Abstract
Among diseases whose cure is still far from being discovered, Alzheimer’s disease (AD) has been recognized as a crucial medical and social problem. A major issue in AD research is represented by the complexity of involved biochemical pathways, including the nature of protein misfolding, which results in the production of toxic species. Considering the involvement of (mis)folding processes in AD aetiology, targeting molecular chaperones represents a promising therapeutic perspective. This review analyses the connection between AD and molecular chaperones, with particular attention toward the most important heat shock proteins (HSPs) as representative components of the human chaperome: Hsp60, Hsp70 and Hsp90. The role of these proteins in AD is highlighted from a biological point of view. Pharmacological targeting of such HSPs with inhibitors or regulators is also discussed.
Keywords: heat shock proteins, chaperones, Alzheimer’s disease, amyloid peptide, protein Tau, Hsp60, Hsp70, Hsp90
1. Introduction
Among neurodegenerative diseases, Alzheimer’s (AD) represents a major concern for public health in the 21st century. AD is mainly characterized by the anomalous processing of two proteins, amyloid-peptides (Aβ) and Tau, leading to the pathological formation of extracellular senile plaques and intracellular neurofibrillary tangles (NFTs). Brains of AD patients present senile plaques formed by insoluble Aβ with a sequence between 38 and 42 amino acids [1]. According to the amyloid hypothesis, Aβ peptides arise from the β-amyloid precursor protein (APP). APP is a trans-membrane protein which is cleaved by β- and γ-secretase. The former—also known as beta-site amyloid precursor protein cleaving enzyme (BACE-1)—produces sAPP β, which is a soluble amyloid precursor and a C-terminal fragment (C99) bound to the membrane [1]. In turn, the cleavage of C99 by γ-secretase releases Aβ40 and Aβ42. Both peptides tend to self-assemble into oligomers and then into fibrils. In particular, Aβ42 is the major component in amyloid plaques and forms the most toxic oligomers. As a consequence, an increased production of Aβ induces cell death, eventually leading to dementia [2]. On the other hand, the intra-cellular NFT lesion results from the pathological hyperphosphorylation of protein Tau and its subsequent misfolding, aggregation and accumulation within the cytoplasm [3].
Currently, the only approved therapy is focused on the limitation of symptoms by inhibiting acetylcholinesterase (AChE) action, thus enhancing cholinergic transmission [4]. Due to the involvement of APP metabolism in Aβ production, the inhibition of secretase enzymes represents a very promising strategy for AD treatment and clinical candidates are in phase 3 trials [5]. On the other hand, many other therapeutic approaches are under evaluation. In particular, Aβ peptide and Tau aggregation inhibitors, photo-therapeutics and metal chelators are among the most promising lead but, currently, these approaches are far from being implemented in clinical practice [6,7,8,9,10,11].
Considering that the main cause of neuron’s damage in AD is due to stress induced by the misfolding of Aβ peptides and Tau, triggering the production of toxic oligomers and eventually plaques and NFTs, the importance of the chaperones in AD and other neurodegenerative diseases has been evidenced in the last two decades. [12]. Among molecular chaperones, Heat Shock Proteins (HSPs) are major constituent of the chaperome and Hsp60 [13], Hsp70 [14,15,16] and Hsp90 [17,18] are considered target [19] of particular relevance in AD [20] and for many other diseases, including cancer [21,22,23,24]. In this review, recent advances regarding the role of these three proteins in AD is highlighted from a biological point of view. The state of the art of their targeting and the development of perspective drugs for future AD therapies is also discussed.
2. Molecular Chaperones and Neurodegenerative Diseases
To face stress, cells use a series of protective mechanisms. One of these biological responses to stress involves an array of highly conserved proteins that have a range of functions with the scope of maintaining cellular homeostasis [25]. These biomolecules include a group of protein named molecular chaperones that play a crucial role within cells, by mediating protein folding, signalling, chaperoning and cell protection. These proteins, that are located inside cells as well as in extracellular environment and in body fluids, are important players in other cellular mechanisms such us protein translocation, protein degradation, cell differentiation and signal transduction [26,27,28]. The expression of many chaperone proteins is induced by stress to assist other proteins in achieving proper folding. These molecular chaperones are included in the family of HSPs however, not all molecular chaperones are stress proteins [29]. There are several classes of HSPs involved in the system to assure the control of protein quality: Hsp60, Hsp70, Hsp90, Hsp40, Hsp100 and Hsp110 as well as the ATP independent small HSPs such as Hsp20 [30]. In this context, chaperonopathies are pathological conditions in which chaperones that are abnormal in composition/structure (e.g., because of mutations or post-translational modifications), quantitative levels, location, or function, play an either primary or auxiliary etiopathogenic role [21].
The accumulation of misfolded proteins and protein aggregation in the human brain is an important characteristic of many neurodegenerative diseases, including AD, Amyotrophic lateral sclerosis, Parkinson’s disease (PD), Huntington’s disease and Creutzfeldt-Jakob disease [31,32] (Table 1). Therefore, neurodegenerative disorders are classified among “proteinopathies,” in which proteins that are misfolded (i.e., conformationally altered) can direct disease progression and are often used as a primary neuropathological biomarker of the disease.
Table 1.
Neurodegenerative diseases due to protein misfolding and aggregation.
Neuronal dysfunction caused by the abnormal aggregation of proteins is a crucial factor for the medical evaluation of these neuronal diseases. The clinical characteristics depend on the affected brain region and may involve disruption of daily activities including sensory, motor and cognitive functions. One hypothesis is that misfolding and protein aggregation cause synaptic loss and neuronal death which are typically observed in various neurodegenerative diseases [31]. The aggregation of misfolded proteins is highly regulated and depends on genetic and environmental factors [31].
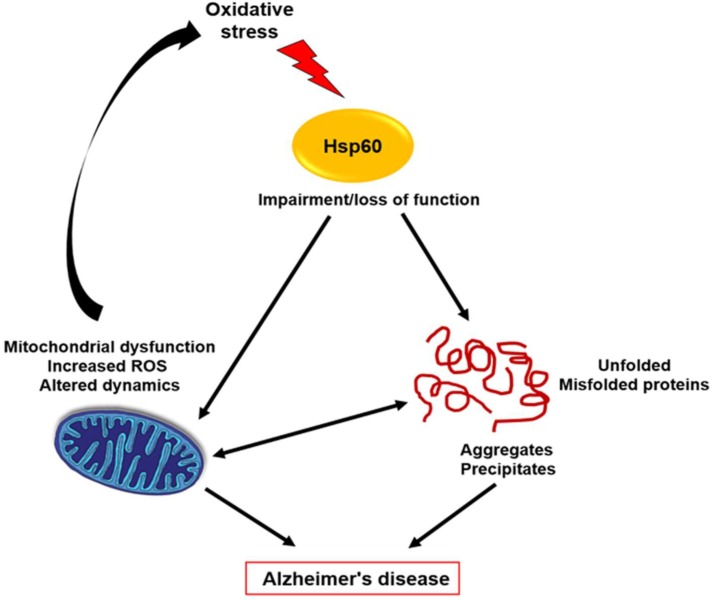
Molecular chaperones regulate protein folding, modulate protein activity and target misfolded or aggregated proteins for refolding or for degradation and translocation. HSPs are essential to efficiently facilitate the protein folding process [36]. They participate in different mechanisms to defend the cells against stress-related events harmful to the cell itself [36]. Therefore, as observed in various neurodegenerative diseases, failure of these cellular control mechanisms can result in pathogenic conditions. There are many data that demonstrated that HSPs regulate protein misfolding in a variety of neurodegenerative diseases, such as AD, probably displaying protective roles and/or acting as pathogenic factors. Indeed, stress-induced proteins like chaperones have been claimed to act as protective molecules for cells of the nervous system [20]. Many evidences demonstrated that oxidative stress is a feature of AD and PD [37]. Mitochondrial dysfunction and abnormal accumulation of Aβ and Tau proteins can contribute to create the imbalance between oxidant and antioxidant mechanisms determining oxidative damage in AD patients [37]. In the brain, oxidative stress can cause damage that contributes to neuronal loss [38]. Reactive oxygen species (ROS) can accumulate inside cells and have negative effects on all biological molecules, determining, for instance, nucleic acid breakage, enzyme inactivation, polysaccharide depolymerisation and lipid peroxidation. Under these stress conditions, the expression of the genes encoding HSPs was induced [38]. Moreover, mitochondrial dysfunction and elevated levels of ROS might create a vicious circle contributing to AD instauration and progression (see Figure 1) [20].
Figure 1.
Hsp60 and Alzheimer’s disease. Oxidative stress may cause Hsp60 structure modifications leading to loss of Hsp60 functions with the consequences of protein misfolding, aggregation and deposition.
Indeed, various HSPs can be transported to synapses and axons to block or hinder the aggregation process of misfolded proteins. Many data demonstrated that HSPs have a role in the direct inhibition of the aggregation of amyloidogenic proteins and also promote bonding to ubiquitin and degradation of aggregated or misfolded proteins [39,40]. Many evidences implicate HSPs in metabolism and the aggregation of both Aβ and Tau [41]. HSPs that are found in the mitochondria matrix can play an important role in protein folding. Alterations in HSPs function affect mitochondria function, such as protein aggregation [42]. It is known that intracellular protein degradation pathways are decreased with aging in many tissue and organs. Indeed, in several neurodegenerative diseases, the protein degradation system is not functional. Moreover, HSPs are involved in a specialized mechanism called chaperone mediated autophagy (CMA). CMA is the only autophagic pathway that allows selective degradation of soluble proteins that contain a consensus peptide motif in lysososmes [43,44].
