Abstract
Biofilm formation in healthcare is an issue of considerable concern, as it results in increased morbidity and mortality, imposing a significant financial burden on the healthcare system. Biofilms are highly resistant to conventional antimicrobial therapies and lead to persistent infections. Hence, there is a high demand for novel strategies other than conventional antibiotic therapies to control biofilm-based infections. There are two approaches which have been employed so far to control biofilm formation in healthcare settings: one is the development of biofilm inhibitors based on the understanding of the molecular mechanism of biofilm formation, and the other is to modify the biomaterials which are used in medical devices to prevent biofilm formation. This review will focus on the recent advances in anti-biofilm approaches by interrupting the quorum-sensing cellular communication system and the multidrug efflux pumps which play an important role in biofilm formation. Research efforts directed towards these promising strategies could eventually lead to the development of better anti-biofilm therapies than the conventional treatments.
Keywords: biofilm formation, healthcare, biofilm inhibition, quorum sensing, multidrug efflux pumps
1. Introduction
Biofilms are surface-attached groups of microbial cells that are embedded in a self-produced extracellular matrix and are highly resistant to antimicrobial agents [1,2,3]. Biofilms can attach to all kinds of surfaces, including metals, plastics, plant and body tissue, medical devices and implant materials [4]. Biofilm formation on indwelling medical devices and implants such as heart valves, pacemakers, vascular grafts, catheters, prosthetic joints, intrauterine devices, sutures and contact lenses poses a critical problem of infection [5]. The use of intravascular catheters for patient care can give rise to central line-associated blood stream infection (CLABSI), and approximately 250,000 cases of primary blood stream infections are reported each year in the USA [6]. Thus, CLABSI results in significant morbidity and mortality and huge increases in healthcare costs. The bacteria most frequently associated with healthcare-associated infections include Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Acinetobacter spp. [5,7]. Among the biofilm-forming bacteria, S. aureus and S. epidermidis are predominantly isolated from cardiovascular devices [8,9]. It has been estimated that S. aureus and S. epidermidis contribute to 40–50% of prosthetic heart valve infections and 50–70% of the catheter biofilm infections [10]. In recent years, Acinetobacter spp. have emerged as the most important nosocomial pathogens involved in a variety of nosocomial infections, including bacteremia, urinary tract infection, soft-tissue infections and secondary meningitis [11,12,13,14]. The Acinetobacter spp. have the ability to colonize and form biofilms on medical devices such as implants, cardiac valves, artificial joints, catheters, etc. [11,15].
Biofilm formation is initiated when the cells attach and adhere to surfaces. The attachment of microbial cells to biomaterials can be facilitated by factors such as bacterial motility, increased shear forces, and hydrodynamic and electrostatic interactions between the microorganism and surface [16]. The adherence of bacteria to biomaterials through cell-surface and biomaterial-surface interactions is mediated by multiple factors, which include cell surface proteins, capsular polysaccharide/adhesin, protein autolysin, etc. [17,18]. For example, Staphylococcal species display cell-surface proteins, namely staphylococcal surface protein-1 and -2 (SSP-1 and SSP-2) [17], which are essential for adhesion of S. epidermidis to polystyrene [19]. In addition, host factors can also mediate the adherence of bacterial cells to implants, as the implant surfaces are usually covered by host plasma and other extracellular components [20]. Once attached to the surfaces, the bacterial cells will proliferate, aggregate and differentiate into biofilm structures [21]. Bacterial cells can detach from mature biofilms and spread to other organ systems, thereby contributing to persistent chronic infections [21,22].
Biofilms are complex structures with customized living environments with differing pH, nutrient availability and oxygen [23]. A worrying feature of biofilm-based infections is the increased tolerance of biofilm cells to biocides compared to planktonic bacteria [24]. The increased drug resistance could be attributed to plasmids containing genes for multidrug resistance, as biofilms form an ideal niche for plasmid exchange [25]. The mechanisms by which biofilms represent increased drug resistance also include slow or incomplete penetration of antimicrobial agents through the extracellular polymeric matrix, the formation of persister or dormant cells in a spore-like non-dividing state, slow growth rate of cells in the biofilm, thereby reducing the number of targets for antimicrobial molecules, etc. [26,27,28]. In addition to the difficulty in treating biofilm with conventional antimicrobial therapy, the treatment is further hindered by increased antibiotic resistance, as bacterial cells acquire resistance under antibiotic selective pressure [29]. For example, it has been reported that more than 70% of hospital isolates of S. epidermidis are methicillin resistant [30]. Thus, there is a high demand for alternative strategies to control biofilm-based infections other than antibiotic therapy. Considering the number of patients suffering from biofilm-based device-related infections, several strategies have been developed in the past few decades. This review will discuss the most successful antibiofilm approaches so far, as well as some of the more promising prospects for the control of these biofilm-based infections.
2. Strategies for the Control of Biofilms
There have been three major strategies considered so far to control biofilm formation or to target different stages of biofilm development. The first approach is inhibiting the initial attachment of bacteria to biofilm-forming surfaces, thereby reducing the chances of biofilm development. The second approach targets the disruption of biofilm during the maturation process [31]. The third strategy is the signal interference approach, in which the bacterial communication system or the quorum sensing (QS) system is interfered with as QS coordinates biofilm formation/maturation in pathogenic bacteria [32]. The different antibiofilm strategies and agents discussed in this review are summarized in Table 1.
Table 1.
Various strategies for the control of biofilms.
| Strategy | Methods/Agents | Examples | References |
|---|---|---|---|
| Inhibition of initial biofilm attachment | (i) Altering chemical properties of biomaterials | (i) Antibiotics, biocides, iron coatings | (i) [33,34,35,36,37,38,39,40,41,42,43] |
| (ii) Changing physical properties of biomaterials | (ii) Use of hydrophilic polymers, superhydrophobic coatings, hydrogel coatings, heparin coatings | (ii) [44,45,46,47,48,49] | |
| Removal of biofilms | (i) Matrix degrading enzymes | (i) Polysaccharide-degrading enzymes (Dispersin B, Endolysins); Nucleases (Deoxyribonuclease I) and Proteases (Proteinase K, trypsin) | (i) [50,51,52,53,54,55] |
| (ii) Surfactants | (ii) Sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), Tween 20 and Triton X-100, surfactin, rhamnolipids | (ii) [56,57,58,59,60] | |
| (iii) Free fatty acids, amino acids and nitric oxide donors | (iii) Cis-2-decenoic acid, d-amino acids, nitric oxide generators such as sodium nitroprusside (SNP), S-nitroso-l-glutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP) | (iii) [61,62,63,64] | |
| Biofilm inhibition by quorum quenching | (i) Degradation of QS signals | (i) Lactonases, acylases and oxidoreductases | (i) [65,66,67,68,69,70,71] |
| (ii) Inhibition of signal synthesis | (ii) Use of analogues of AHL precursor S-adenosyl-methionine (SAM), S-adenosyl-homocysteine (SAH), sinefugin, 5-methylthioadenosine (MTA), butyryl-SAM; SAM biosynthesis inhibitor cycloleucine, AHL synthesis inhibitors such as nickel and cadmium | (ii) [72,73,74,75,76,77,78,79] | |
| (iii) Antagonizing signal molecules | (iii) AHL analogues (bergamottin, dihydroxybergamottin, cyclic sulfur compounds, phenolic compounds including baicalin hydrate and epigallocatechin); AI-2 analogues (ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD); AIP analogues (cyclic peptides such as cyclo (l-Phe-l-Pro) and cyclo(l-Tyr-l-Pro), RNAIII inhibiting peptide (RIP) and its homologues) | (iii) [80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] | |
| (iv) Inhibition of signal transduction | (iv) Use of halogenated furanone or fimbrolide, cinnamaldehyde, virstatin | (iv) [97,98,99,100,101,102,103] | |
| (v) Inhibition of signal transport | (v) Use of copper or silver nanoparticles, Phe-Arg-β-naphthylamide (PAβN) | (v) [104,105,106] |
2.1. Inhibition of Initial Attachment
The initial attachment of cells to the biofilm-forming surfaces happens within an average of the first 2 days of biofilm formation. Inhibition of initial attachment of cells to the surfaces is a potential strategy to prevent biofilm formation rather than targeting the dispersal of established biofilms. The attachment of bacteria to surfaces is mediated by several factors, including adhesion surface proteins, pili or fimbriae, and exopolysaccharides [107,108]. The surfaces that are rough, coated with surface conditioning films and more hydrophobic are prone to ease biofilm formation [109,110,111]. Thus, the initial attachment of cells can be prevented by altering the chemical or physical properties of indwelling medical devices.
2.1.1. By Altering the Chemical Properties of Biomaterials
The commonly used chemical methods to modify the surface of biomedical devices in order to prevent biofilm formation include antibiotics, biocides and ion coatings [33]. Catheters coated with antibiotics such as minocycline and rifampin have been shown to decrease the incidence of biofilm-associated bloodstream infection by S. aureus in healthcare [34]. In addition, catheters impregnated with different antibiotics, including nitrofurazone, gentamicin, norfloxacin, etc., are suggested to have a role in preventing biofilm-associated urinary tract infections [35].
High-throughput screening of chemical libraries has led to the identification of several small chemical molecules as potential drug candidates for controlling biofilm formation and infection. These molecules do not elicit antimicrobial activity, and thus decrease the likelihood of the development of resistance due to the absence of selective pressure against biofilm formation. In Streptococcus pyogenes and S. aureus, a series of small molecules inhibited the expression of many key virulence factors that are involved in biofilm formation and infection [112,113]. The early stages of biofilm formation in S. aureus, S. epidermidis and E. faecalis were inhibited by several aryl rhodamines [114]. In Vibrio cholerae, small molecules inhibited the induction of cyclic di-GMP, which is a second messenger controlling the switch between planktonic and sessile lifestyle of bacteria [115,116]. In addition, N-acetylcysteine, a mucolytic agent, was reported to inhibit the production of exopolysaccharides in biofilms in S. epidermidis [36].
