Abstract
Vitamin C (VC)—a collective term for the different oxidation and protonation forms of ascorbic acid (AscH)—is an essential micronutrient that serves as (i) a potent antioxidant and (ii) a cofactor of a manifold of enzymatic processes. Its role in health is related to redox balance maintenance, which is altered in diseases such as obesity, cancer, neurodegenerative diseases, hypertension, and autoimmune diseases. Despite its importance, VC uptake has been poorly investigated. Available literature values for the passive membrane permeability P of lipid bilayers for AscH scatter by about 10 orders of magnitude. Here, we show by voltage clamp that of AscH’s anionic form (ascorbate ) is negligible. To cross the membrane, picks up a proton in the membrane vicinity and releases it on the other side of the membrane. This leads to a near-membrane pH drop that was visualized by scanning pH microelectrodes. The AscH concentration dependent pH profiles indicated . Thus, AscH’s P is comparable to that of sorbitol and much lower than that of other weak acids like acetic acid or salicylic acid. The observation suggests that the capacity of the passive transcellular transport pathway across the lipid matrix does not suffice to ensure the required VC intake from the gastrointestinal tract.
Keywords: scorbic acid, ascorbate, passive membrane permeability, vitamin C, membrane permeation, weak acid permeation
1. Introduction
Vitamin C (VC) encompasses several vitamers that differ in their protonation and oxidation states and include ascorbic acid (AscH). Vitamin C deficiency results in scurvy [1]. Membrane proteins like the sodium-ascorbate cotransporters SVCT1 and SVCT2 transport reduced ascorbate, thereby contributing to VC homeostasis in the human body [2]. However, SVCT1 knockout only marginally affected intestinal VC adsorption in mice [3], suggesting a role for redundant glucose transporters (GLUT transporters) or significant spontaneous membrane permeability P of AscH that may ensure sufficient intestinal VC uptake. A more quantitative investigation of SVCT’s biological significance requires knowledge of AscH’s passive membrane permeability. Yet, surprisingly little is known. Since AscH is a weak acid with one of its pK values being equal to 4.17 [4], it may be anticipated to passively permeate biological membranes—similarly to other weak acids such as salicylic acid [5] or acetic acid [6,7]. The second proton release reaction with a pK value of 11.57 [8] yields a bivalent anion that due to (i) its extremely low concentration at physiological pH values and (ii) the increased desolvation (Born) energy for bivalent ions cannot make a significant contribution to passive transmembrane VC flux.
With [9] the only available literature value differs by up to 10 orders of magnitude from values that maybe derived from (i) the membrane permeability to the ascorbate anion () [10] or (ii) the oil water partition coefficient of AscH [11]. The goal of the present work was the characterization of AscH passive membrane transport processes. We did so by exploiting scanning electrochemical microscopy in the vicinity of planar lipid bilayers, as this method previously yielded robust values for various acids and bases [5,6,12,13].
Membrane conductivity measurements rendered an upper estimate for , which excludes from making a significant contribution to the passive membrane permeation of VC.
2. Materials and Methods
2.1. Planar Lipid Bilayer
Free standing planar lipid bilayers from Escherichia coli polar lipid extract (PLE; Avanti Polar Lipids, Alabaster, AL, USA) dissolved in n-decane (10 mg/mL) were spread over an aperture (~400 µm in diameter) in a thick Teflon septum which was pretreated with of the lipid solution [14]. At each side of the bilayer, an Ag/AgCl electrode was placed which allowed the application of (i) a triangle AC-voltage (320 Hz, 10 mV peak-to-peak) via an npi-VA-10x current amplifier (npi Elektronik, Tamm, Germany) and (ii) an additional DC bias which is necessary to observe membrane formation.
The membrane conductivity was calculated via Ohm’s law. An A/D converter controlled by the WinWCP 4.0.6 software (University of Strathclyde, Glasgow, UK) digitized the analog output of the current amplifier equipped with a headstage at a sampling rate of . The ampilifier’s 4-pole Bessel low pass filter was set to . All measurements were performed at room temperature (22 °C).
2.2. Ascorbic Acid
Ascorbic Acid spontaneously oxidizes at physiological pH and ambient oxygen concentrations, producing ascorbyl radical and dehydroascorbic acid. This process can be accelerated by light, heat, increasing pH, and the presence of contaminating free iron or copper [15]. To prevent oxidation, we (i) prepared the aqueous AscH containing solutions freshly each day with degassed ultra-pure (Milli-Q, Merck Millipore, Burlington, MA, USA; hence metal-free) water at a pH of 4.25. We were forced to use high millimolar (i.e., physiologically irrelevant) concentrations of ascorbate in our experiments in order to detect transport. Yet the observation of concentration independent P values (see below) indicated the absence of non-standard chemical reactions.
