Abstract
In recent years immune checkpoint inhibitors have garnered attention as being one of the most promising types of immunotherapy on the horizon. There has been particular focus on the immune checkpoint molecules, cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1) which have been shown to have potent immunomodulatory effects through their function as negative regulators of T cell activation. CTLA-4, through engagement with its ligands B7-1 (CD80) and B7-2 (CD86), plays a pivotal role in attenuating the activation of naïve and memory T cells. In contrast, PD-1 is primarily involved in modulating T cell activity in peripheral tissues via its interaction with PD-L1 and PD-L2. The discovery of these negative regulators of the immune response was crucial in the development of checkpoint inhibitors. This shifted the focus from developing therapies that targeted activation of the host immune system against cancer to checkpoint inhibitors, which aimed to mediate tumor cell destruction through the removal of coinhibitory signals blocking anti-tumor T cell responses.
Keywords: checkpoint inhibitor, CTLA-4, PD-1, immunotherapy, cancer
Introduction
The rapidly growing field of cancer immunotherapy has developed largely as result of our increased understanding of the immune system and malignancy[1–2]. One of the early developments in this field occurred when Thomas and Burnett proposed that tumor cells could evoke an immune response and this formed the basis of further research[3]. Following this discovery, the mechanisms of various immune cell responses involved in cancer recognition and elimination; including Forkhead box P3 (FOXP3+) regulatory T cells (T-regs)[4–5], antigen-presenting cells (APCs)[6–7] myeloid-derived suppressor cells (MDSCs)[8] and effector T cell subsets[9–13] have been elucidated.
Another important discovery in the development of checkpoint inhibitors (CIs) is the knowledge that T cell activation requires two signals. The first signal involves specific antigen recognition by lymphocytes and a second co-stimulatory signal is required as well as the existence of negative coinhibitory (costimulatory) signals, while IL-12 and type I IFN (IFNα/β) are the major sources of signal 3 in a variety of T cell activation[14]. Receptors such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) which supply these coinhibitory signals function as immune checkpoints which play an important role in the termination of immune responses following antigen activation; essentially in the maintenance of peripheral tolerance and autoimmunity. Tumours may exploit these immune checkpoints in order to actively avoid immune mediated tumor lysis[15–19]. This research has highlighted the critical role that the immune system plays in controlling tumor growth and the importance of reversing immunosuppressive mechanisms. This review will focus on the mechanisms of action of antibodies that inhibit CTLA-4 and programmed cell death protein 1 (PD-1), encompassing therapies that have already been approved by the FDA and others currently in development[2].
Immune checkpoint inhibitors
The inhibition of immune checkpoints using specially designed checkpoint blocking monoclonal antibodies (mAbs) such as CTLA-4 and PD-1 play an increasingly important role in the treatment against a growing number of malignancies. CTLA-4 attenuates the early activation of naïve and memory T cells through interactions with its ligands B7-1 (CD80) and B7-2 (CD86) (Fig. 1A). PD-1 is an receptor expressed on the surface of activated mature T cells, activated NK cells, B cells, monocytes and multiple normal tissues and plays a crucial role in the maintenance of peripheral tolerance[20–21] (Fig. 1A). In contrast to CTLA-4, PD-1 acts via interactions with its ligands PD-L1 (also known as B7-H1 or CD274) and is involved mainly in T cell activity modulation in peripheral tissues as well as providing a major immune resistance mechanism within the tumor microenvironment. Cells expressing high levels of PD-L1 may include tumor cells, T cells, APCs, epithelial and hematopoetic cells types among others[22–25]. PD-L2 (also known as B7-DC or CD273) is mainly expressed by APCs[26–28].
Fig.1.
