Abstract
Acute pancreatitis (AP) is an inflammatory disorder of pancreatic tissue initiated in injured acinar cells. Severe AP remains a significant challenge due to the lack of effective treatment. The widely-accepted autodigestion theory of AP is now facing challenges, since inhibiting protease activation has negligible effectiveness for AP treatment despite numerous efforts. Furthermore, accumulating evidence supports a new concept that malfunction of a self-protective mechanism, the unfolded protein response (UPR), is the driving force behind the pathogenesis of AP. The UPR is induced by endoplasmic reticulum (ER) stress, a disturbance frequently found in acinar cells, to prevent the aggravation of ER stress that can otherwise lead to cell injury. In addition, the UPR’s signaling pathways control NFκB activation and autophagy flux, and these dysregulations cause acinar cell inflammatory injury in AP, but with poorly understood mechanisms. We therefore summarize the protective role of the UPR in AP, propose mechanistic models of how inadequate UPR could promote NFκB’s pro-inflammatory activity and impair autophagy’s protective function in acinar cells, and discuss its relevance to current AP treatment. We hope that insight provided in this review will help facilitate the research and management of AP.
Keywords: Acute pancreatitis, Endoplasmic reticulum stress, Unfolded protein response, Acinar cell injury, Autophagy
Core tip: The widely-accepted autodigestion theory of acute pancreatitis (AP) has been considerably modified by the recent recognition of endoplasmic reticulum (ER) stress-induced unfolded protein response (UPR) as an essential self-protective activity in acinar cells. Inadequate UPR, however, leads to acinar cell injury in AP with elusive mechanisms. We review the relevant literature and propose mechanistic models with the hope of facilitating the research required for the development of effective AP treatment.
INTRODUCTION
Acute pancreatitis (AP) is one of the most common gastrointestinal disorders leading to hospitalization in the United States, accounting for more than 270000 hospital admissions and costing 2.6 billion dollars per year[1]. More than 75% of AP cases are associated with alcohol consumption and gallstones, and up to 20% of AP patients have a severe form with a mortality rate between 10% to 30%[2]. Severe complications of AP include progression to pancreatic necrosis, bacteremia, sepsis, splenic vein thrombosis, and respiratory failure. Current management strategies for AP treatment, such as aggressive hydration, endoscopic intervention for biliary obstructive disease and pancreatic necrosectomy, have limited beneficial effects on disease progression and results[3]. Therapy that can effectively block the progression of acinar injury before it results in severe complications is still missing. This is largely due to the poor understanding of the molecular dysregulation that leads to irreversible inflammatory injury in acinar cells, despite a wide range of efforts that have been made to define the mechanisms of AP.
In 1896, Hans Chiari, based on his postmortem observations, originally proposed that pancreatitis is a process of autodigestion of the pancreas when “the organ succumbs to its own digestive properties”[4]. Nearly a century later, this concept gained acceptance when elevated levels of trypsin and other proteases were observed in AP animal models, and when mutations in the trypsinogen gene were found in patients with hereditary pancreatitis[5,6]. Further observation of the co-localization of lysozyme with secretory granules in acinar cells also supported the belief that intracellular activation of trypsinogen by lysozyme was the mechanism of autodigestion[7].
Based on the autodigestion theory, several protease inhibitors were developed for AP treatment over the past 50 years[5]. Although few human and animal studies showed beneficial activities of this strategy, including the prophylactic effects of Gabexate on post-endoscopic retrograde cholangiopancreatography AP[8], larger clinical trials failed to demonstrate the effectiveness of these inhibitors in patients with AP. This was thought to be due to the late timing in which these protease inhibitors were provided to patients, which was typically hours after the onset of AP[5].
Animal studies of trypsinogen-deficient mice, however, generated more evidence challenging the trypsinogen-induced autodigestion theory. Despite being deficient in major trypsinogen activity, these mice were still able to develop AP and chronic pancreatitis (CP)[9,10]. Furthermore, in some hereditary pancreatitis patients, trypsinogens encoded by mutated trypsinogen genes had unaltered trypsin activity, but signs of defective protein folding in the endoplasmic reticulum (ER) lumen[11]. Thus, acinar cell injury in AP is not necessarily the result of premature intracellular activation of trypsinogen as previously thought. Other dysregulated cellular activities are likely responsible for triggering injury in acinar cells.
ER STRESS IS A COMMON DISTURBANCE THAT INITIATES ACINAR CELL INJURY
The ER is a multifunctional organelle that stores calcium and metabolizes lipids and carbohydrates, but is principally responsible for protein folding and processing in cells. ER stress is a malfunctioning condition characterized by the accumulation of unfolded and misfolded proteins in the ER lumen[12]. Acinar cells, as the primary producers of digestive enzymes, have abundant ER that enables the highest rate of protein synthesis and processing among the mature cells in the body. This unique feature, however, makes acinar cells particularly susceptible to AP risk factor-induced ER stress[12]. Severe and enduring ER stress can cause irreversible cellular damage associated with an increase of intracellular reactive oxygen species, release of cytochrome c from mitochondria, induction of caspase 12-mediated apoptosis, blockade of autophagic flux, promotion of NFκB-mediated inflammation, and perturbation of calcium-regulated signaling[12-15]. Damaged acinar cells then inevitably promote a local inflammatory response that can attenuate self-protective activities in the remaining intact acinar cells and thereby extend the local injury, which may eventually escalate mild AP to severe AP.
