CCR5 or CXCR4, act as co-receptor. Receptor engagement leads to fusion of the viral and host cell membranes and this cell entry process can be impeded at different stages by antibodies (65). However, the substantial diversity in this RNA virus that is driven by the error-prone reverse transcriptase means that the host antibody response is ultimately ineffective in eliminating and, in most cases, controlling infection once it is established, especially as HIV is a retrovirus. Notwithstanding, vaccine design efforts have been greatly propelled by the discovery of a rapidly growing arsenal of antibodies isolated from infected patients that show extraordinary neutralization potencies against a wide range of clinical isolates (17, 18). One of the major long-term goals in HIV research has been to decipher the structure of antibody-Env complexes to aid in efforts to design immunogens that are able to elicit such broadly neutralizing antibodies (bnAbs) through vaccination (16, 33, 138). Many studies in animal models have demonstrated that, if such antibodies are present prior to infection in high enough concentrations, they are able to provide protection against viral challenge (3, 4, 38, 54, 55, 86, 93, 102, 105, 128).
Until relatively recently, a key limitation in elucidating the epitopes of bnAbs has been the availability of stable recombinant mimics of the viral Env spike. The pre-fusion conformation of the spike is metastable and prone to conformational rearrangements or gp120 shedding. These phenomena are particularly significant as almost all bnAbs specifically recognize the Env native conformation and many are dependent on the quaternary structure of the trimer. Furthermore, the Env spike undergoes furin-mediated maturation during egress through the Golgi apparatus to attain its correct pre-fusion conformation (10, 94). Finally, although accumulating a high density of glycans on the Env surface is a mechanism to evade the host antibody response (143, 148), bnAbs are still able to recognize the dense glycosylation (11, 35, 40, 57, 99, 103, 122, 139) as it differs substantially from that of host proteins. Thus, an active area of investigation has been to determine to what extent recombinant mimics of the viral spike emulate the glycan processing of native infectious virions (25).
Designing, expressing and validating candidate immunogens that exhibit native-like trimeric Env structures has been a key goal in HIV structural biology. Despite the apparent sophistication of current methodologies, significantly divergent structures have been reported for the Env spike over the years. Similarly, divergence has occurred in the analytical characterization of the glycans by chromatographic and mass spectrometric methods. Here, we discuss how rigorous validation processes have led to the unambiguous conclusion that the “SOSIP”platform (117) is the best-in-class soluble, cleaved, recombinant mimic of Env spike. We show how the biophysical methods used for analysis can identify correctly folded Env structures versus those that are misfolded and how the extensive natural sequence diversity of HIV-1 can be accommodated. We also discuss how the conserved overall Env architecture substantially shapes glycan processing and leads to a remarkably homogeneous glycan component of the Env protein that has become a target for antibody recognition.
Biophysical Analysis of the Glycan Shield
Design and Expression of Recombinant Env
The design and expression of recombinant Env has been central to antibody-based vaccine efforts and a prerequisite for biophysical analysis. Unlike influenza, for example, HIV virions display a low density of viral spikes and cannot be extracted from virus particles in appreciable yields. Therefore, strategies such as protease release of native viral glycoprotein that yielded the first generation of influenza hemagglutinin structures (146) cannot be readily applied to HIV. Thus, development of recombinant expression systems to produce properly folded trimers has been a prerequisite for structural analysis. Additionally, the metastable nature of the HIV Env spike (as well as other viral spikes) has meant that initial efforts to isolate trimeric material failed to yield native-like material. The use of trimerization motifs, such as foldon scaffolds, that were highly successful in producing influenza hemagglutinin trimers for example (37, 132, 133), combined with modification of the sequence between gp120 and gp41 to prevent cleavage of gp160, did not result in properly folded trimers. These foldon-based HIV oligomers also displayed poor antigenicity with bnAbs and reacted with antibodies that recognize non-neutralizing epitopes such as at the native oligomerization interface (115). The disordered nature of these foldon-based immunogens was vividly revealed by electron microscopy (114, 149).
To overcome the barriers to recombinant production of native-like trimers, principally that of metastability and gp120 shedding, trimers were developed containing mutations that trap the glycoprotein in its native-like prefusion state. Firstly, a disulphide bond was added between the gp120 and gp41 subunits (termed “SOS”) (9). Remarkably, this was achieved in the absence of any structural information, using PAGE and ELISA binding to the very limited anti-HIV antibodies that were available at the time. Secondly, increased trimer stability was engineered by substituting a proline into gp41 (termed “IP”) in order to destabilize the helical post-fusion conformation and enhance formation of prefusion trimers (119). As the native trimer requires maturation through furin cleavage, furin cleavage was investigated both in a cellular and in vitro context. Maximal cleavage was observed when endogenous cleavage activity was supplemented by co-expression with recombinant furin (10, 32). Negative-stain electron microscopy (ns-EM) proved to be a valuable tool for screening a wide panel of isolates containing these SOSIP mutations and included a truncated at the 664 position prior to the transmembrane region that eliminated the hydrophobic membrane-proximal external region (67, 117). The goal was to determine which possible strains were candidates for producing reasonable quantities of cleaved, stable, recombinant Env glycoprotein for structural studies. The clade A strain, BG505, was selected from this screen and was shown by negative-stain EM to fold into compact, ordered trimers displaying three-fold symmetry (117). The resulting clade A BG505 SOSIP.664 was first in-class and catalysed the structural elucidation of the Env spike initially by negative-stain EM (67, 117), and then at high resolution by cryo-EM and X-ray crystallography (5, 63, 85). Subsequently, the SOSIP format has been applied to a range of trimers of different strains, such as for clade B B41 (115), and clade C DU422 and ZM197M (64); however, the SOSIP mutations are not sufficient to guarantee native-like trimeric structures for all strains and subtypes of HIV and careful antigenic and biophysical analysis is a necessary step in the validation of new oligomeric material. Other recombinant trimer platforms, in part derived from the SOSIP template, have since been developed (51, 52, 71, 127). For review of the history of SOSIP trimers and the current state, please see (118, 120, 142).
Glycoform Engineering
The high density of glycosylation across the Env spike poses special challenges for structural analysis. Glycosylation on proteins is notoriously chemically and conformationally heterogeneous. At best, this can limit the utility of crystallography, and to some extent cryo-EM, which rely on the averaging of signals from many thousands of molecules to reveal the consensus structure. At worst, X-ray crystallographic analysis can be completely precluded if the extensive and diverse glycosylation prevents crystallization or limits the diffraction. Both cryo-EM and crystallographic analysis of Env have been greatly enhanced by the availability of methods to control glycosylation processing. For example, the first structure of the gp120 core was achieved using an insect cell expression system which resulted in paucimannose-type (Man1-3GlcNAc2+/−Fuc) and oligomannose-type (Man5-9GlcNAc2, hereafter referred to simply as Man5-9) and structures that could be readily cleaved by the endoglycosidases Endo H and D (74, 147). Glycan cleavage has aided structural analysis of the core and a variety of other methods have been developed to trap glycans in a cleavable form using mammalian expression systems (23). However, given the importance of glycans within the epitope of bnAbs, it has been increasingly common to attempt to preserve much if not all of the glycan shield.
At one end of the scale, minimally glycosylated scaffolds have been developed that present some glycan epitopes. One example is the engineered outer domain region of gp120 displaying a mini-V3 loop (termed eODmV3) in complex with the Fab of PGT128 that binds glycans (103). As minimally glycosylated structures typically exhibit substantial chemical heterogeneity, the eODmV3 was expressed in 293S cells devoid of GlcNAc transferase I (GnT I) activity (112). This expression system stalls the processing of glycans resulting in oligomannose-type glycans dominated by Man-5. Interestingly, the complex that was crystallized was isolated by size-exclusion chromatography and the resulting structure revealed extensive density for larger Man-9 suggesting that this glycoform had been enriched during purification of the complex.
A similar strategy was employed to determine the structure of the PG16 epitope contained within a V1V2 scaffold construct (99). In this case, the small-molecule inhibitor, swainsonine, was used to impede glycan processing by Golgi α-mannosidase II to generate so-called hybrid-type glycans (99). These glycans have a shared Man-5 core but contain the glycan processing on one arm of the glycan and which can exhibit fucosylation at the GlcNAc proximal to the covalently linked asparagine residue on the protein surface. Similar to the PGT128 complex described above, the PG16 complex enriched for homogeneous glycans, in this case an α2,3-sialylated hybrid-type glycan (99).
At the other end of the scale, the ability to engineer glycans to be sensitive to endoglycosidase was exploited in the elucidation of the first crystal structure of soluble ectodomain of the trimeric Env spike, that of BG505 SOSIP.664 (63). Here, BG505 SOSIP.664 was expressed in GnTI-deficient 293S cells, complexed with antibody PGT122 that binds to a composite glycan and protein epitope, and subjected to glycan cleavage with EndoH, which cleaved those glycans not protected by antibodies in the complex or by the tertiary/quaternary structure. However, a substantial fraction of the glycans can be protected by the bound antibodies, particularly if two or more Fabs are used in the X-ray structure determination (142).
