Abstract
Balo’s concentric sclerosis (BCS) is a rare monophasic demyelinating disease known as multiple sclerosis subtype and seen as a round lesion with variable hyper and hypo-detoxification layers. Characteristic appearance can be seen as “bulb eye” or “onion bulb”. The initial terminology for this neurological disorder was leukoencephalitis periaxialis concentrica; this is defined as a disease in which the white matter of the brain is destroyed in concentric layers in such a way as to leave the axial cylinders intact. This report presents a case of BCS with spontaneous healing of the patient and a mass lesion with concentric rings adjacent to the left lateral ventricle and the posterior portion of the corpus callosum with peripheral vasogenic edema. The neurological lesion of the patient was similar to the magnetic resonance imaging and clinical findings of the BCS.
Keywords: Balo’s concentric sclerosis, Multiple sclerosis, Demyelinating, Magnetic resonance imaging, Diffusion-weighted imaging
Core tip: This case report demonstrates that Balo’s concentric sclerosis (BCS) a patient with a mass lesion containing concentric rings, BCS diagnosis was reported by magnetic resonance imaging. As supported in previously reported clinical trials, BCS is not always a fatal disease and supports the definition that it may be a self-limiting disease.
INTRODUCTION
Balo’s concentric sclerosis (BCS) is characterized radiologically and pathologically by demyelinating lesions with a concentric ring appearance formed by areas of demyelination alternating with relatively preserved myelin[1]. The lesions of BCS often occur in isolation or in association with clinically and radiologically more typical multiple sclerosis (MS). Historically, BCS was thought to be uniformly fatal and diagnosis was post-mortem, but in the magnetic resonance imaging (MRI) era, BCS can be detected intra vitam and, in many cases, has a favorable prognosis[2].
BCS was first described by Marburg in 1906, and in 1928, the Hungarian neuropathologist, Joseph Balo[3] published a report of a student with right hemiparesis followed by optic neuritis, who upon autopsy had demyelinated lesions described as encephalitis periaxialis concentrica. Traditionally, BCS has been grouped under one of the atypical forms of MS, with Marburg’s disease, tumefactive demyelination, Schilder’s disease, and acute haemorrhagic leukoencephalitis, although the contemporary status and usefulness of these categorizations are questionable apart from tumefactive demyelinations contentious. Tumefactive demyelinating lesions are more than 2 cm in size when viewed with MRI and may have an associated mass effect (45%) and/or edema (77%) with larger lesions generally having both more mass effect and edema[4]. Most tumefactive demyelinating lesions are focal and supratentorial, with a predilection for the frontal and parietal lobes, but they can present in other areas of the cerebral hemispheres as well as in the deep gray matter, brainstem, cerebellum, and spinal cord[4-6].
BCS is clinically indicated in clinical trials that may occur in a manner similar to MS. It is known that it can affect young people and children with mild dementia. However, it may be associated with altered behavior and focal central nervous system (CNS) deficits. Clinical trials have reported that BCS exhibits characteristic radiographic findings that aid in ante-mortem diagnosis[7]. BCS is clinically first reported to be a rapidly progressive and lethal condition[8], and subsequently reported clinical trials have demonstrated that anti-inflammatory corticosteroids are efficacious against BCS-associated neurological deficits. Because of this reason, it is known that MRI imaging allows early diagnosis and treatment by significantly affecting the course of the disease.
This acute idiopathic inflammatory demyelinating disease has a unique pathological and radiographic signature of concentric demyelination. The pattern can be quite striking upon MRI, with alternating concentric rings of T2 isointensity and hyperintensity related to advancing waves of demyelination. These may show gadolinium enhancement[9]. Lesions may be small or occupy large sections of a cerebral hemisphere and tend to spare the cortical U-fibers. Pathologically, there are rings of demyelination corresponding to areas of T2 hyperintensity with MRI alternating with rings of normal myelination or partial remyelination corresponding to areas of T2 isointensity. This renders the lesions with an onion bulb appearance[10]. Lesions can also be found in the basal ganglia, pons, cerebellum, and, very infrequently, the spinal cord and optic nerves[2]. Patients with this diagnosis were thought to have a fulminant course that was invariably fatal within a year. However, with the advent of MRI, certain cases detected via MRI have had favorable outcomes[2,11]. The concentric ring appearance is also not specific, with these types of lesions having also been described in the brainstem in a patient with neuromyelitis optica[12] and another with MS[13] as well as in patients with progressive multifocal leukoencephalopathy[14], cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy[15] and concomitant active hepatitis C and human herpes virus 6[16].
In this study, we reported a mass lesion with concentric rings adjacent to the left lateral ventricle and the posterior part of the corpus callosum with a peripheral vasogenic lesion in a patient with spontaneous remission with MRI imaging.
