Abstract
Although cancer cells need more glucose than normal cells to maintain energy demand, chronic hyperglycemia induces metabolic alteration that may dysregulate signaling pathways, including the O-GlcNAcylation and HIF1A (Hypoxia-inducible factor 1-alpha) pathways. Metformin was demonstrated to evoke metabolic stress and induce cancer cell death. The aim of this study was to determine the cytotoxic efficiency of metformin on SKOV-3 cells cultured in hyperglycemia and normoglycemia. To identify the potential mechanism, we assessed the expression of O-linked β-N-acetlyglucosamine transferase (OGT) and glycoside hydrolase O-GlcNAcase (OGA), as well as hypoxia-inducible factor 1-alpha (HIF1A) and glucose transporters (GLUT1, GLUT3). SKOV-3 cells were cultured in normoglycaemia (NG, 5 mM) and hyperglycemia (HG, 25 mM) with and without 10 mM metformin for 24, 48, and 72 h. The proliferation rate, apoptotic and necrotic SKOV-3 cell death were evaluated. Real-Time qPCR was employed to determine mRNA expression of OGT, OGA, GLUT1, GLUT3, and HIF1A. Metformin significantly reduced the proliferation of SKOV-3 cells under normal glucose conditions. Whereas, the efficacy of metformin to induce SKOV-3 cell death was reduced in hyperglycemia. Both hyperglycemia and metformin induced changes in the expression of genes involved in the O-GlcNAcylation status and HIF1A pathway. The obtained results suggest that dysregulation of O-GlcNAcylation, and the related HIF1A pathway, via hyperglycemia, is responsible for the decreased cytotoxic efficiency of metformin in human ovarian cancer cells.
Keywords: metformin, hyperglycemia, ovarian cancer, apoptosis, O-GlcNAcylation, HIF1A gene
1. Introduction
Ovarian cancer is one of the leading cause of death among women. Recently, it was demonstrated that the risk of ovarian cancer is increased among diabetic patients [1]. Diabetes-related pathologies such as insulin resistance, chronic inflammation and high free ovarian steroid hormones are thought to be responsible for this coexistence [2]. Moreover, numerous clinical studies identified that hypoglycemic medications modulate the risk of cancer development. Metformin, the drug of the first choice in type 2 diabetes mellitus treatment, was found to protect from cancer development. Furthermore, experimental studies have revealed that metformin exhibited antiproliferative activity against various cancer cells. The drug was also reported to inhibit the growth of ovarian cancer cells [2,3]. It is widely believed that AMPK (5′AMP-activated protein kinase) activation and inhibition of mTORC (mammalian target of rapamycin complex) are mechanisms of anticancer action of metformin. The drug was also demonstrated to reduce tumorigenesis through metabolic alteration as a result of the mitochondrial respiratory complex I inhibition [3,4].
The typical hallmark of cancer cells is metabolic reprogramming [5]. Cancer cells, to satisfy their energy demand as well as carbon and nitrogen indulgence for macromolecules synthesis, consume an increased amount of glucose and glutamine. This results in the alteration of metabolic and signaling pathways, including inhibition of LKB1 (liver kinase B1)/AMPK and activation of mTORC pathways [6]. Activated mTORC promotes the expression of critical cell growth and metabolic regulators, including hypoxia-inducible factor 1-alpha (HIF1A). This transcription factor favors the expression of numerous genes that contribute to increased consumption of glucose and glutamine [7]. One of the downstream effectors of HIF1A is glucose transporters (GLUT), which are responsible for glucose uptake. In cancer cells, both HIF1A and GLUT1 were found to be upregulated [7,8].
Approximately 3–5% of the total glucose entering a cell is converted into UDP-N-acetylglucosamine (O-GlcNAc), in the hexosamine pathway. O-GlcNAc is used as a substrate for posttranslational modifications of intracellular proteins and thereby regulates their activity. O-GlcNAcylation controls protein function directly or by competing with phosphorylation sites. In response to altered nutrients and metabolic stress, O-GlcNAcylation was found to regulate cellular signaling and transcription regulatory pathways, including the HIF1A pathway [9,10]. Two enzymes regulate the level of O-GlcNAcylation; O-linked β-N-acetlyglucosamine transferase (OGT) and glycoside hydrolase O-GlcNAcase (OGA). An elevated level of O-GlcNAcylated proteins was found in different cancers [11].
Cell metabolism is closely connected with cell death pathways through the mitochondria, which play a crucial role in both metabolism and apoptosis. Metformin was found to affect cellular metabolism by targeting mitochondrial respiratory complex I and exerts direct anticancer action through the induction of cancer cell death or inhibition of cancer cell growth. It is well known that cancer cells are hypersensitive to metabolic stress, such as glucose or glutamine deprivation, and will undergo apoptosis if nutrients are limited [12]. However, little is known about how glucose excess influences the pro-apoptotic action of metformin in tumor cells. To answer this question, we aimed to determine the antiproliferative efficacy of metformin in human ovarian cancer (SKOV-3) cells cultured in normoglycemia (NG) and hyperglycemia (HG). As metformin evokes metabolic alteration, we assessed the expression of OGT and OGA, as well as HIF1A, GLUT1 and GLUT3.
2. Results
2.1. Antiproliferative Efficacy of Metformin in SKOV-3 Cells Depends on the Level of Glycemia
The antiproliferative efficacy of metformin in NG is presented in Figure 1A. According to the method described by Rogalska [13], we determined the values of doubling time of SKOV-3 cells cultured in NG (28 h) and HG (41 h). As displayed in Figure 1A, exposure to 10 mM metformin in NG induced a considerable time-dependent growth inhibition. Figure 1B shows the proliferation of SKOV-3 cells cultured in HG in the presence of metformin. As indicated by the value of doubling time, SKOV-3 cells cultured in HG needed more time to increase their number two times. This indicates that exposure to HG caused a decrease in proliferation efficacy of SKOV-3 cells. The growth rate of SKOV-3 cells cultured in HG in the presence of 10 mM metformin was also low. Furthermore, by comparing proliferation rates (td) in NG and HG, we revealed that HG significantly reduced the antiproliferative efficacy of metformin in SKOV-3 cells, as presented in Figure 1C.
Figure 1.
