Abstract
Luminal short-chain fatty acids (SCFA) are rapidly absorbed from the intestine and subsequently utilized by the host as substrate for metabolic energy production. In pigs, the energy contribution of SCFA is thought to be 30–76%. However, since absorption and blood flow dynamics of SCFA in pigs, particularly during the suckling–weaning period, remain unclear, we aimed to elucidate these phenomena. Thirty-two piglets were used in the present work. Cecal vein blood and digesta, and portal and abdominal vein blood were sampled from suckling (7-, 14-, 21- and 28-day-old) and weaned (weaning at 21 and 28 days of age) piglets. Four piglets from each group were euthanized. SCFA concentrations in blood samples were analyzed by a highly sensitive gas chromatography-mass spectrometry technique. Age at weaning tended to affect SCFA absorption. For example, acetate and propionate concentrations in the cecal vein tended to be higher in piglets weaned at day 21 than at day 28. SCFA concentrations in the abdominal vein tended to differ from those in other veins. Mucosal gene expression analysis suggested that monocarboxylate transporter 1 and occludin were associated in absorption of SCFA from the lumen into the blood of piglets.
Keywords: short-chain fatty acid, hindgut fermentation, absorption from lumen, suckling, weaning
1. Introduction
Short-chain fatty acids (SCFA), particularly acetate, propionate and n-butyrate, are major end-products of gut microbiota [1]. SCFA production depends not only on substrates flowing into the large intestine [1], but also on the organ size and the population and composition of luminal bacteria [2]. Therefore, the large intestine is the major production site of SCFA in hindgut fermenters such as humans and pigs [2]. It is well established that luminal SCFA are rapidly absorbed from the intestine, and subsequently utilized by the host as substrate for metabolic energy production [3]. The energy contribution of SCFA to the basal metabolic rate is thought to be 30–76% [3] and 10% [4] in pigs and humans, respectively. Furthermore, SCFA are useful to the host not only for maintenance of the gut morphology and function [5,6] but also for reduction of appetite and diet-induced obesity [7,8].
During suckling, newborn mammals are fed only maternal milk. In humans [9] and pigs [10], maternal milk contains large amounts of disaccharides and oligosaccharides and thus, hindgut fermentation starts merely a few days after delivery [11]. When hindgut fermentation starts, the concentrations of SCFA in infant feces are approximately 60 mmol/kg [11]. Weaning, which occurs for all newborn mammals, can be a very stressful event. For example, it has been observed that the intestinal structure and function of piglets are drastically affected by weaning [12,13]. Moreover, during weaning, the transition from maternal milk to solid food affects not only the structure and function of the intestine but also the components of the luminal environment such as the intestinal microbiota and its metabolites. In pigs, it has been reported that weaning affects the luminal SCFA concentrations and composition in the large intestine [14,15]. Although the absorption process and blood flow dynamics of SCFA in weaning piglets remain unclear, most SCFA (95%) produced by the luminal microbiota is thought to be quickly absorbed from the mucosa, while only 5% is excreted in the feces [16]. van Beers-Schreurs et al. [17] compared the concentrations of SCFA in the portal and peripheral blood with those in the large intestinal digesta in weaning piglets. They reported that while giving a solid diet during weaning increased the SCFA concentrations in portal blood, the concentrations of SCFA remained unchanged in the intestinal lumen [17]. These data suggest that the concentrations of luminal SCFA do not always reflect the concentrations of absorbed SCFA.
Age at weaning age is one of the important factors for a good post-weaning development of pigs. In our previous study, early weaning at 14 days of age caused functional and morphologic ateliosis in piglets [12,13]. It is worth noting that marked maturation of the mucosal structure and function took place in 14- and 21-day-old suckling piglets [12]. Therefore, it is likely that age at weaning may also affect the ability of the intestine to absorb SCFA.
The aim of the present study was to evaluate the production and influx of SCFA in the cecum of pigs from suckling to weaning using a highly sensitive gas chromatography-mass spectrometry (GC-MS) technique [18]. Gene expression of SCFA transporters and molecules related to the tight junction were also assessed to elucidate the mechanism of SCFA absorption from the lumen into blood of piglets.
