Abstract
Pheromones are chemical signals that induce innate responses in individuals of the same species that may vary with physiological and developmental state. In Drosophila melanogaster, the most intensively studied pheromone is 11-cis-vaccenyl acetate (cVA), which is synthezised in the male ejaculatory bulb and is transferred to the female during copulation. Among other effects, cVA inhibits male courtship of mated females. We found that male courtship inhibition depends on the amount of cVA and this effect is reduced in male flies derived from eggs covered with low to zero levels of cVA. This effect is not observed if the eggs are washed, or if the eggs are laid several days after copulation. This suggests that courtship suppression involves a form of pre-imaginal conditioning, which we show occurs during the early larval stage. The conditioning effect could not be rescued by synthetic cVA, indicating that it largely depends on conditioning by cVA and other maternally-transmitted factor(s). These experiments suggest that one of the primary behavioral effects of cVA is more plastic and less stereotypical than had hitherto been realised.
Keywords: Cis-vaccenyl acetate, Courtship inhibition, Plasticity, Pre-imaginal conditioning
Introduction
Pheromones, first identified as chemicals that release a certain behaviour or physiological response (Karlson & Butenandt, 1959), have recently been more precisely defined as chemical signals that induce innate stereotypical responses in individuals of the same species (Wyatt, 2015). However, the way that pheromones act can depend on the physiological and developmental state of the individual that receives them. One of the most intensely studied pheromones is the Drosophila sex pheromone, 11-cis-vaccenyl acetate (cVA). This compound induces sex-specific effects when it is transferred from males to females during mating, and then introduced into the food substrate during egg-laying. This lipid-derived compound, which is produced in the male ejaculatory bulb, inhibits male courtship of mated females, renders food more attractive to males and females, stimulates females to mate and plays a role in inducing male-male aggression (Bartelt, Jackson & Schaner, 1985a; Butterworth, 1969; Das et al., 2017; Ejima, 2015; Fernandez & Kravitz, 2013; Guiraudie-Capraz, Pho & Jallon, 2007; Jallon, Antony & Benamar, 1981; Kurtovic, Widmer & Dickson, 2007; Laturney & Billeter, 2016; Lebreton et al., 2015; Schaner, Bartelt & Jackson, 1987; Wang et al., 2011; Wertheim et al., 2005; Zawistowski & Richmond, 1986).
Recently, the neuronal circuits involved in processing cVA and producing an appropriate behavioral output have been explored, giving some indication of the circuitry involved in the sex-specific effects. In both sexes, cVA is detected by the antennal sensilla expressing the olfactory receptors Or65a and Or67d (Clyne et al., 1997; Van der Goes Van Naters & Carlson, 2007). These neurons project to the DL3 and DA1 glomeruli, respectively (Couto, Alenius & Dickson, 2005; Fishilevich et al., 2005; Lebreton et al., 2015). Projection neurons leaving DA1 show sexually dimorphic arborization in higher brain centers (Datta et al., 2008; Kohl et al., 2013; Ruta et al., 2010). In males, stimulation by cVA together with the male-specific cuticular hydrocarbons leads to male-male aggression (Fernandez et al., 2010). In both sexes, chronic stimulation by cVA activates the DL3 glomerulus which then inhibits the output of the DA1 glomerulus via the activity of an inhibitory lateral interneuron. In the male, this leads to courtship suppression (Kurtovic, Widmer & Dickson, 2007), whereas in the female it leads to a decline in attraction to cVA (Lebreton et al., 2015).
Common to all these studies is the assumption that responses to cVA are stereotypic and unconditional. Although some studies have described a variable effect of cVA in a early adult conditioning (Liu et al., 2011; Tachibana, Touhara & Ejima, 2015), nothing is known about the potential conditioning effect of cVA during pre-imaginal development (after ‘imago’, the technical term for the adult insect). The term ‘conditioning’ has two meanings in the literature: it can mean treating an animal at one stage with a particular stimulus, and then observing the consequences of that treatment, irrespective of the mechanism involved; this is the sense in which we employ the term here, and is congruent with the earliest studies of changes in Drosophila behaviour following early experience (Manning, 1967; Thorpe, 1939), in which the effect was called pre-imaginal conditioning. The second, more specific use of the term relates to particular models of associative learning (e.g., classical or operant conditioning); we have not investigated any form of learning here, and do not use the term ‘conditioning’ in this sense.
Studying the robust and well-known male courtship suppression phenomenon, we show that adult male responses to cVA are a consequence of exposure to a combination of substances, including cVA, during the larval stage. Furthermore, there is natural variability for this conditioning effect. This finding explains inter-individual response variability to cVA and opens the road to the study of how pre-imaginal conditioning affects nervous system development, and its evolutionary significance.