UPR PROTECTS AGAINST ACINAR CELL INJURY BY RELIEVING ER STRESS
First described by Sambrook’s group in 1988, the unfolded protein response (UPR) is a concerted effort made by the cell to intricately alleviate ER stress that would otherwise significantly threaten normal cellular functions[16]. Failure to counterbalance ER stress by the UPR has been implicated in a broad range of diseases including diabetes, neurodegeneration, cancer, pulmonary fibrosis, cardiac disease and inflammatory disorders such as AP, identifying the UPR as an essential self-protective mechanism[17]. In acinar cells, due to the high susceptibility to ER stress, the UPR is therefore decisive in maintaining cellular homeostasis[12-15].
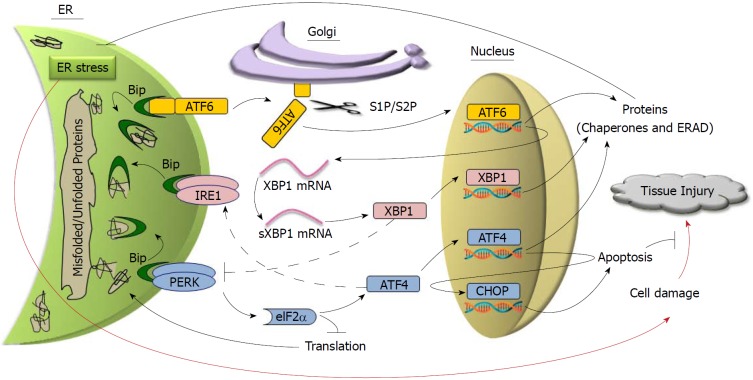
As illustrated in Figure 1, activation of the UPR is initiated by three ER transmembrane proteins, including protein kinase RNA-like ER kinase (PERK), inositol requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6), and each of them activates a different UPR signaling pathway[18]. In the absence of ER stress, these three proteins are bound to a chaperone protein called binding immunoglobulin protein (BiP) that holds them in inactive states on the ER membrane. In stressed ER when unfolded proteins accumulate, however, BiP releases PERK, IRE1 and ATF6 in order to bind to unfolded proteins to help with their folding. The dissociation of BiP triggers activation of PERK and IRE1 via their auto-phosphorylation, and enables ATF6 to translocate to the Golgi apparatus where it is cleavage-activated by proteases. Activated PERK, IRE1 and ATF6 then, via different sequential proceedings, turn on diverse UPR activities, which include: Reducing total protein production by inhibiting translation, eliminating misfolded and unfolded proteins in the ER lumen through ER-associated degradation (ERAD), and increasing the folding capability in the ER by producing more chaperone proteins. Of note, the three UPR regulatory pathways appear to be distinct yet interactive in maintaining homeostasis. Dysregulation of the UPR pathways by AP risk factors, however, is considered as the cause that leads to acinar cell injury[19,20].
Figure 1.
Unfolded protein response protects against tissue injury by relieving endoplasmic reticulum stress. Red arrows represent the pathways that lead to tissue injury. Dotted lines represent the interactions with unclarified mechanisms.
PERK/eIF2/ATF4
PERK signaling controls general protein translation and cell apoptosis in response to ER stress. Activated PERK phosphorylates eukaryotic initiation factor-2α (eIF2α), which inhibits general protein translation by interfering with the formation of the initiation complex at ribosomes. This prevents further accumulation of unfolded and misfolded proteins in the ER lumen[21]. Although it represses global protein translation, phosphorylated eIF2α preferentially promotes the translation of ATF4, which activates the transcription of other UPR genes, including proteins needed for carrying out protein folding and ERAD[22], and enhances the IRE1 pathway[23]. In addition, upregulated ATF4 signaling can activate apoptosis via transcriptional regulation of C/EBP homologous protein (CHOP), a transcription factor that directs ER stress-induced apoptosis[24].
Altered PERK pathway is associated with various disorders including diabetes, metabolic and inflammatory diseases, and cancers[17,25]. Loss-of-function mutations in PERK cause Wolcott-Rallison syndrome manifesting as early onset type 1 diabetes, epiphyseal dysplasia, osteopenia, mental retardation, and hepatic and renal dysfunction[26]. The involvement of multiple organs in Wolcott-Rallison syndrome indicates the broad range of PERK’s protective activities in the body. On the other hand, over-activated PERK can be harmful. In prion-infected mice, excessive and long-term ER stress-induced over-activation of the PERK/eIF2α/ATF4 pathway led to neurodegeneration[27]. Based on these findings, efforts have been made to target PERK as a potential therapeutic strategy in ER stress-related diseases[28].
