Abstract
Trabecular morphogenesis is a key morphologic event during cardiogenesis and contributes to the formation of a competent ventricular wall. Lack of trabeculation results in embryonic lethality. The trabecular morphogenesis is a multistep process that includes, but not limited to, trabecular initiation, proliferation/growth, specification and compaction. Although a number of signaling molecules have been implicated in regulating trabeculation, the cellular processes underlying mammalian trabecular formation are not fully understood. Recent works show that the myocardium displays polarity, and oriented cell division and directional migration of the cardiomyocytes in the monolayer myocardium are required for trabecular initiation and formation. Furthermore, perpendicular oriented cell division is an extrinsic asymmetric cell division that contributes to trabecular specification, and is a mechanism that causes the trabecular cardiomyocytes to be distinct from the cardiomyocytes in compact zone. Once the coronary vasculature system starts to function in the embryonic heart, the trabeculae will coalesce with the compact zone to thicken the heart wall, and abnormal compaction will lead to left ventricular non-compaction (LVNC) and heart failure. There are many reviews about compaction and LVNC. In this review, we will focus on the roles of myocardial polarity and oriented cell division in trabecular initiation, formation, and specification.
Keywords: Myocardial polarity, oriented cell division, trabeculation and trabecular specification
1. Trabeculation and compaction
The heart is the first functional organ formed in mammalian embryonic development. During cardiogenesis, cardiac progenitor cells from the cardiac crescent migrate toward the ventral midline to form a linear heart tube with a smooth inner surface1,2. When the heart tube undergoes looping, myocardium along the outer curvature of the tube will grow inward and form sheet-like structures that extend from the myocardium1,2, which are the newly initiated trabeculae3,4. As the early embryonic heart does not have a coronary circulatory system to perfuse itself, the sheet-like trabeculae function to increase surface area to facilitate nutrients and oxygen exchange. A lack of trabecular formation will result in less availability of oxygen and nutrients in the myocardial tissue and causes embryonic lethality. Trabecular malformation leads to cardiomyopathy and heart failure5,6. Trabeculae are also required for the formation of Purkinje fibers and enable the myocardium to increase its mass in the absence of coronary circulation. Lastly, trabeculae also function to anchor mitral and tricuspid valves via papillary muscles.
During normal cardiac morphogenesis, starting at ~E14.5 in the mouse and about weeks 5–8 of human embryonic life, the coronary plexus gradually forms and delivers blood to the myocardium. The basal portions of the trabeculae will coalesce with the ventricular wall to thicken the compact myocardium, subsequently the inter-trabecular recesses are compressed to capillaries to link to the coronary circulation system3. This trabecular zone coalesce with the compact zone is called the trabecular compaction, starting at the base of the heart and progressing toward the apex3. Disturbed compaction during development results in left ventricular noncompaction (LVNC)3,7,8. About half a million Americans suffer from LVNC9. LVNC is morphologically characterized by excessive trabeculation of the left ventricular walls with deep inter-trabecular recesses that communicate with the ventricular cavity but not with the coronary circulation. The hypoperfusion of the sub-endocardium and abnormal relaxation in LVNC might cause heart failure in patients10,11.
Despite the importance of trabeculation and compaction, the cellular and molecular mechanisms that regulate trabeculation and compaction are still not clear. This review will focus on the cellular and molecular mechanisms underlying trabecular formation and specification.
2. Monolayer myocardium displays transmural polarity.
Cell polarity is defined as asymmetric localization of cellular organelles, transporting lipid vesicles, cytoskeleton and proteins that include signaling receptors, adaptor proteins, and cell fate determinants. Cells establish polarity in response to chemical, electrical, mechanical, or other physical stimuli. Cell polarity is involved in multiple biological processes including directional migration, oriented cell division (OCD), morphogenesis and asymmetric cell division12–15. Loss of cell polarity is associated with uncontrolled cell growth and disrupted morphogenesis16. The roles of cell polarity in cardiac morphogenesis have recently been gradually revealed. We and other groups previously reported that the epicardium and pro-epicardium displayed apical-basal polarity, and polarity loss by tissue specific deletion of polarity genes causes abnormal epicardial and pro-epicardial development17–21. The function of cellular polarity is not fully appreciated in myocardial morphogenesis. The observation that cardiomyocytes of the monolayer of the heart tube migrate inward toward the heart lumen but not outward to the pericardial sac indicates that the myocardial layer displays a transmural polarity. When N-Cadherin, a major component of the adherens junctions of the heart, is disrupted specifically in the heart, the cardiomyocytes migrate randomly with some cells residing in the pericardial sac, which suggests that N-Cadherin is required to establish transmural polarity (Fig. 1). The myocardial layer’s transmural polarity is further demonstrated by the localization of extracellular matrix proteins such as collagen and cardiac jelly, which localize to the lumen side of the myocardial layer (Fig. 2). The extracellular matrix proteins in cardiac jelly might provide essential cues to polarize the cardiomyocytes. Recently several exciting studies show that the monolayer of myocardium displays apical-basal polarity in both zebrafish and mouse22–24 (Fig. 2). These studies found that polarity complex proteins such as Par3, aPKC and Par6 are asymmetrically localized across the monolayer of the myocardial wall. Apical-basal polarity might be essential for oriented cell division during trabecular morphogenesis in the mouse23,24, while apical-basal polarity in zebrafish might maintain the epithelial features of the myocardium and might be essential for apical constriction and promoting the cardiomyocyte entry into the myocardium22.