Several antimicrobial peptides are also known to interfere with biofilm formation in different bacterial pathogens. For example, peptide 1018 is considered to be a biofilm inhibitor in P. aeruginosa, E. coli, A. baumannii, K. pneumoniae, S. aureus, Salmonella typhimurium, Burkholderia cenocepacia [37]. In addition, lantibiotics (nisin, subtilin, epidermin and gallidermin), a class of peptide antibiotics, are reported to inhibit biofilm formation in S. aureus, Lactococcus lactis and S. epidermidis [38,39].
Chelators that interfere with the function of metal ions in biofilm formation are also considered to be biofilm inhibitors [117]. Metallic silver, silver salts, and silver nanoparticles have been widely used as antimicrobial agents in medical implants against bacteria such as E. coli, S. aureus, Klebsiella species, P. aeruginosa, S. typhimurium, and Candida albicans [118,119]. The silver treatment inhibits the replication of DNA, expression of ribosomal and cellular proteins, and respiration process, leading to cell death [40,41,42]. It has been reported that silver ion-coated implants inhibited S. aureus biofilm formation without causing silver accumulation in host tissues [120]. In addition, in the presence of nanoparticles, antibiotics such as penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin displayed increased antibacterial activity against S. aureus [121].
The antibacterial agent coatings on medical devices are typically effective for a short time period due to the leaching of the agent over the course of time [33]. Thus, the immobilization of antimicrobial agents on device surfaces using long, flexible polymeric chains has been an effective contribution in controlling biofilm formation in the long run. For example, the attachment of N-alkylpyridinium bromide, an antibacterial agent, to a polymer, poly(4-vinyl-N-hexylpyridine) was capable of inactivating 99% of S. epidermidis, E. coli, and P. aeruginosa on medical devices [43].
2.1.2. By Changing the Physical Properties of Biomaterials
Biofilm formation begins with a weak reversible adhesion of bacterial cells to the surface of medical devices. If bacteria are not immediately detached from the surface of devices, they anchor permanently, using cell adhesion structures such as pili, and form biofilms [44]. Hydrophobicity and surface charge of implant materials play an important role in determining the ability of bacteria to anchor to surfaces [43]. Thus, modification of the surface charge and hydrophobicity of polymeric materials using several backbone compounds and antimicrobial agents has proven to be effective for biofilm prevention [43]. Hydrophilic polymers such as hyaluronic acid [45] and poly N-vinylpyrrolidone [46] on polyurethane catheters and silicone shuts, respectively, have been known to reduce the adhesion of S. epidermidis. In addition, various hydrogel coatings which reduce bacterial adhesion due to their hydrophilic properties have also been developed especially for ureteral stents [47]. Superhydrophobic surfaces are reported to reduce bacterial adhesion and biofilm formation due to their extremely low wettability [49,122,123]. Tang et al. observed reduced adherence of S. aureus on superhydrophobic titanium surfaces [124]. Also, the adhesion of S. aureus and P. aeruginosa was significantly reduced on superhydrophobic fluorinated silica coating [125]. Crick et al. demonstrated reduced adhesion of S. aureus and E. coli on AACVD (aerosol assisted chemical vapor deposition)-coated superhydrophobic surfaces compared to uncoated plain glass [123]. It has been reported that heparin interferes with bacterial adhesion and colonization [48]. The heparin coating makes the vascular catheter negatively charged, thereby preventing thrombosis and microbial colonization, eventually contributing to reduction of catheter-related infections [126,127]. Surface roughness can also influence biofilm formation, as rough, high-energy surfaces are more conducive to biofilm formation compared to smooth surfaces [128]. It is noted that the surface roughness can alter the hydrophobicity, thus in turn affecting bacterial adherence [128].
2.2. Biofilm Removal
Mature biofilms are highly tolerant to antimicrobials due to the altered growth rate of cells in the biofilm and the emergence of resistant subpopulations [129,130]. Also, biofilms favor the horizontal transfer of antibiotic resistance genes among cells [131]. Thus, it is of utmost importance to understand the antibiotic resistance properties of strains in biofilms when designing new drug treatments. Though conventional antibiotics have been proven to be critical in eliminating bacterial pathogens, they extensively damage the host microbiota, making the environment favorable for opportunistic pathogens. Hence, the agents that interfere with the initial biofilm development or biofilm structure have great potential in controlling biofilm-related infections.
2.2.1. Matrix-Degrading Enzymes
The biofilm matrix is usually composed of exopolysaccharides (EPS), extracellular DNAs (eDNAs), and proteins [132,133,134]. The EPS and eDNAs contribute to antibiotic resistance by preventing the diffusion of antimicrobials or by inducing antibiotic resistance [135,136]. Dissociation of the biofilm matrix is an effective antibiofilm approach, as the matrix accounts for more than 90% of dry mass, and dissociation of the same will expose the sessile cells to antibiotics and host immune defence [137]. Biofilm matrix-degrading enzymes fall into three categories: polysaccharide-degrading enzymes, nucleases and proteases [50]. Dispersin B is a bacterial glycoside hydrolase produced by Actinobacillus actinomycetemcomitans which hydrolyzes poly-N-acetylglucosamine (PNAG), a major matrix exopolysaccharide of Staphylococcus spp. and E. coli [138]. In addition, the application of Dispersin B in combination with triclosan effectively reduced biofilm formation in S. aureus, S. epidermidis, and E. coli [51]. Endolysins, a class of peptidoglycan hydolases produced by bacteriophages are reported to digest the cell wall of bacteria thereby disrupting biofilms [52]. Deoxyribonuclease I which is capable of digesting eDNA is known to disperse biofilms in several bacteria including Staphylococcus strains, A. baumannii, E. coli, Haemophilus influenzae, Klebsiella pneumoniae, Psuedomonas aeruginosa, etc. [53,54]. The matrix proteins can be effectively cleaved by Proteinase K contributing to biofilm prevention and biofilm dispersal [55]. It was demonstrated that the treatment with dispersin B followed by Proteinase K or trypsin successfully eradicated Staphylococcus biofilms [55]. The in vivo application of matrix-degrading enzymes is limited, as the treatment can elicit inflammatory and allergic reactions in the host against these enzymes [139].
2.2.2. Surfactants
Surfactants are reported to have antimicrobial and antibiofilm activities [140]. The surfactants sodium dodecyl sulfate (SDS), cetyltrimethylammonium bromide (CTAB), Tween 20 and Triton X-100 are known to promote either biofilm dispersal or detachment [56,57,58]. A biosurfactant, surfactin, which is a cyclic lipopeptide produced by B. subtilis, is reported to inhibit biofilm formation and induce biofilm dispersal in S. typhimurium, E. coli and P. mirabilis [59]. Rhamnolipids are principal glycolipids produced by many bacteria, including P. aeruginosa, and cause biofilm dispersal in a number bacterial strains [57,60].
2.2.3. Free Fatty Acids, Amino Acids and Nitric Oxide Donors
Free fatty acids are shown to have antibiofilm activity against several pathogenic bacteria. It was reported that P. aeruginosa produces an organic compound cis-2-decenoic acid which is capable of dispersing the already established biofilms by E. coli, K. pneumoniae, P. mirabilis, S. pyogenes, B. subtilis, S. aureus, and C. albicans [61]. The diffusible signal factor, cis-11-methyl-2-decanoic acid produced by Xanthomonas campestris induces biofilm dispersal by controlling the production of exopolysaccharide-degrading enzyme [141]. However, it has also been reported that fatty acids play an important role in the initial stages of biofilm formation in B. subtilis, as the lipids form structural component of extracellular matrix of biofilms [142]. In S. aureus, B. subtilis and P. aeruginosa, a mixture of d-amino acids triggered the disassambly of biofilm by releasing amyloid fibers, which are the proteinaceous component of the extracellular matrix [62,63]. While many l-amino acids promote biofilm formation in P. aeruginosa, in the case of tryptophan, both d- and l-isoforms inhibited biofilm formation and caused biofilm dispersal [143,144]. Nitric oxide (NO) generators such as sodium nitroprusside (SNP), S-nitroso-l-glutathione (GSNO) and S-nitroso-N-acetylpenicillamine (SNAP) are reported to induce biofilm dispersal in P. aeruginosa [64]. A low dose of NO generators dispersed P. aeruginosa biofilms both in vitro and in cystic fibrosis sputum, and enhanced the effect of antibiotics on biofilm-dispersed cells [145].