3. Hsp60
3.1. Biological Role in AD
Hsp60 is a protein that, together with its co-chaperone Hsp10, is considered essential for mitochondrial protein folding [36]. Many studies have demonstrated that Hsp60 can be localized in extra-mitochondrial sites such as in the cytosol, in extracellular vesicles, or on the surface of normal and tumour cells [45,46,47]. Recently, increasing data demonstrated that Hsp60 is localized outside of the cells, where it mediates the interaction between immune cells and other body tissues [48]. Hsp60 can have both pro-survival and pro-death functions depending on the molecules with which it interacts, on the tissue, on the cell type and on the identity of the apoptosis inducers. [27,49,50,51,52,53]. Many evidences have demonstrated that Hsp60 have a role in tumour progression as suggested by its accumulation in the cytosol and plasma membrane of cancerous cells [21]. We also demonstrated that Hsp60 can be secreted in the extracellular space via secretory vesicles that, in turn, can modulate anti-tumour immune responses [45,46,47]. Many researchers have advanced the hypothesis that Hsp60 can be used as a target for anticancer therapy and data in the literature are very encouraging in this regard [21]. For instance, hyperacetylation of Hsp60 in osteosarcoma cells is associated with the anticancer activity of geldanamycin, and Hsp60 nitration is associated with the anti-tumour action of the histone deacetylase inhibitor SAHA in mucoepidermoid cells [26,54]. On the other hand, Hsp60’s role in AD is still unclear. Many data demonstrated that it has a neuroprotective role but other authors have attributed a deleterious effect to the elevated expression of Hsp60 in AD [55,56]. It has been demonstrated that Hsp60 expression by activated microglia is high. Moreover, the extracellular release of Hsp60 increases the production of other pro-inflammatory factors through binding to toll-like receptor 4 (TLR-4) and stimulating neuronal cell death [55]. Over-activation of microglia in response to certain harmful factors, contributes to the progression of several neurodegenerative diseases, including AD [57]. Neurodegenerative diseases are associated with the secretion of various pro-inflammatory and cytotoxic factors by activated microglia in the brain [58,59]. Therefore, inhibiting the activation of microglia and Hsp60 expression/release is an important strategy for the prevention of neurodegeneration [57]. Hsp60 levels were high in lymphocytes from AD patients when compared to controls [60,61]. Indeed, a useful approach would be the test of Hsp60 levels in patients with clinical condition preceding AD, such as mild cognitive impairment, in order to assess the potential value of this protein as an early biomarker of the disease [60,62]. Mitochondrial protein quality control may have a special relevance for the maintenance of neurons. Mitochondrial dysfunction was found in numerous neurodegenerative diseases including AD, HT and Parkinson’s disease [63]. Mutations in mitochondrial genes or nuclear genes encoding mitochondrial proteins are potential causing of neurological diseases and defects of the mitochondrial protein quality control system could represent an important pathogenic factor for neurodegenerative diseases. In particular, mutations in the gene encoding for Hsp60 are associated with hereditary spastic paraplegia (SPG13) [63]. Proteomic analysis of hippocampi of APP-transgenic mice possessed abundant Aβ oligomers from the age of 8 months but no amyloid plaques even at the age of 24 months and showed altered levels of 14 proteins including Hsp70, Hsp60 and Hsp90. In particular, Hsp60 and Hsp70 levels were significantly decreased with respect to control. Aβ oligomers might contribute in changing the expression of the chaperons [56]. Aβ, is the main component of plaques and it accumulates in mitochondria collected from brains of human AD cases and transgenic mouse models of AD [64]. It was demonstrated that HSPs played a protective role in cultured neurons. In particular, Hsp60, Hsp70 and Hsp90 either individually or together, provide protection against intracellular beta-amyloid induced stress through the maintenance of mitochondrial oxidative phosphorylation and functionality of tricarboxylic acid cycle enzymes. Aβ selectively inhibits complex IV activity and such inhibition is selectively neutralized by Hsp60. In this way, the overall effect of HSPs activity resulted in the reduction of free radicals, preservation of ATP generation, reduction of cytochrome C release and prevention of caspase-9 activation, all involved in beta-amyloid-induced neuronal dysfunction and death [65]. On the contrary, it was shown that Hsp60 mediates in vitro the translocation of APP to the mitochondria, leading to dysfunction of this organelle. In particular, Walls et al. found that Hsp60 and APP/Aβ form a molecular association in mitochondria in both transgenic and human AD subjects [41]. Immunoprecipitated APP from human AD mitochondria exhibited a stronger propensity to interact with Hsp60 versus non-demented controls. Mangione et al. [66] demonstrated, in vitro, that Hsp60 inhibits Aβ amyloid aggregation by closing molecular pathways leading to peptide fibrillogenesis. Administration of an Aβ amyloid-Hsp60 peptide-conjugate vaccine led to the induction of anti-Aβ-specific antibodies, associated with a significant reduction of cerebral amyloid accumulation in a mouse model of AD [67]. All these experimental evidences made us hypothesize that the regulation of Hsp60 production could have been a potential therapeutic option for the treatment of AD. However, Hsp60 role in AD remains controversial and further investigations are necessary to better understand if this protein is either a “friend” or a “foe” in the development and progression of the disease [20].
3.2. Targeting and Inhibition
Despite the convincing evidence that supports the involvement of Hsp60 in the development of Alzheimer’s disease [41], there is a lack of studies on its known inhibitors or regulators that could represent potential therapeutic agents in AD. On the other hand, Hsp60’s role in tumours has been assessed and studies regarding the development of inhibitors are related to the opportunity of targeting Hsp60 as a therapeutic anticancer approach [21,22,68]. These compounds are able to modify and regulate Hsp60 expression and functions and, for this reason, their use can be switched from cancer therapy to AD management [20]. In general, various studies pointed out Hsp60 inhibition as a promising therapeutic approach but only a limited number of compounds have been fully characterized and, for most of these inhibitors, the mechanism of action is still undisclosed [69].
In the search of new Hsp60 inhibitors, it is fundamental to consider structural differences between the eukaryotic Hsp60 and its prokaryotic homologue GroEL. Only the first possesses cysteine residues (Cys237, Cys442 and Cys447), which represent ideal drug-interacting sites due to their nucleophilic behaviour and redox potential [21]. Moreover, from a structural point of view, the X-ray structure of Hsp60 was only recently resolved [70] and the models for the Hsp60 folding machine are still under debate considering: the bullet versus football complex with Hsp10 co-chaperones [71,72]; one-ring heptamers versus two-ring tetradecamers [73,74]; the significant differences between crystal and in solution structures [74]. The overall complexity of these aspects accounts for the lack of a model that could be used for drug design and in silico screening, thus slowing down the drug discovery process. Currently, only two modes of action were described for Hsp60 inhibitors: competition with ATP binding site or targeting cysteine residues [21].
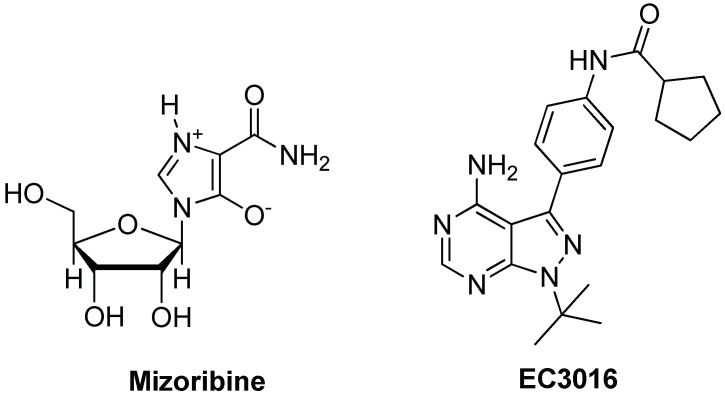
The first example of a compound targeting the ATPase activity was mizoribine, an imidazole-based immunosuppressant (see Figure 2), able to complex with Hsp60, thus affecting its protein-folding activity [75,76]. Interestingly, mizoribine’s activity was also related to the inhibition of the detachment of the co-chaperonin Hsp10 from the Hsp60/Hsp10 complex with a significant difference in the activities observed with the prokaryotic GroEL/GroES system, which is not significantly affected by mizoribine [77]. The pyrazolopyrimidine EC3016 (see Figure 2) was also reported to inhibit the protein-folding function of Hsp60 by blocking ATP binding and hydrolysis [76].
Figure 2.
Chemical structures of Hsp60 inhibitors binding the ATP binding site.
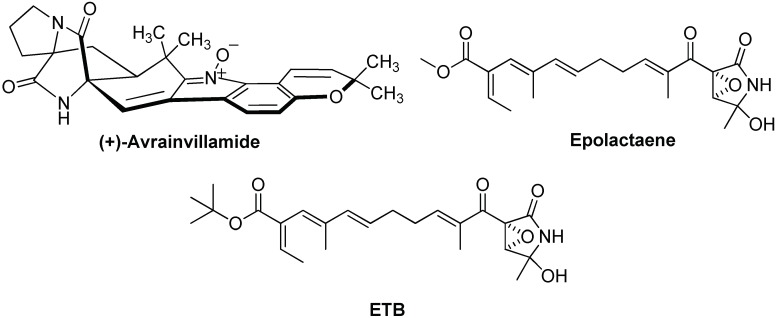
Among compounds that are supposed to interact with cysteine residues, it is worth mentioning natural compounds such as avrainvillamide and epolactaene (see Figure 3). Avrainvillamide is a fungal metabolite supposed to alkylate Hsp60’s cysteine residues through the electrophilic indole-oxide moiety [78]. However, its mode of action has not been demonstrated yet.
Figure 3.
Chemical structures of Hsp60 inhibitors binding the Cys442 residues.
One of the most studied Hsp60 inhibitors is epolactaene, a bacterial metabolite which inhibits the folding activity of human Hsp60, through covalent binding to Cys442 [79]. Epolactaene derivatives, such as the tertiary butyl ester ETB (Figure 3), were also active [80] and SAR studies demonstrated that both the cyclic amide (lactam) and the α,β-unsaturated ketone are critical moieties for inhibiting the chaperone activity of Hsp60 [81]. An in-silico study suggested a putative mode of action for epolactaene, revealing the opening of a binding pocket in proximity of Cys442 after ATP occupies its binding site and that epolactaene covalently binds thiol moiety of Cys 442 through attack at C14 and epoxide ring-opening [82]. MD studies evidenced that epolactaene binding hinders the dynamic conformational changes of the monomer necessary for functional folding process.
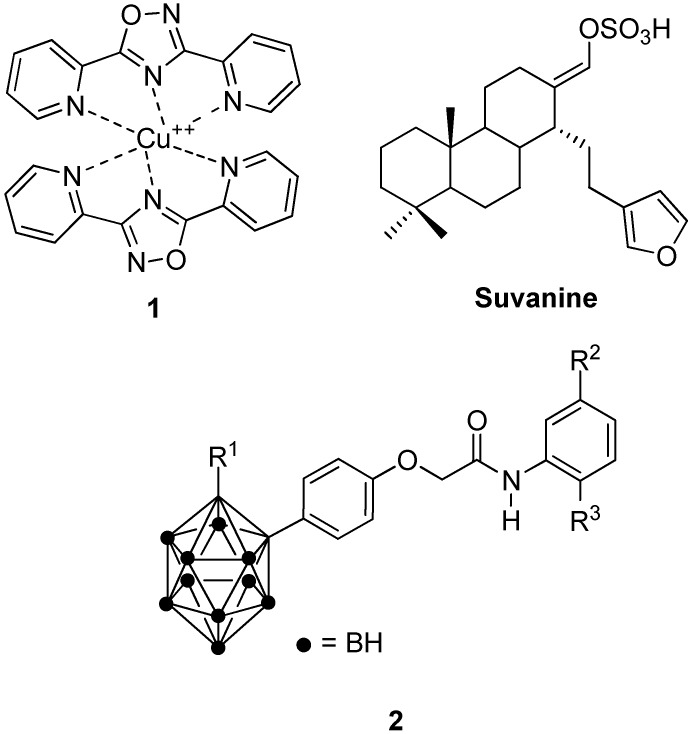
Other representative molecules interacting with Hsp60 or affecting its expression with unknown mechanism of action include copper Complex 1 [27,83], marine sesquiterpene suvanine [84] and carboranylphenoxyacetanilide derivatives 2 [85,86,87] (Figure 4). Other portions of Hsp60 can be investigated to develop new inhibitors; for instance, targeting could be focused on the site of interaction between the mitochondrial Hsp60 and Hsp10. Overall, lack of consensus on the oligomers involved on the folding cycle and the lack of a co-crystallized structure with a known inhibitor, leave several unanswered questions concerning Hsp60’s role in AD. Drug design targeting Hsp60 is therefore a perspective growing field of research and its translation into potential AD therapies is still unexplored.