Antiproliferative action of metformin in SKOV-3 cells is reduced by hyperglycemia. SKOV-3 cells were cultured in normoglycemia (A) and hyperglycemia (B) and simultaneously treated with 10 mM metformin for 24, 48, 72 h. The cell number was determined using the trypan blue method and the proliferation rates were calculated (C). NG + M—cells cultured in normoglycemia and treated with metformin, HG + M—cells cultured in hyperglycemia and treated with metformin, td—proliferation rate. Results are presented as means ± SD of four independent experiments. (*) Statistically significant differences in comparison to untreated cells at time point “0”, p < 0.05; (#) statistically significant differences between the cells exposed to metformin in comparison to unexposed cells at the same time point, p < 0.05.
2.2. Both HG and Metformin Evoked Morphological Changes of SKOV-3 Cells
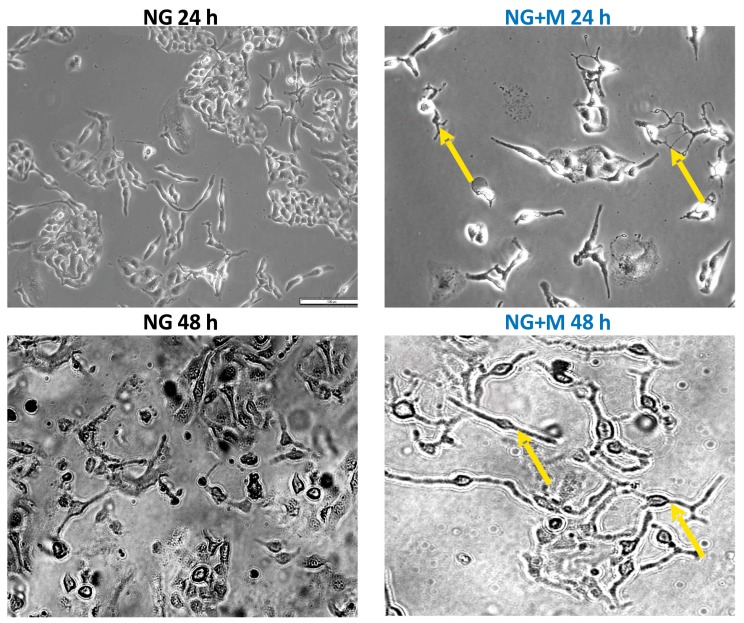
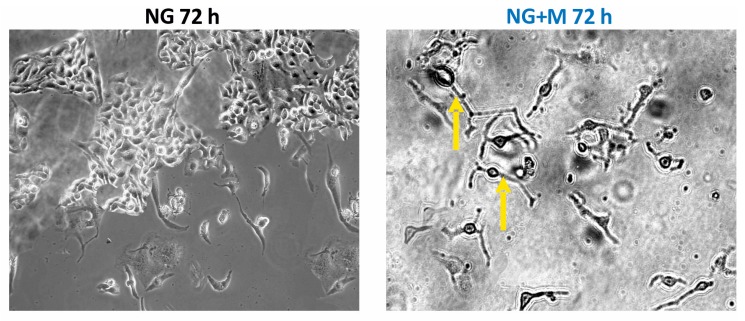
The morphology of SKOV-3 cells cultured in NG and HG treated with 10 mM metformin for 24, 48, and 72 h examined under an inverted optical microscope (Olympus IX70, Tokyo, Japan) [14] is presented in Figure 2 and Figure 3. A small percentage of SKOV-3 cells cultured in NG for 24, 48, and 72 h exhibited morphological changes such as elongation and thinning. In turn, exposure of SKOV-3 cells cultured in NG to metformin pronouncedly increased the number of deteriorated cells in a time-dependent manner. Elongated, single thin cells were detected after just 24 h of exposure to metformin and their number increased after prolonged treatment with the drug (Figure 2). In the case of SKOV-3 cells cultured in HG, morphological changes appeared after 48 h of incubation. Both culture in HG for 48 h and 72 h caused elongation and thinning of the cells, while we also detected slightly smaller cells after 72 h. We found distinctly small, elongated and disintegrated SKOV-3 cells cultured in HG and metformin. Even 24 h exposure to metformin induced deterioration of cells cultured in HG. We also noted that prolonged treatment with metformin (48, 72 h) led to cell disintegration and detachment of the cells from the culture well surface (Figure 3).
Figure 2.
The morphology of SKOV-3 cells treated for 24–72 h with metformin (10 mM) in normal glucose medium examined under an inverted microscope (Olympus IX70, Japan), (scale bar = 100 μm), elongated, thin cells (yellow arrows).
Figure 3.
The morphology of SKOV-3 cells treated for 24–72 h with metformin (10 mM) in high glucose medium examined under an inverted microscope (Olympus IX70, Japan), (scale bar = 100 μm), elongated, thin cells (orange arrows).
2.3. Metformin Induced Mainly Apoptosis in NG, and Both Apoptosis and Necrosis in HG
Figure 4 depicts the typical early apoptotic, late apoptotic, and necrotic morphological changes of SKOV-3 cells cultured in NG and HG in the presence or absence of 10 mM metformin. To discriminate between apoptotic or necrotic SKOV-3 cell death induced by metformin in NG and HG, double staining with Hoechst 33258 and PI with subsequent microscopic analysis was performed. These fluorescent dyes emit several types of fluorescence and differ in their ability to penetrate cells. Blue-fluorescent Hoechst 33258 goes through the intact membrane of live cells and allows for the observation of apoptosis-related chromatin structure changes. It stains the condensed chromatin of apoptotic cells brighter than the looser chromatin of normal cells. In turn, viable and early apoptotic cells with intact cell membranes exclude the red-fluorescent PI. Thus, only late apoptotic and necrotic cells, with the loss of membrane integrity, take up PI. The following morphological changes are detected by the double staining with Hoechst 33258 and PI: Chromatin condensation, cell shrinkage and nuclear fragmentation, apoptotic bodies, plasma membrane and cell disintegration.
Figure 4.