Methods
Flies
We used the D. melanogaster wild-type stock Dijon2000 (Di2 (Houot, Bousquet & Ferveur, 2010); WT1) and the Di2/w1118 line (WT2), derived from the Di2 strain in which we introgressed the genome of the w1118 strain over five repeated backcross generations. This white-eyed line was used to allow us to distinguish adults from this strain and those from the conditioned strain (which had red eyes). No effect due to the mutation was observed. Stocks were raised on yeast / cornmeal / agar medium (6.5 L distilled water, 425 g maize flour, 425 g beer yeast, 60 g agar and 200 ml of 1% solution of nipagin, Sigma-Aldrich, St. Louis, MO, USA, diluted in ethanol). Flies were kept at 24 ± 0.5° and 65 ± 5% humidity on a 12 L: 12 D cycle (subjective day = 8:00 am to 8:00 pm) and were isolated under light CO2 anaesthesia either 0–4 h (for virgin females) or less than 24 h (for virgin males) after eclosion. Adult flies were held for 4 days in 30 ml glass vials filled with four ml fresh plain food. Same sex flies were always kept in small groups except for focal males used in behavioral tests; these were isolated to avoid social interactions affecting their subsequent courtship behavior (Svetec & Ferveur, 2005).
Egg collection and treatment
One h after subjective dawn, 30 male flies and 10 females, all 4-day-old, were placed in a 30 ml glass vial containing four ml fresh plain food. After 3 h, flies were cold-anaesthetized (15 min at four °C) and females were transferred into egg-laying devices. Each device consisted of a 50 mm Petri dish filled with one ml 3% agar striped with fresh yeast to stimulate egg-laying. Cold-anaesthetized mated females were aspirated into the egg-laying device through a small hole on the top of the Petri dish which was closed with a small metal plug. Three hours later, females were discarded and eggs collected. In the case of flies used to produce eggs at 10 days after mating, the females were placed in a tube until the appropriate time.
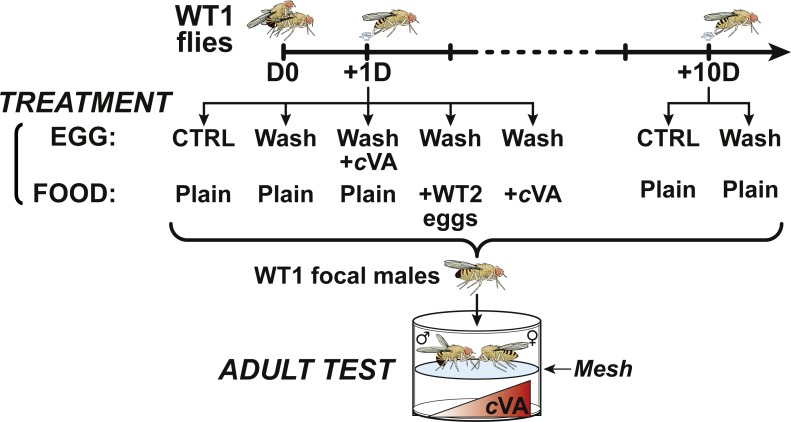
In all control experiments, and when indicated, focal males resulted from eggs laid by females 24 h after mating (+1D), except where focal males originated from eggs laid by the same females 10 days after mating (+10D) (Fig. 1). In some cases, eggs were washed just after collection with a 10% ethanol solution (10%) to remove cVA and other chemicals from the egg surface (“Wash”). Some Wash eggs were submitted to additional treatments, either soaked in a synthetic cVA solution (100 ng cVA/µl pure water; Cayman Chemical, Ann Arbor, MI, USA; 50 mg/ml solution in ethanol; purity > 98%) for 5 min, deposited on food enriched with cVA (15 ng/mm3), or on food seeded with eggs laid by WT2 females 24 h after mating with conspecific males. WT2 flies were discarded at emergence (based on their white eye color) to leave WT1 flies. To determine the critical period for conditioning, eggs (or larvae) were collected and washed with 10% ethanol solution at 10, 20, 40 or 72 h after egg-laying. In all control and experimental tests, Wash eggs (or larvae) were placed in groups of 50 on the food (plain or cVA-rich) contained in glass vials.
Figure 1. Experimental procedure.
At day 0 (D0), male and female 4-day-old WT1 flies were paired for 3 h and mated females then transfered into egg-laying devices were allowed to lay eggs one day (+1D) or 10 days after mating (+10D). For control experiments, +1D and +10D eggs, washed and unwashed were placed on plain food. +1D washed eggs were deposited on plain food either seeded with eggs from a second wild-type strain (+WT2 eggs) or on cVA-rich food (+cVA); other +1D washed eggs were covered with a solution of cVA and placed on plain food. All focal male flies derived from these egg treatments were tested in a double-deck chamber. Each male was paired with a beheaded virgin 4-day-old WT1 female in the upper part of the chamber while a cVA dose was placed in the lower part.