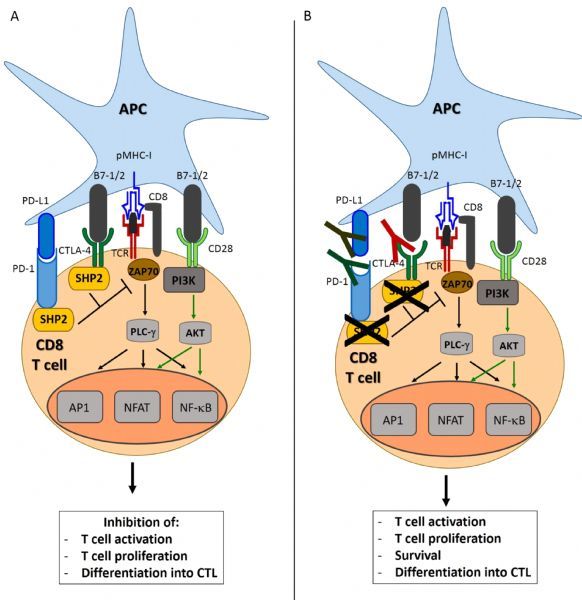
Rationale for the use of immune checkpoint inhibitors in cancer therapy.
The presentation of antigenic peptide on the MHC class I molecule to the CD8 T cell is the first step in the induction of an effective immune response with generation of tumor-specific CTLs. In addition to the recognition of pMHC-I by T cells via TCR, induction of primary T cell response requires co-stimulation of the T cell by interaction of B7.1/2 co-stimulatory molecules on the APC with CD28 on the T cell. This interaction results in downstream signaling that leads to T cell activation and further differentiation into CTLs. Upon activation, T cells express the surface proteins CTLA-4 and PD-1, which bind to B7-1/2 and PD-L1, respectively on the surface of APCs.
Cytotoxic T cells (CTLs) are considered the backbone of immune response against tumor[29]. To recognize and eliminate tumor cells, CTLs require two activating signals. The early immune response, occurring mainly in the lymph nodes is known as the "priming" phase and requires two signals for T cell activation. In the first signal, CD8+ T cells recognize antigenic peptides presented by the major histocompatibility complex (MHC) class I molecules on the surface of cancer cells through their T cell receptor (TCR). The second signal, known as the "costimulatory signal" completes primary T cell activation and is achieved through binding of the T cell costimulatory receptor CD28 with the two costimulatory ligands on APCs; B7-1 and B7-2[28–30]. This leads to downstream signaling and T cell activation and further differentiation into CTLs (Fig. 1A). Of note, CD8 T cells require a third signal, along with antigen and costimulation, to make a productive response and avoid death and/or tolerance induction.
Following activation, CTLs express surface protein receptors, CTLA-4 and PD-1 which function as immune checkpoints. Under usual conditions, the binding between CTLA-4 and B7-1 or B7-2 counteract the costimulatory effects of the CD28 on T cell activation preventing T cell overactivity, as CTLA-4 binds at higher affinity (20 times more)[30]. This balance between inactivity and activity control CTL activity and thus CTLA-4 acts as a negative regulator of T cells[31]. Reports have shown that an important mechanism of tumor evasion is the upregulated expression of CTLA-4 on T cells with the help of TGF-β, enabling cancers to evade the immune effects of CTLs[32–35].
Similarly, the engagement of PD-1 on a T cell with PD-L1 on the tumor cell surface inhibits T cell function and activation. The PD-1 mediated dysfunction of T cells is thought to be due to a number of mechanisms. Following T cell activation, PD-1 binds to PD-L1 on the surface of APCs and this induces T cell apoptosis, anergy, exhaustion or IL-10 production. (Fig. 1A) Further, PD-L1 may act as a barrier to protect tumor associated PD-L1 from CTL lysis[36–37]. Additionally, murine models have demonstrated an interaction between PD-L1 and B7-1. B7-1 may be expressed on activated APCs and T cells and may send out inhibitory signals when bound to PD-L1. Tumours and chronic infections can exploit this pathway to downregulate T cell–mediated immunity against tumors and pathogens[38–39]. In particular, PD-L1 and to a lesser extent, PD-L2 are expressed on many tumors, including urothelial, colon, pancreatic, gastric cancers, ovarian, breast, cervical as well as melanoma glioblastoma and NSCLC[28, 40–48].
Thus, numerous studies have shown that CTLA-4 and PD-1 have an important role in mediating immune evasion in the tumor microenvironment. The administration of mAbs blocking CTLA-4, PD-1 or PD-L1 allows for the generation of a sustained and specific CTL response capable of tumor cell lysis (Fig. 1B)[20, 26, 28]. Clinically successful anti-CTLA-4 antibodies blocking this inhibitory signal such as ipilimumab and tremelimumab have been developed which amplify and prolong an anti-tumoral response[49–50].