A combination of two Fabs, PGT122 and 35O22, which bound to either end of the trimer, were then found that were able to dominate the crystal lattice contacts in X-ray structures of SOSIP.664 trimers (100) and made it possible to solve structures of clade G X1193.c1 SOSIP.665 G459C, clade A BG505 SOSIP.664 T332N, and clade B strain JR-FL trimers with fully intact oligomannose-type glycoforms (134). These structures show extensive density for glycans, although many conserved glycans differ in their apparent conformations across the structures consistent with conformational flexibility of glycans on the Env trimer. The utility in using Fabs to dominate lattice contacts has been further demonstrated by a structure of a BG505 SOSIP.664 trimer expressed in 293F cells with native-like glycosylation, which not only revealed the epitope of a hitherto undisclosed CD4 binding site antibody but also did not require any deliberate glycan engineering (50). Depending on the fraction isolated from the size exclusion purification of the complex, certain glycoforms on the trimer appeared different, suggesting some heterogeneity within a single BG505 SOSIP trimer preparation. Here, the resulting electron density challenged a simplistic understanding of the structure of the glycan shield, not least of which was that the resulting glycan structures differed to those characterized by mass spectrometry of glycopeptides derived from the Env protein (7, 8). Gristick et al. provided appropriate caveats in their analysis (50), but the discrepancies suggest that either there is a substantial enrichment of particular SOSIP glycoforms during crystallization or that the interpretation of glycans not extensively stabilized by interactions with the protein surface can lead to incorrect inferences about the underlying glycan composition.
Because glycans are part of nearly every bnAb epitope, they also influence the choice of bnAb for purification by immune-affinity capture. For example, if a virus is not neutralized by 2G12 or PGT145 (110, 117), two of the most popular antibody reagents used for Env purification, the corresponding soluble SOSIP Env trimer made from that virus is unlikely to be able to be purified using those antibodies, unless their epitopes are engineered into the construct. PGT151 is another alternative purification reagent that is sensitive to the quaternary integrity of the Env trimers (115). Despite differences in these antigenic sites, the way in which one purifies an Env trimer is for the most part independent of the trimer purification strategy and much more dependent on the proper quaternary structure of Env (7, 21, 46, 109). While global trends appear to be consistent, site specific or minor differences may result from use of these different approaches.
X-ray Crystallography of Env
The structure of the HIV-1 Env trimer by X-ray crystallography took many years and studies were first initiated in 2002 with the advent of early versions of the JR-FL SOSIP trimer construct (9, 119). Crystals were obtained with KNH1144 SOSIP.681, but these diffracted to a very low resolution (63). Identification of BG505.664 (117) was the critical breakthrough for obtaining high resolution X-ray and EM structures (63, 85). The resolution of the crystal structure of this third generation BG505 SOSIP.664 Env trimer was 4.7 Å and exhibited anisotropy in the X-ray diffraction that limited the overall resolution (63). Glycan heterogeneity was also a major issue and deglycosylation of the Env trimer in the presence of bnAb PGT122 removed many of the sugars not protected by the bnAb or by quaternary or tertiary contacts in the protein. The overall strategy to determine the Env trimer structure by X-ray crystallography was to use different antibodies, singly or in combination, to enable different crystal packing and lattice formation (63). It was not until around 2008 that bnAbs to HIV-1 started to be discovered in increasing numbers by various groups, such as the Burton, Nussenzweig and Mascola labs (18, 39, 81, 87, 123, 124, 140). Identification of another bnAb, 35O22, in 2013 from Mark Connors lab led to an increase in the resolution of the Env trimer structure to 3.7 Å (100), when used in combination of PGT122. We have tried many combination of antibodies both past and present, but the PGT121- family members combined with 35O22 have proven optimal so far and enabled the resolution of the structures to gradually be increased to 3 Å for the clade A BG505 SOSIP trimers (43); thus, more complete models of the Env trimer have been obtained with greater definition of the structural features, including some well-defined glycans, particularly surrounding the bnAbs. Other constructs including NFL trimers have been crystallized and structures determined for a clade C trimer (52), as well as for clade B and clade G SOSIP trimers (134). For more details, please see (142).
Electron Microscopy of Env Trimers
Electron microscopy was critical in the development of the BG505.664 SOSIP platform as described above. Cryo-electron microscopy (cryo-EM) has, however, played an increasing role in high-resolution structure determination of HIV Env complexes with bNAbs and has emerged as a primary driver for investigating other viral antigens and complexes with antibodies, for example (68, 83, 97, 98). Unlike X-ray crystallography, cryo-EM has the advantage of being independent of crystallization and formation of lattice contacts. Thus, there is no inherent advantage in glycan engineering although analysis of heterogeneous glycosylation sites potentially poses problems in unambiguous structural interpretation. However, the most significant advantage is that the very complexity that can prevent protein crystallization can actually enhance single particle data analysis. Due to the requirement to computationally align and classify cryo-EM images of particles, the more features that the target particle possesses, the greater ability to identify, sort, and align particles with computational fidelity and, therefore, the better the resulting resolution. This approach has been particularly powerful in the HIV field where complexing a target with multiple Fabs can greatly facilitate structure determination of Env and analysis of the bnAb epitopes (78, 85).
Low resolution negative-stain EM is a powerful method for rapidly mapping epitopes of bnAbs, but the limited resolution (~15–25 Å) precludes an explicit atomic-level description. However, hybrid or integrative modelling (141), which can be accomplished by docking crystal structures of components of a given Env trimer-antibody complex into the EM reconstructions can enhance the molecular information that can be obtained. For example, this approach allowed description of the quaternary epitope of the apex targeting antibody, PG9, in complex with BG505 SOSIP.664, revealing that PG9 likely interacts with symmetry-related apex glycans on two protomers of the trimer (62). Modelling cannot however replace explicit high-resolution structure determination. In 2013, we solved the first high-resolution cryo-EM structure of the soluble HIV Env SOSIP.664 trimer (85). This structure was a milestone in the field and began to reveal atomic details of the intact Env trimer and its associated glycans. Nevertheless, the resolution was limited to ~5.8 Å, despite the use of a direct electron detector, which has helped revolutionize cryo-EM (80, 136, 137). The next big advance came when we combined a direct electron detector with the 300keV Titan Krios electron microscope, resulting in a ~4.2 Å resolution reconstruction and the first cryo-EM map with atomic level details of the natively glycosylated trimer (78). Subsequent improvements in sample preparation, data collection, and graphics processing units (GPU) enabled image processing algorithms where we can now achieve ~3.5 Å resolution datasets (95), with many parts of the cryo-EM maps of Env resolved to better than 3 Å and where the atomic features are uncovered in high detail.
Another advantage of cryo-EM is that single particle data can be submitted to rigorous 2D and 3D classification using programs such as Relion (125, 126) to separate out structural heterogeneity or trimers with substoichiometric occupancy of bound antibodies. Thus, a single data set of an Env-bnAb complex typically can be deconvoluted into multiple cryo-EM reconstructions that more completely and accurately portray the actual ensemble of complexes in solution. We used such an approach to show that the CD4-binding site (CD4bs)-directed bnAb PGV04 bound to BG505 SOSIP trimers with variable occupancy, with the average stoichiometry of two per trimer, but where 0–3 Fabs were bound (85). A solution-based approach, isothermal titration calorimetry (ITC), was also used to measure occupancy and resulted in a similar substoichiometric values. The clear advantage of cryo-EM is that the distributions of occupancies and conformational states can be resolved. In this particular case, deglycosylation of the Env trimer increased the stoichiometry, suggesting that glycan heterogeneity, most likely surrounding the CD4bs, led to the protomers with no Fab bound.
The glycans on Env comprise approximately 50% of the mass of the trimer and are by and large flexible and disordered in the absence of bnAbs that partially immobilize the glycans in their cognate epitopes. Hence, we typically observe only the first two sugar residues at any given PNGS in the absence of antibody interaction. The notable exception is PNGS at N262, which, in contrast to all of the other glycans that project off the trimer surface, lays flat against gp120 in a groove and is resolved to Man-5 or Man-6 (78). Further evidence for, and identification of, the more disordered glycans can be observed in the cryo-EM maps contoured at low levels or in low-pass filtered maps. Similar observations can sometimes be made in X-ray maps of glycoproteins contoured at lower resolution.
Resolved glycans that are part of bnAb epitopes are usually consistent with analogous X-ray structures, although most crystallographic structures are derived for Env trimers produced exclusively with high mannose glycans and treated with a glycosidase after antibody complex formation to remove more accessible glycans and promote crystallization (63, 72). Because of the aforementioned flexibility in the glycans, X-ray and cryo-EM maps of Env-antibody complexes are quite similar for the core GlcNAc and mannose moieties that are common to all glycan structures; the complex-specific sugar moieties, such as the core fucose, branching galactose or sialic acid, are rarely resolved in cryo-EM maps, although exceptions include visualization of bi-antennary to tetra-antennary structures (78). Finally, building and refinement of glycan structures into density maps, whether EM or X-ray, remains difficult and there is a great need for improved algorithms and automation (1, 26).
Cryo-EM has clear advantages over X-ray crystallography in the examination of membrane-bound and native viral spikes, where only very small amounts of material can be purified and the hydrophobic transmembrane region tends to be flexible and leads to micelle formation and aggregation (78). These studies have not only demonstrated that membrane-tethering has no measurable impact on the protein structure, but that the SOSIP mutations do not perturb the protein from its native pre-fusion state (78, 85). These recent findings added further structural validation to the use of soluble SOSIPs in vaccination strategies.
