Abstract
Bone morphogenetic proteins (BMPs) are key regulators of cell fate decisions during embryogenesis and tissue homeostasis. BMPs signal through a coordinated assembly of two types of transmembrane serine/ threonine kinase receptors to induce Smad1/5/8 plus non-Smad pathways, such as MAPK and Akt. The recent discovery of BMP receptor inhibitors opened new avenues to study specific BMP signalling and to delineate this effect from TGF-β and Activin signalling. Here we present comprehensive and quantitative analyses on both canonical and non-Smad mediated BMP signalling under Dorsomorphin (DM) and LDN-193189 (LDN) treatment conditions. We demonstrate for the first time, that both compounds affect not only the Smad but also the non-Smad signalling pathways induced by either BMP2, BMP6 or GDF5. The activation of p38, ERK1/2 and Akt in C2C12 cells was inhibited by DM and LDN. In addition “off-target” effects on all branches of BMP non-Smad signalling are presented. From this we conclude that the inhibition of BMP receptors by DM and more efficiently by LDN-193189 affects all known BMP induced signalling cascades.
Keywords: Dorsomorphin, LDN-193189, BMP signalling, p38, Akt
1. Introduction
Bone morphogenetic proteins (BMPs) were originally described as bone morphogens, but in recent years it became evident that they also participate in a variety of processes during embryogenesis, tissue development and tissue repair. BMPs bind to two types of transmembrane Ser/Thr kinase receptors (type I and type II receptors), which upon transphosphorylation activate downstream signalling cascades, such as Smad1/5/8, MAPK and Akt/PKB. Malfunction of the pathways causes diseases ranging form bone disease, vascular diseases, organ dystrophies to cancer. For this reason, nature has selected for complex mechanisms to fine tune and regulate this central signalling network. Secreted antagonists control the accessibility of the ligands for the receptors. Co-receptors modulate the transmission of the extracellular signal into the cytosol while cytoplasmic and nuclear proteins bind and therefore regulate the activity of downstream signalling components (Sieber et al., 2009).
To understand the underlying signalling mechanisms there is a strong need for experimental approaches to manipulate BMP signalling. Classical approaches include the use of wild-type and mutant ligands, antagonists, soluble receptors or their ectodomains, neutralizing antibodies or genetic approaches using gene silencing or protein overexpression. Also specific protein aptamers have been successfully used to inhibit TGF-β signalling (Zhao and Hoffmann, 2006). Another strategy to specifically modulate BMP signalling is the use of small molecule inhibitors (Hong and Yu, 2009). The chemical compound Dorsomorphin (DM), formerly described as Compound C, was identified in a high throughput screen in Zebrafish to lead to a disordered formation of the dorsalventral axis in Zebrafish embryos (Yu et al., 2008a). This dorsalized axial pattern reflected the phenotype of BMP-pathway mutants and therefore strongly indicated that Dorsomorphin abrogated proper BMP signalling (Mullins et al., 1996).
The family of BMP proteins is comprised of over 20 family members, which signal through a limited number of type I and type II receptors. Ligand binding to type I (either Alk1/ActRIb, Alk2/ActRIa, Alk3/BRIa and Alk6/BRIb) and to type II receptors (either BRII, ActRIIa or ActRIIb) leads to an intracellular activation of the Smad signalling cascade as well as to an activation of several non-Smad pathways. For BMP2 it has been shown that the mode of receptor oligomerisation is crucial for the initiation of Smad versus non-Smad signalling. Upon ligand binding to the preformed complex composed of both type I and type II receptor, the constitutive active kinase domain of the type II receptor phosphorylates and thereby activates the GS-Box of the type I receptor (Nohe et al., 2002). Activated type I receptor is able to phosphorylate regulatory Smads (Smad1/5/8), which then form a ternary complex with the common mediator Smad (Smad4) and are selectively retained in the nucleus to regulate BMP target gene transcription (Hill, 2009; Sieber et al., 2009).