2. Materials and Methods
2.1. Animals
The 32 piglets used in the present study are shown in Table 1. The crossbred (Landrace × Large white × Duroc) piglets used in the present experiment were the same as those described in a previous study [13], except for piglets weaned at 14 days of age, which were omitted in the present work because we demonstrated that weaning at such age is not commercially practical for the pig industry [12,13]. All piglets were raised at the Toyohashi Feed Mills Technical Center (Shinshiro, Aichi, Japan). Suckling piglets were fed only maternal milk and weaning piglets were given a typical commercial weaning diet (JustOne Sprout; Toyohashi Feed Mills, Aichi, Japan). The nutrient composition of the diet was as follows (g/kg): crude protein, 214; crude fat, 75; crude fiber, 3; and crude ash, 60. All diets and water were given ad libitum. The animals were handled in accordance with the guidelines for animal studies of the Experimental Animal Committee of Kyoto Prefectural University (approval number KPU240410).
Table 1.
Piglets used in the present study (n = 4) 1.
| Age (Days) | Nutrition Period | Mean Body Weight (kg) | Age at Weaning (Days) | Identification Code |
|---|---|---|---|---|
| 7 | Suckling | 3.7 | – | S7 |
| 14 | Suckling | 5.3 | – | S14 |
| 21 | Suckling | 7.0 | – | S21 |
| 28 | Suckling | 10.4 | – | S28 |
| 28 | Weaned | 9.0 | 21 | W21p7 |
| 35 | Weaned | 13.1 | 21 | W21p14 |
| 35 | Weaned | 12.4 | 28 | W28p7 |
| 42 | Weaned | 15.9 | 28 | W28p14 |
1 Piglets used were the same as those described in a previous study [13], except for piglets weaned at 14 days of age, which were excluded in the present work.
2.2. Dissection and Sampling
Ad libitum feeding was maintained until just before the dissection in all piglets. At the dissection, pigs were intraperitoneally anesthetized with sodium pentobarbital (Somnopentyl; Kyoritsu, Tokyo, Japan). All dissections started at 11:00 a.m. and collection of blood samples from all location of a piglet were finished within 5 min after confirmation of deep anesthesia. Briefly, the abdominal wall of pigs was incised along midline, blood was quickly collected from the cecal, portal, and abdominal veins, and the animals were euthanized by exsanguination. Afterward, the entire intestine was removed, the large intestine separated, and the cecal digesta collected. The cecum was washed several times with sterilized saline, and its middle section of mucosa soaked in RNA-later® solution (Sigma, Tokyo, Japan), and stored first at 4 °C for 24 h, then at −80 °C until use. Blood samples were centrifuged at 1750× g for 10 min at 4 °C and serum was collected. Serum and digesta samples were stored at −80 °C until use.
2.3. Short Chain Fatty Acid Analysis by Ion-Exclusion High-Perfornance Liquid Chromatography
The concentrations of SCFA in the cecal digesta were measured by ion-exclusion high-performance liquid chromatography (HPLC) as previously described [18].
2.4. High-Sensitivity Detection of Short Chain Fatty Acid by Gas Chromatography-Mass Spectrometry
SCFA in serum samples were analyzed by GC-MS using a high-sensitivity detection method as previously described [18].
2.5. Gene Expression Analyses Using Real-Time Polymerase Chain Reaction
Total RNA extraction from cecal mucosa was conducted as described elsewhere [19]. cDNA synthesis and real-time polymerase chain reaction (PCR) were conducted as previously described by Inoue et al. [20]. Gene expressions of monocarboxylate transporter 1 (MCT1), sodium monocarboxylate transporter 1 (SMCT1), and occludin were evaluated by ∆∆Ct methods with reference to glyceraldehyde 3-phosphate dehydrogenase (gapdh) as internal control [21]. The primers and Taqman probes used in the present study are listed in Table 2.
Table 2.
Primers using this study.
| Gene | Sequences (5′-3′) | Accession No. | Probe Number 1 |
|---|---|---|---|
| Solute carrier family 16 member 1 (SLC16A1; MCT1) | F: tttgacactctaggcaatcagg R: gatgagagagaacagttatcggaag |
NM_001128445 | 14 |
| Solute carrier family 5 member 8 (SLC5A8; SMCT1) | F: tgtttgctttggggattttg R: caattccgacccacaaagaa |
NM_001291414 | 20 |
| Occludin | F: ggctaggggtctaaactgagc R: ctcagtgggttgaaggatctg |
NM_001163647 | 5 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F: gtgacactcactcttctacctttga R: tgacaaagtggtcgttgagg |
AF017079 | 45 |
1 Listed probe numbers indicate the product number of the Universal ProbeLibrary Set by Roche Applied Science (Mannheim, Germany).