2.3. Scanning Electrochemical Microscopy
The setup and measurement procedure for scanning electrochemical microscopy are described elsewhere in detail [16]. Briefly, a borosolicate glass capillary is pulled to yield a tip diameter of about After the tip is bent to a right angle, it is silanized inside with bis(dimethylamino)dimethylsilane to create a hydrophobic surface. A protonophore cocktail (Hydrogen ionophore cocktail II, Fluka) filled into the very tip of the pipette renders the electrode pH sensitive. The pipette is filled with KCl solution, and a thin Ag/AgCl wire is inserted. This microelectrode is mounted onto a hydraulic microdrive manipulator (Narishige, Tokyo, Japan), which allows scanning of pH profiles perpendicular to the bilayer surface. The microelectrode and a reference electrode are located in the same compartment of the measurement chamber. Both are connected to a Keithley 6514 electrometer (Keithley Instruments, Cleveland, OH, USA) that is controlled via an IEEE interface and a custom-written LabView program (National Instruments, Austin, TX, USA). The solutions in the chamber are agitated with magnetic stirrer bars to diminish the unstirred layers (USL) in the vicinity of the membrane. Prior to every experiment, the pH-sensitivity of the microelectrode is calibrated.
The buffer for the scanning electrochemical microscopy measurement consists of KCl, β-alanine, KH2PO4 adjusted to pH 4.2. All chemicals were purchased from Sigma-Aldrich (Vienna, Austria). All solutions are sterile filtered. Ascorbic acid gradients are built up adding to the trans-side aliquots from a sodium-l-ascorbate solution (pH 4.2) that is prepared in measurement buffer. At the beginning of the measurement pH on both sides of the membrane is 4.2. Subsequently, pH on the microelectrode side (cis-side) is augmented to 6.5 by KOH addition. The pH gradient acts to decrease the AscH on the cis-side. In turn, the transmembrane AscH flux increases, thereby giving raise to resolvable pH changes within the cis USL (compare Figure 1). After an incubation time of roughly 15 min, the microelectrode is stepwise moved towards the membrane with a velocity of . The position of the membrane is judged from a saltatory increase in microelectrode voltage [17]. Profiles are averaged over at least two scans.
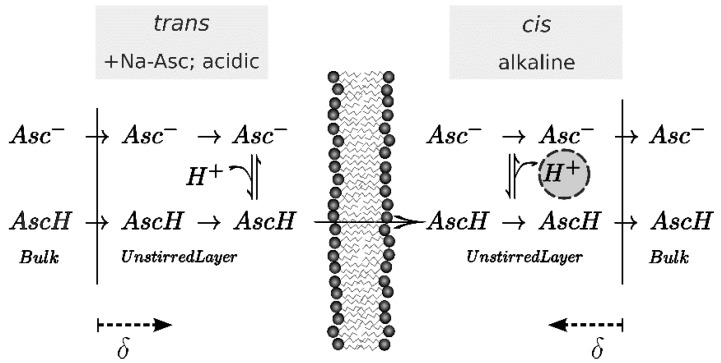
Figure 1.
Scheme of the effect of pH shift upon one-sided addition of Na-Asc to the trans side of the membrane where an acidic pH ensures a high ascorbic acid (AscH) concentration. Once arriving on the alkaline cis-side, almost all acid molecules deprotonate and release a proton (encircled). Hence, the pH drops on the cis in the vicinity of the membrane. Since each compartment is stirred, this effect is occurring only within the unstirred layer of thickness δ. Scanning electrochemical microscopy serves to measure this pH shift.
2.4. Calculation of P− from Membrane Conductivity Measurements
An electrochemical gradient of an ion s of valency zs with membrane permeability Ps across a planar lipid bilayer causes a current of density js. In this study, symmetrical buffer conditions are chosen. The Goldman-Hodgkin-Katz (GHK) flux equation [18,19,20] connects these quantities. We calculate Ps from the specific membrane conductivity induced by the ion s () by using the GHK flux equation (Equation (1)) for symmetrical conditions.
| (1) |
Conductivity measurements were performed for different Na-Asc concentrations at various pH values since around its pK, some weak acids tend to form dimers of the acid and the conjugated base molecule [21]. Because of its charge, the dimer has a presumably lower membrane permeability than the acid form, but a substantially larger membrane permeability than the conjugated base due to its increased volume which lowers the self-solvation energy for entering the lipid environment [5].
The buffer for the conductivity measurement consists of NaCl, HEPES, pH 7.5. The volume in the measurement chamber is partially replaced with a solution of NaAsc, NaCl, HEPES pH 7.5. Additions of HCl lower the pH.
