Abstract
Ceramides are key lipids in energetic-metabolic pathways and signaling cascades, modulating critical physiological functions in cells. While synthesis of ceramides is performed in endoplasmic reticulum (ER), which is altered under overnutrition conditions, proteins associated with ceramide metabolism are located on membrane arrangement of mitochondria and ER (MAMs). However, ceramide accumulation in meta-inflammation, condition that associates obesity with a chronic low-grade inflammatory state, favors the deregulation of pathways such as insulin signaling, and induces structural rearrangements on mitochondrial membrane, modifying its permeability and altering the flux of ions and other molecules. Considering the wide biological processes in which sphingolipids are implicated, they have been associated with diseases that present abnormalities in their energetic metabolism, such as breast cancer. In this sense, sphingolipids could modulate various cell features, such as growth, proliferation, survival, senescence, and apoptosis in cancer progression; moreover, ceramide metabolism is associated to chemotherapy resistance, and regulation of metastasis. Cell–cell communication mediated by exosomes and lipoproteins has become relevant in the transport of several sphingolipids. Therefore, in this work we performed a comprehensive analysis of the state of the art about the multifaceted roles of ceramides, specifically the deregulation of ceramide metabolism pathways, being a key factor that could modulate neoplastic processes development. Under specific conditions, sphingolipids perform important functions in several cellular processes, and depending on the preponderant species and cellular and/or tissue status can inhibit or promote the development of metabolic and potentially breast cancer disease.
Keywords: ceramides, meta-inflammation, breast cancer
1. Introduction
A chronic excess of triglycerides in the body, specifically within adipose tissue, can lead to its saturation and promote the release of free fatty acids (FFA) to blood circulation. These lipids could be transported by serum albumin and lipoproteins, promoting their accumulation in tissues such as liver, pancreas, and skeletal muscle, which triggers alterations in several signaling pathways associated with energetic metabolism and cell cycle regulation [1]. Importantly, saturated fatty acids (SFA) have been described as modulators of gene expression targets involved in the metabolism of sphingolipids [2]. For instance, the increase of cytoplasmic palmitoyl-CoA by lipogenesis and cell internalization could drive fatty acids toward ceramides synthesis in endoplasmic reticulum (ER) through the de novo pathway. Therefore, ceramides modify several metabolic pathways generating a cellular imbalance, for example C16 ceramide could block the activity of the electron transport chain (ECT) by inhibition of complex IV, inducing the generation of reactive oxygen species (ROS) [3]. In addition, an increase in mitochondrial ceramides contributes to the sensitization of the IP3R, favoring the release of Ca2+ from ER to the mitochondria [4].
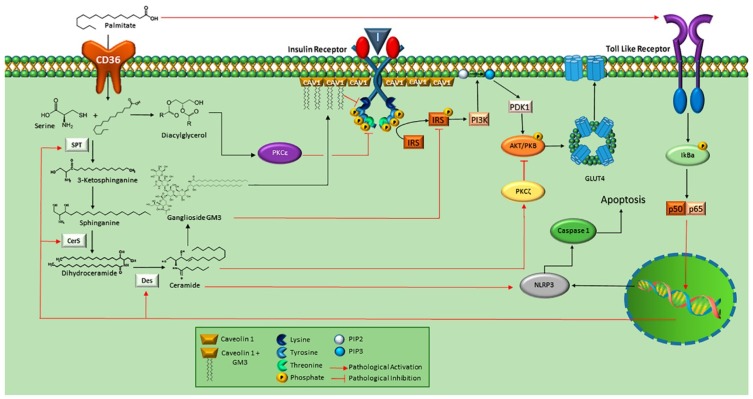
Accumulation of triglycerides and intermediate molecules such as ceramides and diacylglycerols (DAGs) are a consequence of chronic overnutrition [5,6]. Therefore, DAGs, ceramides and SFAs induce chronic inflammatory conditions, originating cellular deleterious effects [7]. An increase in the synthesis of C16-ceramides is associated with obesity and development of diabetes mellitus type 2, inhibiting fatty acid oxidation [8] (Figure 1). Cytosolic DAGs are associated with activation of hepatic PKCε [9], which has been demonstrated to phosphorylate the insulin receptor (INSR) in Threonine-1160 leading to inhibition of IRS kinase activity [10]. Thus, activation of hepatic PKCε induced by DAGs in the pathogenesis of non-alcoholic fatty liver disease has been related to hepatic insulin resistance [9]. In addition, activation of TLR-4 by palmytic acid could trigger a cascade of intracellular signaling mediated by phosphorylation of IkBa, which induces the activation of p50 and p65, and the expression of serin-palmitoyl acyltransferase (SPT), ceramide synthase (CerS) and dihydroceramide desaturase, key enzymes in the synthesis of ceramides [11]. Likewise, the increase in intracellular ceramides could trigger the activation of NOD-like receptor family pyrin domain containing 3 (NLRP3) [12], complex that integrates the inflammasome. Indeed, inflammasome functions as a putative sensor of ceramides; therefore caspase-1 is activated by this mechanism [13]. Evidence shows a close relationship among the role of ceramides with the development of metabolic pathologies (Figure 1).
Figure 1.
Metabolic implications associated to ceramide synthesis. Free fatty acid (FFA) such as palmitic acid is internalized through CD36, and ceramide synthesis is stimulated by serin-palmitoyl acyltransferase (SPT) through the addition of serine to produce sphinganine. Then, is converted into dihydroceramide by CerS and finally to ceramide by Des. Ceramides promote inhibition of Akt/PKB signaling by PKC function. Ceramides can be turned to GM3 which inhibits INRS, blocking insulin signaling. GM3 accumulation promotes the dissociation of IR/Cav-1 complex. Likewise, palmitic acid can function as a ligand for TLR-4, activating its signaling cascade that leads to the expression of genes encoding enzymes such as SPT, CerS, and Des. On the other hand, palmitic acid can be metabolized to DAG, which stimulates PKCε, activated PKCε phosphorylates Thr 1160 of INSR, causing its inhibition. Finally, ceramides could activate NLRP3 and lead to apoptosis by Caspase-1-dependent pathway. Image adapted from references: [9,10,11,12,13].
In addition, an increase in the synthesis of ceramides associated with ER stress promotes the flow of lipids among ER and mitochondria through the structures such as mitochondria-associated ER membranes (MAMs) and multivesicular endosomes (MVEs), moreover these structures are involved in mechanisms such as ceramide synthesis, ATP flux between ER and mitochondria, as well as exosome-secretion [14]. In an important way, exosomes are vesicles that perform key functions in processes of autocrine, paracrine and endocrine communication. Specifically, MVEs are composed of intraluminal vesicles and participate in internalization of macromolecules and vacuolar/lysosomal hydrolase delivery [15]. Once molecules are incorporated in these vesicles, they have two pathways, degradation or secretion as exosomes by fusion with the plasma membrane, a process that is regulated by the endosomal sorting complex required for transport (ESCRT-I). Indeed, exosomes have been described to be enriched in ceramides; consequently, exogenous treatment with a sphingomyelinase inhibitor reduced the releasing of exosomes, as well as in the depletion of neutral sphingomyelinase-2, which suggests that ceramide metabolism is implicated in biogenesis of exosomes from intraluminal vesicles [14]. Furthermore, has been described that MVEs formation, and consequently, exosome secretion can be related with breast cancer, phenomenon proposed as a cellular stress response [16].
Ceramides play critical roles in the alteration of membrane structures, for instance, these lipids are a factor that triggers the formation of channel-like structures in the outer mitochondrial membrane (OMM), modifying conditions such as permeability and ionic concentration; thus, these conditions could facilitate the flux of pro-apoptotic proteins such as cytochrome-C, Smac/Diablo, and Omi/HtrA2, molecules of apoptosis intrinsic pathway. Paradoxically, ceramides function in apoptosis development has allowed a more complex visualization of them, such as their role in cell differentiation and proliferation signaling pathways [17,18]. Ceramides have been described as regulatory lipids that modulate various sides of cell growth, survival, senescence, and apoptosis in cancer [19,20]. Wherein, a correlation between the levels of ceramides and the tumor stage has been demonstrated, ceramides metabolism has also been associated to chemotherapy resistance [19,20]. Then, ceramides play important roles in the regulation of cancer progression, and are linked to their function in meta-inflammation, a condition that associates overnutrition and obesity with a chronic low-grade inflammatory state [21,22], representing a key factor in the regulation of pathological processes. Therefore, in this work, we performed an analysis of the state of the art about the multifaceted role of ceramides in several pathological processes, with a focus on lipid metabolism and breast cancer.
2. Pathways of Ceramide Synthesis
Ceramide synthesis can be carried out by means of hydrolysis of sphingomyelin, de novo synthesis, and the salvage and recycling pathway; therefore, there is a continuous flux of these lipids in cells. For instance, de novo synthesis is activated by increased saturated fatty acid accumulation, wherein palmitoyl-CoA and serine are conjugated by SPT, producing the unstable molecule 3-ketosphinganine, which is rapidly converted to dihydro-sphingosine that undergoes N-acylations by the CerS, producing dihydroceramides. The function of CerS is considered a critical regulatory step, localized in ER and nuclear envelope. Later, the dihydroceramides are transformed by the dihydroceramide desaturase (DES 1 and 2) into ceramides. Subsequently, ceramides are delivered to the Golgi apparatus by vesicle transport, or through carrier proteins such as ceramide transfer protein (CERT) [23,24], which is the only described protein capable of transferring vesicle-independent ceramides among organelles [25].
CerS are composed of six variants whose function lies in the condensation of acyl-CoA of different lengths to its sphinganine. Differences among CerS variants are associated with transmembrane topology, specificity for long chain fatty acids, and tissue distribution [26], each CerS changes during development and is expressed according to the cell type [26]. CerS1 and CerS4 generate C18–C20 ceramides, CerS5 and CerS6 generate C14–C16 ceramides, CerS2 selectively generates C22–C24 ceramides [27,28], and CerS3 mediates the synthesis of very-long chain C28–C32 ceramides with polyunsaturated fatty acids [29]. The activity of each CerS is determined by different post-translational modifications, as well as homo and heterodimerization.
Sphingomyelin is the main sphingolipid constituent of the plasmatic membrane, and ceramides generated by this pathway are produced by the hydrolysis of the phosphocholine head by the action of sphingomyelinase (SMase), which can be activated by extracellular signals such as oxidative stress, inflammation, and ionizing radiation [23]. Likewise, the salvage pathway can be activated by oxidative stress, this process begins in lysosomes or late endosomes wherein sphingomyelin and glycosphingolipids are hydrolyzed by the acid sphingomyelinase and β-glucosidase-1, respectively. Sphingolipids are released from these structures in the form of sphingosine and fatty acids, reaction catalyzed by acid ceramidase, this pathway could be regulated by PKCδ which activates sphingomyelinase. Furthermore, the recycling pathway involves deacylation of exogenous short chain ceramides by ceramidase to produce sphingosine, these pathways converge to obtain a product in common: ceramide produced by ceramide synthase [30]; likewise the recycling pathway has been involved in apoptosis [31]. Evenmore, gangliosides can function as precursors of ceramides, through the function of plasma membrane sialidase, to become sialosyl lactosyl ceramides [23].