Although the Smad pathway has been studied extensively much less is known about transcriptional regulation via non-Smad signalling cascades. BMPs were shown to activate various members of different MAPK pathways, such as p38, ERK1/2 and SAPK/JNK (Gallea et al., 2001; Guicheux et al., 2003). Activation of p38 occurs via TAK1, TAB1 and XIAP, which are all associated to the BMP type I receptor (Yamaguchi et al., 1999). Activation of several MAP kinases results in subsequent phosphorylation and thus in activation of their downstream targets, like ATF2, the c-AMP response element binding protein (CREB), c-Jun or c-Fos and in transcriptional regulation of BMP target genes such as osteopontin, ALP or collagen I (Lai and Cheng, 2002; Barneda-Zahonero et al., 2009). Another important non-Smad signalling pathway includes activation of Akt/PKB, one of the key players regulating e.g. cell survival, proliferation, nutrition metabolism and migration (Manning and Cantley, 2007). This pathway was also shown to be activated in C2C12 cells upon BMP2 stimulation (Gamell et al., 2008).
It was shown previously that Dorsomorphin blocks BMP induced Smad1/5/8 phosphorylation in a dose dependent manner, while having no effect on TGF-β or Activin induced Smad2/3 activation as well as on BMP induced p38 activation (Yu et al., 2008a). The heterocyclic core structure of Dorsomorphin binds with different affinities the ATP binding site in the kinase domain of the type I receptors (Alk1, Alk2, Alk3 and Alk6) and thus inhibits their kinase activity (Yu et al., 2008a; Wrighton et al., 2009). Since Dorsomorphin is known to generally inhibit AMP-activated kinase (AMPK) as well as the receptor tyrosine kinases for PDGF and VEGF (Yu et al., 2008b; Hao et al., 2010), treatment of mammalian cells with this inhibitor shows various “off-target” effects. This disadvantage could be partly overcome by the Dorsomorphin derivative LDN-193189 (LDN) that exhibits a much higher specificity for BMP receptors and can be used at lower concentrations (Yu et al., 2008b).
The murine mesenchymal precursor cell line C2C12 is a common model to study BMP signalling, undergoing rapid osteogenic differentiation under BMP stimulation. In this study, we sought a more detailed understanding of the small molecule inhibitors DM and LDN in these cells. We demonstrate for the first time, that Dorsomorphin and LDN-193189 inhibit both BMP induced Smad1/5/8 phosphorylation and BMP-mediated induction of the p38 MAPK, Erk1/2 and Akt pathway in C2C12 cells.
2. Materials and methods
2.1. Cell culture and reagents
C2C12 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Biochrom AG, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) (Biochrom AG, Berlin, Germany), 2 mM glutamine and penicillin (100 units/ml)/streptomycin (10 µg/ml) (PAA). For experiments cells were seeded into 12-well plates in a density of 7.5 × 105 cells/well. The following day, cells were starved in DMEM supplemented with 0% FCS, 2 mM glutamine and penicillin (100 units/ml)/streptomycin (10 µg/ml) prior to inhibitor and BMP treatment. Therefore cells were washed once with starvation medium and then starved for 5 h in a volume of 500 µl. Cells were incubated with the indicated inhibitor or corresponding vehicle solution for 30 min before adding BMP or vehicle solution. All inhibitors and ligands were dissolved in PBS (PAA) and for each well 25 µl stock solutions at the corresponding concentration were prepared. For time kinetic studies all treatments belonging to one time point were performed on the same plate.
BMP2 was used in a final concentration of 5 nM, BMP6 in a final concentration of 1 nM and GDF5 in a final concentration of 10 nM.
The BMP inhibitor DM was purchased from Calbiochem (Merck, Germany) and LDN-193189 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinoline) was synthesized as a hydrochloride salt as previously described (Cuny et al., 2008). Both inhibitors were solved in DMSO, therefore DMSO concentration in all samples belonging to the experiment was adjusted accordingly. All other inhibitors were purchased from Sigma and administered as follows: p38 inhibitor, PD169316, MEK1/2 inhibitor, UO126, 10 µM; JNK inhibitor, SP600125, 10 µM. The p38 inhibitor SB203580 was purchased from Calbiochem and applied in a concentration of 10 µM.