Figure 4.
Chemical structures of Hsp60 modulators.
4. Hsp70
4.1. Biological Role in AD
The Hsp70 family is composed by 17 members [32], some of which can be induced by stress while others, such as Hsc70, are constitutively expressed. Hsp70 chaperones are found in most cellular compartments, including the nucleus and cytoplasm (Hsc70), mitochondria (mtHsp70, also known as HSPA9 or mortalin) and ER (Grp78, also known as BiP) [32,88]. Hsc70 assists the folding of client proteins via an ATP dependent mechanism and prevents aggregation of the unfolded proteins [88]. Hsp70 can be associated with the co-chaperones Hsp40 and can also collaborate with Hsp90 in various cellular compartments [89]. Overexpression of Hsp70 can determine resistance against apoptosis-inducing agents while downregulation of Hsp70 levels leads to increased sensitivity towards these agents [90,91]. Hsp70 levels were increased in different type of tumours and its presence is associated with poor prognosis in breast and endometrial cancer. Hsp70 binds tumour-suppressor proteins, determining unlimited cellular growth and increased resistance to chemotherapy in breast cancer [88,92]. On the contrary, downregulation of Hsp70 levels in some types of cancers induces differentiation and cell death [93]. Hsp70 can trigger the activation of the immune response by stimulating both innate and adaptive immunities. Moreover, Hsp70 is actively secreted by different types of cells via unusual protein secretory routes, including exosome pathways [94]. Extracellular Hsp70 can exert an immunomodulatory effect, thus playing an important role in the immune response to cancer cells [95,96]. For instance, microvesicles bearing Hsp70 on their surface can activate macrophages or other natural killer cells and play as an indirect regulator of vascular homeostasis [94,97].
Many data demonstrated that Hsp70 is involved in neurodegeneration. In the brains of transgenic mice affected by AD, an increased level in the expression of Hsp70 has been associated with protective effects [98]. Hsp70 may accomplish a neuroprotective role, inhibiting Aβ aggregation suggesting a potential role of Hsp70 in the pathogenesis of this disease [99]. Indeed, Hsp70 can bind with APP and interfere with its secretory route to reduce formation of Aβ [31]. Additionally, Hsp70 can degrade Tau and Aβ oligomers trough the proteasome system [31]. Immunohistochemistry assays and protein expression analyses in AD brain tissues showed high levels of Hsp70 expression in affected regions and these levels appeared to be correlated to the presence of activated glia and dysregulated or stressed neurons [100]. The combination of Hsp70/Hsp40 and Hsp90 induces structural modifications in cytosolic Aβ oligomers but has little effects on fibrils [43]. There are two proposed mechanisms by which HSPs can inhibit the aggregation of Aβ. In one pattern, the chaperone binds misfolded amyloid in an ATP-independent manner, preventing it from aggregation. In a second pattern, the chaperone may bind Aβ in an ATP-dependent manner, changing Aβ conformation to one that is less susceptible to aggregation [101]. Cumulative evidence indicates that Hsp70 has neuroprotection activity against various intracellular amyloids in Drosophila and mouse models [102]. Hsp70 has been associated also with extracellular deposits in AD. In fact, while Hsp70 is normally a cytosolic protein, such an association may be a consequence of release, probably through exosomes to stop the accumulation of proteotoxic assemblies, in agreement with the increased levels of Hsp70 observed in AD. De Mena et al. [102] demonstrated that the engineered form of secreted Hsp70 is highly protective against toxicity induced by extracellular deposition of the Aβ42 in Drosophila. Chaperone proteins, including Hsp70, can bind abnormal Tau directly and reduce its concentration by favouring its degradation and de-phosphorylation [32]. Overall, it is clear that the Hsp70 family is implicated in AD through pathogenic and/or protective mechanisms in which these chaperones (with or without their co-chaperones) participate.
4.2. Targeting and Inhibition
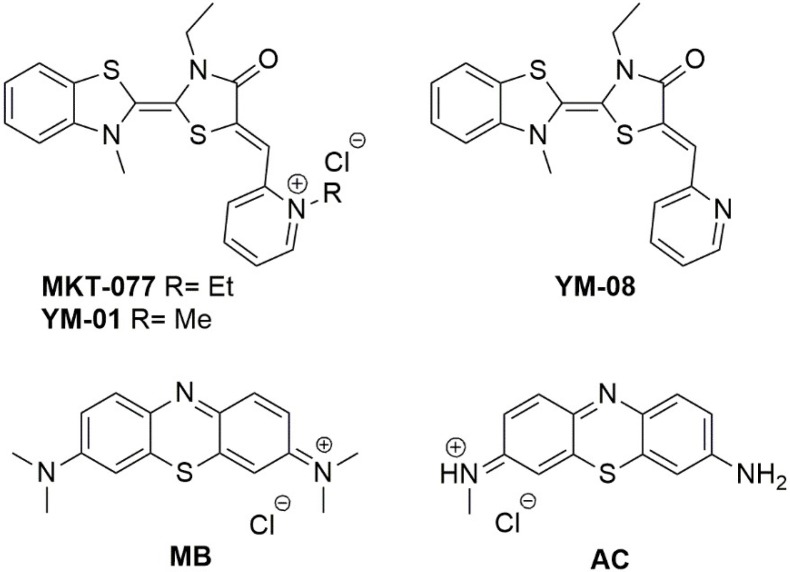
The drug discovery studies targeting Hsp70’s role in AD mainly consists of the transpositions of previous researches in cancer therapy. Moreover, researchers could benefit of well-established screening strategies of new compounds in vitro, as well as in vivo [103]. Hsp70 targeting is related to the design of inhibitors pointing at the ATP-binding site, that is, the allosteric sites in the nucleotide-binding domain (NBD), which is also the substrate-binding domain (SBD) [19]. Compounds bearing the (benzothiazolin-2-yliden)-4-oxothiazolidin-2-ylidene (rhodacyanine) skeleton are reported to bind different allosteric sites of Hsp70 and were previously investigated as anti-cancer compounds [19]. Among these, homologues MKT-077 and YM-01 (Figure 5) were considered as candidate in AD treatment for their ability to rapidly and potently reduce Tau levels in vitro and ex vivo, by targeting Hsp70 [104,105].
Figure 5.
Chemical structures of Hsp70 inhibitors targeting the ATP binding site.
In Tau transgenic brain slices, YM-01 also increased long-term potentiation. Even if its mode of action and binding site was extensively studied by means of NMR and computational techniques [104], MKT-077 was not further considered due to its renal toxicity and low BBB penetration, in general, the rhodacyanine scaffold was considered difficult to improve. Nevertheless, this scaffold was considered as one of the most promising for the development of multimodal drugs able to reduce Tau levels through Hsp70’s modulation to interact with misfolded Tau, thus reducing its toxicity [106]. Indeed, one major improvement of this scaffold was the removal of the pyridinium moiety, as in inhibitor YM-08 (see Figure 5), bearing a neutral pyridine ring [107]. Compared to MKT-077, YM-08 showed a lower activity concerning Hsp70 inhibition and Tau/phospho-Tau reduction but possessed a better PK profile, due the ability to cross the BBB [107].
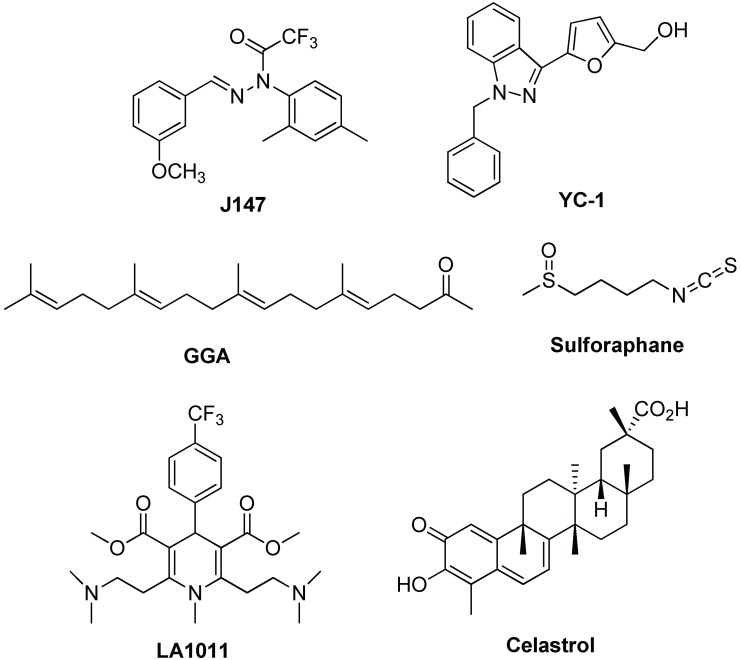
Another important class of molecules is that of phenothiazines (see Figure 5). For instance, Methylene Blue (MB) and Azure C (AC) are able to reduce total Tau and phosphorylated-Tau levels through the inhibition of Hsp70 ATPase function, although with low selectivity [108,109]. Tau toxicity reduction was observed when AC directly interacted with toxic oligomers, by means of induced conformational changes [110], an effect observed also for MB [106]. This suggests the use of phenothiazine derivatives as multimodal drug toward AD. Moreover, the synergistic effect of Hsp70 ATPase activity and Tau aggregation inhibition seems a good way for therapeutic intervention and these two targets should be combined during drug screening [106]. Other representative molecules able to modulate Hsp70 expression are reported in Figure 6.
Figure 6.
Chemical structures of modulators of Hsp70 expression.
