Abstract
Targeted genome editing is a desirable means of basic science and crop improvement. The clustered, regularly interspaced, palindromic repeat (CRISPR)/Cas9 (CRISPR-associated 9) system is currently the simplest and most commonly used system in targeted genomic editing in plants. Single and multiplex genome editing in plants can be achieved under this system. In Arabidopsis, AtWRKY11 and AtWRKY70 genes were involved in JA- and SA-induced resistance to pathogens, in rapeseed (Brassica napus L.), BnWRKY11 and BnWRKY70 genes were found to be differently expressed after inoculated with the pathogenic fungus, Sclerotinia sclerotiorum (Lib.) de Bary. In this study, two Cas9/sgRNA constructs targeting two copies of BnWRKY11 and four copies of BnWRKY70 were designed to generate BnWRKY11 and BnWRKY70 mutants respectively. As a result, twenty-two BnWRKY11 and eight BnWRKY70 independent transformants (T0) were obtained, with the mutation ratios of 54.5% (12/22) and 50% (4/8) in BnWRKY11 and BnWRKY70 transformants respectively. Eight and two plants with two copies of mutated BnWRKY11 and BnWRKY70 were obtained respectively. In T1 generation of each plant examined, new mutations on target genes were detected with high efficiency. The vast majority of BnWRKY70 mutants showed editing in three copies of BnWRKY70 in examined T1 plants. BnWRKY70 mutants exhibited enhanced resistance to Sclerotinia, while BnWRKY11 mutants showed no significant difference in Sclerotinia resistance when compared to non-transgenic plants. In addition, plants that overexpressed BnWRKY70 showed increased sensitivity when compared to non-transgenic plants. Altogether, our results demonstrated that BnWRKY70 may function as a regulating factor to negatively control the Sclerotinia resistance and CRISPR/Cas9 system could be used to generate germplasm in B. napus with high resistance against Sclerotinia.
Keywords: Brassica napus, CRISPR/Cas9, WRKY, Mutation pattern, Sclerotinia sclerotiorum
1. Introduction
The system of clustered, regularly interspaced, palindromic repeats (CRISPR)/Cas (CRISPR-associated) is the latest groundbreaking technology for genome editing and has become the dominant genome editing tool. The CRISPR/Cas system is used by bacteria and archaea as an RNA-guided defense system against invading viruses and plasmids [1,2]. CRISPR/Cas systems can be divided into three major types, namely, types I, II and III and the simplest and most commonly used system is CRISPR/Cas9, a type II system for Streptococcus pyogenes [3,4]. As an RNA-guided nuclease, Cas9 can be loaded into a single gRNA (sgRNA) engineered from two small RNAs (CRISPR RNA and trans-acting CRISPR RNA). The ribonucleoprotein complex formed by the sgRNA and Cas9 protein cleaves genomic DNA that is complementary to a 20 nucleotide stretch of the sgRNA as long as the 5′-NGG-3′ protospacer adjacent motif (PAM) is present in the complementary sequence [2].
Compared with zinc finger nucleases (ZFN) and transcription activator-like effector nucleases (TALEN), due to the ease of sgRNA manipulation, the CRISPR/Cas system presents advantages in terms of simplicity, accessibility, cost and versatility [5,6,7]. This system has been used successfully in many organisms, including animals [8,9,10], plants [11,12], fungi [13] and bacteria [14].
The CRISPR/Cas9 system can efficiently introduce several mutation types, including base substitutions [15,16], insertion mutations and deletion (indel) mutations [17,18] in the target site and deletions or inversions of a large chromatin fragment [19,20]. Unlike its predecessors, the CRISPR/Cas system can introduce a mutation in multiple sites simultaneously and can be used to edit several genes at the same time [21,22]. Therefore, this system is particularly useful for knockout of redundant genes or parallel pathways.
The genomes of model plants and cultivated crops including Arabidopsis thaliana [23,24], tobacco [16], tomato [18], rice [25,26], wheat [25,27], sorghum [21] and B. oleracea [28] have been successfully edited by CRIPSR/Cas9 system. This genetic modification technology does not require the persistent existence of foreign DNA and thus presents strong application prospects in crop breeding [7,11]. A few studies have presented targeted genome editing mediated by the CRISPR/Cas9 system in the important oil crop rapeseed. ALCATRAZ [29] GA1-3, FRUITFULL, DA1, DA2 [30], CLAVATA [31] and SPL3 [32], which are associated with plant or pod development; and BnFAD2, which is responsible for the desaturation of oleic acid to linoleic acid [33], were edited by the CRISPR/Cas9 system in B. napus by different groups. Most of the sgRNAs induced targeted editing, although there were a variety of editing efficiencies (5.3–100%) and the efficiency of multiple mutagenesis was significantly lower than that of single mutagenesis. However, to our knowledge, no attempt has been made to knockout pathogenesis-related genes by the CRISPR/Cas9 system to improve rapeseed resistance to pathogens. S. sclerotiorum is a nonspecific necrotrophic pathogen that causes sclerotinia stem rot in B. napus, resulting yield losses in oilseed Brassicas that vary between 5% and 100% [34]. Creating a new Sclerotinia-resistant variety has become the priority goal of crop breeders [35].
WRKY transcription factors (TFs), defined by their DNA-binding domain, namely, the WRKY domain, have been identified in different plants [36,37] and are widely involved in defense to diverse plant stress conditions, especially in plant immune responses [38,39,40,41]. In Arabidopsis, many WRKY transcription factors have been reported to be associated with disease resistance, including WRKY8 [42], WRKY11 [43], WRKY33 [44,45], WRKY38 and WRKY62 [46], WRKY46 [47], WRKY53 and WRKY70 [48]. Studies have shown that overexpression or loss function of WRKY11 or WRKY70 affects SA and JA-induced disease resistance response to pathogens in Arabidopsis [43,49,50,51]. Previous reports suggest that some BnWRKY genes might be involved in the response to pathogens in B. napus as well [52,53,54,55].
In the present study, we explored the patterns of targeted mutagenesis of the B. napus genome mediated by the CRISPR/Cas9 system. CRISPR/Cas9 vectors with multiple sgRNA expression cassettes were constructed to target the BnWRKY11 and BnWRKY70 genes of B. napus and Agrobacterium-mediated genetic transformation was used to generate transgenic plants. The mutations of targeted sites were then investigated by amplifying and sequencing in the T0 and T1 generations. The mutation pattern was analyzed as well. S. sclerotiorum resistances of the BnWRKY70 knockout and overexpression plants were assessed by detached leaf inoculation and it turned out that loss function of BnWRKY70 enhanced, while overexpression of BnWRKY70 reduced resistance to S. sclerotiorum. Our findings suggested that the CRISPR/Cas9 system can be used to generate multiple homologs mutated plants in B. napus. With the high editing efficiency of this system in T1 plants, homozygous mutants can be generated in limited generations. Therefore, the CRISPR/Cas9 system could be an effective method for theoretical research and could improve rapeseed resistance to pathogens.