Metformin induced mainly apoptosis in NG and both apoptosis and necrosis in HG. (A) The percentage of early apoptotic, late apoptotic and necrotic cells detected at 24, 48, and 72 h of the culture of SKOV-3 cells in NG and HG in the presence and absence of 10 mM metformin. Results are presented as means ± S.D. of four experiments. NG—cells cultured in normoglycemia, NG + M—cells cultured in normoglycemia and treated with metformin, HG—cells cultured in hyperglycemia, HG + M—cells cultured in hyperglycemia and treated with metformin. (*) Statistically significant differences between the cells exposed to metformin in comparison to unexposed cells at the same time points, p < 0.05; (+) statistically significant differences between metformin treated cells in different time points, p < 0.05. (B) The morphological changes of SKOV-3 cells, which were cultured in NG and HG with and without exposure to 10 mM metformin for 48 h and stained with Hoechst 33258 and PI, visualized by fluorescence microscopy (Olympus IX70, Japan; bar 200 µm).
A quantitative analysis of the fractions of early apoptotic, late apoptotic, and necrotic cells, is exhibited in Figure 4A. We found that both SKOV-3 cells cultured in NG and HG did not differ significantly in the percentage of early apoptotic, late apoptotic and necrotic cells. However, a visible increase in the percentage of necrotic cells was noted for SKOV-3 cells culture in HG for 72 h. We found that metformin induced, in a time-dependent manner, both apoptosis and necrosis in SKOV-3 cells cultured in NG. A significant increase in the number of apoptotic and necrotic cells was observed in HG-cultured SKOV-3 cells exposed to metformin for 48 and 72 h. In the case of culture in NG, early and late apoptosis was the major pathway of SKOV-3 cell death caused by metformin. Whereas, a higher percentage of early and late apoptotic, compared to necrotic cells was detected for SKOV-3 cells cultured in HG and exposed to metformin for 48 h. In turn, after 72 h of exposure to metformin, we found a pronouncedly high percentage of necrotic cells in HG-cultured SKOV-3 cells.
The typical early apoptotic, late apoptotic and necrotic morphological changes induced by metformin in SKOV-3 cells cultured in NG and HG for 48 h are presented in Figure 4B. The small percentage of dead SKOV-3 cells cultured in NG for 48 h represented apoptotic cells. It seems that there was an increased percentage of necrotic SKOV-3 cells cultured in HG. To conclude, mainly apoptosis, rather than necrosis, was involved in the metformin-induced death of SKOV-3 cells cultured both in NG and HG.
2.4. Both Metformin and HG Affect Expression of OGT and OGA in SKOV-3 Cells
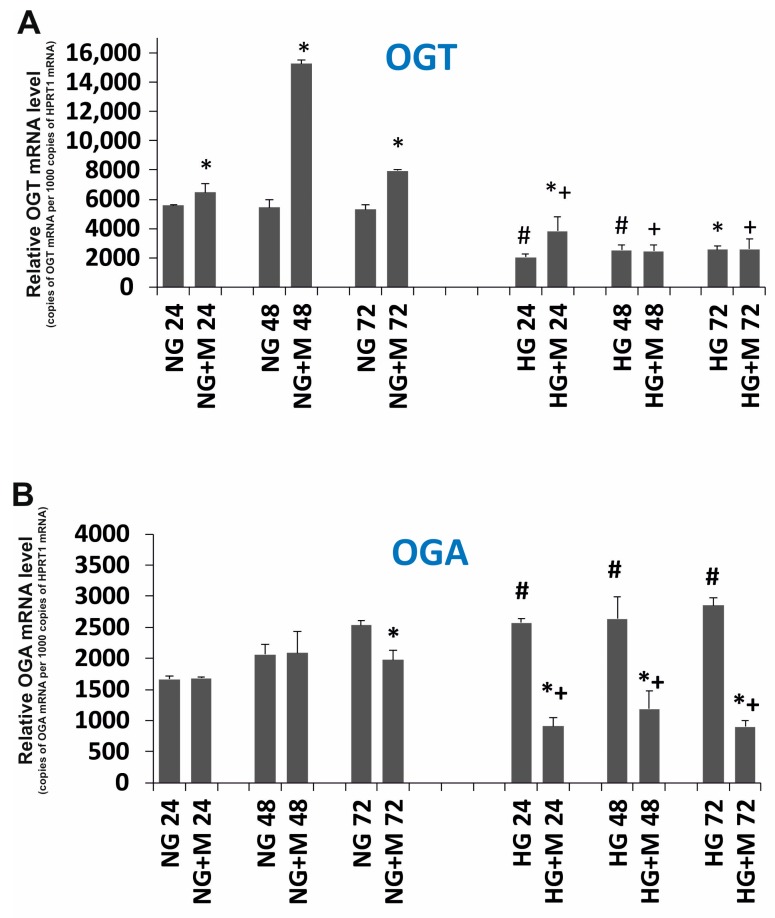
To explore the role of O-GlcNAcylation in SKOV-3 cell death induced by 10 mM metformin upon NG and HG, we evaluated the mRNA expression levels of OGT and OGA. The relative OGT mRNA and OGA mRNA levels are shown in Figure 5. We observed that culture in HG diminished the OGT mRNA level at each time point studied as compared to culture in NG. In NG-cultured SKOV-3 cells, exposed to 10 mM metformin, the level of OGT mRNA at 48 h and 72 h was markedly elevated. Whereas, the level of OGT mRNA seemed to be unaffected by 10 mM metformin in HG-cultured SKOV-3 cells. The obtained results suggest that HG influenced the level of OGT mRNA more than metformin.
Figure 5.
Relative (A) OGT (O-linked β-N-acetlyglucosamine transferase); (B) OGA (O-GlcNAcase) mRNA expression detected after 24, 48, and 72 h in metformin-treated and untreated ovarian cancer cell line SKOV-3 cultured in NG and in HG. NG—cells cultured in normoglycemia, NG + M—cells cultured in normoglycemia and treated with metformin, HG—cells cultured in hyperglycemia, HG + M—cells cultured in hyperglycemia and treated with metformin, (*) Statistically significant differences between the cells exposed to metformin in comparison to unexposed cells at the same time points, p < 0.05; (#) statistically significant differences between cells cultured in HG in comparison to those cultured in NG at the same time-points p < 0.05; (+) statistically significant differences between metformin-treated cells in HG in comparison to metformin-treated cells cultured in NG at the same time points, p < 0.05.
We found that HG did not change significantly the OGA mRNA level in SKOV-3 cells in comparison to NG. Metformin also did not affect the level of OGA mRNA in SKOV-3 cells cultured in NG. However, a noticeable decrease in the OGA mRNA level evoked by metformin was observed in HG at all time points studied.
