Abstract
Prognostic/therapeutic stratification of papillary urothelial cancers is solely based upon histology, despite activated FGFR3-signaling was found to be associated with low grade tumors and favorable outcome. However, there are FGFR3-overexpressing tumors showing high proliferation—a paradox of coexisting favorable and adverse features. Therefore, our study aimed to decipher the relevance of FGFR3-overexpression/proliferation for histopathological grading and risk stratification. N = 142 (n = 82 pTa, n = 42 pT1, n = 18 pT2-4) morphologically G1–G3 tumors were analyzed for immunohistochemical expression of FGFR3 and Ki67. Mutation analysis of FGFR3 and TP53 and FISH for FGFR3 amplification and rearrangement was performed. SPSS 23.0 was used for statistical analysis. Overall FGFR3high/Ki67high status (n = 58) resulted in a reduced ∆mean progression-free survival (PFS) (p < 0.01) of 63.92 months, and shorter progression-free survival (p < 0.01; mean PFS: 55.89 months) in pTa tumors (n = 50). FGFR3mut/TP53mut double mutations led to a reduced ∆mean PFS (p < 0.01) of 80.30 months in all tumors, and FGFR3mut/TP53mut pTa tumors presented a dramatically reduced PFS (p < 0.001; mean PFS: 5.00 months). Our results identified FGFR3high/Ki67high papillary pTa tumors as a subgroup with poor prognosis and encourage histological grading as high grade tumors. Tumor grading should possibly be augmented by immunohistochemical stainings and suitable clinical surveillance by endoscopy should be performed.
Keywords: FGFR3, Ki67, TP53, bladder cancer, prognosis
1. Introduction
Bladder cancer is the second most common genitourinary malignancy [1]. At primary diagnosis, most of the tumors are papillary non-invasive cancers (pTa) which are mostly well differentiated but show a high rate of recurrence. Those tumors are characterized by certain molecular alterations as for example FGFR3 activation [2,3,4,5]. Up to 30% of all patients have invasive disease at diagnosis. These tumors frequently derive from flat carcinoma in situ (CIS) of the urothelium (a high grade lesion, often TP53-mutated) and quickly develop muscle-invasion and metastasis [6,7]. Current prognostic and therapeutic stratification in urothelial cancers is therefore based on tumor staging and grading at histological examination. Staging criteria is the depth of invasion defined by the tumor node metastasis (TNM)-classification of the Union Internationale Contre le Cancer (UICC) [8]. The tumor grading is based upon architectural order and nuclear shape features, which have been thoroughly defined as diagnostic criteria in the 2004 WHO classification of bladder cancer in order to achieve reproducible and comparable diagnoses worldwide. Low grade (LG) tumors show uniform, slightly enlarged nuclei in an orderly, polarized architecture, sometimes with a prominent palisading of the basal layer. Mitotic figures are infrequent [9,10]. High grade (HG) tumors show more pleomorphic nuclei with multiple mitotic features and various extent of architectural disarray [10]. Based on previous genetic analyses and clinical observations, it has been proposed that the histological appearance (grading) of tumors correlates with the underlying genetic alterations, and low grade tumors were regarded genetically stable, whereas high grade tumors, harboring a high number of genetic alterations, were considered genetically “unstable” [7]. Proposed prognostic markers in papillary non-invasive tumors have been the Ki67 labeling index (marker for cell proliferation) and keratin 20 expression (marker for cell differentiation). Tumors with Ki67 ≥ 15% were regarded as highly proliferative [11,12,13] and aberrant expression of keratin 20 was linked to disease recurrence in pTa tumors [14]. Lately, Hurst et al. conducted a comprehensive molecular study on n = 141 papillary non-invasive bladder cancers (low grade, G1 and G2 according to WHO 1973) and found lower overall mutation rates, but more mutations in chromatin modifying genes than in muscle-invasive bladder cancer, and two distinct genomic subgroups of tumors (genomic subtype 1 and 2). The majority of tumors with genomic subtype 1 showed no or only few copy-number alterations. Genomic subtype 2 was characterized by loss of 9q (including the mTORC1 regulator TSC1), increased Ki67 labeling index, upregulated mTORC1 signaling (comprising the overrepresentation of genes in processes that are involved in the unfolded protein response, glycolysis, and cholesterol homeostasis) as well as enrichment for DNA repair and cell-cycle genes [15]. FGFR3 mutations were not found to be significantly different in both subgroups (72% vs. 89%) and TP53 mutations were absent [15]. The authors did not show a correlation of molecular profiles with specific histological features.
However, in routine histological diagnostics, pathologists often see papillary non-invasive tumors with quite uniform, relatively small nuclei, which give a “crowded” impression, but seem to be of “low nuclear grade”. Interestingly, Ki67 labeling in these tumors is often enhanced and from this point of view a reconsideration of a possible “high grade”-biology is implicated. Opposite to the negative predictive impact of a high Ki67 index, these tumors often show a strong expression of FGFR3, which indicates an activation of the signaling pathway resulting in cellular proliferation, but is generally associated with a benign course of disease with higher recurrence rates but less progression [7]. Being aware of this diagnostic-biological “dilemma”, we delineated in this study the immunohistochemical and genetic basis of such FGFR3high/Ki67high papillary bladder cancers in order to reveal their prognostic impact.
2. Results
2.1. Immunohistochemical Combination of Ki67-Index and FGFR3 Levels Defines Worse Patients’ Outcome
Overall, FGFR3 and Ki67 protein expression was analyzed by immunohistochemistry (Figure 1A–I) in n = 142 primary bladder tumors comprising n = 82 papillary non-invasive tumors (for cohort characteristics, see Table S1). In this cohort, 87/142 patients (61.3%) showed a high Ki67-index (≥15% positivity) and 100/142 bladder cancer patients (70.4%) were characterized by strong FGFR3 expression (Tomlinson Score 3) (Figure S1A,C). In papillary non-invasive pTa tumors, 82.9% showed strong FGFR3 and 54.9% increased Ki67 expression (Figure S1B,D).