Behavior
Less than 24 h after eclosion, focal males were screened under light CO2 anaesthesia and individually placed in 30 ml glass vials containing 4ml fresh plain food until the test (4-days old). Behavioral tests were performed under white light in a specially designed mating chamber consisting of two superposed parts (diameter = 11 mm; height = five mm) separated by a fine nylon mesh (Fig. 1). The lower part contained a filter paper (25 mm2) impregnated with a controlled dose of cVA and small amount of yeast (ca. 125 mm3). A single focal male was aspirated into the top part, then after 10 min acclimation a 4-day-old virgin female that had previously been decapitated was aspirated in the top part. We did not use mated females, because they are expected to carry cVA following mating, and the exact amount would vary between females. Virgin CO2-anaesthetized females were decapitated with a razor blade, 1 h before the test. As in many previous studies, we used decapitated virgin females as the target fly; these remained alive, but mostly immobile, for at least 3–4 h. Decapitated virgin females do not produce rejection signals and do not copulate: this allows us to eliminate most female-produced signals that might otherwise bias measures of male activity. Decapitated females were therefore live targets producing stimulatory signals that enabled us to measure the inhibitory effect induced by cVA on male excitation. Total male activity was noted for 10 min, including courtship latency (time from introduction to courtship onset) and the total percentage of time spent courting (courtship index: CI). CI consists of the cumulative duration of all courtship behaviors shown by the subject male (Greenspan & Ferveur, 2000; Lasbleiz, Ferveur & Everaerts, 2006). To estimate the frequency of male courtship we counted the number of males showing a CI>5 divided by the total number of males tested. Males from all treatments were always tested in parallel with control males (derived from sham-control eggs that had been handled, but were otherwise untreated) and in some cases with males derived from washed eggs. The experiments were conducted by individual observers who were unaware of what treatment the flies had been subjected to.
Determination of cVA levels
To quantify the amount of cVA on 4-day-old individual flies, we immersed frozen flies (5 min at −20 °C) for 5 min at room temperature in 30 µl of a hexane solution containing 3.33 ng/µl of two internal standards (n-hexacosane and n-triacontane). We used a similar procedure to determine the amount of cVA in groups of 50 eggs. To evaluate the internal amount of cVA, frozen individuals were soaked in a similar hexane solution for 24 h at 40 °C (Bartelt, Schaner & Jackson, 1985b). The resulting extract and two successive rinses of each individual fly were combined and reduced in volume, under N2 flow, for chromatographic analysis. cVA was quantified by gas chromatography using a Varian CP3380 gas chromatograph fitted with a flame ionization detector, a CP Sil 5CB column (25 m × 0.25 mm internal diameter; 0.1 µm film thickness; Agilent), and a split–splitless injector (60 ml/min split-flow; valve opening 30 s after injection) with helium as carrier gas (velocity = 50 cm/s at 120 °C). The temperature program began at 120 °C, ramping at 10 °C/min to 140 °C, then ramping at two °C/min to 290 °C, and holding for 10 min. The chemical identity of cVA was checked using gas chromatography—mass spectrometry equipped with a CP Sil 5CB column. The amount (in ng) of cVA was calculated on the basis of the data obtained from the internal standards.
Statistics
All statistical analyses were performed using XLSTAT (2012). We used logistic regression to characterize the relationship between cVA amount and courtship inhibition by estimating the active dose 50 (AD50) (Robertson & Preisler, 1992). Courtship frequencies were compared using a Wilks G2 likelihood ratio test completed with a computation of significance by cell (Fisher’s test). Comparisons of courtship indices, courtship latencies or cVA levels were carried out either with a Kruskall-Wallis test with Conover-Iman multiple pairwise comparisons (p = 0.05, with a Bonferroni correction) or with a Mann–Whitney test, after excluding extreme outliers using Tukey’s method (Tukey, 1977).