CASE REPORT
A 19-year-old woman complaining of night-raging nausea, blurred vision, and severe headache for seven days was seen in our clinic. Focal CNS deficiency was not detected in our patient. On cranial MRI, a mass with concentric circles and peripheral vesogenic edema located right lateral to the left lateral ventricle was seen in the posterior part of the corpus callosum (Figure 1). A significant increase was detected in the peripheries and central region of the lesion after contrast material injection (Figure 2). Diffusion-weighted imaging showed circular rings of hyperintensity, similar to the T2-weighted (T2W) images visible diffusion co-efficient maps showed a donut-shaped slow diffusion zone around a central nidus of facilitated diffusion (Figure 3). Single-voxel magnetic resonance spectroscopy was obtained from the left-enhancing centrum semiovale lesion. It indicated a decrease in the choline/N-acetyl aspartate ratio and mild lipid along with lactate peaks (Figure 4). No other lesions were seen. The patient underwent a fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) and there were no pathological findings in favor of malignancy. Characteristic MRI findings suggested the diagnosis of BCS but the patient refused the treatment. After nine months, she was admitted to a neurology clinic for a severe headache. Interestingly, there was only a T2W linear signal intensity on the MRI (Figure 5). This case is very interesting for its spontaneous remission. Cases between 1985-2018 related to BCS can be seen in Table 1.
Figure 1.
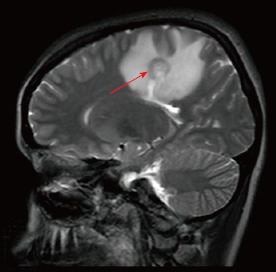
Sagittal T2-weighted magnetic resonance imaging showing a mass lesion with concentric rings located adjacent to the left lateral ventricle and posterior part of the corpus callosum with peripheral vasogenic edema.
Figure 2.
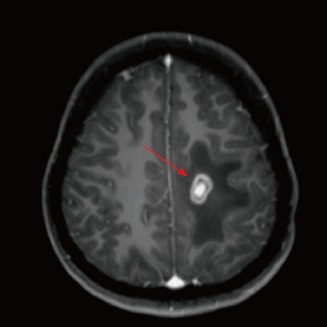
Axial T1-weighted image after administration of gadolinium-diethylenetriaminepentaacetic acid depicts prominent enhancement in the periphery and central area of the lesion.
Figure 3.
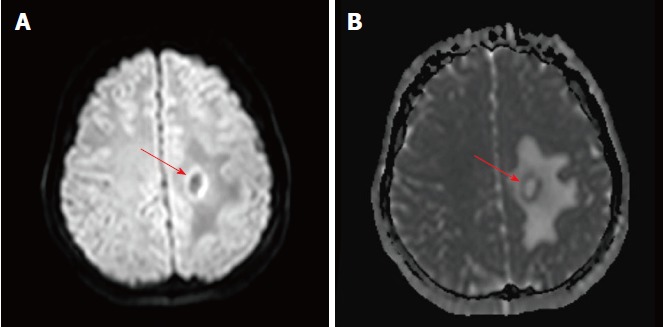
Apparent diffusion co-efficient maps portraying only a thin rim of restricted diffusion at the outer rim of the lesion, with facilitated diffusion centrally and at the outer edema. A: Diffusion weight images shows a thin rim of increased diffusion at the outer rim of the lesion; B: The outer rim is hypointense on the corresponding apparent diffusion coefficient map images, indicating true restriction.
Figure 4.
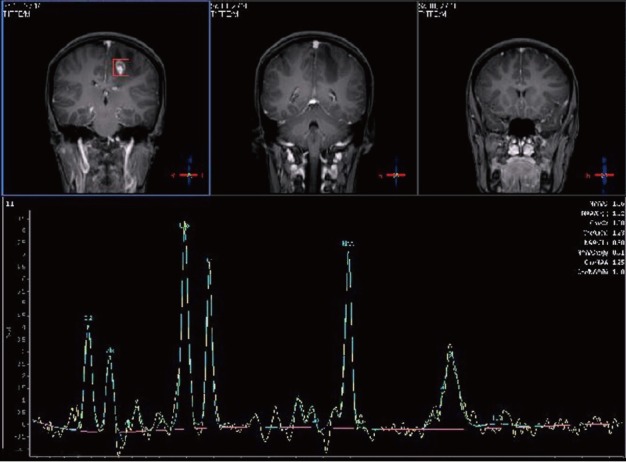
One hundred and forty-four millisecond single-voxel magnetic resonance spectroscopy was obtained from the left-enhancing centrum semiovale lesion. It showed a decrease in the choline/N-acetyl aspartate ratio along with mild lipid and lactate peaks.
Figure 5.
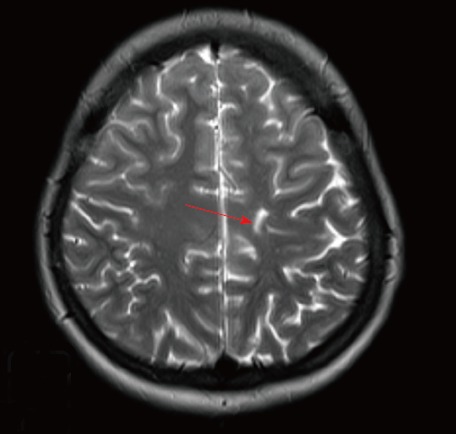
There was only a T2-weighted linear signal intensity with magnetic resonance imaging obtained after nine months.