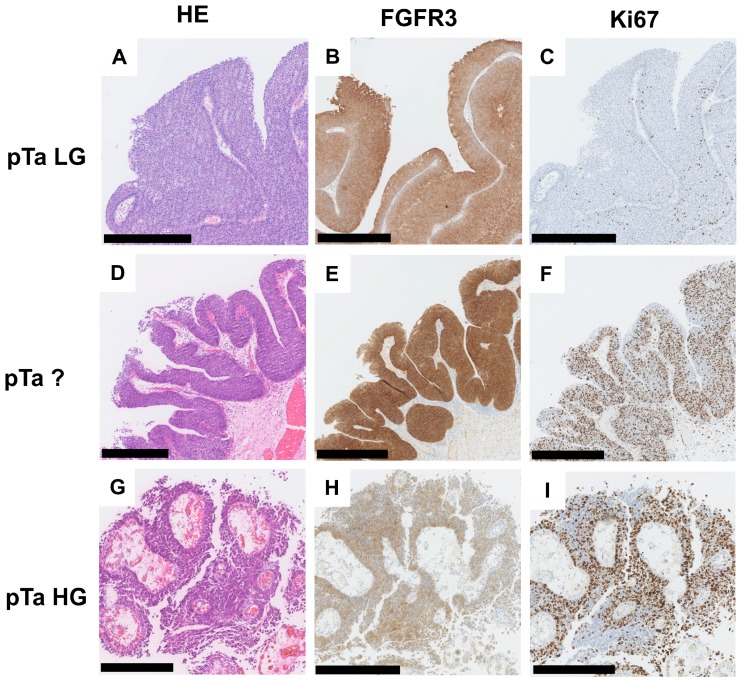
Figure 1.
FGFR3 and Ki67 protein expression in papillary non-invasive (pTa) bladder tumors. Immunohistochemical staining for FGFR3 and Ki67 protein of representative tumors are shown. (A–C) pTa low grade (LG) tumor: (A) Hematoxylin and Eosin (HE) staining; (B) strong FGFR3 immunoreactivity; and (C) only a few cell nuclei are positive for Ki67 expression. (D–F) pTa tumor with “crowded low nuclear grade” (pTa?) morphology: (D) HE staining; (E) strong FGFR3 immunoreactivity; and (F) high nuclear Ki67 protein staining. (G–I) pTa high grade (HG) tumor: (G) HE staining; (H) moderate FGFR3 protein expression; and (I) high nuclear Ki67 staining. Scale bar: 500 µm; original digital magnifications vary from 5× to 7×.
Next, associations between clinico-pathological characteristics and both FGFR3 and Ki67 protein expression were tested. FGFR3 expression and Ki67 index correlated with tumor grading (FGFR3: p < 0.001, Ki67: p < 0.001), but only FGFR3 expression was significantly associated with tumor stage (FGFR3: p < 0.001) (Table 1 and Table 2). No association was found between FGFR3/Ki67 and age at diagnosis or gender.
Table 1.
Clinico-pathological parameters in correlation to FGFR3 protein expression.
| FGFR3 Expression a | |||||
|---|---|---|---|---|---|
| n | 0–2 | 3 | p-Value b | Spearman ρ | |
| Parameter: | |||||
| Age at diagnosis | |||||
| <70 years | 67 | 20 | 47 | 0.946 | 0.006 |
| ≥70 years | 75 | 22 | 53 | ||
| Gender | |||||
| female | 31 | 10 | 21 | 0.372 | 0.031 |
| male | 111 | 32 | 79 | ||
| Histological tumor grade | |||||
| low grade | 49 | 3 | 46 | <0.001 | −0.373 |
| high grade | 93 | 39 | 54 | ||
| Tumor stage | |||||
| pTa | 82 | 14 | 68 | <0.001 | −0.320 |
| pT1–pT4 | 60 | 28 | 32 | ||
a Tomlinson score according to [16]; b Fisher’s exact test; Significant p-values are marked in bold face.
Table 2.
Clinico-pathological parameters in correlation to Ki67 protein expression.
| Ki67 Expression a | |||||
|---|---|---|---|---|---|
| n | <15% | ≥15% | p-Value b | Spearman ρ | |
| Parameter: | |||||
| Age at diagnosis | |||||
| <70 years | 67 | 31 | 36 | 0.083 | 0.146 |
| ≥70 years | 75 | 24 | 51 | ||
| Gender | |||||
| female | 31 | 14 | 17 | 0.408 | 0.070 |
| male | 111 | 41 | 70 | ||
| Histological tumor grade | |||||
| low grade | 49 | 34 | 15 | <0.001 | 0.457 |
| high grade | 93 | 21 | 72 | ||
| Tumor stage | |||||
| pTa | 82 | 37 | 45 | 0.069 | 0.175 |
| pT1–pT4 | 60 | 18 | 42 | ||
a According to [11]; b Fisher’s exact test; Significant p-values are marked in bold face.
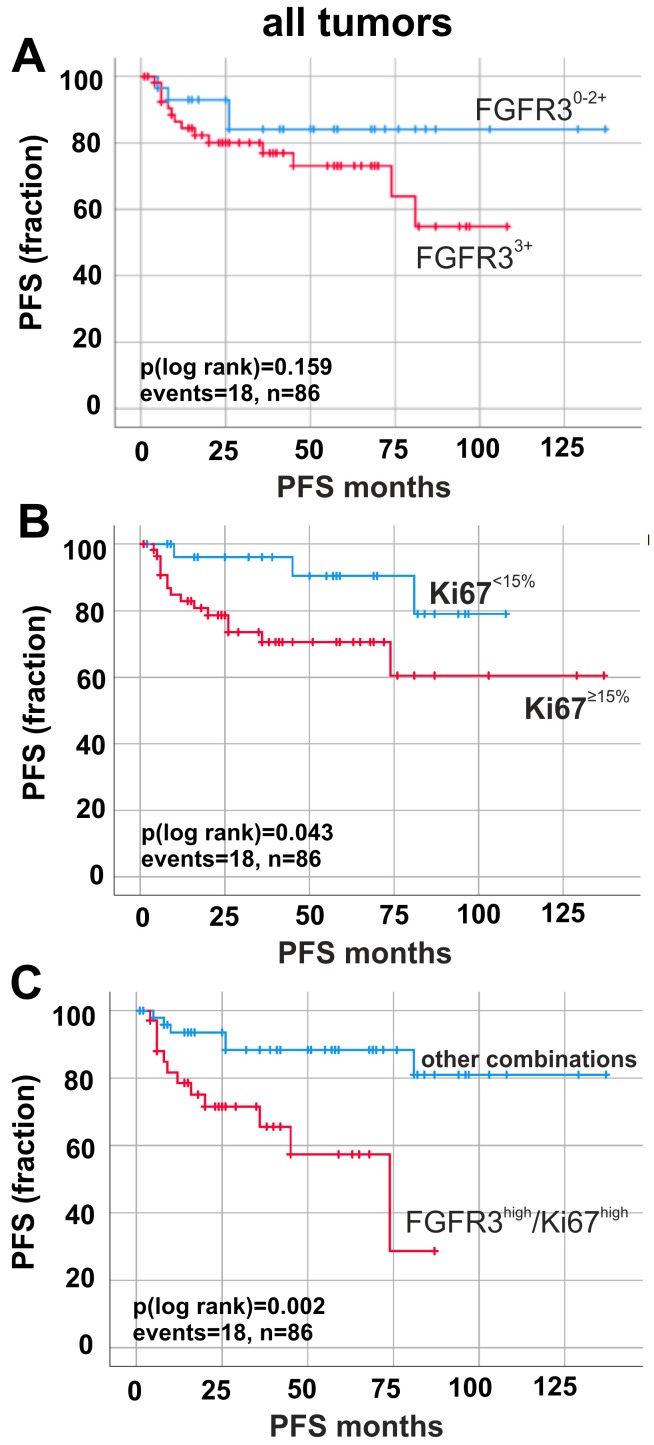
To assess the clinical impact, Kaplan–Meier analyses were performed. FGFR3 expression had no significant impact on progression-free survival (PFS) (Figure 2A). In contrast, enhanced Ki67 expression (≥15%) significantly predicted shorter progression-free survival (∆mean PFS: 2.71 months, p = 0.043). Finally, we aimed to decipher the potential prognostic impact of combined FGFR3 expression and Ki67 index: FGFR3high/Ki67high status was found in n = 58 cases. A combined analysis of FGFR3/Ki67 positivity (Figure 2C and Figure S2A) resulted in a reduced ∆mean PFS (p < 0.01) of 63.92 months when comparing FGFR3high/Ki67high tumors (mean PFS: 54.87 months ± 6.73; 95% CI: 41.78 to 68.05) with all other combinations (mean PFS: 118.78 months ± 6.95; 95% CI: 105.17 to 132.40). If, for example, both markers were expressed at low levels, bladder cancer patients showed no progressive disease at all (Figure S2A). Therefore, our results identify FGFR3high/Ki67high tumors as an aggressive subgroup.