2.5. Calculation of Permeability from pH Profiles in the Unstirred Layers
Shifts in the local pH adjacent to the membrane are indicative of P [6]. In addition to P they are governed by chemical reactions with buffer molecules and the diffusivity Di of all reactants. Consequently, calculation of P should take into account both buffer and acid concentrations. Accordingly, we numerically solved a system of differential equations for both sides of the membrane that describes the diffusion (Equation (2)) and expenditure in chemical reactions (Equation (3)). Membrane permeation is assumed to be so slow that all chemical reactions are in equilibrium. The equilibrium constants of protons and hydroxide anions (Equation (4)), ascorbic acid , and ascorbate (Equation (7)), as well as the protonated and deprotonated forms of the zwitterionic β-alanine and (Equation (5)), and the protonated and deprotonated forms of the phosphate buffer at its second pK, and (Equation (6)) are listed in Table 1 along with the diffusivities of the different species (denoted by index i = 1 … 8) that participate in protonation reactions. The magnitude and unit of the equilibrium constants K are calculated from the respective pK value and type of reaction they describe.
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Table 1.
Parameters used for the calculation of P from pH profiles that have been induced by the transmembrane AscH flux.
| Species | Symbol | Index i | Di (10−10 m²/s) | pK | Equilibrium Constant (Ki) |
|---|---|---|---|---|---|
| Protons | 1 | 93.1 [6] | 14 [23] | ||
| Hydroxide anions | 2 | 52.6 [6] | 14 [23] | ||
| Ascorbic acid, ascorbate | , | 8, 7 | 5.97 [24] | 4.17 [4] | |
| β-alanine zwitterion and cation | , | 3, 4 | 9.36 [25] | 3.63 [4] | |
| Hydrogen phosphate, dihydrogen phosphate | , | 5, 6 | 10.41 [26] | 7.21 [22] |
The calculation considers the indicated species (with index i for nomenclature). Diffusivities Di and pK values are from sources denoted next to the value. Equilibrium constants are recalculated from the pK value with respect to the stoichiometry of the reaction.
Phosphate has three pK values: 2.12, 7.21 and 12.67 [22]. Its buffering capacity is only significant on the cis-side since pK = 7.21 is close to pH ~6.5. The trans side is mainly buffered by β-alanine (pK close to pH). Thus, the low buffering capacity of the phosphate buffer (pK far from pH) can be ignored there. All membrane impermeable substances obey a no-flux-condition at the water-membrane interfaces (x = 0 in nomenclature of the figures) (Equation (8)), except for AscH which flows along its transmembrane gradient, (Equation (9)).
| (8) |
| (9) |
The size of the unstirred layer is defined in terms of the concentration gradient at the membrane water interface (x = 0, Equation (10)).
| (10) |
A linear fit to the profile in the first adjacent to the membrane serves to determine δ. Subsequently, we used Mathematica 9 (Wolfram Research; Champaign, IL, USA) to numerically calculate pH profiles for variable P [6]. Then the resulting profiles were tabulated and interpolated for P. This interpolation function is then used in a ‘FindFit’ routine of Mathematica to extract P from the experimental pH profiles.
3. Results
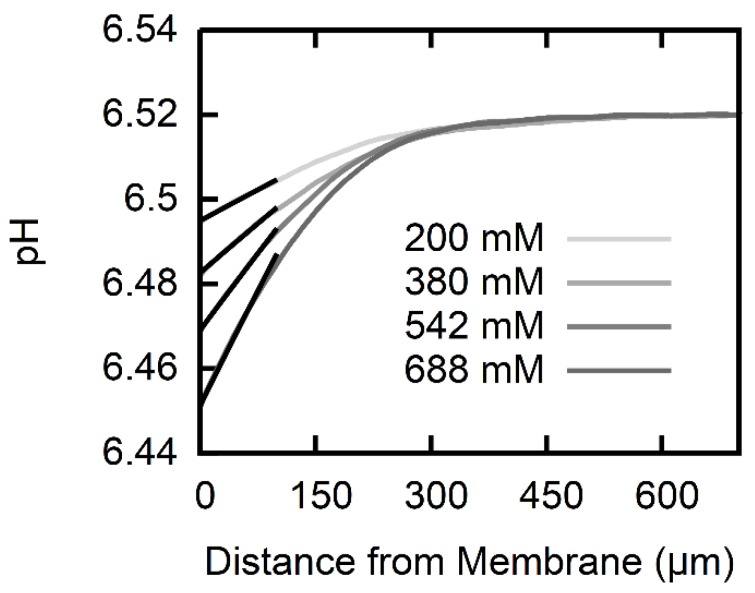
An AscH transmembrane concentration gradient gives rise to an acid flux. In turn, AscH dissociation in the receiving USL gives rise to a pH shift (Figure 2). We optimized the pH profile size by using (i) low buffer concentrations in both compartments, (ii) large Na-l-ascorbate concentrations in the cis compartment, and (iii) a transmembrane pH gradient. As described in Materials and Methods, we first found (Equation (10)) and subsequently fitted the set of differential equations (Equations (2)–(9)) to the pH profiles to obtain P. Both P and δ are listed in Table 2. The USL width reduces with an increasing AscH gradient. This effect can be attributed to an osmotic water flux [27] that builds up due to the asymmetric addition of AscH. The resulting convection is not taken into account in the numerical calculation, but acknowledging the different for the different gradients yields little variance in P ().