Importantly, ceramide synthesis is activated by different cellular stimuli, including increased intracellular concentration of postprandial palmitate, hypoxia, intracellular stress, activation of the neutral sphingomyelinase [24,25], as well as inflammatory stimuli mediated by TLR-4 in response to TNFα and INFγ signaling, inducing the expression of enzymes such as SPT [32]. Therefore, the activation of ceramide synthesis due to meta-inflammation as a result of overnutrition, could generate a deleterious circle by the activation of proinflammatory and pro-apoptotic signaling in metabolic tissues, modifying organelles structure, then the pathological state is amplified [33].
3. Ceramides, Association with Endoplasmic Reticulum and Mitochondria
Ceramide hydrophobicity represents an energetic barrier that prevents free movement among membrane structures; for instance, communication of ER and mitochondria requires physical contact of membrane proteins that facilitate the flow of molecules. Within structure of MAMs, enzymes involved in lipid biosynthetic pathways of ceramides and glycosphingolipids have been described, for instance, ceramide synthase, ceramide glucosyltransferase, glucosylceramide galactosyltransferase, and sialyltransferase [34]. Physiologically, there is a 5–20 % area of contact between ER and mitochondria, which suggests a considerable flow of molecules between these organelles [35,36] (Figure 2).
Figure 2.
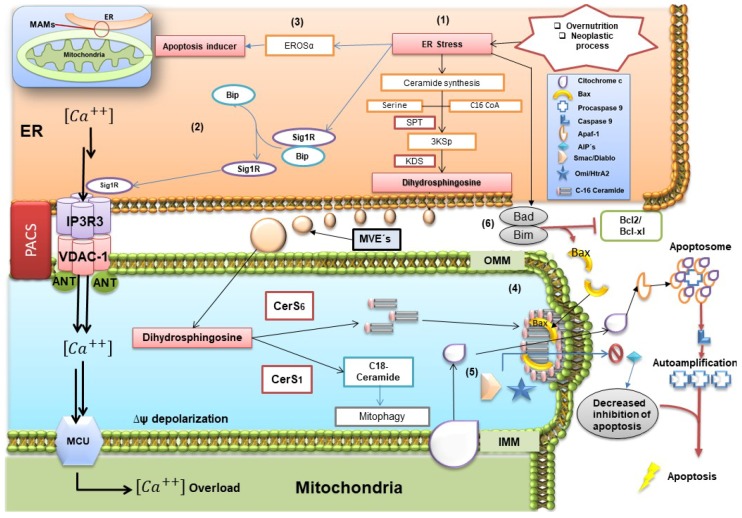
Overview of endoplasmic reticulum (ER) and mitochondria interactions. ER stress phenomenon promotes the activation of the de novo ceramide pathway, producing an increase in the exportation of dyhidrosphingosine and enzymes such as CerS1 and CerS6 through mitochondria-associated ER membranes (MAMs) or multivesicular endosomes (MEVs) to mitochondria (1), this originates the cleavage of Sig1R/Bip complex, Sig1R binds to IP3R3/VDCA-1 allowing the flow of Ca2+ through the ER to the inter-membranal space of the mitochondria by MCU (2), it can cross the inner mitochondrial membrane, producing a supersaturation in the mitochondrial matrix. This condition facilitates the activation of apoptosis by means of CHOP-dependent EROSα (3). In inter-membrane space, C16 ceramide leads to synthesis and assembly of pores that synergistically with Bax are translocated to OMM (4), this modifies the permeability of the membrane and allows the output of cytochrome C and proteins as SMAC/DIABLO and Omi/Htra (5). Later, cytochrome-C is associated with Apaf-1, promoting the change from procaspase-9 to caspase-9, leading to apoptosome formation. The complex Bad/Bim inhibits the antiapoptotic action of Bcl-2 and Bcl-xL, which in turn are associated with preventing the oligomerization of Bax with the pores, as well as inhibition of cytochrome C release (6). Image adapted from references: [4,14,18,37,38,39].
ER-mitochondria associations are not only restricted to synthesis and lipid flow: even more mitochondrial ATP transfer to the ER is critical for chaperone function in the activity of unfolded protein response (UPR). One of chaperones, BiP (Grp78), recruits the adaptor protein TRAF2 in cytoplasmic domains and activate ASK-1 and JNK kinase, which could target mitochondria through various signaling pathways such as phosphorylation of Bim, and Bad [40,41], proteins of the Bcl-2 family involved in cell progression pathways and apoptosis [42]. In an important way, by decreasing the fluidity of the mitochondrial membrane, ceramides attenuate the stability of MAMs, which are structures needed for tumor cells adaptation under conditions of metabolic stress [43] (Figure 2).
In this sense, phosphofurin acidic cluster sorting protein 2 (PACS-2) is implicated in ER-mitochondria tethering, which regulates MAMs formation [37]. The function of PACS-2 is dependent of Akt phosphorylation (Figure 2), which enables PACS-2 to be retained in MAMs [44]. Likewise, PACS-2 interacts with calnexin, a regulator of ER-mitochondria Ca2+ flux [45,46], and blocks BAP31 processing mediated by caspase 8 [37]. This interaction is the basis of PACS-2-mediated ER-mitochondria tethering, wherein BAP31 interacts with the mitochondrial Drp1 docking protein Fis1 [47]. However, this complex is associated with procaspase-8 under conditions of cell stress where the mature form of caspase-8 triggers the formation of the BAP31 p20 fragment, an activator of mitochondrial fission [48]. Likewise, in cytoplasm, p53 can be located within the MAMs, wherein a fraction of p53 could stimulate the activity of the Sarco/ER Ca2+ ATPase pump, regulating Ca2+ levels in ER. In fact, under pro-apoptotic conditions, its downward activity causes an overload of Ca2+ inside the mitochondria, which is a factor of formation of mitochondrial permeability transition pores [4].
Intracellular increase of ceramides modifies several signaling pathways that maintain cell homeostasis [49]. For instance, longer acyl chain ceramides decrease membrane fluidity of mitochondria [50]. Specifically, the presence of two species of ceramides N-acetyl-d-erythro-sphingosine C2 and N-palmitoyl-d-erythrosphingosine C16, has been associated together with other proteins to formation of channel-like structures, then increasing permeability of OMM. These structures could facilitate the movement of proteins from the inter-membrane space to the cytoplasm, such as the inducing factor of apoptosis, cytochrome C, procaspases, and several heat shock proteins [51,52]. Nevertheless, this proposal has been questioned; moreover, Lee et al. (2011) [53] propose that ceramides facilitate the effect of Bax, a pro-apoptotic protein, which directly could trigger the pore formation on OMM [53].
Experimentation on mitochondria of hepatocytes deleted of Bax showed increasing levels of cytochrome C releasing under Bax treatment than stimulation with C16 ceramide alone, even at high doses. In addition, minimal doses of C16 ceramide and recombinant Bax, potentiated the release of cytochrome C. Therefore, C16 ceramide is not considered a direct inducer of increased permeabilization of OMM, while it improves the insertion of Bax on OMM [53]. Although the release of these proteins promotes the activation of several caspases and DNases, other mitochondrial effects appear, such as the increase in ROS, alterations in calcium homeostasis of MAMs, modifications of several components of the ECT, as well as reduction in ATP concentration with the consequent collapse of inner mitochondrial membrane (IMM) potential.
While ceramides are lipids capable of inducing the destabilization of OMM, leading to increased membrane sorting and inducing gel/fluid phase separation possibly to form channels, the characterization of these channel-structures shows that only proteins could have the stability to form these structures. Considering that pore formation, as the main mechanism of mitochondrial permeabilization, has been questioned due to a thermodynamic barrier. Even more, the concept of an increased permeability induced by a surface mismatch between the two mitochondrial monolayers has been proposed [54]. Notwithstanding, in vitro studies with liposomes treated with C16 ceramides and supported by electron microscopy and molecular dynamic simulations, have suggested the pore structure formation [55]. In this proposal, ceramide molecules are organized in columns arranged in anti-parallel way originating a cylindrical shape spanning the hydrophobic interior of the OMM [52,56,57]. These phenomena could be dependent on the long chain and the degree of unsaturation of acyl chain of ceramides [4,38]. Nowadays, the debate on the proposal that explains this process in function of thermodynamic feature, still remains.
Likewise, in mitophagy, a physiological process in which cells eliminate dysfunctional mitochondria, ceramides have been described that induce mitophagy, targeting mitochondria that contains LC3B-II autophagolysosomes [58]. Specifically, C18 ceramides have emerged as tumor suppressors. CERS1 expression generating C18 ceramides mediates the localization of ceramide on the OMM, leading to mitophagy. Target mitochondria through LC3—an important component in the formation of autophagosome which is conjugated with phosphatidyl-ethanolamine through the C-terminal domain forming LC3B-II [58,59]—binds ceramides on the mitochondrial membrane upon DNM1L/DRP1, leading to inhibition of mitochondrial function and oxygen consumption [58]. In this sense, the interaction LC3B-II-ceramide involves the central hydrophobic domain of the protein [60].
4. Role of MAMs in Neoplastic Pathology
The role of MAMs has been considered a fundamental feature of the anti-apoptotic effect in neoplastic cells, mainly due to the control of Ca2+ flux, and the handling of ROS balance, generated by uncontrolled growth and/or antineoplastic therapy [43]. Moreover, the localization of oncogenic and/or tumor suppressor proteins in these special areas of contact ER/mitochondria, participating in the physiological signaling pathways, have direct implications in the development of neoplastic pathology [43]. Currently, several mechanisms have been associated with the function of MAMs in several types of cancer, such as breast, lung, prostate, hematopoietic, and/or lymphoid neoplasias. For instance, the sigma 1 receptor (S1R) in normal tissue is associated with chaperone protein BiP/GRP78 within the area of MAMs [41]; in contrast under conditions of stress it is uncoupled from the BiP, to bind IP3R3, liberating Ca2+ from the ER to the MAMs. On the other hand, S1R is translocated under chronic stress towards the periphery of ER, and thereby attenuates the cell death signal that would generate Ca2+ overload in mitochondria and then, ER Ca2+ depletion during chronic conditions [61].
MAMs perform microzones of anchoring enzymes that maintain oxidative homeostasis, such as endoplasmic reticulum oxidoreductase-1 alpha (ERO-1α), with which overexpression in tumor tissue is associated with poor prognosis [62]. In addition, a strong correlation among the levels of ERO-1α and programmed cell death-ligand 1 (PD-L1) is proposed, allowing the escape of the immunological attack, and confining an immunosuppressive phenotype [63]. However, ERO-1α also participates in cell death mediated by activation of compound 1 activator of procaspase (PAC-1), inducing apoptosis in several tumors [39]. In this sense, ERO-1α shows a dual role in neoplastic pathology, previously mentioned in the implication of oxidative balance and the presentation of immuno-tolerance with anti-apoptotic characteristics, in contrast to cell death via activation of PAC-1 pro-apoptotic feature [39]. In addition, within the structure of MAMs, the PERK arm of UPR is localized [64], its deficiency showed an association with tumors of low proliferation, because PERK triggers mechanisms that maintain redox balance [65].