Interestingly, although PERK, ATF4 and CHOP are sequentially activated in the same pathway, each deficiency causes different phenotypes in the mouse pancreas, indicating that their functions are not fully overlapped in acinar cells. Similar to Wolcott-Rallison syndrome in humans, PERK-deficient mice present with significant pancreatic atrophy associated with increased pancreatic cell death early after their birth[29]. While PERK is required for both secretory homeostasis and survival in β cells, in acinar cells it is only needed for maintaining the viability, but not for enzyme synthesis and secretion[29,30]. In line with this, no ER stress is observed in PERK-deficient acinar cells[29]. ATF4-deficient mice, however, have severely underdeveloped exocrine pancreata with a reduced numbers of acinar cells, indicating a development role of ATF4 in acinar cells[29]. In contrast to PERK-deficient mice and ATF4-deficient mice, CHOP-deficient mice have a completely normal pancreas[31]. Activation of PERK/eIF2α/ATF4 is upregulated in injured acinar cells, leading to the inhibition of general translation and the expression of pro-apoptotic CHOP[32,33]. Increased CHOP is found to be protective in a severe AP animal model, likely because it can direct the fate of injured acinar cells toward less harmful apoptosis instead of more destructive necrosis[31].
IRE1/XBP1
On the membrane of stressed ER, the ribonuclease function of IRE1 is activated to excise an intron from the mRNA of X-box binding protein 1 (XBP1), whose expression is regulated by ATF6[34,35]. Spliced XBP1 mRNA (sXBP1) encodes an active form of XBP1 that activates the transcription of chaperones and ERAD components (Figure 1). Interestingly, in addition to its ribonuclease function, IRE1 has a kinase domain that regulates non-UPR signaling in response to ER stress, such as the activation of nuclear factor kappa light chain enhancer of activated B cells (NFκB)[36].
The IRE1/XBP1 pathway is dysregulated in multiple ER stress-associated human diseases[25], which led to mechanistic studies of the IRE1/XBP1 pathway in different animal models. In neurodegenerative disease models, XBP1 appeared to be pathogenic in amyotrophic lateral sclerosis and Huntington’s disease via the inhibition of autophagy[37,38]. In Alzheimer’s disease and Parkinson’s disease models, however, XBP1-mediated UPR was neuroprotective[39,40]. Interestingly, unlike PERK, XBP1 was dispensable in prion-related disorders[27,41]. In gastrointestinal disorders, IRE1 alleviated ER perturbations in intestinal epithelial cells in inflammatory bowel disease[42], and XBP1 enhanced fibrogenic activity in hepatic stellate cells in a steatosis model[43]. XBP1 was also important for glucose and lipid homeostasis, and linked obesity to type 2 diabetes[44,45]. Thus, the IRE1/XBP1 pathway has distinctive roles in disease progression depending on the pathogenesis. Accordingly, IRE1/XBP1 inhibitors and activators have been developed for disease treatment[28,46]. Still, more extensive and rigorous pre-clinical studies are needed to predict their effectiveness in the clinical setting.
The IRE1/XBP1 pathway is vital for pancreas development, as deficiency of IRE1 or XBP1 impaired exocrine pancreas development in Xenopus and mice[47-49]. In normal pancreatic acinar cells, the IRE1/XBP1 pathway has a basal activity level[50]. Inhibition of IRE1 or XBP1 reduced spontaneous digestive enzyme secretion in acinar cells[13,51], indicating that unlike PERK, the IRE1/XBP1 pathway is required for ordinary digestive function. Notably, inhibition of IRE1/XBP1 led to the over-activation of PERK in acinar cells, and over-activated PERK was associated with diminished XBP1 in AP[32,33]. Although XBP1 expression is transcriptionally regulated by ATF6[35], how XBP1 expression diminishes in AP remains unknown. Intriguingly, unlike in AP, XBP1 is elevated in CP along with other UPR elements[52]. These results suggest that diminished XBP1 could be an early event in the chain of UPR pathway dysregulation in AP.
ATF6
ER stress induces Golgi translocation and cleavage-activation of ATF6. The two proteases that sequentially cleave ATF6 on the Golgi are site one and two proteases (S1P and S2P), which also regulate cholesterol and fatty acid synthesis in the liver via cleavage-activation of sterol regulatory element-binding proteins (SREBPs)[53,54]. Cleaved ATF6 then enters the nucleus and activates the transcription of other genes required for UPR activities[18]. Compared to PERK and IRE1 that regulate diverse cellular activities, ATF6 mainly activates the transcription of chaperones and ERAD components. Notably, ATF6 also activates the transcription of XBP1[35], whose activity could in turn inhibit PERK activation[32,33]. Thus, ATF6 appears to initiate interactions among the three UPR pathways.
