Abstract
Microcystins (MCs) are produced by certain bloom-forming cyanobacteria that can induce toxicity in various organs, including renal toxicity, reproductive toxicity, cardiotoxicity, and immunosuppressive effects. It has been a significant global environmental issue due to its harm to the aquatic environment and human health. Numerous investigators have demonstrated that MC exposure can induce a widespread epidemic of enterogastritis with symptoms similar to food poisoning in areas close to lakes. Both in vivo and in vitro studies have provided evidence of positive associations between MC exposure and gastrointestinal toxicity. The toxicity of MCs on the gastrointestinal tract is multidimensional. MCs can affect gastrointestinal barrier function and shift the structure of gut microbiota in different gut regions. Furthermore, MCs can inhibit the secretion of gastrointestinal digestive enzymes and the release of inflammatory cytokines, which affects the expression of immune-related genes in the intestine. The damage of the intestine is closely correlated to MC exposure because the intestine is the main site for the digestion and absorption of nutrients. The damage to the gastrointestinal tract due to MCs was summarized from different aspects, which can be used as a foundation for further exploration of molecular damage mechanisms.
Keywords: Immunotoxicity, Gastrointestinal toxicity, Intestine, Depuration, Oxidative stress, Microcystins
Core tip: First, the gastrointestinal toxicity of microcystins (MCs) on a population was described. Second, the concentration or localization of MCs in the small intestine after exposure to various concentrations and different time points, as well as after the depuration of MCs in the intestine, was summarized. Third, the change in morphologic pathology and other effects such as oxidative stress, immunotoxicity, digestive enzymes, and gut microbiota in the intestine with exposure to MCs were discussed. Further challenges that need to be addressed were also summarized.
INTRODUCTION
The development of industrialization accompanied by the increase of effluent discharge leads to the occurrence of cyanobacterial bloom and red tide due to nitrogen and phosphorous overload. Previous studies have demonstrated that almost 80% of algal blooms could produce secondary metabolites. This includes microcystins (MCs), which have a general structure of cyclo (-D-Ala1-L-R12-D-erythro-β-methylAsp3-L-R24-Adda5-D-Glu6-N-methyldehydro-Ala7), in which R1 and R2 are the variable L amino acids responsible for most of the congeners (Figure 1)[1]. Furthermore, more than 100 structural variants have been identified, among which microcystin-LR (MC-LR) was the most widespread and virulent type, followed by microcystin-RR (MC-RR), and microcystin-YR (MC-YR)[2,3].
Figure 1.
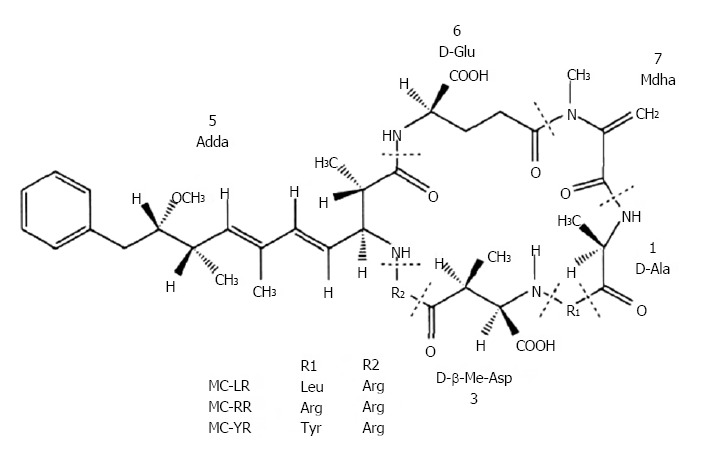
Chemical structure of microcystins.
MCs pose a threat to human health in many ways, such as oral, dermal and inhalation pathways, and exposure may occur during recreation depending on the types of activities undertaken in the water[4,5]. Freshwater aquaculture ponds are important artificially regulated aquatic ecosystems that provide a large number of freshwater fish products in China[6]. Moreover, China’s freshwater aquaculture area and production stands first in the world[7]. Studies have shown that MCs can accumulate in a variety of aquatic organisms, such as zooplankton, bivalves, crustaceans, fish, and aquatic vertebrates[6]. It may exert a potential toxic hazard to humans after the consumption of MC-contaminated aquatic products. Recently, studies have shown that MCs can accumulate in edible crops and soils irrigated with MC-contaminated water[3]. It has a potential risk to be transferred to the human body through the ingestion of these vegetables[3]. Moreover, if MC-contaminated water is applied during medical treatment, this may lead to acute intoxication in patients and even death. Although such incidents seldom happen, this should arouse our attention for its serious consequences.
Studies have indicated that MCs accumulate mainly in the liver, and can be transported to the kidneys, muscle, brain, and intestines through blood circulation[6]. Furthermore, MCs have been confirmed to be transported into cells via organic anion transporting polypeptides (OATPs), which exist in almost every organ[8], while some OATPs are preferentially or even selectively expressed in specific tissues[9]. Thus, the susceptibility among tissues toward MC exposure may be explained by OATP subtypes and the OATP subtype-selective transport of specific MC congeners[8,10]. Therefore, OATPs are the prerequisite for the toxicity that MCs can exert. Previous reports have shown that MCs can cross the intestinal barrier via OATP3A1 and OATP4A1, which are located in the small intestine epithelium[11]. This follows that MCs may pose a potential threat to human health. Therefore, the present study aimed to perform a compilation of increasing information that involves intestinal toxicity with regard to fish, mammals, cells, and group surveys, in order to summarize the present research gaps that should be addressed through further studies.
GASTROINTESTINAL TOXICITY OF MCs ON THE POPULATION
The detriments caused by MCs produced by blue-green algae have attracted worldwide concern. Epidemiological investigation and toxicology research have been used to illuminate the perniciousness of MCs. Epidemiological investigations can reflect the direct association between the health and MC exposure of the population, which is especially important to identify the hazards of MCs on mankind.
Study on enterogastritis with exposure to MC-LR
Widespread epidemics of enterogastritis with symptoms similar to those during food poisoning were found in a series of towns alongside Elk River in Charleston, West Virginia. A survey revealed that the acute gastroenteritis outbreak was on a large scale, and was not caused by bacterial infection but by toxins. After all measures were ineffective in removing toxins, it was speculated that the toxin was most likely MC[12]. Pilotto et al[13] discovered that there was a positive association between the incidence of gastroenteritis and the exposure time and density of cyanobacteria through a prospective investigation. Furthermore, there was a significant trend of the increasing occurrence of enterogastritis when exposed to more than 5000 cyanobacterial cells/mL for more than one hour[13]. Those results show that MCs can lead to acute gastroenteritis in humans.