In colon and breast cancer, ATAD3 has been described as functioning in conjunction with GRP78 within the MAMs, to keep the stability of WASF3, one function of which is the activation of actin polymerization, participating in the metastatic phenomenon, and leading to a bad prognosis [66]. Interestingly, ATAD3 has been pinpoint in the vicinity of the lipid transfer zone between ER and mitochondria [67], the aforementioned is important for maintain the integrity of the lipid bilayer of the mitochondria [68,69]. In lung cancer, the expression of variants of the Bcl-2 family has been described, for instance the presence of Bcl-2 [70,71] and Bcl-XL [72] is associated with an anti-apoptotic phenotype in non-small cell lung carcinoma. On the other hand, myeloid cell leukemia-1 (Mcl-1), another member of the Bcl-2 family within MAMs, activates the voltage-dependent anion-selective channel 1 (VDAC-1), increasing the flux of Ca2+, then cell migration [73].
In addition, hexokinase-2 (H2K) is over-expressed in cancer cells, wherein glycolysis has been described to show an important role in the initiation and neoplastic development in models of mice with K-Ras driven lung cancer and Her2/Neu-driven breast cancer [74]. However, the phosphorylation of H2K dependent of Akt causes the translocation of H2K to the MAMs, wherein maintains interaction with VDAC-1, in this manner H2K takes advantage of the emerging ATP of the channel formed by VDAC-1, catalyzing the first reaction of glycolysis, in this way contributes to the Warburg effect [75], a metabolic adaptation to hypoxic microenvironment due to lack of blood supply, and fundamental in the survival neoplastic during early stages.
In this sense, lactate produced by anaerobic glycolysis in Warburg effect generates an acid microenvironment that activates the VEGF signaling pathway, a proper condition to tumor growing [76]. This hypoxic condition leads to activation of HIFα, which, in collaboration with c-Myc activate pyruvate dehydrogenase kinase 1 [77], reduces mitochondrial oxygen consumption and promotes anaerobic glycolysis, then lactate synthesis [78]. Recently, MAMs have been proposed as regulators of cancer cells metabolism due to their function in Ca2+ exchange between ER and mitochondria, which is needed for oxidative phosphorylation [79]. Therefore, alteration of MAMs could lead to modifications of Ca2+ exchange and ATP production [80]. Moreover, some proteins within the MAMs have been proposed as potential regulators of tumor cell metabolism, for instance, TMX1 is related with glycolysis and oxidative phosphorylation balance [80], mitofusin-2 as an important regulator on MAMs contact has been associated to pro-apoptotic and anti-proliferative signaling [81], and PACS-2 proposed as a tumor suppressor [82].
While MAMs connect membrane surfaces between mitochondria-ER, these associations harbor multiple enzymes and proteins, which allow cell homeostasis preservation and, thus, survival in neoplastic pathology [43]. MAMs represent a focus of therapeutic target for antineoplastic therapy, due to its associations with responses to oxidative stress and harmful microenvironments, favoring resistance to pro-apoptotic stimuli in cancer cells [83]. Indeed, the use of ceramides as coadjuvant therapy with different antineoplastics, is a novel treatment option due to its multifactorial participation in the structure and function of MAMs [84]. In line with this notion, ceramides are bioactive lipids that modulate processes such as proliferation and cell cycle, key events in the development of cancer [19,20]. Various reports have shown that cancer cells strictly modulate ceramide metabolism [19,20,85]. For instance, sphingolipid metabolism has been reported to regulate cancer development, progression, metastasis and resistance to therapy, some mechanisms have been described to explain how ceramides could regulate these processes specifically in breast cancer [19,20].
5. Ceramides and Breast Cancer
Ceramide-1-phosphate and glucosylceramides regulate proliferative processes, while dihydro-sphingosine, ceramides, and sphingosine promote apoptosis [19]. While, the effects of ceramides in various cell types are pleiotropic, functioning as inhibitors of cell proliferation, ceramide levels are down-regulated in most types of cancer via decreasing levels or activities of enzymes involved in ceramide synthesis, and/or increasing the activity of enzymes associated with degradation. For instance, in breast cancer cell lines, ceramides promote cell cycle arrest by mechanisms such as the induction of the dephosphorylation of the Rb retinoblastoma protein, the activation of the cyclin-dependent kinase inhibitor p21, and the inhibition of the cyclin-dependent kinase CDK2 [86,87], additionally to promotion of pore formation mechanisms in OMM [88]. Indeed, taking into account the inducer effect of apoptosis in breast cancer, ceramides are considered a tumor suppressor lipid [86,89,90].
In this sense, acid ceramidase is an enzyme that deacylates ceramides in the ceramide metabolic pathway to sphingosine, which is further phosphorylated by sphingosine kinase (SphK) to generate sphingosine-1-phosphate (S1P). Interestingly, ceramides have been shown to induce apoptosis, whereas S1P could regulate cell survival, proliferation, and angiogenesis in cancer cells [19,91]. S1P is described to exert multiple responses, such as proliferation, survival, and cytoskeletal rearrangement, via its G protein-coupled receptor (GPCR) in several cell types, promoting the activation of Rho and CDC42 GTPases, and MAPK, PI3K/Akt pathways. S1P is synthesized from sphingosine by SphK; two isoforms of mammalian SphK (SphK1 and SphK2) have been characterized. Likewise, the S1P has been shown to accumulate in the tumor microenvironment [19,92] (Figure 3).
Figure 3.
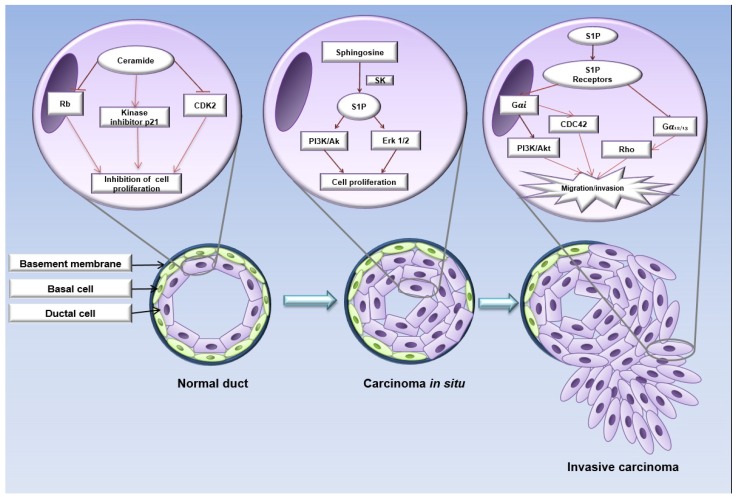
Schematic view of sphingolipid role in breast cancer progression. Structurally, the mammary ducts are composed of basal membrane, a layer of myoepithelial cells and another of luminal ductal cells. In the normal mammary epithelium, ceramides negatively regulate the Rb and CDK2 proteins, inhibiting cell proliferation. In carcinoma in situ, luminal tumor cells present an abnormal metabolism of ceramides, where sphingosine-1-phosphate (S1P) through S1PR promotes activation of the PI3K/Akt and MAPK pathways, inducing cell proliferation. The loss and/or degradation of the basement membrane allow the tumoral cells to migrate and invade the surrounding tissue. In this stage, S1PR promotes GTPases activation such as Rho and CDC42, inducing tumor invasion and consequently, metastasis. Image adapted from references: [12,19,92,102].
Therefore, the role of sphingolipids in the development of breast cancer has been controversial, however cancer cells that maintain an active proliferation specifically present low levels of ceramides, by up-regulating enzymes that metabolize these lipids, resulting in conditions of resistance to apoptosis [85,93]. Previous studies described different expression profiles of the sphingolipids enzymes in cancerous tissue relative to normal tissue. Schiffmann et al. showed that endogenous ceramide level was significantly elevated in malignant breast tissue, indicating that elevation of sphingolipid levels correlates with disease status [94]. Particularly, the mammary tumor tissue showed high levels of C16:0-Cer, C24:1-Cer and C24:0-Cer in comparison with non-tumoral tissue, and this increase was associated with higher expression of ceramide synthases CerS2, CerS4 and CerS6. Interestingly, levels of C18:0-Cer and C20:0-Cer were associated with positive tumors for the estrogen receptor, while high levels of C16:0-Cer were linked with metastasis to lymph nodes [94,95].
On the other hand, increasing ceramide levels using ceramide analogs effectively induce apoptosis of breast cancer cells. The stimulation of breast cancer cell lines MCF7 and MDA-MB-231 with the analog of ceramide (2S,3R)-(4E,6E)-2-octanoylamidooctadecadiene-1,3-diol promotes apoptosis by translocation of phosphatidylserine to the outer monolayer of the cell membrane and deregulation of mitochondrial membrane potential and released of cytochrome C [96,97]. Therefore, ceramides have been proposed as a therapeutic target for specific types of cancer by synergism with several drugs, as potent tumor suppressors, and acting as second messengers in signal transduction and regulation of proliferation, differentiation, senescence, and apoptosis [85,93]. Chemotherapeutic drugs can induce the endogenous accumulation of ceramides, promoting cancer cells apoptosis, evidence suggests that modulation in the lipid metabolism may be effective to improve tumor cells susceptibility to antineoplastic drugs. Indeed, several chemotherapeutic agents increase the synthesis of ceramide through the activation of SMases or de novo pathway, such as gemcitabine, camptothecin, etoposide, cisplatin, fludarabine, and daunorubicin [98,99]. Furthermore, anti-tumoral treatment modulates the expression of the receptors for S1P. In MCF7 breast cancer cells, tamoxifen and medroxyprogesterone treatment induces an increase of S1P2 expression, while this same treatment induces a down-regulation of S1P3 [100]. However, the biological mechanism that causes this phenomenon has not been described. For instance, treatment of P388 and U937 leukemia cells with daunorubicin promotes apoptosis by increasing ceramide levels by de novo pathway [101]. Subsequently, in the next chapters we are going to analyze the critical steps in cancer progression and the role of ceramide metabolism.
6. Involvement of Ceramides and Sphingolipids in the Epithelial-Mesenchymal Transition (EMT)
EMT is an important process involved in embryonic development, wound healing, fibrosis, and breast cancer metastasis [103,104]. The EMT process implies that epithelial cells progressively acquire a mesenchymal phenotype. It is characterized by a decrease in E-cadherin and cytokeratin (CK) 8/18 levels, loss of apical-basal polarity, junctions dissociation, cytoskeleton reorganization, an increase in N-cadherin and vimentin levels [103], as well as matrix degradation and an enhanced ability for cell migration and invasion. Cells that have undergone all these modifications have transitioned from an epithelial to a mesenchymal phenotype [103,104].