J147 is a potent neurotrophic molecule that, in a transgenic AD mouse model, prevents the loss of synaptic proteins and cognitive decline by reducing Hsp70 expression, while inducing Hsp90 overexpression [111]. YC-1, a synthetic small molecule initially developed as an activator of guanylyl cyclase (GC), was proposed as neuroprotective compound due to its ability to suppress Aβ25–35 toxicity in PC12 cells by inducing Hsp70 overexpression [112]. Moreover, geranylgeranylacetone (GGA), a drug approved for ulcer therapy, is able to induce Hsp70 expression with a safe profile. It was tested in an APP23 AD mice model improving its cognitive function and decreasing levels of Aβ, Aβ plaque deposition and synaptic loss [113]. Initially, GGA mode of action was unclear and in some experiments demonstrated to be HSP-independent. More recently, it was verified that amelioration in AD model occurs by regulation of the ERK/p38 MAPK signalling pathway [98]. Oral treatment of a triple transgenic mouse model of AD (3 × Tg-AD) with sulforaphane increases levels of Hsp70 and C-terminus of Hsp70-interacting protein (CHIP), inducing Aβ and Tau clearance and restoring memory deficits [114]. Similar positive effects were also evidenced for 1,4-dihydropyridine candidate LA1011, a synthetic molecule able to upregulate Hsp70 in vitro in SH-SY5Y cells and in vivo with a APPxPS1 mouse model of AD [115]. The extract of Ginkgo biloba leaves, an accepted traditional Chinese medicine, reduce neurotoxicity of the Aβ1–42 oligomer by increasing Hsp70, among other proteins, in SH-SY5Y cells [116]. Similarly, the Hsp70-induced effect was demonstrated in a neuronal cellular model for celastrol [117]. In general, targeting of Hsp70 seems a good strategy in the search of neuroprotective drugs, in particular, for the managing of Tau in AD and in other tauopathies. Further advances in this field could be envisaged in the next future with selective targeting of constitutive protein versus stress-induced ones.
5. Hsp90
5.1. Biological Role in AD
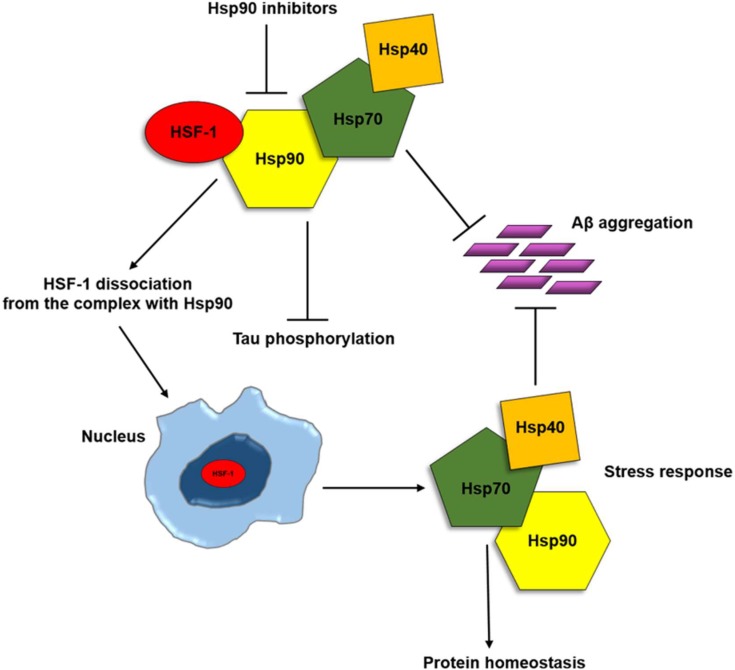
There are at least five types of human Hsp90: HSP90A in cytosol, HSP90alpha, HSP90beta, HSP90B (or Grp94) in the ER and TRAP in mitochondria [118]. Under stress conditions, Hsp90 is the most abundant protein in eukaryotic cells and, like other molecular chaperones, is present in any of its form in most cellular compartments (cytosol, endoplasmic reticulum, mitochondria and chloroplast) [118]. Hsp90 is an ATP-dependent chaperone and plays an important role in the folding of many proteins and in the refolding of denatured proteins after stress [32]. Hsp90 binds several substrates in their native states and targets a specific set of client proteins that are involved in signal transduction [113]. Many of these client proteins are bound to Hsp90 in an inactive state and are activated upon dissociation from Hsp90 [118]. Hsp90 interacts with important client kinases, including cyclin-dependent serine kinases [118]. In cancer cells, Hsp90 is overexpressed and is essential for the malignant transformation and progression of several tumour types such as bladder, breast and lung cancers, as well as leukaemia [30]. Similar to Hsp60 and Hsp70, also Hsp90 has a role in AD. Many data demonstrated that Hsp90 inhibits amyloid aggregation [43], while the complex of Hsp90 with Hsp70/Hsp40 can inhibit Aβ formation [43]. Hsp90 can be released in extracellular environment free or associated with exosomes [94]. When outside the cell, it has a role in activating the immune system [97]. In nervous system, extracellular Hsp90 determines activation of microglial phagocytosis that push Aβ degradation by activation of the Toll-like receptor-4 (TLR4) pathway [119]. From another point of view, chaperone proteins such as Hsp90 form macromolecular complexes with co-chaperones, which can regulate Tau metabolism and Aβ processing [32]. Many data demonstrated that pharmacological inhibition of Hsp90 significantly decreases intracellular levels of the disease-associated phosphorylated Tau species via proteasomal degradation [100]. Administration of Hsp90 inhibitors to primary neurons prevented Aβ induced neurotoxicity [120]. Dickey et al. [121], demonstrated that inhibition of Hsp90 determined a reduction of phosphorylated Tau form and the carboxy terminus of Hsp70-interacting protein (CHIP) is involved in this mechanism. The recruitment of CHIP protein, a co-chaperone with E3 activity, induces the ubiquitination of Tau protein and activates its downstream degradation processes. Many data demonstrated that the combination of chaperones was able to significantly affect the aggregation (see Figure 7).
Figure 7.
Hsp90 inhibition in Alzheimer’s disease. Hsp90 down regulation may induce the reduction of Tau hyperphosphorilation and aggregation and may trigger the so-called stress response. In fact, in the presence of cellular stress and Hsp90 inhibitors, Heat Shock Factor 1 (HSF-1) dissociates from the chaperone, reaches the nucleus, inducing the activation of heat shock genes and of the stress response via the production of Hsp90, Hsp70 and Hsp40, restoring protein homeostasis.
5.2. Targeting and Inhibition
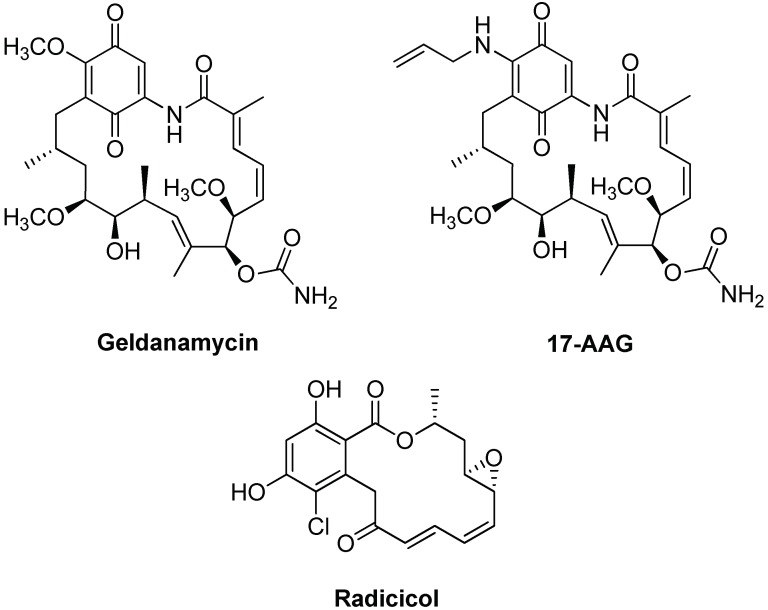
Contrary to Hsp60 and Hsp70, Hsp90 role in AD development and progression seems better defined as reported in the literature cited above. For example, Hsp90 inhibition might be useful in AD treatment counteracting Tau protein hyperphosphorylation and aggregation. However, also in this case, the research of Hsp90 inhibitors in AD could benefit from previous findings regarding anti-cancer drugs [122], with many compounds already tested in clinical trials [123]. The identification of potential Hsp90 inhibitors could be efficiently performed by means of different screening methods including microarray- [124], virtual- [125,126] or cell-based screening [127]. Hsp90 inhibitors mainly interact with the nucleotide-binding pocket, located in the N-terminal domain, where they bind to the ATP-binding site preventing the ADP- and ATP-bound conformational changes necessary for the chaperone activity [19]. This protein site is targeted by many interesting inhibitors, such as Geldanamycin (GA), 17-allylamino-17-desmethoxy-geldanamycin (17-AAG) and radicicol (see Figure 8).
Figure 8.
Chemical structures of Hsp90 inhibitors targeting the ATP binding site.
GA was the first discovered Hsp90 inhibitor; it was isolated from Streptomyces genus and was initially studied as antibiotic and antitumor but toxicity issues stopped further studies [123]. Nevertheless, many GA analogues were developed and 17-AAG was particularly considered as a potent Hsp90 inhibitor with better solubility and safer profile. Pharmacokinetic data obtained from research on 17-AAG as anti-tumoral drug, induced its repurposing as a therapy against AD and other neurodegenerative diseases. The in vivo effects of 17-AGG were demonstrated in a rat model, injected with Aβ25–35 into the hippocampus. [128]. Oral administration of 17-AAG reduces brain injury and improves cognitive processes by inducing HSPs (Hsp27, Hsp40 and, in particular, Hsp70) overexpression at the cellular level. The effect of this inhibitor on the other major target in AD, Tau, was tested in vivo in a mouse model, revealing that high dose of 17-AAG tended to decrease NFTs in transgenic mice [129]. Interestingly, these studies evidenced no effect of 17-AAG on amyloid plaques in Tg2576 mouse model and a significant reduction of NFTs in male tau transgenic (JNPL3) mice. Other authors demonstrated, in Tg2576 mouse model and cultured neurons, that 17-AAG reduces the damage from soluble Aβ and activates the expression of synaptic proteins through HSF1 [130]. In a model of Drosophila larvae expressing human Tau, the protein was reduced in larvae treated with 17-AAG but without the ability to restore locomotion deficit [131]. A similar trend was observed for radicicol, which was proposed for the treatment of neurodegenerative diseases [132].
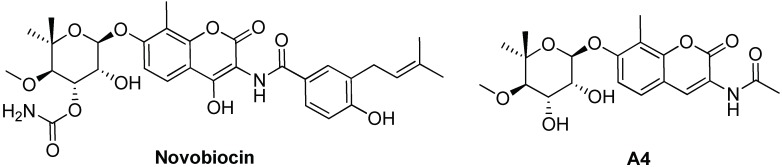
Hsp90 C-terminal domain was also described as an important target, even if few inhibitors are reported in the literature, such as celastrol (see Figure 6), novobiocin and its derivative A4 (see Figure 9) [19]. The protective effect of these compounds was demonstrated on cellular models through Aβ-induced cell death experiments [133,134]. For novobiocin-derived compound A4, the ability to modulate Hsp70 expression as well as a simulation of BBB penetration was also reported [134].
Figure 9.
Chemical structures of Hsp90 inhibitors binding the C-terminal domain.