2.3. Biofilm Inhibition by Quorum Quenching
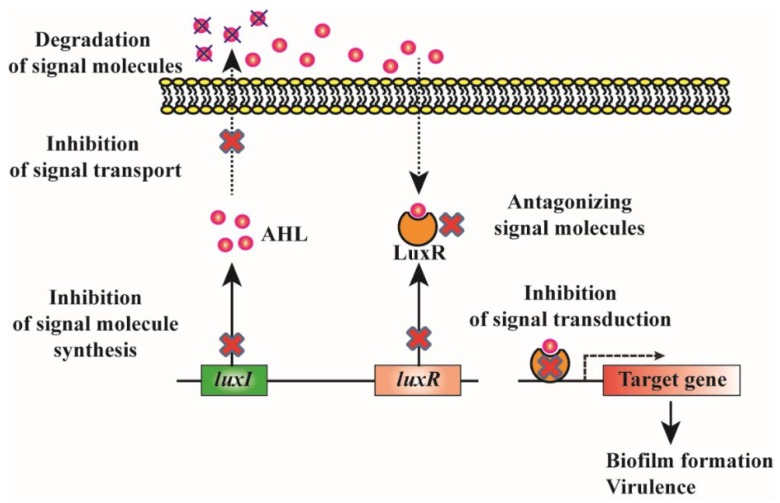
Quorum sensing (QS) is an important cellular communication system in many Gram-negative and Gram-positive bacteria. QS mediates the regulation of various genes according to the density of signaling molecules in the surrounding environment [146]. The signaling molecules of the QS system are denoted as autoinducers [147]. Based on signaling molecules, the QS system is categorized into three; N-acyl homoserine lactones (AHLs)-based (Gram-negative bacteria), autoinducing peptide (AIP)-based (Gram-positive bacteria), and autoinducer-2 (AI-2)-based (both Gram-negative and Gram-positive bacteria) [148,149]. During biofilm formation, following the initial attachment, the cells secrete QS molecules, which modulate bacterial gene expression, transforming planktonic lifestyle into a sessile form [150,151,152]. Since QS plays a crucial role in biofilm formation [153], it has been suggested that QS inhibition (quorum quenching; QQ) would be an interesting strategy to prevent biofilm formation [154]. In addition, QS regulates the production of virulence factors and pathogenesis factors in most pathogens, and thus the QS system can be considered a potential target for the development of new antimicrobial agents [155,156]. The various quorum-quenching strategies that can be beneficial for controlling biofilm formation are depicted in Figure 1. The major advantage of controlling biofilm by QQ is that this strategy reduces the risk of multidrug resistance, making the strategy of great clinical interest for use in the prevention of biofilm-based infections.
Figure 1.
Schematic representation of various quorum-quenching strategies to control biofilm formation. LuxI and luxR genes encode AHL signal synthase and AHL receptor/activator protein respectively. AHL signal synthase is responsible for the production of AHLs, which are diffused (short chain) or pumped (long chain) out of the bacterial cell to the surrounding medium before being taken up into the nearby bacterial cells. The AHL binds to the receptor protein and the AHL-receptor complex activates the expression of quorum-sensing target genes. The quorum-quenching strategies that have been used for attenuating AHL-mediated phenotypes include the inhibition of AHL synthesis, inhibition of signal transport, degradation of signal molecules, inhibition of AHL receptor synthesis, inhibition of AHL-receptor complex formation, inhibition of the binding of AHL-receptor complex to the promoters of target genes etc.
2.3.1. Degradation of QS Signals
AHLs can be degraded by specific enzymes such as lactonases that hydrolyze the lactone ring in the homoserine moiety and acylases that cleave off the acyl side chain, and the activity can be altered by reductases and oxidases [157]. Most of the AHL-degrading enzymes were discovered in bacterial species [158], though some are found in eukaryotes [159,160]. It has been reported that the application of QQ enzymes inhibits biofilm formation in several bacterial strains [65,66,67,68,69,70]. Quorum-quenching enzymes disrupt the biofilm architecture, which increases the antibiotic susceptibility of the cells [71]. Significant reduction of biofilm formation and increased sensitivity to antibiotics was noticed in P. aeruginosa after treatment with lactonase [71]. The oxidoreductases reduced the signaling molecules AHL and AI-2 to QS-inactive hydroxy-derivatives in K. oxytoca and K. pneumoniae [70].
2.3.2. Inhibition of Signal Synthesis
Several reports have shown that mutations affecting AHL synthesis have an adverse effect on biofilm formation. For example, P. aeruginosa strain that lacked the production of 3-oxo-C12-HSL resulted in impaired biofilm formation [161]. The mutation in the gene encoding for AHL synthesis enzyme in B. cenocepacia K56-2, B. cenocepacia J2315, Aeromonas hydrophila and Serratia liquefaciens led to defective biofilm formation [162,163,164,165]. In addition, the mutants of several Vibrio spp., Streptococcus spp. and Staphylococcus spp. that are deficient in AI-2 synthesis were not able to produce biofilms properly. Thus, blocking signal production has been considered as a promising strategy to control biofilm formation. Analogues of AHL precursor molecule, S-adenosyl-methionine (SAM), such as S-adenosyl-homocysteine (SAH), sinefugin, 5-methylthioadenosine (MTA), and butyryl-SAM, are known to inhibit biofilm formation in P. aeruginosa [72]. Also, the SAM biosynthesis inhibitor cycloleucine is reported to inhibit AHL production [73]. The antibiotic azithromycin interferes with signal synthesis in P. aeruginosa, and thus significantly clears biofilm in mouse model of cystic fibrosis [74,75]. In addition, several inhibitors for the key enzymes (5′-methylthioadenosine/S-adenosylhomo-cysteine nucleosidase (MTAN) and S-ribosylhomocysteinase (LuxS) involved in AI-2 synthesis are shown to reduce biofilm formation [76,77]. In B. multivorans, nickel (Ni2+) and cadmium (Cd2+) inhibited the expression of genes responsible for AHL production thereby inhibiting cell-cell signaling and subsequently biofilm formation [79]. The inhibitory effect of Cd2+ in quorum sensing was also reported in Chromobacterium violaceum [78].
2.3.3. Antagonizing the Signal Molecules
Researchers have screened for many signal analogues that antagonize QS signaling, thereby preventing biofilm formation [166,167,168]. AHL analogues in which the lactone ring was replaced by a cyclopentyl or a cyclohexanone ring adversely affected biofilm formation in Serratia marcescens and P. aeruginosa [169,170]. Many natural compounds are also reported to antagonize AHL-based QS signaling, and those include bergamottin and dihydroxybergamottin from grapefruit juice, cyclic sulfur compounds from garlic, patulin, and penicillic acid from a variety of fungi, etc. [80,81,82]. Treatment with patulin, ajoene and garlic extracts resulted in increased antibiotic susceptibility of P. aeruginosa biofilms and increased clearance of P. aeruginosa in in vivo pulmonary infection model [83,84,85]. In addition, some phenolic compounds including baicalin hydrate and epigallocatechin blocked AHL QS and affected biofilm formation of B. cenocepacia, B. multivorans and P. aeruginosa [86,87,88]. It was noted that the antibiotic susceptibility of B. cenocepacia and P. aeruginosa increased after treatment with baicalin hydrate in different in vitro biofilm models [86,87,88]. Thus, the concept of combining QS inhibitor (QSI) and antibiotics would be a better strategy to control biofilm formation by pathogenic bacteria. In addition, it has been noticed that biofilm formation can be effectively controlled by combining QSIs and QQ enzymes. Recently, Fong et al. reported the synergistic effect of a QS inhibitor, G1, which competes with AHL to bind to the response regulator and QQ enzyme, AHL lactonase, to effectively control biofilm formation and virulence by P. aeruginosa [171].
Several compounds that antagonize AI-2 signaling have also been reported to exhibit antibiofilm activity. The AI-2 analogues ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD inhibited biofilm formation and removed preformed biofilms in E. coli and P. aeruginosa [89,90]. Although other compounds, including pyrogallol and its derivatives, some nucleoside analogues, boronic acids, and sulfones have been identified to antagonize AI-2 signaling, only a few have been investigated for their antibiofilm activity [172,173].
Several AIP analogues, such as truncated forms of AIP, and probiotic bacteria-producing natural cyclic dipeptides, such as cyclo(l-Phe-l-Pro) and cyclo(l-Tyr-l-Pro), have been developed to antagonize QS signaling in Gram-positive bacteria. However, the experimental evidence on the anti-biofilm activities of these compounds is highly limited [91,92,93]. The most investigated QS inhibiting peptide is the RNAIII inhibiting peptide (RIP), which is produced by coagulase-negative Staphylococci. RIP interferes with the QS response by inhibiting the production of RNAIII, a key component of QS response in S. aureus [94]. RIP and several RIP homologues have been reported to have anti-QS and anti-biofilm activity against Staphylococcus spp. A RIP analogue, FS3, prevented S. aureus biofilm formation in a rat vascular graft model [95]. In addition, a non-peptide RIP analogue, hamamelitannin, blocked QS in Staphylococcus spp., and potentially inhibited biofilm formation in in vitro and in vivo rat model of graft infection [96]. Several natural compounds, including phytol, anthocyanidins, extracts from Ricinus communis, freshwater bryozoan Hyalinella punctata and selected sponges, and ricinine derivatives, are also known to exhibit anti-biofilm or anti-microbial and anti-quorum sensing activities in P. aeruginosa [174,175,176,177,178]. However, the exact mechanisms by which these compounds display anti-quorum sensing activities are not known.