Table 1.
Cases related to Balo’s concentric sclerosis between 1985-2018
| Reference | Case number | Gender/age | Clinical presentation | Oligoclonal bands | Coexistence with injuries | Histopathological examination | Clinical progression |
| [7] | 1 | F/37 | Monophase | NR | NR | Y | SH |
| 2 | F/56 | Monophase | NR | NR | Y | SH | |
| 3 | M/42 | Monophase | NR | NR | N | SH | |
| 4 | F/33 | Monophase | NR | NR | N | SH | |
| [9] | 1 | F/56 | Relapsing-Remitting | NR | N | Y | MH |
| [11] | 1 | M/51 | Monophase | Negative | N | Y | SH |
| 2 | F/20 | Monophase | Negative | N | N | CH | |
| 3 | M/48 | Monophase | Positive | Y | N | CH | |
| 4 | M/38 | Monophase | Negative | N | N | CH | |
| 5 | F/15 | Monophase | Negative | Y | N | SH | |
| [12] | 1 | F/29 | Relapsing-Remitting | NR | Y | N | MH |
| [13] | 1 | F/45 | Relapsing-Remitting | Negative | N | N | SH |
| [15] | 1 | M/26 | Progressive Primary | NR | N | N | SH |
| [17] | 1 | F/52 | Monophase | NR | N | N | SH |
| 2 | M/31 | Monophase | NR | N | N | CH | |
| 3 | F/40 | Relapsing-Remitting | NR | Y | N | SH | |
| 4 | M/31 | Monophase | NR | N | N | CH | |
| 5 | F/23 | Monophase | NR | Y | N | SH | |
| 6 | F/44 | Relapsing-Remitting | NR | Y | N | SH | |
| 7 | F/43 | Relapsing-Remitting | NR | Y | N | SH | |
| [21] | 1 | M/43 | Relapsing-Remitting | Negative | Y | N | MH |
| [23] | 1 | M/46 | Progressive Primary | NR | NR | Y | D |
| 2 | M/24 | Progressive Primary | NR | NR | Y | D | |
| 3 | M/48 | Progressive Primary | NR | NR | Y | D | |
| 4 | F/40 | Progressive Primary | NR | NR | Y | D | |
| 5 | F/25 | Progressive Primary | NR | NR | Y | D | |
| 6 | F/24 | Progressive Primary | NR | NR | Y | D | |
| [25] | 1 | F/NR | NR | NR | Y | Y | D |
| 2 | F/NR | NR | NR | Y | Y | D | |
| 3 | F/NR | NR | NR | Y | Y | D | |
| 4 | F/NR | NR | NR | Y | Y | D | |
| 5 | F/NR | NR | NR | Y | Y | D | |
| 6 | F/NR | NR | NR | Y | Y | D | |
| 7 | F/NR | NR | NR | Y | Y | D | |
| 8 | F/NR | NR | NR | Y | Y | D | |
| 9 | F/NR | NR | NR | Y | Y | D | |
| 10 | M/NR | NR | NR | Y | Y | D | |
| 11 | M/NR | NR | NR | Y | Y | D | |
| 12 | M/NR | NR | NR | Y | Y | D | |
| 13 | M/NR | NR | NR | Y | Y | D | |
| 14 | M/NR | NR | NR | Y | Y | D | |
| [34] | 1 | F/32 | Monophase | NR | N | N | SH |
| [35] | 1 | F/45 | Monophase | NR | N | N | SH |
| [37] | 1 | F/57 | Progressive Secondary | Negative | Y | N | NH |
| [38] | 1 | F/54 | Progressive Primary | Positive | Y | Y | D |
| [39] | 1 | F/31 | Relapsing-Remitting | NR | Y | N | NR |
| [40] | 1 | M/28 | Relapsing-Remitting | Negative | NR | Y | D |
| [41] | 1 | F/32 | NR | NR | NR | NR | NR |
| [42] | 1 | F/28 | NR | Negative | NR | N | NR |
| [43] | 1 | F/52 | Monophase | Negative | N | Y | SH |
| [44] | 1 | M/4 | Monophase | Negative | N | N | MH |
| [45] | 1 | F/45 | Progressive Primary | Negative | N | N | LH |
| 2 | M/36 | Progressive Primary | Negative | N | N | LH | |
| [46] | 1 | F/24 | Relapsing-Remitting | NR | Y | Y | D |
| [47] | 1 | F/34 | Monophase | NR | N | Y | NR |
| [48] | 1 | M/NR | Monophase | Negative | N | N | SH |
| 2 | F/38 | Progressive Primary | Positive | N | N | D | |
| 3 | M/40 | Monophase | Negative | N | N | SH | |
| [49] | 1 | F/23 | Relapsing-Remitting | Negative | Y | N | MH |
| [50] | 1 | F/13 | Relapsing-Remitting | Positive | N | N | SH |
| [51] | 1 | F/27 | Relapsing-Remitting | Negative | N | Y | LH |
| [52] | 1 | F/37 | Monophase | NR | NR | NR | MH |
| [53] | 1 | F/31 | Monophase | NR | N | N | SH |
| 2 | F/58 | Monophase | NR | N | Y | LH | |
| [54] | 1 | M/26 | Progressive Primary | Positive | N | Y | LH |
| [55] | 1 | F/17 | Relapsing-Remitting | Positive | N | N | MH |
| [56] | 1 | M/37 | Monophase | Negative | Y | N | SH |
| [57] | 1 | M/49 | Progressive Primary | NR | NR | Y | D |
| 2 | M/23 | Progressive Primary | NR | NR | Y | D | |
| 3 | F/28 | Progressive Primary | NR | NR | Y | D | |
| 4 | F/40 | Progressive Primary | NR | NR | Y | D | |
| [58] | 1 | M/52 | Relapsing-Remitting | NR | N | N | D |
| [59] | 1 | F/25 | Progressive Primary | NR | NR | N | SH |
| [60] | 1 | F/21 | Relapsing-Remitting | NR | NR | N | SH |
| 2 | M/45 | Relapsing-Remitting | NR | NR | N | CH | |
| 3 | M/35 | Relapsing-Remitting | NR | NR | N | SH | |
| 4 | F/38 | Progressive Primary | NR | NR | N | D | |
| 5 | M/43 | Progressive Primary | NR | NR | N | MH | |
| 6 | F/33 | Progressive Primary | NR | NR | N | MH | |
| [61] | 1 | M/36 | Monophase | Negative | NR | N | MH |
| 2 | F/52 | Progressive Primary | Negative | NR | N | LH | |
| 3 | M/56 | Progressive Primary | Positive | NR | N | NH | |
| [62] | 1 | M/24 | Progressive Primary | Negative | NR | N | SH |