Effect on gastroenteric carcinomas
Zhou et al[14] investigated the association of MCs in drinking water with the incidence of colorectal cancer through a retrospective cohort. In that study, eight towns in Haining City of Zhejiang Province, China were randomly selected as study sites, and 408 cases of colon and rectum carcinomas were selected for study. The results revealed that the incidence of colorectal cancer was significantly higher in the population that drank river and pond water compared to the population that drank well and tap water, suggesting that MCs may play a causative role in the carcinogenesis of the colon and rectum[14]. Other studies also manifested the positive association between MCs and the incidence of colon and rectum carcinomas. Lin et al[15] discovered that male colorectal cancer mortality increases as the MC content increases in water. Furthermore, a consistent trend between the positive detection rate of MCs and the mortality of colorectal cancer was found. Moreover, Chen et al[16] discovered the positive association between the concentration of MCs and the morbidity of colorectal cancer. In addition, Falconer et al[17] demonstrated that MC exposure is a contributing factor to the increased mortality of gastric cancer.
CONCENTRATION OR LOCALIZATION OF MCs IN THE SMALL INTESTINE
The toxicity of MCs mainly depends on the accumulation dose[2], which can be determined through the intake and depuration of MCs in the body. Furthermore, the location of MCs in cells changes along with the accumulation of MCs thereby causing different degrees of damage.
Localization of MCs in the small intestine
Previous studies have demonstrated that MCs can be detected in the small intestine because a specific OATP membrane transport system can carry it into the enterocytes[11]. After exposure of 0-50 μmol/L of MC-LR concentrations for 24 h in IEC-6 cells, the intracellular localization of MC-LR was in the cytoplasm and around the nucleus. Furthermore, western blot results revealed that there was a dose-dependent content following exposure to MC-LR[18].
Caco-2 cells have microvilli and cellulose associated with brush border epithelium with a similarity in structure and function in small intestine epithelial cells[19]. Therefore, it can be used to simulate intestinal transshipment. Caco-2 cells were treated with concentrations of MC-LR or MC-RR ranging from 1-50 μmol/L for four hours. There was no difference in the subcellular localization of MC-LR or MC-RR among concentrations (Figure 2A). After incubation of Caco-2 cells in a fixed concentration of 20 μmol/L of MC-LR or MC-RR, the intracellular location of MC-LR or MC-RR was observed over a period of time. These results show that staining at the cell membrane could be observed after thirty minutes of treatment, which subsequently progressed to the cytoplasm after two hours and around the nucleus after six hours (Figure 2B). These results confirm that MC-LR and MC-RR could reach the nucleus[11], suggesting that MC-LR may induce DNA damage. In addition, it has been demonstrated that DNA damage can be induced in intestinal tissues after exposure to 50 μg/kg of body weight of MC-LR in mice for 24 h[20].
Figure 2.
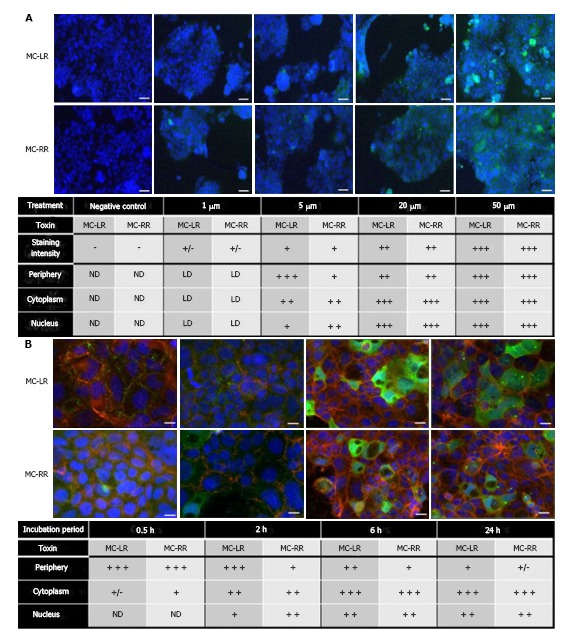
Subcellular localization of MC-LR and MC-RR in Caco-2 cells, which was adapted from a reference[11]. A: Caco-2 cells were treated for four hours with concentrations of MC-LR or MC-RR ranging from 1-50 μmol/L; B: Caco-2 cells were treated for several time points with 20 μmol/L of MC-LR or MC-RR. The cellular localization of these toxins was detected using an anti-ADDA antibody and an Alexa fluor 488 secondary antibody. Nuclei were counterstained with DAPI. The symbols (−), (+), (++) and (+++) represent the relative importance of the staining localization into the cell. Scale bars: A: 20 μm; B: 10 μm. ND: Alexa fluor 488 staining not detected; LD: Low signal of Alexa fluor 488 staining; (+/−) low signal intensity; MC: Microcystin.
In another in vivo study, medaka fish were administered an oral gavage of MC-LR extracts at a dose of 100 mg/L for two hours. The results revealed that the labeled areas were detectable in the intestinal submucosa, and can be seen in the cytoplasm of the submucosal macrophages in adult medaka intestine[21].
Concentration of MCs in the small intestine
OATPs are present in almost every organ or tissue[8]. MCs can be transported to organs such as the kidney, intestine, heart, spleen, and pancreas, and is mediated by blood circulation and OATPs. Various studies have revealed that MC-LR concentrations are higher in the intestines than in other organs[22-26], but MC-RR concentrations are higher in the hepatopancreas than in the intestines under the same condition[7,23,24,27]. The difference in the susceptibility of tissues towards MC-LR and MC-RR exposure may be explained by the OATP subtype-selective transport of specific MC congeners. Moreover, studies have shown that the intestine had the highest MC concentration (MC-LR + MC-RR)[28]. Other studies have also shown that MC content was higher in the hepatopancreas, followed by the intestine and other organs[29,30]. Furthermore, studies manifested that the intestine had relatively low MC content[27,31]. The difference in MC content in the intestine and other organs among species and the different categories of the same species may be explained by the different mechanisms of uptake and depuration among other factors[32].
In studies in vivo, MC concentrations in different parts of the gut were detected by liquid chromatography-mass spectrometry (LC-MS), in which the MC concentration was much higher in the mid-gut walls than in the hind- and fore-gut walls. This clarifies the importance of the mid-gut wall as a major site for MC absorption[32]. The concentration or localization of MCs in the small intestine is summarized in Table 1.
Table 1.