Figure 2.
Prognostic impact of FGFR3 and Ki67 protein expression in all tumors (pTa, pT1 and pT2–4). Kaplan–Meier survival curves display progression-free survival (PFS). (A) Survival curves of patients with high FGFR3 expression (red curve, n = 56) compared to low FGFR3 expression (blue curve, n = 30). (B) Kaplan–Meier analysis of patients with high Ki67 expression (red curve, n = 56) compared to low Ki67 expression (blue curve, n = 30). (C) Survival curve analysis of FGFR3high/Ki67high expression (red curve, n = 34) compared to all other combinations of FGFR3 and Ki67 expression (blue curve, n = 32). n: overall number of cases; events: overall events of tumor progression.
The calculated Cox regression model (including the potentially prognostic parameters stage, grade, age, keratin 20 and keratin 5/6) confirmed independency of the clinical impact of a FGFR3high/Ki67high status on progression-free survival. Patients displaying a combined overexpression of FGFR3 and Ki67 showed an approximately four-fold higher risk for tumor progression (multivariate hazard ratio (HR): 3.943, 95% CI: 1.247 to 12.466, p = 0.019) (Table 3).
Table 3.
Multivariate Cox regression analysis of immunohistochemical markers including all factors potentially influencing PFS.
| Variable | HR | p-Value | 95%CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| FGFR3 high/Ki67high | 3.943 | 0.019 | 1.247 | 12.466 |
| pT status | 0.957 | 0.941 | 0.295 | 3.105 |
| Tumor grade | 0.846 | 0.823 | 0.196 | 3.653 |
| Keratin 5/6 | 0.482 | 0.280 | 0.128 | 1.812 |
| Keratin 20 | 0.424 | 0.115 | 0.146 | 1.232 |
| Age | 1.773 | 0.347 | 0.537 | 5.847 |
2.2. Prognostic Impact of Ki67-Index and FGFR3 Overexpression in Papillary Non-Invasive (pTa) Tumors
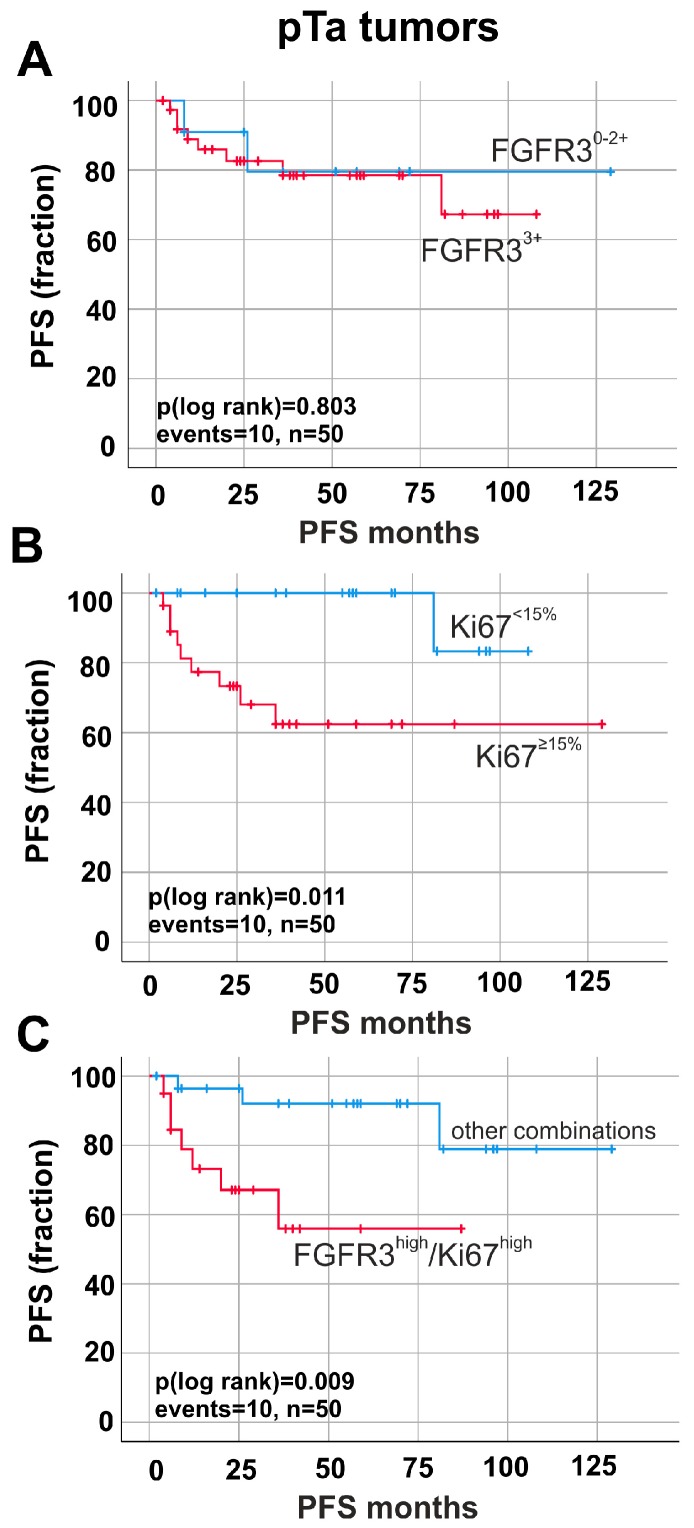
Stratifying our cohort by invasiveness, i.e., into papillary non-invasive (pTa) and invasive tumors (pT1–pT4), FGFR3 overexpression (Tomlinson Score 3) was not associated with tumor progression in pTa bladder cancer (p > 0.05 for PFS) (Figure 3A).