M: Male; F: Female; Y: Yes; N: No; D: Dead; NR: Not reported; CH: Complete healing; SH: Significant healing; MH: Modarate healing; LH: Little healing; NH: No healing.
DISCUSSION
It was stated that the case reports presented about the BCS were seen more in women[2,17-26]. However, it has been pointed out in scientific publications that BCS is more common in East Asian descent[17-20]. According to these studies, genetic and environmental factors should be considered with BCS. Many signs of Balo’s disease are similar to MS symptoms. Headaches, seizures, muscle pain and spasms, muscle weakness, paralysis over time, difficulty speaking, different thinking or understanding, changes in behavior can be seen as clinical manifestations of BCS. And also, BCS symptoms show a similar clinical course, mostly with intracerebral mass lesions[11,17,18].
Preservation of cortical gray matter, cerebral white matter oligodendrocyte loss and demyelination are known pathological findings of BCS[3,20-24]. In the pathology of BCS tissue lesions, the number of oligodendrocytes in the demyelinated areas of the substantia alba layer was reduced, and the lesions were defined as a variation of the immunopathological pattern III of MS[1,22].
The demyelinated ring appearance of BCS has been reported to include foamy macrophages, activated microglia, reactivated astrocytes and axonal loss areas, as is typically found in MS. It has been reported that hypoxia and demyelination of the edge of BCS lesions are related to the production of chemical mediators and cytokines by macrophages or microglia cells. This provides some protection against demyelination at the BCS lesion side, and as the lesion expands, the demyelination area appears to be a relatively preserved myelinated tissue[1].
Hypoxia-inducible factor 1α and heat-shock protein 70 are proteins that protect the myelin structure between the rings demyelinated in BCS lesions[25]. BCS lesions are larger than MS lesions in appearance. Different ring appearances are seen with a shape called onion bulb. The formation of this shape is related to relative myelin preservation and the loss of axon structure[1,26]. The myelin structure in BCS patients is rarely preserved. However, it is stated that this is actually a partial demyelination area[1,27]. When the pathological results of BCS lesions are examined, lymphocytic infiltrates around the vessel and demyelination area at different stages are reported[28]. Histological studies on MS lesions indicated that the areas of demyelination may closely resemble the appearance of BCS patients[22,29,30]. Because of this close anatomical resemblance, some BCS cases have been described as MS cases. Some of the BCS lesions have been found to have lost myelin-associated glycoprotein[1,22].
In MRI studies, BCS lesions may typically be multiple, isolated, and mixed, such as in MS lesions[31]. Concentric rings can be seen the most common alternative augment rings in the outer rings[32]. In T1-weighted MR scans of BCS lesions, the lesions are generally seen as light or dark (isointense or hypointense) concentric rings. However, in the T2W MRI sequences, it was stated that the density of the lamellae appearance around the lesion increased. Apart from these, it has been reported that the images of BCS lesions may have different geometric shapes[33,34]. The image intensity of MR sections on the outer margin of the BCS lesions was found to be higher[27,35]. It has been stated that in the MR sections of the BCS lesions, the flow of the contras material may be in the peripheral direction. However, it was determined that the density of lesion layers increased in T2 weighted sections[7,21]. BCS lesions are frequently seen in the white matter layer (substantia alba) of cerebrum. And, subcortical U-fibres usually initially spared. However, BCS lesions have been reported in rhombencephalon and the basal ganglia[13,36-38]. In our case, T2 images revealed one adjacent hyperintense perioedematous concentric lesion at the left centrum semiovale and periventricular white matter spreading to the corpus callosum (Figure 1). Magnetic resonance spectroscopy of the patient indicated a decrease in the choline/N-acetyl aspartate ratio along with mild lipid and lactate peaks (Figure 4). Clinical studies on long-term follow-up of BCS lesions have shown that these lesions lost their ring appearance and turned into demyelinating areas. It has even been reported that the lesions may have a linear shape[7]. Other studies have shown that the classic concentric view of the BCS lesion can retain its structure for a long time[9], or that BCS lesions may lose their anatomical shape and appear as a classic demyelinating plaque.