2.6. Statistical Analyses
Depending on the results of the Bartlett test, either a complete randomized design, one-way analysis of variance (ANOVA) or the Kruskal–Wallis test was used to analyze the differences in each variable between S7, S14, S21, S28, W21p7, W21p14, W28p7, and W28p14. Tukey–Kramer post hoc (parametric or non-parametric) methods were used for multiple comparisons as needed. Correlation coefficient and its probability between parameters were analyzed by the Pearson’s correlation coefficient test. Differences between means were considered significant at p < 0.05. Values are given as the means ± standard errors. All data were analyzed using STATCEL4 (OMS, Saitama, Japan), an add-in package for Excel® (Microsoft Corp., Redmond, WA, USA).
3. Results
3.1. Concentrations of Short-Chain Fatty Acids in the Cecal Digesta
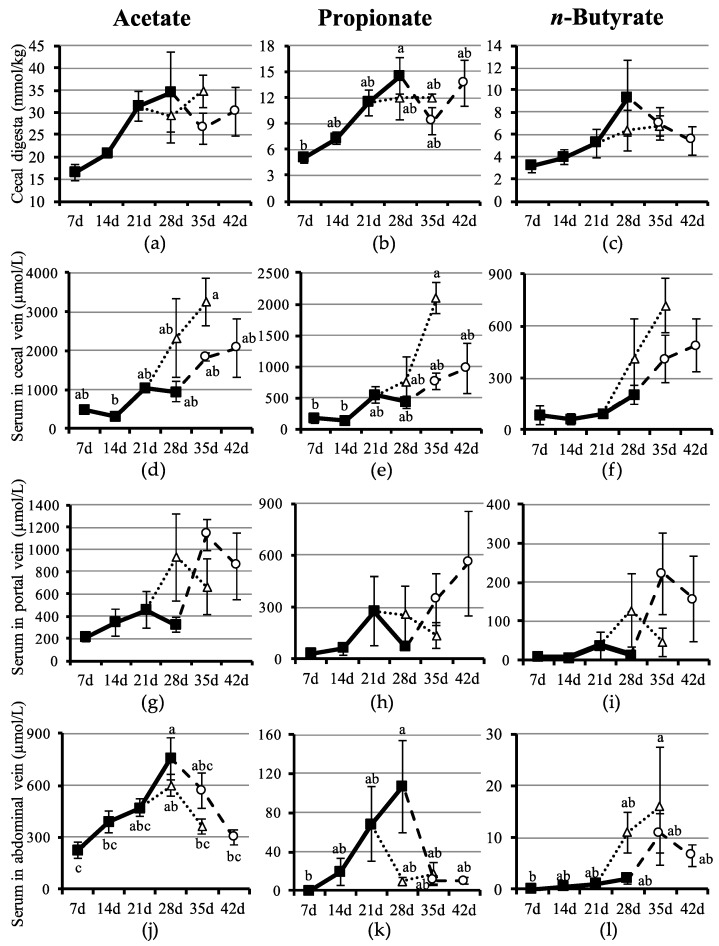
Acetate, propionate and n-butyrate concentrations were found to increase in the cecal digesta of piglets (Figure 1a–c), from day 7 to day 28 after birth, but only the concentration of propionate in S28 piglets was found to be significantly higher than that in S7 piglets (Figure 1b).
Figure 1.
Short-chain fatty acid concentration in the cecal digesta, cecal vein, portal vein and abdominal vein of the piglets. (a) Concentration of acetate in the cecal digesta. (b) Concentration of propionate in the cecal digesta. (c) Concentration of n-butyrate in the cecal digesta. (d) Concentration of acetate in serum in the cecal vein. (e) Concentration of propionate in serum in the cecal vein. (f) Concentration of n-butyrate in serum in the cecal vein. (g) Concentration of acetate in serum in the portal vein. (h) Concentration of propionate in serum in the portal vein. (i) Concentration of n-butyrate in serum in the portal vein. (j) Concentration of acetate in serum in the abdominal vein. (k) Concentration of propionate in serum in the abdominal vein. (l) Concentration of n-butyrate in serum in the abdominal vein. Symbology: closed squares, suckling piglets; open circles, piglets weaned at 28 days of age; open triangles, piglets weaned at 21 days of age. Error bars represent the standard errors. Symbols with different letters (a, b and c) indicate significant differences at p < 0.05.
Age at weaning tended to affect SCFA concentrations in the cecal digesta. For example, although the concentrations of acetate and propionate were unaffected after weaning at day 21, they tended to be affected after weaning at day 28. The concentrations of acetate and propionate detected at weaning at day 28 temporarily decreased within the next seven days (W28p7), before recovering at day 14 post-weaning (W28p14). Nonetheless, these changes in concentrations were not significantly different.