Studies have shown that ATF6-regulated UPR modulates hepatic and neurologic processes. In liver, ATF6 controls gluconeogenesis and blocks ER stress-induced steatosis[55,56]. In the nervous system, ATF6 is neuroprotective in Huntington’s disease via the activation of UPR’s pro-survival activities[57]. Mutations in ATF6 increase the susceptibility to ER stress-induced damage, which underlies the pathogenesis of the visual disorder achromatopsia[58]. Despite the recognized roles of ATF6 in diseases, no drugs have been developed to specifically target ATF6, and only a couple of S1P inhibitors have been used to experimentally reduce lipid synthesis and viral propagation[59,60].
Among PERK, IRE1 and ATF6, ATF6 seems to have the highest sensitivity to ER stress in acinar cells. This is because ATF6 nuclear translocation was observed much earlier than upregulation of BiP, XBP1 mRNA splicing or CHOP expression in a rat AP model[50]. Highly increased ATF6, along with phosphorylation of PERK and elF2 and upregulation of CHOP, was also observed in a mouse binge-drinking model[61]. We consistently found increased cleavage of ATF6 in acinar cells in response to cerulein-induced ER stress, and confirmed that S1P-mediated cleavage-activation of ATF6 was required for the protection of acinar cells in AP[62]. Thus, the ATF6 pathway is a potential target for AP treatment.
UPR IN THE REGULATION OF NFκB-MEDIATED INFLAMMATORY RESPONSE IN AP
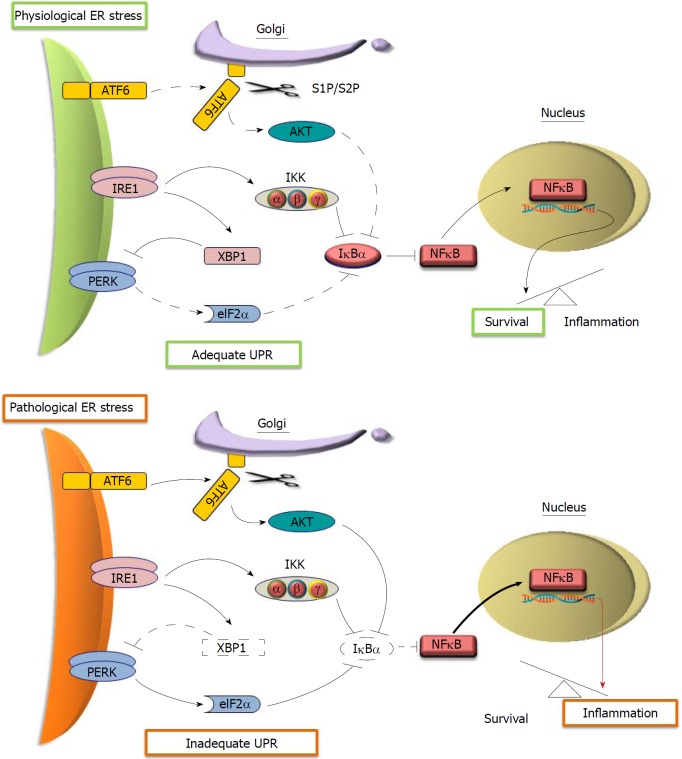
Although knowledge about inflammatory regulation is growing rapidly, how cells initiate inflammation in response to intracellular disturbances is still poorly understood. Interestingly, signaling pathways that control NFκB-mediated inflammatory responses and the UPR were found to be integrated, strongly suggesting that they originate through a common mechanism[63]. As shown in Figure 2, activated eIF2α in the PERK pathway inhibits the translation of both NFκB and its inhibitor IκB, which results in the activation of NFκB since IκB has a much shorter half-life compared to NFκB[63]. Additionally, the kinase function of IRE1 can phosphorylate IκB kinase (IKK) in response to stress, resulting in the degradation of IκBα and subsequent NFκB activation[36]. ATF6 may also activate NFκB via AKT-mediated degradation of IκB[64].
Figure 2.
Proposed models of NFκB activation by the unfolded protein response in response to endoplasmic reticulum stress. In physiological endoplasmic reticulum (ER) stress, adequate unfolded protein response (UPR) activates basal levels of NFκB nuclear translocation that trigger the transcription of pro-survival genes (upper panel). In pathological ER stress, NFκB upregulated by inadequate UPR activates the transcription of pro-inflammatory genes (lower panel). Arrows and lines represent active (solid) and ineffective (dotted) signaling. Thick and red arrows symbolize enhanced interactions and pro-inflammatory signaling, respectively.