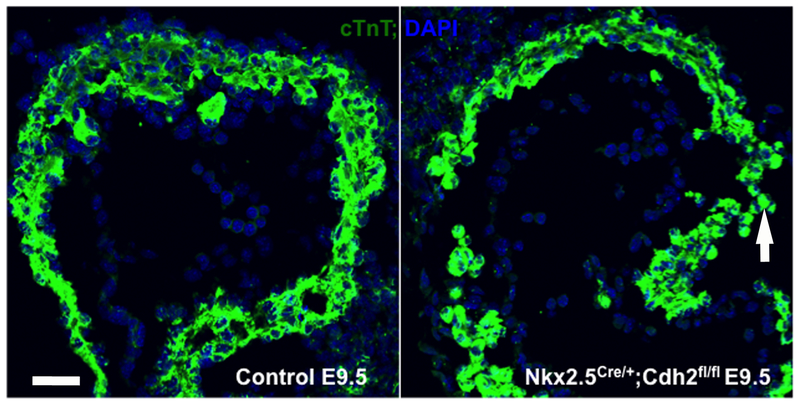
Figure 1. N-Cadherin is required to establish the polarity of the monolayer myocardium.
In the control heart at E9.5, cardiomyocytes migrate inward toward the heart lumen, while some cardiomyocytes in the Nkx2.5Cre/ERT2; Cdh2fl/f migrate outward to the pericardial sac indicated by while arrow. Scale bar is 20 μm.
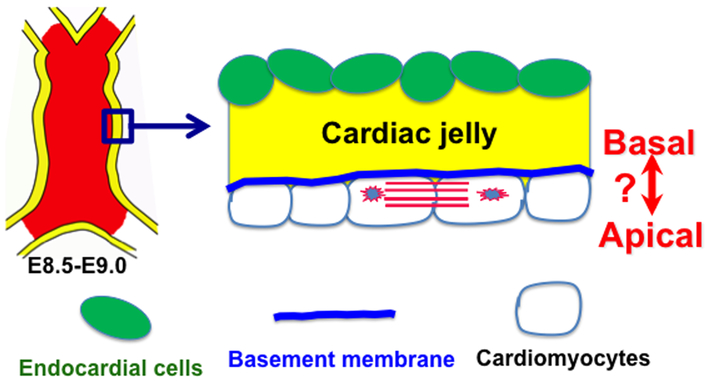
Figure 2. The monolayer of myocardium displays apical-basal polarity.
Mouse embryonic heart tube at E9.5-E9.0 has a smooth inner surface and contains a monolayer myocardium and a monolayer endocardium. The monolayer myocardium displays transmural polarity with basement membrane inside the heart lumen. ?: The orientation of apical-basal polarity shows some controversy, as a group says the domain that abuts the heart lumen is the apical domain or basal domain by another group.
3. Cardiomyocytes in the myocardial epithelium undergo directional migration and OCD
Tissue morphogenesis depends on the spatiotemporal arrangement of cells during development. The most well-known mechanism that contributes to the final shape of a tissue or organ is cellular intercalation in response to chemotactic cues or morphogens25. A less well known but essential mechanism is OCD25. OCD is determined by the alignment of the mitotic spindles to body shape26. OCD is a polarized cell division and a potential asymmetric cell division that contributes to cellular diversity and differentiation during development27. To determine whether OCD occurs during trabecular morphogenesis and trabecular specification, Domian’s and my labs used cellular and genetic tools to examine the cellular behaviors and the underlying genetic basis of trabecular initiation. We found that cardiomyocytes undergo OCD based on immunostaining, with about 40% of the mitotic cells aligning their spindle parallel to the myocardial epithelium, and about 40% of the mitotic cells aligning perpendicularly to the myocardium epithelium at about E8.523,24. Furthermore, individual cardiomyocytes genetically labeled prior to trabeculation via the inducible Cre mediated brainbow / multicolor labeling system were traced and the labeled cells/clones were analyzed to reveal that most labeled cells undergo one round of division to yield two cells by 24 hours after tamoxifen induction. In some clones, one daughter cell is at the surface of the compact zone and the other daughter is in the trabecular or inner compact zones, from which we infer that the two daughter cells resulted from a perpendicular division. In some clones, both daughter cells are at the surface of the compact zone, suggesting a parallel division. While in some clones, the labeled cells migrate away from the outer compact zone, suggesting that the labeled cells migrate and exit the surface of myocardium24. The in vivo single cell labeling further established that the cardiomyocytes in the early myocardial epithelium undergo OCD and directional migration (Fig. 3). These studies show that the trabecular morphogenesis in the mouse is different from zebrafish, in which trabeculae are initiated by directional migration but not OCD28,29. Whether OCD and directional migration contribute to trabecular morphogenesis needs further study, which will be discussed below.