Preclinical data
CTLA-4 blockade
Following in vitro studies that supported CTLA-4 as a key checkpoint molecule in the antitumor immune response, anti-CTLA-4 blocking antibody therapy was initially tested in numerous animal models including breast[51], prostate[52], lymphoma[53], colon[54] and melanoma[55]. The first study, carried out by Alison and colleagues demonstrated that CTLA-4 blockade enhances the anti-tumor immune response[31]. Although this efficacy was limited to a few cancer cell lines that only responded to CTLA-4 when combined with a transduced granulocyte-macrophage colony-stimulating factor (GM-CSF) producing cellular vaccine[51, 56]. These findings suggested that CTLA-4 blockade could result in significant anti-tumor activity through stimulation of the endogenous antitumor response through enhancement of naturally or vaccine-induced tumor-specific T cells. Further, in the case of poorly immunogenic tumors, which have a limited endogenous immune response, the combination of CTLA-4 antibody with a vaccine has the potential to establish an immune response to hinder tumor growth and lead to tumor regression in certain cases[2, 28].
These studies have paved the way toward CTLA-4 blockade in human clinical trials. A significant phase III trial was published in 2010 for ipilimumab[57] which together with tremelimumab[58] are the two most clinically successful anti-CTLA-4 mAbs[50]. Ipilimumab was found to have a significant increase in survival, for patients with previously treated unresectable metastatic melanoma and was the first drug in this class approved by the FDA for use as first or second line therapy for advanced melanoma[50, 59–61].
Further, as well as enhancing overall survival, ipilimumab treatment was associated with a durable response (>2.5 years) with the potential to achieve long-term control of disease in a significant proportion (15-20%) of individuals[49–50]. The median duration of response was two years, compared with 4–8 months for chemotherapy regimens and oncogene-targeted therapy[62]. The results showed that stable patients at ≥24 weeks were followed up and continued to be stable beyond 48 weeks. Improved durability was associated with improved survival outcomes with one year survival at 42% and 2 year survival at 14%[63–64]. Considering the advanced inoperable stage of disease in this patient group, this outcome is encouraging[49–50, 65]. These durable responses suggest lasting adaptations in the immune system, supporting the belief that immunomodulating therapy may alter the patient’s intrinsic tumor-specific T cell function[64–65].
PD-1 blockade
The role of PD-1 as an important regulator of immunity within the tumor microenvironment through inhibition of T cells has been shown[60, 66–67]. It was predicted that PD-1/PD-L1 blockade would have a greater anticancer effect than CTLA-4 inhibitors with fewer unwanted side effects due to the selective immunosuppressive signals delivered by cancer cells[2, 20]. Effective antitumor T cell responses have been shown by testing PD-1 blockade together with GM-CSF in murine models such as CT26 colon carcinoma, murine B16 melanoma and pancreatic ductal adenocarcinoma models[68–69]. Numerous clinical trials have therapeutically exploited the PD-1/L1 pathway to considerable effect, with durable response rates between 20% to 50% in multiple types of cancer[21, 60]. These successes led to FDA approval of the anti-PD-1 antibodies, pembrolizumab (humanized IgG4, Merck) followed by nivolumab (fully human IgG4, Bristol-Myers Squibb, Ono Pharmaceuticals) in 2014, for patients with advanced melanoma who had not responded to anti-CTLA-4[70–72].
Multiple trials have demonstrated that blockade of the PD-1/L1 pathway has effective anti-tumor activity in a number of different malignancies including bladder cancer[73], breast cancer[66, 74], colorectal cancer[60, 65–67, 75], diffuse large B cell lymphoma[76], follicular lymphoma[77], gastric cancer[66], head and neck squamous cell carcinoma[74], Hodgkin’s lymphoma[78], melanoma[79–84], ovarian cancer[66, 74], non–small cell lung cancer (NSCLC)[8, 60, 65–66, 85–88], pancreatic cancer[8, 27, 66, 74], renal cell carcinoma (RCC)[60, 74, 89], prostate cancer[60, 65], sarcoma[74], small cell lung cancer (SCLC) and uterine cancer[74]. Further trials are investigating anti-PD-1/L1 administration in other cancers such as lung [90], bladder[91–93], renal cancers[74, 94], breast[95–96] and chemotherapy-refractory Hodgkin disease[78].