3.2. Concentrations of Short-Chain Fatty Acids in the Cecal Vein
In serum in the cecal vein, SCFA concentrations did not change from day 7 to day 28 after birth, although weaning tended to increase the concentrations of acetate, propionate, and n-butyrate (Figure 1d–f). Particularly, acetate and propionate concentrations in W21p14 piglets increased significantly than those in S14 piglets.
3.3. Concentrations of Short-Chain Fatty Acids in the Portal Vein
In serum in the portal vein, changes in SCFA concentration were not detected (Figure 1g,h,j), due to the wide range of individual values.
3.4. Concentrations of Short-Chain Fatty Acids in the Abdominal Vein
The concentrations of SCFA in serum in the abdominal vein tended to differ from those in other veins (Figure 1j–l). For example, the concentrations of acetate and propionate gradually increased from day 7 onward, being those detected at day 28 significantly higher than those at day 7. It is worth noting that the concentration of n-butyrate was barely detected from day 7 to day 28 after birth (Figure 1l).
Age at weaning also tended to affect SCFA concentrations in the abdominal vein after weaning. For example, after weaning at day 21, the concentration of acetate initially tended to increase (W21p7), before showing a tendency to decrease at day 14 post-weaning (W21p14). In contrast, after weaning at day 28 a decrease in the concentration of acetate was detected, which by day 14 post-weaning (W28p14) became significant, when compared with S28 piglets. In addition, the concentration of propionate tended to decrease in piglets weaned at days 21 and 28. Regarding the concentration of n-butyrate, a non-significant increase was detected in all weaned piglets, regardless of age at weaning.
3.5. Gene Expressions of SCFA Transporters and Occludin in the Cecal Mucosa
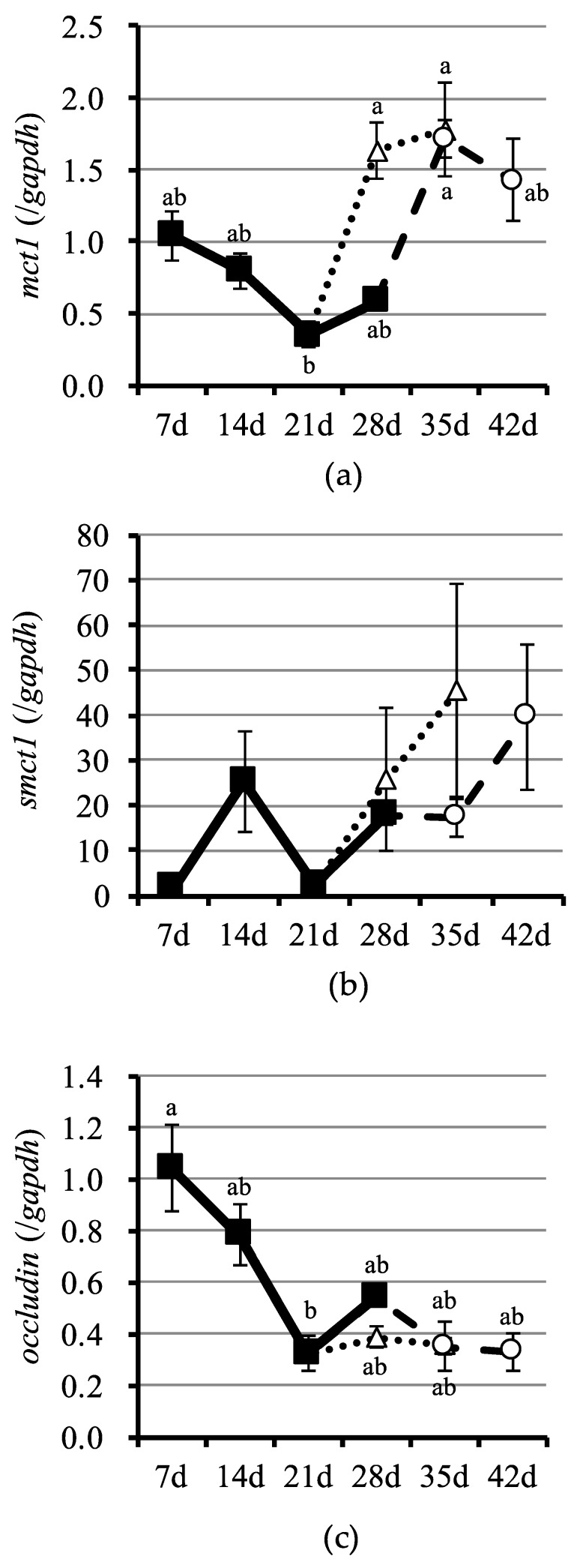
Gene expression of SCFA transporters MCT1 and SMCT1, and occludin—a molecule related to the tight junction—is shown in Figure 2. While the gene expression of MCT1 decreased during suckling, it increased after weaning (Figure 2a). Indeed, there were significant differences between the gene expression of MCT1 observed at weaning days and after weaning (S21 vs. W21p7, W21p14, and W28p7). The highest expression of smct1 was detected in S14 piglets (Figure 2b). In addition, smct1 expression showed non-significant increases after weaning at days 21 and 28. Gene expression of occludin was the highest in S7 piglets, but it decreased over time (Figure 2c). While a significant difference was observed between S7 and S21 piglets, weaning did not affect the gene expression of occludin.