Figure 2.
pH profiles induced by AscH transmembrane flux. The pH in the cis compartment (100 mM KCl, 0.5 mM ß-alanine, 0.3 mM KH2PO4 adjusted to pH ~6.5) acidifies within the USL, since part of the AscH molecules that arrive from the trans-compartment (100 mM KCl, 0.5 mM ß-alanine, 0.3 mM KH2PO4 adjusted to pH 4.25) deprotonate. The different concentrations of Na-l-ascorbate in the trans-compartment (see inset) range from 200 mM (light gray) to 688 mM (dark gray). Numerically calculated pH profiles (black lines) that take into account both diffusion and expenditure in protonation reactions for ascorbic acid, and the two buffering agents are fitted to the experimental data within the first . A membrane permeability of the polar lipid extract (PLE) lipid membrane for ascorbic acid is obtained.
Table 2.
Unstirred layer width and AscH acid permeability P for various gradients of Na-Asc.
| Na-l-Ascorbate Gradient (mM) | USL Width | AscH Membrane Permeability P (cm/s) |
|---|---|---|
| 200 | 260 | |
| 380 | 245 | |
| 542 | 215 | |
| 688 | 195 | |
| Average | ||
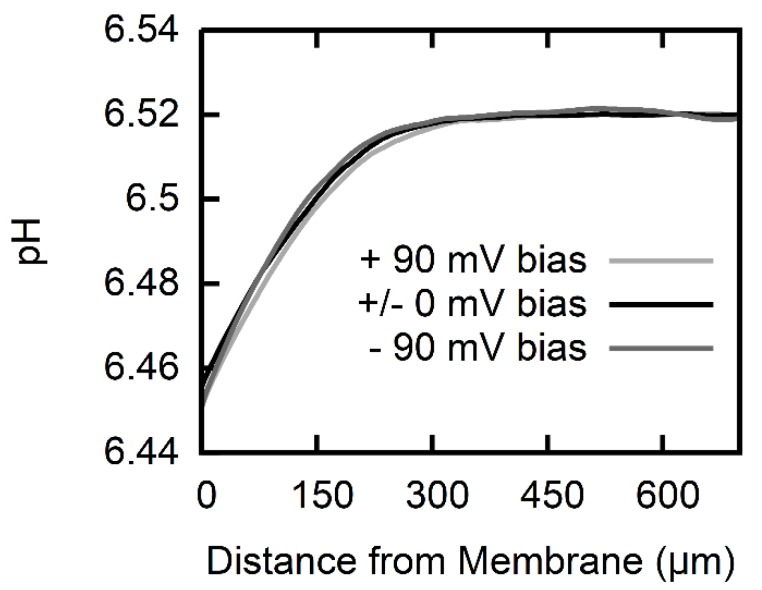
The pH profiles are not susceptible to the application of a transmembrane potential (Figure 3). This observation suggests that the ionic form does not permeate the membrane on a large scale. Otherwise, the anions that were driven by external voltage across the membrane would have affected the deprotonation of the also permeating AscH, that is, the equilibrium AscH concentration adjacent to the receiving interface would have increased simply because the concentration of increased. In turn, the transmembrane AscH concentration gradient would have diminished, resulting in a smaller flux and smaller pH profiles.
Figure 3.
pH profiles at a constant AscH gradient (trace for 688 mM Na-l-ascorbate in Figure 2) under varying transmembrane voltage bias (black: no bias, light gray: +90 mV, dark gray: −90 mV). The pH profiles are independent of the applied transmembrane voltage within the borders of resolution which attributes the pH profiles to the permeation of a neutral species.
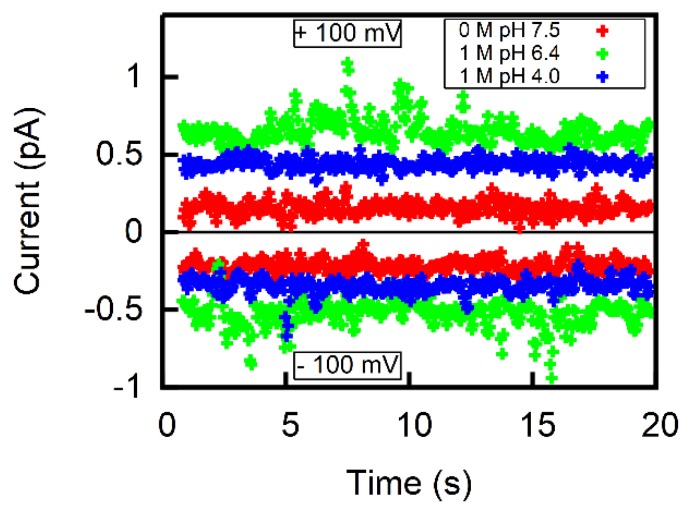
Membrane conductivity measurements (Figure 4) confirmed the assumption that the flux is much lower than the AscH flux. Even though the concentration was raised to 1 M and pH was decreased to match acid’s pK, we did not observe an increment in current > at of DC voltage. This translates into a specific membrane conductivity g < for a membrane that is in diameter. Such g value is on the lower end of values reported for freestanding lipid bilayers (, [14,28]). Attributing g in its entirety to the permeation of yields an upper limit for of (Equation (1)). Thus, is at least four orders of magnitude smaller than P.