Summary of concentrations or locations of microcystins in experimental models
| Model | Toxins | Exposure | Dose | Time | Experiments | Con. or loc. | Ref. |
| Caco-2 cell | MC-LRMC-RR | Incubation | 1, 5, 20, 50 μmol/L | 0.5, 2, 6, 24 h | Immunofluorescence | Periphery, cytoplasm, nucleus | [11] |
| IEC-6 cell | MC-LR | Incubation | 6.25, 12.5, 25, 50 μmol/L | 6, 12, 24 h | Immunofluorescence WB | Cytoplasm, around the nucleus | [18] |
| Medaka fish | MC-LR | Gavage | 100 mg/L | 2 h | Immunohistochemistry | In the cytoplasm of the submucosal macrophages | [21] |
| Cyprinus carpio, anguilla anguilla | MC-LR MC-RR | Immersion | Lake Oubeira | 12 mo | HPLC | Intestine > hepatopancreas liver > intestine | [22] |
| Aristichthys nobilis | MC-LR MC-RR | i.p. | 50, 200 μg/kg body weight | 1, 3, 12, 24, 48, 72 h | LC–ESI-MS | Higher in intestine than in other organs liver > intestine | [23] |
| Silver carp | MC-LR/MC-RR | Immersion | 40 mm plankton net | 40, 80 d | HPLC | 49.2 and 115.3 (average 78.8) μg/g DW | [24] |
| Crayfish | MC-LR | Immersion | 0.1, 1, 10, 100 μg/L | 8 h, 1, 3, 4, 7 d | HPLC | Higher in intestine than in other organs | [25] |
| Bellamya aeruginosa | MC-LR MC-RR MC-YR | Immersion | Lake Taihu | 12 mo | LC-MS | Intestine > hepatopancreas | [26] |
| Jenynsia multidentata, corydoras paleatus | MC-RR | Immersion | 50 μg/L | 24 h | HPLC LC-ESI-TOF–MS | Liver > intestine | [27] |
| Aristichthys nobilis | MC-LR | Immersion | Lake Taihu | 12 mo | LC-MS/HPLC-UV | 85.67 mg/g DW | [28] |
| Freshwater mussels | MC-LR MC-RR MC-YR | Immersion | Lake Taihu | 12 mo | LC-MS HPLC | 20.65 μg/g DW | [30] |
| Carassius auratus | MC-LRMC-RR | i.p. | 200 μg/kg body weight | 1, 3, 12, 24, 48 h | LC-MS | Less than 0.1% of the injected MCs | [31] |
| Bivalves | MC-LR MC-RR MC-YR | Immersion | Lake Taihu | 6 mo | LC-MS | Hepatopancreas > intestine | [32] |
| Wistar rat | MC-LR | i.v. | 80 μg/kg body weight | 1, 2, 4, 6, 12, 24 h | LC-MS | Less than 0.2% of injected MCs | [33] |
| Carassius carassius | MC-LR MC-RR | i.p. | 50 μg/kg body weight | 1, 3, 12, 24, 48, 168 h | LC-MS | Less than 0.05% of injected MCs | [34] |
| Freshwater fish at different trophic levels | MC-LR/MC-RR | Immersion | Lake Chaohu | - | HPLC/LC/ESI-MS | Higher in intestine than in other organs | [35] |
“Con.”, “loc.” and “-” represent the concentration, location and no data, respectively. MC: Microcystin; LC-MS: Liquid chromatography-mass spectrometry; HPLC: High-performance liquid chromatography.
Depuration of MCs in the intestine
MCs can accumulate and metabolize in various tissues through the blood and exert toxic effects. Studies have shown that no MC-LR was detectable despite the abundant presence of MC-RR in the intestine[24], while Wang and Yuan et al[25] found that MC-LR content was much higher in the intestine than in other organs, which may be the result of the selective absorption of MCs in small intestine epithelial cells, or the difference between the elimination and inhibition in the intestine among species[23,25]. Chen et al[29] demonstrated that the highest concentration of MCs was found in the kidneys, and two peaks were observed, indicating that MCs can be directly excreted via the kidney in rats. The kidneys function as an important excretory organ and play a significant role in filtering to form urine and discharge metabolic wastes, which regulate the balance between electrolytes and acid-base. Moreover, Lei et al[31] found that a significant negative correlation was present between MC-RR concentrations in blood and in the kidneys, confirming that the blood plays an important role in the transportation of MC-RR to the kidneys for excretion. From the above, it can be found that MCs may accumulate in the intestines. Studies have revealed that MCs exist mainly in two forms in animal tissues: Covalently bound MCs and methanol-extractable forms[25]. MCs accumulate rapidly, but are slowly eliminated in the intestine[25,31], which is probably due to the presence of MCs in the intestine with a covalently bound form[25].
PATHOLOGICAL EFFECT OF MCs IN THE INTESTINES
MCs can not only induce damage to enterocytes in vitro, but also induce pathological injury in vivo, leading to the production of oxides, the parasecretion of immunocytokines and digestive enzymes, and disorders in intestinal secretion of water, electrolytes, and intestinal flora.
Effect of MCs on Enterocytes
Previous studies indicated that MCs can be transported across enterocyte membranes via OATPs[2]. Moreover, studies have manifested that cell viability significantly decreased, while the ratio of apoptotic cells increased, after intestinal epithelial cells (IEC-6) and human intestinal Caco-2 cells were exposed to MCs[18]. MCs can affect the integrity of the intestinal barrier by decreasing its transepithelial electrical resistance (TEER) values and inducing the cytoskeletal protein expression of occludin and ZO-1 in IECs in a dose-dependent decrease[18]. Tight junctions (TJs) are composed of occludin and ZO-1. The dysfunction of the intestinal TJ barrier is an important event in the pathogenesis of enteropathies. MCs may influence the barrier function of the intestine by affecting the TJs of IEC-6. Furthermore, MCs can increase intracellular ROS production and promote proinflammatory cytokine secretion, including IL-6 and IL-8[36], which contributes to the damage to the small intestinal epithelial cells. Humpage et al[37] confirmed that MCs could induce the transformation of normal crypt cells (NCC). This follows that MCs can induce damage to enterocytes.