2. Results
2.1. Sequence Identification and Expression Analysis of BnWRKY11 and BnWRKY70 Genes in B. napus
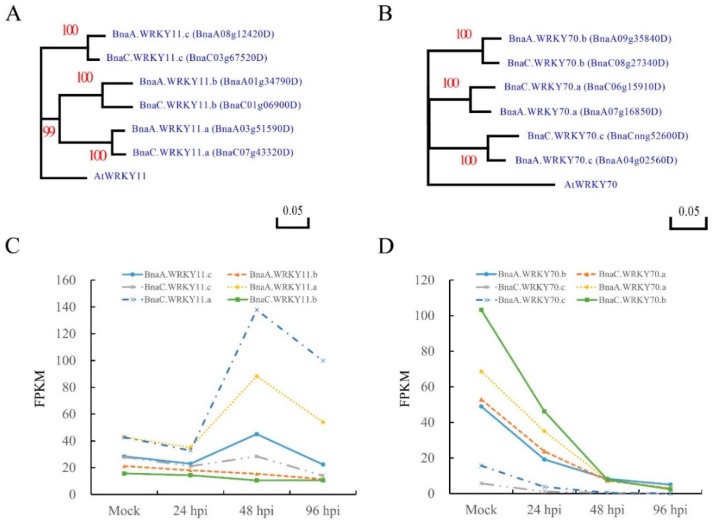
Wu et al. [56] analyzed the transcriptome of B. napus lines to investigate the defense responses to S. sclerotiorum using in-depth RNA sequencing (RNA-seq), results showed that BnWRKY11 and BnWRKY70 genes differentially expressed in resistant B. napus lines J964 after inoculated by S. sclerotiorum. Both AtWRKY11 and AtWRKY70 genes have one copy in Arabidopsis [57]. Depending on the AtWRKY11 and AtWRKY70 gene sequence, we found the reference genome of Darmor-bzh [58] comprised six homoeologs of BnWRKY11 and BnWRKY70 genes respectively by BlastP (E-value ≤ 1 × 10−5, identity ≥ 50% and coverage ≥ 50%) (Figure 1A,B). Depending on the naming conventions of Østergaard et al. [59], the copies of BnWRKY11 and BnWRKY70 were named BnaA.WRKY11.a, BnaA.WRKY11.b, BnaA.WRKY11.c, BnaC.WRKY11.a, BnaC.WRKY11.b, BnaC.WRKY11.c (Figure 1A), and BnaA.WRKY70.a, BnaA.WRKY70.b, BnaA.WRKY70.c, BnaC.WRKY70.a, BnaC.WRKY70.b, BnaC.WRKY70.c (Figure 1B) respectively. According to the transcriptomics sequencing data published by Wu et al. [56], we found that three of the six copies (BnaA.WRKY11.a, BnaC.WRKY11.a and BnaA.WRKY11.c) were significantly up-regulated at 48 h post-inoculation (hpi) (Figure 1C), BnaA.WRKY11.a and BnaC.WRKY11.a not only showed the greatest expression change after inoculation but also had highest expression level before inoculation than those of the other four copies (Figure 1C). The expression of six BnWRKY70 homologue genes were significantly down-regulated after inoculated by S. sclerotiorum and the expression level were getting lower and lower over inoculation time (Figure 1D). The expression level of BnaA.WRKY70.c and BnaC.WRKY70.c were significantly lower than that of other four copies. Among the BnWRKY11 and BnWRKY70 genes, BnaC.WRKY11.a and BnaC.WRKY70.b had the highest expression levels before inoculation with S. sclerotiorum and also most significantly induced (BnaC.WRKY11.a) or suppressed (BnaC.WRKY70.b) after inoculation. Because of the difficulty in simultaneously targeted editing to up to six copies, the copies of BnWRKY11 and BnWRKY70 that have high initial expression level and most dramatically induced or suppressed after inoculation with S. sclerotiorum were chosen as candidate genes to knockout by CRISPR/Cas9 system. For BnWRKY11, BnaA.WRKY11.a and BnaC.WRKY11.a were chosen and for BnWRKY70, BnaA.WRKY70.a, BnaA.WRKY70.b, BnaC.WRKY70.a and BnaC.WRKY70.b were chosen.
Figure 1.
Phylogenetic tree of WRKY11 and WRKY70 and the expression level of BnWRKY11 and BnWRKY70 in response to S. sclerotiorum inoculation. (A) Phylogenetic tree of BnWRKY11 and the homologs from Arabidopsis; (B) Phylogenetic tree of BnWRKY70 and the homologs from Arabidopsis; (C,D) The expression level of BnWRKY11 and BnWRKY70 in response to S. sclerotiorum inoculation [51]. The tree was generated using the DNAMAN program by maximum likelihood (ML) methods. Bootstrap values are displayed with red numbers. hpi, hours post-inoculation.
2.2. CRISPR/Cas9 Binary Vector Construction, Rapeseed Transformation and Screening of Positive Transformants
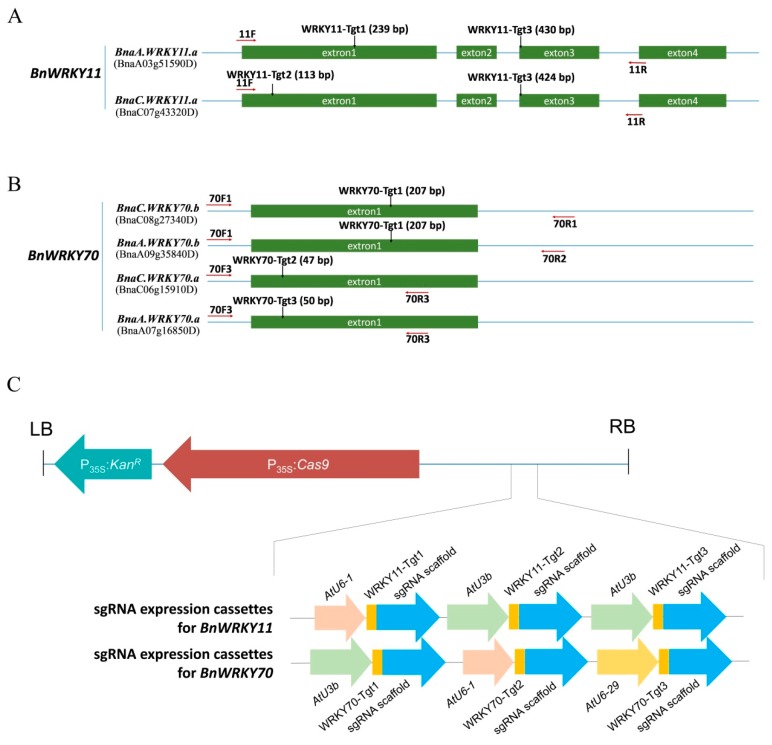
We targeted BnWRKY11 and BnWRKY70 genes in B. napus to test the CRISPR/Cas9 system for genome editing (Figure 2). For BnWRKY11, we designed two sgRNAs targeting BnaA.WRKY11.a and BnaC.WRKY11.a. WRKY11-Tgt1 (Target1) and WRKY11-Tgt3 targeted the first and third exons of BnaA.WRKY11.a and WRKY11-Tgt2 and WRKY11-Tgt3 targeted to the first and third exons of BnaC.WRKY11.a, respectively (Figure 2A). All three WRKY70-Tgt targeted the first exon of the WRKY70 genes and WRKY70-Tgt1 targeted BnaC.WRKY70.b and BnaA.WRKY70.b, while WRKY70-Tgt2 targeted BnaC.WRKY70.a and WRKY70-Tgt3 targeted BnaA.WRKY70.a (Figure 2B). CRISPR/Cas9 constructs that targeted to BnWRKY11 and BnWRKY70 with three sgRNA expression cassettes were generated (Figure 2C). The binary expression vector pLYCRISPR/Cas9P35S-N containing a neomycin phosphotransferase gene was used for genetic transformation. With G418 as the selection agent and as confirmed by polymerase chain reaction (PCR) (primers: Cas9-F: GAAGTACCCCACTATCTACCAC, Cas9-R: ATGAAGAGCTTGTCCACGTC), we obtained 30 transgenic plants with 22 BnWRKY11 transformants (CRI-W11) and 8 BnWRKY70 transformants (CRI-W70).
Figure 2.
Position of target sites and primers on BnWRKY11 and BnWRKY70 and physical maps of the T-DNA regions of Cas9/sgRNA constructs. (A,B) the target sites for BnWRKY11 and BnWRKY70 respectively and the primers for the amplification were shown as well. Tgt1-Tgt3 means the chosen target sites, the locations of target sites are marked with black arrows; primers are shown in red arrows. (C) Physical maps of the T-DNA regions of Cas9/sgRNA constructs. LB/RB, left/right border of T-DNA; P35S:Cas9, Cas9 gene which driven by CMV35S promoter; P35S:KanR, NTP gene which driven by CMV35S promoter. AtU3/AtU6, Arabidopsis U3/U6 promoter.