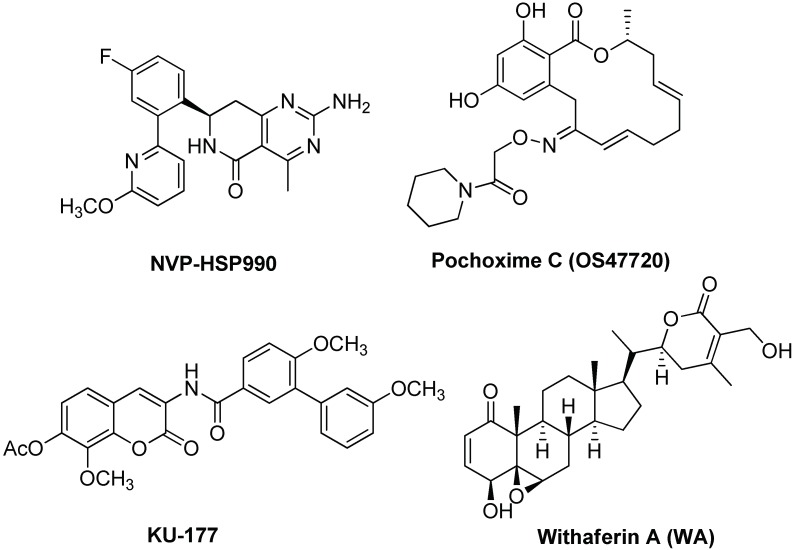
Other Hsp90 inhibitors or modulators were recently investigated (see Figure 10). Reversal of synaptic impairments in a rTg4510 transgenic AD mouse model was obtained with compound NVP-HSP990 which has a high Hsp70 induction capacity and is probably able to induce Tau clearance [135]. Pochoxime C (OS47720), a CNS-permeable and non-toxic Hsp90 inhibiting compound, restores synaptic dysfunction and memory loss in vivo in a Tg2576 mice AD model [136]. The effects of OS47720 depend upon HSF-1 activation and are followed by HSF1-mediated transcriptional events on synaptic genes. This study points out the importance of using Hsp90 inhibitors with a safe profile for an actual application toward neurodegenerative diseases and suggests their use in association with other drugs, such as β-secretase inhibitors, for a perspective multiple drug therapy approach.
Figure 10.
Chemical structures of Hsp90 inhibitors/modulators.
Finally, a recent trend is the modulation of Hsp90 functions through co-chaperones modulations. In fact, the co-chaperone activator of Hsp90 ATPase homolog 1 (Aha1) increased the production of aggregated Tau [137]. Treatment with KU-177, a novobiocin-based Aha1 inhibitor, reduced the accumulation of insoluble Tau in rTg4510 transgenic mouse model [137]. Similarly, a potential application for AD treatment was suggested for withaferin A (WA), a potent inhibitor of the Hsp90/Cdc37 interaction by regulation of LRRK2, like celastrol [138].
6. Conclusions
The study of connections between AD and HSPs is a research area of great interest and therapeutic potential in the next future (Table 2).
Table 2.
HSPs localization, functions and involvement in AD and neurodegeneration.
In the last decade, many results were obtained mainly from research on anti-cancer agents. Some compounds of potential therapeutic interest were highlighted but clinical trials are not in due course. Therefore, gaining further knowledge is fundamental and many issues should be clarified, such as: (i) AD biochemical pathways involving HSPs; (ii) mode of action of HSPs inhibitors; (iii) selective targeting of constitutive versus stress-induced HSPs; (iv) understanding of client/HSPs protein-protein interactions at the molecular level [139,140]. In general, HSPs targeting could be a keystone for perspective drugs in the context of multitargeted drug discovery and polypharmacological approach toward a complex disease such as AD.
Funding
This research and APC were funded by the Italian MIUR within the “FIRB-Futuro in Ricerca 2012” Program-Grant Project RBFR12SIPT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hamley I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 2.Parodi J., Sepúlveda F.J., Roa J., Opazo C., Inestrosa N.C., Aguayo L.G. β-Amyloid causes depletion of synaptic vesicles leading to neurotransmission failure. J. Biol. Chem. 2010;285:2506–2514. doi: 10.1074/jbc.M109.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerson J.E., Castillo-Carranza D.L., Kayed R. Advances in therapeutics for neurodegenerative tauopathies: Moving toward the specific targeting of the most toxic tau species. ACS Chem. Neurosci. 2014;5:752–769. doi: 10.1021/cn500143n. [DOI] [PubMed] [Google Scholar]
- 4.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar D., Ganeshpurkar A., Kumar D., Modi G., Gupta K.S., Singh K.S. Secretase inhibitors for the treatment of Alzheimer’s disease: Long road ahead. Eur. J. Med. Chem. 2018;148:436–452. doi: 10.1016/j.ejmech.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Martorana A., Giacalone V., Bonsignore R., Pace A., Gentile C., Pibiri I., Buscemi S., Lauria A., Palumbo Piccionello A. Heterocyclic scaffolds for the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2016;22:3971–3995. doi: 10.2174/1381612822666160518141650. [DOI] [PubMed] [Google Scholar]
- 7.Gerson J.E., Kayed R. Therapeutic approaches targeting pathological tau aggregates. Curr. Pharm. Des. 2016;22:4028–4039. doi: 10.2174/1381612822666160518142226. [DOI] [PubMed] [Google Scholar]
- 8.Battistini A., Palumbo Piccionello A., Sgarbossa A., Vilasi S., Ricci C., Ghetti F., Spinozzi F., Marino Gammazza A., Giacalone V., Martorana A., et al. Curcumin-like compounds designed to modify amyloid beta peptide aggregation patterns. RSC Adv. 2017;7:31714. doi: 10.1039/C7RA05300B. [DOI] [Google Scholar]
- 9.Mangione M.R., Palumbo Piccionello A., Marino C., Ortore M.G., Picone P., Vilasi S., Di Carlo M., Buscemi S., Bulone D., San Biagio P.L. Photo-inhibition of Aβ fibrillation mediated by a newly designed fluorinated oxadiazole. RSC Adv. 2015;5:16540–16548. doi: 10.1039/C4RA13556C. [DOI] [Google Scholar]
- 10.Yang X., Cai P., Liu Q., Wu J., Yin Y., Wang X., Long L. Novel 8-hydroxyquinoline derivatives targeting β-amyloid aggregation, metal chelation and oxidative stress against Alzheimer’s disease. Bioorg. Med. Chem. 2018;26:3191–3201. doi: 10.1016/j.bmc.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Spinello A., Bonsignore R., Barone G., Keppler B.K., Terenzi A. Metal ions and metal complexes in Alzheimer’s disease. Curr. Pharm. Des. 2016;22:3996–4010. doi: 10.2174/1381612822666160520115248. [DOI] [PubMed] [Google Scholar]
- 12.Macario A.J., De Macario E.C. Molecular chaperones and age-related degenerative disorders. Adv. Cell. Aging Gerontol. 2001;7:131–162. doi: 10.1016/S1566-3124(01)07018-3. [DOI] [Google Scholar]
- 13.Cappello F., Marino Gammazza A., Vilasi S., Ortore M.G., San Biagio P.L., Campanella C., Pace A., Palumbo Piccionello A., Taglialatela G., De Macario E.C., et al. Chaperonotherapy for Alzheimer’s Disease: Focusing on HSP60. In: Asea A.A.A., Almasoud N.N., Krishnan S., Kaur P., editors. Heat Shock Protein-Based Therapies. Volume 9. Springer; Cham, Switzerland: 2015. pp. 51–76. [DOI] [Google Scholar]
- 14.Manos-Turvey A., Brodsky J.L., Wipf P. The Effect of Structure and Mechanism of the Hsp70 Chaperone on the Ability to Identify Chemical Modulators and Therapeutics. Top. Med. Chem. 2016;19:81–130. doi: 10.1007/7355_2015_90. [DOI] [Google Scholar]
- 15.Turturici G., Sconzo G., Geraci F. Hsp70 and its molecular Role in nervous system diseases. Biochem. Res. Int. 2011;2011:618127. doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontaine S.N., Martin M.D., Dickey C.A. Neurodegeneration and the Heat Shock Protein 70 Machinery: Implications for Therapeutic Development. Curr. Top. Med. Chem. 2016;16:2741–2752. doi: 10.2174/1568026616666160413140741. [DOI] [PubMed] [Google Scholar]
- 17.Blair L.J., Sabbagh J.J., Dickey C.A. Targeting HSP90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin. Ther. Targets. 2014;18:1219–1232. doi: 10.1517/14728222.2014.943185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inda C., Bolaender A., Tai W., Gandu S.R., Koren J., III Stressing out Hsp 90 in Neurotoxic Proteinopathies. Curr. Top. Med. Chem. 2016;16:2829–2838. doi: 10.2174/1568026616666160413141350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taldone T., Ochiana S.O., Patel P.D., Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 2014;35:592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino Gammazza A., Caruso Bavisotto C., Barone R., Macario E.C., Macario A.J. Alzheimer’s disease and Molecular Chaperones: Current Knowledge and the Future of Chaperonotherapy. Curr. Pharm. Des. 2016;22:4040–4049. doi: 10.2174/1381612822666160518141437. [DOI] [PubMed] [Google Scholar]
- 21.Cappello F., Marino Gammazza A., Palumbo Piccionello A., Campanella C., Pace A., De Macario E.C., Macario A.J. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert Opin. Ther. Targets. 2014;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- 22.Pace A., Barone G., Lauria A., Martorana A., Palumbo Piccionello A., Pierro P., Terenzi A., Almerico A.M., Buscemi S., Campanella C., et al. Hsp60, a novel target for antitumor therapy: Structure-function features and prospective drugs design. Curr. Pharm. Des. 2013;19:2757–2764. doi: 10.2174/1381612811319150011. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S., Burns T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approch. Int. J. Mol. Sci. 2017;18:1978. doi: 10.3390/ijms18091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Liu T., Rios Z., Mei Q., Lin X., Cao S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2016;38:226–256. doi: 10.1016/j.tips.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Macario A.J.L., De Macario E.C. Chaperonopathies and chaperonotherapy. FEBS Lett. 2007;581:3681–3688. doi: 10.1016/j.febslet.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 26.Campanella C., D’Anneo A., Marino Gammazza A., Caruso Bavisotto C., Barone R., Emanuele S., Lo Cascio F., Mocciaro E., Fais S., De Macario E.C., et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget. 2016;7:28849–28867. doi: 10.18632/oncotarget.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso Bavisotto C., Nikolic D., Marino Gammazza A., Barone R., Lo Cascio F., Mocciaro E., Zummo G., De Macario E.C., Macario A.J., Cappello F., et al. The dissociation of the Hsp60/pro-Caspase-3- complex by bis (pyrydil)oxadiazole complex (CubipyOXA) leads to cell death in NCl-H292 cancer cells. J. Inorg. Biochem. 2017;170:8–16. doi: 10.1016/j.jinorgbio.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Vilasi S., Bulone D., Caruso Bavisotto C., Campanella C., Marino Gammazza A., San Biagio P.L., Cappello F., De Macario E.C., Macario A.J.L. Chaperonin of group I: Oligomeric spectrum and biochemical and biological implications. Front. Mol. Biosc. 2018;4:99. doi: 10.3389/fmolb.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macario A.J.L., De Macario E.C. Molecular chaperones: Multiple functions, pathologies, and potential applications. Front. Biosci. 2007;12:2588–2600. doi: 10.2741/2257. [DOI] [PubMed] [Google Scholar]