Effect of MCs on morphology
The intestines hollow body comprises of four concentric layers: mucosa, submucosa, muscle and serosa[38]. MCs may have different effects on these layers. MCs can induce the pathological lesions on the small intestinal mucosa in a dose-dependent manner. A large quantity of macrophages has been found below the epithelium[38], illustrating the loss of adhesion between loose connective tissues and the tract. Large amounts of macrophages were noted in the submucosa with prolonged exposure time and concentration[21,38]. Erythrocyte cells, such as goblet cells, play a significant role in the protection and digestion function of the intestine. Several zones of lysis and fewer goblet cells were detected in the intestinal epithelium in MC-LR-treated fish[39]. Nevertheless, the most prominent characteristic was the loss of microvilli and exfoliation of epithelial cells[38-41]. Intestinal pathological changes were characterized by hyperplasia, which thickened at several points in the lining of the intestinal mucosa, resulting in an undulating surface[42,43]. Fish treated with 25 μg and 50 μg of MC-LR exhibited a catarrhal enteritis process and necrotic enteritis, respectively[40]. An ultrastructural study revealed that in severe damage zones of the intestine, the vacuolization of enterocytes and hemorrhaging vessels in the submucosa were observed, as well as loss of normal architecture in smooth muscle fibers and the necrosis of fibers[38,40]. The pathological damage on the intestine is presented in Figure 3.
Figure 3.
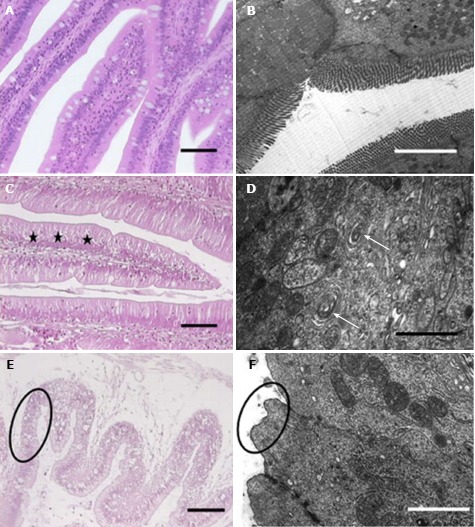
Histopathological and ultrastructural observations in the gastrointestinal tract of Tenca fish exposed to MCs. Adapted from a ref.[40]. H and E staining (A) and ultrastructural observations (B). A and B: Control groups; C and D: 25 mg of MC-LR/fish group; E and F: 55 mg of MC-LR/fish groups. Vacuolated enterocytes (star). Vacuolization of the endoplasmic reticulum (arrow). Pycnotic nuclei, vacuolated cytoplasm (E, circle). Total microvilli loss (F, circle).
Oxidative stress related to MC exposure
The toxicity of MCs is primarily due to the irreversible inhibition of serine/threonine protein phosphatase 1 and 2A[44,45], which leads to disruptions in the cytoskeleton, necrosis, cytoplasmic vacuolization, and consequent apoptosis. Furthermore, recent reports have shown that oxidative stress induced by MCs is the initial factor that causes other various injuries[4]. MCs can induce the oxidative stress of mitochondria, leading to the increase of mitochondrial permeability transition and the release of cytochrome c. This would consequently increases the protein expression of Bax and caspases-3, -8 and -9 (P < 0.01), and inhibits the protein expression of Bcl-2[46,47]. The toxicity of MC-LR is involved in alterations in oxidation and the antioxidant system[40,48,49]. Studies have shown that oxidative stress biomarkers (ROS, TBARS, and MDA) have a dose-dependent increase with exposure to MCs, while antioxidase (GSH, SOD, GPx, GR, and GST) activities in general increase at lower concentrations, and decreased at higher concentrations[48-50]. The results above suggest that the production of excessive oxidative substances and the dysfunction of the anti-oxidative system induced by MC-LR can lead to small intestinal lesions.
Immunotoxicity of MCs on the intestines
The mucosa plays important pleiotropic roles, including absorption, secretion and barrier functions. Furthermore, the mucosa has a lot of lymphocytes and macrophages, and a decrease in intraepithelial lymphocytes in the mucous of the intestine was detected with exposure to 50 μg/kg and 100 μg/kg of MC-LR for 48 h[49]. Furthermore, the mRNA levels of IFN-1, IL-1b, IL-8, TGF-b, and TNF-a dramatically increased in the intestine in all MC-LR treated groups[51]. Moreover, the destruction of the intestinal mucosal structure induced by MC-LR may be responsible for the dysfunction of mucosal immunity.
Effect of MCs on digestive enzymes
Digestive enzymes exist in the brush border of the intestinal mucosa and play an important role in the final digestion phase. Its activity is closely correlated to the integrity of the intestinal mucosa. Liu et al[52] observed that the activity of disaccharides, alkaline phosphatase and gamma-glutamyltransferase (γ-glutamyltransferase) declined after the intraperitoneal injection of MC-LR for 28 d. Yao et al[53] discovered that the activity of diastase and protease significantly declined in the intestines of silver carp in water contaminated by MCs. However, Moreno et al[50] were able to catch sight that the activity of diastase decreased, while other intestinal apical membrane enzymes (lactase, maltase, and alkaline phosphatase) were not modified after intravenous injection of MC-LR for eight hours. The results reported by Liu and Yao are not completely consistent, which was possibly due to the discrepancy in exposure dose and methods. Above all, MCs can affect the digestive function through altering the integrity of the intestinal mucosa.
Effect of MCs on the intestinal secretion of water and electrolytes
MCs can inhibit protein phosphatase and stimulate macrophagocytes to secrete the corresponding cytokines. Rocha et al[54] observed that MC-LR can induce the release of interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) through peritoneal macrophages in vitro and the secretion of electrolytes in rabbit ileal mucosa. Nobre et al[55] detected that MC-LR promoted the secretion of water and electrolytes (potassium, chloride, and sodium). The phosphorylation of residues in the N-terminus of the Na-K-Cl cotransporter is the mechanism that regulates the activation of intestinal fluid secretion in diarrheal toxicity[55]. A previous study has implicated protein phosphatase 1 (PP1) in the dephosphorylation of the Na-K-Cl cotransporter[55]. It has been recognized that MC-LR is a potent inhibitor of PP1 and protein phosphatase 2A (PP2A), which demonstrates that MC-LR may indirectly inhibit the dephosphorylation of the Na-K-Cl cotransporter by inhibiting the activity of PP1[55].
Effects of MCs on gut microbiota and other aspects
The small intestine consists of the duodenum, jejunum and ileum. The intestinum crassum can be divided into the cecum, colon and rectum. The toxicity of MCs on the gut significantly depends on the absorption capacity, which is determined by the structure of the gut. MCs have a higher affinity with OATPs and can be absorbed rapidly and efficiently eliminated with bile acids. Dahlem et al[56] revealed that MCs are absorbed mainly in the ileum but are relatively low in the jejunum because the ileum is an active site for the transport of bile salt. The gut microbiome is a diverse and carefully balanced ecosystem of great importance in keeping the intestine healthy. If this balance is broken, many diseases can occur. Studies found that MC-LR can increase the microbial species richness as well as the microbial diversity in the cecum and colon with no effect in the jejunum and ileum. The increase of Barnesiella was most remarkable. Therefore, the toxicological effects of MC-LR varied between the jejunum and ileum and the other two gut regions[2]. However, there are few studies on the effects of MC-LR on the gut microbiome. The gut microbiome has a significant impact on human health as the first defense line in the intestine. Therefore, the effect of MC-LR on the intestinal flora needs to be further studied. Furthermore, studies have also shown that MC-LR could change the glycosylation pattern of the intestinal wall, and suppress the efflux activity of multidrug resistance proteins[57].