In the pancreas, evidence has been mounting in support of a dual role of NFκB in the regulation of survival and inflammation in acinar cells. Basal NFκB activity is considered as prosurvival in acinar cells, while highly active NFκB favors the proinflammatory “arm”[65]. The prosurvival activity of NFκB in acinar cells is evidenced by worsened AP in the mouse pancreas with a loss-of-function mutation in the NFκB subunit p65[66], as well as the ameliorated AP in IκBα-mutated mice that have increased basal NFκB activity[67]. However, the proinflammatory effect becomes dominant when NFκB is over-activated in AP[68,69]. As shown in Figure 2, we propose that adequate UPR induces basal NFκB activity to enhance the survival of acinar cells, since besides IRE1/IKK, neither PERK/eIF2α nor ATF6/AKT are effective in inducing the degradation of IκBa. In dysregulated UPR, however, all three pathways are activated to effectively promote IκBα degradation. This results in significantly upregulated NFκB activity, which promotes the inflammatory response in AP. In support of this model, a study has shown that maximized NFκB activation can be induced by the cooperation between PERK/eIF2α-mediated translation repression and IRE1-mediated phosphorylation of IKK in response to ER stress[70]. In addition, the inhibition of AKT attenuated pancreas inflammation in a severe AP model associated with reduced activation of NFκB[71], supporting the possible role of ATF6-regulated AKT in the over-activation of NFκB in acinar cells.
UPR IN THE REGULATION OF AUTOPHAGY IN AP
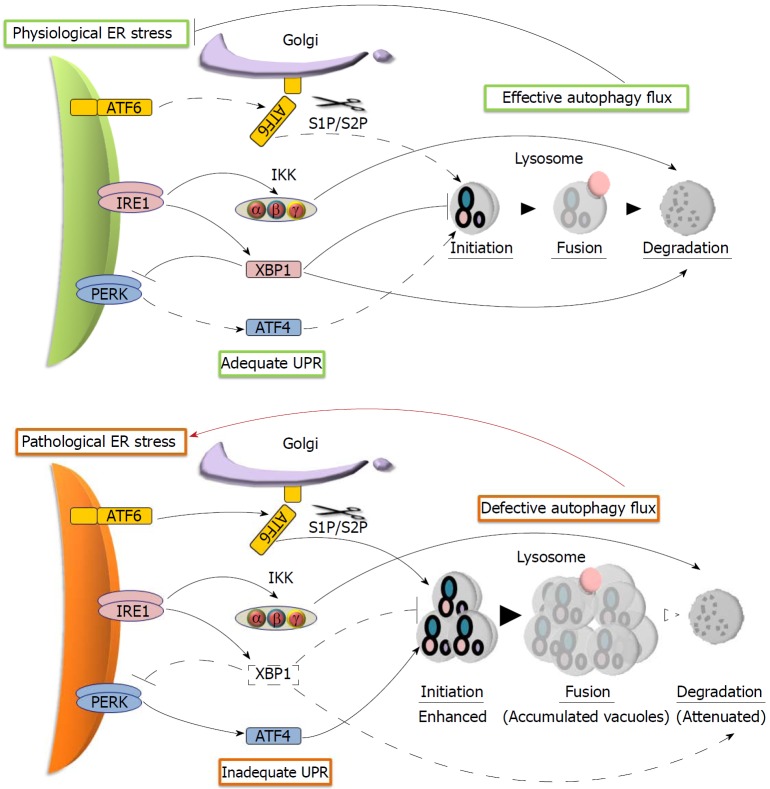
Autophagy is another fundamental protective activity, whose impairment has been considered as a point of convergence in the multiple deranged pathways of AP. Autophagy helps relieve ER stress by regulating cellular degradation[72]. Impaired autophagy in AP is characterized by defective autophagic flux, with the accumulation of large autophagic vacuoles manifesting as vacuolization in acinar cells. As shown in Figure 3, the pathways in adequate UPR help maintain autophagic flux. XBP1 prevents the accumulation of autophagic vacuoles by repressing the induction of autophagy and facilitating the processing of cathepsin, a lysosomal protease required for the activation of acid hydrolases in autophagic vacuoles[37,38,73,74]. The promotion of autophagic protein degradation by IRE1-activated IKK also facilitates autophagic flux in acinar cells, since both IRE1-deficient mice and IKK-deficient mice have spontaneous acinar cell vacuolization[49,75]. In addition to IRE1/XBP1, the role of ATF4 and ATF6 in the promotion of autophagy cannot be excluded, since they activate the transcription of autophagy genes[76,77]. In AP, however, we propose that the initiation of autophagy is significantly enhanced by a combined effect of diminished XBP1 with upregulated ATF6 and ATF4, while the protein degradation is attenuated because of the lack of enough XBP1 to effectively process cathepsins. These dysregulations of autophagic flux could synergistically induce the vacuolization of acinar cells, which further aggravates pathogenic ER stress in AP (Figure 3).
Figure 3.
Proposed models of autophagy flux regulation by the unfolded protein response in response to endoplasmic reticulum stress. In physiological endoplasmic reticulum (ER) stress, adequate unfolded protein response (UPR) maintains effective autophagy flux that relieves ER stress (upper panel). In pathological ER stress, however, inadequate UPR causes the accumulation of intracellular vacuoles, which further aggravates ER stress (lower panel). Arrows and lines represent active (solid) and ineffective (dotted) signaling. Thick and red arrows symbolize enhanced interactions and harmful activity, respectively.
Thus, multiple lines of evidence support a model of AP in which the protective UPR is undesirably transformed into a driving force behind pathogenic ER stress, proinflammatory NFκB activation and defective autophagy in injured acinar cells. Further validation of this model could help elucidate the pathogenesis of AP.