Overall, X-ray crystallographic and cryo-EM analyses of Env trimers that displayed engineered glycans to those where no glycan engineering was used has suggested that the protein structure is independent of glycan processing. Hence, the Env trimer can tolerate a significant degree of glycan heterogeneity.
Glycan and glycopeptide analytics
The prevalence of glycans within the epitopes of bnAbs on Env has motivated the examination of the precise chemical composition of the Env glycans. Central to any vaccination strategy that aims to elicit glycan-targeting antibodies is that the recombinant immunogen displays glycans that sufficiently resemble their individual processed states on the native virion so that any resulting anti-glycan antibody response will cross-react with and neutralize the virus. It is an established tenet of glycobiology that the fine processing of complex-type glycosylation is heavily influenced by the producer cell type and prevailing physiological conditions (111, 116). However, it is extremely challenging to isolate native virions from infected individuals for glycan and glycopeptide analysis. For this reason, analysis was initially restricted to recombinant monomeric gp120 (30, 48, 79, 96, 152), before eventually proceeding to trimeric native-like SOSIPs (7, 8, 53), membrane-tethered Env trimers (47), and viruses produced in different cell systems (13, 36, 101, 106). The antigenic cross-reactivity between the leading recombinant trimers and glycan-binding bnAbs indicates that the identity of glycans on recombinant immunogens can be used to infer the glycan structure on the native virus. While the cross-reactivity does not formally identify the native glycan target, it does, however, demonstrate that recombinant immunogens bearing such glycans could conceivably elicit similar cross-reactive anti-glycan antibodies.
Two main approaches have been used in understanding the composition of the glycan shield of immunogens and virus: analysis of released glycans and analysis of glycopeptides. The latter approach provides site-specific information and is necessary to reveal the chemical conformation of precise antibody epitopes. However, much information can be garnered from the initial evaluation of a pool of enzymatically released glycans. Analysis of glycans from gel bands of pseudo-virions and peripheral blood mononuclear cell (PBMC)-derived virions showed that the virus is dominated by oligomannose-type glycans (13, 36, 106) and provided an important benchmark for evaluation of candidate immunogens.
Mass spectrometry can be influenced by glycan charge and is not necessarily quantitative. In contrast, ultra- and high-performance liquid chromatography (UPLC and HPLC) of fluorescently labelled glycans enable the unbiased profiling of glycans (34). In addition, the abundance of oligomannose-type glycans (or other features) can be readily assessed by comparing the parent glycan pool with that of glycans digested, in this case, with oligomannose-specific endoglycosidases, such as Endo H. These methods have been used to demonstrate that SOSIP.664 format has significantly higher content of oligomannose-type glycans compared to other oligomeric Env formats, such as foldons or uncleaved trimers (115).
The analysis of glycopeptides requires the use of mass spectrometry. However, the methodology can yield results that contradict with what is known of glycan structures from structural and antigenic information. When these discrepancies arise, it is likely due to lack of quantitative ionization and the over-representation of minor species. While differential ionization can be one source of error, the principles adopted by the data analysis software can also impede quantitative analysis. For example, many software packages utilize ion counts rather than ion intensities in the evaluation of ion abundance. In the site-specific analysis of BG505 SOSIP.664, the in-line LC-MS analysis that we implemented was benchmarked to the MS of isolated glycopeptide fractions (8). Overall, the analyses were in close agreement from the two approaches in the analysis of glycosylation sites that contained exclusively oligomannose-type glycans, those with almost exclusively complex-type glycans, and those with a range of structures (termed ‘mixed’ sites). The ability to quantify the abundance of glycans has led to the assessment of the impact of different protein architectures on glycan processing, such as the impact of trimerization on glycosylation of gp120, or the impact of lack of furin-cleavage on the maturation of the glycan shield (7, 115). Non-quantitative analysis of glycopeptides has also found utility in capturing the major species present on virion-derived material. Panico et al. also showed that virions have comparable glycan compositions at all sites on gp120 with the exception of the membrane-proximal N88 position (101).
While glycopeptide analysis offers a great level of detail regarding the precise glycans displayed at individual sites on an immunogen or virus, there are some advantages afforded by the simplification of the material to reveal basic classes of glycosylation status. For example, Cao et al. used protein N-glycosidase and Endo H to determine the relative abundance of non-glycosylated peptide, peptide displaying oligomannose/hybrid-type glycans, and the percentage of glycan displaying Endo H-resistant complex-type glycans (21). This method is sensitive and more reliably achieves complete coverage in comparison to the analysis of the parent glycopeptides. Reassuringly, there is close agreement between the validated glycopeptide analysis of Behrens et al. (8) and that of the deglycosylation approach of Cao et al. (21) (Figure 1).
Figure 1.
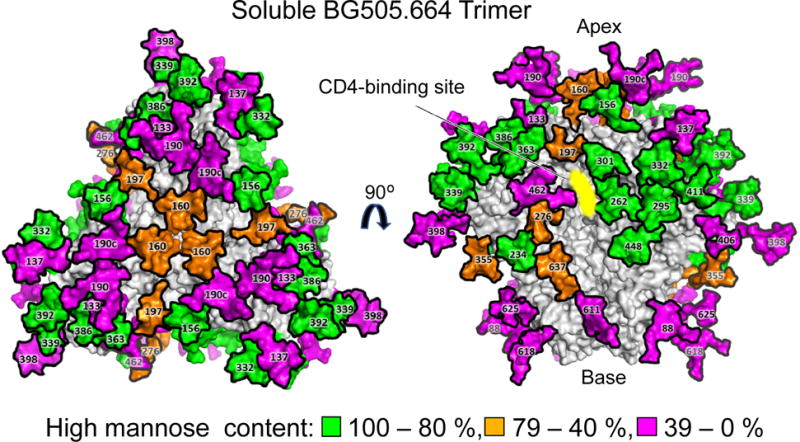
Model of the processing status of individual N-linked glycosylation sites on the BG505 SOSIP.664 trimer. The model is adapted from Behrens et al. (7, 8) and is constructed from the cryo-EM structure of glycosylated SOSIP (78) with terminal glycans modelled in that are not apparent in the cryo-EM maps. The protein surface is depicted in gray and the surface of the glycans are coored according to the percentage of high-mannose gycans.
Glycan Binding Analyses of Anti-HIV bnAbs
In another important development, glycan arrays have been particularly useful in assessing the glycan binding properties of bnAbs. The current arrays have a large assortment of glycans that can be interrogated for binding to receptors and other proteins (45). Several arrays are available and the one that is most commonly used was developed by Jim Paulson, Chi-Huey Wong and others (12, 15) and made generally available through the the Consortium for Functional Glycomics. The most recent versions contains extended airway glycans that include extended poly-N-acetyl-lactosamine (poly-LacNAc) chains (104) and other presentations for high- throughput analysis (22). Other glycan arrays such as the neoglycolipid array (42, 75) and aluminum oxide-coated glass slide array (22) have also been developed. When the glycan is a major component of the bnAb epitope, then binding can be seen with the bnAb on the array. On the other hand, even though glycans are typically part of the bnAb epitopes, they are sometimes not readily visualized on the glycan array if their binding is weak or requires glycan clustering (76), or if the epitope intimately involves the glycan-protein interface (66, 72). One of the first examples in the HIV field was that of antibody 2G12 that binds exclusively to high mannose glycans (12, 19, 121). The next example was for PGT128 that also binds high mannose sugars (103). Recognition of more complex glycans has also been observed for antibodies, such as PG9/PG16 at the Env trimer apex (78, 90, 99) and PGT151 (11, 40, 78, 90, 99), which binds lower down the trimer where the glycans are less dense and can be processed more readily to completeness to complex-type sugars. Indeed, branched bi- to tetra-antennary glycans have been visualized by cryo-EM on JR-FL trimers (78).
Biosynthesis of the Glycan Shield
Env Structure
In mammals, both the humoral and cellular innate immune systems can readily recognise and be activated by exposed oligomannose-type glycans (49). The conversion in the Golgi apparatus of oligomannose- to complex-type glycans on self glycoproteins is consequently highly efficient. Only rare examples have been documented of secreted or cell-surface self glycoproteins presenting oligomannose-type glycans (2, 27). These typically occur in structurally hindered sites or in some disease pathologies where the integrity of the secretory system may have been compromised (84). In contrast to self glycoproteins, analysis of the glycans of recombinant gp120 identified a minor population of oligomannose structures (92). Extensive analysis has repeatedly confirmed such a population on a variety of recombinant gp120 monomers (7, 30, 48, 79, 96, 107, 152) and the oligomannose-type glycans cluster within a heavily glycosylated region on the outer domain, which has been coined the ‘intrinsic mannose patch’ (IMP) (6, 13, 25, 36). The abundance of oligomannose-type glycans in the IMP is less than observed on virions-derived Env and has led to the hypothesis of an additional ‘trimer-associated mannose patch’ (TAMP) (6, 13, 25, 36).