2.5. Expression Changes of GLUT1, GLUT3 and HIF1A Evoked by HG and Metformin
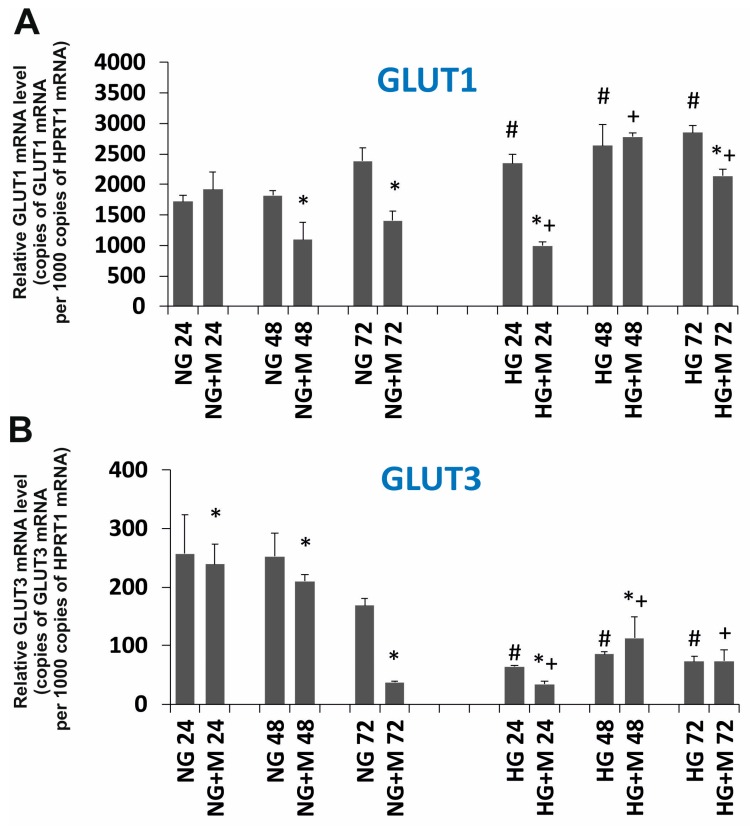
The relative GLUT1, GLUT3 and HIF1A mRNA levels are shown in Figure 6A–C. As presented in Figure 6A,B, it seems that the expression of GLUT1 is significantly higher than the expression of GLUT3 in SKOV-3 cells. This observation may suggest that GLUT1 plays a dominant role in glucose transport in SKOV-3 cells. The relative expression of GLUT1 did not change significantly in HG compared to NG. However, exposure to metformin for 48 and 72 h caused a distinct decreased expression of GLUT1 in SKOV-3 cell cultured in NG. SKOV-3 cells cultured in HG treated with metformin presented a decreased expression of GLUT1 after 24 h and unchanged expression at the rest of the time points. The relative GLUT3 expression was diminished in SKOV-3 cells cultured in HG compared to NG (Figure 6B). Metformin evoked a significant decrease in the level of GLUT3 mRNA in SKOV-3 cells cultured in NG for 48 and 72 h. It seems that the drug did not influence the expression of GLUT3 in HG-cultured cells.
Figure 6.
Relative (A) SLC2A1 (GLUT1); (B) SLC2A3 (GLUT3); (C) HIF1A mRNA expression detected after 24, 48, and 72 h in a metformin-treated and untreated ovarian cancer cell line (SKOV-3) cultured in NG and in HG. NG—cells cultured in normoglycemia, NG + M—cells cultured in normoglycemia and treated with metformin, HG—cells cultured in hyperglycemia, HG + M—cells cultured in hyperglycemia and treated with metformin. (*) Statistically significant differences between the cells exposed to metformin in comparison to unexposed cells at the same time points, p < 0.05; (#) statistically significant differences between cells cultured in HG in comparison to those cultured in NG at the same time points p < 0.05; (+) statistically significant differences between metformin-treated cells in HG in comparison to metformin-treated cells cultured in NG at the same time points, p < 0.05.
We observed that the culture of SKOV-3 cells in HG affected the expression of HIF1A in comparison to the culture in NG. We found that the level of HIF1A mRNA diminished in SKOV-3 cells in NG, especially at 72 h. On the contrary, in SKOV-3 cells cultured in HG for 72 h, the HIF1A level was elevated (Figure 6C). Interestingly, both in the case of SKOV-3 cells cultured in NG and HG, metformin reduced the expression of HIF1A, in the normoglycemic condition after 24 and 48 h of exposure to metformin and in the hyperglycemic condition after 48 and 72 h of treatment with metformin.
3. Discussion
According to the American Cancer Society, ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system. This cancer mainly develops among older women, especially after menopause. The incidence of type 2 diabetes grows with age, therefore, the coexistence of ovarian cancer and diabetes also increases with age. The major symptom of diabetes—chronic hyperglycemia—may significantly influence the effectiveness of ovarian cancer chemo- and radiotherapy. Metformin, the first line hypoglycemic agent in the therapy of Type 2 diabetes mellitus (T2DM), was demonstrated to inhibit the growth of various cancers. Therefore, we aimed to evaluate the anticancer efficacy of metformin toward human ovarian cancer cells cultured in normoglycemia and hyperglycemia. Since glucose exposure causes metabolic alteration that affects O-GlcNAcylation and HIF1A pathway, we examined the expression of OGT, OGA, HIF1A, GLUT1, and GLUT3.
Interestingly, we found that the proliferation of SKOV-3 cells cultured in hyperglycemia was significantly lower in comparison to normoglycemia. This is unusual for cancer cells because it is widely accepted that high glucose stimulates proliferation by satisfying energy and carbon needs [15]. Since O-GlcNAcylation is sensitive to metabolic state and is involved in tumor growth in vitro [16], we determined the expression of OGT and OGA mRNA. We found that HG caused a significant decrease in the expression of OGT and a slight increase in OGA in SKOV-3 cells. Thus, our results suggest that lower proliferation of SKOV-3 cells exposed to HG may be associated with a decrease in O-GlcNAcylation. It is contrary to the general view that by reducing O-GlcNAcylation (via a decrease in OGT) tumor growth is inhibited [10].