2.3.4. Inhibition of Signal Transduction by Interfering with Response Regulator Activity
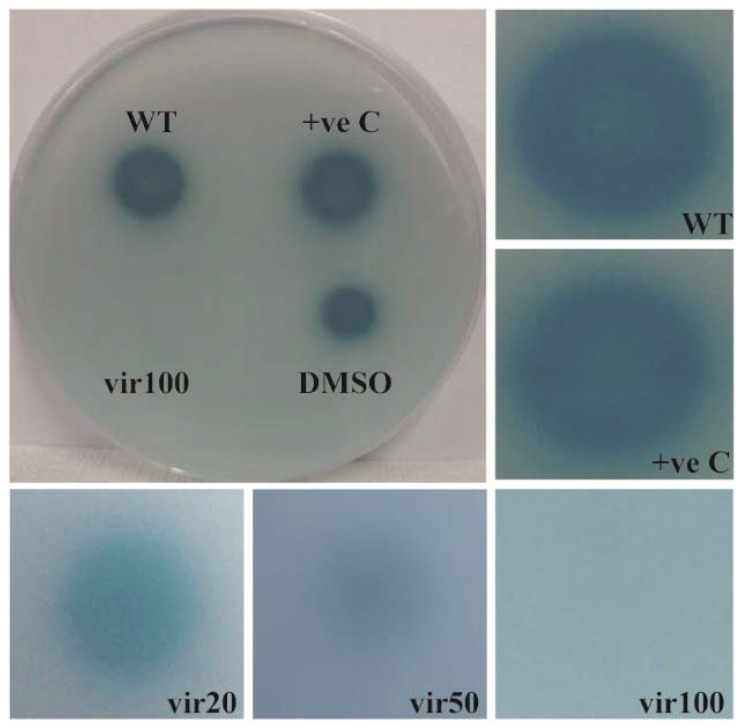
The QS system can also be hindered at the level of signal transduction cascade. The natural compounds, halogenated furanone or fimbrolide and cinnamaldehyde which are isolated from red algae Delisea pulchra and cinnamon bark, respectively, interfere with signal transduction and affect biofilm formation, thereby increasing antibiotic susceptibility in several pathogenic bacteria [97,98,99,100]. Both compounds block AI-2 and AHL-type QS systems, and thereby affect biofilm formation in V. harveyi [101,102]. The halogenated furanone and cinnamaldehyde inhibits AI-2 QS and AHL QS by decreasing the DNA-binding ability of the response regulator LuxR, which is important for the signal transduction cascade, or by displacing AHL from its receptor, respectively [101,102,179,180]. In addition, the natural furanone inactivates LuxS and accelerates LuxR turnover, thereby blocking AI-2 and AHL QS signaling system, respectively [181,182]. Cinnamaldehyde is widely used as a flavoring agent in food and beverages, while the application of furanones is limited because of their toxicity [100,183]. We reported previously that virstatin, a small organic molecule, prevents biofilm formation by interfering with the QS system in A. nosocomialis [103]. It was noticed that virstatin inhibits the expression of the response regulator, AnoR, which is a positive regulator of the AHL synthase gene, anoI in A. nosocomialis [103]. The repression of AnoR leads to decreased synthesis of AHL (Figure 2), adversely affecting the signal transduction cascade. Virstatin or its derivatives can be considered potential agents to inhibit the QS system and to control biofilm-based infections, and further studies in this direction could lead to the development of better antibacterial therapeutics.
Figure 2.
Effect of virstatin on the production of AHL. Bioassay was carried out to check the effect of virstatin on the production of AHLs in A. nosocomialis. For this, the strain was cultivated overnight in Luria Bertani (LB) medium at 30 °C, and the cells were washed with LB and diluted to an OD600 of 1. The cells were treated with different concentrations of virstatin (20, 50, 100 mM), which was dissolved in dimethyl sulfoxide (DMSO), and 5 µL of the samples were spotted onto chromoplate overlaid with A. tumefaciens NT1 (pDCI41E33) [184,185]. Synthetic N-(3-hydroxy-dodecanoyl)-l-homoserine lactone (OH-dDHL) was spotted as a positive control. The plates were incubated at 30 °C for 22 h, followed by the detection of the color zone surrounding the bacteria. A representative chromoplate image with 100 mM virstatin and images of color zones from different concentrations of virstatin are shown. WT, A. nosocomialis wild type; +ve C, OH-dDHL; vir20, vir50 and vir100; wild-type cells treated with 20, 50 and 100 mM virstatin respectively.
2.3.5. Inhibition of Signal Transport
The signaling molecules need to be exported and released into the extracellular space to be sensed by other bacteria for effective cell-to-cell communication. The role of multidrug-resistant (MDR) efflux pumps in signal traffic was first reported in P. aeruginosa, in which AHLs with long side chains are actively transported across the cell membrane through the MexAB-OprM efflux pump [186]. In P. aeruginosa, the expression of the autoinducer-producing gene and the genes encoding the virulence factors is limited by the intracellular concentration of the autoinducer [187]. The involvement of the MDR efflux pump in the QS system has also been reported in E. coli, in which the overexpression of the QS regulator SdiA led to the increased expression of the AcrAB efflux pump [188]. In Bacteroides fragilis, an opportunistic pathogen of the gastrointestinal tract, the BmeB efflux pump controls the intracellular AHL concentration by effluxing AHL outside of cells [189]. In addition, the expression of the MDR efflux pump, BpeAB-OprB, was reported to be essential for the export of six AHL inducers to the extracellular environment in B. pseudomallei [190,191]. Thus, the inhibition of the efflux pump would be a promising strategy to alter QS signaling cascade, thereby preventing biofilm formation and virulence.
Several studies have provided evidence to show the link between the physiological function of efflux pump and biofilm formation. In E. coli and Klebsiella strains, the inhibition of the efflux pump activity using efflux pump inhibitors (EPIs) reduced biofilm formation [192]. The genetic inactivation or the chemical inhibition of efflux pump activity resulted in impaired biofilm formation in S. enterica serovar typhimurium [193]. The effect of efflux pump inhibitors to prevent biofilm formation was also demonstrated in P. aeruginosa and S. aureus [105], in which copper nanoparticles work well as EPI and anti-biofilm agents [104]. In addition, in P. aeruginosa, the MDR efflux pump, MexAM-OPrM was disrupted by silver nanoparticles [194]. Recently, it was observed that the well-characterized EPI, Phe-Arg-β-naphthylamide (PAβN) alter the expression of QS molecules and QS-dependent virulence phenotypes in P. aeruginosa PAO1, as well as in clinical isolates [106]. The application of EPIs not only helps to reduce the biofilm-forming capacity of bacteria, but also to revive the bactericidal effect of conventional antibiotics [195].
It has been reported previously that AHLs with long side chains are exported out through the MexAB-OprM pump in P. aeruginosa [186], and the expression of the pump is modulated by the intracellular concentration of autoinducer molecules [196]. In addition, we have identified previously that A. nosocomialis produces AHL with long side chain, N-(3-hydroxy-dodecanoyl)-l-homoserine lactone (3OH-C12-AHL) as signaling molecules [103], and these AHLs might be actively transported through the efflux pumps. Thus, it can be postulated that the QS system controls the activity of the efflux system, contributing to the effective transport of AHLs across the cell membrane, in turn contributing to virulence and biofilm formation. Further studies in this direction would unravel in depth the role of the QS system in controlling the activity of these efflux pumps. The MDR efflux pumps and the regulators modulating them would be potential targets for the development of better therapeutics for biofilm-based infections.
3. Conclusions
In this review, we discuss the current strategies and future perspectives for developing improved therapeutics for controlling biofilm-based infections. The various approaches for modulating biofilm formation on medical devices are addressed in detail, with special emphasis on quorum-quenching strategies. Significant advances have been made in understanding the role of quorum sensing in biofilm formation in the past few years. In addition, several studies have shown that multidrug efflux pumps play a potential role in controlling biofilm formation. However, the fundamental mechanisms by which the QS systems exert the regulatory functions on biofilm formation are poorly understood. In this review, we postulate that QS systems regulate the activity of multidrug efflux pumps in transporting QS molecules across the cell membrane, thereby affecting biofilm formation. We propose that the transcriptional factors modulating the QS system and/or efflux pumps would be potential targets for developing QSIs. It is of utmost importance to improve our understanding of the molecular mechanism by which QS systems regulate biofilm formation and multidrug efflux pumps, as it will eventually be of help in developing better therapeutics for the treatment of problematic biofilm-related infections. Furthermore, detailed research is needed to understand the effect of these QSIs on different stages of biofilm formation and to validate their applicability on humans. Since QSIs do not induce any antibiotic resistance, they can be of great potential in the future for the treatment of biofilm-based infections in healthcare settings.