In support of a TAMP, profiling of released glycans from a panel of oligomeric immunogens has demonstrated that only those trimers with a compact structure displaying three-fold symmetry along the trimer axis exhibit elevated level of oligomannose-type glycans (7, 115). This finding established that quaternary structural constraints are important in driving the formation of the native mannose patch on Env. At one extreme, BG505 SOSIP.664 displays ~65% oligomannose-type glycans, whereas the CZA97 foldon trimer has ~30% suggesting little or no quaternary constraints on glycan processing are imposed by foldon-induced oligomerization (115). This notion is consistent with the EM analysis that suggests the abundance of native-like material in foldon trimers is negligible (115). Despite these convincing observations, a contradictory cryo-EM analysis has been reported that has been used to argue that the CZA97 foldon adopts a native-like fold (82). The conflict is likely to be a result of the imposition of three-fold symmetry in the data processing that is not reflective of the foldon trimer population and may also explain why high-resolution reconstructions could not be achieved (82). In one controlled experiment to shed further light on this issue, quantitative site-specific analysis was performed on BG505 monomer, uncleaved BG505 oligomer and BG505 SOSIP.664 (7). This analysis revealed the conservation of the IMP across the different formats indicative of similar processing of glycans, but showed that only the BG505 SOSIP.664 trimers displayed significantly elevated content of oligomannose-type glycans and a defined TAMP along the protomer interface (7).
Glycan analysis therefore has a considerable role in complementing cryo-EM and X-ray crystallography in the evaluation of candidate trimeric immunogens. High content of oligomannose-type glycans together with observed ordered compact structures is the hallmark of correctly folded native-like Env material. This complementary approach has been used to assess a growing number of candidate immunogens and to assess the impact of further protein engineering strategies (31, 103, 115, 134, 135, 150).
The conservation of the mannose patch has important implications in immunogen design. Not only does evaluation of the glycosylation enable triaging of immunogen candidates, but the conservation of the mannose patch suggests that it is largely insensitive to mutation that effect the frequency and distribution of glycans (24). So why is the mannose patch so resilient to changes in glycosylation position and frequency? The answer lies in the extent of the substrate recognition interface of the ER and Golgi α-mannosidases (6, 107). The crystal structure of a deactivated ER α-mannosidase I in complex with its oligomannose substrate was docked onto a structure of Env containing the N332 glycan, which resides at the centre of the IMP. The enzyme in the resulting model of the complex completely enveloped three neighboring glycans (N295, N137 and N411) and supports the simple model that enzyme inaccessibility prevents canonical glycan maturation (6). Importantly, the extent and redundancy of these clashes suggests that mutation would unlikely impact the processing of neighboring glycan structures. This model is supported by glycan profiling and selected site-specific analysis of gp120 monomers containing glycan site mutations (107). Finally, enzymatically inaccessible glycans have been revealed in an in vitro model of gp120 maturation where gp120 with engineered Man9GlcNAc2 glycoforms was incubated with ER α-mannosidase I (36).
Overall, we can conclude that mimicry in protein structure between recombinant trimers and functional Env spikes similarly drives mimicry between their glycan shields (115). But whereas this may be true for glycosylation sites presenting sterically protected oligomannose-type structures, does this hold for those sites displaying complex-type glycans? The enzymatic answer to this question has two hierarchies: one at the level of branching (for example bi-antennary versus tri-antennary glycans) and the other in the types and abundance of terminal structures (such as sialylation, fucosylation, or even polylactosamine extensions). Analysis of PBMC-derived Env by ion-mobility MS and HPLC suggests that, while the content of oligomannose-type glycans is similar to that observed in SOSIP.664 formats, the processed glycans diverge in their degree of processing (106). In particular, the abundance of sialylated structures was notable and also dominated by α2,6-linkages rather than α2,3-linkages typically observed in human embryonic kidney (HEK) 293T/F or Chinese hamster ovary (CHO) expression systems (106). Importantly, recombinant SOSIP.664 has negligible polylactosamine structures that are characteristic of macrophage-derived Env (145). The significant structural divergence that results from polylactosamine extensions underscores the conclusion that recombinant trimers may only reliably recapitulate the mannose patch of native trimers and that the cell-dependent variation in complex-type structures may limit the utility of targeting epitopes containing complex-type glycans. This concern is underscored by the reduced sensitivity to antibody-mediated neutralization of isogenic virus derived from macrophages compared to PBMCs (145). Nevertheless, bnAbs by definition are able to target multiple strains and subtypes and also can also by and large cope with glycan heterogeneity. For the bnAbs with the greatest breadth, the likely explanation is that the components of the glycans that are recognized are those that are common in all glycoforms, namely the GlcNAc-mannose core. In several cases, this is borne out by interactions seen with these core sugars with bnAbs in cryo-EM and X-ray structures (44, 50, 61, 72, 73, 76, 77, 134, 142).
In other cases, the limitation in dealing with glycan heterogeneity is underscored by the observation of neutralization plateaus in which increasing bnAb concentration fails to completely neutralize a virus (88). This finding has been interpreted as evidence that glycan heterogeneity can lead to a population of resistant viruses. In support of this notion, these neutralization plateaus can often be influenced by glycan engineering of the target virus. For example, even antibodies targeting the IMP can display incomplete neutralization plateaus (88). Virions displaying Man-9, in the presence of kifunensine, are rendered resistant to PGT135 neutralization (88). In addition, binding of PGT135 is substantially impaired by engineering all of the glycoforms of gp120 to Man-9 (108). Thus, in a vaccination setting whether for natural viruses or for designed immunogens, subtle heterogeneity of a glycan target can affect the potency of certain antibody lineages to particular epitopes.
In the future, designed Env immunogens may be explored bearing a greater range of complex-type structures. This will entail obtaining a much more detailed understanding of the fine glycan structures presented by virions in a native infectious setting coupled with concomitant advances in the expression of Env immunogens displaying bespoke glycoforms.
Structural Biology of the Glycan Shield
BnAbs interact with glycans in a variety of ways, from core N-acetylglucosamines to the extended branches of high mannose or complex glycoforms (Figure 2). The N332 glycan, for which there is the most structural information, is typically a Man-8 or Man-9 that is completely resolved in several structures with bound bnAbs (e.g. PGT121, PGT124, PGT128, 10-1074) (43, 44, 50, 77, 103, 134). While there is some flexibility within this glycan, the structures demonstrate its relative rigidity, as it is located at the center of the densely glycosylated mannose patch on the outer domain of gp120 (Figure 3). In fact, it is so densely glycosylated that sugar processing enzymes are sterically excluded from accessing these glycans (6, 107), thereby endowing its restricted character to a high mannose patch. This patch is conserved across multiple HIV Env subtypes (13, 46), is a function of the trimeric quaternary structure of Env (7, 115), and independent of the purification method as long as native-like, prefusion Env constructs are expressed (21, 115).
Figure 2.
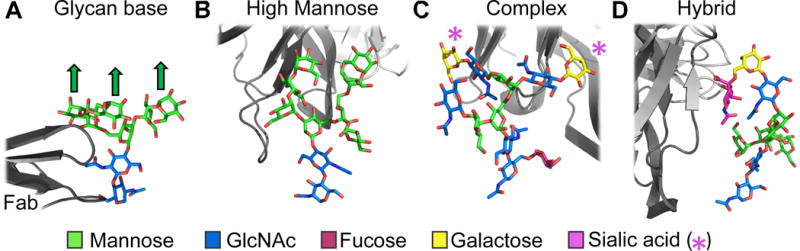
Examples of bnAb recognition of glycans. A. The light chain (dark gray) of 3BNC117 (PDB 5V8L), a CD4bs-directed bnAb, interacts primarily with the core GlcNAc moieties (blue) at the base of the glycan and less with the branched Man glycans (green). Note that the glycans are only resolved to Man-6 with the terminal branching glycans (indicated by arrows) disordered in the cryoEM map. B. The heavy chain (dark gray) of PGT128 (PDB 5ACO), an N332 supersite bnAb, interacts with extended Man-8 (shown) or Man-9 glycans. Because these glycan moieties interact strongly with the bnAb heavy (dark gray) and light (light gray) chains, they are typically well resolved in crystallographic or cryoEM maps. In fact, PGT128 binds high mannose so well that the structure of the Fab and Man-9 alone was solved by crystallography (PDB 3TV3) (103). Man-8 is resolved in the figure shown. C. The heavy chain (dark gray) of PGT151 (PDB 5FUU), a gp120/gp41 interface bnAb interacts with minimally a tri-antennary complex glycan. Glycan array binding data suggest that this N611 glycan may be contain terminal sialic acid residues (indicated by magenta *), but these were not resolved in the cryo-EM map. D. The heavy chain (dark gray) of PG16 (PDB 4DQO) interacts with a terminal sialic acid of a hybrid-type glycan at N173.
Figure 3.