We demonstrated that cytotoxic action of metformin on SKOV-3 cells was significantly reduced in HG in comparison to NG. This result is in agreement with findings reported by others, who found a decreased anticancer efficacy of metformin in breast cancer cells cultured in HG (MCF7, MDAMB231 and SKBR3) [17]. In addition, the reduced efficacy of other chemotherapeutics was also reported for liver cancer cells (HepG2, Bel-7402) cultured in HG [16]. Ikemura noted that in diabetic mice, the growth of adenocarcinoma in the colon was slower and that chemotherapy with oxaliplatin and fluorouracil was less effective [18]. According to Zhuang, high glucose provides fuel for glycolytic metabolism that maintains ATP levels in the cell despite inhibition of mitochondrial oxidative metabolism by metformin. As glucose is consumed and AMPK is not effectively activated by metformin, cancer cells do not have enough fuel to maintain glycolytic metabolism. As a result, ATP drops in the cell, leading to energy collapse and cell death [19]. In turn, Karnevi provided molecular evidence that the decreased anticancer action of metformin in pancreatic cancer cells exposed to HG involved the insulin/insulin-like growth factor-1 (IGF1) pathway. Exposure to high glucose levels promoted IGF-1-mediated PKB (protein kinase B, Akt) activation, which correlated with stimulated AMPKSer485 phosphorylation and impaired AMPKThr172 phosphorylation, resulting in reduced anti-proliferative and apoptotic effects by metformin [20].
The next step of our research was to identify the potential mechanism responsible for different types of metformin’s cytotoxicity toward SKOV-3 cells in NG and HG. As O-GlcNAcylation is sensitive to metabolic alteration, we examined the expression of O-GlcNAcylation regulators. We found a significant increase in the level of OGT mRNA in SKOV-3 cells cultured in NG and exposed to metformin. This observation may be a result of AMPK activation by metformin. Evidence indicated that AMPK directly phosphorylates OGT and increases its level [21,22]. Interestingly, our results revealed that the expression of OGT in cells exposed to both HG and metformin or only exposed to HG was pronouncedly diminished. These observations may suggest that HG changes O-GlcNAcylation status in SKOV-3 cells more than metformin. The O-GlcNAcylation status strictly depends on the balance between the level of OGT and OGA. The effect of metformin alone and HG alone on the expression of OGA was small. However, Pagesy detected a significantly elevated OGA level in diabetic patients [23]. In turn, simultaneous exposure to HG and metformin of SKOV-3 cells was associated with a marked decreased expression of OGA in comparison to SKOV-3 cultured only in NG or cultured in NG and metformin. These results may imply that metabolic stress caused by HG and metformin affects OGA level and thereby may play a significant role in the O-GlcNAcylation status in SKOV-3 cells.
A cancer cell presents metabolic reprogramming, which is manifested by alterations in numerous signaling pathways. One of them is the HIF1A pathway that is launched by the hyperactive mTOR pathway in different cancer cells, including ovarian cancer [6,7,24]. The HIF1A pathway was also found to be elevated as a result of increased O-GlcNAcylation (via increased OGT) in response to metabolic alteration [9,10]. HIF1A mediates the adaptation of cells to low oxygen, mainly through upregulation of its effectors participating in glycolytic metabolism with the glucose transporter family (GLUT) at the head. The influence of glucose concentration on HIF1A expression was reported to be dependent on the cell type [25]. In our study, we demonstrated that SKOV-3 cells cultured in HG displayed lower expression of HIF1A and GLUT3 and an unchanged expression of GLUT1 in comparison to cells cultured in NG. The obtained results indicate that the effect of metformin on the HIF1A pathway was dependent on glycemic condition. In SKOV-3 cells cultured in NG we observed that metformin decreased the expression of HIF1A, GLUT1 and GLUT3. This is consistent with the result found by Alves in Sertoli cells, which revealed metformin enhanced glycolytic flux [26]. Wang found that by reducing HIF1A, the level of GLUT1 also decreased in human glioblastoma cells [27]. In turn, it was demonstrated, that metformin inhibited HIF1A and suppressed the expression of glucose transporters (GLUT1, GLUT3) and regulatory enzymes of the glycolytic pathway in cervical tumor cells [28]. Oppositely, in SKOV-3 cells cultured in HG, metformin slightly diminished HIF1A expression and did not influence GLUT1 and GLUT3 expression. Consistently, Qi demonstrated that in oral squamous cell carcinoma cells metformin significantly reduced the expression of HIF1A [29]. In agreement with our data, Ece reported a significantly decreased level of HIF1A in patients with type 2 diabetes and breast cancer receiving metformin [30]. It is believed that metformin specifically reduces HIF1A expression as a result of the inhibition of ATP synthesis and this action is independent of AMPK activation [31]. Interestingly, Xiao indicated that HG, via the generation of reactive oxygen species, is responsible for the reduced expression and activation of HIF1A [25]. It is clearly seen that the influence of metformin on the expression of GLUT1 and GLUT3 in SKOV-3 cells cultured in HG is negligible. Taken together, these observations suggest that, despite decreasing HIF1A expression, metformin may not run glycolytic flux in SKOV-3 cells cultured in HG.
Multiple clinical trials indicate that metformin reduces the risk of cancer development in diabetic patients [32]. Furthermore, metformin was found to inhibit the progression of various cancers, including ovarian cancer. Kumar showed in a case-control study that patients with ovarian cancer treated with metformin had a significantly better 5-year survival (disease recurrences and cancer-specific mortalities) in comparison to patients with ovarian cancer not taking metformin [33]. However, little is known about metformin action in patients suffering from ovarian cancer without diabetes. Currently, a phase II clinical trial is ongoing to assess whether the addition of metformin to standard chemotherapy improves survival in non-diabetic ovarian cancer patients (NCT02122185; clinicaltrials.gov). The results of our current in vitro study revealed that metformin inhibited proliferation and promoted apoptosis of SKOV-3 cells more efficiently in normoglycemia than in hyperglycemia. Our findings are in line with previously reported evidence showing that low glucose intensifies metformin cytotoxicity toward breast and thyroid cancer cells [34,35]. The mechanism by which the glucose level influences metformin action is being investigated. Zhuang suggested that low glucose in the medium triggered metformin mediated ATP depletion and cell death by reducing metformin-stimulated glycolysis [19]. Furthermore, recently, Tang demonstrated that metformin induced apoptosis and inhibition of ovarian cancer cell growth was associated with a stronger AMPK activation by metformin in a low glucose medium, in comparison to a high glucose medium. They showed that metformin action involved epigenetic alteration since the treatment with the drug significantly reduced histone H3 lysine 27 trimethylation and polycomb repressor complex 2 (PRC2) levels [36]. All together, these findings support the need for the evaluation of metformin efficacy for the treatment of patients with ovarian cancer without diabetes.