Figure 3.
Prognostic impact of FGFR3 and Ki67 protein expression in papillary non-invasive (pTa) tumors. Kaplan–Meier survival curves demonstrate progression-free survival (PFS). (A) Survival curves of patients with high FGFR3 expression (red curve, n = 39) compared to low FGFR3 expression (blue curve, n = 11). (B) Kaplan–Meier analysis of patients with high Ki67 expression (red curve, n = 28) compared to low Ki67 expression (blue curve, n = 22). (C) Impact of combined markers on risk stratification of tumor progression is shown. Survival curve analysis of FGFR3high/Ki67high expression (red curve, n = 20) compared to all other combinations of FGFR3 and Ki67 expression (blue curve, n = 30) in pTa tumors. n, overall number of cases; events, overall events of tumor progression.
Single marker analysis of high Ki67-index correlated with progression-free survival (∆mean PFS: 17.06 months, p = 0.011) (Figure 3B). Now, combining the two immunohistochemical markers, univariate Kaplan–Meier curve revealed a significant impact of FGFR3high/Ki67high expression on patients’ outcome only in pTa tumors. In fact, patients with high FGFR3high/Ki67high showed a significantly (p < 0.01) shorter progression-free survival (mean PFS: 55.89 months ± 9.23; 95% CI: 37.82 to 73.98) compared to those patients with all other combinations of FGFR3/Ki67 expression (mean PFS: 113.85 months ± 8.12; 95% CI: 97.94 to 129.77, p = 0.009) (Figure 3C).
2.3. Altered Molecular FGFR3/TP53 Status Predicts Worse Patients’ Survival
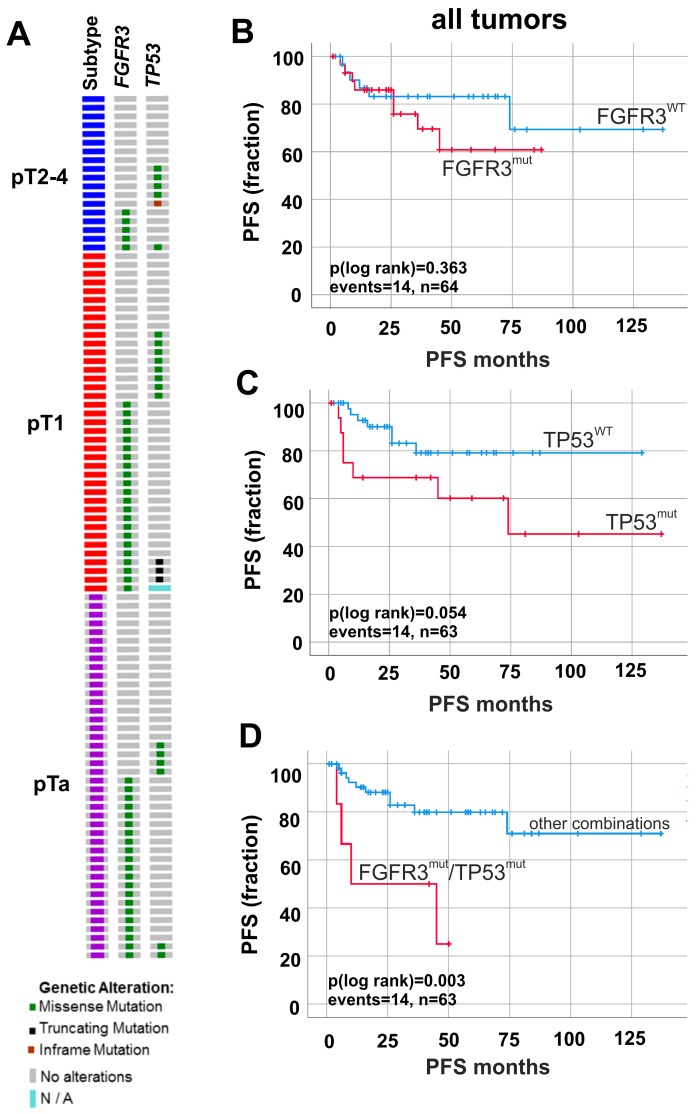
Since we hypothesized that FGFR3-overexpression and high cell proliferation might indicate a higher risk for progression in papillary non-invasive tumors, we further investigated the molecular status of our cohort by studying both mutations for FGFR3 as papillary and TP53 as invasive markers (for detailed mutation data, see Table S2). In total, 48 out of 99 (48.5%) analyzed patients harbored mutations within the FGFR3 gene (Figure 4A).
Figure 4.
FGFR3 and TP53 mutation frequency and prognostic impact on tumor progression. (A) Oncoprint graph for FGFR3 and TP53 mutation analysis. (B–D) Kaplan–Meier survival curves display progression-free survival (PFS). (B) Survival curves of tumors with detected FGFR3 mutations (red curve, n = 30) compared to non-mutated FGFR3 gene status (blue curve, n = 34). (C) Kaplan–Meier analysis of tumors with mutated TP53 (red curve, n = 17) compared to wildtype TP53 (blue curve, n = 46). (D) Impact of double mutations on risk stratification of tumor progression is demonstrated. Univariate analysis of double mutations (red curve, n = 6) compared to all other combinations of mutated and non-mutated FGFR3 and TP53 genes (blue curve, n = 57). n, overall number of cases; events, overall events of tumor progression.
The most frequent mutation was p.S249C (pTa: 13/21, pT1: 10/22, pT2–4: 2/5). FGFR3 mutations showed no significant association with clinico-pathological parameters like tumor stage or grade (Table S3). TP53 mutations were present in n = 23/98 (23.5%) patients (Figure 4A). There were n = 18/23 (78.3%) tumors which solely showed missense mutations (pTa: 6/6, pT1: 7/11, pT2–4: 5/6) and n = 5/23 (21.7%) tumors with mutations leading to a premature transcription stop either due to the appearance of a stop codon or a frameshift (pTa: 0/6, pT1: 4/11, pT2–4: 1/6). TP53 mutations correlated with tumor grade (p < 0.05) but not with stage (Table S4). Mutations in both genes (referred to as double mutations) were found in n = 6/99 (6.1%) patients.
Survival analysis revealed no significant association between single mutations, i.e., FGFR3 or TP53, with patient’s outcome for PFS (Figure 4B,C). However, mutations in both genes (FGFR3mut/TP53mut) predicted unfavorable prognosis for PFS. Double mutations led to a reduced ∆mean PFS (p < 0.01) of 80.30 months: FGFR3mut/TP53mut tumors (mean PFS: 27.08 months ± 8.41; 95% CI: 10.59 to 43.57) showed shorter PFS in contrast with all other combinations (mean PFS: 107.83 months ± 8.62; 95% CI: 90.49.17 to 124.28) (Figure 4D and Figure S2B).