Funding
This research was funded by the research fund of Chungnam National University (2017). Also, this research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A61029617).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Costerton J.W., Irvin R.T., Cheng K.J. The bacterial glycocalyx in nature and disease. Annu. Rev. Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- 2.Kunin C.M., Steele C. Culture of the surfaces of urinary catheters to sample urethral flora and study the effect of antimicrobial therapy. J. Clin. Microbiol. 1985;21:902–908. doi: 10.1128/jcm.21.6.902-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickel J.C., Ruseska I., Wright J.B., Costerton J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985;27:619–624. doi: 10.1128/AAC.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donlan R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donlan R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddadin Y., Regunath H. Central Line Associated Blood Stream Infections (CLABSI) StatPearls Publishing; Treasure Island, FL, USA: 2017. [PubMed] [Google Scholar]
- 7.Tien H.C., Battad A., Bryce E.A., Fuller J., Mulvey M., Bernard K., Brisebois R., Doucet J.J., Rizoli S.B., Fowler R., et al. Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect. Dis. 2007;7:95. doi: 10.1186/1471-2334-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto M. Staphylococcal biofilms. In: Romeo T., editor. Bacterial Biofilms. Volume 322. Springer; Berlin, Germany: 2008. pp. 207–228. (Current Topics in Microbiology and Immunology book series). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A., Singh K.P., Jain A. Medical significance and management of staphylococcal biofilm. FEMS Immunol. Med. Microbiol. 2010;58:147–160. doi: 10.1111/j.1574-695X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Pour N.K., Dusane D.H., Dhakephalkar P.K., Zamin F.R., Zinjarde S.S., Chopade B.A. Biofilm formation by Acinetobacter baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011;62:328–338. doi: 10.1111/j.1574-695X.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 12.Dijkshoorn L., Nemec A., Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 13.Patwardhan R.B., Dhakephalkar P.K., Niphadkar K.B., Chopade B.A. A study on nosocomial pathogens in ICU with special reference to multiresistant Acinetobacter baumannii harbouring multiple plasmids. Indian J. Med. Res. 2008;128:178–187. [PubMed] [Google Scholar]
- 14.Bergogne-Bérézin E., Towner É.K.J. Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical and epidemiological features. Clin. Microiol. Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litzler P.Y., Benard L., Barbier-Frebourg N., Vilain S., Jouenne T., Beucher E., Bunel C., Lemeland J.F., Bessou J.P. Biofilm formation on pyrolytic carbon heart valves: Influence of surface free energy, roughness, and bacterial species. J. Thorac. Cardiovasc. Surg. 2007;134:1025–1032. doi: 10.1016/j.jtcvs.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Percival S.L., Suleman L., Vuotto C., Donelli G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015;64:323–334. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- 17.Von Eiff C., Heilmann C., Peters G. New aspects in the molecular basis of polymer-associated infections due to Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 1999;18:843–846. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 18.Muller E., Hübner J., Gutierrez N., Takeda S., Goldmann D.A., Pier G.B. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect. Immun. 1993;61:551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veenstra G.J., Cremers F.F., van Dijk H., Fleer A. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J. Bacteriol. 1996;178:537–541. doi: 10.1128/jb.178.2.537-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Götz F. Staphylococcus and biofilms. Mol. Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 21.Stoodley P., Sauer K., Davies D.G., Costerton J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 22.Fey P.D., Olson M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5:917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence J.R., Korber D.R., Wolfaardt G.M. Heterogeneity of natural biofilm communities. Cells Mater. 1996;6:175–191. [Google Scholar]
- 24.Aslam S. Effect of antibacterials on biofilms. Am. J. Infect. Control. 2008;36:S175.e9–S175.e11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Francolini I., Donelli G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010;59:227–238. doi: 10.1111/j.1574-695X.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 26.Stewart P.S., Davison W.M., Steenbergen J.N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 2009;53:3505–3507. doi: 10.1128/AAC.01728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borriello G., Werner E., Roe F., Kim A.M., Ehrlich G.D., Stewart P.S. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2004;48:2659–2664. doi: 10.1128/AAC.48.7.2659-2664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 29.Martínez J.L., Baquero F. Interactions among strategies associated with bacterial infection: Pathogenicity, epidemicity, and antibiotic resistance. Clin. Microbiol. Rev. 2002;15:647–679. doi: 10.1128/CMR.15.4.647-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diekema D.J., Pfaller M.A., Schmitz F.J., Smayevsky J., Bell J., Jones R.N., Beach M., Group S.P. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY antimicrobial surveillance program, 1997–1999. Clin. Infect. Dis. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 31.Kalia V.C., Purohit H.J. Quenching the quorum sensing system: Potential antibacterial drug targets. Crit. Rev. Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 32.Wright J.S., III, Lyon G.J., George E.A., Muir T.W., Novick R.P. Hydrophobic interactions drive ligand-receptor recognition for activation and inhibition of staphylococcal quorum sensing. Proc. Natl. Acad. Sci. USA. 2004;101:16168–16173. doi: 10.1073/pnas.0404039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dror N., Mandel M., Hazan Z., Lavie G. Advances in microbial biofilm prevention on indwelling medical devices with emphasis on usage of acoustic energy. Sensors. 2009;9:2538–2554. doi: 10.3390/s90402538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos E.R., Reitzel R., Jiang Y., Hachem R.Y., Chaftari A.M., Chemaly R.F., Hackett B., Pravinkumar S.E., Nates J., Tarrand J.J., et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: More than 0.5 million catheter days and 7 years of clinical experience. Crit. Care Med. 2011;39:245–251. doi: 10.1097/CCM.0b013e3181feb83e. [DOI] [PubMed] [Google Scholar]
- 35.Schumm K., Lam T.B. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst. Rev. 2008:CD004013. doi: 10.1002/14651858.CD004013.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Giraldo C., Rodríguez-Benito A., Morán F.J., Hurtado C., Blanco M.T., Gómez-García A.C. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997;39:643–646. doi: 10.1093/jac/39.5.643. [DOI] [PubMed] [Google Scholar]
- 37.De la Fuente-Núñez C., Reffuveille F., Haney E.F., Straus S.K., Hancock R.E. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10:e1004152. doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parisot J., Carey S., Breukink E., Chan W.C., Narbad A., Bonev B. Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic. Antimicrob. Agents Chemother. 2008;52:612–618. doi: 10.1128/AAC.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saising J., Dube L., Ziebandt A.K., Voravuthikunchai S.P., Nega M., Götz F. Activity of gallidermin on Staphylococcus aureus and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2012;56:5804–5810. doi: 10.1128/AAC.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Q.L., Wu J., Chen G.Q., Cui F.Z., Kim T.N., Kim J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka M., Hara K., Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klasen H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26:131–138. doi: 10.1016/S0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 43.Jansen B., Kohnen W. Prevention of biofilm formation by polymer modification. J. Ind. Microbiol. 1995;15:391–396. doi: 10.1007/BF01569996. [DOI] [PubMed] [Google Scholar]
- 44.Marlow V.L., Porter M., Hobley L., Kiley T.B., Swedlow J.R., Davidson F.A., Stanley-Wall N.R. Phosphorylated DegU manipulates cell fate differentiation in the Bacillus subtilis biofilm. J. Bacteriol. 2014;196:16–27. doi: 10.1128/JB.00930-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassinelli C., Morra M., Pavesio A., Renier D. Evaluation of interfacial properties of hyaluronan coated poly(methylmethacrylate) intraocular lenses. J. Biomater. Sci. Polym. Ed. 2000;11:961–977. doi: 10.1163/156856200744138. [DOI] [PubMed] [Google Scholar]
- 46.Boelens J.J., Tan W.F., Dankert J., Zaat S.A. Antibacterial activity of antibiotic-soaked polyvinylpyrrolidone-grafted silicon elastomer hydrocephalus shunts. J. Antimicrob. Chemother. 2000;45:221–224. doi: 10.1093/jac/45.2.221. [DOI] [PubMed] [Google Scholar]
- 47.John T., Rajpurkar A., Smith G., Fairfax M., Triest J. Antibiotic pretreatment of hydrogel ureteral stent. J. Endourol. 2007;21:1211–1216. doi: 10.1089/end.2007.9904. [DOI] [PubMed] [Google Scholar]
- 48.Appelgren P., Ransjö U., Bindslev L., Espersen F., Larm O. Surface heparinization of central venous catheters reduces microbial colonization in vitro and in vivo: Results from a prospective, randomized trial. Crit. Care Med. 1996;24:1482–1489. doi: 10.1097/00003246-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Falde E.J., Yohe S.T., Colson Y.L., Grinstaff M.W. Superhydrophobic materials for biomedical applications. Biomaterials. 2016;104:87–103. doi: 10.1016/j.biomaterials.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X.H., Lee J.H. Antibiofilm agents: A new perspective for antimicrobial strategy. J. Microbiol. 2017;55:753–766. doi: 10.1007/s12275-017-7274-x. [DOI] [PubMed] [Google Scholar]