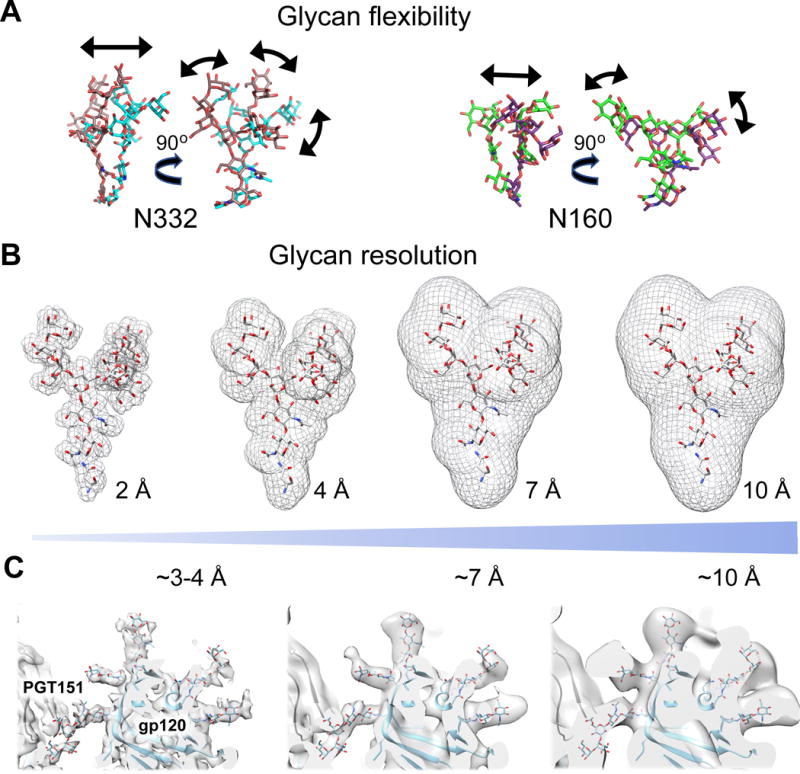
Glycan flexibility and resolution. A. Glycans exhibit differing degrees of flexibility wherein the base is constrained and the two faces of the glycan remain, whereas the branched glycans have additional degrees of freedom. Shown are example conformations for Man-5 (purple, PG9 bound; PDB 3U4E) or Man-6 (green, PGT145 bound; PDB 5V8L) of N160 and Man-8 (brown, PGT128 bound; PDB 5ACO) and Man-8 (cyan, 10-1074 bound; PDB 5T3X) of N332. B. Ideal density (mesh) for a Man-9 (white sticks) glycan filtered to resolutions ranging from 2-20 Å. C. Cryo-EM density map (EMD-3308) filtered to different resolutions highlighting several gp120 glycans. At the lowest resolution (3–4 Å), only the first 2–3 glycan moieties are observed, due to conformational variability in the extended sugars. In the case of the glycan at N448, which interacts with the bnAb PGT151, extended branching is observed. As the cryo-EM density map is filtered to progressively higher resolutions, more of the glycan density is observed and the map captures some of the conformational heterogeneity. Glycosylation sites that contain a heterogeneous population of glycoforms (as confirmed by MS) can further blur the resolution. Even at the highest filtered resolution cryo-EM maps (10 Å), the full extent of glycans is not resolved to the same extent as the idealized density shown in Panel B. This supports a high degree of conformational flexibility of the branched portions of glycans.
N332 Examples
The prototypic example of binding to high mannose glycan at N332 is PGT128 where almost all of the sugars in the Man-9 glycan interact with the antibody. In crystal structures of Fabs PGT 127 and 128, a bound Man-9 glycan could be resolved to ultra-high resolution of 1.65 and 1.29 Å, respectively (103) and indicated that the glycan bound in it low energy conformation with associated stabilizing water molecules between the sugar moieties of the glycan. A complex structure with an engineered outer domain of gp120 (eOD) further illustrated that PGT128 bound not only to the glycan at N332 but also at N301, but in this case only to the core sugars (Figure 4A). Only a comparatively small linear region of the protein at the base of the V3 loop (Ile323-Arg327) interacted specifically with the Fab. Other antibody crystal structures where the V3 loop was observed include PGT135 (72), PGT121 (63), 10-1074 (50) and PGT124 (44), the latter two recognizing only a single glycan at N332 compared to the other antibodies that interact with two or more glycans. The N332 glycan has also been observed in EM reconstructions with N332-targeting bnAb PGT128 (77).
Figure 4.
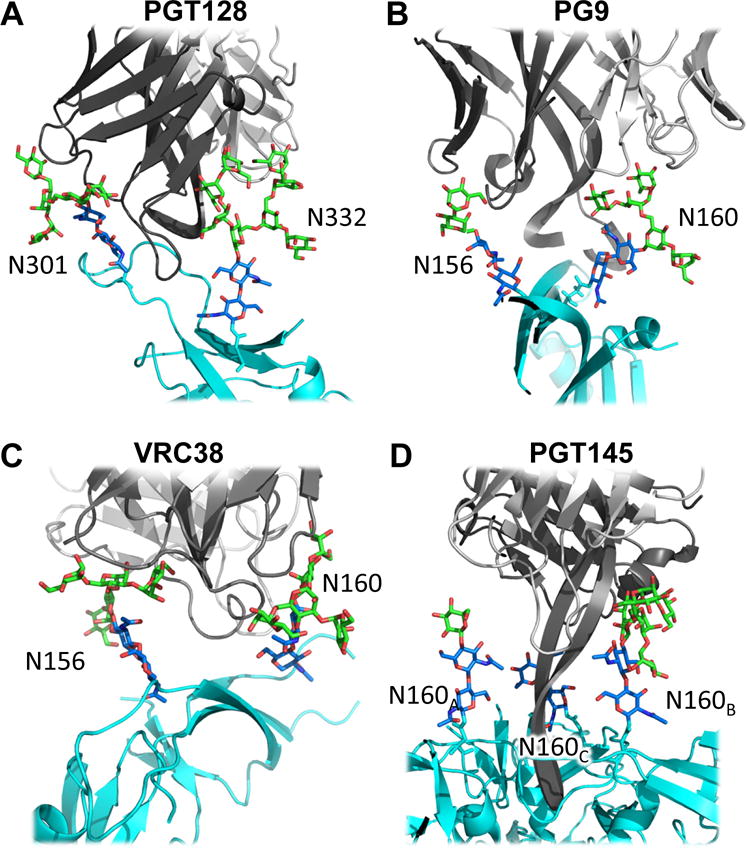
Penetrating the glycan shield. Early structures of bnAbs bound to small portions of gp120 revealed how bnAbs deployed long heavy chain CDR3 loops to insert between glycans and access the underlying peptide surface of Env. A. In PGT128, an N332 supersite bnAb, CDRH3 is sandwiched between glycans at N301 and N332. B. In PG9, an apex bnAb, CDRH3 is sandwiched between N156 and N160. C. VRC38 also targets N156 and N160 Man-5 glycans (20). D. PGT145, another apex bnAb inserts its very long CDRH3 between three symmetrically related N160 glycans at the three-fold axis. In all cases, one glycan more intimately interacts with the bnAb and is more fully resolved in the structures.
N160 Examples
The trimer apex is characterized by the conserved N160 and N156 (or N173) glycans that project upward and are critical components of bnAbs such as PG9/PG16, PGT145/PGMD1400, CAP256.VRC26, and others. Early crystal structures of PG9 and PG16 in complex with a small, scaffolded piece of the Env apex V1/V2 revealed glycans Man-4/N156 and Man-5/N160, and Man-5/N160 and sialated hybrid-N156, respectively (Figure 4B) (90, 99). The structure of VRC38, another apex-targeting bnAb, in complex with the same scaffold but expressed in 293S GnTI-deficient cell line, similar to the PG9 structures, revealed yet another view of the N156 and N160 Man-5 glycans (Figure 4C) (20). The recent cryo-EM structure of BG505 SOSIP, expressed in 293F cells, in complex with PGT145 interpreted Man-6 at the glycan at N160 (76). The long, anionic, beta-hairpin of PGT145 heavy chain complementarity determining region-3 (CDR3) penetrates through the N160 glycan triad, interacting primarily with one N160 glycan (Figure 4D). These examples illustrate the exquisite subtleties of our present understanding of glycans, and how the binding of a bnAb can help resolve glycans and/or induce a conformational difference in their orientation (71, 72).
Complex Glycans in gp41
N611 and N637 are modified tri- or tetra-antennary complex glycans with core fucosylation. In the JR-FL deltaCT complex with PGT151, there are extensive interactions with the terminal branches of the glycans, as well as core sugar moieties including the fucose attached to the first GlcNAc sugar (Figure 3). The diversity/heterogeneity and complexity of these interactions may be responsible for the lower breadth achieved by these bnAbs and the plateaus observed in incomplete neutralization curves. Hence, bnAbs evolve to accommodate glycans that include engaging the conserved base of the glycans (Man-3) and negotiating the more flexible and heterogeneous branches (Figures 2 and 3). After extensive somatic hypermutation, many bnAbs become dependent on glycans for binding, and removal of the glycans can result in increased off-rates and weaker binding affinity (e.g. 3BC315, 3BNC117) (70, 76). Others, like the PGT151 family or N332 bnAbs are obligate glycan binders with little to no affinity or ability to neutralize in the absence of certain glycans (40). While there are trends across subtypes, glycan dependence does not hold across all subtypes and there are examples where glycans can be removed or a proximal glycan can act as a surrogate (e.g. PGT128) (77, 129).