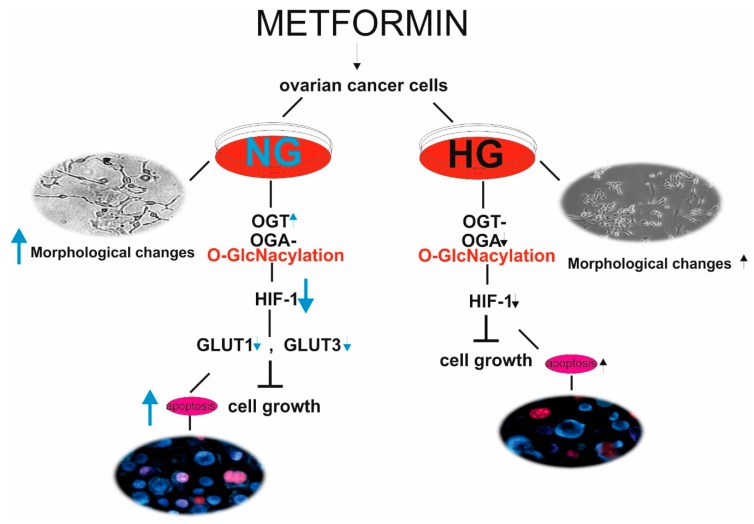
To conclude, our results indicate that metformin exhibits direct antitumor action on ovarian cancer SKOV-3 cells. The anticancer efficacy of metformin is higher when ovarian cancer cells were cultured in NG in comparison to culture in HG. To the best of our knowledge, this is the first study presenting that the response of SKOV-3 cells to metformin depends on the level of glycemia, which affects diversely OGT and OGA expression and O-GlcNAcylation status. Therefore, HG dysregulates O-GlcNAcylation and the related HIF1A pathway, thus preventing metformin-stimulated inhibition of growth and proliferation of SKOV-3 cells. The summary of our findings is presented in Figure 7.
Figure 7.
Metformin acts through a HIF1A/GLUT-dependent mechanism in SKOV-3 cells. The response of ovarian cancer cells to metformin depends on the level of glycemia, which affects diversely OGT and OGA expression and O-GlcNAcylation status. In NG, metformin increases the expression of OGT and downregulates HIF1A and GLUT expression leading to SKOV-3 cell apoptosis. HG dysregulates OGT and OGA expression and related HIF1A and GLUT expression preventing metformin-stimulated inhibition of growth and proliferation of SKOV-3 cells. Black arrows (HG conditions), blue arrows (NG conditions)—the height of arrow indicates intensification and direction of apoptotic changes, morphological changes and expression of the examined genes.
4. Material and Methods
4.1. Reagents
Metformin, trypsin-EDTA, and Trizol® Reagent were supplied by Sigma (St. Louis, MO, USA). Glucose free RPMI 1640 and fetal bovine serum (FBS) were supplied by Cambrex (Basel, Switzerland) and Life Technologies (Carlsbad, NM, USA). High Capacity cDNA Reverse Transcription Kit, TaqMan® Gene Expression Assays, and TaqMan Universal PCR MasterMix were obtained from Life Technologies USA. All other chemicals and solvents were of high analytical grade and were supplied by Sigma or POCH S.A. (Gliwice, Poland).
4.2. Cell Culture and Treatment
The human SKOV-3 cell line used in the experiments was obtained from the American Type Culture Collection (ATCC) based in Rockville, MD, USA. The cells were grown in a monolayer at 37 °C in a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% heat inactivated FBS, penicillin (10 U/mL) and streptomycin (50 μg/mL). In order to obtain normal glucose conditions (NG, 5 mM) and hyperglycemic conditions (HG, 25 mM), D-glucose was added to glucose-free RPMI 1640. Cells were cultured in growth medium with 5 mM glucose for 24 h before switching to 25 mM glucose. Then, the SKOV-3 cells were maintained for at least 1 day in NG or for 2 days in HG to achieve a logarithmic growth phase. The effect of metformin on the viability of SKOV-3 cells was determined by the MTT test (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) as described previously. We found that the ≥5 mM concentration of metformin evoked a significant decrease in the viability of SKOV-3 cells and the IC50 value was designated as 14 mM [13]. For further experiments, we chose a metformin concentration of 10 mM, which corresponded to a SKOV-3 survival rate of more than 60% of cells in relation to untreated cells. Finally, the cells were cultured under normo- and hyperglycemic conditions with or without metformin. The medium was changed every 24 h. The cells were routinely screened for Mycoplasma contamination.
4.3. Determination of Proliferation Rate
To determine the SKOV-3 cell proliferation rate we employed the trypan blue exclusion method [37]. Live cells possess intact cell membranes that are impermeable to trypan blue and exclude the dye. After mixing the cell suspension with the trypan blue solution, a viable cell will have a clear cytoplasm whereas a nonviable cell will have a blue cytoplasm. The SKOV-3 cells were seeded at a density of 2 × 105 per sample in growth media with 5 mM glucose for 24 h. Hyperglycemia was achieved by transferring cells from a 5 mM glucose medium to a medium containing 25 mM glucose. The adaptation time to the glycemic condition was 24 h. Then, the cells were cultured in NG for 24 h and HG for 48 h to reach the logarithmic growth phase. Then, metformin was added to obtain a final concentration of 10 mM. To calculate the proliferation rate, the cells were counted after 24, 48, and 72 h of exposure to metformin. Briefly, 4% trypan blue solution was mixed with the cell suspension in a ratio of 1:1, transferred to the chamber of Thoma and viable/nonviable cells were counted under an optical microscope. Based on the number of cells at the beginning and at each studied time point, we calculated the doubling time using the following formula td = t/log2 (Nt/N0), where td is time required for duplication of cell number, t is the time interval between the initial and final calculation of cell number, N0 and Nt are cell numbers at the beginning and the end of the experiment, respectively [38,39].