For instance, in mouse mammary epithelial cells under the EMT process, a modulation in the metabolism of gangliosphingolipids has been determined. Galpβ1-3GalpNAcβ1-4Galpβ1-4Glcpβ (Gg4) is involved in the regulation of EMT, since the concentration of this lipid is reduced in transdifferentiate cells into mesenchymal cells. In addition, Gg4 forms complexes with proteins such as E-cadherin and β-catenin, preventing its degradation and favoring the epithelial phenotype [99]. Additionally, the differential expression of enzymes related to the ganglioside metabolism observed in EMT is associated with the regulation of transcription factors such as Snail1, ZEB1, and Twist, key EMT-inducing proteins [105].
Likewise, mammary epithelial cells under EMT showed a decrease in the levels of Ceramide C16:0, as well as CerS6. Mammary epithelial cells treated with TGF-β have decreased levels of CerS6, a phenomenon observed in patients with triple negative breast cancer (TNBC) mesenchymal cells [106]. The decrease of C16:0 ceramide levels in patients with TNBC are associated with migratory and invasive capacity of tumor cells. In addition, the down-regulation of CerS6 in tumors is associated with mesenchymal phenotype, as well as aggressive malignancies and poor prognosis [106].
As previously described, S1P is a bioactive lipid that regulates several responses including inflammation, survival, and cell migration [102]. Forming complex with their respective receptors (GPCRs), S1P promotes the activation of non-receptor kinases such as FAK, Src, as well as GTPase Rac, regulatory proteins of cell migration and invasion [102]. In human mammary non-tumorigenic epithelial cells MCF10A, a cellular model for the study of EMT, treatment with S1P induces the activation of the S1P3 receptor, promoting the activation of Erk ½, Rac, Akt, and PI3K, and an increase in the expression and secretion of MMP-9. Moreover, S1P induces an increase in migratory and invasive capacity of mammary epithelial cells, promoting an EMT-like process in MCF10A cells [102] (Figure 4).
Figure 4.
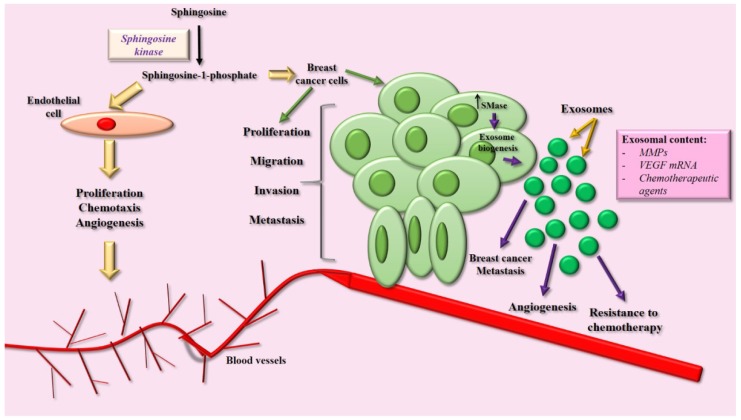
Ceramides and sphingolipids in breast cancer progression. S1P is a critical molecule involved in the modulation of angiogenesis. In endothelial cells, it has been demonstrated that S1P promotes proliferation, migration, and chemotaxis, resulting in the generation of intra-tumoral blood vessels that facilitate the process of metastasis. Moreover, S1P induces migration and invasion of breast cancer cells, promoting the process of tumor intravasation. Finally, in tumor cells, the SMase enzyme shows high activity, promoting the biogenesis and secretion of exosomes, extracellular vesicles that modulate angiogenesis, metastasis, and resistance to chemotherapy. Image adapted from references: [14,120,132,136].
Recently, a study performed in human breast cancer patients showed that ceramide levels are elevated in breast tumor tissue compared to adjacent tissue and/or non-tumor tissue [90]. In addition, the enzymes involved in the de novo pathway, in the salvage and the sphingomyelin pathways are overexpressed in cancerous tissues, accompanied by an increase in the levels of multiple ceramides (C14: 0, C16: 0, C18: 1, C18: 0, C20: 0, C22: 0, C24: 1, C24: 0, C26: 1 and C26: 0), increase in the levels of monohexocylceramide species (C14: 0, C16: 0, C18: 1, C18: 0, C20: 0, C22: 0, C24: 1, C24: 0, C26: 1 and C26: 0), and an increase in sphingomyelin levels (C14: 0, C16: 0, C18: 1, C18: 0, C20: 0, C22: 0, C24: 1, C24: 0, C26: 1 and C26: 0), as previously mentioned these are related with an aggressive phenotype [90].
Interestingly, the metabolism of ceramides is associated with invasiveness and metastasis. For example, key regulatory enzymes in the ceramides and sphingolipid metabolism such as glucosylceramide synthase [26,107], acid ceramidase [108,109], and ceramide kinase [110,111] are overexpressed in mammary tumor tissue and/or breast cancer cell lines, suggesting a strong association between ceramides and their intermediates on tumor progression. Therefore, this broadens the understanding of the role of this class of lipids.
7. Ceramides and Sphingolipids and Their Implication in Breast Cancer Metastasis
In mammary tumor progression, the development of new blood vessels, denominated tumor angiogenesis, is a critical process. In this sense, S1P and S1P receptors have been described as key regulators of this process [112]. It has been shown that S1P induces an increase in intracellular calcium concentrations and migration of HUVECs cells through the activation of the S1P receptor [113]. Additionally, S1P promotes an increase in the proliferation and chemotaxis of HUVEC cells accompanied with enhanced in vitro angiogenesis [113,114]. This data confirms the involvement of molecules such as S1P and its receptors as modulators of angiogenesis and reflects its importance in tumor vascularization.
Considering the importance of ceramides in the regulation of multiple tumor processes, recently the ceramide nanoliposomes effect on mammary tumor cells cultures has been described. In MDA-MB-231 cells, the treatment of C6 ceramides encapsulated in liposomes (ceramide nanoliposomes) was able to significantly increase apoptosis under non-adherent conditions (anoikis), accompanied by an increase in caspase 3/7 activity [115]. In addition, the ceramide nanoliposomes promoted a decrease in cell migration, associated with a diminution in the secretion of IL-6 and IL-8 in MDA-MB-231 cells [115].
Therefore, the interrelation between growth factors and the metabolism of sphingolipids in breast cancer cells has been demonstrated. For example, it has been shown that the treatment of MCF7 mammary tumor cells with epidermal growth factor (EGF) promotes an increase in the expression of sphingosine kinase 1, an enzyme required for cell proliferation and migration [116,117]. Interestingly, in the same cell line, the oestrogen-induced transactivation of EGFR depends on the sphingosine kinase pathway and S1P, a process accompanied by the activation of Src and matrix secretion of metalloproteinases, proteins highly involved in the regulation of migration, invasion, and tumor metastasis [117].
Finally, it is important to highlight the differences between events regulated by ceramides, sphingolipid-metabolites, and S1P. For instance, the presence of high levels of ceramides and their producing enzymes (CerS2) leads to a decrease in the secretion and activity of metalloproteinases, resulting in poorly invasive phenotypes of breast cancer cell lines [118]. In the other hand, the role of S1P as a modulator of cell invasion and metastasis has been demonstrated. In human glioblastoma cells, S1P induces an increase in the secretion and activity of metalloproteinases, leading to the degradation of extracellular matrix, invasion of local tissue and finally, metastasis [119]. Additionally, S1P receptors play an important role in breast tumor development. It has been determined that sphingosine-1-phosphate receptor 1 (S1P1) is over-expressed in multiple breast cancer cell lines, positively regulating processes such as tumor migration and invasion. In line with this notion, the down-regulation of S1P1 inhibits cell proliferation, colony formation, migration and invasion [120].
Studies show that sphingosine-1-phosphate receptor 3 (S1P3) is over-expressed in invasive breast cancer cell lines, resulting in an increase in the expression of COX-2 and in microsomal prostaglandin (PG) E2 synthase, accompanied by a high synthesis of PGE2 and favoring tumor metastasis [121]. S1P3 has been involved as a regulator of blood-brain barrier permeability. In the breast cancer brain metastasis, the surrounding astrocytes express high levels of S1P3, which regulates and promotes the secretion of CCL2 and IL-6, leading to the relaxation of endothelial cell adhesion and increases the permeability of the blood-brain barrier [122].
This data strongly suggests that the subtle balance between the metabolites and enzymes producing ceramides/sphingolipids in tumor cells leads to the modulation of mammary tumor progression, highlighting the role of these molecules and their receptors as potential targets for breast cancer intervention, specifically associated to metastasis.
8. Exosome-Ceramides and the Role in Tissue Communication
Extracellular vesicles are spherical structures surrounded by a bilayer lipid membrane that are found in several body fluids and are secreted by a wide variety of cell types, including tumor cells [123]. According to the International Society for extracellular vesicles, these structures are classified depending on their size and biogenesis in apoptotic bodies, microvesicles, and exosomes [124,125]. Particularly, the exosomes are originated from MVEs, presented average size of 30–100 nm. In different types of cancer, altered levels of exosome release are observed, and their number, composition, and cellular origin depend on the state of the disease [124]. Moreover, it has been shown that exosomes regulate processes of tumor progression, such as proliferation, migration, invasion, metastasis, immunosurveillance, and evasion [126], as well as resistance to chemotherapy, denoting them as an important feature in the study of breast cancer progression [123]. Once secreted, exosomes mediate intercellular communication in autocrine, paracrine, and endocrine pathways. The exosomes modulate the functions of target cells by interacting with surface proteins of vesicles with membrane receptors and being endocyted by the target cell. In this way, the target cells uptake the set of charged molecules present in the exosomes, resulting in modulation of the cellular responses [123,124]. For instance, exosomes secreted by glioma cells transport and transfer EGFRvIII, a constitutively active oncogenic form of EGFR, then acceptor tumor cells show an increase in cell proliferation and survival [127]. Several studies have shown that cancer cells resistant to doxorubicin and cisplatin release high amounts of these molecules through exosomes, therefore their release contributes to tumor cell survival [128,129]. Moreover, cancer cell-derived exosomes contain MMP-2 and MMP-9, enzymes that degrade components of the extracellular matrix, facilitating the invasion of tumor cells into the surrounding tissue (Figure 4) [130]. Furthermore a recent study shows that tumor-secreted exosomes could facilitate metastasis even for tumour cells that lack the capacity to metastasize and this could be regulated by their pattern of integrins expression [131].
Exosome-composition depends on the cells from which they originate, including proteins, nucleic acids (RNA, DNA), and lipids [123,132]. Interestingly, sphingolipids have been associated with the biogenesis and secretion of exosomes. Accumulation of sphingolipids and the high activity of sphingomyelin phosphodiesterase (SMase) generates an increase in levels of free ceramides, associated with the formation of exosomes [124,125,133]. Compared to their parental cells, exosomes secreted by various cell types are enriched with various bioactive lipids such as eicosanoids, fatty acids, ceramides, and sphingomyelin [134,135], therefore, these molecules can be transferred to the acceptor cells. For instance, it has been determined that sphingosine-1-phosphate receptor 2 (S1P2) is secreted and transported in exosomes derived from MDA-MB-231 breast cancer cells. Indeed, the exosomes that transport S1P2 are taken-up by cancer-associated fibroblasts (CAFs), resulting in an increase in CAFs proliferation dependent on ERK ½ signaling [136].