In conclusion, in a patient with a mass lesion containing concentric rings, BCS diagnosis was reported by MRI imaging. As supported in previously reported clinical trials, BCS is not always a fatal disease and supports the definition that it may be a self-limiting disease. Although BCS is usually known to possess a fulminant demyelinating course, there are cases in the literature with favorable prognoses and occasionally cases with spontaneous remission[21]. The unexpected finding of spontaneous remission without any treatment was noted in this case. A mass lesion with concentric rings that we determined more than nine months later were seen with a linear signal intensity without any treatment during MRI (Figure 5).
ARTICLE HIGHLIGHTS
Case characteristics
In a 19-year-old woman complaining of night-raging nausea, blurred vision, and severe headache ongoing for a week was admitted in our clinic.
Clinical diagnosis
The patient underwent magnetic resonance imaging (MRI) examination at our hospital, which indicated a mass with concentric circles and peripheral vesogenic edema located right lateral to the left lateral ventricle was seen in the posterior part of the corpus callosum.
Differential diagnosis
The patient underwent a fluorodeoxyglucose positron emission tomography/computed tomography and there were no pathological findings in favor of malignancy.
Imaging diagnosis
MRI and single-voxel magnetic resonance spectroscopy were used in this case.
Treatment
The patient refused the treatment.
Related reports
Balo’s concentric sclerosis (BCS) was first described by Marburg in 1906, and in 1928, the Hungarian neuropathologist, Joseph Balo, published a report of a student. Cases related to BCS between 1985-2018 were presented in this case report together with clinical findings and results.
Term explanation
BCS is a rare monophasic demyelinating disease known as multiple sclerosis subtype. BCS may rapidly progress to become severe and fatal.
Experiences and lessons
The unexpected finding of spontaneous remission without any treatment was reported by MRI in this case. Clinicians should consider BCS is not always a fatal disease.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient prior to all procedures described in the report as well as for the use of the patient’s clinical information and images for published scientific works.
Conflict-of-interest statement: All of the authors report no relationships that could be construed as a conflict of interest.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Peer-review started: May 19, 2018
First decision: July 8, 2018
Article in press: August 6, 2018
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Demonacos C, Lin GM, Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Song H
Contributor Information
Özgür Ertuğrul, Department of Radiology, Memorial Hospital, Diyarbakır 21100, Turkey.
Esra Çiçekçi, Department of Physiotherapy, University of Health Sciences, Gazi Yaşargil Education and Research Hospital, Diyarbakır 21100, Turkey.
Mehmet Cudi Tuncer, Department of Anatomy, Faculty of Medicine, University of Dicle, Diyarbakır 21280, Turkey. drcudi@hotmail.com.
Mehmet Ufuk Aluçlu, Department of Neurology, Faculty of Medicine, University of Dicle, Diyarbakır 21280, Turkey.