Toxicities
CTLA-4 and PD-1 inhibitors are the most clinically successful checkpoint inhibitors nevertheless, there are a number of concerns including autoimmunity, unique adverse effects and toxicity related to checkpoint blockade mAbs[97]. Although direct comparisons have not been carried out, clinical response levels and toxicities are generally consistent between PD-1 and PD-L1. Due to the role of the PD-1 pathway in the maintenance of self-tolerance, the inhibition of this pathway can cause problems, resulting in adverse immunologic responses termed "immune related adverse events" (IRAEs). IRAEs may cause toxicity to tissues and organs which are usually protected by the immune system, resulting in autoimmune-like diseases and inflammation.
However, the most common side effect of PD-1 blockade is fatigue which is not necessarily a limiting factor in treatment duration and does not require medical treatment[84]. Other common side effects associated with CIs were decreased appetite (12%) and diarrhea (10%)[60]. However, less frequently observed toxicities can occur in pulmonary (inflammatory pneumonitis), endocrine, mucocutaneous and renal (interstitial nephritis) sites and even immunologically privileged sites such as the eye resulting in damage. These events are rare and may be life threatening such as the case of inflammatory pneumonitis, requiring cessation of therapy and treatment with immunosuppressants such as corticosteroids[98]. Interestingly, clinical responses persist despite treatment cessation and immunosuppression which suggests that the ideal duration of PD-1 checkpoint blockade has yet to be determined[99].
In one trial 60% of patients treated with anti-CTLA-4 experienced adverse events of which 10%-15% were classed as severe (grade 3/4)[50]. IRAEs are less frequent in anti-PD-1 treated patients than in those treated with CTLA-4 blockade (13.3% as opposed to 19.9% in anti-CTLA-4 treated patients) leading to approval of anti-PD-1 treatment as first line for advanced melanoma in the USA and the EU[2, 98]. Understanding the adverse effects associated with checkpoint blockade as well as having effective treatment plans for their management are crucial to optimise the efficacy of anti-PD-1 and anti-CTLA-4 therapy.
Neoantigens
Questions still remain about the degree to which individual host and tumor characteristics determine therapeutic responsiveness and whether these can be used to predict durability and responsiveness. Whole-genome sequencing of tumors has revealed that growing tumors acquire hundreds of somatic tumor specific mutations, which form new antigens designated "neoantigens" which have been seen in mouse tumor models and in CTLA-4 and PD-1 treated patients[100–102].These neoantigens are key determinants in the response of patients to PD-1 and CTLA-4 checkpoint immunotherapy[102–103]. Despite neoantigen specific T cells being generated in growing tumors they are unable to produce an effective antitumor immune response. However, several studies have shown that neoantigen specific T cells were reactivated following CIs administration and formed an antitumor response[101–103]. The genomics of individual tumors therefore goes some way in explaining the variable responses among patients who have undergone CIs treatment.
Combination therapy
Notably, preclinical studies of anti-CTLA-4 and anti-PD-1 mAb combinations demonstrated promising results in a range of cancers[92, 104–105]. The first phase I clinical trial, combining ipilimumab and nivolumab was updated in an ASCO annual meeting in 2014 showing a 2 year survival of 79% (objective response rate of 43%) among patients with advanced melanoma. However, combination therapy was shown to have increased adverse effects compared to administration of the agents alone (63% of grade 3/4 toxicities)[106].
Further, a recent phase III study assigned untreated patients (n = 945) with metastatic melanoma to combination treatment with nivolumab and ipilimumab. The median progression free survival was 11.5 months in the combination treatment in comparison to 2.9 months for ipilimumab and 6.9 months with nivolumab. The study found that patients with PD-L1 negative tumors responded more effectively to a combination of PD-1 and CTLA-4 blockade (11.2 months) as opposed to nivolumab alone (5.3 months)[106]. Similar to the 2014 study, treatment related toxicity was higher in the nivolumab-plus-ipilimumab group (55%) as opposed to nivolumab (16.3%) or ipilimumab (27.3%) monotherapy[82, 106]. Treatment related adverse events in the combination therapy group are consistent with side effects seen in previous trials[50, 64, 84] and were managed primarily with immune-modulatory agents. Thus, despite the higher incidence of adverse effects in the combination group, the toxicity profile is consistent with anti-CTLA-4/PD-1 monother- apy[104–105].