CD4 Binding Site Glycans
The receptor CD4bs glycans are clear impediments to binding the Env trimer. The CD4 receptor itself contains an immunoglobulin fold and resembles the heavy chain of an antibody. Many CD4bs bnAbs therefore use receptor mimicry for recognition. However, the presence of a light chain creates an additional steric hurdle and severely restricts the permissible angle of approach and binding to the CD4bs (28, 58, 63, 85, 130, 151). Efforts to circumvent these hurdles and elicit CD4bs bnAbs include minimal epitope presentation or outright removal of CD4bs glycans (14, 29, 58, 69, 144). While several glycans act as steric barriers, N276 is the most critical. The N276 glycan interacts with the light chain of VRC01-class bnAbs, and requires an unusually short (5aa) CDR3 to accommodate the glycan (60). Most VRC01-class bnAbs become dependent on this glycan and deletion results in loss of affinity and neutralization (41). However, elicitation of the germlines (GL) of VRC01-like antibodies requires removal of the N276 from GL trimers (91) or on epitope-scaffolded proteins such as eOD6/8 (58, 59). Immunization then requires adding back of these deleted glycans to enable evolution of the elicited GL antibodies to achieve recognition of native virions with their full constellation of glycans (59, 131).
Glycans are truly steric barriers that normally silence or suppress immune responses to Env. This was beautifully shown in rabbit vaccination experiments using the BG505.664 SOSIP trimer. The primary neutralizing responses to these trimers were to a rare “glycan hole” on the Env trimer (29, 69, 89). The exposed, peptidic trimer base, a neo-epitope on the soluble SOSIP trimers, was also preferentially targeted by non-neutralizing antibodies (56). These studies highlight the preference of the adaptive immune response to protein epitopes over glycan-containing epitopes, and the hurdles inherent in HIV vaccine design. Strategies to knock-out particular glycans in the priming immunogen and reintroduce them in subsequent boosting immunogens is an active area of research to help overcome these challenges.
Conclusions and Perspectives
Substantial progress has been made recently in characterizing the extensive glycan shield of HIV (8, 21). It was thought for many years that this glycan coat provided an impenetrable barrier to the immune system. However, the density of the N-linked glycosylation on the HIV trimer far exceeds anything on human proteins and furthermore limits the heterogeneity of the glycans on HIV. Because of the exceedingly high density, many of the glycans are stalled as incompletely processed high mannose sugars that are relatively homogeneous on the Env surface. As a result, the glycans in the more exposed gp120 domain are relatively conserved particularly in the IMP. Although these self glycans should be relatively immunosilent, and indeed can be used to diminish immunodominant epitopes (113), their overwhelming density and their proximity to the viral protein components has rendered them as targets for the immune system. Although immune responses against heavily glycosylated proteins, such as HIV, are relatively weak, over time antibodies can develop that recognize both the sugar and peptide components of the glycoprotein. Indeed, almost all of the bnAbs to HIV contact or attempt to circumnavigate these glycans (72) and they become integral parts of the bnAb epitopes. These glycans have aided in elucidation of the Env structures as complexes with bnAbs that recognize these glycans were instrumental for determination of the initial X-ray and EM structures for crystal lattice contacts and for adding mass and features for cryo-EM particle alignment and resolution (63, 85). Such approaches have been extensively used since then to decipher the wide range of broadly neutralizing epitopes on the viral surface. Characterization of these glycans has proved challenging not only in the context of the Env protein structure, but also in analytically dissecting the composition of the glycans at each glycosylation site in the protein. Methods have now advanced such that a whole new chapter has been written recently in analysis of the HIV glycan shield (7, 8, 21). The question of whether the soluble SOSIP proteins express the same glycoforms as the membrane-bound version has also recently been addressed (46, 106), as well as the more challenging question of what glycoforms are expressed on Env on the virus in different cell lines and, in particular, in CD4+ T cells (106). Some subtle differences do seem to arise in Env glycosylation (106), but it is not known whether such variation will be critical for eliciting broadly neutralizing responses against soluble immunogens or whether some membrane-bound construct will ultimately be required. Notwithstanding, bnAbs isolated from HIV-infected individuals bind strongly to the soluble Env constructs, which can be explained in part by antibody recognition of the core component of the glycans that is essentially identical for high mannose, hybrid and complex glycans. Some differences do arise such as the fucosylation on the first N-acetylglucosamine in complex glycans, and the heterogeneity of the complex glycans themselves can play a role in not achieving 100% neutralization, where a plateauing effect in the neutralization assays is observed with certain bnAbs and certain viruses (88, 108).
However, when we look back and consider how difficult and challenging it was to gain even limited information on the glycan shield, we have come a very long way in the last few years. Progress is now very rapid and more and more Env constructs are being analysed to look for similarities and differences in glycosylation in different strains and subtypes and for comparison with the same Env proteins on the virus itself. The glycan shield is still a barrier to overcome for natural infection and for vaccination. Vaccine trials in animal models have already illustrated that small breaches in the glycan shield can be exploited by the immune system (29, 69, 89, 144), but these holes tend to be viral isolate specific and do not give rise to neutralization breadth. Filling these glycan holes as well as creating new holes for engagement of desired germline antibodies is currently being explored (29, 69, 89, 144). Without question, the quality and quantity of information amassed in the last few years on the glycan shield through use of a variety of biophysical methods has been quite astounding. We now have to harness that ever increasing compendium of structural and functional knowledge on the HIV Env glycoprotein to aid in design of a truly effective HIV vaccine.
Acknowledgments
The authors are supported by the Scripps CHAVI-ID (1UM1 AI100663), the International AIDS Vaccine Initiative Neutralizing Antibody Consortium (IAVI NAC) through the Collaboration for AIDS Vaccine Discovery (CAVD), CAVD grant OPP1084519. ABW is also supported by CAVD grant OPP1115782 and M.C. by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 681137.
Contributor Information
Max Crispin, Email: max.crispin@soton.ac.uk.
Andrew B. Ward, Email: andrew@scripps.edu.
Ian A. Wilson, Email: wilson@scripps.edu.
Literature Cited
- 1.Agirre J, Davies GJ, Wilson KS, Cowtan KD. Carbohydrate structure: the rocky road to automation. Curr Opin Struct Biol. 2017;44:39–47. doi: 10.1016/j.sbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Arnold JN, Radcliffe CM, Wormald MR, Royle L, Harvey DJ, et al. The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J Immunol. 2004;173:6831–40. doi: 10.4049/jimmunol.173.11.6831. [DOI] [PubMed] [Google Scholar]
- 3.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 4.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartesaghi A, Merk A, Borgnia MJ, Milne JL, Subramaniam S. Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat Struct Mol Biol. 2013;20:1352–7. doi: 10.1038/nsmb.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens AJ, Crispin M. Structural principles controlling HIV envelope glycosylation. Curr Opin Struct Biol. 2017;44:125–33. doi: 10.1016/j.sbi.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens AJ, Harvey DJ, Milne E, Cupo A, Kumar A, et al. Molecular architecture of the cleavage-dependent mannose patch on a soluble HIV-1 envelope glycoprotein trimer. J Virol. 2017;91:e01894–16. doi: 10.1128/JVI.01894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behrens AJ, Vasiljevic S, Pritchard LK, Harvey DJ, Andev RS, et al. Composition and antigenic effects of individual glycan sites of a trimeric HIV-1 envelope glycoprotein. Cell Rep. 2016;14:2695–706. doi: 10.1016/j.celrep.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binley JM, Sanders RW, Clas B, Schuelke N, Master A, et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–43. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binley JM, Sanders RW, Master A, Cayanan CS, Wiley CL, et al. Enhancing the proteolytic maturation of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2002;76:2606–16. doi: 10.1128/JVI.76.6.2606-2616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity. 2014;40:669–80. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–8. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek Ra, et al. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS ONE. 2011;6:e23521. doi: 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briney B, Sok D, Jardine JG, Kulp DW, Skog P, et al. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell. 2016;166:1459–70.e11. doi: 10.1016/j.cell.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryan MC, Fazio F, Lee HK, Huang CY, Chang A, et al. Covalent display of oligosaccharide arrays in microtiter plates. J Am Chem Soc. 2004;126:8640–1. doi: 10.1021/ja048433f. [DOI] [PubMed] [Google Scholar]
- 16.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol. 2016;34:635–59. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–6. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 20.Cale EM, Gorman J, Radakovich NA, Crooks ET, Osawa K, et al. Virus-like particles identify an HIV V1V2 apex-binding neutralizing antibody that lacks a protruding loop. Immunity. 2017;46:777–91. doi: 10.1016/j.immuni.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao L, Diedrich JK, Kulp DW, Pauthner M, He L, et al. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat Commun. 2017;8:14954. doi: 10.1038/ncomms14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SH, Han JL, Tseng SY, Lee HY, Lin CW, et al. Glycan array on aluminum oxide-coated glass slides through phosphonate chemistry. J Am Chem Soc. 2010;132:13371–80. doi: 10.1021/ja1046523. [DOI] [PubMed] [Google Scholar]