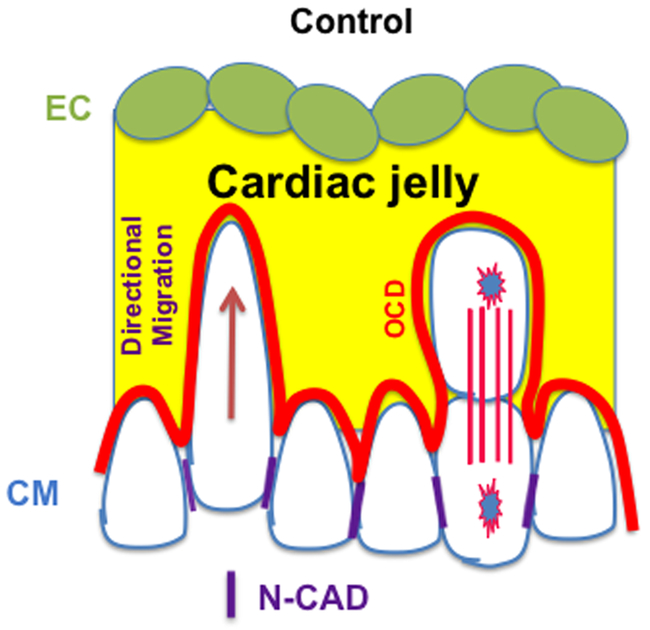
Figure 3. Directional migration and OCD contribute to trabecular initiation.
Both directional migration and OCD contribute to trabecular initiation, and N-Cadherin might be essential to establish the apical-basal polarity for directional migration and OCD. This figure is adapted from a published figure24.
4. Cardiomyocytes’ directional migration and OCD contribute to trabecular formation.
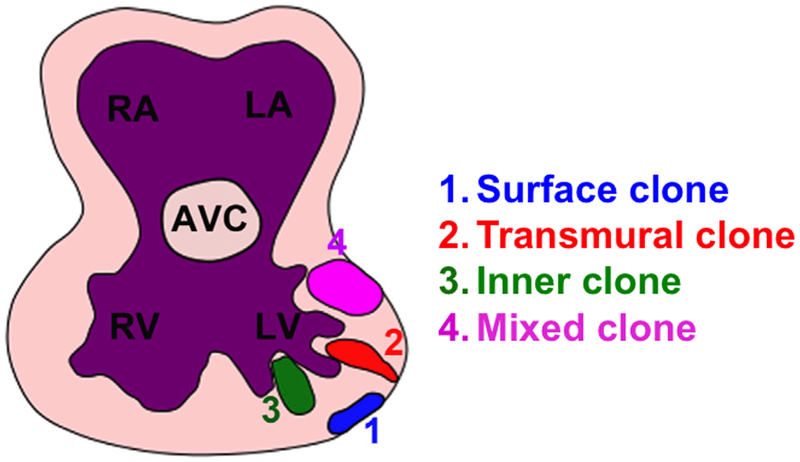
The observed perpendicular OCD of the cardiomyocytes in the myocardial epithelium can potentially send a daughter cell into cardiac jelly, which might result in trabecular initiation. Another potential mechanism of trabecular initiation is directional migration in which some cardiomyocytes undergo cytoskeleton rearrangement, become elongated, orient perpendicularly to the heart wall, and eventually migrate into the cardiac jelly to result in trabecular initiation. In zebrafish, the trabeculae are initiated by an epithelial-mesenchymal like transition and then migrate into the cardiac lumen to initiate trabeculation22,28. Although OCD occurs in the myocardial epithelium of the zebrafish, it does not contribute to trabecular initiation based on observations with variety tools, including time-lapse imaging22,28,30. However, time-lapse observation of the cardiomyocyte cellular dynamics during mouse trabecular morphogenesis is technically challenging, and is not feasible at this moment. Instead, single cell lineage tracing was used to infer cellular dynamics during trabecular formation in our study24. To determine whether directional migration and perpendicular OCD contribute to trabecular formation in the mouse, cardiomyocytes were labeled at E8.0-E8.5, a stage when the myocardium consists of a single cell layer and trabeculation has not yet initiated. 72 hours after induction, the labeled single cell has undergone several rounds of cell divisions to form a clonal cluster that exhibits specific geometric patterns. Based on the geometric distribution and anatomical annotation of each clone, the clones are categorized into four different patterns (Fig. 4). In the transmural clone, labeled cells are localized to the inner compact and trabecular zones, with one cell of the clone remaining in the outer compact zone. It can be inferred that the transmural clone is likely derived from a perpendicular OCD. In the surface clone, all the cells are in the outer compact layer of the myocardium, and are derived from parallel OCD. In the third clonal type, all the cells are in the inner compact zone and/or trabecular zone, and are defined as an inner clone, and were derived from directional migration. The forth type is the mixed clone, which displays two or more cells in the outer compact zone and some cells in the inner compact or trabecular zones, which might be derived from directional migration and OCD. These results suggest that inner clone, transmural clone and mixed clones contribute to trabecular formation, and directional migration and OCD contribute to trabecular initiation (Fig. 4)24. Details of the trabecular formation based single cell lineage tracing studies can be found in the original study24. Our study is consistent with previous work using intragenic recombination or virus showing that labeled