Co-stimulatory molecules
Similar to immune checkpoint molecules, agonistic antibodies for co-stimulatory pathways such as CD137 (4-1BB), CD27, OX40 are showing promise as they augment T cell activation, and therefore may have a role in the antitumor T cell response[99]. A CD137 agonist antibody was tested in combination with anti-PD-L1 antibody in a murine breast cancer model which overcame resistance to immune mediated rejection and showed improvements in T cell immunity in other mouse models[107–108]. Based on combined treatment with agonistic anti-OX40 antibodies and anti-CTLA-4 antibodies, which induced tumor regression and improved survival[109], further early phase trials investigating combinations of OX40 and PD-L1 and OX40 and CTLA-4 are currently underway in advanced solid tumors (NCT02221960 & NCT02205333). Subsequent trials in solid tumor models have been tested; OX40 and anti-CTLA-4 in ovarian carcinoma (ID8), prostate cancer (TRAMP1), anti-CD137 and CTLA-4 blockade in MC38 colon cancer and GL261 glioblastoma, demonstrating synergy between CD137, PD-1, and CTLA-4[109–113]. Based on these promising results, two current phase I/II trials are investigating the combination of anti-PD therapy with anti-CD137 in advanced solid tumors (NCT02554812 and NCT0217-9918).
Conclusion
The introduction of CIs in the arsenal of immunotherapeutics against cancer has ushered in a new era in the treatment of many cancers. Unprecedented responses have been seen among patients with advanced cancers including melanoma, lung, bladder, RCC and Hodgkin’s disease, treated with anti-CTLA-4, PD-1/L1. However, only less than 25% of patients treated with these agents have got benefit. The future of immunotherapy depends upon identifying and developing ideal combinations of immunotherapies in order to optimise and enhance the efficacy of treatment, as well as achieving a durable anti-cancer effect. More research needs to be conducted into immune checkpoint combination approaches based on individual tumor genetics to be able to predict responses to treatment and increase the number of patients that respond to therapy. Although many challenges still remain, there is a sense of hope that checkpoint inhibitors have heralded a new era in the treatment of many cancers. Combining immune checkpoint antibodies with other immune-stimulating agents such as conventional drugs, targeted agents and most promisingly tumor-targeted oncolytic virus, may open a new avenue for cancer patients in which a durable clinical benefit can be achieved.
Acknowledgments
This work was supported by The MRC DPFS grant (MR/M015696/1) and Ministry of Sciences and Technology of China (2013DFG32080).
Contributor Information
Ming Yuan, Email: m.yuan@qmul.ac.uk.
Yaohe Wang, Email: yaohe.wang@qmul.ac.uk.