References
- 1.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 2.Hardy TA, Miller DH. Baló’s concentric sclerosis. Lancet Neurol. 2014;13:740–746. doi: 10.1016/S1474-4422(14)70052-3. [DOI] [PubMed] [Google Scholar]
- 3.Balo J. Encephalitis periaxialis concentrica. Arch Neur Psych. 1928;19:242–264. [Google Scholar]
- 4.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, Thomsen K, Mandrekar J, Altintas A, Erickson BJ, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altintas A, Petek B, Isik N, Terzi M, Bolukbasi F, Tavsanli M, Saip S, Boz C, Aydin T, Arici-Duz O, et al. Clinical and radiological characteristics of tumefactive demyelinating lesions: follow-up study. Mult Scler. 2012;18:1448–1453. doi: 10.1177/1352458512438237. [DOI] [PubMed] [Google Scholar]
- 6.Wallner-Blazek M, Rovira A, Fillipp M, Rocca MA, Miller DH, Schmierer K, Frederiksen J, Gass A, Gama H, Tilbery CP, et al. Atypical idiopathic inflammatory demyelinating lesions: prognostic implications and relation to multiple sclerosis. J Neurol. 2013;260:2016–2022. doi: 10.1007/s00415-013-6918-y. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, Chu NS, Lu CS, Sung CY. Serial magnetic resonance imaging in patients with Balò’s concentric sclerosis: natural history of lesion development. Ann Neurol. 1999;46:651–656. doi: 10.1002/1531-8249(199910)46:4<651::aid-ana15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Ro LS, Wang LJ, Wong YC. Balò’s concentric sclerosis: MRI. Neuroradiology. 1996;38:322–324. doi: 10.1007/BF00596578. [DOI] [PubMed] [Google Scholar]
- 9.Ng SH, Ko SF, Cheung YC, Wong HF, Wan YL. MRI features of Balo’s concentric sclerosis. Br J Radiol. 1999;72:400–403. doi: 10.1259/bjr.72.856.10474505. [DOI] [PubMed] [Google Scholar]
- 10.Darke M, Bahador FM, Miller DC, Litofsky NS, Ahsan H. Baló’s concentric sclerosis: imaging findings and pathological correlation. J Radiol Case Rep. 2013;7:1–8. doi: 10.3941/jrcr.v7i6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaarslan E, Altintas A, Senol U, Yeni N, Dincer A, Bayindir C, Karaagac N, Siva A. Baló’s concentric sclerosis: clinical and radiologic features of five cases. AJNR Am J Neuroradiol. 2001;22:1362–1367. [PMC free article] [PubMed] [Google Scholar]
- 12.Graber JJ, Kister I, Geyer H, Khaund M, Herbert J. Neuromyelitis optica and concentric rings of Baló in the brainstem. Arch Neurol. 2009;66:274–275. doi: 10.1001/archneurol.2008.539. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto R, Yabe I, Niino M, Sato K, Tsuji S, Kikuchi S, Sasaki H. Baló’s concentric sclerosislike lesion in the brainstem of a multiple sclerosis patient. J Neurol. 2008;255:760–761. doi: 10.1007/s00415-008-0795-9. [DOI] [PubMed] [Google Scholar]
- 14.Markiewicz D, Adamczewska-Goncerzewicz Z, Dymecki J, Goncerzewicz A. A case of primary form of progressive multifocal leukoencephalopathy with concentric demyelination of Baló type. Neuropatol Pol. 1977;15:491–500. [PubMed] [Google Scholar]
- 15.Chitnis T, Hollmann TJ. CADASIL mutation and Balo concentric sclerosis: a link between demyelination and ischemia? Neurology. 2012;78:221–223. doi: 10.1212/WNL.0b013e31823fcd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira D, Castro S, Nadais G, Dias Costa JM, Fonseca JM. Demyelinating lesions with features of Balo’s concentric sclerosis in a patient with active hepatitis C and human herpesvirus 6 infection. Eur J Neurol. 2011;18:e6–e7. doi: 10.1111/j.1468-1331.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 17.Chaodong Wang, Zhang KN, Wu XM, Gang Huang, Xie XF, Qu XH, Xiong YQ. Balo’s disease showing benign clinical course and co-existence with multiple sclerosis-like lesions in Chinese. Mult Scler. 2008;14:418–424. doi: 10.1177/1352458507084036. [DOI] [PubMed] [Google Scholar]
- 18.Tabira T. Concentric sclerosis (Balo’s disease) In: Lisak RP, Truong DD, Carroll WM, Bhidayasiri R, editors. International neurology a clinical approach. Sussex: Blackwell Publishing; 2009. pp. 389–390. [Google Scholar]
- 19.Capello E, Mancardi GL. Marburg type and Balò’s concentric sclerosis: rare and acute variants of multiple sclerosis. Neurol Sci. 2004;25 Suppl 4:S361–S363. doi: 10.1007/s10072-004-0341-1. [DOI] [PubMed] [Google Scholar]
- 20.Kira J. Astrocytopathy in Balo’s disease. Mult Scler. 2011;17:771–779. doi: 10.1177/1352458511400475. [DOI] [PubMed] [Google Scholar]
- 21.Kastrup O, Stude P, Limmroth V. Balo’s concentric sclerosis. Evolution of active demyelination demonstrated by serial contrast-enhanced MRI. J Neurol. 2002;249:811–814. doi: 10.1007/s00415-002-0718-0. [DOI] [PubMed] [Google Scholar]