cardiomyocyte clones grow in the orientation of the transmural axis or the myocardial planar axis31–34, which might result from perpendicular OCD and parallel division, respectively. Furthermore, our study answers the question whether the trabecular and compact cardiomyocytes specified before trabecular formation. The lineage tracing data show that some clones such as transmural clones and mixed clones contain cardiomyocytes in both compact and trabecular zone, suggesting that the compact cardiomyocytes and trabecular cardiomyocytes might be derived from the same population during cardiogenesis and are not specified before trabecular initiation.
Figure 4. The transmural, inner and mixed clones contribute to trabecular formation.
Cells of surface clones localize to the surface of the myocardium. Cells of inner, transmural and mixed clones contribute to the formation of trabeculae and inner compact zone. This figure is adapted from a published figure24.
5. Molecular mechanisms that regulate directional migration and trabecular formation.
The machinery that orients the spindle orientation is conserved from nematodes to mammals and involves an evolutionarily conserved adaptor protein LGN that binds lipid-anchored Gai at the cell cortex35–37. LGN interacts and recruits NuMA to form a complex at the cell cortex. This complex anchors spindle astral microtubules to the cell cortex and applies a pulling force on those microtubules through associated dynein to orient the mitotic spindle35,36,38–40. For the daughter cells to be properly positioned within the tissue, the position of the mitotic spindle must be tightly coordinated with the cortical polarity, and the LGN/NuMA/Gai complex has to interact with the polarity complex Par3/Par6/aPKC via Inscuteable. To establish the correct orientation of the mitotic spindle, cells respond to instructive spatial cues from their local environment to establish asymmetric localization of polarity complex: Par3/Par6/aPKC. This polarity complex binds to LGN/NuMA/Gai to establish the mitotic spindle orientation. Another essential complex required to establish mitotic spindle orientation is the adherens junction. Adherens junctions are the primary sites of epithelial cell attachment, and are essential for maintaining epithelial sheet integrity, apical-basal polarity and cytoskeleton. This complex includes E-Cadherin or N-Cadherin and cellular adaptor proteins such as different catenins and p12041. Previously, it was shown that loss of adherens adhesion disrupts the orientation of cell division in different tissues and species42–46. Our work shows that Numb Family Proteins are required to stabilize the adherens junctions and establish epicardial polarity. Epicardial cell specific deletion of Numb Family Proteins or β-catenin disrupts the epicardial cell’s polarity, randomizes the epicardial cell’s mitotic spindle orientation, causes less perpendicular OCD, and fewer epicardial cells entering into the myocardium17. Later it was found that cell–cell adhesion proteins such as E-cadherin function as an instructive cue for orientation of cell division by directing interaction with the LGN/NuMA/Gai complex. This mediates the stabilization of cortical associations of astral microtubules at cell–cell adhesions to orient the mitotic spindle 47.