Figure 2.
Relative gene expression of MCT1, SMCT1, and occludin in the cecal mucosa. (a) Monocarboxylate transporter 1 (mct1). (b) Sodium monocarboxylate transporter 1 (smct1). (c) Occludin. Symbology: closed squares, suckling piglets; open circles, piglets weaned at 28 days of age; open triangles, piglets weaned at 21 days of age. Error bars represent the standard errors. Symbols with different letters (a and b) indicate significant differences at p < 0.05.
3.6. Correlation Analysis of Short-Chain Fatty Acids Concentration in the Cecal Vein
Correlations of the concentrations of SCFA in the cecal vein with those in other samples and gene expression associated with SCFA transporters and the tight junction are shown in Table 3.
Table 3.
Correlation analysis between short-chain fatty acids (SCFA) concentrations in the cecal vein and other variables.
| SCFA in the Cecal Vein | Other Variables | Correlation Coefficient | Probability 1 |
|---|---|---|---|
| Acetate | Cecal digesta | 0.56 | <0.001 |
| Portal vein | 0.46 | 0.002 | |
| Abdominal vein | 0.13 | 0.39 | |
| mct1 | 0.44 | 0.003 | |
| smct1 | −0.02 | 0.88 | |
| occludin | −0.43 | 0.003 | |
| Propionate | Cecal digesta | 0.40 | 0.01 |
| Portal vein | 0.15 | 0.31 | |
| Abdominal vein | −0.01 | 0.96 | |
| mct1 | 0.48 | 0.001 | |
| smct1 | 0.26 | 0.08 | |
| occludin | −0.40 | 0.01 | |
| n-Butyrate | Cecal digesta | 0.20 | 0.19 |
| Portal vein | −0.02 | 0.88 | |
| Abdominal vein | −0.01 | 0.96 | |
| mct1 | 0.40 | 0.01 | |
| smct1 | 0.16 | 0.28 | |
| occludin | −0.38 | 0.01 |
1 When probability was less than 0.05, we considered significant correlation was observed between the parameters.
While acetate and propionate concentrations in the cecal vein positively correlated with those in cecal digesta (p < 0.05), n-butyrate concentration in the cecal vein did not. Moreover, while the concentration of acetate in the cecal vein also positively correlated with that in the portal vein (p < 0.05), the concentrations of propionate and n-butyrate did not. Finally, the concentrations of SCFA in the cecal and the abdominal veins did not correlate. Interestingly, all SCFA concentrations in the cecal vein significantly correlated with the gene expression of MCT1 and occludin (p < 0.05). However, while the correlation with MCT1 gene expression was positive, the correlation with occludin gene expression was negative.
4. Discussion
SCFA are produced mainly in the hindgut of non-ruminant mammals [22]. In pigs, the cecum contains larger concentrations of SCFA in comparison with those found in the colon and rectum [6,22]. Therefore, in the present work cecum was chosen as the organ to evaluate SCFA production.
SCFA were readily detected in the cecal digesta of piglets, and their concentrations were detected to increase in suckling piglets from day 7 to day 28 after birth, but significant difference was not detected in acetate and n-butyrate (Figure 1a–c). Regarding the acetate, however, significant difference was observed between S14 and S21 when statistical analysis was performed on the values for only suckling piglets. Due to piglets ingested only maternal milk during suckling, the increase in SCFA concentrations in the cecal digesta may have been caused mainly by changes in milk composition and consequently in microbiota composition. For example, Noblet & Etienne [23] reported that the lactose concentration in milk gradually increases after parturition. Therefore, carbohydrates flowing into the cecum at higher concentrations are likely to further stimulate fermentation. The data from our previous study seem to validate this hypothesis because we showed that the gut microbiota of neonatal piglets undergoes changes even during suckling [24]. It has been well documented that weaning is one of the most stressful events for newborn mammals in general [25]. Indeed, the transition from liquid to solid food during weaning drastically affects the morphology and functions of the intestine [12,13], which in turn causes the gut microbiota to undergo profound changes [24]. In that context, in our study it was unexpected that SCFA concentrations in the cecal digesta did not change with weaning (Figure 1a–c). One possible explanation for the lack of change in SCFA concentration may be the size of cecum, which naturally increases after weaning [26]. The enlargement of cecum likely concealed an increase in the total amount of SCFA occurring in cecal digesta. Another fact that may help explain this unexpected outcome may be an increase in SCFA absorption after weaning, which is further discussed below.