- 51.Darouiche R.O., Mansouri M.D., Gawande P.V., Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and dispersinb combination. J. Antimicrob. Chemother. 2009;64:88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 52.Shen Y., Köller T., Kreikemeyer B., Nelson D.C. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013;68:1818–1824. doi: 10.1093/jac/dkt104. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan J.B., LoVetri K., Cardona S.T., Madhyastha S., Sadovskaya I., Jabbouri S., Izano E.A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in Staphylococci. J. Antibiot. 2012;65:73–77. doi: 10.1038/ja.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tetz G.V., Artemenko N.K., Tetz V.V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 2009;53:1204–1209. doi: 10.1128/AAC.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaignon P., Sadovskaya I., Ragunah C., Ramasubbu N., Kaplan J.B., Jabbouri S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 2007;75:125–132. doi: 10.1007/s00253-006-0790-y. [DOI] [PubMed] [Google Scholar]
- 56.Simões M., Pereira M.O., Vieira M.J. Action of a cationic surfactant on the activity and removal of bacterial biofilms formed under different flow regimes. Water Res. 2005;39:478–486. doi: 10.1016/j.watres.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Boles B.R., Thoendel M., Singh P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005;57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- 58.Chen X., Stewart P.S. Biofilm removal caused by chemical treatments. Water Res. 2000;34:4229–4233. doi: 10.1016/S0043-1354(00)00187-1. [DOI] [Google Scholar]
- 59.Mireles J.R., 2nd, Toguchi A., Harshey R.M. Salmonella enterica serovar typhimurium swarming mutants with altered biofilm-forming abilities: Surfactin inhibits biofilm formation. J. Bacteriol. 2001;183:5848–5854. doi: 10.1128/JB.183.20.5848-5854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdel-Mawgoud A.M., Lépine F., Déziel E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies D.G., Marques C.N. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolodkin-Gal I., Romero D., Cao S., Clardy J., Kolter R., Losick R. d-amino acids trigger biofilm disassembly. Science. 2010;328:627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jermy A. Biofilms: Disassembly instructions included. Nat. Rev. Microbiol. 2012;10:376. doi: 10.1038/nrmicro2808. [DOI] [Google Scholar]
- 64.Barraud N., Schleheck D., Klebensberger J., Webb J.S., Hassett D.J., Rice S.A., Kjelleberg S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009;191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.dos Reis Ponce A., Martins M.L., de Araujo E.F., Mantovani H.C., Vanetti M.C. Aiia quorum-sensing quenching controls proteolytic activity and biofilm formation by Enterobacter cloacae. Curr. Microbiol. 2012;65:758–763. doi: 10.1007/s00284-012-0226-0. [DOI] [PubMed] [Google Scholar]
- 66.Ivanova K., Fernandes M.M., Francesko A., Mendoza E., Guezguez J., Burnet M., Tzanov T. Quorum-quenching and matrix-degrading enzymes in multilayer coatings synergistically prevent bacterial biofilm formation on urinary catheters. ACS Appl. Mater. Interfaces. 2015;7:27066–27077. doi: 10.1021/acsami.5b09489. [DOI] [PubMed] [Google Scholar]
- 67.Jo S.J., Kwon H., Jeong S.Y., Lee S.H., Oh H.S., Yi T., Lee C.H., Kim T.G. Effects of quorum quenching on the microbial community of biofilm in an anoxic/oxic mbr for wastewater treatment. J. Microbiol. Biotechnol. 2016;26:1593–1604. doi: 10.4014/jmb.1604.04070. [DOI] [PubMed] [Google Scholar]
- 68.Lee J., Lee I., Nam J., Hwang D.S., Yeon K.M., Kim J. Immobilization and stabilization of acylase on carboxylated polyaniline nanofibers for highly effective antifouling application via quorum quenching. ACS Appl. Mater. Interfaces. 2017;9:15424–15432. doi: 10.1021/acsami.7b01528. [DOI] [PubMed] [Google Scholar]
- 69.Vinoj G., Vaseeharan B., Thomas S., Spiers A.J., Shanthi S. Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar. Biotechnol. 2014;16:707–715. doi: 10.1007/s10126-014-9585-9. [DOI] [PubMed] [Google Scholar]
- 70.Weiland-Bräuer N., Kisch M.J., Pinnow N., Liese A., Schmitz R.A. Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme. Front. Microbiol. 2016;7:1098. doi: 10.3389/fmicb.2016.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiran S., Sharma P., Harjai K., Capalash N. Enzymatic quorum quenching increases antibiotic susceptibility of multidrug resistant Pseudomonas aeruginosa. Iran. J. Microbiol. 2011;3:1–12. [PMC free article] [PubMed] [Google Scholar]
- 72.Parsek M.R., Val D.L., Hanzelka B.L., Cronan J.E., Jr., Greenberg E.P. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanzelka B.L., Greenberg E.P. Quorum sensing in Vibrio fischeri: Evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J. Bacteriol. 1996;178:5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Favre-Bonté S., Köhler T., Van Delden C. Biofilm formation by Pseudomonas aeruginosa: Role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 2003;52:598–604. doi: 10.1093/jac/dkg397. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann N., Lee B., Hentzer M., Rasmussen T.B., Song Z., Johansen H.K., Givskov M., Høiby N. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 2007;51:3677–3687. doi: 10.1128/AAC.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen G., Rajan R., Zhu J., Bell C.E., Pei D. Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase. J. Med. Chem. 2006;49:3003–3011. doi: 10.1021/jm060047g. [DOI] [PubMed] [Google Scholar]
- 77.Gutierrez J.A., Crowder T., Rinaldo-Matthis A., Ho M.C., Almo S.C., Schramm V.L. Transition state analogs of 5′-methylthioadenosine nucleosidase disrupt quorum sensing. Nat. Chem. Biol. 2009;5:251–257. doi: 10.1038/nchembio.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thornhill S.G., Kumar M., Vega L.M., McLean R.J.C. Cadmium ion inhibition of quorum signalling in Chromobacterium violaceum. Microbiology. 2017;163:1429–1435. doi: 10.1099/mic.0.000531. [DOI] [PubMed] [Google Scholar]
- 79.Vega L.M., Mathieu J., Yang Y., Pyle B.H., McLean R.J.C., Alvarez P.J.J. Nickel and cadmium ions inhibit quorum sensing and biofilm formation without affecting viability in Burkholderia multivorans. Int. Biodeterior. Biodegrad. 2014;91:82–87. doi: 10.1016/j.ibiod.2014.03.013. [DOI] [Google Scholar]
- 80.Kim C., Kim J., Park H.Y., Park H.J., Lee J.H., Kim C.K., Yoon J. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008;80:37–47. doi: 10.1007/s00253-008-1474-6. [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen T.B., Skindersoe M.E., Bjarnsholt T., Phipps R.K., Christensen K.B., Jensen P.O., Andersen J.B., Koch B., Larsen T.O., Hentzer M., et al. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology. 2005;151:1325–1340. doi: 10.1099/mic.0.27715-0. [DOI] [PubMed] [Google Scholar]
- 82.Galloway W.R., Hodgkinson J.T., Bowden S.D., Welch M., Spring D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 83.Bjarnsholt T., Jensen P.Ø., Rasmussen T.B., Christophersen L., Calum H., Hentzer M., Hougen H.P., Rygaard J., Moser C., Eberl L., et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 84.Rasmussen T.B., Bjarnsholt T., Skindersoe M.E., Hentzer M., Kristoffersen P., Köte M., Nielsen J., Eberl L., Givskov M. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005;187:1799–1814. doi: 10.1128/JB.187.5.1799-1814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jakobsen T.H., van Gennip M., Phipps R.K., Shanmugham M.S., Christensen L.D., Alhede M., Skindersoe M.E., Rasmussen T.B., Friedrich K., Uthe F., et al. Ajoene, a sulfur rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012;56:2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brackman G., Cos P., Maes L., Nelis H.J., Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011;55:2655–2661. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brackman G., Hillaert U., Van Calenbergh S., Nelis H.J., Coenye T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009;160:144–151. doi: 10.1016/j.resmic.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Huber B., Eberl L., Feucht W., Polster J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch. C. 2003;58:879–884. doi: 10.1515/znc-2003-11-1224. [DOI] [PubMed] [Google Scholar]
- 89.Ren D., Zuo R., González Barrios A.F., Bedzyk L.A., Eldridge G.R., Pasmore M.E., Wood T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71:4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roy V., Meyer M.T., Smith J.A., Gamby S., Sintim H.O., Ghodssi R., Bentley W.E. AI-2 analogs and antibiotics: A synergistic approach to reduce bacterial biofilms. Appl. Microbiol. Biotechnol. 2013;97:2627–2638. doi: 10.1007/s00253-012-4404-6. [DOI] [PubMed] [Google Scholar]
- 91.George E.A., Novick R.P., Muir T.W. Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J. Am. Chem. Soc. 2008;130:4914–4924. doi: 10.1021/ja711126e. [DOI] [PubMed] [Google Scholar]
- 92.Li J., Wang W., Xu S.X., Magarvey N.A., McCormick J.K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in Staphylococci. Proc. Natl. Acad. Sci. USA. 2011;108:3360–3365. doi: 10.1073/pnas.1017431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott R.J., Lian L.Y., Muharram S.H., Cockayne A., Wood S.J., Bycroft B.W., Williams P., Chan W.C. Side-chain-to-tail thiolactone peptide inhibitors of the staphylococcal quorum-sensing system. Bioorg. Med. Chem. Lett. 2003;13:2449–2453. doi: 10.1016/S0960-894X(03)00497-9. [DOI] [PubMed] [Google Scholar]
- 94.Gov Y., Bitler A., Dell’Acqua G., Torres J.V., Balaban N. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: Structure and function analysis. Peptides. 2001;22:1609–1620. doi: 10.1016/S0196-9781(01)00496-X. [DOI] [PubMed] [Google Scholar]
- 95.Cirioni O., Mocchegiani F., Cacciatore I., Vecchiet J., Silvestri C., Baldassarre L., Ucciferri C., Orsetti E., Castelli P., Provinciali M., et al. Quorum sensing inhibitor FS3-coated vascular graft enhances daptomycin efficacy in a rat model of staphylococcal infection. Peptides. 2013;40:77–81. doi: 10.1016/j.peptides.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 96.Kiran M.D., Adikesavan N.V., Cirioni O., Giacometti A., Silvestri C., Scalise G., Ghiselli R., Saba V., Orlando F., Shoham M., et al. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008;73:1578–1586. doi: 10.1124/mol.107.044164. [DOI] [PubMed] [Google Scholar]