- 23.Chang VT, Crispin M, Aricescu AR, Harvey DJ, Nettleship JE, et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure. 2007;15:267–73. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coss KP, Vasiljevic S, Pritchard LK, Krumm SA, Glaze M, et al. HIV-1 glycan density drives the persistence of the mannose patch within an infected individual. J Virol. 2016;90:11132–44. doi: 10.1128/JVI.01542-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispin M, Doores KJ. Targeting host-derived glycans on enveloped viruses for antibody-based vaccine design. Curr Opin Virol. 2015;11:63–69. doi: 10.1016/j.coviro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crispin M, Stuart DI, Jones EY. Building meaningful models of glycoproteins. Nat Struct Mol Biol. 2007;14:354. doi: 10.1038/nsmb0507-354a. discussion 54-5. [DOI] [PubMed] [Google Scholar]
- 27.Crispin MD, Ritchie GE, Critchley AJ, Morgan BP, Wilson IA, et al. Monoglucosylated glycans in the secreted human complement component C3: implications for protein biosynthesis and structure. FEBS Lett. 2004;566:270–4. doi: 10.1016/j.febslet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Crooks ET, Osawa K, Tong T, Grimley SL, Dai YD, et al. Effects of partially dismantling the CD4 binding site glycan fence of HIV-1 Envelope glycoprotein trimers on neutralizing antibody induction. Virology. 2017;505:193–209. doi: 10.1016/j.virol.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, et al. Vaccine-elicited tier 2 HIV-1 neutralizing antibodies bind to quaternary epitopes involving glycan-deficient patches proximal to the CD4 binding site. PLoS Pathog. 2015;11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutalo JM, Deterding LJ, Tomer KB. Characterization of glycopeptides from HIV-I(SF2) gp120 by liquid chromatography mass spectrometry. J Am Soc Mass Spectrom. 2004;15:1545–55. doi: 10.1016/j.jasms.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, et al. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell. 2015;163:1702–15. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decroly E, Vandenbranden M, Ruysschaert JM, Cogniaux J, Jacob GS, et al. The convertases furin and PC1 can both cleave the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp160 into gp120 (HIV-1 SU) and gp41 (HIV-I TM) J Biol Chem. 1994;269:12240–7. [PubMed] [Google Scholar]
- 33.Dimitrov DS. Therapeutic antibodies, vaccines and antibodyomes. mAbs. 2010;2:347–56. doi: 10.4161/mabs.2.3.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domann PJ, Pardos-Pardos AC, Fernandes DL, Spencer DI, Radcliffe CM, et al. Separation-based glycoprofiling approaches using fluorescent labels. Proteomics. 2007;7(Suppl 1):70–6. doi: 10.1002/pmic.200700640. [DOI] [PubMed] [Google Scholar]
- 35.Doores KJ. The HIV glycan shield as a target for broadly neutralizing antibodies. FEBS J. 2015;282:4679–91. doi: 10.1111/febs.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, et al. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107:13800–5. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emini EA, Schleif WA, Nunberg JH, Conley AJ, Eda Y, et al. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–30. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 39.Escolano A, Dosenovic P, Nussenzweig MC. Progress toward active or passive HIV-1 vaccination. J Exp Med. 2017;214:3–16. doi: 10.1084/jem.20161765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, et al. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40:657–68. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freund NT, Horwitz JA, Nogueira L, Sievers SA, Scharf L, et al. A New glycan-dependent CD4-binding site neutralizing antibody exerts pressure on HIV-1 in vivo. PLoS Pathog. 2015;11:e1005238. doi: 10.1371/journal.ppat.1005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol. 2002;20:1011–7. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- 43.Garces F, Lee JH, de Val N, de la Pena AT, Kong L, et al. Affinity maturation of a potent family of HIV antibodies is primarily focused on accommodating or avoiding glycans. Immunity. 2015;43:1053–63. doi: 10.1016/j.immuni.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garces F, Sok D, Kong L, McBride R, Kim HJ, et al. Structural evolution of glycan recognition by a family of potent HIV antibodies. Cell. 2014;159:69–79. doi: 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geissner A, Seeberger PH. Glycan arrays: From basic biochemical research to bioanalytical and biomedical applications. Annu Rev Anal Chem. 2016;9:223–47. doi: 10.1146/annurev-anchem-071015-041641. [DOI] [PubMed] [Google Scholar]
- 46.Go EP, Ding H, Zhang S, Ringe RP, Nicely N, et al. Glycosylation benchmark profile for HIV-1 envelope glycoprotein production based on eleven Env trimers. J Virol. 2017;91:e02428–16. doi: 10.1128/JVI.02428-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Go EP, Herschhorn A, Gu C, Castillo-Menendez L, Zhang S, et al. Comparative analysis of the glycosylation profiles of membrane-anchored HIV-1 envelope glycoprotein trimers and soluble gp140. J Virol. 2015;89:8245–57. doi: 10.1128/JVI.00628-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Go EP, Liao H-X, Alam SM, Hua D, Haynes BF, Desaire H. Characterization of host-cell line specific glycosylation profiles of early transmitted/founder HIV-1 gp120 envelope proteins. J Proteome Res. 2013;12:1223–34. doi: 10.1021/pr300870t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green RS, Stone EL, Tenno M, Lehtonen E, Farquhar MG, Marth JD. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity. 2007;27:308–20. doi: 10.1016/j.immuni.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Gristick HB, von Boehmer L, West AP, Jr, Schamber M, Gazumyan A, et al. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat Struct Mol Biol. 2016;23:906–15. doi: 10.1038/nsmb.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guenaga J, Dubrovskaya V, de Val N, Sharma SK, Carrette B, et al. Structure-guided redesign increases the propensity of HIV Env to generate highly stable soluble trimers. J Virol. 2015;90:2806–17. doi: 10.1128/JVI.02652-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guenaga J, Garces F, de Val N, Stanfield RL, Dubrovskaya V, et al. Glycine substitution at helix-to-coil transitions facilitates the structural determination of a stabilized subtype C HIV envelope glycoprotein. Immunity. 2017;46:792–803. doi: 10.1016/j.immuni.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guttman M, Garcia NK, Cupo A, Matsui T, Julien JP, et al. CD4-induced activation in a soluble HIV-1 Env trimer. Structure. 2014;22:974–84. doi: 10.1016/j.str.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–13. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu JK, Crampton JC, Cupo A, Ketas T, van Gils MJ, et al. Murine antibody responses to cleaved soluble HIV-1 envelope trimers are highly restricted in specificity. J Virol. 2015;89:10383–98. doi: 10.1128/JVI.01653-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J, Kang BH, Pancera M, Lee JH, Tong T, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41–gp120 interface. Nature. 2014;515:138–42. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–6. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–63. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–61. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jardine JG, Sok D, Julien JP, Briney B, Sarkar A, et al. Minimally mutated HIV-1 broadly neutralizing antibodies to guide reductionist vaccine design. PLoS Pathog. 2016;12:e1005815. doi: 10.1371/journal.ppat.1005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Julien J-P, Lee JH, Cupo A, Murin CD, Derking R, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci USA. 2013;110:4351–6. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–83. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Julien JP, Lee JH, Ozorowski G, Hua Y, Torrents de la Pena A, et al. Design and structure of two HIV-1 clade C SOSIP.664 trimers that increase the arsenal of native-like Env immunogens. Proc Natl Acad Sci USA. 2015;112:11947–52. doi: 10.1073/pnas.1507793112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250:180–98. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, et al. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog. 2013;9:e1003342. doi: 10.1371/journal.ppat.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khayat R, Lee JH, Julien JP, Cupo A, Klasse PJ, et al. Structural characterization of cleaved, soluble HIV-1 envelope glycoprotein trimers. J Virol. 2013;87:9865–72. doi: 10.1128/JVI.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–21. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klasse PJ, LaBranche CC, Ketas TJ, Ozorowski G, Cupo A, et al. Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from Clades A, B and C. PLoS Pathog. 2016;12:e1005864. doi: 10.1371/journal.ppat.1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, et al. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med. 2012;209:1469–79. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong L, He L, de Val N, Vora N, Morris CD, et al. Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Nat Commun. 2016;7:12040. doi: 10.1038/ncomms12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong L, Torrents de la Pena A, Deller MC, Garces F, Sliepen K, et al. Complete epitopes for vaccine design derived from a crystal structure of the broadly neutralizing antibodies PGT128 and 8ANC195 in complex with an HIV-1 Env trimer. Acta Crystallogr D Biol Crystallogr. 2015;71:2099–108. doi: 10.1107/S1399004715013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwong PD, Wyatt R, Desjardins E, Robinson J, Culp JS, et al. Probability analysis of variational crystallization and its application to gp120, the exterior envelope glycoprotein of type 1 human immunodeficiency virus (HIV-1) J Biol Chem. 1999;274:4115–23. doi: 10.1074/jbc.274.7.4115. [DOI] [PubMed] [Google Scholar]
- 75.Lawson AM, Hounsell EF, Stoll MS, Feeney J, Chai WG, et al. Characterisation of minor tetra- to hepta-saccharides O-linked to human meconium glycoproteins by t.l.c.-m.s. microsequencing of neoglycolipid derivatives in conjunction with conventional m.s. and 1H-n.m.r. spectroscopy. Carbohydr Res. 1991;221:191–208. doi: 10.1016/0008-6215(91)80056-s. [DOI] [PubMed] [Google Scholar]
- 76.Lee JH, Andrabi R, Su CY, Yasmeen A, Julien JP, et al. A broadly neutralizing antibody targets the dynamic HIV envelope trimer apex via a long, rigidified, and anionic β-hairpin structure. Immunity. 2017;46:690–702. doi: 10.1016/j.immuni.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JH, de Val N, Lyumkis D, Ward AB. Model building and refinement of a natively glycosylated HIV-1 Env protein by high-resolution cryoelectron microscopy. Structure. 2015;23:1943–51. doi: 10.1016/j.str.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JH, Ozorowski G, Ward AB. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science. 2016;351:1043–8. doi: 10.1126/science.aad2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–82. [PubMed] [Google Scholar]