- 22.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Yao DL, Webster HD, Hudson LD, Brenner M, Liu DS, Escobar AI, Komoly S. Concentric sclerosis (Baló): morphometric and in situ hybridization study of lesions in six patients. Ann Neurol. 1994;35:18–30. doi: 10.1002/ana.410350105. [DOI] [PubMed] [Google Scholar]
- 24.Weinshenker B, Miller D. “Multiple sclerosis: one disease or many?”. In: Thompson AB, Siva A, Kesselring J, editors. Frontiers in multiple sclerosis. 2nded. London: Taylor Francis Group; 1998. pp. 37–46. [Google Scholar]
- 25.Stadelmann C, Ludwin S, Tabira T, Guseo A, Lucchinetti CF, Leel-Ossy L, Ordinario AT, Brück W, Lassmann H. Tissue preconditioning may explain concentric lesions in Baló’s type of multiple sclerosis. Brain. 2005;128:979–987. doi: 10.1093/brain/awh457. [DOI] [PubMed] [Google Scholar]
- 26.Hu W, Lucchinetti CF. The pathological spectrum of CNS inflammatory demyelinating diseases. Semin Immunopathol. 2009;31:439–453. doi: 10.1007/s00281-009-0178-z. [DOI] [PubMed] [Google Scholar]
- 27.Wiendl H, Weissert R, Herrlinger U, Krapf H, Küker W. Diffusion abnormality in Balo’s concentric sclerosis: clues for the pathogenesis. Eur Neurol. 2005;53:42–44. doi: 10.1159/000084264. [DOI] [PubMed] [Google Scholar]
- 28.Garbern J, Spence AM, Alvord EC Jr. Balo’s concentric demyelination diagnosed premortem. Neurology. 1986;36:1610–1614. doi: 10.1212/wnl.36.12.1610. [DOI] [PubMed] [Google Scholar]
- 29.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 30.Barnett MH, Henderson AP, Prineas JW. The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult Scler. 2006;12:121–132. doi: 10.1191/135248506ms1304rr. [DOI] [PubMed] [Google Scholar]
- 31.Charil A, Yousry TA, Rovaris M, Barkhof F, De Stefano N, Fazekas F, Miller DH, Montalban X, Simon JH, Polman C, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol. 2006;5:841–852. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- 32.Gray F, Léger JM, Duyckaerts C, Bor Y. [Baló’s concentric sclerosis: lesions restricted to the pons] Rev Neurol (Paris) 1985;141:43–45. [PubMed] [Google Scholar]
- 33.Courville CB. Concentric sclerosis. In: Vinken P, Bruyn GW, editors. Handbook of clinical neurology. Amsterdam, North Holland; 1970. pp. 437–451. [Google Scholar]
- 34.Wang L, Liu YH. Balo’s concentric sclerosis. Lancet. 2010;376:189. doi: 10.1016/S0140-6736(09)61876-6. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh EC, Heran MK, Fenton DM, Lapointe JS, Nugent RA, Graeb DA. Diffusion-weighted imaging findings in Balo concentric sclerosis. Br J Radiol. 2006;79:e28–e31. doi: 10.1259/bjr/36636301. [DOI] [PubMed] [Google Scholar]
- 36.Itoyama Y, Tateishi J, Kuroiwa Y. Atypical multiple sclerosis with concentric or lamellar demyelinated lesions: two Japanese patients studied post mortem. Ann Neurol. 1985;17:481–487. doi: 10.1002/ana.410170511. [DOI] [PubMed] [Google Scholar]
- 37.Kreft KL, Mellema SJ, Hintzen RQ. Spinal cord involvement in Balo’s concentric sclerosis. J Neurol Sci. 2009;279:114–117. doi: 10.1016/j.jns.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 38.Moore GR, Neumann PE, Suzuki K, Lijtmaer HN, Traugott U, Raine CS. Balo’s concentric sclerosis: new observations on lesion development. Ann Neurol. 1985;17:604–611. doi: 10.1002/ana.410170614. [DOI] [PubMed] [Google Scholar]
- 39.Iannucci G, Mascalchi M, Salvi F, Filippi M. Vanishing Balò-like lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;69:399–400. doi: 10.1136/jnnp.69.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nandini M, Gourie-Devi M, Shankar SK, Mustare VB, Ravi V. Balo’s concentric sclerosis diagnosed intravitam on brain biopsy. Clin Neurol Neurosurg. 1993;95:303–309. doi: 10.1016/0303-8467(93)90106-q. [DOI] [PubMed] [Google Scholar]
- 41.Gharagozloo AM, Poe LB, Collins GH. Antemortem diagnosis of Baló concentric sclerosis: correlative MR imaging and pathologic features. Radiology. 1994;191:817–819. doi: 10.1148/radiology.191.3.8184071. [DOI] [PubMed] [Google Scholar]
- 42.Sekijima Y, Tokuda T, Hashimoto T, Koh CS, Shoji S, Yanagisawa N. Serial magnetic resonance imaging (MRI) study of a patient with Balo’s concentric sclerosis treated with immunoadsorption plasmapheresis. Mult Scler. 1997;2:291–294. doi: 10.1177/135245859700200605. [DOI] [PubMed] [Google Scholar]