- 97.Ren D., Sims J.J., Wood T.K. Inhibition of biofilm formation and swarming of Bacillus subtilis by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Lett. Appl. Microbiol. 2002;34:293–299. doi: 10.1046/j.1472-765x.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 98.Hume E.B.H., Baveja J., Muir B.W., Schubert T.L., Kumar N., Kjelleberg S., Griesser H.J., Thissen H., Read R., Poole-Warren L.A., et al. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials. 2004;25:5023–5030. doi: 10.1016/j.biomaterials.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 99.He Z.Y., Wang Q., Hu Y.J., Liang J.P., Jiang Y.T., Ma R., Tang Z.S., Huang Z.W. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int. J. Antimicrob. Agents. 2012;40:30–35. doi: 10.1016/j.ijantimicag.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 100.Janssens J.C., Steenackers H., Robijns S., Gellens E., Levin J., Zhao H., Hermans K., De Coster D., Verhoeven T.L., Marchal K., et al. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2008;74:6639–6648. doi: 10.1128/AEM.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niu C., Afre S., Gilbert E.S. Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett. Appl. Microbiol. 2006;43:489–494. doi: 10.1111/j.1472-765X.2006.02001.x. [DOI] [PubMed] [Google Scholar]
- 102.Defoirdt T., Miyamoto C.M., Wood T.K., Meighen E.A., Sorgeloos P., Verstraete W., Bossier P. The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein luxR. Environ. Microbiol. 2007;9:2486–2495. doi: 10.1111/j.1462-2920.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 103.Oh M.H., Choi C.H. Role of LuxIR homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J. Microbiol. Biotechnol. 2015;25:1390–1400. doi: 10.4014/jmb.1504.04069. [DOI] [PubMed] [Google Scholar]
- 104.Christena L.R., Mangalagowri V., Pradheeba P., Ahmed K.B., Shalini B.I., Vidyalakshmi M., Anbazhagan V., Sai subramanian N. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015;5:12899–12909. doi: 10.1039/C4RA15382K. [DOI] [Google Scholar]
- 105.Baugh S., Phillips C.R., Ekanayaka A.S., Piddock L.J., Webber M.A. Inhibition of multidrug efflux as a strategy to prevent biofilm formation. J. Antimicrob. Chemother. 2014;69:673–681. doi: 10.1093/jac/dkt420. [DOI] [PubMed] [Google Scholar]
- 106.Rampioni G., Pillai C.R., Longo F., Bondì R., Baldelli V., Messina M., Imperi F., Visca P., Leoni L. Effect of efflux pump inhibition on Pseudomonas aeruginosa transcriptome and virulence. Sci. Rep. 2017;7:11392. doi: 10.1038/s41598-017-11892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conrady D.G., Brescia C.C., Horii K., Weiss A.A., Hassett D.J., Herr A.B. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. USA. 2008;105:19456–19461. doi: 10.1073/pnas.0807717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maira-Litrán T., Kropec A., Abeygunawardana C., Joyce J., Mark G., 3rd, Goldmann D.A., Pier G.B. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 2002;70:4433–4440. doi: 10.1128/IAI.70.8.4433-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mittelman M.W. Adhesion to biomaterials. In: Fletcher M., editor. Bacterial Adhesion: Molecular and Ecological Diversity. Wiley-Liss, Inc.; New York, NY, USA: 1996. [Google Scholar]
- 110.Characklis W.G., McFeters G.A., Marshall K.C. Physiological ecology in biofilm systems. In: Characklis W.G., Marshall K.C., editors. Biofilms. John Wiley & Sons; New York, NY, USA: 1990. [Google Scholar]
- 111.Fletcher M., Loeb G.I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 1979;37:67–72. doi: 10.1128/aem.37.1.67-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun H., Xu Y., Sitkiewicz I., Ma Y., Wang X., Yestrepsky B.D., Huang Y., Lapadatescu M.C., Larsen M.J., Larsen S.D., et al. Inhibitor of streptokinase gene expression improves survival after group A Streptococcus infection in mice. Proc. Natl. Acad. Sci. USA. 2012;109:3469–3474. doi: 10.1073/pnas.1201031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma Y., Xu Y., Yestrepsky B.D., Sorenson R.J., Chen M., Larsen S.D., Sun H. Novel inhibitors of Staphylococcus aureus virulence gene expression and biofilm formation. PLoS ONE. 2012;7:e47255. doi: 10.1371/journal.pone.0047255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Opperman T.J., Kwasny S.M., Williams J.D., Khan A.R., Peet N.P., Moir D.T., Bowlin T.L. Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrob. Agents Chemother. 2009;53:4357–4367. doi: 10.1128/AAC.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jenal U., Dorman C.J. Small molecule signaling. Curr. Opin. Microbiol. 2009;12:125–128. doi: 10.1016/j.mib.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 116.Römling U., Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 117.Abraham N.M., Lamlertthon S., Fowler V.G., Jefferson K.K. Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: Role for clumping factor B. J. Med. Microbiol. 2012;61:1062–1070. doi: 10.1099/jmm.0.040758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chernousova S., Epple M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 119.Besinis A., Hadi S.D., Le H.R., Tredwin C., Handy R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology. 2017;11:327–338. doi: 10.1080/17435390.2017.1299890. [DOI] [PubMed] [Google Scholar]
- 120.Secinti K.D., Özalp H., Attar A., Sargon M.F. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J. Clin. Neurosci. 2011;18:391–395. doi: 10.1016/j.jocn.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 121.Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 122.Liu T., Yin B., He T., Guo N., Dong L., Yin Y. Complementary effects of nanosilver and superhydrophobic coatings on the prevention of marine bacterial adhesion. ACS Appl. Mater. Interfaces. 2012;4:4683–4690. doi: 10.1021/am301049v. [DOI] [PubMed] [Google Scholar]
- 123.Crick C.R., Ismail S., Pratten J., Parkin I.P. An investigation into bacterial attachment to an elastomeric superhydrophobic surface prepared via aerosol assisted deposition. Thin Solid Films. 2011;519:3722–3727. doi: 10.1016/j.tsf.2011.01.282. [DOI] [Google Scholar]
- 124.Tang P., Zhang W., Wang Y., Zhang B., Wang H., Lin C., Zhang L. Effect of superhydrophobic surface of titanium on Staphylococcus aureus adhesion. J. Nanomater. 2011;2011:2. doi: 10.1155/2011/178921. [DOI] [Google Scholar]
- 125.Privett B.J., Youn J., Hong S.A., Lee J., Han J., Shin J.H., Schoenfisch M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir. 2011;27:9597–9601. doi: 10.1021/la201801e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Russell P.B., Kline J., Yoder M.C., Polin R.A. Staphylococcal adherence to polyvinyl chloride and heparin-bonded polyurethane catheters is species dependent and enhanced by fibronectin. J. Clin. Microbiol. 1987;25:1083–1087. doi: 10.1128/jcm.25.6.1083-1087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdelkefi A., Achour W., Ben Othman T., Ladeb S., Torjman L., Lakhal A., Ben Hassen A., Hsairi M., Ben Abdeladhim A. Use of heparin-coated central venous lines to prevent catheter-related bloodstream infection. J. Support. Oncol. 2007;5:273–278. [PubMed] [Google Scholar]
- 128.Meiron T.S., Saguy I.S. Adhesion modeling on rough low linear density polyethylene. J. Food Sci. 2007;72:E485–E491. doi: 10.1111/j.1750-3841.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 129.Donlan R.M., Costerton J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ito A., Taniuchi A., May T., Kawata K., Okabe S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 2009;75:4093–4100. doi: 10.1128/AEM.02949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Savage V.J., Chopra I., O’Neill A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013;57:1968–1970. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 133.Mann E.E., Wozniak D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whitchurch C.B., Tolker-Nielsen T., Ragas P.C., Mattick J.S. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 135.Mulcahy H., Charron-Mazenod L., Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mah T.F.C., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 137.Kaplan J.B. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs. 2009;32:545–554. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- 138.Ramasubbu N., Thomas L.M., Ragunath C., Kaplan J.B. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J. Mol. Biol. 2005;349:475–486. doi: 10.1016/j.jmb.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 139.Chen M., Yu Q., Sun H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013;14:18488–18501. doi: 10.3390/ijms140918488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Hamme J.D., Singh A., Ward O.P. Physiological aspects. Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006;24:604–620. doi: 10.1016/j.biotechadv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 141.Deng Y., Lim A., Lee J., Chen S., An S., Dong Y.H., Zhang L.H. Diffusible signal factor (DSF) quorum sensing signal and structurally related molecules enhance the antimicrobial efficacy of antibiotics against some bacterial pathogens. BMC Microbiol. 2014;14:51. doi: 10.1186/1471-2180-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pedrido M.E., de Oña P., Ramirez W., Leñini C., Goñi A., Grau R. Spo0a links de novo fatty acid synthesis to sporulation and biofilm development in Bacillus subtilis. Mol. Microbiol. 2013;87:348–367. doi: 10.1111/mmi.12102. [DOI] [PubMed] [Google Scholar]
- 143.Bernier S.P., Ha D.G., Khan W., Merritt J.H., O’Toole G.A. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res. Microbiol. 2011;162:680–688. doi: 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brandenburg K.S., Rodriguez K.J., McAnulty J.F., Murphy C.J., Abbott N.L., Schurr M.J., Czuprynski C.J. Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;57:1921–1925. doi: 10.1128/AAC.00007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Howlin R.P., Cathie K., Hall-Stoodley L., Cornelius V., Duignan C., Allan R.N., Fernandez B.O., Barraud N., Bruce K.D., Jefferies J., et al. Low-dose nitric oxide as targeted anti-biofilm adjunctive therapy to treat chronic Pseudomonas aeruginosa infection in cystic fibrosis. Mol. Ther. 2017;25:2104–2116. doi: 10.1016/j.ymthe.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Socransky S.S., Haffajee A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 147.Frias J., Olle E., Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immun. 2001;69:3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eberhard A., Burlingame A.L., Eberhard C., Kenyon G.L., Nealson K.H., Oppenheimer N.J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 149.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 150.Brözel V.S., Strydom G.M., Cloete T.E. A method for the study of de novo protein synthesis in Pseudomonas aeruginosa after attachment. Biofouling. 1995;8:195–210. doi: 10.1080/08927019509378272. [DOI] [Google Scholar]