FURTHER CHALLENGES
The concentration of MCs in the intestines vary among species
The present study comprehensively summarized the increasing information on the influence of MCs on the intestines. A positive association between intestinal toxicity and MC exposure were observed. The concentration or localization of MCs in the small intestine is summarized in Table 1. MCs can be absorbed into the bloodstream and transported to the intestine through blood circulation, and with the mediation of OATPs, MCs can be transferred from blood to enterocytes. No differences were observed in the localization of MC-LR or MC-RR among concentrations. Nevertheless, MCs can cross from the cell membrane to reach the cytoplasm and subsequently the nucleus with prolonged MC exposure. Hence, DNA damage may be induced by MCs and the apoptotic process would be triggered when DNA damage exceeds its repair capacity. This is perhaps one of the mechanisms for MCs to induce enterocyte apoptosis. However, further studies are needed to confirm this. There was a discrepancy in MC content in various organs within species. This may be the result of the different doses and methods used. Furthermore, the distribution of MCs in the intestines differs among tissues across separate species. The difference in MC content in the intestine across separate species may be explained by the differential expression of OATP subtypes. The difference in uptake capacity of the intestine between MC-LR and MC-RR may be the result of the OATP subtype-selective transport of specific MC congeners. The uptake ability of MCs varies in different sites of the intestine. There may be some substances (e.g., bile acids) that competed with MCs in the transport by OATPs. All of these explanations are speculation and further research is needed to confirm these hypotheses.
The majority of reports have mainly focused on the uptake and transport of MCs in the jejunum and ileum and rarely focused on other parts of the gut. Moreover, it remains uncertain whether the uptake mechanism of MCs in other parts of the intestine is the same as that in the jejunum, ileum, and colon. Simultaneously, there are few reports with regard to the depuration of MCs in the intestine. Studies have shown that MCs can be excreted directly via the kidneys, since a negative correlation was present between MC-RR concentration in the blood and in the kidneys[29,31]. It has long been recognized that glutathione (GSH) and cysteine (Cys) conjugation play an important role in the detoxification of MCs in animal organs[23]. However, further validation and exploration are needed to reduce the physical toxic effects of MCs. Otherwise, whether the intestine is an accumulation organ or intestinal microflora has a role in the degradation of MCs remains to be further studied.
Other mechanisms that could damage the intestines may exist
MCs can induce pathological damage of the small intestine, including the loss of microvilli, cytoplasmic vacuolization, the exfoliation of epithelial cells, the hyperplasia of the intestinal mucosa, the hemorrhage of vessels in the submucosa, fibrosis of smooth muscle, disruption of cytoskeleton, and necrosis. These molecular mechanisms may be involved in the disruption of cytoskeleton-associated proteins, the production of reactive oxygen species (ROS) and suppression of anti-oxidation resistance, the activation of pro-apoptotic proteins and inhibition of anti- apoptotic proteins, the release of IL-1β and TNF-α by peritoneal macrophages, and the inhibition of PP1 activity, which would eventually lead to changes in morphology, alteration in digestive enzyme activity, shifts in gut microbial patterns, the prohibition of multidrug resistance protein efflux activity, the decline in the immunity of the small intestine mucosa, disorder in water and electrolytes, and even DNA damage and carcinogenesis. Studies have mainly focused on the pathological injury induced by MCs in the intestine but rarely on its molecular mechanisms. The most important molecular toxic mechanism of MCs in eukaryotes is it can strongly and specifically inhibit the activity of serine and threonine PP1 and PP2A, which are involved in many important intracellular processes such as cell growth, differentiation, protein synthesis, cell signaling, etc. Studies have confirmed that there is an irreversible covalent bond between MCs and PP1/PP2A[58]. The toxic mechanisms reported in the small intestine are related to oxidative stress and the expression of apoptosis-related proteins after exposure to MC-LR. Therefore, further studies are needed to explore the specific cell signaling or receptors in MC-LR-induced intestinal damage. Furthermore, other mechanisms such as the endoplasmic reticulum stress pathway, caspase-dependent pathway, and mitochondrial-dependent pathway still require further investigation.
Long-term exposure experiments and cohort studies are necessary
As it is known, the confirmation of human carcinogen requirement is as follows: (1) a rigorous design, reliable method to exclude the confounding epidemiological survey; (2) dose-response relationship; and (3) other survey data validation or animal experiments support. There is a certain correlation between the pollution of MCs and the occurrence of gastrointestinal cancer according to existing population research data. However, the majority of present population studies are retrospective investigations with more confounding factors and biases. Therefore, the causal relationship between the exposure of MCs and the occurrence of cancer remains unclear. More appropriate methods (cohort study) should be taken to determine its causal relationship. Present animal experiments are mostly acute or subacute experiments, and tumorigenesis is usually triggered by long term exposure to low doses of carcinogens. Therefore, long-term animal exposure experiments with low-dose MCs are needed.
The intestine is the main site for digestion and absorption, and directly determines the physical nutritional status. In previous studies conducted by the investigators, weight decreased significantly after intraperitoneal injection of 25 μg/kg of body weight of MC-LR for 14 days (data not shown). The damage induced by MCs to the gastrointestinal tract and further challenges that need to be addressed are summarized in Figure 4.
Figure 4.
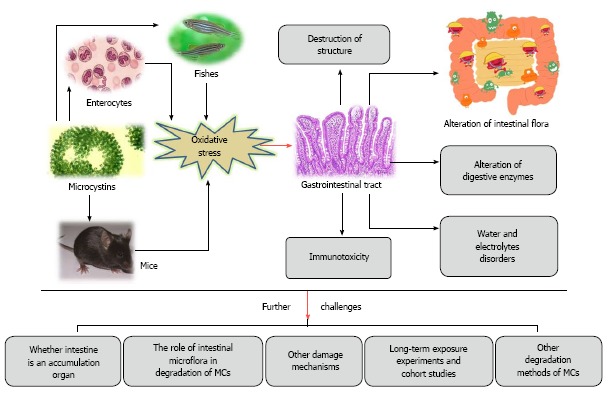
Summarization of the damages of microcystins to the gastrointestinal tract and further challenges that need to be addressed. MCs: Microcystins.