- 30.Rappa F., Sciume C., Lo Bello M., Bavisotto Caruso C., Marino Gammazza A., Barone R., Campanella C., David S., Carini F., Zarcone F., et al. Comparative analysis of hsp10 and hsp90 expression in healthy mucosa and adenocarcinoma of the large bowel. Anticancer Res. 2014;34:4153–4159. [PubMed] [Google Scholar]
- 31.Maiti P., Manna J., Veleri S., Frautschy S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed. Res. Int. 2014;2014:495091. doi: 10.1155/2014/495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lackie R.E., Maciejewski A., Ostapchenko V.G., Marques-Lopez J., Choy W.Y., Duennwald M.L., Prado V.F., Prado M.A.M. The Hsp70/Hsp90 cheperone machinery in neurodegenerative diseases. Front. Neurosci. 2017;11:254. doi: 10.3389/fnins.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum D., Chern Y., Domenici M.R., Buée L., Lin C.Y., Rea W., Ferré S., Popoli P. The Role of Adenosine Tone and Adenosine Receptors in Huntington’s Disease. J. Caffeine Adenosine Res. 2018;8:43–58. doi: 10.1089/caff.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarty A.K., Jarosz D.F. More than Just a Phase: Prions at the Crossroads of Epigenetic Inheritance and Evolutionary Change. J. Mol. Biol. 2018 doi: 10.1016/j.jmb.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexopoulou Z., Lang J., Perrett R.M., Elschami M., Hurry M.E., Kim H.T., Mazaraki D., Szabo A., Kessler B.M., Goldberg A.L., et al. Deubiquitinase Usp8 regulates α-synuclein clearance and modifies its toxicity in Lewy body disease. Proc. Natl. Acad. Sci. USA. 2016;113:E4688–E4697. doi: 10.1073/pnas.1523597113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czarnecka A.M., Campanella C., Zummo G., Cappello F. Mitochondrial chaperones in cancer: From molecular biology to clinical diagnostics. Cancer Biol. Ther. 2006;5:714–720. doi: 10.4161/cbt.5.7.2975. [DOI] [PubMed] [Google Scholar]
- 37.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdul H.M., Butterfield D.A. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by co-treatment of acetyl-l-carnitine and α-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: Implications for Alzheimer’s disease. Free Radical Biomol. 2007;42:371–384. doi: 10.1016/j.freeradbiomed.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mateju D., Franzmann T.M., Patel A., Kopach A., Boczek E.E., Maharana S., Lee H.O., Carra S., Hyman A.A., Simon A. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017;36:1669–1687. doi: 10.15252/embj.201695957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meriin A.B., Narayanan A., Meng L., Alexandrov I., Varelas X., Cissé I.I., Sherman M.Y. Hsp70-Bag3 complex is a hub for proteotoxicity-induced signaling that controls protein aggregation. Proc. Natl. Acad. Sci. USA. 2018;115:E7043–E7052. doi: 10.1073/pnas.1803130115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walls K.C., Coskun P., Gallegos-Perez J.L., Zadourian N., Freude K., Rasool S., Blurton-Jones M., Green K.N., LaFerla F.M. Swedish Alzheimer mutation induces 877 mitochondrial dysfunction mediated by HSP60 mislocalization of amyloid precursor protein 878 (APP) and beta-amyloid. J. Biol. Chem. 2012;287:30317–30327. doi: 10.1074/jbc.M112.365890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R.S., Ramachandra N.B., Bowes T., Singh B. Unusual cellular disposition of the mitochondrial molecular chaperones Hsp60, Hsp70 and Hsp10. Novartis Found. Symp. 2008;291:59–68. doi: 10.1002/9780470754030.ch5. [DOI] [PubMed] [Google Scholar]
- 43.Luo G.R., Le W.D. Collective roles of molecular chaperones in protein degradation pathways associated with neurodegenerative diseases. Curr. Pharm. Biotechnol. 2010;11:180–187. doi: 10.2174/138920110790909740. [DOI] [PubMed] [Google Scholar]
- 44.Cuervo A.M., Wong E. Chaperone-mediated autophagy: Roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merendino A.M., Bucchieri F., Campanella C., Marcianò V., Ribbene A., David S., Zummo G., Burgio G., Corona D.F., De Macario C.E., et al. Hsp60 is actively secreted by human tumor cells. PLoS ONE. 2010;5:e9247. doi: 10.1371/journal.pone.0009247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campanella C., Bucchieri F., Merendino A.M., Fucarino A., Burgio G., Corona D.F., Barbieri G., David S., Farina F., Zummo G., et al. The odyssey of hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS ONE. 2012;7:e42008. doi: 10.1371/journal.pone.0042008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campanella C., Rappa F., Sciumè C., Marino Gammazza A., Barone R., Bucchieri F., David S., Curcurù G., Caruso Bavisotto C., Pitruzzella A., et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121:3230–3239. doi: 10.1002/cncr.29499. [DOI] [PubMed] [Google Scholar]
- 48.Caruso Bavisotto C., Marino Gammazza A., Rappa F., Fucarino A., Pitruzzella A., David S., Campanella C. Exosomes: Can doctors still ignore their existence? EuroMediterr. Biomed. J. 2013;8:137–139. doi: 10.3269/1970-5492.2013.8.22. [DOI] [Google Scholar]
- 49.Chandra D., Choy G., Tang D.G. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: Evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J. Biol. Chem. 2007;282:31289–31301. doi: 10.1074/jbc.M702777200. [DOI] [PubMed] [Google Scholar]
- 50.Campanella C., Bucchieri F., Ardizzone N.M., Marino Gammazza A., Montalbano A., Ribbene A., Di Felice V., Bellafiore M., David S., Rappa F., et al. Upon oxidative stress, the antiapoptotic Hsp60/procaspase-3 complex persists in mucoepidermoid carcinoma cells. Eur. J. Histochem. 2008;52:221–228. doi: 10.4081/1220. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh J.C., Dohi T., Kang B.H., Altieri D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S., Knowlton A.A. HSP60, Bax, apoptosis and the heart. J. Cell. Mol. Med. 2005;9:51–58. doi: 10.1111/j.1582-4934.2005.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xanthoudakis S., Roy S., Rasper D., Hennessey T., Aubin Y., Cassady R., Tawa P., Ruel R., Rosen A., Nicholson D.W. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999;18:2049–2056. doi: 10.1093/emboj/18.8.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorska M., Marino Gammazza A., Zmijewski M.A., Campanella C., Cappello F., Wasiewicz T., Kuban-Jankowska A., Daca A., Sielicka A., Popowska U., et al. Geldanamycin-induced osteosarcoma cell death is associated with hyperacetylation and loss of mitochondrial pool of heat shock protein 60 (hsp60) PLoS ONE. 2013;8:e71135. doi: 10.1371/journal.pone.0071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y., Zheng J., Xu Y., Zhang X. Paraquat-induced inflammatory response of microglia through HSP60/TLR4 signaling. Hum. Exp. Toxicol. 2018;1:960327118758152. doi: 10.1177/0960327118758152. [DOI] [PubMed] [Google Scholar]
- 56.Takano M., Yamashita T., Nagano K., Otani M., Maekura K., Kamada H., Tsunoda S., Tsutsumi Y., Tomiyama T., Mori H., et al. Proteomic analysis of the hippocampus in Alzheimer’s disease model mice by using two-dimensional fluorescence difference in gel electrophoresis. Neurosci. Lett. 2013;534:85–89. doi: 10.1016/j.neulet.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Zhang R., Li Y., Hou X., Miao Z., Wang Y. Neuroprotective effect of heat shock protein 60 on matrine-suppressed microglial activation. Exp. Ther. Med. 2017;14:1832–1836. doi: 10.3892/etm.2017.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Block M.L., Zecca L., Hong J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 59.Liu B., Hong J.S. Role of microglia in inflammation-mediated neurodegenerative disease: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 60.Cappello F., De Macario C.E., Marino Gammazza A., Bonaventura G., Carini F., Czarnecka A.M., Farina F., Zummo G., Macario A.J.L. Hsp60 and human aging: Les liaisons dangereuses. Front. Biosci. 2013;18:626–637. doi: 10.2741/4126. [DOI] [PubMed] [Google Scholar]
- 61.Wojsiat J., Prandelli C., Laskowska-Kaszub K., Martín-Requero A., Wojda U. Oxidative Stress and Aberrant Cell Cycle in Alzheimer’s Disease Lymphocytes: Diagnostic Prospects. J. Alzheimers Dis. 2015;46:329–350. doi: 10.3233/JAD-141977. [DOI] [PubMed] [Google Scholar]
- 62.Gleixner A.M., Pulugulla S.H., Pant D.B., Posimo J.M., Crum T.S., Leak R.K. Impact of aging on heat shock protein expression in the substantia nigra and striatum of the female rat. Cell Tissue Res. 2014;357:43–54. doi: 10.1007/s00441-014-1852-6. [DOI] [PubMed] [Google Scholar]
- 63.Bross P., Magnoni R., Bie A.S. Molecular chaperone disorders: Defective Hsp60 in neurodegeneration. Curr. Top. Med. Chem. 2012;12:2491–2503. doi: 10.2174/1568026611212220005. [DOI] [PubMed] [Google Scholar]
- 64.Cardoso S.M., Santana I., Swerdlow R.H., Oliveira C.R. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Aβ toxicity. J. Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 65.Veereshwarayya V., Kumar P., Rosen K.M., Mestril R., Querfurth H.W. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J. Biol. Chem. 2006;281:29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- 66.Mangione M.R., Vilasi S., Marino C., Librizzi F., Canale C., Spigolon D., Bucchieri F., Fucarino A., Passantino R., Cappello F., et al. Hsp60, amateur chaperone in amyloid-beta fibrillogenesis. Biochim. Biophys. Acta. 2016;1860:2474–2483. doi: 10.1016/j.bbagen.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Nemirovsky A., Fisher Y., Baron R., Cohen I.R., Monsonego A. Amyloid beta-HSP60 peptide conjugate vaccine treats a mouse model of Alzheimer’s disease. Vaccine. 2011;29:4043–4050. doi: 10.1016/j.vaccine.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 68.Kondoh Y., Osada H. High-throughput screening identifies small molecule inhibitors of molecular chaperones. Curr. Pharm. Des. 2013;19:473–492. doi: 10.2174/138161213804143743. [DOI] [PubMed] [Google Scholar]