In addition, S1P is associated with several cellular processes such as angiogenesis, cell growth, motility, and in an important manner can be contained and transported by lipoproteins, the high density lipoproteins (HDL) being the main carrier. Approximately 60% of circulating S1P is associated to HDL, likewise HDL can carry on other sphingolipids such as sphingomyelins and ceramides (C:24) [137,138,139]. HDL is known for its anti-atherogenic function due to its composition of apolipoproteins, among which ApoM is found, whose function is to bind to S1P [140]; both ApoM and S1P are synthesized and secreted by the liver [141]. Although S1P has been considered as a cardioprotective molecule, S1P is described as a possible mediator of HDL-dependent angiogenesis, due to its interaction with receptor S1P3, whose activation promotes the expression of VEGFR2 [142]. Therefore, S1P is a moonlighting molecule, although it has a cardioprotective role through angiogenesis activation, this same effect can be detrimental favoring tumor proliferation. In this sense, S1P3 has been found up-regulated in breast cancer cells, a condition associated to increased migration and invasion of metastatic cells induced by an inflammatory environment in a COX-2-PGE2-dependent pathway [121].
On the other hand, S1P is capable of activating the matriptase, a serine protease with an important role in tissue remodeling [143], whose overexpression found in breast cancer tissue is associated with a poor prognosis [144] through activation of PAR-2-NF-κB and PI3K-Akt-mTOR, pro-inflammatory and pro-oncogenic signaling, respectively [145]. Likewise, matriptase has been implicated in invasiveness and metastasis in prostate cancer [146,147]. Recently, M69 antibody has been employed in TNBC cells with overexpressed matriptase [148] indicating that this S1P-activated enzyme has an important role on breast cancer therapy.
9. Role of Ceramides in Chemoresistance
Ceramides have been proposed as key regulatory molecules in breast cancer chemoresistance, being even considered as sensitizers to chemotherapy. The poor response in chemotherapy is a phenomenon commonly observed in breast cancer, however, is exacerbated in TNBC cells, a breast cancer subtype with a poor prognosis [149,150]. Several cell pathways have been proposed as triggers of neoplastic processes in TNBC, such as CD73, CD133, IMP3, ABCG2, HSF1 and PI3K/AKT/mTOR [150,151,152,153,154,155]. In breast cancer patients studied by Che J., et al., it has been found that after exposition to doxorubicin, cyclophosphamide, fluorouracil or its combination, ceramides levels decreased, indicating that its down-regulation is a common intrinsic response against breast cancer chemotherapy [20]. This subside response of ceramides concentration was determined in mammary tumor tissue, while in untreated women the levels remain unchanged [5]. Specific enzymes of ceramide metabolism have been characterized, UDP-glucose ceramide glucosyltransferase (UGCG) levels were higher after exposition to chemotherapeutic agents as doxorubicin, which lead to decreased ceramide levels [148]. Thus, specific inhibition of UGCG led to higher ceramide levels and this enhanced the sensitivity to doxorubicin and cyclophosphamide in MDA-MB-231 cells [20]. Zhang X. et al., reported that doxorubicin treatment induced the expression of UGCG in MCF-7 cells, an estrogen receptor (ERα) positive model, while this phenomenon is absent in MDA-MB-231 (ERα negative), suggesting an association between the ERα and the UGCG [156].
Moreover, UGCG is involved in multidrug resistance processes, especially in breast and colon cancer [157,158]. For instance, in one study the effects of UGCG on multidrug resistance protein 1 (MDR1) levels was characterized, which concluded that UGCG, specifically Gb3 and Gb4 up-regulates expression of MDR1 through β-catenin signaling, granting doxorubicin and paclitaxel resistance to murine breast cancer cells [159]. In addition, hyper-methylation of CpG islands in 5’ regions of UGCG promoter leads to its inhibition and subsequently to a major sensitivity to chemotherapy in breast cancer cells. In this investigation, authors demonstrated that methylation of UGCG promoter is performed by DNA methyltransferases (DNMTs). The UGCG promoter can be demethylated after the effect of DNMTs inhibitor 5-Aza-dc (Azacitidine), which has been used for therapy at hematological neoplastic processes, indicating the direct relationship between UGCG methylation and cell chemo-sensitivity [160].
Tamoxifen treatments have been reported to increase levels of C16, dHC16, C18, C20, dHC20, dHC22 ceramides in 4T1, MCF-7 and MDA-MB-231 cells, processes accompanied to JNK phosphorylation, caspase-3 and PARP cleavage, both conditions known as markers for cell death, suggesting that tamoxifen also induces cell death in a ceramide-dependent manner [161]. Moreover, tamoxifen shows an antagonist effect over P-gp, a protein with a flippase activity which transports glucosylceramides into Golgi lumen, and this inhibition results in a decreased ceramide glycosylation that leads to increased ceramide levels. Likewise, inhibition of the activity of acid ceramidase by tamoxifen has been reported. Therefore, tamoxifen effects on activation of cell death markers, diminution of ceramide glycosylation, and acid ceramidase inhibition explain its role at enhancing sensitivity to chemotherapy in breast cancer cells [162]. In addition, RIP2, an important mediator of innate immunity, has been recently studied as a modulator of breast cancer survival by Jafaar R., et al., whose results indicate that this kinase confers resistance to ceramide-induced apoptosis in triple-negative breast cancer cells treated with paclitaxel [163]. Thus, RIP2 inhibition can be proposed as a novel target to improve ceramide-induced apoptosis and then, a better antineoplastic drugs response.
10. Final Considerations
Ceramides are a species of sphingolipids whose functions have become relevant due to their various intracellular and signaling functions, some of them involved in pathological processes. Sphingolipid metabolism presents moonlighting effects on neoplastic processes due to its multiple metabolites, for instance ceramides being the proapoptotic intermediates, glucosylceramides the inducers of chemoresistance, and S1P the mitogenic sphingolipid [162]. Thus, it is critical to deepen the knowledge about why the increase in ceramides during initial stages of oncogenic processes favors the survival of the tumoral cells, but later becomes cytotoxic, opposed to the early phases. Evidence suggests that ceramide metabolism is associated with the efficacy of breast cancer therapeutics, several reports have evaluated the possibility to use ceramides as a vehicle in cancer treatments. Therefore, a better understanding of the mechanisms by which sphingolipids regulate cancer cell signaling and metabolism will improve the development of therapeutics.
The aim of this work was to describe an integral relationship between ceramide metabolism and breast cancer development and progression, highlighting its importance in the modulation of key processes such as apoptosis, proliferation, EMT, migration, invasion, and metastasis, resulting in a highly comprehensive and novel work that contributes to the better understanding of tumor ceramides metabolism. Therefore, this work represents a new approach to mechanisms that trigger cancer development and the role of ceramides.
Ceramides function on cell apoptosis has been demonstrated, then these lipids are closely related to mitochondrial structure alterations, facilitating the flow of pro-apoptotic proteins from the intermembrane space into the cytoplasm; also, they exert intracellular effects on insulin signaling induced by meta-inflammation. Therefore, ceramides can be visualized as a hub in the cellular metabolism by functioning as molecules with dual roles, as under stress conditions, which lead to inflammation, they regulate several functions such as apoptosis, and even subtle phenomena in the regulation of cellular cancer signaling mechanisms. The specific conditions in which these lipids will become pathological molecules is a subject pending elucidation.
Acknowledgments
The authors recognize the helpful contribution of Gisel Ivonne Aceves-Franco, who critically reviewed and edited the manuscript, and José Antonio Uribe Vizcarra for contribution in the design of graphical abstract.
Abbreviations
| ER | Endoplasmic reticulum |
| FFA | Free fatty Acids |
| SFA | Saturated Fatty Acids |
| ECT | Electron transport chain |
| ROS | Reactive oxygen specie |
| DAGs | Diacylglycerols |
| INSR | Insulin receptor |
| SPT | Serin-palmitoyl acyltransferase |
| CerS | Ceramide synthase |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| MVEs | Multivesicular endosome |
| ESCRT-I | Endosomal sorting complex required for transport |
| OMM | Outer mitochondrial membrane |
| DES | Dihydroceramide desaturase |
| CERT | Ceramide transfer protein |
| SMase | Sphingomyelinase |
| UPR | Unfolded protein response |
| PACS-2 | Phosphofurin acidic cluster sorting protein 2 |
| IMM | Inner mitochondrial membrane |
| ERO-1α | Endoplasmic reticulum oxidoreductase-1 alpha |
| PD-L1 | Programmed cell death-ligand 1 |
| PAC-1 | Compound 1 activator of procaspase |
| Mcl-1 | Myeloid cell leukemia-1 Mcl-1 |
| VDAC-1 | Voltage-dependent anion-selective channel 1 |
| H2K | Hexokinase-2 |
| GPCR | G Protein-coupled receptor |
| S1P | Sphingosine-1-phosphate |
| SphK | Sphingosine kinase |
| EMT | Epithelial-mesenchymal transition |
| CK | Cytokeratin |
| Gg4 | Galpβ1-3GalpNAcβ1-4Galpβ1-4Glcpβ |
| TNBC | Triple negative breast cancer |
| EGF | Epidermal growth factor |
| Erα | Estrogen receptor |
| UGCG | UDP-glucose ceramide glucosyltransferase |
| 5-Aza-dc | Azacitidine |
| MAMs | Mitochondria-associated ER membranes |
| MDR1 | Multidrug resistance protein 1 |
| DNMTs | DNA methyltransferases |
Author Contributions
Conceived the idea: V.G.-G. and O.G.-H. Wrote the manuscript: V.G.-G., I.M.-N., J.F.D.-V., O.G.-H., G.H.-U. and A.A.P-A. Figures preparation: I.M.-N., J.F.D.-V., O.G.-H., G.H.-U. and A.A.P-A. Critically reviewed manuscript and figures: V.G.-G., J.F.D.-V. and O.G.-H. All authors approved the final manuscript.