2.2. Antibodies and Western blotting
Protein lysates were subjected to SDS-PAGE and transferred on PVDF membranes by Western blotting. Membranes were blocked for 1 h in 0.1% TBS-T containing 5% dry milk powder, washed three times in 0.1% TBS-T and incubated with indicated primary antibodies overnight at 4 °C following manufacturer’s protocols. The following antibodies were used: GAPDH (#2118, Cell Signaling), β-tubulin (#T5201, Sigma), p-Smad1/5/8 (#9511, Cell Signaling), p-p38 Thr180/Tyr182 (#V1211, Promega), p-ERK 1/2 (pp42/p44 MAPK Thr202/Thy204, #9101, Cell Signaling), p-ATF2 Thr71 (#9221, Cell Signaling), p-CREB Ser133 (#4276, Cell Signaling), p-Akt Ser 473 (#4051, Cell Signaling). To guarantee highly quantitative Western Blots, we avoided stripping the membranes and applied lysates on several gels. Each blot was separately probed for proper loading control by either GAPDH or β-tubulin (as indicated).
3. Results
3.1. Activation of Smad1/5/8, p38 and Akt by different BMP family members is inhibited by Dorsomorphin and LDN-193189 in a dose dependent manner
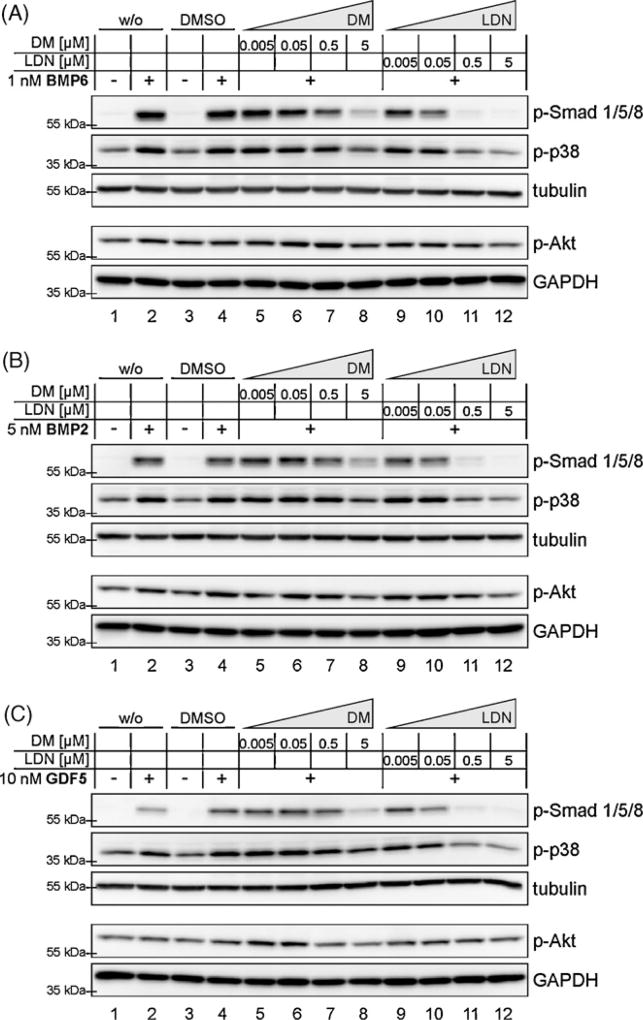
The discovery of pharmacological tools to selectively target the BMP signalling pathway has been very useful in discriminating TGF-β and BMP signalling inputs in physiological processes. LDN-193189 was the result of a structure-activity relationship study of Dorsomorphin (Cuny et al., 2008), which was discovered previously as an inhibitor of BMP induced Smad1/5/8 phosphorylation (Yu et al., 2008a). It has been reported, that in pulmonary arterial smooth muscle cells (PASMCs) both DM and LDN abolish BMP4 induced Smad1/5/8 phosphorylation, while the BMP induced non-Smad signalling pathway via p38 is unaffected by these compounds (Hong and Yu, 2009). To prove these findings in C2C12 cells, we stimulated cells with three different BMP ligands (BMP2, BMP6 or GDF5) in the presence of increasing concentrations of DM and LDN (Fig. 1A–C). These ligands were selected on the basis of their known BMP type I receptor utilization in C2C12 and other cell types. The choice for these different BMPs was according to their binding preferences regarding the different type I receptors. BMP6 signals via the type I receptor Alk2, BMP2 via Alk3 and GDF5 via Alk6. Different ligand concentrations were chosen to consider their individual activity on C2C12 cells. All three BMP ligands induced phosphorylation of Smad1/5/8 after 60 min and this phosphorylation was reduced by both DM and LDN in a dose dependent manner. As expected, LDN showed an improved efficacy as compared to DM and abolished almost completely the generation of p-Smad1/5/8 at a concentration of 0.5 µM (Fig. 1A–C, lanes 11). This correlates with the findings in PASMCs (Yu et al., 2008b). Inhibition by DM was less efficient and even at a concentration of 5 µM not complete (Fig. 1A–C, lanes 8). All three ligands induced in C2C12 cells a strong increase in phosphorylated p38 MAPK levels and also a slight increase in p-Akt. These BMP induced signalling events were reduced to background levels by both DM and LDN, each at concentrations needed for efficient p-Smad inhibition. This finding is partly in contrast to the previous study in PASMCs treated with BMP4 (Yu et al., 2008a,b) and suggests a wider range of potency as previously described for both compounds.