2.3. Confirmation of Cas9-Induced Mutagenesis in Transgenic Plants of B. napus
To detect mutagenesis at the targeted site, we cut and mixed several leaves from the transgenic plants for DNA extraction. Using locus-specific primers (Table S2), we amplified and sequenced the flanking sequences in given target sites. As expected, a double-peak phenomenon occurred 3–4 bp upstream of PAM in the sequence chromatograms of amplicons (Figure S1).
The Sanger chromatograms of the PCR products of the targeted DNA were analyzed by the online tool TIDE (Tracking of Indels by Decomposition, https://tide.deskgen.com) [60] to evaluate the existence of editing events and mutation efficiency with p-value < 0.001 (Tables S4 and S5). Among the twenty-two T0 transgenic lines of CRI-W11, genomes of twelve and ten plants were edited at WRKY11-Tgt2 and WRKY11-Tgt3 sites in BnaC.WRKY11.a respectively, while eight plants among them showed mutated in both copies of BnWRKY11 (Table 1 and Table S4). No editing events were detected at WRKY11-Tgt1 site. Among the eight CRI-W70 transgenic plants, three independent mutagenesis were induced by WRKY70-Tgt2 and WRKY70-Tgt3 in the BnaA.WRKY70.b and BnaA.WRKY70.a loci, respectively (Table 1, Figure S1). This represents that mutation frequencies were 54.5% at WRKY11-Tgt2 (BnaC.WRKY11.a), 31.8% at WRKY11-Tgt3 (BnaA.WRKY11.a) and 40.9% at WRKY11-Tgt3 (BnaC.WRKY11.a) in T0 plants of CRI-W11. 37.5% plants were mutated by WRKY70-Tgt2 and WRKY70-Tgt3 at BnaA.WRKY70.b and BnaA.WRKY70.a respectively (Table 1). Two of the CRI-W70 plants showed mutated in both BnaA.WRKY70.b and BnaA.WRKY70.a (Table 1 and Table S5).
Table 1.
The targets and primers designed for BnWRKY11 and BnWRKY70 and mutation rates in T0 plants.
| Target Gene (Number of Transformants) | Copies | Target | Amplification Primer | No. of Plants with Mutations | Mutation Frequency (%) |
|---|---|---|---|---|---|
| BnWRKY1122 | BnaA.WRKY11.a | WRKY11-Tgt1, WRKY11-Tgt3 |
11subF/11subR→11F/11R | 0, 7 | 0, 31.8% |
| BnaC.WRKY11.a | WRKY11-Tgt2, WRKY11-Tgt3 |
11F/11R | 12, 9 | 54.5%, 40.9% |
|
| BnWRKY708 | BnaC.WRKY70.b | WRKY70-Tgt1 | 70F3/70R3 | 0 | 0 |
| BnaA.WRKY70.b | WRKY70-Tgt1 | 70F3/70R3 | 3 | 37.5% | |
| BnaC.WRKY70.a | WRKY70-Tgt2 | 70F1/70R2 | 0 | 0 | |
| BnaA.WRKY70.a | WRKY70-Tgt3 | 70F1/70R1 | 3 | 37.5% |
Tgt, the target sequence used to generate sgRNA expression cassette. The amplify of BnaA.WRKY11.a was performed with the primer pair 11subF1/11subR1 first, then subcloned the products with primer pair 11F/11R.
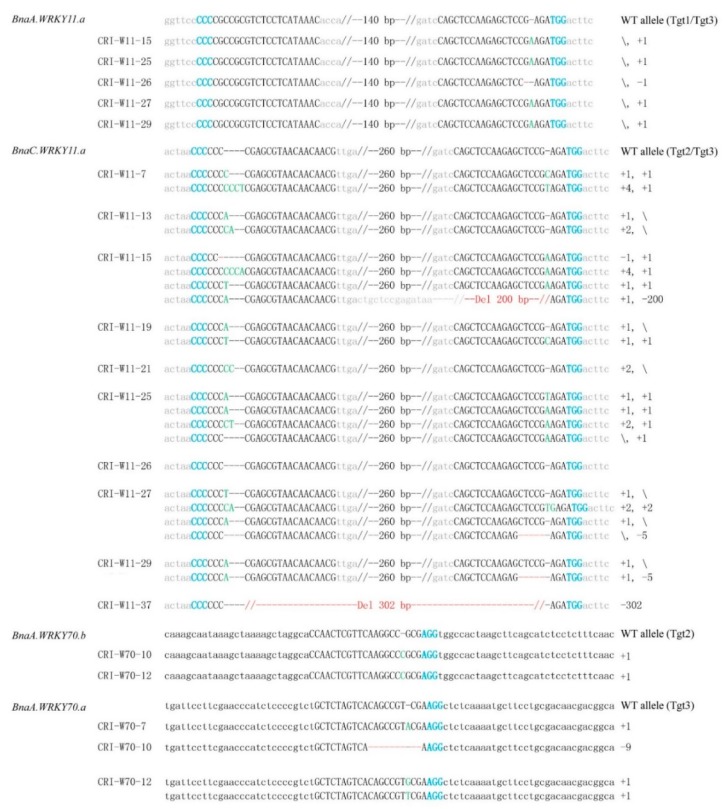
To identify the mutation type, we cloned the mutated amplification products and then randomly sequenced six clones. Depending on the mutation efficiencies assessed by TIDE, some samples with low mutation efficiency were not analyzed by sequencing. The results showed that one or more editing events occurred at the target sites of these transgenic lines (Figure 3). Four alleles were detected in the transgenic plants CRI-W11-15, CRI-W11-25 and CRI-W11-27 and 3 different alleles were detected in CRI-W11-7, CRI-W11-13, CRI-W11-19 and CRI-W11-29 including the WT allele, indicating that the plants were chimeric. In addition, a deletion of 302 bp in BnaC.WRKY11.a of the CRI-W11-37 plant was detected (Figure 3). Notably, the potential double-strand breaks at WRKY11-Tgt2 and WRKY11-Tgt3 sites in BnaC.WRKY11.a were 302 bp distant and therefore, targeted genomic deletion was achieved between Cas9 cut sites. The sequencing results showed that three types of BnaA.WRKY70.a alleles existed in CRI-W70-12, including a WT allele (Figure 3). Among the 6 targets, 4 of them (WRKY11-Tgt2, WRKY11-Tgt3, WRKY70-Tgt1 and WRKY70-Tgt3) induced mutations with different editing efficiencies, whereas the other 2 targets did not. These results suggest that the CRISPR/Cas9 system can be used to edit more than one gene simultaneously in B. napus and that targeted genomic deletion can be achieved by multiplex editing with a relatively low efficiency.
Figure 3.
Multiplex mutagenesis of B. napus genome in T0 generation. The protospace adjacent motif (PAM) is shown in bold blue letters; red dashes mark the deletions; the inserted nucleotide is marked by a green letter. The numbers on the right show the type of mutation and how many nucleotides are involved, with “−” and “+” indicating deletion or insertion of the given number of nucleotides, respectively. Tgt1-Tgt3 means the target sequence used to generate sgRNA expression cassette.
2.4. Variety and Frequency of Mutations
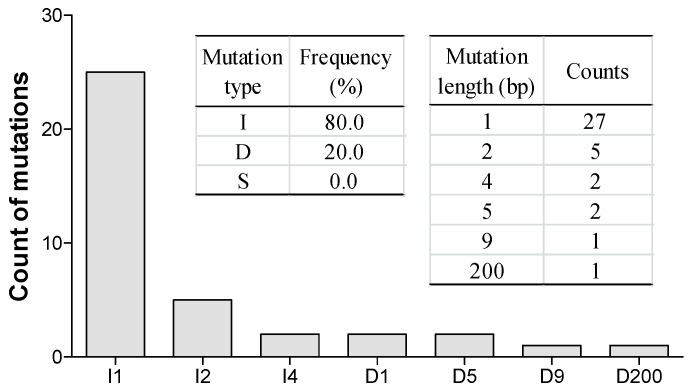
In the current study on B. napus, the mutation types and frequencies were surveyed in the T0 generation of transgenic plants (Figure 4). Using the limited number of editing events in T0 plants, we summarized the mutation types induced by the sgRNA we used in this research. Results showed that, among the detected mutations, 80% (32/40) were insertions and the remaining 20% (8/40) were deletions; no substitutions were found. Most of the insertions were 1 bp (25/40). Six deletions that ranged from 1–200 bp were detected. 27 of 40 all mutations we detected in T0 plants changed by only 1 bp. All identified mutations occurred between bases 3 and 4 upstream of the PAM of the given target site.