Funding
This study was supported by Coordinación de Posgrado e Investigación-UABC grant 106/2/N/57/1 (1983), Convocatoria Especial para PTC 2015/2016 UABC grant 1984, PRODEP UABC-PTC-487, and XVI Convocatoria de Apoyo a la Movilidad Académica 2018-ID1286.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Acosta-Montano P., Garcia-Gonzalez V. Effects of dietary fatty acids in pancreatic beta cell metabolism, implications in homeostasis. Nutrients. 2018;10:393. doi: 10.3390/nu10040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavez J.A., Summers S.A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Gudz T.I., Tserng K.Y., Hoppel C.L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 4.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F., Pozzan T., Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: Significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amati F., Dube J.J., Alvarez-Carnero E., Edreira M.M., Chomentowski P., Coen P.M., Switzer G.E., Bickel P.E., Stefanovic-Racic M., Toledo F.G., et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung J.O., Koutsari C., Blachnio-Zabielska A.U., Hames K.C., Jensen M.D. Effects of meal ingestion on intramyocellular ceramide concentrations and fractional de novo synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 2017 doi: 10.1152/ajpendo.00153.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engin A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017;960:197–220. doi: 10.1007/978-3-319-48382-5_8. [DOI] [PubMed] [Google Scholar]
- 8.Turpin S.M., Nicholls H.T., Willmes D.M., Mourier A., Brodesser S., Wunderlich C.M., Mauer J., Xu E., Hammerschmidt P., Bronneke H.S., et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Ter Horst K.W., Gilijamse P.W., Versteeg R.I., Ackermans M.T., Nederveen A.J., la Fleur S.E., Romijn J.A., Nieuwdorp M., Zhang D., Samuel V.T., et al. Hepatic diacylglycerol-associated protein kinase cepsilon translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep. 2017;19:1997–2004. doi: 10.1016/j.celrep.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., Marcucci M.J., Zhang D., Abulizi A., Zhang X.M., et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L., Ravussin E., Stephens J.M., Dixit V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda N. Ceramide-induced apoptosis in renal tubular cells: A role of mitochondria and sphingosine-1-phoshate. Int. J. Mol. Sci. 2015;16:5076–5124. doi: 10.3390/ijms16035076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 15.Morvan J., Rinaldi B., Friant S. Pkh1/2-dependent phosphorylation of Vps27 regulates ESCRT-I recruitment to endosomes. Mol. Biol. Cell. 2012;23:4054–4064. doi: 10.1091/mbc.e12-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar D., Gupta D., Shankar S., Srivastava R.K. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget. 2015;6:3280–3291. doi: 10.18632/oncotarget.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abou-Ghali M., Stiban J. Regulation of ceramide channel formation and disassembly: Insights on the initiation of apoptosis. Saudi J. Biol. Sci. 2015;22:760–772. doi: 10.1016/j.sjbs.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesan V., Perera M.N., Colombini D., Datskovskiy D., Chadha K., Colombini M. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis. 2010;15:553–562. doi: 10.1007/s10495-009-0449-0. [DOI] [PubMed] [Google Scholar]
- 19.Ponnusamy S., Meyers-Needham M., Senkal C.E., Saddoughi S.A., Sentelle D., Selvam S.P., Salas A., Ogretmen B. Sphingolipids and cancer: Ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6:1603–1624. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Che J., Huang Y., Xu C., Zhang P. Increased ceramide production sensitizes breast cancer cell response to chemotherapy. Cancer Chemother. Pharmacol. 2017;79:933–941. doi: 10.1007/s00280-017-3292-y. [DOI] [PubMed] [Google Scholar]
- 21.Kumari M., Wang X., Lantier L., Lyubetskaya A., Eguchi J., Kang S., Tenen D., Roh H.C., Kong X., Kazak L., et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Investig. 2016;126:2839–2854. doi: 10.1172/JCI86080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Gonzalez V., Gutierrez-Quintanar N., Mas-Oliva J. The C-terminal Domain Supports a Novel Function for CETPI as a New Plasma Lipopolysaccharide-Binding Protein. Sci. Rep. 2015;5:16091. doi: 10.1038/srep16091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanada K., Kumagai K., Tomishige N., Yamaji T. CERT-mediated trafficking of ceramide. Biochim. Biophys. Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Leonardini A., D’Oria R., Incalza M.A., Caccioppoli C., Buccheri V.A., Cignarelli A., Paparella D., Margari V., Natalicchio A., Perrini S., et al. GLP-1 receptor activation inhibits palmitate induced apoptosis via ceramide in human cardiac progenitor cells. J. Clin. Endocrinol. Metab. 2017 doi: 10.1210/jc.2017-00970. [DOI] [PubMed] [Google Scholar]
- 25.Crivelli S.M., Paulus A., Markus J., Bauwens M., Berkes D., de Vries H.E., Mulder M.T., Walter J., Mottaghy F.M., Losen M., et al. Synthesis, radiosynthesis, and preliminary in vitro and in vivo evaluation of the fluorinated ceramide trafficking inhibitor (HPA-12) for brain applications. J. Alzheimers Dis. 2017;60:783–794. doi: 10.3233/JAD-161231. [DOI] [PubMed] [Google Scholar]
- 26.Mullen T.D., Hannun Y.A., Obeid L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012;441:789–802. doi: 10.1042/BJ20111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraveka J.M., Li L., Szulc Z.M., Bielawski J., Ogretmen B., Hannun Y.A., Obeid L.M., Bielawska A. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J. Biol. Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raichur S., Wang S.T., Chan P.W., Li Y., Ching J., Chaurasia B., Dogra S., Ohman M.K., Takeda K., Sugii S., et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U., Bayerle A., van der Hoeven F., Imgrund S., Kirsch J., et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 30.Kitatani K., Idkowiak-Baldys J., Hannun Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda S., Mitsutake S., Tsuji K., Igarashi Y. Apoptosis occurs via the ceramide recycling pathway in human HaCaT keratinocytes. J. Biochem. 2006;139:255–262. doi: 10.1093/jb/mvj026. [DOI] [PubMed] [Google Scholar]
- 32.Miller L.G., Jr., Young J.A., Ray S.K., Wang G., Purohit S., Banik N.L., Dasgupta S. Sphingosine toxicity in EAE and MS: Evidence for ceramide generation via serine-palmitoyltransferase activation. Neurochem. Res. 2017 doi: 10.1007/s11064-017-2280-2. [DOI] [PubMed] [Google Scholar]
- 33.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ardail D., Popa I., Bodennec J., Louisot P., Schmitt D., Portoukalian J. The mitochondria-associated endoplasmic-reticulum subcompartment (MAM fraction) of rat liver contains highly active sphingolipid-specific glycosyltransferases. Biochem. J. 2003;371:1013–1019. doi: 10.1042/bj20021834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 36.Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmen T., Aslan J.E., Blagoveshchenskaya A.D., Thomas L., Wan L., Xiang Y., Feliciangeli S.F., Hung C.H., Crump C.M., Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samanta S., Stiban J., Maugel T.K., Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim. Biophys. Acta. 2011;1808:1196–1201. doi: 10.1016/j.bbamem.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seervi M., Sobhan P.K., Joseph J., Ann Mathew K., Santhoshkumar T.R. ERO1α-dependent endoplasmic reticulum-mitochondrial calcium flux contributes to ER stress and mitochondrial permeabilization by procaspase-activating compound-1 (PAC-1) Cell Death Dis. 2013;4:e968. doi: 10.1038/cddis.2013.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Hsu S.Y., Kaipia A., Zhu L., Hsueh A.J. Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11. Mol. Endocrinol. 1997;11:1858–1867. doi: 10.1210/mend.11.12.0023. [DOI] [PubMed] [Google Scholar]
- 43.Morciano G., Marchi S., Morganti C., Sbano L., Bittremieux M., Kerkhofs M., Corricelli M., Danese A., Karkucinska-Wieckowska A., Wieckowski M.R., et al. Role of Mitochondria-associated ER membranes in calcium regulation in cancer-specific settings. Neoplasia. 2018;20:510–523. doi: 10.1016/j.neo.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. Feature article: MTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. USA. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myhill N., Lynes E.M., Nanji J.A., Blagoveshchenskaya A.D., Fei H., Carmine Simmen K., Cooper T.J., Thomas G., Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell. 2008;19:2777–2788. doi: 10.1091/mbc.e07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynes E.M., Raturi A., Shenkman M., Ortiz Sandoval C., Yap M.C., Wu J., Janowicz A., Myhill N., Benson M.D., Campbell R.E., et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J. Cell Sci. 2013;126:3893–3903. doi: 10.1242/jcs.125856. [DOI] [PubMed] [Google Scholar]
- 47.Iwasawa R., Mahul-Mellier A.L., Datler C., Pazarentzos E., Grimm S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011;30:556–568. doi: 10.1038/emboj.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breckenridge D.G., Stojanovic M., Marcellus R.C., Shore G.C. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J. Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz-Villanueva J.F., Diaz-Molina R., Garcia-Gonzalez V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015;16:17193–17230. doi: 10.3390/ijms160817193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kogot-Levin A., Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2014;100:88–94. doi: 10.1016/j.biochi.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Bernardi P., Scorrano L., Colonna R., Petronilli V., di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 52.Siskind L.J., Davoody A., Lewin N., Marshall S., Colombini M. Enlargement and contracture of C2-ceramide channels. Biophys. J. 2003;85:1560–1575. doi: 10.1016/S0006-3495(03)74588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H., Rotolo J.A., Mesicek J., Penate-Medina T., Rimner A., Liao W.C., Yin X., Ragupathi G., Ehleiter D., Gulbins E., et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Artetxe I., Ugarte-Uribe B., Gil D., Valle M., Alonso A., Garcia-Saez A.J., Goni F.M. Does ceramide form channels? The ceramide-induced membrane permeabilization mechanism. Biophys. J. 2017;113:860–868. doi: 10.1016/j.bpj.2017.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colombini M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim. Biophys. Acta. 2010;1797:1239–1244. doi: 10.1016/j.bbabio.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Siskind L.J., Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stiban J., Fistere D., Colombini M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis. 2006;11:773–780. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- 58.Sentelle R.D., Senkal C.E., Jiang W., Ponnusamy S., Gencer S., Selvam S.P., Ramshesh V.K., Peterson Y.K., Lemasters J.J., Szulc Z.M., et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012;8:831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanida I., Ueno T., Kominami E. LC3 and Autophagy. Methods Mol. Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 60.Bollinger C.R., Teichgraber V., Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T., Su T.P. Intracellular dynamics of ε-1 receptors (ε-1 binding sites) in NG108-15 cells. J. Pharmacol. Exp. Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 62.Kutomi G., Tamura Y., Tanaka T., Kajiwara T., Kukita K., Ohmura T., Shima H., Takamaru T., Satomi F., Suzuki Y., et al. Human endoplasmic reticulum oxidoreductin 1-α is a novel predictor for poor prognosis of breast cancer. Cancer Sci. 2013;104:1091–1096. doi: 10.1111/cas.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka T., Kutomi G., Kajiwara T., Kukita K., Kochin V., Kanaseki T., Tsukahara T., Hirohashi Y., Torigoe T., Okamoto Y., et al. Cancer-associated oxidoreductase ERO1-α promotes immune escape through up-regulation of PD-L1 in human breast cancer. Oncotarget. 2017;8:24706–24718. doi: 10.18632/oncotarget.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Vliet A.R., Giordano F., Gerlo S., Segura I., Van Eygen S., Molenberghs G., Rocha S., Houcine A., Derua R., Verfaillie T., et al. The ER Stress Sensor PERK Coordinates ER-Plasma Membrane Contact Site Formation through Interaction with Filamin-A and F-Actin Remodeling. Mol. Cell. 2017;65:885–899. doi: 10.1016/j.molcel.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 65.Blais J.D., Addison C.L., Edge R., Falls T., Zhao H., Wary K., Koumenis C., Harding H.P., Ron D., Holcik M., et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol. Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teng Y., Ren X., Li H., Shull A., Kim J., Cowell J.K. Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein. Oncogene. 2016;35:333–343. doi: 10.1038/onc.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Issop L., Fan J., Lee S., Rone M.B., Basu K., Mui J., Papadopoulos V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology. 2015;156:334–345. doi: 10.1210/en.2014-1503. [DOI] [PubMed] [Google Scholar]