References
- 1. Page DB, Postow MA, Callahan MK, et al. Immune modulation in cancer with antibodies[J]. Annu Rev Med, 2014, 65: 185–202 . [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy[J]. Nat Rev Cancer, 2012, 12(4): 252–264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnet M. Cancer; a biological approach. I. The processes of control[J]. Br Med J, 1957, 1(5022): 779–786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system[J]. Nat Rev Immunol, 2010, 10(7): 490–500 . [DOI] [PubMed] [Google Scholar]
- 5. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival[J]. Nat Med, 2004, 10(9): 942–949 . [DOI] [PubMed] [Google Scholar]
- 6. Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma[J]. J Exp Med, 2006, 203(4): 871–881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity[J]. Nat Med, 2003, 9(5): 562–567 . [DOI] [PubMed] [Google Scholar]
- 8. Cui TX, Kryczek I, Zhao L, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2[J]. Immunity, 2013, 39(3): 611–621 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer[J]. N Engl J Med, 2003, 348(3): 203–213 . [DOI] [PubMed] [Google Scholar]
- 10. Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer[J]. N Engl J Med, 2005, 353(25): 2654–2666 . [DOI] [PubMed] [Google Scholar]
- 11. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome[J]. Science, 2006, 313(5795): 1960–1964 . [DOI] [PubMed] [Google Scholar]
- 12. Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells[J]. Sci translat med, 2011;3:104ra0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao E, Maj T, Kryczek I, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction[J]. Nat Immunol, 2016, 17(1): 95–103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen L, Linsley PS, Hellström KE. Costimulation of T cells for tumor immunity[J]. Immunol Today, 1993, 14(10): 483–486 . [DOI] [PubMed] [Google Scholar]
- 15. Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4[J]. Immunity, 1995, 3(5): 541–547 . [DOI] [PubMed] [Google Scholar]
- 16. Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice[J]. Science, 2001, 291(5502): 319–322 . [DOI] [PubMed] [Google Scholar]
- 17. Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor[J]. Immunity, 1999, 11(2): 141–151 . [DOI] [PubMed] [Google Scholar]
- 18. Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4[J]. Science, 1995, 270(5238): 985–988 . [DOI] [PubMed] [Google Scholar]
- 19. Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell responses[J]. Curr Opin Immunol, 1995, 7(5): 682–686 . [DOI] [PubMed] [Google Scholar]
- 20. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application[J]. Int Immunol, 2007, 19(7): 813–824 . [DOI] [PubMed] [Google Scholar]
- 21. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway[J]. N Engl J Med, 2016, 375(18): 1767–1778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion[J]. Nat Med, 1999, 5(12): 1365–1369 . [DOI] [PubMed] [Google Scholar]
- 23. Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells[J]. J Exp Med, 2001, 193(7): 839–846 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation[J]. J Exp Med, 2000, 192(7): 1027–1034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation[J]. Nat Immunol, 2001, 2(3): 261–268 . [DOI] [PubMed] [Google Scholar]
- 26. Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses[J]. Immunity, 2007, 27(1): 111–122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion[J]. Nat Med, 2002, 8(8): 793–800 . [DOI] [PubMed] [Google Scholar]
- 28. Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next[J]? Curr Opin Immunol, 2015, 33: 23–35 . [DOI] [PubMed] [Google Scholar]
- 29. Aldarouish M, Wang C. Trends and advances in tumor immunology and lung cancer immunotherapy[J]. Journal of experimental & clinical cancer research: CR. 2016;35:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited[J]. Annu Rev Immunol, 2005, 23: 515–548 . [DOI] [PubMed] [Google Scholar]
- 31. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade[J]. Science, 1996, 271(5256): 1734–1736 . [DOI] [PubMed] [Google Scholar]
- 32. Erfani N, Mehrabadi SM, Ghayumi MA, et al. Increase of regulatory T cells in metastatic stage and CTLA-4 over expression in lymphocytes of patients with non-small cell lung cancer (NSCLC)[J]. Lung Cancer, 2012, 77(2): 306–311 . [DOI] [PubMed] [Google Scholar]
- 33. Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment[J]. Cell Death Dis, 2015, 6: e1792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Funt SA, Page DB, Wolchok JD, et al. CTLA-4 antibodies: new directions, new combinations[J]. Oncology (Williston Park), 2014, 28(Suppl 3): 6–14 . [PubMed] [Google Scholar]
- 35. Li L, Chao QG, Ping LZ, et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC[J]. Cancer Biother Radiopharm, 2009, 24(3): 357–367 . [DOI] [PubMed] [Google Scholar]
- 36. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment[J]. Nat Rev Immunol, 2008, 8(6): 467–477 . [DOI] [PubMed] [Google Scholar]