Figure 4.
Representative current traces for ±100 mV applied to freestanding lipid bilayers of comparable sizes (diameter of aperture 395.5 µm) in the absence of Na-Asc at pH 7.5 (red) and in the presence of 1 M Na-Asc at pH 6.4 (green) and at pH 4.0 (blue). The aqueous solution contained 100 mM NaCl and 10 mM HEPES. If all conductivity is attributed to Asc−, one obtains an upper limit for on the order of .
4. Discussion
With P of about AscH permeates fluid membranes much slower than other weak acids of comparable size—like salicylic acid [5,29] or acetic acid [6]—but is comparable to the membrane permeability of other neutral substances, for example, sorbitol [30]. The transport rate of the deprotonated species is at least four orders of magnitude lower, being characterized by . It is thus even smaller than to the slow transport rate of the smaller chloride ions, for which were reported [31].
Our study is not in line with data reported by a nuclear magnetic resonance (NMR) study, where and have been deduced for and AscH effluxes from fluid dipalmitoyl-lecithin (DPPC) vesicles at 52 °C [9]. The small difference between P and in the NMR study seems to violate membrane electrostatics: the latter imposes a penalty for placing a monovalent ion with a gyration radius r = into the bilayer [32] of about 16.5 kcal/mol [33]. Substracting (i) 5.4 kcal/mol with which the dipole potential favors anion permeation and (ii) 2.5 kcal/mol for the image energy [34], we find an additional penalty of 8.6 kcal/mol for the anion, which translates into a drop of roughly six orders of magnitude in permeabilities as compared to the neutral species. In contrast, the NMR data favor the permeation of the neutral species by only a factor of 100.
A comparison with our data suggests that P has been underestimated and overestimated in the NMR study: the NMR-based AscH rate transforms into P for a fluid bilayer at room temperature, which amounts to only 1/10 of the permeability of our planar bilayers. The calculation assumes that the activation energy EA scales with P [35], that is, amounts to EA ≈ 20 kcal/mol for AscH—a value similar to that of tetraphenylborate [34] that translocates at [36]. The rate of the NMR study is equivalent to for a fluid bilayer at room temperature, that is, it matches the value of the upper permeability limit of planar bilayers. The calculation assumes EA 30 kcal/mol [34]—as has been reported for Cl- that permeates at 30 kcal/mol [34]—as has been reported for Cl- that permeates at [34].
Attempts to derive P and from the reaction of with paramagnetic spin probes that were intercalated in oriented lipid multilayers [10] resulted in a large overestimation of the transport rate . It was estimated to be . If the calculation was correct, would appear inside lipid vesicles of diameter d = 100 nm after time [37]
| (11) |
had elapsed subsequent to addition to the outer solution. Yet, both a recent time resolved electron paramagnetic resonance(EPR) immersion depth study [38] and the original EPR study [10] indicate that it takes 20–30 min to penetrate to a probe that is buried at a depth of 20 Å of a fluid lipid bilayer. The of would completely rule out the possibility of using ascorbate to monitor lipid flip-flop [39], the more so, the neutral species AscH would permeate 106 times faster (see Equation (11)).
According to Overton’s rule, both P and correlate well with the partition coefficients Koct/w and Koil/w between water and octanol or olive oil, respectively [40]:
| (12) |
| (13) |
Inserting published Koct/w and Koil/w into the empirical relations Equations (12) and (13) yields overestimated P values (Table 3). The neglect of the acid base equilibrium is a major reason for the failure. For example, Oldendorf [11] obtains Koil/w for carbon-radiolabeled AscH in a biphasic system of Ringer’s solution buffered to pH 7.55–7.58 and refined olive oil. Dissecting the contributions of AscH and by using acid’s pK and the pH in the aqueous phase may be misleading: P of AscH appears in the cm/s range (see Table 3). As pointed out before, such high permeability can be excluded. The measurements leading to = −2.67 by HPLC with a stationary hydrocarbon phase and a mobile solvent phase [41] may have encountered the same problem: pH is not indicated. Moreover, the range of of the calibration substances was limited to 0.9–6.5 [41]. Since it does not embrace for AscH, we doubt the accuracy of the reported value.