Routine clinical practice
MCs can enter the body through the mouth, inhalation, skin contact, medical treatment, uptake of aquatic products contaminated by MCs, etc. Epidemiological surveys showed that MCs in drinking water sources are one of the major causes of the high incidence of primary liver cancer in some areas of southern China. The gastroenteritis of children occurs every year in an area of a specific reservoir water supply in Harare, Zimbabwe. However, children who used other water supplies in the city did not develop gastroenteritis[59,60]. MCs were detected in many drinking water sources especially in the Taihu River of Wuxi, China in 2007[26]. MCs pose a serious threat to human health. Thus, we advise in daily life to not drink river water directly, to not participate in water activities in bodies of water contaminated by water blooms, to not eat aquatic products contaminated with MCs, and to not use water contaminated with MCs as medical water in medical treatment. These recommendations will play a vital role in preventing the damage of MCs.
CONCLUSION
From the above discussion, it could be concluded that MCs induced damage to the gastrointestinal tract and caused various kinds of pathogenesis in the gastrointestinal tract, all of which poses a threat to human health. However, the mechanisms involved are unknown, and a broad number of issues raised in the present indicate the need to explore toxic mechanisms and seek detoxification methods. All of those proposed further challenges needs to be addressed.
Footnotes
Conflict-of-interest statement: The authors declare that no conflicts of interest exist in this study.
Manuscript source: Unsolicited manuscript
Peer-review started: April 23, 2018
First decision: May 23, 2018
Article in press: June 28, 2018
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent):
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Amornyotin S, Chow WK, Slomiany BL, Teramoto-Matsubara OT S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
Contributor Information
Jin-Xia Wu, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Hui Huang, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Lei Yang, Department of Nutriology, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Xiao-Feng Zhang, Department of Nutriology, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Shen-Shen Zhang, Department of Nutriology, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Hao-Hao Liu, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Yue-Qin Wang, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Le Yuan, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Xue-Min Cheng, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Dong-Gang Zhuang, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China.
Hui-Zhen Zhang, Department of Environmental Hygiene, College of Public Health, Zhengzhou University, Zhengzhou 450001, Henan Province, China. huizhenzhang@zzu.edu.cn.
References
- 1.Carmichael WW, Beasley V, Bunner DL, Eloff JN, Falconer I, Gorham P, Harada K, Krishnamurthy T, Yu MJ, Moore RE. Naming of cyclic heptapeptide toxins of cyanobacteria (blue-green algae) Toxicon. 1988;26:971–973. doi: 10.1016/0041-0101(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Giesy JP, Xie P. The dose makes the poison. Sci Total Environ. 2018;621:649–653. doi: 10.1016/j.scitotenv.2017.11.218. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Jiang X, Manubolu M, Riedl K, Ludsin SA, Martin JF, Lee J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res Int. 2017;102:234–245. doi: 10.1016/j.foodres.2017.09.079. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Li Y, Duan H, Sivakumar R, Li X. Chronic exposure of nanomolar MC-LR caused oxidative stress and inflammatory responses in HepG2 cells. Chemosphere. 2018;192:305–317. doi: 10.1016/j.chemosphere.2017.10.158. [DOI] [PubMed] [Google Scholar]
- 5.Vidal F, Sedan D, D’Agostino D, Cavalieri ML, Mullen E, Parot Varela MM, Flores C, Caixach J, Andrinolo D. Recreational Exposure during Algal Bloom in Carrasco Beach, Uruguay: A Liver Failure Case Report. Toxins (Basel) 2017;9:pii: E267. doi: 10.3390/toxins9090267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Zhang R, Ye J, Wu X, Zhang Y, Wu C. Monitoring and research of microcystins and environmental factors in a typical artificial freshwater aquaculture pond. Environ Sci Pollut Res Int. 2018;25:5921–5933. doi: 10.1007/s11356-017-0956-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Bleeker A, Liu J. Nutrient discharge from china’s aquaculture industry and associated environmental impacts. Environ Res Lett. 2015;10:045002. [Google Scholar]
- 8.Steiner K, Zimmermann L, Hagenbuch B, Dietrich D. Zebrafish Oatp-mediated transport of microcystin congeners. Arch Toxicol. 2016;90:1129–1139. doi: 10.1007/s00204-015-1544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J, Wang J, Xiang Z, Diao W, Su M, Shi W, Wan T, Han X. The organic anion transporting polypeptide 1a5 is a pivotal transporter for the uptake of microcystin-LR by gonadotropin-releasing hormone neurons. Aquat Toxicol. 2017;182:1–10. doi: 10.1016/j.aquatox.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Feurstein D, Kleinteich J, Heussner AH, Stemmer K, Dietrich DR. Investigation of microcystin congener-dependent uptake into primary murine neurons. Environ Health Perspect. 2010;118:1370–1375. doi: 10.1289/ehp.0901289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeller P, Clément M, Fessard V. Similar uptake profiles of microcystin-LR and -RR in an in vitro human intestinal model. Toxicology. 2011;290:7–13. doi: 10.1016/j.tox.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Miller AP, Tisdale ES. Public health engineering: epidemic of intestinal disorders in charlestion, W. VA., occurring simultaneously with unprecedented water supply conditions. Am J Public Health Nations Health. 1931;21:198–200. doi: 10.2105/ajph.21.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilotto LS, Douglas RM, Burch MD, Cameron S, Beers M, Rouch GJ, Robinson P, Kirk M, Cowie CT, Hardiman S, et al. Health effects of exposure to cyanobacteria (blue-green algae) during recreational water-related activities. Aust N Z J Public Health. 1997;21:562–566. doi: 10.1111/j.1467-842x.1997.tb01755.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Yu H, Chen K. Relationship between microcystin in drinking water and colorectal cancer. Biomed Environ Sci. 2002;15:166–171. [PubMed] [Google Scholar]