- 37. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future[J]. J Clin Invest, 2015, 125(9): 3384–3391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sauce D, Almeida JR, Larsen M, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status[J]. AIDS, 2007, 21(15): 2005–2013 . [DOI] [PubMed] [Google Scholar]
- 39. Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses[J]. Eur J Immunol, 2003, 33(10): 2706–2716 . [DOI] [PubMed] [Google Scholar]
- 40. Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target[J]. Proc Natl Acad Sci U S A, 2004, 101(49): 17174–17179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression[J]. Cancer, 2007, 109(8): 1499–1505 . [DOI] [PubMed] [Google Scholar]
- 42. Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer[J]. Proc Natl Acad Sci U S A, 2007, 104(9): 3360–3365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance[J]. Acta Histochem, 2006, 108(1): 19–24 . [DOI] [PubMed] [Google Scholar]
- 44. Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2007;13:2151–2157. [DOI] [PubMed] [Google Scholar]
- 45. Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production[J]. J Immunol, 2003, 170(3): 1257–1266 . [DOI] [PubMed] [Google Scholar]
- 46. Konishi J, Yamazaki K, Azuma M, et al. B7–H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2004;10:5094–5100. [DOI] [PubMed] [Google Scholar]
- 47. Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-gamma and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway[J]. Blood, 2007, 110(1): 296–304 . [DOI] [PubMed] [Google Scholar]
- 48. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy[J]. FEBS Lett, 2014, 588(2): 368–376 . [DOI] [PubMed] [Google Scholar]
- 49. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma[J]. N Engl J Med, 2011, 364(26): 2517–2526 . [DOI] [PubMed] [Google Scholar]
- 50. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma[J]. N Engl J Med, 2010, 363(8): 711–723 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation[J]. J Exp Med, 1999, 190(3): 355–366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer[J]. Proc Natl Acad Sci U S A, 1997, 94(15): 8099–8103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Ginderachter JA, Liu Y, Geldhof AB, et al. B7-1, IFN gamma and anti-CTLA-4 co-operate to prevent T-cell tolerization during immunotherapy against a murine T-lymphoma[J]. Int J Cancer, 2000, 87(4): 539–547 . [PubMed] [Google Scholar]
- 54. Saha A, Chatterjee SK. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer[J]. Scand J Immunol, 2010, 71(2): 70–82 . [DOI] [PubMed] [Google Scholar]
- 55. Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses[J]. J Exp Med, 2001, 194(6): 823–832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4[J]. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5238–42. [DOI] [PubMed] [Google Scholar]
- 57. Boasberg P, Hamid O, O’Day S. Ipilimumab: unleashing the power of the immune system through CTLA-4 blockade[J]. Semin Oncol, 2010, 37(5): 440–449 . [DOI] [PubMed] [Google Scholar]
- 58. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab[J]. Semin Oncol, 2010, 37(5): 450–454 . [DOI] [PubMed] [Google Scholar]
- 59. Sondak VK, Smalley KS, Kudchadkar R, et al. Ipilimumab[J]. Nat Rev Drug Discov, 2011, 10(6): 411–412 . [DOI] [PubMed] [Google Scholar]
- 60. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. N Engl J Med, 2012, 366(26): 2443–2454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2012;18:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson JA, Hamid O, Minor D, et al. Ipilimumab in treatment-naive and previously treated patients with metastatic melanoma: retrospective analysis of efficacy and safety data from a phase II trial[J]. J Immunother, 2012, 35(1): 73–77 . [DOI] [PubMed] [Google Scholar]
- 63. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment[J]. Nat Rev Cancer, 2012, 12(4): 237–251 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma[J]. J Clin Oncol, 2015, 33(17): 1889–1894 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med, 2012, 366(26): 2455–2465 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates[J]. J Clin Oncol, 2010, 28(19): 3167–3175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2013;19:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li B, VanRoey M, Wang C, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors[J]. Clinical cancer research: an official journal of the American Association for Cancer Research, 2009;15:1623–1634. [DOI] [PubMed] [Google Scholar]
- 69. Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors[J]. J Immunother, 2015, 38(1): 1–11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial[J]. Lancet, 2014, 384(9948): 1109–1117 . [DOI] [PubMed] [Google Scholar]
- 71. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials[J]. JAMA Oncol, 2015, 1(4): 433–440 . [DOI] [PubMed] [Google Scholar]
- 72. Ascierto PA, Marincola FM. 2015: The Year of Anti-PD-1/PD-L1s Against melanoma and beyond[J]. EBio Medicine, 2015, 2(2): 92–93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer[J]. Nature, 2014, 515(7528): 558–562 . [DOI] [PubMed] [Google Scholar]