4.4. RNA Isolation and cDNA Synthesis
Total RNA was isolated using Trizol® Reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. An RNA ratio of 260/A280 ≈ 2.0 was considered as pure. Complementary DNA (cDNA) was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Grand Island, NY, USA) following the manufacturer’s instructions. cDNA synthesis was performed in a 20 μL volume that included 10 μL of total RNA (2 μg), 2 μL 10× RT Buffer, 0.8 μL of 25× dNTP Mix (100 mM), 2 μL 10× RT Random Primers, 1 μL of RNase Inhibitor, 1 μL of MultiScribe™ Reverse Transcriptase and 3.2 μL of nuclease-free water. The profile of time and temperature was as follows: 10 min at 25 °C, 120 min at 37 °C and 5 min at 85 °C.
4.5. Quantitative Real-Time RT-PCR (RT-qPCR)
Real-time gene expression analysis of target genes: OGT, OGA, SLC2A1 (GLUT1), SLC2A3 (GLUT3), HIF1A was performed using TaqMan® Gene Expression Assays according to the manufacturer’s instructions. The HPRT1 (hypoxanthine phosphoribosyltransferase 1) gene was used as an internal control. The following assays were used to determine gene expression: OGT—Hs00269228_m1; OGA—Hs00201970_m1; SLC2A1-Hs00892681_m1; SLC2A3—Hs00359840_m1; HIF1A—Hs00153153_m1; HPRT1—Hs02800695_m1. PCR reactions were performed in a 10 µL volume that included 5 µL of 2× TaqMan Universal PCR MasterMix, 3.5 µL of water, 1 µL cDNA template (50 ng) and 0.5 µL of TaqMan® Gene Expression Assay. The RT-qPCR reaction was carried out using the Mastercycler ep realplex. Relative RNA quantification was performed using the ΔCt method. ΔCt (Cttargeted gene − CtHPRT1) values were recalculated into relative copy number values (number of target gene mRNA copies per 1000 copies of HPRT1 mRNA).
4.6. Morphological Assessment of Apoptosis and Necrosis
To determine the ratio between viable apoptotic and necrotic cell fractions, simultaneous cell staining with Hoechst 33258 and PI (propidium iodide) was conducted. These fluorescent dyes vary in their spectral characteristics and ability to penetrate cells. The analysis was performed with a fluorescence microscope (Olympus IX70, Tokyo, Japan). The morphological changes were detected at 24, 48, and 72 h of exposure to normoglycaemia and hyperglycemia with or without 10 mM metformin. At each time point studied, cells were removed from culture dishes by trypsinization, centrifuged and suspended in PBS to obtain 1 × 105 cells/mL. To 100 µL of cell suspension, 1 µL of Hoechst 33258 (0.13 mM) and 1 µL of PI (0.23 mM) were added and the cells were incubated at room temperature for 10 min in the darkness [40]. At least 300 cells were counted on each slide and each experiment was done in triplicate.
4.7. Statistical Analysis
Data were expressed as mean ± SD. Two-way analysis of variance was used. Statistical calculations were performed using STATISTICA ver.11. A p-value of <0.05 or <0.01 was considered as significant.
Acknowledgments
The authors wish to thank Marzena Pacholska for valuable technical assistance.
Author Contributions
A.R., E.F., M.B., A.S., A.M. formed the hypothesis and designed the study. A.R., E.F. participated in experimental design and conducted most of the experiments and collected data. A.R. and E.F. analyzed the results, and A.R. with A.S. and A.M. wrote the manuscript draft. All authors contributed to manuscript preparation.
Funding
This work was supported by grant no B1511000001025.02 for young scientists (Ministry of Science and Higher Education) Poland, by statutory research admitted for Department of Medical Biophysics (B1611000000190.01), and by grant no. 503/2-159-01/503-21-003 from the Medical University of Lodz.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Zhang D., Li N., Xi Y., Zhao Y., Wang T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017;130:43–52. doi: 10.1016/j.diabres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Anastasi E., Filardi T., Tartaglione S., Lenzi A., Angeloni A., Morano S. Linking type 2 diabetes and gynecological cancer: An introductory overview. Clin. Chem. Lab. Med. 2018;56:1413–1425. doi: 10.1515/cclm-2017-0982. [DOI] [PubMed] [Google Scholar]
- 3.Andrzejewski S., Gravel S.P., Pollak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw R.J., Cantley L.C. Decoding key nodes in the metabolism of cancer cells: Sugar & spice and all things nice. F1000 Biol. Rep. 2012;4:2. doi: 10.3410/B4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown R.S., Wahl R.L. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::AID-CNCR2820721020>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer C.M., Lynch T.P., Sodi V.L., Falcone J.N., Schwab L.P., Peacock D.L., Vocadlo D.J., Seagroves T.N., Reginato M.J. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol. Cell. 2014;54:820–831. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch T.P., Ferrer C.M., Jackson S.R., Shahriari K.S., Vosseller K., Reginato M.J. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J. Biol. Chem. 2012;287:11070–11081. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Mjiyad N., Caro-Maldonado A., Ramirez-Peinado S., Munoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30:253–264. doi: 10.1038/onc.2010.466. [DOI] [PubMed] [Google Scholar]
- 13.Rogalska A., Wojcik-Krowiranda K., Forma E., Ciesielski P., Bienkiewicz A., Brys M., Krzeslak A., Marczak A. Effects of metformin on the survival of the SKOV-3 ovarian cancer cell line and the expression of genes encoding enzymes involved in O-Glcnacylation. Ginekol. Pol. 2014;85:521–526. doi: 10.17772/gp/1764. [DOI] [PubMed] [Google Scholar]
- 14.Okumura M., Yamamoto M., Sakuma H., Kojima T., Maruyama T., Jamali M., Cooper D.R., Yasuda K. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: Reciprocal involvement of PKC-alpha and PPAR expression. Biochim. Biophys. Acta. 2002;1592:107–116. doi: 10.1016/S0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 15.Vasconcelos-Dos-Santos A., de Queiroz R.M., da Costa Rodrigues B., Todeschini A.R., Dias W.B. Hyperglycemia and aberrant O-GlcNAcylation: Contributions to tumor progression. J. Bioenerg. Biomembr. 2018;50:175–187. doi: 10.1007/s10863-017-9740-x. [DOI] [PubMed] [Google Scholar]