Figure 4.
Mutation types and frequency in transgenic plants. Mutation types and frequency from combined data of four different targets at T0 generation. Left insert, occurrence of insertion (I), deletion (D) and substitution (S) mutation types. Right insert, counts of different mutation length. In x-axis: I#, # of bp inserted at target site; D#, # of bp deleted from target site.
2.5. Mutagenesis in T1 Plants
The alleles of the targeted genes of the T1 plants were examined by sequence analysis of the T1 plants CRI-W11-6, CRI-W11-10, CRI-W70-6, CRI-W70-7, CRI-W70-9 and CRI-W70-10. For the T0 generation of the transgenic plants we chose, genome editing events were not detected at all targets in CRI-W11-6, CRI-W11-10, CRI-W70-6 and CRI-W70-9 plants (data not shown), while CRI-W70-7 showed heterozygous mutations at WRKY70-Tgt3 (targeting BnaA.WRKY70.a) and CRI-W70-10 showed heterozygous mutations at both WRKY70-Tgt2 (targeting BnaA.WRKY70.b) and WRKY70-Tgt3 (targeting BnaA.WRKY70.a) (Figure 3).
Results of mutation detection showed that many new editing events occurred in T1 plants (Table S3). In T1 plants of the CRI-W11-6 and CRI-W11-10 lines, we detected mutation efficiencies of WRKY11-Tgt2 (targeted to BnaC.WRKY11.a) and WRKY11-Tgt3 (targeted to BnaA.WRKY11.a and BnaC.WRKY11.a) reaching 100% (Table 2). All 4 lines of CRI-W70 showed a high proportion of mutagenesis in BnaA.WRKY70.b (8/10–10/10), BnaC.WRKY70.a (8/10–10/10) and BnaA.WRKY70.a (7/10–10/10) (Table 2). However, no mutagenesis was mediated by WRKY11-Tgt1 (targeting BnaA.WRKY11.a) or WRKY70-Tgt3 in any of the T1 plants. TA cloning and sequencing of the targeted sequences were performed in T1 plants as well. The results showed some T1 plants of the CRI-W11 and CRI-W70 lines were chimeras (Table S3). These results indicated that compare to T0 plant, additional mutations happened in T1 plants.
Table 2.
Sum of the edited T1 plants of CRI-W11 and CRI-W70.
| Line | Number of Examined Plants | Cas9:sgRNA Constructs a | Number of Edited Plants b | |||
|---|---|---|---|---|---|---|
| BnWRKY11 | ||||||
| BnaA.WRKY11.a (WRKY11-Tgt1) | BnaA.WRKY11.a (WRKY11-Tgt3) | BnaC.WRKY11.a (WRKY11-Tgt2) | BnaC.WRKY11.a (WRKY11-Tgt3) | |||
| CRI-W11-6 | 10 | 10 | 10 | 10 | 0 | 10 |
| CRI-W11-10 | 10 | 10 | 10 | 10 | 0 | 10 |
| BnWRKY70 | ||||||
| BnaA.WRKY70.b | BnaA.WRKY70.a | BnaC.WRKY70.a | BnaC.WRKY70.b | |||
| CRI-W70-6 | 10 | 8 | 8 | 8 | 0 | 8 |
| CRI-W70-7 | 10 | 8 | 8 | 8 | 0 | 8 |
| CRI-W70-9 | 10 | 10 | 10 | 10 | 0 | 10 |
| CRI-W70-10 | 10 | 8 | 10 | 10 | 0 | 10 |
Tgt means the target sequence used to generate sgRNA expression cassette. a Cas9:sgRNA construct in the plants was identified by PCR, with the primer pair: Cas9-F/Cas9-R; b Detailed mutation types for every plant were listed on Table S3.
The existence of the CRISPR/Cas9 component in T1 plants was also examined. Ten T1 transgenic plants were randomly selected and DNA of the leaves was extracted and amplified. Among the T1 plants examined (Table 2), segregation of the CRISPR/Cas9 components was detected in the CRI-W70-6, CRI-W70-7 and CRI-W70-10 lines, while the CRISPR/Cas9 component was detected in all of the CRI-W11-6, CRI-W11-10 and CRI-W70-9 T1 plants. TA cloning and sequencing analysis of targeted DNA demonstrated that, the T1 plants with CRISPR/Cas9 components, BnaC.WRKY11.a and BnaC.WRKY70.b were mutated in all of the examined plants, except for BnaA.WRKY70.a that showed editing in 7 of 8 plants. We further found that the CRISPR/Cas9 component was crossed out in CRI-W70-10-2 and CRI-W70-3 plants. In CRI-W70-10-2 plants, BnaA.WRKY70.b and BnaC.WRKY70.b were heterozygous, and both copies showed a “C” insertion in one of the alleles, while BnaA.WRKY70.a showed biallelic mutation; Similarly, in CRI-W70-10-3 plants, BnaA.WRKY70.b and BnaA.WRKY70.a were heterozygous and showed a “C” and “T” insertion in one of the alleles respectively, while BnaC.WRKY70.b showed biallelic mutation type with a “C” insertion and combined mutation (2 bp insertion and 3 bp deletion). These results suggested that the genetic mutations in T0 plants could be inherited to next generation.
2.6. BnWRKY70 Mutants Enhance Resistance to S. sclerotiorum
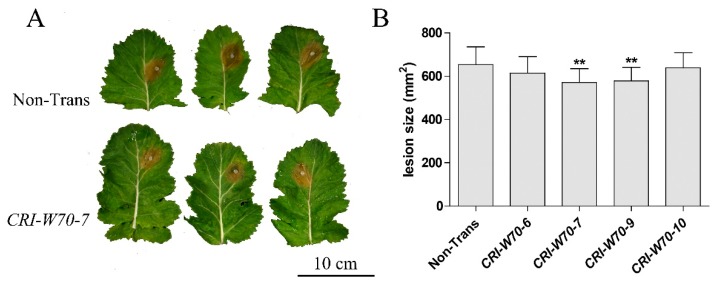
To evaluate the Sclerotinia resistance of transgenic plants, S. sclerotiorum infection was performed on detached leaves of CRI-W70 T1 generation plants. The T1 plants that mutated three copies of BnWRKY70 (Table 2) were chose for Sclerotinia resistance assessment. Lesion area was measured at 48 hpi. The results showed that, compared with the non-transgenic lines, the lesion areas on the detached leaves of CRI-W70-7 and CRI-W70-9 plants were significantly decreased (Figure 5A,B).
Figure 5.
Lesion area on leaves of BnWRKY70 knockout B. napus lines inoculated with S. sclerotiorum. (A) Representatives of disease symptom on the Non-Transgenic (Non-Trans), BnWRKY70 knockout lines. Leaves of 6-week-old plants were inoculated with S. sclerotiorum. Photos were taken 48 h post-inoculation. (B) Lesion area on leaves of BnWRKY70 overexpression lines. ** indicate that the means are statistically different (p < 0.01).