Table 3.
Partition coeffcients (log K or K, the respective value is underlined) available in literature. Permeabilites based on these log K or K are estimated with the correlation (see Equations (12) and (13)) of Walter and Gutknecht [40].
| Year and Source | Solvents | Method | Log K | K | Log P (log cm/s) | P (cm/s) |
|---|---|---|---|---|---|---|
| 1974 [11] | Water/olive oil | Shake-flask method and radioactively labeled AscH/, fraction of radioactivity in both phases | −2.34 | 0.0046 | −3.33 | |
| Ibidem with correction for uncharged fraction | 1.04 | 10.96 | −0.3 | 0.49 | ||
| 1990 [49] | Water/octanole | Calculated with quantitative structure-activity relation [43] | −2.0482 | 0.0089 | −4.55 | 2.8 × 10−5 |
| 2012 [50] | Water/octanole | Calculated with quantitative structure-activity relation [42] | −2.41 | 0.0039 | −4.97 | |
| 2016 [41] | Water/octanole | HPLC-based assay | −2.67 | 0.0021 | −5.27 | 5.3 × 10−6 |
| 2017 [44] | Water/octanole | Calculated with Linear Solvation Energy Relationship (LSER) approach | −2.61 | 0.0025 | −5.2 | |
| 2017 [44] | Water/olive oil | Calculated with LSER | −4.4 | 0.00004 | −5.7 | |
Unfortunately, computations relying on structural similarity (structure-activity-relation, SAR [42,43]) or other descriptors (Linear Solvation Energy Relationship, LSER [44,45]) include substances with erroneous experimental partition coefficients, which may bias the prediction. Nevertheless, if the scatter of the empirical relations (Equations (12) and (13)) is taken into account, the two computed values based on SAR [42,43] and the LSER value of about 0.15 [45] yield a P prediction that is reasonably close to our experimentally determined value.
Our P value allows a very rough estimation of the AscH transport capacity of the intestinal tract. The flux (Φ) through the intestinal barrier:
| (14) |
depends on its area Aint = 32 m2 [46] and AscH’s concentration difference Δclb between lumen and blood. Assuming that the total luminal concentration (AscH + Asc−) may be represented by the gastric juice concentration of 90 µmol/L and a plasma concentration of 30 µmol/L [47] yields Δclb = 60 µmol/L × 104.17–7.4 = 35 nmol/L. The P value of 10−8 cm/s that was obtained at room temperature corresponds to P ≈ 5 × 10−8 cm/s at 37 °C—considering an activation energy of 20 kcal/mol. The resulting Φ = 6·10−13 mol/s is likely to be an overestimation since the number neglects the significant jejunal fluid secretion. Such Φ is clearly insufficient as it corresponds to only about 1 µg AscH per day. The required uptake of 30–180 mg [48] per day requires facilitated transport.
5. Conclusions
We were able to determine the membrane permeability of PLE to ascorbic acid to be by using scanning electrochemical microscopy. The surprisingly low membrane permeability underpins the need for facilitated transport in human physiology. Furthermore, we showed that with an upper limit of , the membrane permeability of ascorbate is negligible as compared to the membrane permeability of ascorbic acid. This clarifies a long lasting discrepancy among available literature data for P and partition coefficients. The extremely low membrane permeability of is in line with (i) ’s application as quencher of reactive oxygen species that is active in aqueous solutions and (ii) its use as “membrane impermeable” quencher of EPR probes.
Acknowledgments
Supported by Johannes Kepler Open Access Publishing Fund.