- 15.Lin YD, Zhang YS, Xu M, Yang JB, Chen Y, Hu L, Shen WA. Study on the relationship between Wuxi Taihu waters pollution by algae toxin and health ofthe population (In Chinese) Sh J Prev Med. 2003;15:435–437. [Google Scholar]
- 16.Chen K, Jiao DA, Lu L. Study on the relationship between the type of drinkingwater and the incidence of colorectal cancer (In Chinese) Chinese Journal of Public Health; 1991;10:324–326. [Google Scholar]
- 17.Falconer IR. Toxic cyanobacterial bloom problems in Australian waters: risks andimpacts on human health. Phycologia. 2001;40:228–233. [Google Scholar]
- 18.Zhou Y, Xu X, Yu B, Yu G. Characterization of in vitro effects of microcystin-LR on intestinal epithelial cells. Environ Toxicol. 2017;32:1539–1547. doi: 10.1002/tox.22375. [DOI] [PubMed] [Google Scholar]
- 19.Markowska M, Oberle R, Juzwin S, Hsu CP, Gryszkiewicz M, Streeter AJ. Optimizing Caco-2 cell monolayers to increase throughput in drug intestinal absorption analysis. J Pharmacol Toxicol Methods. 2001;46:51–55. doi: 10.1016/s1056-8719(01)00161-7. [DOI] [PubMed] [Google Scholar]
- 20.Gaudin J, Huet S, Jarry G, Fessard V. In vivo DNA damage induced by the cyanotoxin microcystin-LR: comparison of intra-peritoneal and oral administrations by use of the comet assay. Mutat Res. 2008;652:65–71. doi: 10.1016/j.mrgentox.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Djediat C, Malécot M, de Luze A, Bernard C, Puiseux-Dao S, Edery M. Localization of microcystin-LR in medaka fish tissues after cyanotoxin gavage. Toxicon. 2010;55:531–535. doi: 10.1016/j.toxicon.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Amrani A, Nasri H, Azzouz A, Kadi Y, Bouaïcha N. Variation in cyanobacterial hepatotoxin (microcystin) content of water samples and two species of fishes collected from a shallow lake in Algeria. Arch Environ Contam Toxicol. 2014;66:379–389. doi: 10.1007/s00244-013-9993-2. [DOI] [PubMed] [Google Scholar]
- 23.He J, Chen J, Xie P, Zhang D, Li G, Wu L, Zhang W, Guo X, Li S. Quantitatively evaluating detoxification of the hepatotoxic microcystins through the glutathione and cysteine pathway in the cyanobacteria-eating bighead carp. Aquat Toxicol. 2012;116-117:61–68. doi: 10.1016/j.aquatox.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Xie L, Xie P, Ozawa K, Honma T, Yokoyama A, Park HD. Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut. 2004;127:431–439. doi: 10.1016/j.envpol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Yuan J, Gu Z, Zheng Y, Zhang Y, Gao J, Chen S, Wang Z. Accumulation and detoxification dynamics of microcystin-LR and antioxidant responses in male red swamp crayfish Procambarus clarkii. Aquat Toxicol. 2016;177:8–18. doi: 10.1016/j.aquatox.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Xie P, Liu Y, Chen J, Liang G. Bioaccumulation of the hepatotoxic microcystins in various organs of a freshwater snail from a subtropical Chinese lake, Taihu Lake, with dense toxic Microcystis blooms. Environ Toxicol Chem. 2007;26:171–176. doi: 10.1897/06-222r.1. [DOI] [PubMed] [Google Scholar]
- 27.Cazenave J, Wunderlin DA, de Los Angeles Bistoni M, Amé MV, Krause E, Pflugmacher S, Wiegand C. Uptake, tissue distribution and accumulation of microcystin-RR in Corydoras paleatus, Jenynsia multidentata and Odontesthes bonariensis. A field and laboratory study. Aquat Toxicol. 2005;75:178–190. doi: 10.1016/j.aquatox.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Xie P, Zhang D, Lei H. In situ studies on the distribution patterns and dynamics of microcystins in a biomanipulation fish--bighead carp (Aristichthys nobilis) Environ Pollut. 2007;147:150–157. doi: 10.1016/j.envpol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Xie P, Guo L, Zheng L, Ni L. Tissue distributions and seasonal dynamics of the hepatotoxic microcystins-LR and -RR in a freshwater snail (Bellamya aeruginosa) from a large shallow, eutrophic lake of the subtropical China. Environ Pollut. 2005;134:423–430. doi: 10.1016/j.envpol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Xie P. Seasonal dynamics of the hepatotoxic microcystins in various organs of four freshwater bivalves from the large eutrophic lake Taihu of subtropical China and the risk to human consumption. Environ Toxicol. 2005;20:572–584. doi: 10.1002/tox.20146. [DOI] [PubMed] [Google Scholar]
- 31.Lei H, Xie P, Chen J, Liang G, Dai M, Zhang X. Distribution of toxins in various tissues of crucian carp intraperitoneally injected with hepatotoxic microcystins. Environ Toxicol Chem. 2008;27:1167–1174. doi: 10.1897/07-522.1. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Xie P. Microcystin accumulation in freshwater bivalves from Lake Taihu, China, and the potential risk to human consumption. Environ Toxicol Chem. 2007;26:1066–1073. doi: 10.1897/06-423r1.1. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Xie P, Chen J, Liang G. Distribution of microcystins in various organs (heart, liver, intestine, gonad, brain, kidney and lung) of Wistar rat via intravenous injection. Toxicon. 2008;52:721–727. doi: 10.1016/j.toxicon.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Lei H, Xie P, Chen J, Liang G, Yu T, Jiang Y. Tissue distribution and depuration of the extracted hepatotoxic cyanotoxin microcystins in crucian carp (Carassius carassius) intraperitoneally injected at a sublethal dose. ScientificWorldJournal. 2008;8:713–719. doi: 10.1100/tsw.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, Xie P, Guo L, Li L, Miyabara Y, Park HD. Organ distribution and bioaccumulation of microcystins in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ Toxicol. 2005;20:293–300. doi: 10.1002/tox.20120. [DOI] [PubMed] [Google Scholar]
- 36.Huguet A, Henri J, Petitpas M, Hogeveen K, Fessard V. Comparative cytotoxicity, oxidative stress, and cytokine secretion induced by two cyanotoxin variants, microcystin LR and RR, in human intestinal Caco-2 cells. J Biochem Mol Toxicol. 2013;27:253–258. doi: 10.1002/jbt.21482. [DOI] [PubMed] [Google Scholar]