Multivariate analysis confirmed the prognostic impact of FGFR3mut/TP53mut tumors. Double mutated tumors exhibited a 6.6 times higher risk for tumor progression (multivariate hazard ratio (HR): 6.563, 95% CI: 1.694 to 25.425, p = 0.006) (Table 4).
Table 4.
Multivariate Cox regression analysis of molecular markers including all factors potentially influencing PFS.
| Variable | HR | p-Value | 95%CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| FGFR3mut/TP53mut | 6.563 | 0.006 | 1.694 | 25.425 |
| pT status | 1.179 | 0.821 | 0.284 | 4.896 |
| Tumor grade | 0.241 | 0.138 | 0.037 | 1.580 |
| Keratin 5/6 | 0.714 | 0.621 | 0.188 | 2.712 |
| Keratin 20 | 0.872 | 0.814 | 0.279 | 2.730 |
| Age | 1.41 | 0.584 | 0.412 | 2.809 |
2.4. Prognostic Impact of FGFR3 and TP53 Mutations in Papillary Non-Invasive (pTa) Tumors
Next, we focused on pTa tumors, in particular those with FGFR3-overexpression and high cell proliferation. In pTa tumors, the following distribution was found: n = 17/42 (40.5%) FGFR3wt/TP53wt, n = 19/42 (45.2%) FGFR3mut/TP53wt, n = 4/42 (9.5%) FGFR3wt/TP53mut and n = 2/42 (4.8%) FGFR3mut/TP53mut. On the contrary, pT1 tumors showed n = 9/39 (23.1%) FGFR3wt/TP53wt, n = 19/39 (48.7%) FGFR3mut/TP53wt, n = 8/39 (20.5%) FGFR3wt/TP53mut and n = 3/39 (7.7%) FGFR3mut/TP53mut. pT2–4 tumors represented with the following mutational pattern: n = 8/18 (44.4%) FGFR3wt/TP53wt, n = 4/18 (22.2%) FGFR3mut/TP53wt, n = 5/18 (27.8%) FGFR3wt/TP53mut and n = 1/18 (5.6%) FGFR3mut/TP53mut.
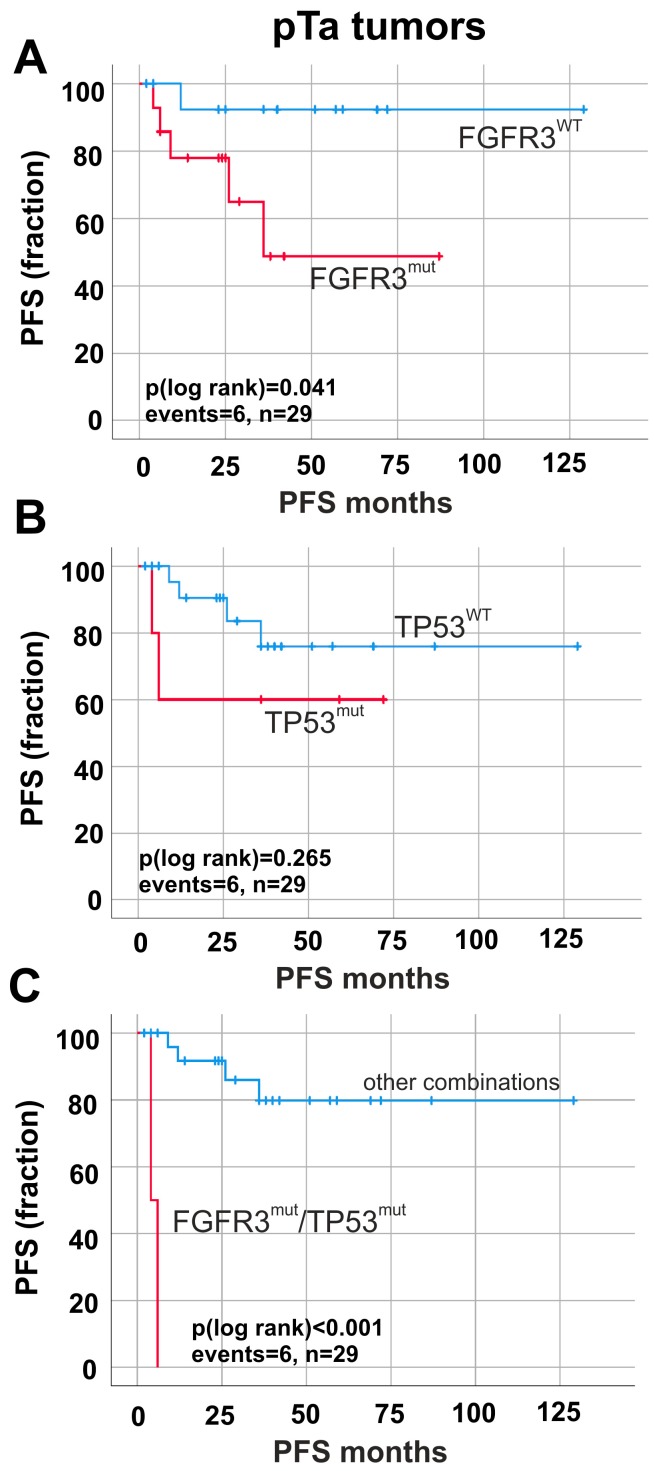
Survival analyses revealed a correlation between FGFR3 mutations and shorter PFS (p = 0.041) in pTa tumors (Figure 5A). TP53 mutations did not show any effects (p > 0.05) on PFS (Figure 5B). Interestingly, tumors exhibiting double mutation status FGFR3mut/TP53mut (n = 6) presented a dramatically reduced ∆mean PFS (p < 0.001) of 102.52 months (mean PFS: 5.00 months ± 1.00; 95% CI: 3.04 to 6.96) in pTa tumors (Figure 5C) compared with all other combinations (mean PFS: 107.52 months ± 9.72; 95% CI: 88.46 to 126.57).
Figure 5.
Prognostic impact of FGFR3 and TP53 mutations on tumor progression in papillary non-invasive (pTa) tumors. Progression-free survival (PFS) is shown. (A) Univariate survival analysis illustrates that detected FGFR3 mutations (red curve, n = 14) predict shorter PFS compared to non-mutated FGFR3 gene status (blue curve, n = 15). (B) Kaplan–Meier analysis of tumors with mutated TP53 (red curve, n = 5) compared to wildtype TP53 (blue curve, n = 24). (C) Impact of double mutations on risk stratification of tumor progression is shown. Survival analysis of double mutations (red curve, n = 2) compared to all other combinations of mutated and non-mutated FGFR3 and TP53 genes (blue curve, n = 27) in pTa tumors. n: overall number of cases; events: overall events of tumor progression.