During cardiogenesis, deletion of Cdh2, the gene encoding N-Cadherin, which is the major cadherin in cardiomyocytes, causes cardiomyocytes to be loosely associated48, and the hearts of the knockout have an abnormal morphology and exhibit an absence of trabeculae24. The mitotic spindle orientation pattern of the Cdh2 null cardiomyocytes in the heart is random and significantly different from that of the controls. However, the random mitotic orientation of the Cdh2 null cardiomyocytes might be due to cardiac growth arrest of the knockout, and whether OCD defect contributes to the trabeculation defects is not clear. To avoid cardiac growth arrest and further study how N-Cadherin regulates trabeculation, Cdh2 within some individual cells of Rosa26CreERT2; Rosa26Conf; Cdh2fl/fl mosaic hearts were deleted by inducing Cre activation at E7.75 and the clonal patterns and distributions of iCdh2 clones examined 72 hours later. The Cdh2 null clone display more surface and mixed clones, but less transmural and inner clones. In addition, the overall geometric location of Cdh2 null clones is different from that of the control clones as the Cdh2 null clones primarily localized to the heart surface and failed to invade as deeply into the heart as control clones, suggesting that N-Cadherin is required for invasion/migration. The reduced number of transmural, inner and mixed clones, and reduced invasion of all the clones might contribute to the trabeculation defects of the Cdh2 heart specific knockout and indicates that N-Cadherin regulates OCD and directional migration in a cardiomyocyte autonomous manner24. In zebrafish, although OCD does not contribute to trabeculation, the dynamic localization of N-Cadherin is required for cardiomyocyte entry toward the cardiac lumen to initiate trabeculation49. Whether N-Cadherin relocalizes during mouse trabeculation is not clear. In zebrafish, apical constriction mediated directional migration does not take place in the absence of Nrg/ErbB signaling, blood flow and cardiac contractility22. Whether Nrg1/ErbB2/4 regulates cardiomyocytes entry into myocardium via cardiomyocyte polarity and OCD in the mouse is unknown.
6. Compact and trabecular cardiomyocytes display different expression profiles, which might contribute to the differential maturation of the trabecular and compact cardiomyocytes.
Trabecular cardiomyocytes, which take the major responsibility for pumping at early stages of cardiac development, are more differentiated than compact zone cardiomyocytes3. Trabecular and compact cardiomyocytes display different features with trabecular cardiomyocytes exhibiting a lower proliferation rate and being more molecularly mature than cardiomyocytes of the compact zone50. These zones can also be distinguished by the differential expression of many genes. For example, p57, Irx3, BMP10, Sphingosine 1-phosphate receptor-1 and Cx40 are highly expressed in the trabecular zone, while Tbx20, Hey2, and N-Myc are highly expressed in the compact zone3,24,51–55. While these genes are specifically expressed in trabecular or compact cardiomyocytes at certain stages, the functions of each gene in the two zones are not clearly elucidated so far.
We adapted an RNAscope ISH/IFS system, in which single mRNAs and proteins can be detected simultaneously, to determine that BMP10 and Hey2 are expressed in trabecular zone and compact zone, respectively24. BMP10 is required to regulate cardiomyocytes proliferation via inhibiting the expression of p57, a major cell cycle inhibitor in the embryonic heart, to regulate cardiomyocyte proliferation53. Therefore, the finding that BMP10 is expressed in the trabecular zone, where cardiomyocytes proliferate at a slower rate comparing to cardiomyocytes in the compact zone, is surprising and suggests that differential BMP10 expression is not responsible for the differential proliferation rates between the compact and trabecular zones. The mechanism for how BMP10 regulates cardiomyocyte proliferation will need further study.
The asymmetric expression of Hey2 might contribute to the different features of trabecular and compact cardiomyocytes. Recent work using the heart specific Hey2 knockout shows that Hey2 plays a role in cardiomyocyte specification or maturation. Cardiac specific Hey2 knockout hearts display ectopic atrial gene expression56,57. Hey2 null cardiomyocytes displayed abnormal mitochondria, abnormal accumulation of glycogen particles, disorganized myofibrils, and increased expression of β-MHC and ANF genes 58, indicating defects in differentiation and maturation. These defects in Hey2 null cardiomyocytes might be the cause of the dilated left ventricular chamber with markedly diminished fractional shortening of the left ventricle observed in these mice 58. These studies indicate that Hey2 might be involved in cardiomyocyte maturation during heart development and trabecular specification, specifically to repress cardiomyocyte maturation. This is supported by the observation that Hey2 deletion in cardiomyocytes promotes cardiomyocyte maturation and is consistent with the observation that compact cardiomyocytes are less mature than cardiomyocytes in the trabecular zone.