SCFA found in blood serum in the cecal vein are those absorbed from the lumen following a partial utilization by the mucosal cells [27]. In the present work, absorption of SCFA was relatively low during suckling, but increased after weaning (Figure 1d–f). By contrast, although the concentrations of SCFA in the cecal vein did not change during suckling, they gradually increased in the cecal digesta during the same period (Figure 1a–c). These contrasting results seem to indicate that some sort of impaired absorption of SCFA took place in the lumen of suckling piglets. Nonetheless, it was detected that after weaning, SCFA concentrations in the cecal vein were high, which may indicate that absorption from the hindgut mucosa substantially increased in this period. When the correlation coefficient between the concentrations of SCFA in the cecal digesta and cecal vein were evaluated, it was found that acetate and propionate correlated positively, but n-butyrate did not (Table 3). These results seem to indicate that a considerable amount of n-butyrate was not absorbed into the cecal vein but remained within the cecal mucosa, perhaps utilized by the cells, especially the epithelial cells. The fact that n-butyrate is the major energy source of the epithelial cells in the large intestine [28] seems to validate our results. Interestingly, unlike in the suckling period, during the post-weaning period a higher concentration of n-butyrate was detected in the cecal vein (Figure 1f), which suggests that in weaned piglets, more n-butyrate flowed into blood and reached the liver through the portal vein (Figure 1i). In comparison, n-butyrate and other SCFA are transported through the portal vein to the liver of humans [29], implying that the dynamics of n-butyrate may be a similar feature in both humans and pigs.
When the host absorbs SCFA from the lumen, two routes have been observed: a ‘passive’ diffusion and an ‘active’ transport, but their contribution rate remains unclear [3], particularly during the suckling–weaning period. Passive diffusion, also known as gut permeability, permits the entry not only of SCFA but also of pathogenic microorganisms [30], which is generally problematic for the pig farming industry if it occurs after weaning [31]. The active transport of SCFA is a carrier-mediated transport dependent on metabolic energy, and it is believed that some transporters expressed by the epithelial cells are involved in the active transport of SCFA [32]. Indeed, HCO3/monocarboxylate exchange proteins, MCT and SMCT have been suggested to be facilitators of the influx of SCFA in the large intestine [16]. However, the HCO3/monocarboxylate exchange proteins in the large intestine are yet to be fully identified. For the present study, we selected two well-known transporters; MCT1 and SMCT1 as possible candidates involved in the active transport of SCFA in the epithelial cells of the intestine [16]. In the present work, although expression of mct1 was induced by weaning (Figure 2a), that of smct1 was not (Figure 2b). Moreover, mct1 expression positively correlated with the concentrations of acetate, propionate and n-butyrate in the cecal vein (Table 3), suggesting that MCT1 plays an important role as active transport in suckling-weaned SCFA absorption. With regard to the passive diffusion of SCFA, weaning seems to increase gut permeability, because the expression of genes associated with the tight junction such as occludin, zonula occludens protein-1 and claudin-1 are downregulated by weaning [30,31]. Indeed, a downregulated expression of these genes induces an increased passive diffusion in a lactulose/mannitol tolerance test [30]. In the present study, while gene expression of occludin decreased during suckling, the expression of this gene was unaffected by weaning (Figure 2c). Furthermore, occludin expression negatively correlated with the concentrations of acetate, propionate and n-butyrate in the cecal vein (Table 3), suggesting that passive diffusion may also contribute to SCFA absorption in developing piglets.
