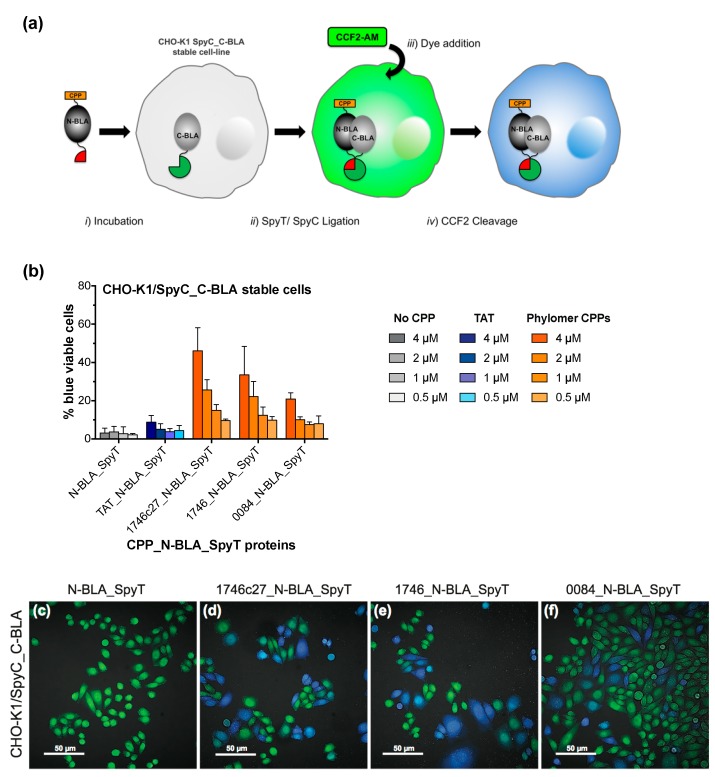
Figure 3.
A CPP-mediated split β-lactamase cytosolic delivery assay. (a) Live-cell functional uptake can be measured when N-terminal β-lactamase (N-BLA), fused to a CPP sequence, penetrates the cell membrane to complement C-terminal β-lactamase protein (C-BLA) in the cytosol (step i). Expression of the split β-lactamase moieties as fusions with either SpyTag (CPP_N-BLA_SpyT) or SpyCatcher (SpyC_C-BLA) facilitates rapid formation of functional β-lactamase proteins through SpyT/SpyC ligation inside the cells (step ii). This internalization is detected after addition of dye substrate (step iii) through cleavage of cytosol-retained CCF2 (step iv), which is measured via a change in fluorescence signal from green (520 nm) to blue (450 nm). (b) Internalization of CPP_N-BLA_SpyT and β-lactamase complementation in CHO-K1/SpyC_C-BLA cells is dose-dependent, measured by CCF2 cleavage (increase in % blue viable cells as estimated by flow cytometry). N-BLA_SpyT protein (No CPP negative control) shows a negligible effect on the β-lactamase complementation signal over cell-line background at all concentrations tested. Error bars represent standard error of the mean of two independent experiments (duplicate samples). (c–f) Live cell confocal microscopy visually confirms the CPP-mediated cytosolic delivery of CPP_N-BLA_SpyT (4 µM) in CHO-K1/SpyC_C-BLA cells and subsequent β-lactamase complementation signal. N-BLA_SpyT protein (c, negative control) shows minimal evidence of internalization. β-Lactamase activity is dependent on cytosolic delivery of N-BLA_SpyT with phylomer CPPs 1746c27_N-BLA_SpyT (d), 1746_N-BLA_SpyT (e), or 0084_N-BLA_SpyT (f). Bar scale is 50 µm.