Fig. 1.
Activation of Smad1/5/8, p38 and Akt by different BMP family members is inhibited by Dorsomorphin (DM) and LDN-193189 (LDN) in a dose dependent manner. Serum starved C2C12 cells were treated with DM or LDN for 30 min prior to ligand addition. Samples were incubated for 1 h with 1 nM BMP6 (A), 5 nM BMP2 (B) or 10 nM GDF5 (C). Different ligand concentrations were chosen to consider their individual activity on C2C12 cells. Final DMSO concentration was adjusted to 0.05% (excepting w/o DMSO controls). After cell lysis, samples were immunobloted against p-Smad1/5/8, p-Akt, p-p38 as well as for GAPDH or tubulin as loading control.
3.2. High concentrations of Dorsomorphin and LDN-193189 affect Akt and p38 activity in unstimulated cells
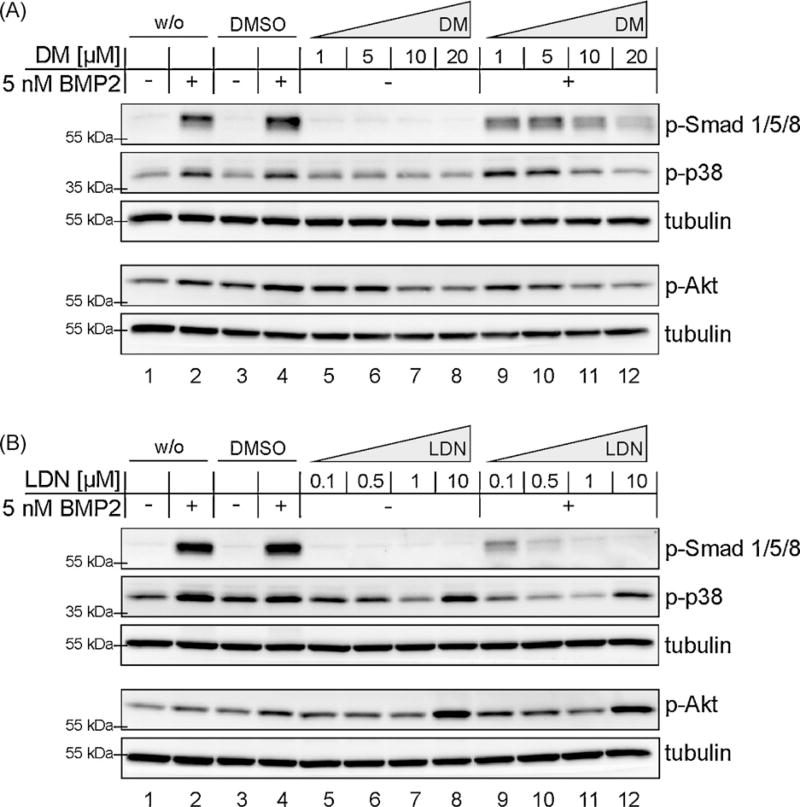
Next we addressed the question, whether DM and LDN exhibit at higher concentrations “off-target” effects on different signalling molecules along the BMP pathways. Concentrations up to 20 µM DM and 10 µM LDN were applied on C2C12 cells under stimulation and non-stimulation conditions (Fig. 2A and B). Ligand independent effects of these compounds were strikingly different. The concentration of 10 µM LDN induced after 60 min a strong ligand independent p38 and Akt phosphorylation (Fig. 2B, lanes 8 and 12). Concentrations lower than 1 µM did not show any influence on these targets. DM however showed even at low concentrations ligand independent “off-target” effects on p-Akt (Fig. 2A, lanes 5–8; quantification Fig S1A).