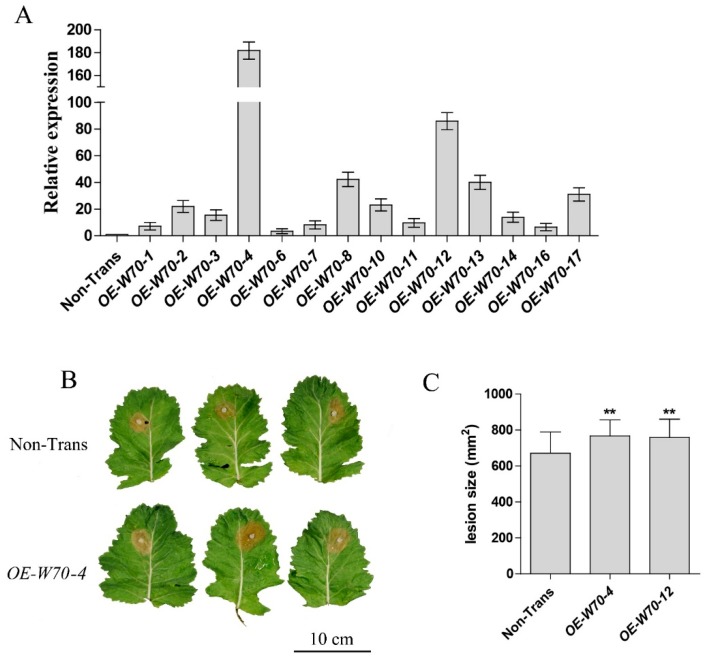
To confirm that the expression level of BnWRKY70 could affect the Sclerotinia resistance in B. napus, 35S:BnWRKY70 overexpression plants were generated and assessed for Sclerotinia resistance. We constructed the binary expression vector pMDC83-BnWRKY70-GFP and used Agrobacterium-mediated genetic transformation to obtain overexpressed plants. The copy BnaC.WRKY70.b was cloned and overexpressed. The expression level of BnaC.WRKY70.b in overexpression plants (OE-W70) was detected by RT-qPCR with specific primers qW70C08-F and qW70C08-R (Table S2) and 14 overexpression lines were obtained (Figure 6A). The most highly expressed lines OE-W70-4 and OE-W70-12 were selected for detached leaves inoculation with S. sclerotiorum, with the results showing that the lesion areas of the two lines were significantly larger than those of the non-transgenic plants (Figure 6B,C). The above results indicate that BnWRKY70 plays a negative regulatory role in the defense against S. sclerotiorum in B. napus. The resistance of CRI-W11 plants to S. sclerotiorum was also tested and no significant difference in S. sclerotiorum resistance was found between BnWRKY11 knockout mutants and non-transgenic plants (Figure S2).
Figure 6.
Expression analysis and lesion area on leaves of BnWRKY70 overexpression B. napus lines inoculated with S. sclerotiorum. (A) RT-qPCR analysis of BnWRKY70 expression in overexpression plants. BnActin7 was used as reference gene. The data shown are the mean of three independent experiments ± standard error (SE). (B) Representatives of disease symptom on the Non-Transgenic (Non-Trans), BnWRKY70 knockout lines. Leaves of 6-week-old plants were inoculated with S. sclerotiorum. Photos were taken 48 h post-inoculation. (C) Lesion area on leaves of BnWRKY70 overexpression lines. ** indicate that the means are statistically different (p < 0.01).
3. Discussion
Many researchers have reported that the CRISPR/Cas9 system mediates targeted genome editing in plants [11,29,30,61,62]. The efficiency of mutations varies depending on the species and constructions of Cas9/sgRNA [22,63]. Ma et al. [64] believed that selection of target with GC contents of approximately 50–70% and with minimal or no base pairing with the sgRNA sequence is desirable. The targets we designed followed these guidelines.
In this research, we demonstrated that the CRISPR/Cas9 system can be an effective tool for multiplex genome editing in B. napus. As an allotetraploid crop, B. napus carries two or more copies of one gene in most cases. Thus, multiplex genome editing is necessary for gene knockout plants. Here, we designed 6 targets and constructed 2 gene knockout vectors targeting 6 loci of the BnWRKY11 and BnWRKY70 genes. Although both of the Cas9/sgRNA constructions we generated introduced genome editing in T0 transgenic B. napus plants, 2 of the sgRNAs were nonfunctional. Both of the sgRNAs were driven by AtU6-1, while other four were driven by AtU6-29 and AtU3b respectively. If not functioning of the sgRNAs was caused by AtU6-1 promoter need to be confirmed by further experiment. Changing the sgRNA promoters to the B. napus endogenous promoters and prescreening for functional and efficient sgRNAs might be good solutions to this problem [63].
Three or more independent editing events occurred at the WRKY11-Tgt2 target site BnaC.WRKY11.a in some CRI-W11 plants. This result indicated that the regenerated plants were chimeras or the Cas9/sgRNA complexes functioned weakly and continuously after the plant was regenerated from callus. Only one allele of BnaC.WRKY11.a in CRI-W11-37 showed targeted genomic deletion even though many mutagenesis occurred at the WRKY11-Tgt2 and WRKY11-Tgt3 target sites simultaneously. Eight BnWRKY11 transformants with both loci mutated were generated and two BnWRKY70 transformants with two loci (BnaA.WRKY11.a and BnaA.WRKY11.b) mutated were obtained in T0 plants. This probably because the number of transgenic plants we obtained was insufficient. Nonetheless, mutagenesis might be induced during the growth of plants and all-knockout plants could be generated by selfing or hybridization of transgenic plants for the T1 generation. Considering the existence of nonfunctional sgRNA, multiple sgRNAs designed to a given gene are highly recommended for successful editing of targeted genes.
Theoretically, the CRISPR/Cas9 system should continuously function as it exists in a cell until the WT alleles undergo mutation. In our research, we found that the number of editing events induced by the CRISPR/Cas9 system was lower in T0 transgenic plants than in T1 plants. This result is in accordance with the inference, considering the continuous functioning of CRISPR/Cas9 component, the T0 plants should have been developed into complicated chimeras at adult stage. But, when the DNA was sampled from leaves at seedling stage, the editing events detected in T0 plants do not complete the genotype of the chimeric plants. Hence, this can explain the detection of new editing events in CRI-W70-10-2 and CRI-W70-10-3 plants, which the CRISPR/Cas9 component were crossed out, showing that the mutations might have been inherited from CRI-W70-10 plants. In addition, the frequent appearance of chimeras in T1 plants indicated that most of the mutations occurred after the seed development.
For the transgenic plants with unedited targeted homoeolog(s), screening for plants containing CRISPR/Cas9 component during breeding for continuous editing could be a feasible approach.
Extensive evidence has shown that suppression of the expression of specific genes through RNAi silencing or T-DNA insertion alters the sensitivity to pathogens in plants [49,51,65,66]. Therefore, changing the expression levels of genes could be an effective means to study their functions in disease resistance or for breeding new disease-resistant varieties. Previous studies have found that WRKY70 is involved in the regulation of leaf senescence [67,68] and BR signaling processes [69] and can participate in plant immune processes by regulating important members of the JA and SA signaling pathways in the plant defense response in Arabidopsis [50,70,71,72]. In the present study, except for BnaC.WRKY70.a, the other three copies of BnWRKY70 were mutated in the T1 plants of CRI-W70 that we tested. Although homozygous BnWRKY70 knockout mutants were not obtained in T1 generation, mutations of each copy were either homozygous or biallelic for those plants that contain Cas9/sgRNA component, even though in some samples the mutations were chimeric. S. sclerotiorum infection tests demonstrated that the BnWRKY70 mutants increased resistance to S. sclerotiorum. To confirm the negative effects of BnWRKY70 in S. sclerotiorum resistance, we constructed BnWRKY70 overexpression plants. Infection test demonstrated that BnWRKY70-overexpressing plants showed a more sensitive phenotype, indicating that the BnWRKY70 gene may play a negative regulatory role in the response to S. sclerotiorum in B. napus. The molecular mechanism of how the BnWRKY70 gene participates in the disease resistance of rapeseed remains to be further studied.
Because off-targeting has rarely been reported in plants [30,63,73,74], off-target effects were not studied in this study. The risk of off-targeting in transgenic plants that are generated by Agrobacterium-mediated transformation could be much lower than in animal cells because the copies of imported foreign genes are fewer in plant cells. Moreover, the targets we designed were highly conserved (data not shown) in the seed sequences [5]. Beyond that, unwanted off-target mutations in plants could be eliminated by crossing the mutant plants with their parental lines [64].