UPR AND AP MANAGEMENT
Recognition of the failure of inadequate UPR to relieve ER stress as an initiation fact of acinar cell injury in AP can make clinicians more cautious of using the medications that impair the UPR in patients with AP risk. For example, Bortezomib, an ERAD inhibitor used in the treatment of patients with multiple myeloma, induced AP[78].
Understanding the UPR in AP helps address the concern of the replacement of total parenteral nutrition with enteral nutrition in current AP management. Total parenteral nutrition was a universal management therapy for both mild and severe AP in the 1980s and 1990s. This was because total parenteral nutrition was thought to alleviate the burden on injured acinar cells in AP, since acinar secretion in healthy individuals induced by enteral nutrition can be avoided with parenteral nutrition[79]. In AP patients, however, enteral nutrition may not necessarily increase the enzyme production in acinar cells, since the PERK pathway that blocks the synthesis of digestive enzymes is highly activated in acinar cells. Indeed, multiple studies have proven that enteral nutrition does not worsen the pancreatic injury in AP patients, but has significantly decreased the risk of intestinal infection associated with total parenteral nutrition[3].
In addition, some strategies in current AP management alleviate the inflammatory microenvironment that otherwise could worsen ER stress and dysregulated UPR in AP. For example, early aggressive hydration is somehow effective in preventing serious complications, such as pancreatic necrosis[3]. The considered underlying mechanisms include resolving the hypoxia, nutrient deprivation, and pH changes in the inflamed AP tissues that may aggravate the dysregulation of the UPR in injured acinar cells. Moreover, the shifting concept of surgical management of pancreatic necrosis also supports the importance of the microenvironment in acinar injury. Open necrosectomy was previously practiced widely for necrotizing pancreatitis. However, studies have shown that the mortality in stable patients with infected necrosis can be significantly reduced if necrosectomy is delayed until the necrosis is walled-off by fibrous tissue. This favorable outcome is likely associated with the recovery of the UPR in residual acinar cells. Similarly, the minimally-invasive step-up approach that can efficiently minimize the surgical trauma and stress in residual acinar cells has been shown to be superior to open necrosectomy for necrotizing pancreatitis[80].
The finding of dysregulated UPR in AP also provides potential targets for new pharmacological intervention. During the pathogenesis of AP, the initial chain of events in the acinar cells that lead to the clinical presentation of AP are quite distant to the patient presenting in the emergency room. Hours later, when the patient presents, it may seem to be too late for pancreatic function-targeted interventions to be beneficial[5]. However, this is not likely to be true for several reasons. The majority of patients have mild disease upon admission and only progress to severe disease over the next 24-48 h[3]. Additionally, few patients who develop necrosis of the pancreas have this finding on admission computed tomography. Most complications of the disease, such as pulmonary edema, sepsis and renal failure, develop later in the course of the disease. Considering the pattern of clinical progression and the ongoing acinar cell destruction seen as pancreatic necrosis evolves, the events in the acinar cell that cause AP represent an important target for pharmacological intervention. This is supported by the fact that up to 80% of AP cases are self-limited by self-protective mechanisms[2,3], such as the UPR that alleviates the disturbances in acinar cells. Therefore, targeting the UPR seems to be a reasonable strategy to prevent the aggravation of pancreatic injury and inflammation in AP when the patient presents.
CONCLUSION
In summary, we have proposed that dysregulated UPR plays a decisive role in the pathogenesis of AP. Of note, in comparison to the rapidly-growing research on other ER stress-associated disorders such as neurodegenerative diseases, studies of how AP risk factors impair the UPR and lead to acinar cell injury are very limited. In order to improve AP management, more efforts and resources are needed to identify the UPR pathway as a potential target for therapeutic intervention in AP.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: June 21, 2018
First decision: July 23, 2018
Article in press: August 21, 2018
P- Reviewer: Mann O, Nagahara H, Zhu X, Zhu X S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Kaylene Barrera, Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Albert Stanek, Department of Surgery and Pathology, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Kei Okochi, College of Medicine, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Zuzanna Niewiadomska, Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Cathy Mueller, Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Peiqi Ou, School of Graduate Studies, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Devon John, Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Antonio E Alfonso, Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States.
Scott Tenner, Greater New York Endoscopy Surgical Center, State University of New York, Brooklyn, NY 11235, United States.
Chongmin Huan, Department of Surgery and Cell Biology, State University of New York, Downstate Medical Center, Brooklyn, NY 11203, United States. chongmin.huan@downstate.edu.