- 16.Xu X., Si M., Lou H., Yan Y., Liu Y., Zhu H., Lou X., Ma J., Zhu D., Wu H., et al. Hyperglycemia decreases anti-cancer efficiency of Adriamycin via AMPK pathway. Endocr. Relat. Cancer. 2018;25:955–966. doi: 10.1530/ERC-18-0036. [DOI] [PubMed] [Google Scholar]
- 17.Zou L., Zhu-Mauldin X., Marchase R.B., Paterson A.J., Liu J., Yang Q., Chatham J.C. Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent. J. Biol. Chem. 2012;287:34419–34431. doi: 10.1074/jbc.M112.393207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikemura M., Hashida T. Effect of Hyperglycemia on Antitumor Activity and Survival in Tumor-bearing Mice Receiving Oxaliplatin and Fluorouracil. Anticancer Res. 2017;37:5463–5468. doi: 10.21873/anticanres.11975. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Y., Chan D.K., Haugrud A.B., Miskimins W.K. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE. 2014;9:e108444. doi: 10.1371/journal.pone.0108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnevi E., Said K., Andersson R., Rosendahl A.H. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer. 2013;13:235. doi: 10.1186/1471-2407-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Q., Yang C., Du Y., Chen Y., Liu H., Deng M., Zhang H., Zhang L., Liu T., Liu Q., et al. AMPK regulates histone H2B O-GlcNAcylation. Nucleic Acids Res. 2014;42:5594–5604. doi: 10.1093/nar/gku236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J., Wang S., Viollet B., Zou M.H. Regulation of the proteasome by AMPK in endothelial cells: The role of O-GlcNAc transferase (OGT) PLoS ONE. 2012;7:e36717. doi: 10.1371/journal.pone.0036717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Pagesy P., Tachet C., Mostefa-Kara A., Larger E., Issad T. Increased OGA Expression and Activity in Leukocytes from Patients with Diabetes: Correlation with Inflammation Markers. Exp. Clin. Endocrinol. Diabetes. 2018 doi: 10.1055/a-0596-7337. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H., Feng Y. Hypoxia-inducible factor 1alpha (HIF-1alpha) correlated with tumor growth and apoptosis in ovarian cancer. Int. Gynecol. Cancer Soc. 2006;16(Suppl. S1):405–412. doi: 10.1111/j.1525-1438.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 25.Xiao H., Gu Z., Wang G., Zhao T. The possible mechanisms underlying the impairment of HIF-1alpha pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int. J. Med. Sci. 2013;10:1412–1421. doi: 10.7150/ijms.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves M.G., Martins A.D., Vaz C.V., Correia S., Moreira P.I., Oliveira P.F., Socorro S. Metformin and male reproduction: Effects on Sertoli cell metabolism. Br. J. Pharmacol. 2014;171:1033–1042. doi: 10.1111/bph.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang E., Zhang C., Polavaram N., Liu F., Wu G., Schroeder M.A., Lau J.S., Mukhopadhyay D., Jiang S.W., O’Neill B.P., et al. The role of factor inhibiting HIF (FIH-1) in inhibiting HIF-1 transcriptional activity in glioblastoma multiforme. PLoS ONE. 2014;9:e86102. doi: 10.1371/journal.pone.0086102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyszka-Czochara M., Bukowska-Strakova K., Kocemba-Pilarczyk K.A., Majka M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients. 2018;10:841. doi: 10.3390/nu10070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi X., Xu W., Xie J., Wang Y., Han S., Wei Z., Ni Y., Dong Y., Han W. Metformin sensitizes the response of oral squamous cell carcinoma to cisplatin treatment through inhibition of NF-kappaB/HIF-1alpha signal axis. Sci. Rep. 2016;6:35788. doi: 10.1038/srep35788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ece H., Cigdem E., Yuksel K., Ahmet D., Hakan E., Oktay T.M. Use of oral antidiabetic drugs (metformin and pioglitazone) in diabetic patients with breast cancer: How does it effect serum Hif-1 alpha and 8Ohdg levels? Asian Pac. J. Cancer Prev. 2012;13:5143–5148. doi: 10.7314/APJCP.2012.13.10.5143. [DOI] [PubMed] [Google Scholar]
- 31.Viollet B., Guigas B., Sanz Garcia N., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandini S., Puntoni M., Heckman-Stoddard B.M., Dunn B.K., Ford L., DeCensi A., Szabo E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. Phila. 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S., Meuter A., Thapa P., Langstraat C., Giri S., Chien J., Rattan R., Cliby W., Shridhar V. Metformin intake is associated with better survival in ovarian cancer: A case-control study. Cancer. 2013;119:555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menendez J.A., Oliveras-Ferraros C., Cufi S., Corominas-Faja B., Joven J., Martin-Castillo B., Vazquez-Martin A. Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle. 2012;11:2782–2792. doi: 10.4161/cc.20948. [DOI] [PubMed] [Google Scholar]
- 35.Bikas A., Jensen K., Patel A., Costello J., Jr., McDaniel D., Klubo-Gwiezdzinska J., Larin O., Hoperia V., Burman K.D., Boyle L., et al. Glucose-deprivation increases thyroid cancer cells sensitivity to metformin. Endocr. Relat. Cancer. 2015;22:19–32. doi: 10.1530/ERC-15-0402. [DOI] [PubMed] [Google Scholar]
- 36.Tang G., Guo J., Zhu Y., Huang Z., Liu T., Cai J., Yu L., Wang Z. Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation. Int. J. Oncol. 2018;52:1899–1911. doi: 10.3892/ijo.2018.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaltenbach J.P., Kaltenbach M.H., Lyons W.B. Nigrosin as a dye for differentiating live and dead ascites cells. Exp. Cell Res. 1958;15:112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- 38.Korzyńska A., Zychowicz M. A method of estimation of the cell doubling time on basis of the cell culture monitoring data. Biocybern. Biomed. Eng. 2008;28:75–82. [Google Scholar]
- 39.Uzbekov R.E. Analysis of the cell cycle and a method employing synchronized cells for study of protein expression at various stages of the cell cycle. Biochem. Mosc. 2004;69:485–496. doi: 10.1023/B:BIRY.0000029845.11184.30. [DOI] [PubMed] [Google Scholar]
- 40.Gasiorowski K., Brokos B., Kulma A., Ogorzalek A., Skorkowska K. A comparison of the methods applied to detect apoptosis in genotoxically-damaged lymphocytes cultured in the presence of four antimutagens. Cell. Mol. Biol. Lett. 2001;6:141–159. [PubMed] [Google Scholar]