Fig. 2.
High concentrations of Dorsomorphin (DM) and LDN-193189 (LDN) not only inhibit BMP2-induced activation of Smad1/5/8, p38 and Akt, but also affect their activity in non stimulated cells. Serum starved C2C12 cells were treated with DM (A) and LDN (B) for 30 min prior to ligand addition. Samples were incubated with 5 nM BMP2 for 60 min. Final DMSO concentration was adjusted to 0.2% in DM treated and 0.1% in LDN treated samples respectively (excepting w/o DMSO controls). After cell lysis, samples were immunobloted against p-Smad1/5/8, p-Akt, p-p38 as well as for tubulin as loading control.
3.3. Dorsomorphin and LDN-193189 inhibit BMP2 mediated activation of Smad and non-Smad signalling cascades including their downstream targets
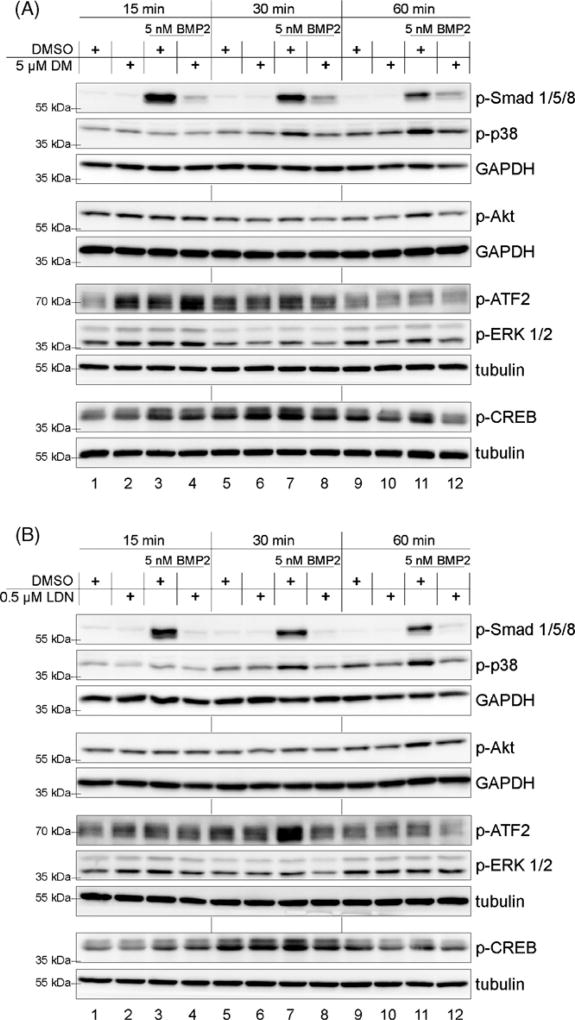
To gain further insights into how Dorsomorphin and LDN-193189 influence BMP signalling, we performed detailed time kinetic experiments to follow phosphorylation events during the first hour of BMP2 stimulation (Fig. 3). The amount of p-Smad1/5/8 was strongly elevated within the first 15 min of BMP stimulation and decreased subsequently (Fig. 3A and B, lanes 3, 7 and 11). Induction of p-p38 could be detected earliest at 30 min of stimulation, the induction of p-Akt at 60 min (Fig. 3A and B, lanes 7 and 11). All these BMP effects were clearly abrogated by DM (Fig. 3A) and in the case of p-Smad even more efficiently by treatment with LDN (Fig. 3B, quantification Fig S1B).
Fig. 3.
Dorsomorphin (DM) and LDN-193189 (LDN) inhibit BMP2 mediated activation of Smad and non-Smad signalling cascades. Serum starved C2C12 cells were treated with 5 µM DM (A) and 0.5 µM LDN (B) for 30 min prior to addition of 5 nM BMP2. Final DMSO concentration was adjusted to 0.05% in DM treated and 0.005% in LDN treated samples. Cells were lysed at indicated timepoints and subsequently probed for western blotting using different antibodies against p-Smad1/5/8 and the non-Smad signalling targets p-p38, p-Akt, p-ERK1/2, p-ATF2 and p-CREB. Each membrane was also probed against tubulin or GAPDH as loading control.