In summary, we demonstrated that the CRISPR/Cas9 system is an effective tool for multiple genome editing in B. napus. The efficiencies of different sgRNA-induced mutations vary greatly and the mutation types and frequencies induced by CRISPR/Cas9 in B. napus are similar to those in Arabidopsis and rice. Targeted editing of the pathogenic gene can change the defense response in B. napus to pathogens. Therefore, the CRISPR/Cas9 system is useful for both basic research and disease resistance breeding in B. napus.
4. Materials and Methods
4.1. Target Design and Vector Construction for Targeted Gene Mutation
The CRISPR/Cas9-related vectors we used in this research included a CRISPR/Cas9 binary vector and several sgRNA vectors provided by Yaoguang Liu (South China Agricultural University, Guangzhou). The target sequences used to generate sgRNA expression cassettes were selected with the assistance of an online tool called the Optimized CRISPR Plant Design Tool (http://cbi.hzau.edu.cn/cgi-bin/CRISPR) [75] and by referring to common rules [7,75,76]. sgRNA folding was predicted with RNA Folding Form (version 2.3, Energies) [77].
The minimum amount of base pairing formed between the target sequence and sgRNA scaffold or the target sequence itself was selected for genome editing. When the selected target sequences started with the nucleotides “C” or “T”, an extra “A” or “G” nucleotide was added at the 5′ end of the target sequence. To test whether multiple targeted editing can be induced simultaneously by the CRISPR/Cas9 system in transgenic B. napus plants, we created 2 and 3 gRNA expression cassettes targeting the exon of different copies of BnWRKY11 and BnWRKY70, respectively. In each copy of BnWRKY11 and BnWRKY70, we selected one or two targeting site(s) and designed sgRNAs to target these sites (listed in Table S1). All the target sequences were located in the exon of the open reading frame [78], except for WRKY11-Tgt3, which was located across an exon and an intron.
For mutant identification, we designed one primer pair to amplify a specific locus in most cases or two loci if the identities of two sequences are too similar to distinguish and if the sequences before the target sites share the same length. The construction of CRISPR/Cas9 vectors containing Cas9 and multiple sgRNA expression cassettes followed the procedure described previously [64]. Briefly, double-stranded target sequences were introduced to the sgRNA expression cassettes by overlapping PCR. Then, the purified PCR products were integrated into pLYCRISPR/Cas9P35S-N by a Golden Gate clone [79]. The Cas9/sgRNA constructions were directly used to transform E. coli competent cells. The positive colonies were selected for sequence identification. The expression of sgRNAs was driven by the AtU3 and/or AtU6 promoter. The ORF of the Cas9 gene was Gramineae codon optimized and driven by the cauliflower mosaic virus 35S promoter (P35S). The CRISPR/Cas9 constructs were introduced to the Agrobacterium tumefaciens strain GV3101 through the freezing and thawing method.
4.2. Genetic Transformation of B. napus
B. napus line “J9712” was used as the receptor, which was kindly provided by Professor Yongming Zhou (National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University). Transformation of B. napus was performed as described by De Block et al. [80] with some modification. Briefly, certified, uniform, healthy seeds were surface sterilized with a sodium hypochlorite solution and subsequently rinsed in sterile distilled water. The seeds were germinated on 1/2 MS basal medium with 2% sucrose in darkness. The seedlings were grown at 25 °C in the dark for seven days. Afterward, the hypocotyl (~15 mm) was cut and the explants were made to float in an infection medium [MS medium supplemented with 3% sucrose and 100 μM acetosyringone (AS); pH 5.8] for 20 min. Then, the explants were transferred to a co-cultivation medium (MS medium supplemented with 3% sucrose, 1 mg/L of 2,4-D, 0.3 mg/L of kinetin, 100 μM of AS, 5 mg/L of AgNO3 and 8 g/L of agar; pH 5.8) for 3 days. Subsequently, the explants were transferred to a callus induction medium [MS medium supplemented with 3% sucrose, 1 mg/L of 2,4-D, 0.3 mg/L of kinetin, 5 mg/L of AgNO3, 500 mg/L of cefotaxime (Cef), 25 mg/L of G418 and 8 g/L of agar; pH 5.8] and incubated at 25 °C. The explants were then transferred to a shoot differentiation medium (MS medium supplemented with 1% glucose, 100 μM of AgNO3, 2.0 mg/L of zeatin, 0.1 mg/L of IAA, 500 mg/L of Cef, 25 mg/L of G418 and 8 g/L of agar; pH 5.8) until shoots initialized. Finally, healthy green shoots were transferred to bottles containing a root initiation medium (MS medium supplemented with 1% sucrose and 8 g/L of agar; pH 5.8). Plantlet acclimatization and establishment were performed. The BnWRKY70 gene BnaC08g27340D (BnaC.WRKY70.b) was cloned for overexpression and P35S:BnWRKY70-GFP was constructed to generate BnWRKY70 overexpression plants. The binary expression vector pMDC83 (see vector map on Figure S3) was used in this research.
4.3. Mutation Analysis
Genomic DNA was extracted from the transgenic B. napus plants and wild-type plants using the hexadecyl trimethyl ammonium bromide (CTAB) method [81]. We designed the PCR primers in the flanking region of the Cas9/sgRNA targets and analyzed the targeted mutagenesis by PCR amplification and Sanger sequencing. PCR was performed using Phanta Max Super-Fidelity DNA Polymerase (Vazyme, Nanjing, China). For the regenerated plants, the presence of CRISPR/Cas9 constructs was investigated by PCR with Cas9 gene primers. For the transformed B. napus plants, the DNA fragments spanning the Cas9/gRNA target sequences were amplified by PCR and the products were analyzed by TA cloning and sequencing. The primers used for PCR amplification are listed in Table S2.
4.4. S. sclerotiorum Infection Assay
B. napus plants were grown in a field of the experimental farm of Yangzhou University, Jiangsu, China. The S. sclerotiorum (Lib.) de Bary isolate SS-1 was maintained and cultured on potato dextrose agar (PDA) medium [82]. The uniform agar disk with fungal hyphae was placed on detached leaf surface of 6-week-old B. napus plants. During inoculation, leaves were kept in a growth tray with a transparent cover to maintain high humidity. The inoculated leaves were transferred to a growth chamber and the lesion sizes were measured at 48-h post-inoculation as descripted in Wu et al. [82].
Acknowledgements
We sincerely thank Professor Yaoguang Liu (South China Agricultural University, Guangzhou, China) for providing the CRISPR/Cas9 binary vector and several sgRNA vectors.
Abbreviations
| CRISPR | Clustered, regularly interspaced, palindromic repeat |
| sgRNA | Single guide RNA |
| PAM | Protospacer adjacent motif |
| ZFN | Zinc finger nucleases |
| TALEN | Transcription activator-like effector nucleases |
| TFs | Transcription factors |
| hpi | hours post-inoculation |
| AS | Acetosyringone |
| Cef | Cefotaxime |
| CTAB | Hexadecyl trimethyl ammonium brom |
Supplementary Materials
The supplementary materials are available online at http://www.mdpi.com/1422-0067/19/9/2716/s1.