- 37.Humpage AR, Hardy SJ, Moore EJ, Froscio SM, Falconer IR. Microcystins (cyanobacterial toxins) in drinking water enhance the growth of aberrant crypt foci in the mouse colon. J Toxicol Environ Health A. 2000;61:155–165. doi: 10.1080/00984100050131305. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira MF, Oliveira VM, Oliveira R, da Cunha PV, Grisolia CK, Pires OR Jr. Histopathological effects of [D-Leu(1)]Microcystin-LR variants on liver, skeletal muscle and intestinal tract of Hypophthalmichthys molitrix (Valenciennes, 1844) Toxicon. 2010;55:1255–1262. doi: 10.1016/j.toxicon.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Trinchet I, Djediat C, Huet H, Dao SP, Edery M. Pathological modifications following sub-chronic exposure of medaka fish (Oryzias latipes) to microcystin-LR. Reprod Toxicol. 2011;32:329–340. doi: 10.1016/j.reprotox.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Atencio L, Moreno I, Jos A, Pichardo S, Moyano R, Blanco A, Cameán AM. Dose-dependent antioxidant responses and pathological changes in tenca (Tinca tinca) after acute oral exposure to Microcystis under laboratory conditions. Toxicon. 2008;52:1–12. doi: 10.1016/j.toxicon.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Djediat C, Moyenga D, Malécot M, Comte K, Yéprémian C, Bernard C, Puiseux-Dao S, Edery M. Oral toxicity of extracts of the microcystin-containing cyanobacterium Planktothrix agardhii to the medaka fish (Oryzias latipes) Toxicon. 2011;58:112–122. doi: 10.1016/j.toxicon.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Huynh-Delerme C, Edery M, Huet H, Puiseux-Dao S, Bernard C, Fontaine JJ, Crespeau F, de Luze A. Microcystin-LR and embryo-larval development of medaka fish, Oryzias latipes. I. Effects on the digestive tract and associated systems. Toxicon. 2005;46:16–23. doi: 10.1016/j.toxicon.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Cameán A. Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon. 2005;46:725–735. doi: 10.1016/j.toxicon.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Lin W, Hou J, Guo H, Li L, Wang L, Zhang D, Li D, Tang R. The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish. Environ Pollut. 2018;235:197–206. doi: 10.1016/j.envpol.2017.12.059. [DOI] [PubMed] [Google Scholar]
- 45.Martins ND, Yunes JS, Monteiro DA, Rantin FT, Kalinin AL. Microcystin-LR leads to oxidative damage and alterations in antioxidant defense system in liver and gills of Brycon amazonicus (SPIX & AGASSIZ, 1829) Toxicon. 2017;139:109–116. doi: 10.1016/j.toxicon.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 46.La-Salete R, Oliveira MM, Palmeira CA, Almeida J, Peixoto FP. Mitochondria a key role in microcystin-LR kidney intoxication. J Appl Toxicol. 2008;28:55–62. doi: 10.1002/jat.1251. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Cai C, Wu Y, Shao D, Ye B, Zhang Y, Liu J, Wang J, Jia X. Mitochondrial and endoplasmic reticulum pathways involved in microcystin-LR-induced apoptosis of the testes of male frog (Rana nigromaculata) in vivo. J Hazard Mater. 2013;252-253:382–389. doi: 10.1016/j.jhazmat.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Pavagadhi S, Gong Z, Hande MP, Dionysiou DD, de la Cruz AA, Balasubramanian R. Biochemical response of diverse organs in adult Danio rerio (zebrafish) exposed to sub-lethal concentrations of microcystin-LR and microcystin-RR: a balneation study. Aquat Toxicol. 2012;109:1–10. doi: 10.1016/j.aquatox.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Sedan D, Laguens M, Copparoni G, Aranda JO, Giannuzzi L, Marra CA, Andrinolo D. Hepatic and intestine alterations in mice after prolonged exposure to low oral doses of Microcystin-LR. Toxicon. 2015;104:26–33. doi: 10.1016/j.toxicon.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Moreno IM, Mate A, Repetto G, Vázquez CM, Cameán AM. Influence of microcystin-LR on the activity of membrane enzymes in rat intestinal mucosa. J Physiol Biochem. 2003;59:293–299. doi: 10.1007/BF03179887. [DOI] [PubMed] [Google Scholar]
- 51.Chen C, Liu W, Wang L, Li J, Chen Y, Jin J, Kawan A, Zhang X. Pathological damage and immunomodulatory effects of zebrafish exposed to microcystin-LR. Toxicon. 2016;118:13–20. doi: 10.1016/j.toxicon.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 52.Liu LY, Xie P. Effect of microcystin - LR on intestinal digestive enzymes in mice (In Chinese) Acta Hdrobiologia Sinica. 2014;38:533–539. [Google Scholar]
- 53.Yao YH, Yu LN, He WH, Li G, Luo XS. Effects of toxic microcystis on the growth, activities of digestive enzymes and accumulation of microcystin in Carassius auratus. Zhongguo Shuichan Kexue. 2007;14:969–973. [Google Scholar]
- 54.Rocha MF, Sidrim JJ, Soares AM, Jimenez GC, Guerrant RL, Ribeiro RA, Lima AA. Supernatants from macrophages stimulated with microcystin-LR induce electrogenic intestinal response in rabbit ileum. Pharmacol Toxicol. 2000;87:46–51. doi: 10.1111/j.0901-9928.2000.870108.x. [DOI] [PubMed] [Google Scholar]
- 55.Nobre AC, Nunes-Monteiro SM, Monteiro MC, Martins AM, Havt A, Barbosa PS, Lima AA, Monteiro HS. Microcystin-LR promote intestinal secretion of water and electrolytes in rats. Toxicon. 2004;44:555–559. doi: 10.1016/j.toxicon.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Dahlem AM, Hassan AS, Swanson SP, Carmichael WW, Beasley VR. A model system for studying the bioavailability of intestinally administered microcystin-LR, a hepatotoxic peptide from the cyanobacterium Microcystis aeruginosa. Pharmacol Toxicol. 1989;64:177–181. doi: 10.1111/j.1600-0773.1989.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 57.Bieczynski F, Torres WD, Painefilu JC, Castro JM, Bianchi VA, Frontera JL, Paz DA, González C, Martín A, Villanueva SS, et al. Alterations in the intestine of Patagonian silverside (Odontesthes hatcheri) exposed to microcystin-LR: Changes in the glycosylation pattern of the intestinal wall and inhibition of multidrug resistance proteins efflux activity. Aquat Toxicol. 2016;178:106–117. doi: 10.1016/j.aquatox.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Svircev Z, Baltić V, Gantar M, Juković M, Stojanović D, Baltić M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2010;28:39–59. doi: 10.1080/10590500903585382. [DOI] [PubMed] [Google Scholar]
- 59.Zilberg B. Gastroenteritis in Salisbury. European children--a five-year study. Cent Afr J Med. 1966;12:164–168. [PubMed] [Google Scholar]
- 60.Pouria S, de Andrade A, Barbosa J, Cavalcanti RL, Barreto VT, Ward CJ, Preiser W, Poon GK, Neild GH, Codd GA. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet. 1998;352:21–26. doi: 10.1016/s0140-6736(97)12285-1. [DOI] [PubMed] [Google Scholar]