Finally, we assessed the clinical impact of immunohistochemical and mutational status as a combined approach. Survival analysis displayed that FGFR3mut/TP53mut double mutated tumors were significantly associated with worse patients’ outcome only in FGFR3 and Ki67 overexpressing tumors: reduced PFS in FGFR3high/Ki67high double mutated tumors compared with all other combinations of molecular status of TP53 and FGFR3 (FGFR3wt/TP53wt p = 0.001; FGFR3mut/TP53wt p < 0.001; FGFR3wt/TP53mut p = 0.116) (data not shown). However, it has to be noted that the number of double mutations is very low, and, hence, statistical validity should be enhanced in future studies.
2.5. FGFR3high/Ki67high Tumors Define a Subset of pTa Tumors Including Lesions with Molecular FGFR3 Pathway Activation
Prognostic stratification of bladder cancer patients in routine histopathological diagnostics claims simple and cost-effective means, therefore, we evaluated the concordance of immunohistochemical staining results and molecular status.
The majority of FGFR3high/Ki67high tumors was characterized by conjunct FGFR3 mutations with a significant correlation only in papillary non-invasive tumors (pTa n = 19/27 (70.4%), p < 0.001) but not in pT1 and pT2–4 tumors (Table S5). There was no significant correlation between FGFR3high/Ki67high tumors and TP53 mutations independently of the given tumor stage (data not shown).
To evaluate the diagnostic potential of immunohistochemical markers covering the molecular FGFR3 pathway, we performed ROC (Receiver operating characteristics) curve statistics to calculate sensitivity and specificity. Accordingly, both immunohistochemical markers detect FGFR3 mutations with 90.5% sensitivity and 61.9% specificity (area under curve (AUC): 0.776, p = 0.004, positive predictive value (PPV): 70.4%, negative predictive value (NPV): 85.7%). These data show that FGFR3high/Ki67high tumors include papillary lesions with mutation-based altered FGFR3 signaling, but also tumors without molecular alterations (pTa n = 8/27 (29.6%)). Hence, our data give evidence that FGFR3high/Ki67high tumors define a subset of pTa tumors associated with poor prognosis potentially decoupled from the described protective effect of FGFR3 activation [7].
3. Discussion
In our study, we systematically analyzed papillary non-invasive and invasive tumors for distinct prognostic immunohistochemical and molecular markers. We focused on a subgroup of immunohistochemically FGFR3high/Ki67high tumors in order to reveal their prognostic impact on patient survival and re-evaluate their histological classification/grading.
Although, according to nuclear and architectural criteria, these papillary tumors appear to be orderly and more “nuclear low grade”, we found them associated with worse PFS compared with FGFR3high/Ki67low tumors. This was especially evident in pTa tumors, where mean progression-free survival was reduced to 55 instead of 113 months. Therefore, we asked whether these tumors harbor a special molecular phenotype turning them into aggressive ones. In literature FGFR3 and TP53 mutations were initially thought to be mutually exclusive as FGFR3 mutations were associated with pTa and LG tumors (“papillary pathway”), whereas the TP53 mutations were often found in invasive and HG carcinomas (“CIS/invasive pathway”) [17,18]. Notwithstanding, Hernandez et al. reported FGFR3 and TP53 mutations to be independently distributed in a large series of pT1G3 tumors, that were consequently interpreted as a particular group of bladder tumors that could not be classified into either one pathway or the other [4]. In our study, we saw a similar trend with well-known inverse relationships between FGFR3 and TP53 mutations for both stage and grade, while mutations in FGFR3 and TP53 revealed an independent but not mutually exclusive assignment (six tumors with double mutations). Biologically activated FGFR3 signaling promotes cell proliferation and tumor growth, however interestingly, highest numbers of FGFR3-alterations are found in benign papillary or low grade papillary tumors with usually low proliferation (Ki67) index [19,20,21]. TP53 inactivation results in reduced cellular apoptosis and thus maintains tumor growth via reduced cell death [22,23,24,25]. We hypothesized that a FGFR3high/Ki67high phenotype might be resulting from inactivated p53, however we found no sufficient molecular evidence for this theory in our cohort. Recent comprehensive sequencing data of papillary non-invasive bladder tumors revealed a genomic subtype 2, which is characterized by loss of 9q (including TSC1), increased Ki67 labeling index, upregulated mTORC1 signaling, glycolysis, features of the unfolded protein response, altered cholesterol homeostasis and DNA repair [15]. Therefore, high proliferation might be explained by mutations in DNA repair genes or the deletion/mutation of TSC1, which consequently leads towards an upregulation of mTORC1 and PIK3CA mutations. Further analyses to strengthen this theory have to be performed in the future.
Comprehensive molecular data of bladder cancer has been gained in the recent years [15,26,27], however, complex multigene analysis and RNA expression analysis are costly and laborious, and therefore cost-effective simple analyses for routine histological examination are needed. In our study, we analyzed whether fast and simple immunohistochemical analyses are suitable to detect a more aggressive molecular subtype. We found a highly significant correlation between strong FGFR3/Ki67 immunohistochemical staining and FGFR3 mutation status, which indicates that FGFR3 protein expression is more frequent than mutational activation [16]. Moreover, our FGFR3high/Ki67high subgroup also comprises those neoplasms without any molecular (FGFR3 and/or TP53) alterations defining in this combination a subset of pTa tumors with poor prognosis, i.e., FGFR3 overexpression was associated with unfavorable outcome as previously shown, for instance, for invasive bladder tumors treated with adjuvant chemotherapy [28]. Thus, our data support the proposed clinical significance of these two immunohistochemical markers for diagnostic and prognostic stratification of more aggressive papillary non-invasive bladder tumors.
Taken together, we found immunohistochemically FGFR3high/Ki67high pTa tumors associated with worse prognosis/survival, despite appearing histologically of “lower nuclear grade”/G2. Even if these tumors appear to be “low grade” (according to the 2004 WHO classification), we recommend classifying them as “high grade” pTa tumors. In light of our findings, we suggest immunohistochemical staining for FGFR3 and Ki67 in order to gain evidence for this more aggressive molecular subgroup with worse prognosis. These patients probably could profit from close endoscopic follow-up, as especially urine cytology might also be challenging/less sensitive due to their minimal nuclear changes.