Author Contributions
J.W. and Y.W. designed the experiment and refined the research; Q.S. drafted the manuscript; Q.S., L.L. and D.L. carried out the experiments and analyzed the data; D.W. and Y.F. writing-review & editing the manuscript. All authors discussed the results and comments on the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2016YFD0102000), National Key Basic Research Program of China (2015CB150201), National Natural Science Foundation of China (31330057, 31601330), China Postdoctoral Science Foundation (2015M581867, 2016T90514) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ishino Y., Shinagawa H., Makino K., Amemura M., Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli and identification of the gene product. J. Bacteriol. 1987;169:5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P., Moineau S., Mojica F.J., Wolf Y.I., Yakunin A.F., et al. Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison M.M., Jenkins B.V., O’Connor-Giles K.M., Wildonger J. A CRISPR view of development. Genes Dev. 2014;28:1859–1872. doi: 10.1101/gad.248252.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barakate A., Stephens J. An overview of CRISPR-based tools and their improvements: New opportunities in understanding plant-pathogen interactions for better crop protection. Front. Plant Sci. 2016;7:765. doi: 10.3389/fpls.2016.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malina A., Mills J.R., Cencic R., Yan Y.F., Fraser J., Schippers L.M., Paquet M., Dostie J., Pelletier J. Repurposing CRISPR/Cas9 for in situ functional assays. Genes Dev. 2013;27:2602–2614. doi: 10.1101/gad.227132.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortesi L., Fischer R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015;33:41–52. doi: 10.1016/j.biotechadv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Bassett A.R., Tibbit C., Ponting C.P., Liu J.L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan J.Z., Lu G.Q., Xie Z., Lou M.L., Luo J., Guo L., Zhang Y. Genome-wide identification of CRISPR/Cas9 off-targets in human genome. Cell Res. 2014;24:1009–1012. doi: 10.1038/cr.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho T.T., Zhou N., Huang J., Koirala P., Xu M., Fung R., Wu F., Mo Y.Y. Targeting non-coding RNAs with the CRISPR/Cas9 system in human cell lines. Nucleic Acids Res. 2014;43:e47. doi: 10.1093/nar/gku1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belhaj K., Chaparro-Garcia A., Kamoun S., Patron N.J., Nekrasov V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015;32:76–84. doi: 10.1016/j.copbio.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Minkenberg B., Xie K.B., Yang Y.N. Discovery of rice essential genes by characterizing a CRISPR-edited mutation of closely related rice MAP kinase genes. Plant J. 2017;89:636–648. doi: 10.1111/tpj.13399. [DOI] [PubMed] [Google Scholar]
- 13.Arazoe T., Miyoshi K., Yamato T., Ogawa T., Ohsato S., Arie T., Kuwata S. Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol. Bioeng. 2015;112:2543–2549. doi: 10.1002/bit.25662. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y., Chen B., Duan C.L., Sun B.B., Yang J.J., Yang S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2015;81:2506–2514. doi: 10.1128/AEM.04023-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W.Z., Yang B., Weeks D.P. Efficient CRISPR/Cas9-mediated gene editing in Arabidopsis thaliana and inheritance of modified genes in the T2 and T3 generations. PLoS ONE. 2014;9:e99225. doi: 10.1371/journal.pone.0099225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J.P., Wang G.H., Ma S.Y., Xie X.D., Wu X.W., Zhang X.T., Wu Y.Q., Zhao P., Xia Q.Y. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 2015;87:99–110. doi: 10.1007/s11103-014-0263-0. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama T., Fish M.B., Fisher M., Oomen-Hajagos J., Thomsen G.H., Grainger R.M. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ron M., Kajala K., Pauluzzi G., Wang D., Reynoso M.A., Zumstein K., Garcha J., Winte S., Masson H., Inagaki S., et al. Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol. 2014;166:455–469. doi: 10.1104/pp.114.239392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao A., Wang Z.X., Hu Y.Y., Wu Y.D., Luo Z., Yang Z.P., Zu Y., Li W.Y., Huang P., Tong X.J., et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 2013;41:e141. doi: 10.1093/nar/gkt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H.B., Liu B., Weeks D.P., Spalding M.H., Yang B. Large chromosomal deletions and heritable small genetic changes induced by CRISPR/Cas9 in rice. Nucleic Acids Res. 2014;42:10903–10914. doi: 10.1093/nar/gku806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W.Z., Zhou H.H., Bi H.H., Fromm M., Yang B., Weeks D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013;41:e188. doi: 10.1093/nar/gkt780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z.P., Xing H.L., Dong L., Zhang H.Y., Han C.Y., Wang X.C., Chen Q.J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015;16:144. doi: 10.1186/s13059-015-0715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Z.Y., Mao Y.F., Xu N.F., Zhang B.T., Wei P.L., Yang D.L., Wang Z., Zhang Z.J., Zheng R.Z., Yang L., et al. Multigeneration analysis reveals the inheritance, specificity and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111:4632–4637. doi: 10.1073/pnas.1400822111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn F., Mantegazza O., Greiner A., Hegemann P., Eisenhut M., Weber A.P. An efficient visual screen for CRISPR/Cas9 activity in Arabidopsis thaliana. Front. Plant Sci. 2017;8:39. doi: 10.3389/fpls.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan Q.W., Wang Y.P., Li J., Gao C.X. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014;9:2395–2410. doi: 10.1038/nprot.2014.157. [DOI] [PubMed] [Google Scholar]
- 26.Nieves-Cordones M., Mohamed S., Tanoi K., Kobayashi N.I., Takagi K., Vernet A., Guiderdoni E., Périn C., Sentenac H., Véry A.-A. Production of low-Cs+ rice plants by inactivation of the K+ transporter OsHAK1 with the CRISPR-Cas system. Plant J. 2017;91:43–56. doi: 10.1111/tpj.13632. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Liang Z., Zong Y., Wang Y.P., Liu J.X., Chen K.L., Qiu J.L., Gao C.X. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016;7:12617. doi: 10.1038/ncomms12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrenson T., Shorinola O., Stacey N., Li C.D., Ostergaard L., Patron N., Uauy C., Harwood W. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 2015;16:258. doi: 10.1186/s13059-015-0826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braatz J., Harloff H.J., Mascher M., Stein N., Himmelbach A., Jung C. CRISPR-Cas9 targeted mutagenesis leads to simultaneous modification of different homoeologous gene copies in polyploid oilseed rape (Brassica napus) Plant Physiol. 2017;174:935–942. doi: 10.1104/pp.17.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H., Wu J.J., Tang T., Liu K.D., Dai C. CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci. Rep. 2017;7:7489. doi: 10.1038/s41598-017-07871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y., Zhu K.Y., Li H.L., Han S.Q., Meng Q.W., Khan S.U., Fan C.C., Xie K.B., Zhou Y.M. Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 2018;16:1322–1355. doi: 10.1111/pbi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., Hao M.Y., Wang W.X., Wang H., Chen F., Chu W., Zhang B.H., Mei D.S., Cheng H.T., Hu Q. An efficient CRISPR/Cas9 platform for rapidly generating simultaneous mutagenesis of multiple gene homoeologs in allotetraploid oilseed rape. Front. Plant Sci. 2018;9:442. doi: 10.3389/fpls.2018.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuzaki A., Ogawa T., Koizuka C., Kaneko K., Inaba M., Imamura J., Koizuka N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol. Biochem. 2018;131:63–69. doi: 10.1016/j.plaphy.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Saharan G.S., Mehta N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management. Springer; Heidelberg, Germany: 2008. pp. 1–485. [Google Scholar]