Author Contributions
Data curation, C.H.; Investigation, C.H.; Project administration, P.P.; Resources, P.P.; Writing—original draft, C.H.; Writing—review & editing, P.P.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hediger M.A. New view at C. Nat. Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 2.Tsukaguchi H., Tokui T., Mackenzie B., Berger U.V., Chen X.Z., Wang Y., Brubaker R.F., Hediger M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 3.Corpe C.P., Tu H., Eck P., Wang J., Faulhaber-Walter R., Schnermann J., Margolis S., Padayatty S., Sun H., Wang Y., et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Investig. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windholz M. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 10th ed. Merck & Co.; Rahway, NJ, USA: 1983. p. 2179. [Google Scholar]
- 5.Saparov S.M., Antonenko Y.N., Pohl P. A new model of weak acid permeation through membranes revisited: Does overton still rule? Biophys. J. 2006;90:L86–L88. doi: 10.1529/biophysj.106.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonenko Y.N., Denisov G.A., Pohl P. Weak acid transport across bilayer lipid membrane in the presence of buffers. Theoretical and experimental pH profiles in the unstirred layers. Biophys. J. 1993;64:1701–1710. doi: 10.1016/S0006-3495(93)81542-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Missner A., Kugler P., Antonenko Y.N., Pohl P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc. Natl. Acad. Sci. USA. 2008;105:E123. doi: 10.1073/pnas.0809606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamim M., Khoo S. Some physical properties of aqueous L-ascorbic acid solutions. Aust. J. Chem. 1979;32:2293–2295. doi: 10.1071/CH9792293. [DOI] [Google Scholar]
- 9.Sapper H., Roth K.D., Lohmann W. The diffusion of L(+)-ascorbic acid across DPPC vesicle membranes. J. Microencapsul. 1985;2:23–30. doi: 10.3109/02652048509049574. [DOI] [PubMed] [Google Scholar]
- 10.Schreier-Muccillo S., Marsh D., Smith I.C. Monitoring the permeability profile of lipid membranes with spin probes. Arch. Biochem. Biophys. 1976;172:1–11. doi: 10.1016/0003-9861(76)90041-2. [DOI] [PubMed] [Google Scholar]
- 11.Oldendorf W.H. Lipid Solubility and Drug Penetration of the Blood Brain Barrier. Exp. Biol. Med. 1974;147:813–816. doi: 10.3181/00379727-147-38444. [DOI] [PubMed] [Google Scholar]
- 12.Mathai J.C., Missner A., Kügler P., Saparov S.M., Zeidel M.L., Lee J.K., Pohl P. Membrane Transport of Hydrogen Sulfide: No Facilitator Required. Biophys. J. 2010;98 doi: 10.1016/j.bpj.2009.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonenko Y.N., Pohl P., Denisov G.A. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys. J. 1997;72:2187–2195. doi: 10.1016/S0006-3495(97)78862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller P., Rudin D.O., Tien H.T., Wescott W.C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- 15.Buettner G.R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: Ascorbate as a test for catalytic metals. J. Biochem. Biophys. Methods. 1988;16:27–40. doi: 10.1016/0165-022X(88)90100-5. [DOI] [PubMed] [Google Scholar]
- 16.Pohl P., Saparov S.M. Solvent drag across gramicidin channels demonstrated by microelectrodes. Biophys. J. 2000;78:2426–2434. doi: 10.1016/S0006-3495(00)76786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonenko Y.N., Bulychev A.A. Measurements of Local pH Changes near Bilayer Lipid-Membrane by Means of a pH Microelectrode and a Protonophore-Dependent Membrane-Potential. Comparison of the Methods. Biochim. Biophys. Acta. 1991;1070:279–282. doi: 10.1016/0005-2736(91)90176-9. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkin A.L., Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates; Sunderland, MA, USA: 1984. [Google Scholar]
- 20.Goldman D.E. Potential, Impedance, and Rectification in Membranes. J. Gen. Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim. Biophys. Acta. 1970;205:1–6. doi: 10.1016/0005-2728(70)90055-1. [DOI] [PubMed] [Google Scholar]
- 22.Lide D. Handbook of Chemistry and Physics. 72nd ed. CRC Press; Boca Raton, FL, USA: 1991–1992. [Google Scholar]
- 23.Marshall W.L., Franck E.U. Ion Product of Water Substance, 0 °C–1000 °C, 1–10,000 Bars New International Formulation and Its Background. J. Phys. Chem. Ref. Data. 1981;10:295–304. doi: 10.1063/1.555643. [DOI] [Google Scholar]
- 24.Robinson D., Anderson J.E., Lin J.L. Measurement of Diffusion-Coefficients of Some Indoles and Ascorbic-Acid by Flow-Injection Analysis. J. Phys. Chem. 1990;94:1003–1005. doi: 10.1021/j100365a092. [DOI] [Google Scholar]
- 25.Donoian H.C., Kegeles G. Diffusion of Beta-Alanine in Water at 25 Degrees. J. Am. Chem. Soc. 1961;83:255–259. doi: 10.1021/ja01463a001. [DOI] [Google Scholar]
- 26.Edwards O.W., Huffman E.O. Diffusion of Aqueous Solutions of Phosphoric Acid at 25-Degrees. J. Phys. Chem. 1959;63:1830–1833. doi: 10.1021/j150581a011. [DOI] [Google Scholar]