- 80.Li X, Mooney P, Zheng S, Booth CR, Braunfeld MB, et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat Methods. 2013;10:584–90. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y, Pan J, Cai Y, Grigorieff N, Harrison SC, Chen B. Conformational states of a soluble, uncleaved HIV-1 envelope trimer. J Virol. 2017;91:e00175–17. doi: 10.1128/JVI.00175-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Pan J, Jenni S, Raymond DD, Caradonna T, et al. CryoEM structure of an influenza virus receptor-binding site antibody-antigen interface. J Mol Biol. 2017;429:1829–39. doi: 10.1016/j.jmb.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Loke I, Kolarich D, Packer NH, Thaysen-Andersen M. Emerging roles of protein mannosylation in inflammation and infection. Mol Aspects Med. 2016;51:31–55. doi: 10.1016/j.mam.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 85.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–90. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCoy LE, Falkowska E, Doores KJ, Le K, Sok D, et al. Incomplete neutralization and deviation from sigmoidal neutralization curves for HIV broadly neutralizing monoclonal antibodies. PLoS Pathog. 2015;11:e1005110. doi: 10.1371/journal.ppat.1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCoy LE, van Gils MJ, Ozorowski G, Messmer T, Briney B, et al. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep. 2016;16:2327–38. doi: 10.1016/j.celrep.2016.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–43. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medina-Ramírez M, Garces F, Escolano A, Skog P, de Taeye SW, et al. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J Exp Med. 2017;214:2573–90. doi: 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mizuochi T, Spellman MW, Larkin M, Solomon J, Basa LJ, Feizi T. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem J. 1988;254:599–603. doi: 10.1042/bj2540599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–5. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moulard M, Decroly E. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim Biophys Acta. 2000;1469:121–32. doi: 10.1016/s0304-4157(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 95.Ozorowski G, Pallesen J, de Val N, Lyumkis D, Cottrell CA, et al. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature. 2017;547:360–63. doi: 10.1038/nature23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pabst M, Chang M, Stadlmann J, Altmann F. Glycan profiles of the 27 N-glycosylation sites of the HIV envelope protein CN54gp140. Biol Chem. 2012;393:719–30. doi: 10.1515/hsz-2012-0148. [DOI] [PubMed] [Google Scholar]
- 97.Pallesen J, Murin CD, de Val N, Cottrell CA, Hastie KM, et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat Microbiol. 2016;1:16128. doi: 10.1038/nmicrobiol.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci USA. 2017;114:E7348–E57. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20:804–13. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514:455–61. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panico M, Bouche L, Binet D, O’Connor MJ, Rahman D, et al. Mapping the complete glycoproteome of virion-derived HIV-1 gp120 provides insights into broadly neutralizing antibody binding. Sci Rep. 2016;6:32956. doi: 10.1038/srep32956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang P-S, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng W, de Vries RP, Grant OC, Thompson AJ, McBride R, et al. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe. 2017;21:23–34. doi: 10.1016/j.chom.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci USA. 2012;109:15859–64. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pritchard LK, Harvey DJ, Bonomelli C, Crispin M, Doores KJ. Cell- and protein-directed glycosylation of native cleaved HIV-1 envelope. J Virol. 2015;89:8932–44. doi: 10.1128/JVI.01190-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pritchard LK, Spencer DI, Royle L, Bonomelli C, Seabright GE, et al. Glycan clustering stabilizes the mannose patch of HIV-1 and preserves vulnerability to broadly neutralizing antibodies. Nat Commun. 2015;6:7479. doi: 10.1038/ncomms8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pritchard LK, Spencer DI, Royle L, Vasiljevic S, Krumm SA, et al. Glycan microheterogeneity at the PGT135 antibody recognition site on HIV-1 gp120 reveals a molecular mechanism for neutralization resistance. J Virol. 2015;89:6952–9. doi: 10.1128/JVI.00230-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pritchard LK, Vasiljevic S, Ozorowski G, Seabright GE, Cupo A, et al. Structural constraints determine the glycosylation of HIV-1 envelope trimers. Cell Rep. 2015;11:1604–13. doi: 10.1016/j.celrep.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pugach P, Ozorowski G, Cupo A, Ringe R, Yasmeen A, et al. A native-like SOSIP.664 trimer based on an HIV-1 subtype B env gene. J Virol. 2015;89:3380–95. doi: 10.1128/JVI.03473-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 112.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci USA. 2002;99:13419–24. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ringe RP, Ozorowski G, Rantalainen K, Struwe WB, Matthews K, et al. Reducing V3 antigenicity and immunogenicity on soluble, native-like HIV-1 Env SOSIP trimers. J Virol. 2017;91:e00677–17. doi: 10.1128/JVI.00677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ringe RP, Sanders RW, Yasmeen A, Kim HJ, Lee JH, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proc Natl Acad Sci USA. 2013;110:18256–61. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ringe RP, Yasmeen A, Ozorowski G, Go EP, Pritchard LK, et al. Influences on the design and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers. J Virol. 2015;89:12189–210. doi: 10.1128/JVI.01768-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rudd PM, Dwek RA. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol. 1997;32:1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 117.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanders RW, Moore JP. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol Rev. 2017;275:161–82. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sanders RW, Vesanen M, Schuelke N, Master A, Schiffner L, et al. Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J Virol. 2002;76:8875–89. doi: 10.1128/JVI.76.17.8875-8889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanders RW, Wilson IA, Moore JP. HIV’s Achilles’ Heel. Sci Am. 2016;315:50–55. doi: 10.1038/scientificamerican1216-50. [DOI] [PubMed] [Google Scholar]
- 121.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1–>2 mannose residues on the outer face of gp120. J Virol. 2002;76:7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scharf L, Scheid JF, Lee JH, West AP, Chen C, et al. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep. 2014;7:785–95. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 124.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–7. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scheres SH. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–30. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scheres SH. Processing of structurally heterogeneous cryo-EM data in RELION. Methods Enzymol. 2016;579:125–57. doi: 10.1016/bs.mie.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 127.Sharma SK, de Val N, Bale S, Guenaga J, Tran K, et al. Cleavage-independent HIV-1 Env trimers engineered as soluble native spike mimetics for vaccine design. Cell Rep. 2015;11:539–50. doi: 10.1016/j.celrep.2015.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–10. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 129.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, et al. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med. 2014;6:236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sok D, Pauthner M, Briney B, Lee JH, Saye-Francisco KL, et al. A prominent site of antibody vulnerability on HIV envelope incorporates a motif associated with CCR5 binding and its camouflaging glycans. Immunity. 2016;45:31–45. doi: 10.1016/j.immuni.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, et al. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity. 2016;45:483–96. doi: 10.1016/j.immuni.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–10. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 133.Stevens J, Corper AL, Basler CF, Taubenberger JK, Palese P, Wilson IA. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science. 2004;303:1866–70. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- 134.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, et al. Trimeric HIV-1-Env structures define glycan shields from Clades A, B, and G. Cell. 2016;165:813–26. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van Gils MJ, van den Kerkhof TL, Ozorowski G, Cottrell CA, Sok D, et al. An HIV-1 antibody from an elite neutralizer implicates the fusion peptide as a site of vulnerability. Nat Microbiol. 2016;2:16199. doi: 10.1038/nmicrobiol.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Veesler D, Campbell MG, Cheng A, Fu CY, Murez Z, et al. Maximizing the potential of electron cryomicroscopy data collected using direct detectors. J Struct Biol. 2013;184:193–202. doi: 10.1016/j.jsb.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vinothkumar KR, Henderson R. Single particle electron cryomicroscopy: trends, issues and future perspective. Q Rev Biophys. 2016;49:e13. doi: 10.1017/S0033583516000068. [DOI] [PubMed] [Google Scholar]
- 138.Walker LM, Burton DR. Rational antibody-based HIV-1 vaccine design: current approaches and future directions. Curr Opin Immunol. 2010;22:358–66. doi: 10.1016/j.coi.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward AB, Sali A, Wilson IA. Integrative structural biology. Science. 2013;339:913–5. doi: 10.1126/science.1228565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ward AB, Wilson IA. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev. 2017;275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei X, Decker JM, Wang S, Hui H, Kappes JC, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 144.Wibmer CK, Gorman J, Anthony CS, Mkhize NN, Druz A, et al. Structure of an N276-dependent HIV-1 neutralizing antibody targeting a rare V5 glycan hole adjacent to the CD4 binding site. J Virol. 2016;90:10220–35. doi: 10.1128/JVI.01357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Willey RL, Shibata R, Freed EO, Cho MW, Martin MA. Differential glycosylation, virion incorporation, and sensitivity to neutralizing antibodies of human immunodeficiency virus type 1 envelope produced from infected primary T-lymphocyte and macrophage cultures. J Virol. 1996;70:6431–6. doi: 10.1128/jvi.70.9.6431-6436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature. 1981;289:366–73. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 147.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–11. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 148.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 149.Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou T, Doria-Rose NA, Cheng C, Stewart-Jones GBE, Chuang GY, et al. Quantification of the impact of the HIV-1-glycan shield on antibody elicitation. Cell Rep. 2017;19:719–32. doi: 10.1016/j.celrep.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–58. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry. 2000;39:11194–204. doi: 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]