- 74. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J]. Nature, 2014, 515(7528): 563–567 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency[J]. N Engl J Med, 2015, 372(26): 2509–2520 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial[J]. J Clin Oncol, 2013, 31(33): 4199–4206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial[J]. Lancet Oncol, 2014, 15(1): 69–77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma[J]. N Engl J Med, 2015, 372(4): 311–319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma[J]. N Engl J Med, 2013, 369(2): 134–144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab[J]. J Clin Oncol, 2014, 32(10): 1020–1030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Robert C, Schachter J, Long GV, et al. , and the KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma[J]. N Engl J Med, 2015, 372(26): 2521–2532 . [DOI] [PubMed] [Google Scholar]
- 82. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma[J]. N Engl J Med, 2015, 373(1): 23–34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation[J]. N Engl J Med, 2015, 372(4): 320–330 . [DOI] [PubMed] [Google Scholar]
- 84. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial[J]. Lancet Oncol, 2015, 16(4): 375–384 . [DOI] [PubMed] [Google Scholar]
- 85. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer[J]. N Engl J Med, 2015, 372(21): 2018–2028 . [DOI] [PubMed] [Google Scholar]
- 86. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2015, 373(2): 123–135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer[J]. J Clin Oncol, 2015, 33(18): 2004–2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial[J]. Lancet Oncol, 2015, 16(3): 257–265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McDermott DF, Drake CG, Sznol M, et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab[J]. J Clin Oncol, 2015, 33(18): 2013–2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer[J]. Clin Pharmacol Ther, 2014, 96(2): 214–223 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance[J]. Nature, 2014, 515(7528): 568–571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mangsbo SM, Sandin LC, Anger K, et al. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy[J]. J Immunother, 2010, 33(3): 225–235 . [DOI] [PubMed] [Google Scholar]
- 93. Raval RR, Sharabi AB, Walker AJ, et al. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer[J]. J Immunother Cancer, 2014, 2: 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tykodi SS. PD-1 as an emerging therapeutic target in renal cell carcinoma: current evidence[J]. Onco Targets Ther, 2014, 7: 1349–1359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol, 2013, 5(3): 169–181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer[J]. Oncoimmunology, 2014, 3: e29288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brahmer JR, Topalian S, Wollner I, et al. Safety and activity of MDX-1106 (ONO-4538), an anti-PD-1 monoclonal antibody, in patients with selected refractory or relapsed malignancies[J]. J Clin Oncol, 2008, 26: 3006 . 18458044 [Google Scholar]
- 98. Swart M, Verbrugge I, Beltman JB. Combination Approaches with Immune-Checkpoint Blockade in Cancer Therapy[J]. Front Oncol, 2016, 6: 233 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies[J]. Annals of oncology: official journal of the European Society for Medical Oncology, 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer[J]. Nature, 2013, 500(7463): 415–421 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma[J]. N Engl J Med, 2014, 371(23): 2189–2199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer[J]. Science, 2015, 348(6230): 124–128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens[J]. Nature, 2014, 515(7528): 577–581 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors[J]. Proc Natl Acad Sci U S A, 2010, 107(9): 4275–4280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy[J]. J Leukoc Biol, 2013, 94(1): 25–39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sznol MKH, Callahan MK, Postow MA, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma[J]. ASCO Annual Meeting ASCO Annual Meeting 2014. [Google Scholar]
- 107. Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity[J]. Cancer Res, 2005, 65(3): 1089–1096 . [PubMed] [Google Scholar]
- 108. Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors[J]. Nat Med, 1997, 3(6): 682–685 . [DOI] [PubMed] [Google Scholar]
- 109. Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity[J]. Cancer Immunol Res, 2014, 2(2): 142–153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guo Z, Wang X, Cheng D, et al. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer[J]. PLoS One, 2014, 9(2): e89350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kocak E, Lute K, Chang X, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity[J]. Cancer Res, 2006, 66(14): 7276–7284 . [DOI] [PubMed] [Google Scholar]
- 112. Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model[J]. PLoS One, 2014, 9(7): e101764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dai M, Wei H, Yip YY, et al. Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation[J]. J Immunother, 2013, 36(4): 248–257 . [DOI] [PMC free article] [PubMed] [Google Scholar]