4. Materials and Methods
4.1. Patient Samples, Tissue Microarrays and DNA
We retrospectively selected urothelial bladder cancer cases (mutational analysis: n = 42 pTa, n = 39 pT1, n = 18 pT2–4; immunohistochemical analysis: n = 82 pTa, n = 42 pT1, n = 18 pT2–4) from our pathology archive and from the archive of the Institute of Pathology in Erlangen. Formalin-fixed paraffin-embedded (FFPE) surgical specimens were used to construct tissue microarrays (all samples) and extract DNA (n = 99 samples) using Qiagen kits (Qiagen, Hilden, Germany) as previously described [29,30,31]. Patient information was obtained by the Department of Urology and the local ethics committee approved a retrospective, pseudonymized study of archival tissues (RWTH EK 009/12). Histological tumor grade and stage was classified according to WHO 2004 classification [8].
4.2. Immunohistochemistry
For immunohistochemical stainings, TMA sections were pretreated with DAKO PT-Link heat induced antigen retrieval with Low pH (pH 6) or High pH (pH 9) Target Retrieval Solution (DAKO, Hamburg, Germany) and incubated for 30 min at room temperature with respective antibodies in a DAKO Autostainer (DAKO). For stainings anti-FGFR3 (clone B9, PTlink pH6, dilution 1:25, Flex+M; Santa Cruz Biotechnology, Heidelberg, Germany), anti-Ki67 (clone MIB-1, PTlink pH 6, dilution 1:400, Flex+M; DAKO), anti-CK 20 (clone Ks20.8, PTlink pH 6, dilution 1:200, Flex+M; DAKO), and anti-CK5/6 (clone D5/16 B4, PTlink pH 9, dilution 1:100, Flex+M; DAKO) were used. Appropriate linker molecules EnVisionTMFLEX+ (mouse/rabbit), EnVision FLEX/HRP detection system and counterstaining with EnVision FLEX Hematoxylin were applied. Stainings were evaluated by an experienced uropathologist (NTG) who was blinded for patient identity, diagnosis and clinical follow-up results. FGFR3 positivity was reported according to a semiquantitative scoring system developed by Tomlinson et al. [16]. All other stainings were evaluated for staining intensities (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining) and percentages of positive stained tumor cells. Results were judged as follows: Keratin 20 positive ≥10% stained cells [14,32], Keratin 5/6 positive ≥10% [32], Ki67 positive ≥15% [11,13] stained cells.
4.3. Fluorescence In Situ Hybridization
ZytoLight Dual Color Probe SPEC FGFR3/CEN 4 and ZytoLight Dual Color Break Apart Probe SPEC FGFR3 (Zytovision, Bremerhaven, Germany) were hybridized onto 3 µm TMA sections according to the manufacturer’s protocols. Slides were evaluated with a Zeiss Axiovert 135 fluorescence microscope (Carl Zeiss, Oberkochen, Germany), and Diskus Software (MIL 7.5, 4.80) (Büro Hilgers, Königswinter, Germany) using appropriate channels/filters (AHF ZyGreen F36-720, AHF ZyOrange F36-740, AHF DAPI, AHF F56-700). Signals of 60 nuclei of tumor cells were counted at high magnification (×1000) and judged as described previously [33].
4.4. Sanger Sequencing
PCR-amplification of exons 7, 10 and 15 of the FGFR3 gene and exons 5, 6, 7, 8 and 9 of TP53 were carried out using routine protocols. Primers and annealing temperatures are given in Table S6. PCR products were purified by either ExoSAP-IT (Affymetrix, Lahr/Schwarzwald, Germany) or a PCR purification kit (PerkinElmer Chemagen, Baesweiler, Germany) according to the manufacturer’s instructions. Sanger sequencing of both strands was run on an ABI PRISM 3500 Genetic Analyzer (Applied Biosystems, Weiterstadt, Germany) using the Big dye Terminator kit (Applied Biosystems), the same primer sets and the seq purification kit (PerkinElmer Chemagen).
4.5. Statistical Analysis
Statistical analysis was performed using SPSS (Statistical Package for the Social Sciences) software version 23.0 (SPSS Inc., Chicago, IL, USA). p-values < 0.05 were considered significant. Statistical associations between clinico-pathological and molecular factors were determined by Fisher’s exact test. Correlation analysis was performed by calculating a Spearman’s rank correlation coefficient. Survival (progression-free survival (PFS)) was calculated using the Kaplan–Meier method with log-rank statistics. Survival was measured from surgery until relapse, death or progression and was censored for patients alive without evidence of event at the last follow-up date. Multivariate Cox-regression analysis was performed to test for an independently prognostic value of FGFR3-Ki67 protein expression and FGFR3-TP53 mutations. Receiver operating characteristics (ROC) curves were calculated to assess biomarker performance of immunohistochemical markers regarding molecular alterations.
Acknowledgments
The authors appreciate the excellent technical support of Ursula Schneider, Inge Losen, Patrick Kühl and Oliver Dohmen.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/19/9/2548/s1.
Author Contributions
Conceptualization, R.K. and N.T.G; Methodology, M.G., A.B., A.M. and M.R.; Software, M.R.; Validation, R.S.; Resources, T.G. and C.B.; Writing—Original Draft Preparation, N.T.G., M.G. and M.R.; Writing—Review and Editing, A.M., A.B., R.S., T.G., and C.B.; Visualization, M.R.; and Supervision, R.K. and N.T.G.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Van Rhijn B.W., Lurkin I., Radvanyi F., Kirkels W.J., Van der Kwast T.H., Zwarthoff E.C. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001;61:1265–1268. [PubMed] [Google Scholar]
- 3.Billerey C., Chopin D., Aubriot-Lorton M.H., Ricol D., Gil Diez de Medina S., van Rhijn B., Bralet M.P., Lefrere-Belda M.A., Lahaye J.B., Abbou C.C., et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 2001;158:1955–1959. doi: 10.1016/S0002-9440(10)64665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez S., Lopez-Knowles E., Lloreta J., Kogevinas M., Jaramillo R., Amoros A., Tardón A., García-Closas R., Serra C., Carrato A., et al. FGFR3 and Tp53 mutations in T1G3 transitional bladder carcinomas: Independent distribution and lack of association with prognosis. Clin. Cancer. Res. 2005;11:5444–5450. doi: 10.1158/1078-0432.CCR-05-0122. [DOI] [PubMed] [Google Scholar]
- 5.Neuzillet Y., van Rhijn B.W., Prigoda N.L., Bapat B., Liu L., Bostrom P.J., Fleshner N.E., Gallie B.L., Zlotta A.R., Jewett M.A., et al. FGFR3 mutations, but not FGFR3 expression and FGFR3 copy-number variations, are associated with favourable non-muscle invasive bladder cancer. Virchows. Arch. 2014;465:207–213. doi: 10.1007/s00428-014-1596-4. [DOI] [PubMed] [Google Scholar]
- 6.Wu X.R. Urothelial tumorigenesis: A tale of divergent pathways. Nat. Rev. Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 7.Knowles M.A., Hurst C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 8.Brierley J., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours. 8th ed. John Wiley & Sons Inc.; Chichester, UK: Hoboken, NJ, USA: 2017. [Google Scholar]