Although no significance was found, the dynamics of SCFA in the portal vein bore a resemblance to those in the cecal vein (Figure 1g–i). Conversely, the dynamics of the concentrations of acetate and propionate in the abdominal vein were different from those in other veins (Figure 1j,k). Acetate and propionate concentrations increased in the abdominal vein during suckling, but sharply decreased after weaning (Figure 1j,k). After flowing into the liver, SCFA are readily metabolized by hepatocytes [33]. For example, propionate is almost all metabolized to glucogenic and lipogenetic substrates in the liver, hence it is hardly detected in the abdominal vein afterward [33]. In the present study, however, a relatively high amount of propionate was still observed in the abdominal vein of 28-day-old suckling piglets (Figure 1k). Two scenarios can be proposed to explain this apparent discrepancy: (1) under-development prevents the liver from completely metabolizing propionate during suckling; and (2) additional acetate and propionate may have directly flown in from the lymph fluid via the thoracic duct. To corroborate this possibility, we confirmed that the lymph fluid of suckling piglets contained higher concentrations of acetate (125 µmol/L) and propionate (2.0 µmol/L) than did that of weaned piglets (acetate: 83 µmol/L; propionate: undetected). Therefore, it can be cautiously asserted that the concentrations of acetate and propionate in the lymph fluid likely contributed to an increase in the concentrations of these SCFA in the abdominal vein during suckling.
5. Conclusions
In the present study, the dynamics of SCFA production and influx in piglets were evaluated from suckling to weaning. It was observed that while that the concentrations of SCFA in the cecal digesta changed from day 7 to day 28 after birth (suckling), those in the cecal vein did not. Our results suggested that some sort of impaired absorption of SCFA took place during suckling. Moreover, while the concentrations of SCFA in the cecal digesta were unaffected by weaning, those in the cecal vein substantially increased after weaning. Based on these results, it can be hypothesized that absorption from the hindgut mucosa likely begins after weaning. Gene expression analysis seemed to support this hypothesis, as it was observed in the cecal mucosa that while SCFA transporter mct1 was upregulated, occuludin—a protein associated with the tight junction—was downregulated. It may be concluded that the present work demonstrated that age at weaning affected SCFA absorption, especially an earlier weaning at 21 days of age rather than a later weaning at 28 days of age. Nonetheless, because limited numbers of piglets were used in this study, further study is needed to determine our hypothesis regarding the effect of weaning.
Acknowledgments
The authors would like to thank S. Ishizuka and T. Hira of Hokkaido University for their excellent suggestions and comments. Special thanks to H. Hatanaka, Y. Kawada and T. Nagino, and T. Harayama and Y. Shimizu of Kyoto Prefectural University, and M. Nishikawa and N. Matsukawa of the Kyoto Institute of Nutrition and Pathology for their technical assistance.
Author Contributions
Conceptualization, T.T. and R.I.; Methodology, T.T., R.I. and M.N.; Validation, T.T. and R.I.; Formal Analysis, T.T.; Investigation, M.N., R.I., S.T., K.F. and T.T.; Resources, K.F.; Data Curation, T.T.; Writing—Original Draft Preparation, M.N.; Writing—Review and Editing, T.T. and R.I; Visualization, T.T.; Supervision, T.T.; Project Administration, T.T., Funding Acquisition, T.T. All authors have read and approved the final manuscript.
Funding
This research was funded by JSPS KAKENHI grant number 23780269 and 26660214.
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Macfarlane G.T., Gibson G.R. Microbiological aspects of the production of short-chain fatty acids in the large bowel. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 87–105. [Google Scholar]
- 2.Hume I.D. Flow dynamics of digesta and colonic fermentation. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 119–132. [Google Scholar]
- 3.Engelhardt W.V. Absorption of short-chain fatty acids from the large intestine. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 149–170. [Google Scholar]
- 4.Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 5.Scheppach W. Effects of short-chain fatty acids on gut morphology and function. Gut. 1994;35:S35–S38. doi: 10.1136/gut.35.1_Suppl.S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukahara T., Iwasaki Y., Nakayama K., Ushida K. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa. J. Nutr. Sci. Vitaminol. 2003;49:414–421. doi: 10.3177/jnsv.49.414. [DOI] [PubMed] [Google Scholar]
- 7.Byrne C.S., Chambers E.S., Morrison D.J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015;39:1131–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J. Nutr. 2006;136:2127–2130. doi: 10.1093/jn/136.8.2127. [DOI] [PubMed] [Google Scholar]