References
- 1.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 3.Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 4.Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde. 1896;17:69–96. [Google Scholar]
- 5.Singh VP, Chari ST. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology. 2005;128:2172–2174. doi: 10.1053/j.gastro.2005.03.087. [DOI] [PubMed] [Google Scholar]
- 6.Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 7.Willemer S, Bialek R, Adler G. Localization of lysosomal and digestive enzymes in cytoplasmic vacuoles in caerulein-pancreatitis. Histochemistry. 1990;94:161–170. doi: 10.1007/BF02440183. [DOI] [PubMed] [Google Scholar]
- 8.Cavallini G, Tittobello A, Frulloni L, Masci E, Mariana A, Di Francesco V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy--Italian Group. N Engl J Med. 1996;335:919–923. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- 9.Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217.e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sah RP, Dudeja V, Dawra RK, Saluja AK. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144:1076–1085.e2. doi: 10.1053/j.gastro.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kereszturi E, Szmola R, Kukor Z, Simon P, Weiss FU, Lerch MM, Sahin-Tóth M. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat. 2009;30:575–582. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logsdon CD, Ji B. The role of protein synthesis and digestive enzymes in acinar cell injury. Nat Rev Gastroenterol Hepatol. 2013;10:362–370. doi: 10.1038/nrgastro.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waldron RT, Pandol S, Lugea A, Groblewski G. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Exocrine Pancreas Physiology and Pancretitis. Pancreapedia: Exocrine Pancreas Knowledge Base; 2015. [Google Scholar]
- 14.Lugea A, Waldron RT, Pandol SJ. Pancreatic adaptive responses in alcohol abuse: Role of the unfolded protein response. Pancreatology. 2015;15:S1–S5. doi: 10.1016/j.pan.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sah RP, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol. 2012;28:507–515. doi: 10.1097/MOG.0b013e3283567f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 17.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 18.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 19.Lugea A, Gerloff A, Su HY, Xu Z, Go A, Hu C, French SW, Wilson JS, Apte MV, Waldron RT, et al. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology. 2017;153:1674–1686. doi: 10.1053/j.gastro.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandol SJ, Gorelick FS, Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function - a hypothesis. Front Physiol. 2011;2:8. doi: 10.3389/fphys.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 22.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Tsuru A, Imai Y, Saito M, Kohno K. Novel mechanism of enhancing IRE1α-XBP1 signalling via the PERK-ATF4 pathway. Sci Rep. 2016;6:24217. doi: 10.1038/srep24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis. 2010;5:29. doi: 10.1186/1750-1172-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5:206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 28.Maly DJ, Papa FR. Druggable sensors of the unfolded protein response. Nat Chem Biol. 2014;10:892–901. doi: 10.1038/nchembio.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi: 10.1186/1471-2121-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Sartori DJ, Li C, Yu QC, Kushner JA, Simon MC, Diehl JA. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol Cell Biol. 2012;32:5129–5139. doi: 10.1128/MCB.01009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng TI, Wu HY, Chen BL, Jhuang JY, Huang KH, Chiang CK, Liu SH. C/EBP homologous protein deficiency aggravates acute pancreatitis and associated lung injury. World J Gastroenterol. 2013;19:7097–7105. doi: 10.3748/wjg.v19.i41.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldron RT, Javaherizadeh P, Gerloff A, Zeng Q, Patterson JB, Pandol SJ, Lugea A. Differential modulation of XBP1 and PERK ER stress pathways in acute pancreatitis. Pancreatology. 2013;13:e83. [Google Scholar]
- 33.Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 36.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, Caballero B, Kiffin R, Segura-Aguilar J, Cuervo AM, et al. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum Mol Genet. 2012;21:2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Hum Mol Genet. 2011;20:2144–2160. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sado M, Yamasaki Y, Iwanaga T, Onaka Y, Ibuki T, Nishihara S, Mizuguchi H, Momota H, Kishibuchi R, Hashimoto T, et al. Protective effect against Parkinson’s disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 41.Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci USA. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim RS, Hasegawa D, Goossens N, Tsuchida T, Athwal V, Sun X, Robinson CL, Bhattacharya D, Chou HI, Zhang DY, et al. The XBP1 Arm of the Unfolded Protein Response Induces Fibrogenic Activity in Hepatic Stellate Cells Through Autophagy. Sci Rep. 2016;6:39342. doi: 10.1038/srep39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 46.Jiang D, Niwa M, Koong AC. Targeting the IRE1α-XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol. 2015;33:48–56. doi: 10.1016/j.semcancer.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan L, Li X, Feng J, Yin C, Yuan F, Wang X. IRE1α is essential for Xenopus pancreas development. J Biomed Res. 2014;28:123–131. doi: 10.7555/JBR.28.20130076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwawaki T, Akai R, Kohno K. IRE1α disruption causes histological abnormality of exocrine tissues, increase of blood glucose level, and decrease of serum immunoglobulin level. PLoS One. 2010;5:e13052. doi: 10.1371/journal.pone.0013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kubisch CH, Sans MD, Arumugam T, Ernst SA, Williams JA, Logsdon CD. Early activation of endoplasmic reticulum stress is associated with arginine-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G238–G245. doi: 10.1152/ajpgi.00471.2005. [DOI] [PubMed] [Google Scholar]