- 9.Montironi R., Lopez-Beltran A., Scarpelli M., Mazzucchelli R., Cheng L. Morphological classification and definition of benign, preneoplastic and non-invasive neoplastic lesions of the urinary bladder. Histopathology. 2008;53:621–633. doi: 10.1111/j.1365-2559.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 10.Moch H., Humphrey P.A., Ulbright T.M., Reuter V.E. International Agency for Research on Cancer. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. International Agency for Research on Cancer; Lyon, France: 2016. [Google Scholar]
- 11.Cina S.J., Lancaster-Weiss K.J., Lecksell K., Epstein J.I. Correlation of Ki-67 and p53 with the new World Health Organization/International Society of Urological Pathology Classification System for Urothelial Neoplasia. Arch. Pathol. Lab. Med. 2001;125:646–651. doi: 10.1043/0003-9985(2001)1252.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Hentic O., Couvelard A., Rebours V., Zappa M., Dokmak S., Hammel P., Maire F., O’Toole D., Lévy P., Sauvanet A., et al. Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factors in patients with liver metastases of digestive endocrine carcinomas. Endocr. Relat. Cancer. 2011;18:51–59. doi: 10.1677/ERC-09-0319. [DOI] [PubMed] [Google Scholar]
- 13.Bertz S., Otto W., Denzinger S., Wieland W.F., Burger M., Stohr R., Link S., Hofstädter F., Hartmann A. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur. Urol. 2014;65:218–226. doi: 10.1016/j.eururo.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 14.Harnden P., Mahmood N., Southgate J. Expression of cytokeratin 20 redefines urothelial papillomas of the bladder. Lancet. 1999;353:974–977. doi: 10.1016/S0140-6736(98)05383-5. [DOI] [PubMed] [Google Scholar]
- 15.Hurst C.D., Alder O., Platt F.M., Droop A., Stead L.F., Burns J.E., Burghel G.J., Jain S., Klimczak L.J., Lindsay H., et al. Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell. 2017;32:701–715. doi: 10.1016/j.ccell.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomlinson D.C., Baldo O., Harnden P., Knowles M.A. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J. Pathol. 2007;213:91–98. doi: 10.1002/path.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Rhijn B.W., van der Kwast T.H., Vis A.N., Kirkels W.J., Boeve E.R., Jobsis A.C., Zwarthoff E.C. FGFR3 and P53 characterize alternative genetic pathways in the pathogenesis of urothelial cell carcinoma. Cancer. Res. 2004;64:1911–1914. doi: 10.1158/0008-5472.CAN-03-2421. [DOI] [PubMed] [Google Scholar]
- 18.Bakkar A.A., Wallerand H., Radvanyi F., Lahaye J.B., Pissard S., Lecerf L., Kouyoumdjian J.C., Abbou C.C., Pairon J.C., Jaurand M.C., et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer. Res. 2003;63:8108–8112. [PubMed] [Google Scholar]
- 19.Hernandez S., Lopez-Knowles E., Lloreta J., Kogevinas M., Amoros A., Tardon A., Carrato A., Serra C., Malats N., Real F.X. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J. Clin. Oncol. 2006;24:3664–3671. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 20.Junker K., van Oers J.M., Zwarthoff E.C., Kania I., Schubert J., Hartmann A. Fibroblast growth factor receptor 3 mutations in bladder tumors correlate with low frequency of chromosome alterations. Neoplasia. 2008;10:1–7. doi: 10.1593/neo.07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Rhijn B.W., Zuiverloon T.C., Vis A.N., Radvanyi F., van Leenders G.J., Ooms B.C., Kirkels W.J., Lockwood G.A., Boevé E.R., Jöbsis A.C., et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur. Urol. 2010;58:433–441. doi: 10.1016/j.eururo.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Sigal A., Rotter V. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer. Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 23.Zuckerman V., Wolyniec K., Sionov R.V., Haupt S., Haupt Y. Tumour suppression by p53: The importance of apoptosis and cellular senescence. J. Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]
- 24.Oren M., Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb. Perspect Biol. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivlin N., Brosh R., Oren M., Rotter V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein J.N., Akbani R., Broom B.M., Wang W., Verhaak R.G., McConkey D., Lerner S., Morgan M., Creighton C.J., Smith C., et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedegaard J., Lamy P., Nordentoft I., Algaba F., Hoyer S., Ulhoi B.P., Vang S., Reinert T., Hermann G.G., Mogensen K., et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell. 2016;30:27–42. doi: 10.1016/j.ccell.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Sung J.Y., Sun J.M., Chang J.B., Seo S.I., Soo J.S., Moo L.H., Yong C.H., Young K.S., Choi Y.L., Young K.G. FGFR3 overexpression is prognostic of adverse outcome for muscle-invasive bladder carcinoma treated with adjuvant chemotherapy. Urol. Oncol. 2014;32:e23–e31. doi: 10.1016/j.urolonc.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Gaisa N.T., Graham T.A., McDonald S.A., Canadillas-Lopez S., Poulsom R., Heidenreich A., Jakse G., Tadrous P.J., Knuechel R., Wright N.A. The human urothelium consists of multiple clonal units, each maintained by a stem cell. J Pathol. 2011;225:163–171. doi: 10.1002/path.2945. [DOI] [PubMed] [Google Scholar]
- 30.Fischbach A., Rogler A., Erber R., Stoehr R., Poulsom R., Heidenreich A., Schneevoigt B.S., Hauke S., Hartmann A., Knuechel R., et al. Fibroblast growth factor receptor (FGFR) gene amplifications are rare events in bladder cancer. Histopathology. 2015;66:639–649. doi: 10.1111/his.12473. [DOI] [PubMed] [Google Scholar]
- 31.Molitor M., Junker K., Eltze E., Toma M., Denzinger S., Siegert S., Knuechel R., Gaisa N.T. Comparison of structural genetics of non-schistosoma-associated squamous cell carcinoma of the urinary bladder. Int. J. Clin. Exp. Pathol. 2015;8:8143–8158. [PMC free article] [PubMed] [Google Scholar]
- 32.Gaisa N.T., Braunschweig T., Reimer N., Bornemann J., Eltze E., Siegert S., Toma M., Villa L., Hartmann A., Knuechel R. Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer. Virchows. Arch. 2011;458:301–312. doi: 10.1007/s00428-010-1017-2. [DOI] [PubMed] [Google Scholar]
- 33.Baldia P.H., Maurer A., Heide T., Rose M., Stoehr R., Hartmann A., Williams S.V., Knowles M.A., Knuechel R., Gaisa N.T. Fibroblast growth factor receptor (FGFR) alterations in squamous differentiated bladder cancer: A putative therapeutic target for a small subgroup. Oncotarget. 2016;7:71429–71439. doi: 10.18632/oncotarget.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.