- 151.Davies D.G., Chakrabarty A.M., Geesey G.G. Exopolysaccharide production in biofilms: Substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1993;59:1181–1186. doi: 10.1128/aem.59.4.1181-1186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sauer K., Camper A.K. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 2001;183:6579–6589. doi: 10.1128/JB.183.22.6579-6589.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parsek M.R., Greenberg E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 154.Brackman G., Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015;21:5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 155.Hentzer M., Riedel K., Rasmussen T.B., Heydorn A., Andersen J.B., Parsek M.R., Rice S.A., Eberl L., Molin S., Høiby N., et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 156.Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N., Kumar N., Schembri M.A., Song Z., Kristoffersen P., et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uroz S., Chhabra S.R., Cámara M., Williams P., Oger P., Dessaux Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology. 2005;151:3313–3322. doi: 10.1099/mic.0.27961-0. [DOI] [PubMed] [Google Scholar]
- 158.Tang K., Zhang X.H. Quorum quenching agents: Resources for antivirulence therapy. Mar. Drugs. 2014;12:3245–3282. doi: 10.3390/md12063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang F., Wang L.H., Wang J., Dong Y.H., Hu J.Y., Zhang L.H. Quorum quenching enzyme activity is widely conserved in the sera of mammalian species. FEBS Lett. 2005;579:3713–3717. doi: 10.1016/j.febslet.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 160.Uroz S., Heinonsalo J. Degradation of N-acyl homoserine lactone quorum sensing signal molecules by forest root-associated fungi. FEMS Microbiol. Ecol. 2008;65:271–278. doi: 10.1111/j.1574-6941.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 161.Bjarnsholt T., Tolker-Nielsen T., Høiby N., Givskov M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 2010;12:e11. doi: 10.1017/S1462399410001420. [DOI] [PubMed] [Google Scholar]
- 162.Tomlin K.L., Malott R.J., Ramage G., Storey D.G., Sokol P.A., Ceri H. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl. Environ. Microbiol. 2005;71:5208–5218. doi: 10.1128/AEM.71.9.5208-5218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huber B., Riedel K., Hentzer M., Heydorn A., Gotschlich A., Givskov M., Molin S., Eberl L. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology. 2001;147:2517–2528. doi: 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- 164.Lynch M.J., Swift S., Kirke D.F., Keevil C.W., Dodd C.E., Williams P. The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environ. Microbiol. 2002;4:18–28. doi: 10.1046/j.1462-2920.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 165.Labbate M., Queck S.Y., Koh K.S., Rice S.A., Givskov M., Kjelleberg S. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J. Bacteriol. 2004;186:692–698. doi: 10.1128/JB.186.3.692-698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith K.M., Bu Y., Suga H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 2003;10:81–89. doi: 10.1016/S1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 167.Smith K.M., Bu Y., Suga H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 2003;10:563–571. doi: 10.1016/S1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 168.Kim C., Kim J., Park H.Y., Park H.J., Kim C.K., Yoon J., Lee J.H. Development of inhibitors against TraR quorum-sensing system in Agrobacterium tumefaciens by molecular modeling of the ligand-receptor interaction. Mol. Cells. 2009;28:447–453. doi: 10.1007/s10059-009-0144-6. [DOI] [PubMed] [Google Scholar]
- 169.Ishida T., Ikeda T., Takiguchi N., Kuroda A., Ohtake H., Kato J. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 2007;73:3183–3188. doi: 10.1128/AEM.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morohoshi T., Shiono T., Takidouchi K., Kato M., Kato N., Kato J., Ikeda T. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl. Environ. Microbiol. 2007;73:6339–6344. doi: 10.1128/AEM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fong J., Zhang C., Yang R., Boo Z.Z., Tan S.K., Nielsen T.E., Givskov M., Wu B., Su H., Yang L. Synergy of quorum quenching enzyme and quorum sensing inhibitor in inhibiting P. aeruginosa quorum sensing. bioRxiv. 2017:182543. doi: 10.1101/182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ni N.T., Choudhary G., Li M.Y., Wang B.H. Pyrogallol and its analogs can antagonize bacterial quorum sensing in Vibrio harveyi. Bioorg. Med. Chem. Lett. 2008;18:1567–1572. doi: 10.1016/j.bmcl.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 173.Brackman G., Celen S., Baruah K., Bossier P., Van Calenbergh S., Nelis H.J., Coenye T. AI-2 quorum-sensing inhibitors affect the starvation response and reduce virulence in several Vibrio species, most likely by interfering with LuxPQ. Microbiology. 2009;155:4114–4122. doi: 10.1099/mic.0.032474-0. [DOI] [PubMed] [Google Scholar]
- 174.El-Naggar M.H., Elgaml A., Abdel Bar F.M., Badria F.A. Antimicrobial and antiquorum-sensing activity of Ricinus communis extracts and ricinine derivatives. Nat. Prod. Res. 2018;32:1–7. doi: 10.1080/14786419.2017.1423306. [DOI] [PubMed] [Google Scholar]
- 175.Pejin B., Ciric A., Dimitric Markovic J., Glamoclija J., Nikolic M., Sokovic M. An insight into anti-biofilm and anti-quorum sensing activities of the selected anthocyanidins: The case study of Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2017;31:1177–1180. doi: 10.1080/14786419.2016.1222386. [DOI] [PubMed] [Google Scholar]
- 176.Pejin B., Ciric A., Glamoclija J., Nikolic M., Sokovic M. In vitro anti-quorum sensing activity of phytol. Nat. Prod. Res. 2015;29:374–377. doi: 10.1080/14786419.2014.945088. [DOI] [PubMed] [Google Scholar]
- 177.Pejin B., Talevska A., Ciric A., Glamoclija J., Nikolic M., Talevski T., Sokovic M. Anti-quorum sensing activity of selected sponge extracts: A case study of Pseudomonas aeruginosa. Nat. Prod. Res. 2014;28:2330–2333. doi: 10.1080/14786419.2014.934239. [DOI] [PubMed] [Google Scholar]
- 178.Pejin B., Ciric A., Karaman I., Horvatovic M., Glamoclija J., Nikolic M., Sokovic M. In vitro antibiofilm activity of the freshwater bryozoan Hyalinella punctata: A case study of Pseudomonas aeruginosa PAO1. Nat. Prod. Res. 2016;30:1847–1850. doi: 10.1080/14786419.2015.1072714. [DOI] [PubMed] [Google Scholar]
- 179.Brackman G., Defoirdt T., Miyamoto C., Bossier P., Van Calenbergh S., Nelis H., Coenye T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8:149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Manefield M., de Nys R., Kumar N., Read R., Givskov M., Steinberg P., Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 181.Kuehl R., Al-Bataineh S., Gordon O., Luginbuehl R., Otto M., Textor M., Landmann R. Furanone at subinhibitory concentrations enhances staphylococcal biofilm formation by luxS repression. Antimicrob. Agents Chemother. 2009;53:4159–4166. doi: 10.1128/AAC.01704-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Manefield M., Rasmussen T.B., Henzter M., Andersen J.B., Steinberg P., Kjelleberg S., Givskov M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology. 2002;148:1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 183.Han Y., Hou S., Simon K.A., Ren D., Luk Y.Y. Identifying the important structural elements of brominated furanones for inhibiting biofilm formation by Escherichia coli. Bioorg. Med. Chem. Lett. 2008;18:1006–1010. doi: 10.1016/j.bmcl.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 184.Zhang L., Murphy P.J., Kerr A., Tate M.E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 185.Park S.Y., Lee S.J., Oh T.K., Oh J.W., Koo B.T., Yum D.Y., Lee J.K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology. 2003;149:1541–1550. doi: 10.1099/mic.0.26269-0. [DOI] [PubMed] [Google Scholar]
- 186.Pearson J.P., Van Delden C., Iglewski B.H. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1999;181:1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Evans K., Passador L., Srikumar R., Tsang E., Nezezon J., Poole K. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1998;180:5443–5447. doi: 10.1128/jb.180.20.5443-5447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rahmati S., Yang S., Davidson A.L., Zechiedrich E.L. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 2002;43:677–685. doi: 10.1046/j.1365-2958.2002.02773.x. [DOI] [PubMed] [Google Scholar]
- 189.Pumbwe L., Skilbeck C.A., Wexler H.M. Presence of quorum-sensing systems associated with multidrug resistance and biofilm formation in Bacteroides fragilis. Microb. Ecol. 2008;56:412–419. doi: 10.1007/s00248-007-9358-3. [DOI] [PubMed] [Google Scholar]
- 190.Chan Y.Y., Chua K.L. The Burkholderia pseudomallei BpeAB-OprB efflux pump: Expression and impact on quorum sensing and virulence. J. Bacteriol. 2005;187:4707–4719. doi: 10.1128/JB.187.14.4707-4719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chan Y.Y., Bian H.S., Tan T.M., Mattmann M.E., Geske G.D., Igarashi J., Hatano T., Suga H., Blackwell H.E., Chua K.L. Control of quorum sensing by a Burkholderia pseudomallei multidrug efflux pump. J. Bacteriol. 2007;189:4320–4324. doi: 10.1128/JB.00003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kvist M., Hancock V., Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl. Environ. Microbiol. 2008;74:7376–7382. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baugh S., Ekanayaka A.S., Piddock L.J., Webber M.A. Loss of or inhibition of all multidrug resistance efflux pumps of Salmonella enterica serovar typhimurium results in impaired ability to form a biofilm. J. Antimicrob. Chemother. 2012;67:2409–2417. doi: 10.1093/jac/dks228. [DOI] [PubMed] [Google Scholar]
- 194.Nallathamby P.D., Lee K.J., Desai T., Xu X.H. Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry. 2010;49:5942–5953. doi: 10.1021/bi100268k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gupta D., Singh A., Khan A.U. Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res. Lett. 2017;12:454. doi: 10.1186/s11671-017-2222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maseda H., Sawada I., Saito K., Uchiyama H., Nakae T., Nomura N. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004;48:1320–1328. doi: 10.1128/AAC.48.4.1320-1328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]