- 35.Wu J., Zhao Q., Liu S., Shahid M., Lan L., Cai G.Q., Zhang C.Y., Fan C.C., Wang Y.P., Zhou Y.M. Genome-wide association study identifies new loci for resistance to Sclerotinia stem rot in Brassica napus. Front. Plant Sci. 2016;7:1418. doi: 10.3389/fpls.2016.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eulgem T., Rushton P.J., Robatzek S., Somssich I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 37.Ulker B., Somssich I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Eulgem T., Somssich I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007;10:366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Pandey S.P., Somssich I.E. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150:1648–1655. doi: 10.1104/pp.109.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishihama N., Yoshioka H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 2012;15:431–437. doi: 10.1016/j.pbi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J.J., Ma S.H., Ye N.H., Jiang M., Cao J.S., Zhang J.H. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017;59:86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- 42.Chen L.G., Zhang L.P., Li D.B., Wang F., Yu D.Q. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2013;110:E1963–E1971. doi: 10.1073/pnas.1221347110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Journot-Catalino N., Somssich I.E., Roby D., Kroj T. The transcription factors WRKY11 and WRKY17 act as negative regulators of basal resistance in Arabidopsis thaliana. Plant Cell. 2006;18:3289–3302. doi: 10.1105/tpc.106.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao G.H., Meng X.Z., Liu Y.D., Zheng Z.Y., Chen Z.X., Zhang S.Q. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell. 2011;23:1639–1653. doi: 10.1105/tpc.111.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sham A., Moustafa K., Al-Shamisi S., Alyan S., Iratni R., AbuQamar S. Microarray analysis of Arabidopsis WRKY33 mutants in response to the necrotrophic fungus Botrytis cinerea. PLoS ONE. 2017;12:e0172343. doi: 10.1371/journal.pone.0172343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K.C., Lai Z.B., Fan B.F., Chen Z.X. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20:2357–2371. doi: 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheikh A.H., Eschen-Lippold L., Pecher P., Hoehenwarter W., Sinha A.K., Scheel D., Lee J. Regulation of WRKY46 transcription factor function by mitogen-activated protein kinases in Arabidopsis thaliana. Front. Plant Sci. 2016;7:61. doi: 10.3389/fpls.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y.R., Dong Q.Y., Yu D.Q. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci. 2012;185–186:288–297. doi: 10.1016/j.plantsci.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Brader G., Palva E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shim J.S., Jung C., Lee S., Min K., Lee Y.W., Choi Y., Lee J.S., Song J.T., Kim J.K., Choi Y.D. AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling. Plant J. 2013;73:483–495. doi: 10.1111/tpj.12051. [DOI] [PubMed] [Google Scholar]
- 51.Jiang C.H., Huang Z.Y., Xie P., Gu C., Li K., Wang D.C., Yu Y.Y., Fan Z.H., Wang C.J., Wang Y.P., et al. Transcription factors WRKY70 and WRKY11 served as regulators in rhizobacterium Bacillus cereus AR156-induced systemic resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. J. Exp. Bot. 2016;67:157–174. doi: 10.1093/jxb/erv445. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z., Fang H.D., Chen Y., Chen K.P., Li G.Y., Gu S.L., Tan X.L. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol. Plant Pathol. 2014;15:677–689. doi: 10.1111/mpp.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu S.A., Kracher B., Ziegler J., Birkenbihl R.P., Somssich I.E. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife. 2015;4:e07295. doi: 10.7554/eLife.07295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu F., Li X.X., Wang M.R., Wen J., Yi B., Shen J.X., Ma C.Z., Fu T.D., Tu J.X. Interactions of WRKY15 and WRKY33 transcription factors and their roles in the resistance of oilseed rape to Sclerotinia infection. Plant Biotechnol. J. 2018;16:911–925. doi: 10.1111/pbi.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman H., Raman R., Coombes N., Song J., Diffey S., Kilian A., Lindbeck K., Barbulescu D.M., Batley J., Edwards D., et al. Genome-wide association study identifies new loci for resistance to leptosphaeria maculans in canola. Front. Plant Sci. 2016;7:1513. doi: 10.3389/fpls.2016.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J., Zhao Q., Yang Q.Y., Liu H., Li Q.Y., Yi X.Q., Cheng Y., Guo L., Fan C.C., Zhou Y.M. Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci. Rep. 2016;6:19007. doi: 10.1038/srep19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Initiative A.G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 58.Chalhoub B., Denoeud F., Liu S., Parkin I.A.P., Tang H., Wang X., Chiquet J., Belcram H., Tong C., Samans B., et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- 59.Ostergaard L., King G.J. Standardized gene nomenclature for the Brassica genus. Plant Methods. 2008;4:10. doi: 10.1186/1746-4811-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brinkman E.K., Chen T., Amendola M., van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168. doi: 10.1093/nar/gku936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain M. Function genomics of abiotic stress tolerance in plants: A CRISPR approach. Front. Plant Sci. 2015;6:375. doi: 10.3389/fpls.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang G., Zhang H.M., Lou D.J., Yu D.Q. Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci. Rep. 2016;6:21451. doi: 10.1038/srep21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J.J., Song N., Sun S.L., Yang W.L., Zhao H.M., Song W.B., Lai J.S. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genomics. 2016;43:25–36. doi: 10.1016/j.jgg.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Ma X.L., Zhang Q.Y., Zhu Q.L., Liu W., Chen Y., Qiu R., Wang B., Yang Z.F., Li H.Y., Lin Y.R., et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Encinas-Villarejo S., Maldonado A.M., Amil-Ruiz F., de los Santos B., Romero F., Pliego-Alfaro F., Munoz-Blanco J., Caballero J.L. Evidence for a positive regulatory role of strawberry (Fragaria x ananassa) Fa WRKY1 and Arabidopsis At WRKY75 proteins in resistance. J. Exp. Bot. 2009;60:3043–3065. doi: 10.1093/jxb/erp152. [DOI] [PubMed] [Google Scholar]
- 66.Lai Z.B., Wang F., Zheng Z.Y., Fan B.F., Chen Z.X. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011;66:953–968. doi: 10.1111/j.1365-313X.2011.04553.x. [DOI] [PubMed] [Google Scholar]
- 67.Ulker B., Shahid Mukhtar M., Somssich I.E. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta. 2007;226:125–137. doi: 10.1007/s00425-006-0474-y. [DOI] [PubMed] [Google Scholar]
- 68.Besseau S., Li J., Palva E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012;63:2667–2679. doi: 10.1093/jxb/err450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J.N., Nolan T., Ye H.X., Zhang M.C., Tong H.N., Xin P.Y., Chu J.F., Chu C.C., Li Z.H., Yin Y.H. Arabidopsis WRKY46, WRKY54 and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought response. Plant Cell. 2017;29:1425–1439. doi: 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J., Brader G., Kariola T., Palva E.T. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006;46:477–491. doi: 10.1111/j.1365-313X.2006.02712.x. [DOI] [PubMed] [Google Scholar]
- 71.Ren C.M., Zhu Q., Gao B.D., Ke S.Y., Yu W.C., Xie D.X., Peng W. Transcription factor WRKY70 displays important but no indispensable roles in jasmonate and salicylic acid signaling. J. Integr. Plant Biol. 2008;50:630–637. doi: 10.1111/j.1744-7909.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 72.Zhou M., Lu Y., Bethke G., Harrison B.T., Hatsugai N., Katagiri F., Glazebrook J. WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. N. Phytol. 2017;217:700–713. doi: 10.1111/nph.14846. [DOI] [PubMed] [Google Scholar]
- 73.Mahfouz M.M., Piatek A., Stewart C.N., Jr. Genome engineering via TALENs and CRISPR/Cas9 systems: Challenges and perspectives. Plant Biotechnol. J. 2014;12:1006–1014. doi: 10.1111/pbi.12256. [DOI] [PubMed] [Google Scholar]
- 74.Feng C., Yuan J., Wang R., Liu Y., Birchler J.A., Han F.P. Efficient targeted genome modification in maize using CRISPR/Cas9 system. J. Genet. Genomics. 2016;43:37–43. doi: 10.1016/j.jgg.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Lei Y., Lu L., Liu H.Y., Li S., Xing F., Chen L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant. 2014;7:1494–1496. doi: 10.1093/mp/ssu044. [DOI] [PubMed] [Google Scholar]
- 76.Miao J., Guo D.S., Zhang J.Z., Huang Q.P., Qin G.J., Zhang X., Wan J.M., Gu H.Y., Qu L.J. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013;23:1233–1236. doi: 10.1038/cr.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rietz S., Bernsdorff F.E., Cai D.G. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 2012;63:5507–5519. doi: 10.1093/jxb/ers203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engler C., Kandzia R., Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Block M., De Brouwer D., Tenning P. Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 1989;91:694–701. doi: 10.1104/pp.91.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray M.G., Thompson W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J., Cai G.Q., Tu J.Y., Li L.X., Liu S., Luo X.P., Zhou L.P., Fan C.C., Zhou Y.M. Identification of QTLs for resistance to sclerotinia stem rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus. PLoS ONE. 2013;8:e67740. doi: 10.1371/journal.pone.0067740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.