- 68.Van Vliet A.R., Verfaillie T., Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim. Biophys. Acta. 2014;1843:2253–2262. doi: 10.1016/j.bbamcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Szymanski J., Janikiewicz J., Michalska B., Patalas-Krawczyk P., Perrone M., Ziolkowski W., Duszynski J., Pinton P., Dobrzyn A., Wieckowski M.R. Interaction of mitochondria with the endoplasmic reticulum and plasma membrane in calcium homeostasis, lipid trafficking and mitochondrial structure. Int. J. Mol. Sci. 2017;18:1576. doi: 10.3390/ijms18071576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karczmarek-Borowska B., Filip A., Wojcierowski J., Smolen A., Korobowicz E., Korszen-Pilecka I., Zdunek M. Estimation of prognostic value of Bcl-xL gene expression in non-small cell lung cancer. Lung Cancer. 2006;51:61–69. doi: 10.1016/j.lungcan.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Malkinson A.M. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp. Lung Res. 1998;24:541–555. doi: 10.3109/01902149809087385. [DOI] [PubMed] [Google Scholar]
- 72.Park D., Magis A.T., Li R., Owonikoko T.K., Sica G.L., Sun S.Y., Ramalingam S.S., Khuri F.R., Curran W.J., Deng X. Novel small-molecule inhibitors of Bcl-xL to treat lung cancer. Cancer Res. 2013;73:5485–5496. doi: 10.1158/0008-5472.CAN-12-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang H., Shah K., Bradbury N.A., Li C., White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W., Chandel N., Laakso M., Muller W.J., Allen E.L., et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf A., Agnihotri S., Micallef J., Mukherjee J., Sabha N., Cairns R., Hawkins C., Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beckert S., Farrahi F., Aslam R.S., Scheuenstuhl H., Konigsrainer A., Hussain M.Z., Hunt T.K. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–324. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 77.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Raturi A., Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM) Biochim. Biophys. Acta. 2013;1833:213–224. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Bittremieux M., Parys J.B., Pinton P., Bultynck G. ER functions of oncogenes and tumor suppressors: Modulators of intracellular Ca2+ signaling. Biochim. Biophys. Acta. 2016;1863:1364–1378. doi: 10.1016/j.bbamcr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Ma L.I., Chang Y., Yu L., He W., Liu Y. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via PI3K/Akt signaling in breast cancer cells. Oncol. Lett. 2015;10:3816–3822. doi: 10.3892/ol.2015.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herrera-Cruz M.S., Simmen T. Cancer: Untethering mitochondria from the endoplasmic reticulum? Front. Oncol. 2017;7:105. doi: 10.3389/fonc.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patergnani S., Missiroli S., Marchi S., Giorgi C. Mitochondria-associated endoplasmic reticulum membranes microenvironment: Targeting autophagic and apoptotic pathways in cancer therapy. Front. Oncol. 2015;5:173. doi: 10.3389/fonc.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morad S.A., Cabot M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer. 2013;13:51–65. doi: 10.1038/nrc3398. [DOI] [PubMed] [Google Scholar]
- 86.Paschall A.V., Zimmerman M.A., Torres C.M., Yang D., Chen M.R., Li X., Bieberich E., Bai A., Bielawski J., Bielawska A., et al. Ceramide targets xIAP and cIAP1 to sensitize metastatic colon and breast cancer cells to apoptosis induction to suppress tumor progression. BMC Cancer. 2014;14:24. doi: 10.1186/1471-2407-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haynes T.A., Filippov V., Filippova M., Yang J., Zhang K., Duerksen-Hughes P.J. DNA damage induces down-regulation of UDP-glucose ceramide glucosyltransferase, increases ceramide levels and triggers apoptosis in p53-deficient cancer cells. Biochim. Biophys. Acta. 2012;1821:943–953. doi: 10.1016/j.bbalip.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X., Li B., Zhang Y., Liu J. Ceramide induces release of mitochondrial proapoptotic proteins in caspase-dependent and -independent manner in HT-29 cells. Sci. China Life Sci. 2008;51:66–71. doi: 10.1007/s11427-008-0015-y. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y.Y., Hill R.A., Li Y.T. Ceramide glycosylation catalyzed by glucosylceramide synthase and cancer drug resistance. Adv. Cancer Res. 2013;117:59–89. doi: 10.1016/B978-0-12-394274-6.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moro K., Kawaguchi T., Tsuchida J., Gabriel E., Qi Q., Yan L., Wakai T., Takabe K., Nagahashi M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget. 2018;9:19874–19890. doi: 10.18632/oncotarget.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roh J.L., Kim E.H., Park J.Y., Kim J.W. Inhibition of glucosylceramide synthase sensitizes head and neck cancer to cisplatin. Mol. Cancer Ther. 2015;14:1907–1915. doi: 10.1158/1535-7163.MCT-15-0171. [DOI] [PubMed] [Google Scholar]
- 92.Roh J.L., Park J.Y., Kim E.H., Jang H.J. Targeting acid ceramidase sensitises head and neck cancer to cisplatin. Eur. J. Cancer. 2016;52:163–172. doi: 10.1016/j.ejca.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 93.Henry B., Moller C., Dimanche-Boitrel M.T., Gulbins E., Becker K.A. Targeting the ceramide system in cancer. Cancer Lett. 2013;332:286–294. doi: 10.1016/j.canlet.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Schiffmann S., Sandner J., Birod K., Wobst I., Angioni C., Ruckhaberle E., Kaufmann M., Ackermann H., Lotsch J., Schmidt H., et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
- 95.Nagahashi M., Tsuchida J., Moro K., Hasegawa M., Tatsuda K., Woelfel I.A., Takabe K., Wakai T. High levels of sphingolipids in human breast cancer. J. Surg. Res. 2016;204:435–444. doi: 10.1016/j.jss.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flowers M., Fabrias G., Delgado A., Casas J., Abad J.L., Cabot M.C. C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast Cancer Res. Treat. 2012;133:447–458. doi: 10.1007/s10549-011-1768-8. [DOI] [PubMed] [Google Scholar]
- 97.Vethakanraj H.S., Babu T.A., Sudarsanan G.B., Duraisamy P.K., Ashok Kumar S. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2015;464:833–839. doi: 10.1016/j.bbrc.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 98.Ogretmen B., Hannun Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 99.Guan F., Handa K., Hakomori S.I. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. USA. 2009;106:7461–7466. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosal P., Sukocheva O.A., Wang T., Mayne G.C., Watson D.I., Hussey D.J. Effects of chemotherapy agents on Sphingosine-1-Phosphate receptors expression in MCF-7 mammary cancer cells. Biomed. Pharmacother. 2016;81:218–224. doi: 10.1016/j.biopha.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 101.Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 102.Kim E.S., Kim J.S., Kim S.G., Hwang S., Lee C.H., Moon A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J. Cell Sci. 2011;124:2220–2230. doi: 10.1242/jcs.076794. [DOI] [PubMed] [Google Scholar]
- 103.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nistico P., Bissell M.J., Radisky D.C. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012;4:a011908. doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathow D., Chessa F., Rabionet M., Kaden S., Jennemann R., Sandhoff R., Grone H.J., Feuerborn A. Zeb1 affects epithelial cell adhesion by diverting glycosphingolipid metabolism. EMBO Rep. 2015;16:321–331. doi: 10.15252/embr.201439333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edmond V., Dufour F., Poiroux G., Shoji K., Malleter M., Fouque A., Tauzin S., Rimokh R., Sergent O., Penna A., et al. Downregulation of ceramide synthase-6 during epithelial-to-mesenchymal transition reduces plasma membrane fluidity and cancer cell motility. Oncogene. 2015;34:996–1005. doi: 10.1038/onc.2014.55. [DOI] [PubMed] [Google Scholar]
- 107.Levy M., Futerman A.H. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–356. doi: 10.1002/iub.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bleicher R.J., Cabot M.C. Glucosylceramide synthase and apoptosis. Biochim. Biophys. Acta. 2002;1585:172–178. doi: 10.1016/S1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 109.Grosch S., Schiffmann S., Geisslinger G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 110.Bornancin F. Ceramide kinase: The first decade. Cell Signal. 2011;23:999–1008. doi: 10.1016/j.cellsig.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Gomez-Munoz A. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim. Biophys. Acta. 2006;1758:2049–2056. doi: 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 112.Kim C.W., Lee H.M., Lee T.H., Kang C., Kleinman H.K., Gho Y.S. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312–6317. [PubMed] [Google Scholar]
- 113.Paik J.H., Chae S., Lee M.J., Thangada S., Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J. Biol. Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- 114.Wang F., Van Brocklyn J.R., Hobson J.P., Movafagh S., Zukowska-Grojec Z., Milstien S., Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 115.Haakenson J.K., Khokhlatchev A.V., Choi Y.J., Linton S.S., Zhang P., Zaki P.M., Fu C., Cooper T.K., Manni A., Zhu J., et al. Lysosomal degradation of CD44 mediates ceramide nanoliposome-induced anoikis and diminished extravasation in metastatic carcinoma cells. J. Biol. Chem. 2015;290:8632–8643. doi: 10.1074/jbc.M114.609677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarkar S., Maceyka M., Hait N.C., Paugh S.W., Sankala H., Milstien S., Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 117.Sukocheva O., Wadham C., Holmes A., Albanese N., Verrier E., Feng F., Bernal A., Derian C.K., Ullrich A., Vadas M.A., et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: The role of sphingosine kinase-1. J. Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fan S.H., Wang Y.Y., Lu J., Zheng Y.L., Wu D.M., Zhang Z.F., Shan Q., Hu B., Li M.Q., Cheng W. CERS2 suppresses tumor cell invasion and is associated with decreased V-ATPase and MMP-2/MMP-9 activities in breast cancer. J. Cell Biochem. 2015;116:502–513. doi: 10.1002/jcb.24978. [DOI] [PubMed] [Google Scholar]