Results
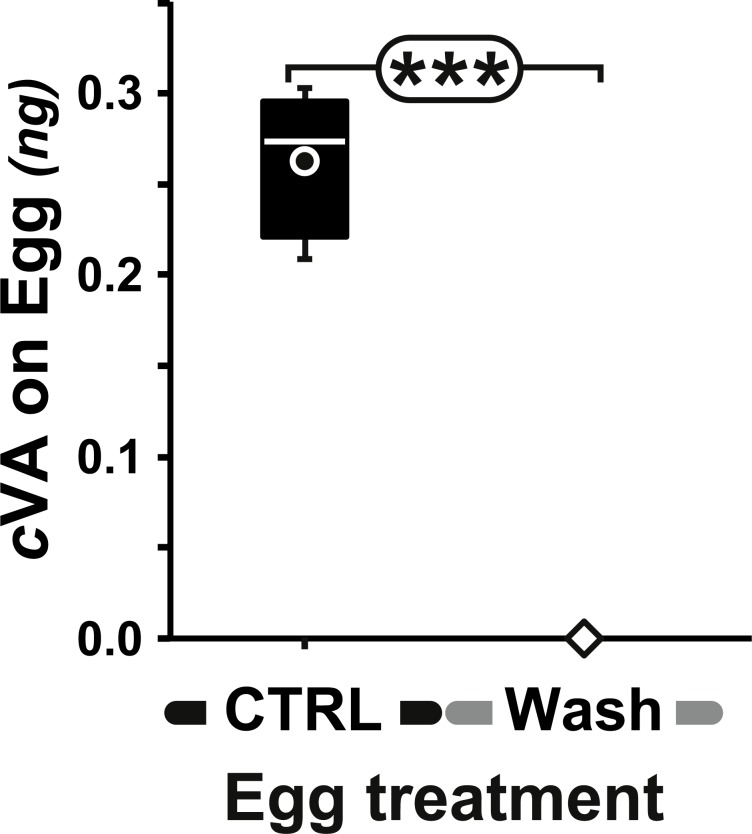
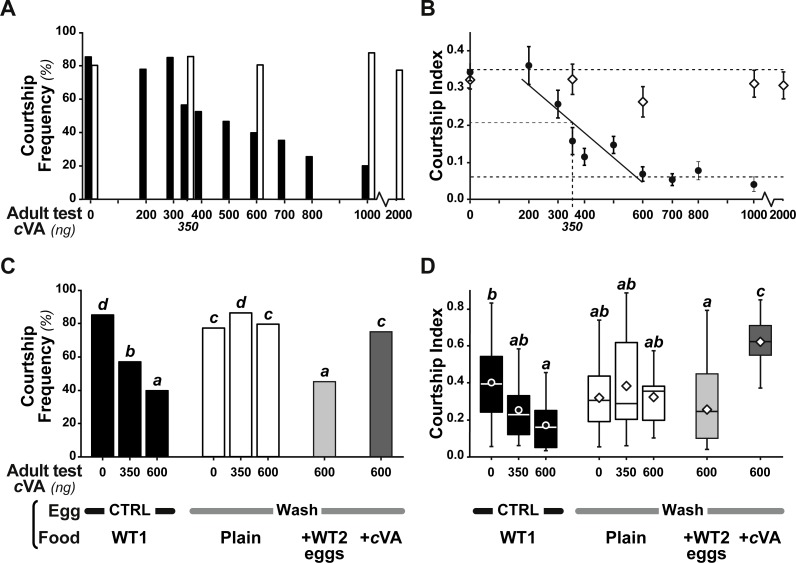
Measurable amounts of cVA are transferred to eggs laid one day after mating (0.21 ± 0.02 ng; +1D) and can be completely eliminated by washing the eggs with a 10% ethanol solution (0 ng; Fig. 2). Males derived from control, unwashed eggs showed a clear dose–response courtship suppression effect when their courtship towards an immobilised virgin female was observed in a chamber placed above a source of varying amounts of cVA (Fig. 1). Both courtship frequency (Khi29df = 89.4, p < 10−4; filled bars in Fig. 3A) and courtship index (CI; KW9df = 129.4, p < 10−4; filled circles in Fig. 3B) showed clear negative correlations with cVA levels. When no cVA was present below the mating chamber, control males courted females with a high frequency (85%) and a strong courtship index (CI = 0.34 ± 0.02); in the presence of 600 ng cVA these figures declined to 40% courtship and a CI = 0.07 ± 0.02. Maximum courtship suppression was induced with 600 ng, and 50% inhibition was observed with 350 ng; subsequent tests were performed with these two cVA doses. These levels are of the same order of magnitude as the total amount of cVA present in male flies (974 ± 186 ng; Fig. S1), or on the cuticle of a mated female (313 ± 23 ng). Virtually no cVA can be detected on the cuticle of a virgin female (0.4 ± 0.04 ng).
Figure 2. Amount of cVA detected on washed eggs.
Amount (ng/egg) of cVA detected on eggs laid one day after mating (+1D) either control (n = 19) or washed with a 10% ethanol solution (“Wash”; n = 20). Amounts were significantly different at p ≤ 10−4. Amounts are shown as box-and-whisker plots indicating the 25th and 75th percentiles (boxes), the median (line within box) and the limits (whiskers) beyond which values were considered anomalous. ***: p < 0.001.
Figure 3. Behavioral effect of cis-vaccenyl acetate (cVA) manipulation on eggs.
The courtship of focal males paired with beheaded females was measured for 10 min, with different cVA doses (0–2,000 ng). We noted the frequency of males courting (%; A, C) and the total proportion of time spent courting (courtship index; B, D; the corresponding courtship latencies are shown on Fig. S2). (A, B) We first compared the courtship of control focal males (filled bars or circles) and males derived from washed eggs (empty bars or diamonds) raised on plain food (n = 15 − 91). (C, D) Using three cVA doses (0, 350, 600 ng), we compared the courtship performance of males derived from control and washed eggs with those of males derived from washed eggs placed on food with WT2 eggs (light gray bars) or cVA-rich food (15 ng/mm3; dark gray bars). Courtship indices (D) are shown as box-and-whisker and significant differences are indicated by different letters (for example, “a” differs from “b” but not from “ab”; n = 20 − 107). Corresponding values for courtship latency are shown in Fig. S4.