- 10.Xu R.J. Composition of porcine milk. In: Xu R.-J., Cranwell P., editors. The Neonatal Pig, Gastrointestinal Physiology and Nutrition. Nottingham University Press; Nottingham, UK: 2003. pp. 213–246. [Google Scholar]
- 11.Lifschitz C.H. Colonic short-chain fatty acids in infants and children. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 525–535. [Google Scholar]
- 12.Inoue R., Tsukahara T., Nakatani M., Okutani M., Nishibayashi R., Ogawa S., Harayama T., Nagino T., Hatanaka H., Fukuta K., et al. Weaning markedly affects transcriptome profiles and Peyer’s patch formation in piglet ileum. Front. Immunol. 2015;6:630. doi: 10.3389/fimmu.2015.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsukahara T., Inoue R., Nakatani M., Fukuta K., Kishino E., Ito T., Ushida K. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim. Sci. J. 2016;87:67–75. doi: 10.1111/asj.12399. [DOI] [PubMed] [Google Scholar]
- 14.Mathew A.G., Franklin M.A., Upchurch W.G., Chattin S.E. Effect of weaning on ileal short-chain fatty acid concentrations in pigs. Nutr. Res. 1996;16:1689–1698. doi: 10.1016/0271-5317(96)00188-1. [DOI] [Google Scholar]
- 15.Franklin M.A., Mathew A.G., Vickers J.R., Clift R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs. 24 days of age. J. Anim. Sci. 2002;80:2904–2910. doi: 10.2527/2002.80112904x. [DOI] [PubMed] [Google Scholar]
- 16.Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Beers-Schreurs H.M.G., Nabuurs M.J.A., Vellenga L., Kalsbeek-van der Valk H.J., Wensing T., Breukink H.J. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J. Nutr. 1998;128:947–953. doi: 10.1093/jn/128.6.947. [DOI] [PubMed] [Google Scholar]
- 18.Tsukahara T., Matsukawa N., Tomonaga S., Inoue R., Ushida K., Ochiai K. High-sensitivity detection of short-chain fatty acids in porcine ileal, cecal, portal and abdominal blood by gas chromatography-mass spectrometry. Anim. Sci. J. 2014;85:494–498. doi: 10.1111/asj.12188. [DOI] [PubMed] [Google Scholar]
- 19.Ushida K., Yoshida Y., Tsukahara T., Watanabe T., Inoue R. Oral administration of Enterococcus faecalis cell preparation improves villous atrophy after weaning through enhancement of growth factor expression in mice. Biomed. Res. 2010;31:191–198. doi: 10.2220/biomedres.31.191. [DOI] [PubMed] [Google Scholar]
- 20.Inoue R., Tsuruta T., Nojima I., Nakayama K., Tsukahara T., Yajima T. Postnatal changes in the expression of genes for cryptdins1-6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol. Med. Microbiol. 2008;52:407–416. doi: 10.1111/j.1574-695X.2008.00390.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa S., Okutani M., Tsukahara T., Nakanishi N., Kato Y., Fukuta K., Romero-Perez G.A., Ushida K., Inoue R. Gene expression profiles of porcine T cells are characteristically distinct in colostrum compared with blood. Am. J. Vet. Res. 2016;77:961–968. doi: 10.2460/ajvr.77.9.961. [DOI] [PubMed] [Google Scholar]
- 22.Breves G., Stück K. Short-chain fatty acids in the hindgut. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 73–85. [Google Scholar]
- 23.Noblet J., Etienne M. Effect of energy level in lactating sows on yield and composition of milk and nutrient balance of piglets. J. Anim. Sci. 1986;63:1888–1896. doi: 10.2527/jas1986.6361888x. [DOI] [PubMed] [Google Scholar]
- 24.Inoue R., Tsukahara T., Nakanishi N., Ushida K. Development of the intestinal microbiota in the piglet. J. Gen. Appl. Microbiol. 2005;51:257–265. doi: 10.2323/jgam.51.257. [DOI] [PubMed] [Google Scholar]
- 25.Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013;4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo M., Martin-Orue S.M., Nofrarias M., Manzanilla E.G., Gasa J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet. Microbiol. 2007;124:239–247. doi: 10.1016/j.vetmic.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Livesey G., Elia M. Short-chain fatty acids as an energy source in the colon: Metabolism and clinical implications. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 427–481. [Google Scholar]
- 28.Young G.P., Gibson P.R. Butyrate and the human cancer cell. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 319–335. [Google Scholar]
- 29.Bloemen J.G., Venema K., van de Poll M.C., Olde Damink S.W., Buurman W.A., Dejong C.H. Short chain fatty acids exchange across the gut and the liver in humans measured at surgery. Clin. Nutr. 2009;28:657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B., Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- 31.Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- 32.Iwanaga T., Takebe K., Kato I., Karaki S., Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed. Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- 33.Remesy C., Demigne C., Morand C. Metabolism of short-chain fatty acid in the liver. In: Cummings J.H., Rombeau J.L., Sakata T., editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. pp. 171–190. [Google Scholar]