- 51.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sah RP, Garg SK, Dixit AK, Dudeja V, Dawra RK, Saluja AK. Endoplasmic reticulum stress is chronically activated in chronic pancreatitis. J Biol Chem. 2014;289:27551–27561. doi: 10.1074/jbc.M113.528174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, Mori K. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naranjo JR, Zhang H, Villar D, González P, Dopazo XM, Morón-Oset J, Higueras E, Oliveros JC, Arrabal MD, Prieto A, et al. Activating transcription factor 6 derepression mediates neuroprotection in Huntington disease. J Clin Invest. 2016;126:627–638. doi: 10.1172/JCI82670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohl S, Zobor D, Chiang WC, Weisschuh N, Staller J, Gonzalez Menendez I, Chang S, Beck SC, Garcia Garrido M, Sothilingam V, et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet. 2015;47:757–765. doi: 10.1038/ng.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins JL, Robbins MD, Warren LC, Xia D, Petras SF, Valentine JJ, Varghese AH, Wang IK, Subashi TA, Shelly LD, et al. Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J Pharmacol Exp Ther. 2008;326:801–808. doi: 10.1124/jpet.108.139626. [DOI] [PubMed] [Google Scholar]
- 60.Urata S, Yun N, Pasquato A, Paessler S, Kunz S, de la Torre JC. Antiviral activity of a small-molecule inhibitor of arenavirus glycoprotein processing by the cellular site 1 protease. J Virol. 2011;85:795–803. doi: 10.1128/JVI.02019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren Z, Wang X, Xu M, Yang F, Frank JA, Ke ZJ, Luo J. Binge ethanol exposure causes endoplasmic reticulum stress, oxidative stress and tissue injury in the pancreas. Oncotarget. 2016;7:54303–54316. doi: 10.18632/oncotarget.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrera K, Okochi K, Stanek A, Mueller C, Ou P, Alfonso A, Huan C. Site-1-Protease mediated unfolded protein response protects against pancreatic injury in acute pancreatitis. Gastroenterology. 2017;152:S894–895. [Google Scholar]
- 63.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, et al. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gukovsky I, Gukovskaya A. Nuclear factor-κB in pancreatitis: Jack-of-all-trades, but which one is more important? Gastroenterology. 2013;144:26–29. doi: 10.1053/j.gastro.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Algül H, Treiber M, Lesina M, Nakhai H, Saur D, Geisler F, Pfeifer A, Paxian S, Schmid RM. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J Clin Invest. 2007;117:1490–1501. doi: 10.1172/JCI29882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neuhöfer P, Liang S, Einwächter H, Schwerdtfeger C, Wartmann T, Treiber M, Zhang H, Schulz HU, Dlubatz K, Lesina M, et al. Deletion of IκBα activates RelA to reduce acute pancreatitis in mice through up-regulation of Spi2A. Gastroenterology. 2013;144:192–201. doi: 10.1053/j.gastro.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 68.Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G, Wirth T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117:1502–1513. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang H, Liu Y, Daniluk J, Gaiser S, Chu J, Wang H, Li ZS, Logsdon CD, Ji B. Activation of nuclear factor-κB in acinar cells increases the severity of pancreatitis in mice. Gastroenterology. 2013;144:202–210. doi: 10.1053/j.gastro.2012.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7:e45078. doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu P, Wang J, Yang ZW, Lou XL, Chen C. Regulatory roles of the PI3K/Akt signaling pathway in rats with severe acute pancreatitis. PLoS One. 2013;8:e81767. doi: 10.1371/journal.pone.0081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gukovskaya AS, Gukovsky I. Autophagy and pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G993–G1003. doi: 10.1152/ajpgi.00122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan D, Wang HW, Bowman RL, Joyce JA. STAT3 and STAT6 Signaling Pathways Synergize to Promote Cathepsin Secretion from Macrophages via IRE1α Activation. Cell Rep. 2016;16:2914–2927. doi: 10.1016/j.celrep.2016.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Lee J, Fisher SJ, White MF, Biddinger SB, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li N, Wu X, Holzer RG, Lee JH, Todoric J, Park EJ, Ogata H, Gukovskaya AS, Gukovsky I, Pizzo DP, et al. Loss of acinar cell IKKα triggers spontaneous pancreatitis in mice. J Clin Invest. 2013;123:2231–2243. doi: 10.1172/JCI64498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.B'chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41:7683–7699. doi: 10.1093/nar/gkt563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zalckvar E, Berissi H, Mizrachy L, Idelchuk Y, Koren I, Eisenstein M, Sabanay H, Pinkas-Kramarski R, Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solakoglu T, Akyol P, Guney T, Dilek I, Atalay R, Koseoglu H, Akin E, Demirezer Bolat A, Buyukasik NS, Ersoy O. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189–190. doi: 10.1016/j.pan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 79.O'Keefe SJ, Lee RB, Anderson FP, Gennings C, Abou-Assi S, Clore J, Heuman D, Chey W. Physiological effects of enteral and parenteral feeding on pancreaticobiliary secretion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G27–G36. doi: 10.1152/ajpgi.00155.2002. [DOI] [PubMed] [Google Scholar]
- 80.van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]