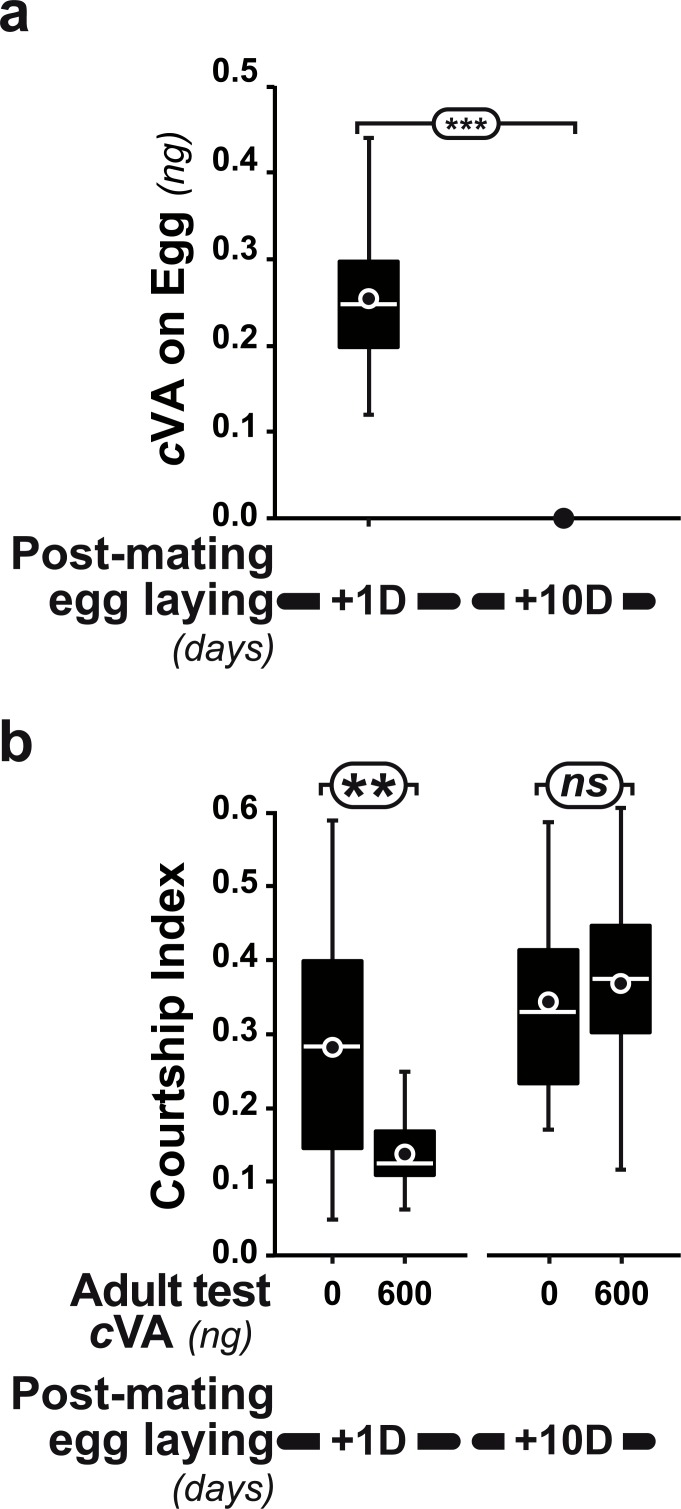
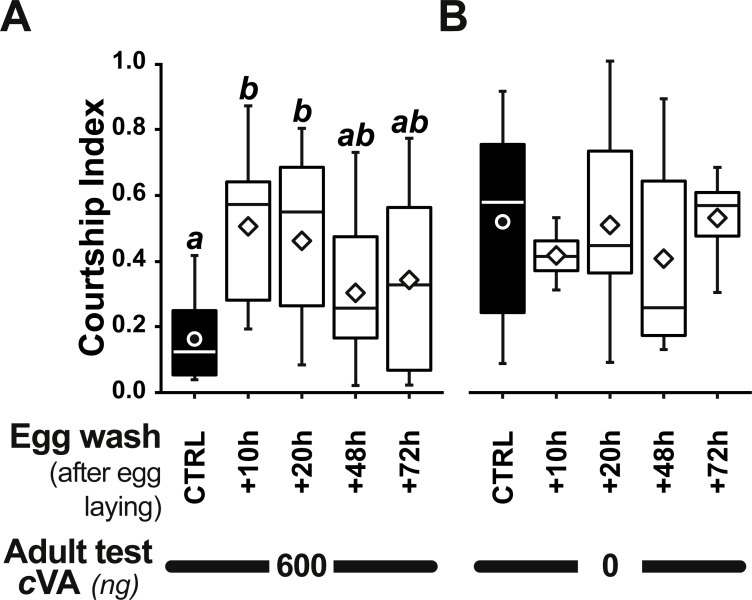
Strikingly, if cVA was removed from eggs by washing them immediately after laying, males derived from these eggs showed no dose–response courtship suppression, even with very high cVA doses (e.g., 2,000 ng; frequency = 78%; CI = 0.31 ± 0.02; empty bars and diamonds in Figs. 3A, 3B). Egg-washing had no effect on male behavior (Fig. 3D). This suggests that cVA-based courtship suppression is produced through a pre-imaginal conditioning effect on the developing fly. Variation in the amount of cVA present on eggs occurs naturally, as revealed by a comparison of eggs laid by the same females one day (+1D) and 10 days (+10D) after mating. At +10D, eggs were covered in no detectable cVA (0 ng, as compared to 0.25 ± 0.03 ng +1D flies; U12,10 = 120, p = 0.005; Fig. 4A). Males derived from +10D eggs showed no courtship suppression (U10,8 = 35, p = 0.70; Fig. 2B and Fig. S2), unlike males derived from +1D eggs, which showed significantly less intense courtship in the presence of cVA (U19,14 = 209, p = 0.005). Taken together, these data show that male courtship suppression by cVA involves a conditioning effect that is mediated by the presence of a substance or substances on eggs which is correlated with the level of cVA. To reveal whether there is a critical period for conditioning, eggs were washed at different periods after laying (10 h, 20 h), and larvae were washed 48 h and 72 h after egg-laying; Fig. 5). No courtship suppression was observed if eggs were washed 10 h or 20 h after egg laying, while control levels of suppression (indicating the existence of pre-imaginal conditioning) were seen when older larvae were washed (48 h or 72 h after egg-laying) (KW4df = 15.6; p = 0.004; Fig. 5A and Fig. S3A). No effect of egg wash was detected in the absence of cVA below the courtship chamber (Fig. 5B and Fig. S3B).