Among analyses of Smad1/5/8, p38 and Akt activation, we extended our studies on two p38 downstream targets: ATF2 and CREB (Deak et al., 1998; Morton et al., 2004). As it is known that both are also phosphorylated by activated ERK1/2 MAPK signalling (Deak et al., 1998; Arthur and Cohen, 2000), we also included ERK1/2 activation kinetics in our study (Figs. 3 and 4). Consistently with the kinetics of p38 activation, both, ATF2 and CREB were activated after 30 and 60 min of BMP2 stimulation (Fig. 3, lanes 7 and 11.). Both phosphorylation events were inhibited by DM and LDN (Fig. 3, lanes 8 and 12). Surprisingly, p-ATF2 and p-CREB were already strongly induced after 15 min of ligand addition, even though p38 induction occurs later, i.e. at 30 min stimulation. This phosphorylation coincides with a BMP2 mediated activation of ERK1/2 (Fig. 3A and B, lanes 1 and 3), which also is slightly sensitive to LDN inhibition (Fig. 3B, lanes 3, 4 and 7, 8). However, both DM and LDN show also ligand-independent effects in directly activating p-ERK1/2 after 15 min (Fig. 3A and B, lanes 1 and 2).
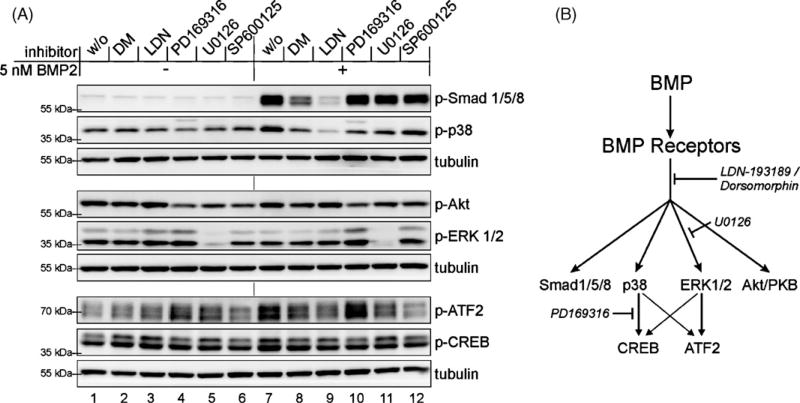
Fig. 4.
BMP dependent activation of ATF2 occurs downstream of ERK1/2, while CREB is activated via p38. (A) Serum starved C2C12 cells were treated with different inhibitors against BMP type I receptors 5 µM Dorsomorphin (DM) or 0.5 µM LDN-193189 (LDN), p38 (PD169316, 10 µM), ERK1/2 (U0126, 10 µM) or against JNK (SP600125, 10 µM) for 30 min prior to ligand addition. After inhibitor pretreatment cells were stimulated by adding 5 nMBMP2 for 30 min. Final DMSO concentration was adjusted to 0.2% in all samples. Cells were lysed at indicated timepoints and subsequently probed for western blotting using different antibodies against p-Smad1/5/8 or the non-Smad signalling targets p-p38, p-AKT, p-ERK1/2, p-ATF2 and p-CREB. Each membrane was also probed against tubulin as loading control. (B) Schematic presentation of BMP-mediated Smad and non-Smad pathways; the point of interference of all used inhibitors is indicated.
Taken together we can conclude that DM and more efficiently LDN inhibit four branches of BMP2-induced signalling, Smad1/5/8, p38, Akt/PKB and ERK1/2. This was shown by analyzing both the lack of activation through phosphorylation and the effect on their respective downstream signalling molecule.