7. OCD contributes to trabecular specification via a mechanism of extrinsic asymmetric cell division
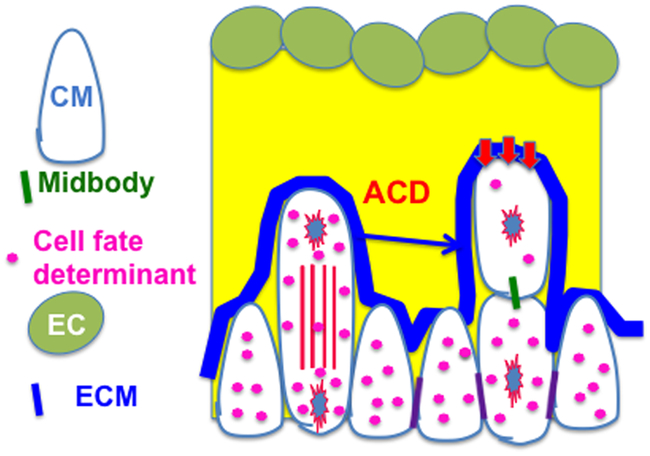
The differential expression profiles of trabecular and compact markers might contribute to their distinct differentiation and maturation as discussed above; however, the underlying mechanism of how the markers are differentially expressed is unknown 3,51,52. One potential mechanism contributing to the differential expression of Hey2 and Bmp10 in trabecular and compact cardiomyocytes would be the asymmetric distribution of Hey2 and Bmp10 in cardiomyocytes that undergo perpendicular OCD. To determine whether perpendicular OCD contributes to regional specification, E9.5 heart sections were stained with acetylated α-Tubulin and p120 (an adherens junction-associated protein that marks the membrane) and hybridized with probes to Bmp10 or Hey2 mRNA24. The number of signal dots or signal intensity in dividing cells were counted and the ratio between the two domains of the dividing cell at telophase or the two daughter cells after the division was calculated. We found that the mitotic cells undergoing perpendicular divisions did not display asymmetric distribution of Hey2. Our work showed that asymmetric expression of Hey2 and Bmp10 occurs after but not before cytokinesis in perpendicular OCD, and that their asymmetric distributions are due to the differences in geometric location between the two daughter cells. In parallel divisions, asymmetric distribution of Hey2 and BMP10 is not observed. The daughter cell closer to cardiac jelly in perpendicular oriented cell division displays less Hey2 and more BMP10 compared to the other daughter cell that is relatively closer to the surface of the heart, indicating that potential instructive cues for trabecular regional specification might lie in the cardiac jelly and endocardium (Fig. 5).
Figure 5. Perpendicular OCD is an extrinsic asymmetric cell division.
Cell fate determinants are symmetrically distributed to a dividing cell at telophase, but asymmetrically between the two daughter cells that are still linked by midbody, indicating the extrinsic asymmetric cell division. This figure is adapted from a published figure24.
8. Future directions.
With the essential functions of trabeculae well established but the mechanisms of trabecular formation and compaction unclear, there are many questions that need to be further studied. For instance, are the cardiomyocytes that undergo OCD or directional migration are predetermined before trabecular initiation? Many signaling pathways or molecules, including NRG1/ErbB2,46,59,60, Notch signaling61, Hand262, YAP63,64, DAAM165, and Numb Family Proteins66,67 regulate trabecular initiation. Recent work shows that signaling pathway such as Notch1 and NRG1/ErbB2 regulates the expression level of Hey2, but not the asymmetric distribution between the compact and trabecular zones55. The signaling pathways regulate the asymmetric distribution of Hey2 or other genes with differential expression between the two zones remains unknown. Also unknown is whether the expression of different trabecular markers are regulated by the same signaling pathways. Furthermore, in zebrafish, apical constriction or cardiomyocyte depolarization does not take place in the absence of Nrg and ErbB signaling or blood flow and cardiac contractility. Whether Nrg1/ErbB2/4 regulate cardiomyocytes polarity to control their entry into myocardium in the mouse is unknown. With the genetic tools such as, Hey2CreERT2, which is specifically active in compact zone at early embryonic stage, and NppaCreERT2, which is specifically active in trabecular cardiomyocytes68, to separate trabecular and compact cells being available, more definitive experiments can be designed to reveal the mechanisms of trabeculation and compaction in the future.
Acknowledgement
We thank the Wu laboratory members for scientific discussion, and Dr. John Schwarz for critical reading.
Funding: This study was funded by American Heart Association [13SDG16920099] and by National Heart, Lung, and Blood Institute grant [R01HL121700] to MW.
Footnotes
Conflict of Interest: None declared.
Disclosures: None.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Ethical approval: This article does not contain any studies with human participants performed by any of the authors.