Figure 4. Post-mating effect on cVA conditioning.
(A) Comparison of cVA on eggs laid by the same females either one day (+1D) or 10 days (+10D) after mating (n = 12 and 10, respectively). (B) Courtship indices were compared in males derived from +1D and +10D eggs tested with either 0 or 600 ng cVA (n = 49 − 144). ***: p < 0.001; **: p < 0.01; ns, non-significant. For more details, see Fig. 1. Corresponding values for courtship frequency and latency are shown in Fig. S2.
Figure 5. Identifying a critical period for cVA conditioning.
Bars and box-plots show the courtship frequency (top) and courtship index (bottom) of focal males derived from +1D eggs. These eggs were either control (filled bars) or were washed at different periods after egg-laying (+10, +20, +48, +72 h; empty bars) and then placed on plain food. Males derived from these eggs were tested either with 600 ng cVA (A) or without cVA (B). For more details see Figs. 1 and 3. n = 10–45. Corresponding values for courtship latency are shown in Fig. S3.
We were able to rescue courtship suppression by placing washed eggs on food containing unwashed eggs (and therefore natural levels of female-deposited compounds). Males derived from these eggs showed a courtship suppression similar to control males (frequency = 45%, Khi27df = 55.5, p < 10−4; CI = 0.22 ± 0.045, KW 7df = 34.3, p < 10−4; light gray bars in Figs. 3C, 3D). This shows that an apparent critical period for pre-imaginal conditioning occurs after larval eclosion from the egg, which occurs around 20–24 h after egg-laying. This may be a true critical period requiring the nervous system to be in a particular state, or it may simply reveal that pre-imaginal conditioning requires the larva to come into contact with substances on the outside of the egg.