- 43.Kim MO, Lee SA, Choi CG, Huh JR, Lee MC. Balo’s concentric sclerosis: a clinical case study of brain MRI, biopsy, and proton magnetic resonance spectroscopic findings. J Neurol Neurosurg Psychiatry. 1997;62:655–658. doi: 10.1136/jnnp.62.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakami Y, Matsuishi T, Shimizu T, Yamashita Y, Nagamitsu S, Kojima K, Kato H, Tabira T. Baló’s concentric sclerosis in a 4-year-old Japanese infant. Brain Dev. 1998;20:250–252. doi: 10.1016/s0387-7604(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 45.Singh S, Kuruvilla A, Alexander M, Korah IP. Balo’s concentric sclerosis: value of magnetic resonance imaging in diagnosis. Australas Radiol. 1999;43:400–404. doi: 10.1046/j.1440-1673.1999.433700.x. [DOI] [PubMed] [Google Scholar]
- 46.Moore GR, Berry K, Oger JJ, Prout AJ, Graeb DA, Nugent RA. Baló’s concentric sclerosis: surviving normal myelin in a patient with a relapsing-remitting dinical course. Mult Scler. 2001;7:375–382. doi: 10.1177/135245850100700606. [DOI] [PubMed] [Google Scholar]
- 47.Caracciolo JT, Murtagh RD, Rojiani AM, Murtagh FR. Pathognomonic MR imaging findings in Balo concentric sclerosis. AJNR Am J Neuroradiol. 2001;22:292–293. [PMC free article] [PubMed] [Google Scholar]
- 48.Gu J, Wang R, Lin J, Fang S. Concentric sclerosis: imaging diagnosis and clinical analysis of 3 cases. Neurol India. 2003;51:528–530. [PubMed] [Google Scholar]
- 49.Airas L, Kurki T, Erjanti H, Marttila RJ. Successful pregnancy of a patient with Balo’s concentric sclerosis. Mult Scler. 2005;11:346–348. doi: 10.1191/1352458505ms1158oa. [DOI] [PubMed] [Google Scholar]
- 50.Pohl D, Rostasy K, Krone B, Hanefeld F. Baló’s concentric sclerosis associated with primary human herpesvirus 6 infection. J Neurol Neurosurg Psychiatry. 2005;76:1723–1725. doi: 10.1136/jnnp.2004.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball T, Malik O, Roncaroli F, Quest RA, Aviv RI. Apparent diffusion coefficient changes and lesion evolution in Balo’s type demyelination-correlation with histopathology. Clin Radiol. 2007;62:498–503. doi: 10.1016/j.crad.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Mowry EM, Woo JH, Ances BM. Baló’s concentric sclerosis presenting as a stroke-like syndrome. Nat Clin Pract Neurol. 2007;3:349–354. doi: 10.1038/ncpneuro0522. [DOI] [PubMed] [Google Scholar]
- 53.Khiat A, Lesage J, Boulanger Y. Quantitative MRS study of Baló’s concentric sclerosis lesions. Magn Reson Imaging. 2007;25:1112–1115. doi: 10.1016/j.mri.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Lindquist S, Bodammer N, Kaufmann J, König F, Heinze HJ, Brück W, Sailer M. Histopathology and serial, multimodal magnetic resonance imaging in a multiple sclerosis variant. Mult Scler. 2007;13:471–482. doi: 10.1177/1352458506071329. [DOI] [PubMed] [Google Scholar]
- 55.Dreha-Kulaczewski SF, Helms G, Dechent P, Hofer S, Gärtner J, Frahm J. Serial proton MR spectroscopy and diffusion tensor imaging in infantile Balo’s concentric sclerosis. Neuroradiology. 2009;51:113–121. doi: 10.1007/s00234-008-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Xie P, Fan X, Tang H. Balò’s concentric sclerosis presenting with benign clinical course and multiple sclerosis-like lesions on magnetic resonance images. Neurol India. 2009;57:66–68. doi: 10.4103/0028-3886.48815. [DOI] [PubMed] [Google Scholar]
- 57.Matsuoka T, Suzuki SO, Iwaki T, Tabira T, Ordinario AT, Kira J. Aquaporin-4 astrocytopathy in Baló’s disease. Acta Neuropathol. 2010;120:651–660. doi: 10.1007/s00401-010-0733-7. [DOI] [PubMed] [Google Scholar]
- 58.Brown JW, Coles AJ, Jones JL. First use of alemtuzumab in Balo’s concentric sclerosis: a case report. Mult Scler. 2013;19:1673–1675. doi: 10.1177/1352458513498129. [DOI] [PubMed] [Google Scholar]
- 59.Purohit B, Ganewatte E, Schreiner B, Kollias S. Balo’s Concentric Sclerosis with Acute Presentation and Co-Existing Multiple Sclerosis-Typical Lesions on MRI. Case Rep Neurol. 2015;7:44–50. doi: 10.1159/000380813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen F, Liu T, Li J, Xing Z, Huang S, Wen G, Lu G. Eccentric development of Balo’s concentric sclerosis: detected by magnetic resonance diffusion-weighted imaging and magnetic resonance spectroscopy. Int J Neurosci. 2015;125:433–440. doi: 10.3109/00207454.2014.946563. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal M, Ulmer JL, Klein AP, Mark LP. Why Is This Auntminnie a Diagnostic Conundrum?: A Knowledge-Based Approach to Balo’s Concentric Sclerosis From Reports of 3 Cases and Pooled Data From 68 Other Patients in the Literature. Curr Probl Diagn Radiol. 2018;pii:S0363–0188(17)30191-3. doi: 10.1067/j.cpradiol.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Sagduyu Kocaman A, Yalinay Dikmen P, Karaarslan E. Cocaine-induced multifocal leukoencephalopathy mimicking Balo’s concentric sclerosis: A 2-year follow-up with serial imaging of a single patient. Mult Scler Relat Disord. 2018;19:96–98. doi: 10.1016/j.msard.2017.11.011. [DOI] [PubMed] [Google Scholar]