Reference,
- 1.Manasek FJ Embryonic development of the heart. I. A light and electron microscopic study of myocardial development in the early chick embryo. Journal of morphology 125, 329–365, doi: 10.1002/jmor.1051250306 (1968). [DOI] [PubMed] [Google Scholar]
- 2.Van Mierop LH Embryology of the univentricular heart. Herz 4, 78–85 (1979). [PubMed] [Google Scholar]
- 3.Sedmera D, Pexieder T, Vuillemin M, Thompson RP & Anderson RH Developmental patterning of the myocardium. Anat Rec 258, 319–337 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Icardo JM & Fernandez-Teran A Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat (Basel) 130, 264–274 (1987). [DOI] [PubMed] [Google Scholar]
- 5.Jenni R, Rojas J & Oechslin E Isolated noncompaction of the myocardium. N Engl J Med 340, 966–967, doi: 10.1056/NEJM199903253401215 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Gassmann M et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378, 390–394, doi: 10.1038/378390a0 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Jefferies JL et al. Cardiomyopathy Phenotypes and Outcomes for Children With Left Ventricular Myocardial Noncompaction: Results From the Pediatric Cardiomyopathy Registry. J Card Fail 21, 877–884, doi: 10.1016/j.cardfail.2015.06.381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towbin JA & Jefferies JL Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ Res 121, 838–854, doi: 10.1161/CIRCRESAHA.117.310987 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Finsterer J Left ventricular non-compaction and its cardiac and neurologic implications. Heart failure reviews 15, 589–603, doi: 10.1007/s10741-010-9175-5 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Hussein A, Karimianpour A, Collier P & Krasuski RA Isolated Noncompaction of the Left Ventricle in Adults. J Am Coll Cardiol 66, 578–585, doi: 10.1016/j.jacc.2015.06.017 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Weiford BC, Subbarao VD & Mulhern KM Noncompaction of the ventricular myocardium. Circulation 109, 2965–2971, doi: 10.1161/01.CIR.0000132478.60674.D0 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Horvitz HR & Herskowitz I Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell 68, 237–255, doi:0092–8674(92)90468-R [pii] (1992). [DOI] [PubMed] [Google Scholar]
- 13.Jan YN & Jan LY Polarity in cell division: what frames thy fearful asymmetry? Cell 100, 599–602, doi:S0092–8674(00)80695–9 [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 14.Neumuller RA & Knoblich JA Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23, 2675–2699, doi:23/23/2675 [pii] 10.1101/gad.1850809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu M & Herman MA A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev Biol 293, 316–329, doi: 10.1016/j.ydbio.2005.12.024 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Bryant DM & Mostov KE From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9, 887–901, doi:nrm2523 [pii] 10.1038/nrm2523 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu M et al. Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell 19, 114–125, doi: 10.1016/j.devcel.2010.06.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J et al. CDC42 is required for epicardial and pro-epicardial development by mediating FGF receptor trafficking to the plasma membrane. Development 144, 1635–1647, doi: 10.1242/dev.147173 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose T et al. PAR3 is essential for cyst-mediated epicardial development by establishing apical cortical domains. Development 133, 1389–1398, doi:dev.02294 [pii] 10.1242/dev.02294 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Rhee DY et al. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 136, 3185–3193, doi:136/18/3185 [pii] 10.1242/dev.032334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengbusch JK, He W, Pinco KA & Yang JT Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol 157, 873–882, doi: 10.1083/jcb.200203075 jcb.200203075 [pii] (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez-Amilburu V et al. In Vivo Visualization of Cardiomyocyte Apicobasal Polarity Reveals Epithelial to Mesenchymal-like Transition during Cardiac Trabeculation. Cell reports 17, 2687–2699, doi: 10.1016/j.celrep.2016.11.023 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Passer D, van de Vrugt A, Atmanli A & Domian IJ Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation. Cell reports 14, 1662–1672, doi: 10.1016/j.celrep.2016.01.030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J et al. Single-Cell Lineage Tracing Reveals that Oriented Cell Division Contributes to Trabecular Morphogenesis and Regional Specification. Cell reports 15, 158–170, doi: 10.1016/j.celrep.2016.03.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanon I & Gonzalez-Gaitan M Oriented cell division in vertebrate embryogenesis. Curr Opin Cell Biol 23, 697–704, doi: 10.1016/j.ceb.2011.09.009 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Wei Y & Mikawa T Formation of the avian primitive streak from spatially restricted blastoderm: evidence for polarized cell division in the elongating streak. Development 127, 87–96 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Baena-Lopez LA, Baonza A & Garcia-Bellido A The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15, 1640–1644, doi:S0960–9822(05)00846–8 [pii] 10.1016/j.cub.2005.07.062 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Liu J et al. A dual role for ErbB2 signaling in cardiac trabeculation. Development 137, 3867–3875, doi:137/22/3867 [pii] 10.1242/dev.053736 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han P et al. Coordinating cardiomyocyte interactions to direct ventricular chamber morphogenesis. Nature 534, 700–704, doi: 10.1038/nature18310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudt DW et al. High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 141, 585–593, doi: 10.1242/dev.098632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meilhac SM, Esner M, Kerszberg M, Moss JE & Buckingham ME Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J Cell Biol 164, 97–109, doi: 10.1083/jcb.200309160 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meilhac SM, Esner M, Kelly RG, Nicolas JF & Buckingham ME The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6, 685–698 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Meilhac SM et al. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130, 3877–3889 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Mikawa T, Cohen-Gould L & Fischman DA Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. III: Polyclonal origin of adjacent ventricular myocytes. Dev Dyn 195, 133–141, doi: 10.1002/aja.1001950208 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Du Q, Stukenberg PT & Macara IG A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat Cell Biol 3, 1069–1075, doi: 10.1038/ncb1201-1069 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Du Q & Macara IG Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516, doi: 10.1016/j.cell.2004.10.028 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Mauser JF & Prehoda KE Inscuteable regulates the Pins-Mud spindle orientation pathway. PLoS One 7, e29611, doi: 10.1371/journal.pone.0029611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laan L et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148, 502–514, doi: 10.1016/j.cell.2012.01.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seldin L, Muroyama A & Lechler T NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. eLife 5, doi: 10.7554/eLife.12504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendricks AG et al. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr Biol 22, 632–637, doi: 10.1016/j.cub.2012.02.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yap AS, Brieher WM & Gumbiner BM Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13, 119–146, doi: 10.1146/annurev.cellbio.13.1.119 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Inaba M, Yuan H, Salzmann V, Fuller MT & Yamashita YM E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One 5, e12473, doi: 10.1371/journal.pone.0012473 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Elzen N, Buttery CV, Maddugoda MP, Ren G & Yap AS Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Molecular biology of the cell 20, 3740–3750, doi: 10.1091/mbc.E09-01-0023 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Borgne R, Bellaiche Y & Schweisguth F Drosophila E-cadherin regulates the orientation of asymmetric cell division in the sensory organ lineage. Curr Biol 12, 95–104, doi:S0960982201006480 [pii] (2002). [DOI] [PubMed] [Google Scholar]
- 45.Lu B, Roegiers F, Jan LY & Jan YN Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409, 522–525, doi: 10.1038/35054077 35054077 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- 46.Yamashita YM, Jones DL & Fuller MT Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550, doi: 10.1126/science.1087795 301/5639/1547 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 47.Gloerich M, Bianchini JM, Siemers KA, Cohen DJ & Nelson WJ Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nature communications 8, 13996, doi: 10.1038/ncomms13996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Y et al. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development 128, 459–469 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Cherian AV, Fukuda R, Augustine SM, Maischein HM & Stainier DY N-cadherin relocalization during cardiac trabeculation. Proc Natl Acad Sci U S A 113, 7569–7574, doi: 10.1073/pnas.1606385113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sedmera D et al. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec A Discov Mol Cell Evol Biol 274, 773–777, doi: 10.1002/ar.a.10085 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Chen H, Qu X, Chang CP & Shou W Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC). Am J Med Genet C Semin Med Genet 163, 144–156, doi: 10.1002/ajmg.c.31369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochilas LK, Li J, Jin F, Buck CA & Epstein JA p57Kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatr Res 45, 635–642 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Chen H et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 131, 2219–2231, doi: 10.1242/dev.01094 dev.01094 [pii] (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clay H et al. Sphingosine 1-phosphate receptor-1 in cardiomyocytes is required for normal cardiac development. Dev Biol 418, 157–165, doi: 10.1016/j.ydbio.2016.06.024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miao L et al. Notch signaling regulates Hey2 expression in a spatiotemporal dependent manner during cardiac morphogenesis and trabecular specification. Sci Rep 8, 2678, doi: 10.1038/s41598-018-20917-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xin M et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A 104, 7975–7980, doi: 10.1073/pnas.0702447104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koibuchi N & Chin MT CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res 100, 850–855, doi: 10.1161/01.RES.0000261693.13269.bf (2007). [DOI] [PubMed] [Google Scholar]
- 58.Kokubo H et al. Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ Res 95, 540–547, doi: 10.1161/01.RES.0000141136.85194.f0 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Lee KF et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378, 394–398, doi: 10.1038/378394a0 (1995). [DOI] [PubMed] [Google Scholar]
- 60.Meyer D & Birchmeier C Multiple essential functions of neuregulin in development. Nature 378, 386–390, doi: 10.1038/378386a0 (1995). [DOI] [PubMed] [Google Scholar]
- 61.Grego-Bessa J et al. Notch signaling is essential for ventricular chamber development. Dev Cell 12, 415–429, doi: 10.1016/j.devcel.2006.12.011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.VanDusen NJ et al. Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell reports 9, 2071–2083, doi: 10.1016/j.celrep.2014.11.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin M et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Science signaling 4, ra70, doi: 10.1126/scisignal.2002278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Gise A et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A 109, 2394–2399, doi: 10.1073/pnas.1116136109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li D et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 138, 303–315, doi: 10.1242/dev.055566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao C et al. Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation. Development 141, 281–295, doi: 10.1242/dev.093690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu M & Li J Numb family proteins: novel players in cardiac morphogenesis and cardiac progenitor cell differentiation. Biomolecular concepts 6, 137–148, doi: 10.1515/bmc-2015-0003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian X et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nature communications 8, 87, doi: 10.1038/s41467-017-00118-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]