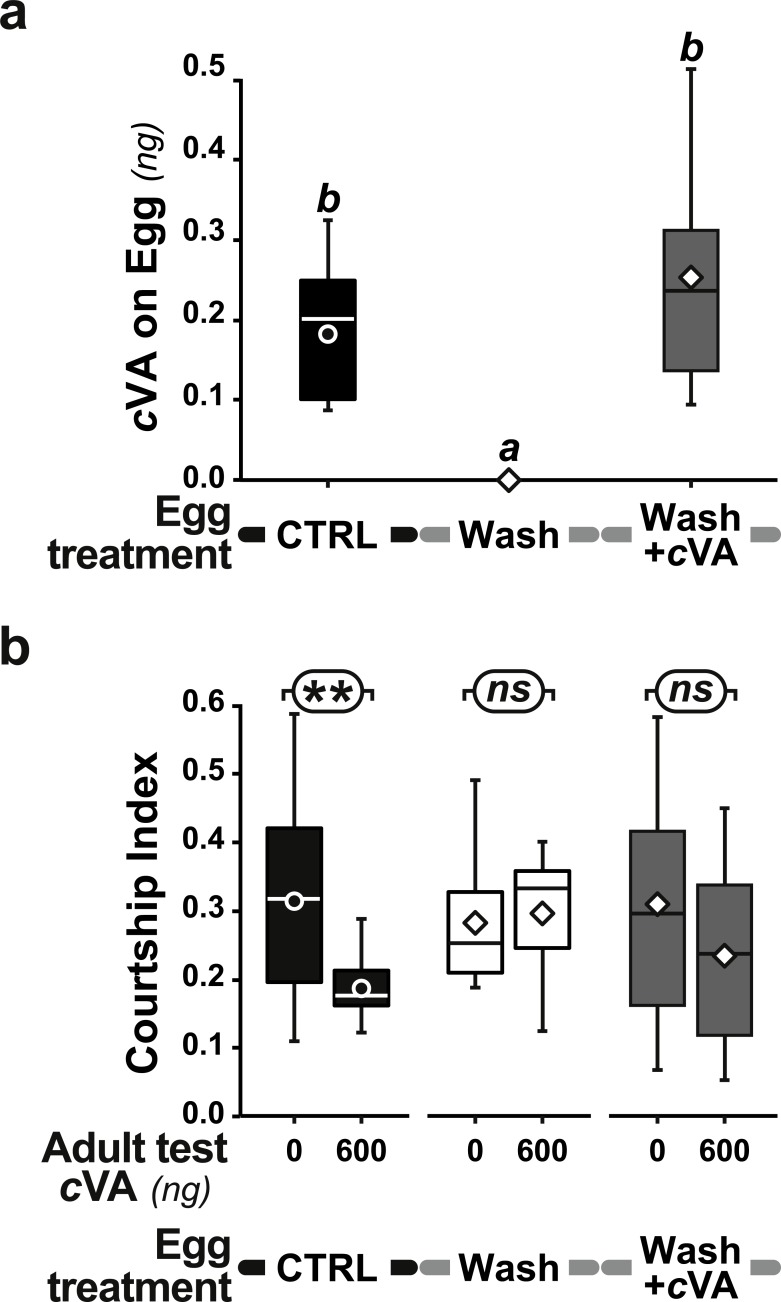
However, when we placed washed eggs on food enriched with synthetic cVA (15 ng/mm3), males derived from these eggs showed no courtship suppression (frequency = 75%; CI = 0.53 ± 0.06; dark gray bars in Figs. 3C, 3D and Fig. S4); similar results were obtained when washed eggs were covered with synthetic cVA (Fig. 6). Although the amount of synthetic cVA recovered from eggs in these two experiments was similar to the levels of natural cVA found on control eggs (0.29 ± 0.05 and 0.21 ± 0.02 ng, respectively, P = 0.126; Fig. 6A), both of these sets of males behaved like males derived from washed eggs (Fig. 6B, Fig. S5). Assuming that synthetic cVA is chemically identical to its natural equivalent, we conclude that other compounds coating the egg, or forming the egg coat, are at least partly responsible for the larval conditioning effect on cVA-mediated courtship suppression, perhaps in synergy with cVA.
Figure 6. Effect of synthetic cVA on eggs.
+1D control eggs (filled bars and box-plots) were compared with washed eggs (empty bars and box plots), and washed eggs covered with a cVA solution (shaded bars and box-plots). All eggs were placed on plain food. We measured (A) the amount of cVA on eggs (n = 19 for control, n = 8 for Wash and n = 12 for Wash+cVA), and (B) the courtship index of derived males tested with 0 or 600 ng cVA (n = 61 − 203). For more details see Figs. 1 and 5. Corresponding values for courtship frequency and latency are shown in Fig. S5.
Discussion
The rich behavioral effects induced by cVA in male and female flies have become the focus of a series of neurobiological studies that have begun to reveal the neuronal circuitry involved. Here we show that the longest-established of these effects—male courtship suppression—is a conditional phenotype, dependent not only upon the apparent learning effect occurring during adult life (Siegel & Hall, 1979) but also on exposure to the substance during the first instar larval stage—pre-imaginal conditioning. In addition to an effect imputable to cVA, this conditioning is induced by unknown stimuli that are introduced onto the egg by the female, or which are present on the chorion and are detected by the larva on eclosion. This conclusion is based on the following results: the effect is blocked by washing the eggs, can be rescued by putting washed eggs on food containing normal eggs, but cannot be rescued by adding cVA alone (Figs. 3C, 3D).