- 27.Pohl P., Saparov S.M., Antonenko Y.N. The effect of a transmembrane osmotic flux on the ion concentration distribution in the immediate membrane vicinity measured by microelectrodes. Biophys. J. 1997;72:1711–1718. doi: 10.1016/S0006-3495(97)78817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutsmann T., Heimburg T., Keyser U., Mahendran K.R., Winterhalter M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 2015;10:188–198. doi: 10.1038/nprot.2015.003. [DOI] [PubMed] [Google Scholar]
- 29.Gutknecht J., Tosteson D.C. Diffusion of weak acids across lipid bilayer membranes: Effects of chemical reactions in the unstirred layers. Science. 1973;182:1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- 30.Wood R.E., Wirth F.P., Jr., Morgan H.E. Glucose permeability of lipid bilayer membranes. Biochim. Biophys. Acta. 1968;163:171–178. doi: 10.1016/0005-2736(68)90095-3. [DOI] [PubMed] [Google Scholar]
- 31.Hauser H., Oldani D., Phillips M.C. Mechanism of ion escape from phosphatidylcholine and phosphatidylserine single bilayer vesicles. Biochemistry. 1973;12:4507–4517. doi: 10.1021/bi00746a032. [DOI] [PubMed] [Google Scholar]
- 32.Rashin A.A., Honig B. Reevaluation of the Born model of ion hydration. J. Phys. Chem. 1985;89:5588–5593. doi: 10.1021/j100272a006. [DOI] [Google Scholar]
- 33.Lomize A.L., Pogozheva I.D., Mosberg H.I. Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes. J. Chem. Inf. Model. 2011;51:930–946. doi: 10.1021/ci200020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honig B.H., Hubbell W.L., Flewelling R.F. Electrostatic interactions in membranes and proteins. Annu. Rev. Biophys. Biophys. Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- 35.De Gier J., Mandersloot J.G., Hupkes J.V., McElhaney R.N., Van Beek W.P. On the mechanism of non-electrolyte permeation through lipid bilayers and through biomembranes. Biochim. Biophys. Acta. 1971;233:610–618. doi: 10.1016/0005-2736(71)90160-X. [DOI] [PubMed] [Google Scholar]
- 36.Flewelling R.F., Hubbell W.L. Hydrophobic ion interactions with membranes. thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys. J. 1986;49:531–540. doi: 10.1016/S0006-3495(86)83663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missner A., Pohl P. 110 years of the Meyer-Overton rule: Predicting membrane permeability of gases and other small compounds. ChemPhysChem. 2009;10:1405–1414. doi: 10.1002/cphc.200900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nusair N.A., Mayo D.J., Dorozenski T.D., Cardon T.B., Inbaraj J.J., Karp E.S., Newstadt J.P., Grosser S.M., Lorigan G.A. Time-resolved EPR immersion depth studies of a transmembrane peptide incorporated into bicelles. Biochim. Biophys. Acta. 2012;1818:821–828. doi: 10.1016/j.bbamem.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kornberg R.D., McConnell H.M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971;10:1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- 40.Walter A., Gutknecht J. Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 1986;90:207–217. doi: 10.1007/BF01870127. [DOI] [PubMed] [Google Scholar]
- 41.Bharate S.S., Kumar V., Vishwakarma R.A. Determining Partition Coefficient (Log P), Distribution Coefficient (Log D) and Ionization Constant (pKa) in Early Drug Discovery. Comb. Chem. High Throughput Screen. 2016;19:461–469. doi: 10.2174/1386207319666160502123917. [DOI] [PubMed] [Google Scholar]
- 42.Petrauskas A.A., Kolovanov E.A. ACD/Log P method description. Perspect. Drug Discov. Des. 2000;19:99–116. doi: 10.1023/A:1008719622770. [DOI] [Google Scholar]
- 43.Klopman G., Namboodiri K., Schochet M. Simple Method of Computing the Partition-Coefficient. J. Comput. Chem. 1985;6:28–38. doi: 10.1002/jcc.540060106. [DOI] [Google Scholar]
- 44.Ulrich N., Endo S., Brown T.N., Watanabe N., Bronner G., Abraham M.H., Goss K.U. UFZ-LSER Database V 3.2 [Internet] [(accessed on 7 June 2018)]; Available online: http://www.ufz.de/lserd.
- 45.Sprunger L.M., Achi S.S., Acree W.E., Abraham M.H. Development of correlations for describing solute transfer into acyclic alcohol solvents based on the Abraham model and fragment-specific equation coefficients. Fluid Phase Equilib. 2010;288:139–144. doi: 10.1016/j.fluid.2009.10.028. [DOI] [Google Scholar]
- 46.Helander H.F., Fandriks L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 47.Waring A.J., Drake I.M., Schorah C.J., White K.L., Lynch D.A., Axon A.T., Dixon M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut. 1996;38:171–176. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob R.A., Sotoudeh G. Vitamin C function and status in chronic disease. Nutr. Clin. Care. 2002;5:66–74. doi: 10.1046/j.1523-5408.2002.00005.x. [DOI] [PubMed] [Google Scholar]
- 49.McCoy G.D., Rosenkranz H.S., Klopman G. Non-mutagenic carcinogens are primarily hydrophobic. Carcinogenesis. 1990;11:1111–1117. doi: 10.1093/carcin/11.7.1111. [DOI] [PubMed] [Google Scholar]
- 50.Dörwald F.Z. Lead Optimization for Medicinal Chemists: Pharmacokinetic Properties of Functional Groups and Organic Compounds. John Wiley & Sons; Hoboken, NJ, USA: 2012. [Google Scholar]