3.4. BMP dependent activation of ATF2 occurs predominantly downstream of ERK1/2, while CREB is mainly activated via p38
For a better understanding of MAPKs involved in ATF2 and CREB activation after BMP stimulation, we used specific inhibitors for each branch of the MAPK cascades (Fig. 4). We selected the p38 inhibitor PD169316, the c-jun N-terminal kinase (JNK) 1/2/3 inhibitor SP600125 and the MEK1/2 inhibitor U0126. The latter affects the MAPKKs upstream of ERK1/2. All these inhibitors were applied in a concentration of 10 µM, commonly recommended for cell culture experiments. The inhibitors were added 30 min before ligand stimulation or vehicle treatment. Consistent with the results presented above, DM and LDN inhibited BMP-mediated Smad1/5/8, p38, ATF2 and CREB phosphorylation after 30 min of stimulation (Fig. 4, lanes 7–9). Note that, p-38 was activated and ERK1/2 was not activated at this time point (Fig. 4, lanes 1 and 7). However inhibition of phosphorylated p38 by PD169316led to decreased amounts of p-CREB but highly increased amounts of pATF2 (Fig. 4, lane 10). This observation can be explained by the strong induction of p-ERK upon PD169316 treatment, an effect that was even enhanced by BMP2 stimulation (Fig. 4A, lanes 4 and 10). The same was seen in two independent experiments by using SB203580 instead of PD169316 (data not shown). In agreement with this result the inhibition of activated ERK1/2 by U0126 abolished the BMP-mediated induction of p-ATF2 while effects on CREB phosphorylation were neglectable (Fig. 4A, lanes 7 and 11). The treatment with the JNK inhibitor SP600125 showed reduced activation of both MAPK downstream targets (Fig. 4A, lane 12).
From this we conclude that DM and LDN, by targeting the BMP receptors, act upstream of all known BMP induced non-Smad signalling pathways.
4. Discussion
The molecular mechanism by which BMPs, in particular BMP2, induces the Smad pathway is well understood and very distinct from the mechanism, by which the same ligand induces non-Smad signalling pathways, such as MAPK and Akt (Nohe et al., 2002; Hartung et al., 2006; Sieber et al., 2009). Small molecule inhibitors targeting the receptors are therefore extremely valuable tools to dissect these complex signalling structures. For their use in therapeutic interventions it is of special importance to understand their efficacy and specificity in detail in order to eliminate potential “offtarget” effects. We demonstrate here for the first time, that the BMP receptor inhibitors Dorsomorphin and its derivative from a structure-activity relationship study LDN-193189 are able to block the activation of Smad1/5/8 as well as the non-Smad pathways through MAPKs p38, ERK1/2 and the Akt pathway. In this comprehensive study we have included three different BMP ligands, all representing one individual subgroup of the ligands with distinct type I receptor preference. BMP2 binds with high affinity to BMPRIa/Alk3, GDF5 to BMPRIb/Alk6 receptor and BMP6 signals predominantly via ActRI/Alk2 (Nickel et al., 2009). All these receptors are present in different quantities in C2C12 cells, mesenchymal precursor cells, which differentiate towards the osteoblast lineage upon BMP stimulation.
Application of BMP6, BMP2 or GDF5 to C2C12 cells results in C-terminal phosphorylation of Smad1/5/8. A concentration of 5 µM DM or 0.5 µM LDN is necessary to efficiently inhibit phosphorylation of Smad 1/5/8 for 1 h. It had been shown before that the type I receptors used by these ligands (Alk2, Alk3 and Alk6) are inhibited by DM and LDN with decreasing efficacy (Yu et al., 2008a; Yu et al., 2008b). In this study we included a quantitative measure in DM and LDN effectiveness and observed that BMP6 mediated Smad1/5/8 phosphorylation was most efficiently blocked by LDN when compared to BMP2 and GDF5 (Fig. 1). Consistently with the known preferences of DM and LDN for different type I receptors, this might suggest, Alk2 was blocked most efficiently in this cellular context. For all further studies BMP2 was used in order to compare the results to the published data obtained from BMP4 the most related member of the ligand family (Kawabata et al., 1998; Lavery et al., 2008).
Under the same inhibitor concentrations BMP-mediated p38 and Akt activation were inhibited, clearly demonstrating that both DM and LDN show impact on non-Smad signalling pathways as well. A detailed and comprehensive study on the “off-target” effects of both inhibitors leads us to the assumption to use LDN preferentially for studies on non-Smad BMP signalling. These “off-target” effects might include toxic effects and stress response signals. Furthermore vehicle treated controls both for inhibitor as well as for ligand addition are indispensable to figure out true BMP effects.