- 69.Qianli M., Bingbing X.L., Xiangshu X. Toward Developing Chemical Modulators of Hsp60 as potential Therapeutics. Front. Mol. Biosci. 2018;5:35. doi: 10.3389/fmolb.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nisemblat S., Parnas A., Yaniv O., Azem A., Frolow F. Crystallization and structure determination of a symmetrical ‘football’ complex of the mammalian mitochondrial Hsp60-Hsp10 chaperonins. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014;70:116–119. doi: 10.1107/S2053230X1303389X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nisemblat S., Parnas A., Yaniv O., Azem A. Crystal structure of the human mitochondrial chaperonin symmetrical football complex. Proc. Natl. Acad. Sci. USA. 2015;112:6044–6049. doi: 10.1073/pnas.1411718112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida R., Okamoto T., Motojima F., Kubota H., Takahashi H., Tanabe M., Oka T., Kitamura A., Kinjo M., Yoshida M., et al. Physicochemical properties of the mammalian molecular chaperone HSP60. Int. J. Mol. Sci. 2018;19:489. doi: 10.3390/ijms19020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vilasi S., Carrotta R., Mangione M.R., Campanella C., Librizzi F., Randazzo L., Martorana V., Marino Gammazza A., Ortore M.G., Vilasi A., et al. Human Hsp60 with its mitochondrial import signal occurs in solution as heptamers and tetradecamers remarkably stable over a wide range of concentrations. PLoS ONE. 2014;9:e97657. doi: 10.1371/journal.pone.0097657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spinello A., Ortore M.G., Spinozzi F., Ricci C., Barone G., Marino Gammazza A., Palumbo Piccionello A. Quaternary structures of GroEL and naïve-Hsp60 chaperonins in solution: A combined SAXS-MD study. RCS Adv. 2015;5:49871–49879. doi: 10.1039/C5RA05144D. [DOI] [Google Scholar]
- 75.Itoh H., Komatsuda A., Wakui H., Miura A.B., Tashima Y. Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J. Biol. Chem. 1999;274:35147–35151. doi: 10.1074/jbc.274.49.35147. [DOI] [PubMed] [Google Scholar]
- 76.Chapman E., Farr G.W., Fenton W.A., Johnson S.M., Horwich A.L. Requirement for binding multiple ATPs to convert a GroEL ring to the folding-active state. Proc. Natl. Acad. Sci. USA. 2008;105:19205–19210. doi: 10.1073/pnas.0810657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanabe M., Ishida R., Izuhara F., Komatsuda A., Wakui H., Sawada K., Otaka M., Nakamura N., Itoh H. The ATPase activity of molecular chaperone HSP60 is inhibited by immunosuppressant mizoribine. Am. J. Mol. Biol. 2012;2:93–102. doi: 10.4236/ajmb.2012.22010. [DOI] [Google Scholar]
- 78.Wulff J.E., Herzon S.B., Siegrist R., Myers A.G. Evidence for the rapid conversion of Stephacidin b into the electrophilic monomer avrainvillamide in cell culture. J. Am. Chem. Soc. 2007;129:4898–4899. doi: 10.1021/ja0690971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagumo Y., Kakeya H., Shoji M., Hayashi Y., Dohmae N., Osada H. Epolactaene binds human Hsp60 Cys442 resulting in the inhibition of chaperone activity. Biochem. J. 2005;387:835–840. doi: 10.1042/BJ20041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun W., Wang L., Jiang H., Murchie A.I. Targeting mitochondrial transcription in fission yeast with ETB, an inhibitor of HSP60, the chaperone that binds to the mitochondrial transcription factor Mtf1. Genes Cells. 2012;17:122–131. doi: 10.1111/j.1365-2443.2011.01578.x. [DOI] [PubMed] [Google Scholar]
- 81.Nagumo Y., Kakeya H., Yamaguchi J., Uno T., Shoji M., Hayashi Y., Osada H. Structure-activity relationships of epolactaene derivatives: Structural requirements for inhibition of Hsp60 chaperone activity. Bioorg. Med. Chem. Lett. 2004;14:4425–4429. doi: 10.1016/j.bmcl.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 82.Spinello A., Barone G., Cappello F., Pace A., Buscemi S., Palumbo Piccionello A. The Binding Mechanism of Epolactaene to Hsp60 Unveiled by in Silico Modelling. ChemistrySelect. 2016;4:759–765. doi: 10.1002/slct.201600125. [DOI] [Google Scholar]
- 83.Terenzi A., Barone G., Palumbo Piccionello A., Giorgi G., Portanova P., Calvaruso G., Buscemi S., Vivona N., Pace A. Synthesis, characterization, cellular uptake and interaction with native DNA of a bis (pyridyl)-1,2,4-oxadiazole copper(II) complex. Dalton Trans. 2010;39:9140–9145. doi: 10.1039/c0dt00266f. [DOI] [PubMed] [Google Scholar]
- 84.Cassiano C., Monti M.C., Festa C., Zampella A., Riccio R., Casapullo A. Chemical proteomics reveals heat shock protein 60 to be the main cellular target of the marine bioactive sesterterpene Suvanine. ChemBioChem. 2012;13:1953–1958. doi: 10.1002/cbic.201200291. [DOI] [PubMed] [Google Scholar]
- 85.Ban H.S., Shimizu K., Minegishi H., Nakamura H. Identification of HSP60 as a primary target of o-carboranylphenoxyacetanilide, an HIF1alpha inhibitor. J. Am. Chem. Soc. 2010;132:11870–11871. doi: 10.1021/ja104739t. [DOI] [PubMed] [Google Scholar]
- 86.Ban H.S., Shimizu K., Minegishi H., Nakamura H. Identification of heat shock protein 60 as the regulator of the hypoxia-inducible factor subunit HIF-1. Pure Appl. Chem. 2012;84:2325–2337. doi: 10.1351/PAC-CON-11-11-03. [DOI] [Google Scholar]
- 87.Nakamura H., Yasui Y., Maruyama M., Minegishi H., Ban H.S., Sato S. Development of hypoxia-inducible factor (HIF)-1a inhibitors: Effect of ortho-carborane substituents on HIF transcriptional activity under hypoxia. Bioorg. Med. Chem. Lett. 2013;23:806–810. doi: 10.1016/j.bmcl.2012.11.081. [DOI] [PubMed] [Google Scholar]
- 88.Murphy M.E. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jores T., Lawatscheck J., Beke V., Franz-Wachtel M., Yunoki K., Fitzgerald J.C., Macek B., Endo T., Kalbacher H., Buchner J., et al. Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial β-barrel proteins. J. Cell. Biol. 2018;217:jcb.201712029. doi: 10.1083/jcb.201712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tatsuta T., Hosono M., Ogawa Y., Inage K., Sugawara S., Nitta K. Downregulation of Hsp70 inhibits apoptosis induced by sialic acid-binding lectin (leczyme) Oncol. Rep. 2014;31:13–18. doi: 10.3892/or.2013.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alani B., Salehi R., Sadeghi P., Khodagholi F., Digaleh H., Jabbarzadeh-Tabrizi S., Zare M., Korbekandi H. Silencing of Hsp70 intensifies 6-OHDA-induced apoptosis and Hsp90 upregulation in PC12 cells. J. Mol. Neurosci. 2015;55:174–183. doi: 10.1007/s12031-014-0298-3. [DOI] [PubMed] [Google Scholar]
- 92.Nadin S.B., Sottile M.L., Montt-Guevara M.M., Gauna G.V., Daguerre P., Leuzzi M., Gago F.E., Ibarra J., Cuello-Carrión F.D., Ciocca D.R., et al. Prognostic implication of HSPA (HSP70) in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Cell Stress Chaperones. 2014;19:493–505. doi: 10.1007/s12192-013-0475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jagadish N., Agarwal S., Gupta N., Fatima R., Devi S., Kumar V., Suri V., Kumar R., Suri V., Sadasukhi T.C., et al. Heat shock protein 70-2 (HSP70-2) overexpression in breast cancer. J. Exp. Clin. Cancer Res. 2016;35:150. doi: 10.1186/s13046-016-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campanella C., Caruso Bavisotto C., Marino Gammazza A., Nikolic D., Rappa F., David S., Cappello F., Bucchieri F., Fais S. Exosomal Heat Shock Proteins as New Players in Tumour Cell-to-Cell Communication. J. Circ. Biomark. 2014;3:4. doi: 10.5772/58721. [DOI] [Google Scholar]
- 95.Bausero M.A., Gastpar R., Multhoff G., Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J. Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gastpar R., Gehrmann M., Bausero M.A., Asea A., Gross C., Schroeder J.A., Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cappello F., Logozzi M., Campanella C., Caruso Bavisotto C., Marcilla A., Properzi F., Fais S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. 2017;96:93–98. doi: 10.1016/j.ejps.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 98.Sun Y., Zhang J.R., Chen S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med. 2017;14:5267–5274. doi: 10.3892/etm.2017.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoshino T., Murao N., Namba T., Takehara M., Adachi H., Katsuno M., Sobue G., Matsushima T., Suzuki T., Mizushima T. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J. Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koren J., Jinwal U.K., Lee D.C., Jones J.R., Shults C.L., Johnson A.G., Anderson L.J., Dickey C.A. Chaperone signalling complexes in Alzheimer’s disease. J. Cell. Mol. Med. 2009;13:619–630. doi: 10.1111/j.1582-4934.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Evans C.G., Wisen S., Gestwicki J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J. Biol. Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 102.De Mena L., Chhangani D., Fernandez-Funez P., Rincon-Limas D.E. secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids. Fly. 2017;11:179–184. doi: 10.1080/19336934.2017.1291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Repalli J., Meruelo D. Screening strategies to identify HSP70 modulators to treat Alzheimer’s disease. Drug Des. Dev. Ther. 2015;9:321–331. doi: 10.2147/DDDT.S72165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rousaki A., Miyata Y., Jinwal U.K., Dickey C.A., Gestwicki J.E., Zuiderweg E.R. Allosteric drugs: The interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J. Mol. Biol. 2011;411:614–632. doi: 10.1016/j.jmb.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abisambra J., Jinwal U.K., Miyata Y., Rogers J., Blair L., Li X., Seguin S.P., Wang L., Jin Y., Bacon J., et al. Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biol. Psychiatry. 2013;74:367–374. doi: 10.1016/j.biopsych.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin D.M., Baker J.D., Suntharalingam A., Nordhues B.A., Shelton L.B., Zheng D., Sabbagh J.J., Haystead T.A.J., Gestwicki J.E., Dickey C.A. Inhibition of Both Hsp70 Activity and Tau Aggregation in Vitro Best Predicts Tau Lowering Activity of Small Molecules. ACS Chem. Biol. 2016;11:2041–2048. doi: 10.1021/acschembio.6b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miyata Y., Li X., Lee H.F., Jinwal U.K., Srinivasan S.R., Seguin S.P., Young Z.T., Brodsky J.L., Dickey C.A., Sun D., et al. Synthesis and initial evaluation of YM-08, a blood-brain barrier permeable derivative of the heat shock protein 70 (Hsp70) inhibitor MKT-077, which reduces tau levels. ACS Chem. Neurosci. 2013;4:930–939. doi: 10.1021/cn300210g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jinwal U.K., Koren J., O’Leary J.C., Jones J.R., Abisambra J.F., Dickey C.A. Hsp70 ATPase Modulators as Therapeutics for Alzheimer’s and other Neurodegenerative Diseases. Mol. Cell Pharmacol. 2010;2:43–46. doi: 10.4255/mcpharmacol.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Congdon E.E., Wu J.W., Myeku N., Figueroa Y.H., Herman M., Marinec P.S., Gestwicki J.E., Dickey C.A., Yu W.H., Duff K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lo Cascio F., Kayed R., Azure C. Targets and Modulates Toxic Tau Oligomers. ACS Chem. Neurosci. 2018;9:1317–1326. doi: 10.1021/acschemneuro.7b00501. [DOI] [PubMed] [Google Scholar]