- 119.Van Brocklyn J.R., Young N., Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/S0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 120.Wu H.X., Wang G.M., Lu X., Zhang L. miR-542-3p targets sphingosine-1-phosphate receptor 1 and regulates cell proliferation and invasion of breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2017;21:108–114. [PubMed] [Google Scholar]
- 121.Filipenko I., Schwalm S., Reali L., Pfeilschifter J., Fabbro D., Huwiler A., Zangemeister-Wittke U. Upregulation of the S1P3 receptor in metastatic breast cancer cells increases migration and invasion by induction of PGE2 and EP2/EP4 activation. Biochim. Biophys. Acta. 2016;1861:1840–1851. doi: 10.1016/j.bbalip.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 122.Gril B., Paranjape A.N., Woditschka S., Hua E., Dolan E.L., Hanson J., Wu X., Kloc W., Izycka-Swieszewska E., Duchnowska R., et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat. Commun. 2018;9:2705. doi: 10.1038/s41467-018-05030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu W., Tan C., He Y., Zhang G., Xu Y., Tang J. Functional miRNAs in breast cancer drug resistance. OncoTargets Ther. 2018;11:1529–1541. doi: 10.2147/OTT.S152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Galindo-Hernandez O., Villegas-Comonfort S., Candanedo F., Gonzalez-Vazquez M.C., Chavez-Ocana S., Jimenez-Villanueva X., Sierra-Martinez M., Salazar E.P. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch. Med. Res. 2013;44:208–214. doi: 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 125.Galindo-Hernandez O., Gonzales-Vazquez C., Cortes-Reynosa P., Reyes-Uribe E., Chavez-Ocana S., Reyes-Hernandez O., Sierra-Martinez M., Salazar E.P. Extracellular vesicles from women with breast cancer promote an epithelial-mesenchymal transition-like process in mammary epithelial cells MCF10A. Tumour Biol. 2015;36:9649–9659. doi: 10.1007/s13277-015-3711-9. [DOI] [PubMed] [Google Scholar]
- 126.Xu J., Camfield R., Gorski S.M. The interplay between exosomes and autophagy—Partners in crime. J. Cell Sci. 2018;131:jcs215210. doi: 10.1242/jcs.215210. [DOI] [PubMed] [Google Scholar]
- 127.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 128.Shedden K., Xie X.T., Chandaroy P., Chang Y.T., Rosania G.R. Expulsion of small molecules in vesicles shed by cancer cells: Association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 129.Safaei R., Larson B.J., Cheng T.C., Gibson M.A., Otani S., Naerdemann W., Howell S.B. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol. Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 130.Rilla K., Mustonen A.M., Arasu U.T., Harkonen K., Matilainen J., Nieminen P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2017 doi: 10.1016/j.matbio.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 131.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weidle U.H., Dickopf S., Hintermair C., Kollmorgen G., Birzele F., Brinkmann U. The role of micro RNAs in breast cancer metastasis: preclinical validation and potential therapeutic targets. Cancer Genom. Proteom. 2018;15:17–39. doi: 10.21873/cgp.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galindo-Hernandez O., Serna-Marquez N., Castillo-Sanchez R., Salazar E.P. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot. Essent. Fatty Acids. 2014;91:299–310. doi: 10.1016/j.plefa.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 134.Choi D.S., Kim D.K., Kim Y.K., Gho Y.S. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–1571. doi: 10.1002/pmic.201200329. [DOI] [PubMed] [Google Scholar]
- 135.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 136.El Buri A., Adams D.R., Smith D., Tate R.J., Mullin M., Pyne S., Pyne N.J. The sphingosine 1-phosphate receptor 2 is shed in exosomes from breast cancer cells and is N-terminally processed to a short constitutively active form that promotes extracellular signal regulated kinase activation and DNA synthesis in fibroblasts. Oncotarget. 2018;9:29453–29467. doi: 10.18632/oncotarget.25658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karliner J.S. Sphingosine kinase and sphingosine 1-phosphate in the heart: A decade of progress. Biochim. Biophys. Acta. 2013;1831:203–212. doi: 10.1016/j.bbalip.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tran-Dinh A., Diallo D., Delbosc S., Varela-Perez L.M., Dang Q.B., Lapergue B., Burillo E., Michel J.B., Levoye A., Martin-Ventura J.L., et al. HDL and endothelial protection. Br. J. Pharmacol. 2013;169:493–511. doi: 10.1111/bph.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Denimal D., Pais de Barros J.P., Petit J.M., Bouillet B., Verges B., Duvillard L. Significant abnormalities of the HDL phosphosphingolipidome in type 1 diabetes despite normal HDL cholesterol concentration. Atherosclerosis. 2015;241:752–760. doi: 10.1016/j.atherosclerosis.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 140.Liu M., Seo J., Allegood J., Bi X., Zhu X., Boudyguina E., Gebre A.K., Avni D., Shah D., Sorci-Thomas M.G., et al. Hepatic apolipoprotein M (apoM) overexpression stimulates formation of larger apoM/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J. Biol. Chem. 2014;289:2801–2814. doi: 10.1074/jbc.M113.499913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alemany R., van Koppen C.J., Danneberg K., Ter Braak M., Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch. Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 142.Jin F., Hagemann N., Sun L., Wu J., Doeppner T.R., Dai Y., Hermann D.M. High-density lipoprotein (HDL) promotes angiogenesis via S1P3-dependent VEGFR2 activation. Angiogenesis. 2018;21:381–394. doi: 10.1007/s10456-018-9603-z. [DOI] [PubMed] [Google Scholar]
- 143.Benaud C., Oberst M., Hobson J.P., Spiegel S., Dickson R.B., Lin C.Y. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J. Biol. Chem. 2002;277:10539–10546. doi: 10.1074/jbc.M109064200. [DOI] [PubMed] [Google Scholar]
- 144.Kang J.Y., Dolled-Filhart M., Ocal I.T., Singh B., Lin C.Y., Dickson R.B., Rimm D.L., Camp R.L. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101–1105. [PubMed] [Google Scholar]
- 145.Sales K.U., Friis S., Konkel J.E., Godiksen S., Hatakeyama M., Hansen K.K., Rogatto S.R., Szabo R., Vogel L.K., Chen W., et al. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2015;34:346–356. doi: 10.1038/onc.2013.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ko C.J., Huang C.C., Lin H.Y., Juan C.P., Lan S.W., Shyu H.Y., Wu S.R., Hsiao P.W., Huang H.P., Shun C.T., et al. Androgen-induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75:2949–2960. doi: 10.1158/0008-5472.CAN-14-3297. [DOI] [PubMed] [Google Scholar]
- 147.Tsai C.H., Teng C.H., Tu Y.T., Cheng T.S., Wu S.R., Ko C.J., Shyu H.Y., Lan S.W., Huang H.P., Tzeng S.F., et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene. 2014;33:4643–4652. doi: 10.1038/onc.2013.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rather G.M., Lin S.Y., Lin H., Banach-Petrosky W., Hirshfield K.M., Lin C.Y., Johnson M.D., Szekely Z., Bertino J.R. Activated matriptase as a target to treat breast cancer with a drug conjugate. Oncotarget. 2018;9:25983–25992. doi: 10.18632/oncotarget.25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu T., Wang X., Li J., Song X., Wang Y., Zhang L., Li Z., Tian J. Identification of personalized chemoresistance genes in subtypes of basal-like breast cancer based on functional differences using pathway analysis. PLoS ONE. 2015;10:e0131183. doi: 10.1371/journal.pone.0131183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schwentner L., Wolters R., Koretz K., Wischnewsky M.B., Kreienberg R., Rottscholl R., Wockel A. Triple-negative breast cancer: The impact of guideline-adherent adjuvant treatment on survival—A retrospective multi-centre cohort study. Breast Cancer Res. Treat. 2012;132:1073–1080. doi: 10.1007/s10549-011-1935-y. [DOI] [PubMed] [Google Scholar]
- 151.Loi S., Pommey S., Haibe-Kains B., Beavis P.A., Darcy P.K., Smyth M.J., Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Elnashar A.T., Ali el S.M., Gaber A. The prognostic value of triple negative in stage II/III breast cancer. J. Oncol. Pharm. Pract. 2012;18:68–75. doi: 10.1177/1078155211398299. [DOI] [PubMed] [Google Scholar]
- 153.Nadal R., Ortega F.G., Salido M., Lorente J.A., Rodriguez-Rivera M., Delgado-Rodriguez M., Macia M., Fernandez A., Corominas J.M., Garcia-Puche J.L., et al. CD133 expression in circulating tumor cells from breast cancer patients: Potential role in resistance to chemotherapy. Int. J. Cancer. 2013;133:2398–2407. doi: 10.1002/ijc.28263. [DOI] [PubMed] [Google Scholar]
- 154.Desai S., Liu Z., Yao J., Patel N., Chen J., Wu Y., Ahn E.E., Fodstad O., Tan M. Heat shock factor 1 (HSF1) controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 (ATG7) J. Biol. Chem. 2013;288:9165–9176. doi: 10.1074/jbc.M112.422071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beelen K., Hoefnagel L.D., Opdam M., Wesseling J., Sanders J., Vincent A.D., van Diest P.J., Linn S.C. PI3K/AKT/mTOR pathway activation in primary and corresponding metastatic breast tumors after adjuvant endocrine therapy. Int. J. Cancer. 2014;135:1257–1263. doi: 10.1002/ijc.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang X., Wu X., Su P., Gao Y., Meng B., Sun Y., Li L., Zhou Z., Zhou G. Doxorubicin influences the expression of glucosylceramide synthase in invasive ductal breast cancer. PLoS ONE. 2012;7:e48492. doi: 10.1371/journal.pone.0048492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gouaze V., Yu J.Y., Bleicher R.J., Han T.Y., Liu Y.Y., Wang H., Gottesman M.M., Bitterman A., Giuliano A.E., Cabot M.C. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol. Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 158.Gouaze-Andersson V., Yu J.Y., Kreitenberg A.J., Bielawska A., Giuliano A.E., Cabot M.C. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim. Biophys. Acta. 2007;1771:1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu Y.Y., Gupta V., Patwardhan G.A., Bhinge K., Zhao Y., Bao J., Mehendale H., Cabot M.C., Li Y.T., Jazwinski S.M. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and β-catenin signaling. Mol. Cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu J., Zhang X., Liu A., Zhang D., Su Y., Liu Y., You D., Yuan L., Kong X., Wang X., et al. Altered methylation of glucosylceramide synthase promoter regulates its expression and associates with acquired multidrug resistance in invasive ductal breast cancer. Oncotarget. 2016;7:36755–36766. doi: 10.18632/oncotarget.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bekele R.T., Venkatraman G., Liu R.Z., Tang X., Mi S., Benesch M.G., Mackey J.R., Godbout R., Curtis J.M., McMullen T.P., et al. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: Implications for tamoxifen therapy and resistance. Sci. Rep. 2016;6:21164. doi: 10.1038/srep21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morad S.A., Cabot M.C. Tamoxifen regulation of sphingolipid metabolism—Therapeutic implications. Biochim. Biophys. Acta. 2015;1851:1134–1145. doi: 10.1016/j.bbalip.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jaafar R., Mnich K., Dolan S., Hillis J., Almanza A., Logue S.E., Samali A., Gorman A.M. RIP2 enhances cell survival by activation of NF-κB in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2018;497:115–121. doi: 10.1016/j.bbrc.2018.02.034. [DOI] [PubMed] [Google Scholar]