Time kinetic studies are essential for improving our understanding of signalling dynamics. For this reason we investigated in detail the early signalling events initiated by BMP2 within 1 h upon stimulation (Fig. 3). With this we could pinpoint how the efficacy of p-Smad1/5/8 inhibition by DM and LDN depends on time. After 15 min of stimulation also DM exhibits an excellent performance to inhibit Smad1/5/8 phosphorylation, while after 60 min the superior advantages of LDN are obvious.
The temporal analysis showed that Smad and ERK1/2 MAPK pathways are already activated 15 min upon stimulation with BMP2. But while ERK1/2 activation is terminated shortly after, p-p38 and p-Smads are still present 60 min after ligand addition. A robust signal of p-Akt downstream of BMP2 could be detected only at a later time point, i.e. after 60 min. The activation of all these key players of different signalling pathways was inhibited by DM and LDN. Reinforcing this result, we also showed that the MAPK downstream factors ATF2 and CREB are targeted by DM and LDN (Figs. 3 and 4).
In consequence our findings indicate, that the BMP induced immediate early phosphorylation of ATF2 depends on activated ERK1/2, whereas CREB is predominantly phosphorylated by p38 (Fig. 4B) via its downstream effector kinase MSK1 (Deak et al., 1998). Nevertheless, because the JNK inhibitor also displays considerable effects on these MAPK downstream targets, further analyses of the impact of the BMP/JNK pathway are necessary.
Taken together our comprehensive signalling studies show that the BMP receptor inhibitors Dorsomorphin and LDN-193189 inhibit both Smad and non-Smad signalling and show distinct “off-target” effects in the herein studied cell system. The potency of the effects of LDN-193189 in particular upon MAPK p38 and p-Akt activation suggests an “on-target” effect via inhibition of BMP type I receptors. Moreover, the use of these inhibitors allowed us to define ATF2 and CREB as the downstream effectors of BMP2 mediated ERK1/2 and p-38 signalling in C2C12 cells (Fig. 4B).
Supplementary Material
Acknowledgments
We thank members of the Knaus lab for vivid discussions and support and Matheusz Kolanczyk for helpful advice. BMP2 was a generous gift from Walter Sebald (Würzburg), BMP6 from Slobodan Vukicevic (Zagreb) and GDF5 (BioPharm, Heidelberg). This work was supported by the Deutsche Forschungsgemeinschaft (research grant SFB760 to PK and a BSRT fellowship to JK).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biocel.2010.07.018.
References
- Arthur JS, Cohen P. MSK1 is required for CREB phosphorylation in response to mitogens in mouse embryonic stem cells. FEBS Lett. 2000;482:44–8. doi: 10.1016/s0014-5793(00)02031-7. [DOI] [PubMed] [Google Scholar]
- Barneda-Zahonero B, Minano-Molina A, Badiola N, Fado R, Xifro X, Saura CA, et al. Bone morphogenetic protein-6 promotes cerebellar granule neurons survival by activation of the MEK/ERK/CREB pathway. Mol Biol Cell. 2009;20:5051–63. doi: 10.1091/mbc.E09-05-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–92. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–41. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–8. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- Gamell C, Osses N, Bartrons R, Ruckle T, Camps M, Rosa JL, et al. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J Cell Sci. 2008;121:3960–70. doi: 10.1242/jcs.031286. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–8. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–53. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–18. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Lai CF, Cheng SL. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J Biol Chem. 2002;277:15514–22. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–58. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton S, Davis RJ, Cohen P. Signalling pathways involved in multisite phosphorylation of the transcription factor ATF-2. FEBS Lett. 2004;572:177–83. doi: 10.1016/j.febslet.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Nickel J, Sebald W, Groppe JC, Mueller TD. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 2009;20:367–77. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, et al. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–8. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–55. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Lin X, Yu PB, Feng XH. Transforming growth factor β can stimulate smad1 phosphorylation independently of bone morphogenic protein receptors. J Biol Chem. 2009;284:9755–63. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–87. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008b;14:1363–9. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008a;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BM, Hoffmann FM. Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol Biol Cell. 2006;17:3819–31. doi: 10.1091/mbc.E05-10-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.