- 111.Chen Q., Prior M., Dargusch R., Roberts A., Riek R., Eichmann C., Chiruta C., Akaishi T., Abe K., Maher P., et al. A Novel Neurotrophic Drug for Cognitive Enhancement and Alzheimer’s Disease. PLoS ONE. 2011;6:e27865. doi: 10.1371/journal.pone.0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai Y.C., Lee Y.M., Lam K.K., Lin J.F., Wang J.J., Yen M.H., Cheng P.Y. The Role of Heat Shock Protein 70 in the Protective Effect of YC-1 on β-Amyloid-Induced Toxicity in Differentiated PC12 Cells. PLoS ONE. 2013;8:e69320. doi: 10.1371/journal.pone.0069320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoshino T., Suzuki K., Matsushima T., Yamakawa N., Suzuki T., Mizushima T. Suppression of Alzheimer’s Disease-Related Phenotypes by Geranylgeranylacetone in Mice. PLoS ONE. 2013;8:e76306. doi: 10.1371/journal.pone.0076306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S., Choi B.R., Kim J., LaFerla F.M., Yoon Park J.H., Han J.S., Lee K.W., Kim J. Sulforaphane upregulates the heat shock protein co-chaperone CHIP and clears amyloid-β and tau in a mouse model of Alzheimer’s disease. Mol. Nutr. Food. Res. 2018;62:1800240. doi: 10.1002/mnfr.201800240. [DOI] [PubMed] [Google Scholar]
- 115.Kasza Á., Hunya Á., Frank Z., Fülőp F., Tőrők Z., Balogh G., Sántha M., Bálind A., Bernáth S., Blundell L.I.M.K., et al. Dihydropyridine Derivatives Modulate Heat Shock Responses and have a Neuroprotective Effect in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2016;53:557–571. doi: 10.3233/JAD-150860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu L., Zhang C., Kalionis B., Wan W., Murthi P., Chen C., Li Y., Xia S. EGb761 protects against Aβ1−42 oligomer-induced cell damage via endoplasmic reticulum stress activation andHsp70 protein expression increase in SH-SY5Y cells. Exp. Gerontol. 2016;75:56–63. doi: 10.1016/j.exger.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Chow M.A., Tang D.W.F., Hanif A., Brown I.R. Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones. 2013;18:155–160. doi: 10.1007/s12192-012-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ou J.R., Tan M.S., Xie A.M., Yu J.T., Tan L. Heat shock protein 90 in Alzheimer’s disease. Biomed. Res. Int. 2014;2014:796869. doi: 10.1155/2014/796869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kakimura J., Kitamura Y., Takata K., Umeki M., Suzuki S., Shibagaki K., Taniguchi T., Nomura Y., Gebicke-Haerter P.J., Smith M.A., et al. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16:601–603. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- 120.Woo J.A., Liu T., Zhao X., Trotter C., Yrigoin K., Cazzaro S., Narvaez E., Khan H., Witas R., Bukhari A., et al. Enhanced tau pathology via RanBP9 and Hsp90/Hsc70 chaperone complexes. Hum. Mol. Genet. 2017;26:3973–3988. doi: 10.1093/hmg/ddx284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dickey C.A., Kamal A., Lundgren K., Klosak N., Bailey R.M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C.B., et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Investig. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alam Q., Alam M.Z., Sait K.H.W., Anfinan N., Noorwali A.W., Kamal M.A., Khan M.S.A., Haque A. Translational Shift of HSP90 as a Novel Therapeutic Target from Cancer to Neurodegenerative Disorders: An Emerging Trend in the Cure of Alzheimer’s and Parkinson’s Diseases. Curr. Drug Metabol. 2017;18:868–876. doi: 10.2174/1389200218666170728115606. [DOI] [PubMed] [Google Scholar]
- 123.Biamonte M.A., Van de Water R., Arndt J.W., Scannevin R.H., Perret D., Lee W.C. Heat shock protein 90: Inhibitors in clinical trials. J. Med. Chem. 2010;14:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 124.Shax E., Walter J.G., Märzhäuser H., Sthal F., Scheper T., Agard D.A., Eichner S., Kirschning A., Zeilinger C. Microarray-based screening of heat shock protein inhibitors. J. Biotechnol. 2014;180:1–9. doi: 10.1016/j.jbiotec.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 125.Lauria A., Abbate I., Gentile C., Angileri F., Martorana A., Almerico A.M. Synthesis and biological activities of a new class of heat shock protein 90 inhibitors, designed by energy-based pharmacophore virtual screening. J. Med. Chem. 2013;56:3424–3428. doi: 10.1021/jm4002023. [DOI] [PubMed] [Google Scholar]
- 126.Shumaila K., Subhankar P. Identifying a C-terminal ATP-binding sites-based novel Hsp90-Inhibitor in silico: A plausible therapeutic approach in Alzheimer’s disease. Med. Hypotheses. 2014;83:39–46. doi: 10.1016/j.mehy.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 127.Dickey C.A., Eriksen J., Kamal A., Burrows F., Kasibhatla S., Eckman C.B., Hutton M., Petrucelli L. Development of a High Throughput Drug Screening Assay for the Detection of Changes in Tau Levels—Proof of Concept with HSP90 inhibitors. Curr. Alzheimer Res. 2005;2:231–238. doi: 10.2174/1567205053585927. [DOI] [PubMed] [Google Scholar]
- 128.Ortega L., Calvillo M., Luna F., Pérez-Severiano F., Rubio-Osornio M., Guevara G., Limon I.D. 17-AAG improves cognitive process and increases heat shock protein response in a model lesion with Aβ25-35. Neuropeptides. 2014;48:221–232. doi: 10.1016/j.npep.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 129.Ho S.W., Tsui Y.T.C., Wong T.T., Cheung S.K.K., Goggins W.B., Yi L.M., Cheng K.K., Baum L. Effect of 17-allylamino-17-demethoxygeldanamy-cin(17-AAG) in transgenic mouse models of frontotemporal lobar degeneration and Alzheimer’s disease. Transl. Neurodegener. 2013;2:24. doi: 10.1186/2047-9158-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y., Wang B., Liu D., Li J.J., Xue Y., Sakata K., Zhu L.Q., Heldt S.A., Xu H., Liao F.F. Hsp90 Chaperone Inhibitor 17-AAG Attenuates Aβ-Induced Synaptic Toxicity and Memory Impairment. J. Neurosci. 2014;34:2464–2470. doi: 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sinadinos C., Quraishe S., Sealey M., Samson P.B., Mudher A., Wyttenbach A. Low Endogenous and Chemical Induced Heat Shock Protein Induction in a 0N3Rtau-Expressing Drosophila Larval Model of Alzheimer’s Disease. J. Alzheimers Dis. 2013;33:1117–1133. doi: 10.3233/JAD-2012-121534. [DOI] [PubMed] [Google Scholar]
- 132.Pillay I., Nakano H., Sharma S.V. Radicicol inhibits tyrosine phosphorylation of the mitotic Src substrate Sam68 and retards subsequent exit from mitosis of Src-transformed cells. Cell Growth Differ. 1996;7:1487–1499. [PubMed] [Google Scholar]
- 133.Lu Y., Ansar S., Michaelis M.L., Blagg B.S.J. Neuroprotective activity and evaluation of Hsp inhibitors in an immortalized neuronal cell line. Bioorg. Med. Chem. 2009;17:1709–1715. doi: 10.1016/j.bmc.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ansar S., Burlison J.A., Kyle Hadden M., Ming Yu X., Desino K.E., Bean J., Neckers L., Audus K.L., Michaelis M.L., Blagg B.S.J. A non-toxic Hsp90 inhibitor protects neurons from Aβ-induced toxicity. Bioorg. Med. Chem. Lett. 2007;17:1984–1990. doi: 10.1016/j.bmcl.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Thirstrup K., Sotty F., Pereira Montezinho L.C., Badolo L., Thougaard A., Kristjansson M., Jensen T., Watson S., Nielsen S.M. Linking HSP90 target occupancy to HSP70 induction and efficacy in mouse brain. Pharmacol. Res. 2016;104:197–205. doi: 10.1016/j.phrs.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 136.Wang B., Liu Y., Huang L., Chen J., Li J.J., Wang R., Kim E., Chen Y., Justicia C., Sakata K., et al. A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer’s mouse model via an HSF1-mediated mechanism. Mol. Psychiatry. 2017;22:990–1001. doi: 10.1038/mp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shelton B.L., Baker J.D., Zheng D., Sullivan L.E., Solanki P.K., Webster J.M., Sun Z., Sabbagh J.J., Nordhues B.A., Koren J., III, et al. Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc. Natl. Acad. Sci. USA. 2017;144:9707–9712. doi: 10.1073/pnas.1707039114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayan M., Zhang J., Braswell K., Gibson C., Zitnyar A., Lee D.C., Varghese-Gupta S., Jinwal U.K. Withaferin A regulates LRRK2 levels by interfering with the Hsp90-Cdc37 chaperone complex. Curr. Aging Sci. 2015;8:259–265. doi: 10.2174/1874609808666150520111109. [DOI] [PubMed] [Google Scholar]
- 139.Oroz J., Kim J.H., Chang B.J., Zweckstetter M. Mechanistic basis for the recognition of a misfolded protein by the molecular chaperone Hsp90. Nat. Stuct. Mol. Biol. 2017;24:407–413. doi: 10.1038/nsmb.3380. [DOI] [PubMed] [Google Scholar]
- 140.Radli M., Rüdiger S.G.D. Dancing with the Diva: Hsp90- Client interactions. J. Mol. Biol. doi: 10.1016/j.jmb.2018.05.026. in press. [DOI] [PubMed] [Google Scholar]