The pre-imaginal conditioning described here appears to occur in the first larval stage—washing of eggs at 10 h and 20 h after egg-laying prevented conditioning as shown by the abolition of cVA-based courtship suppression, while washing of larvae at 48 h or 72 h after egg-laying had no effect on courtship suppression, revealing control levels of conditioning. Moreover, males derived from washed eggs deposited on food containing control eggs also showed courtship suppression, suggesting that pre-imaginal conditioning does not occur in the egg itself but immediately after eclosion of the first instar larva.
The absence of courtship suppression in males derived from washed eggs reared in the presence of synthetic cVA could be taken to indicate that cVA is not involved at all in this effect. However, this would be to overlook the clear dose–response effect of cVA shown on normal males in the presence of cVA, which demonstrates a role for cVA in courtship suppression. We conclude that the simultaneous presence of another factor or factors, along with cVA, is required during the conditioning exposure phase to induce the modulation of cVA perception in adult males. Washing the eggs removed both cVA and these unknown factors. Covering washed eggs with synthetic cVA did not rescue the adult response to cVA because those other factor(s) are necessary to create an association or synergy with cVA.
A diverse range of substances, unmeasured in our experiments, could conceivably act additively with cVA: for example, sex-peptides which are transferred during mating and disappear from the female genital tract after several days (Peng et al., 2005), metabolites produced by the microbes present on the chorion and which disappear after egg-washing (Farine et al., 2017), or cuticular hydrocarbons which are found in trace levels on eggs, but which not no detectable variation with female age post-mating (total CHs amount = 11.9 ± 0.8 ng at D1 and 13.9 ± 1.4 ng at D10; U10,10 = 32, p = 0.186; Fig. S7). Whatever the case, our data firmly indicate that cVA is involved since we measured and compared the adult male response in the presence/absence of this substance only. It is also possible that the synthetic cVA that we used does not exactly cover the chorion structure as in the natural situation.
This effect occurs pre-pupation, whereas the phenotype we have studied is expressed in the adult. Changes to adult behavior consequent on larval experience—generally involving olfaction—have been widely reported in Drosophila and in other holometabolous insects, going back to the 1930s and are known as pre-imaginal conditioning (Manning, 1967; Thorpe, 1939); for a review see Barron (2001) and Barron & Corbet (1999). This phenomenon is distinct from the sparse claims for transfer of memory through pupation in holometabolous insects (e.g., Tully, Cambiazo & Kruse, 1994). The most parsimonious explanation of many of the examples of pre-imaginal conditioning reported in Drosophila involves a ‘chemical legacy’ of odoriferous particles on the outside of the pupa that are detected on adult emergence, altering the activity of the adult olfactory system and inducing a change in behavior (Barron, 2001). We cannot exclude this possibility, but the levels of cVA used in our experiments are far lower than the levels of substances such as menthol which are traditionally used in pre-imaginal conditioning experiments, particles of which are present on the pupa. Furthermore, the pre-imaginal conditioning effect observed here will have a different neurobiological basis than classical learning.
Neither of the receptors that are currently known to detect cVA (Or65a and Or67d) are expressed in larvae (Fishilevich et al., 2005), indicating that cVA must be perceived by an as-yet unidentified larval receptor. Moreover, we found no evidence of attraction to any dose of cVA in larval olfactory tests (Fig. S6). If conditioning works through altered activity of larval neural circuits, conserved through metamorphosis and affecting adult neurons, by definition the unidentified stimuli that are involved with cVA in producing the conditioning effect must be detected by larvae.
The plasticity of pheromonally-induced courtship suppression we describe here in male flies contrasts with the widespread assumption that pheromones induce stereotypical and unconditional behaviors (Wyatt, 2015). Modulation of an innate pheromonal response occurs in C. elegans, where early exposure to the repellent pheromone asc-Δ9 increases adult responses to this substance through increased expression of the odr-2 glycosylated phosphatidylinositol (GPI)-linked signaling gene (Hong et al., 2017). Given that cVA is found in a range of Drosophila species (Hedlund et al., 1996; Symonds & Wertheim, 2005), conditioning may also affect male courtship inhibition in these species. Equally, the widespread behavioral effects of cVA on both sexes of D. melanogaster may be based on conditioning, and thereby susceptible to showing greater levels of variability than is currently assumed.
The variation in the responses shown by males derived from eggs laid 1 and 10 days after mating, which correlates with the level of cVA on these eggs, reveals an unexpected source of phenotypic variability in the behavior of Drosophila siblings. Males derived from eggs laid by females 10 days after mating showed no cVA-based courtship suppression, unlike their brothers derived from eggs laid 1 day after mating. The population-level variability in the courtship suppression phenotype produced by this effect would mean that some males would reduce their courtship of mated females, whereas others would not.
An adaptationist explanation of the courtship suppression phenotype needs to be nuanced to take this into account: it is not sufficient to suggest that by suppressing courtship following contact with cVA, Drosophila males avoid ‘wasteful’ courtship that does not lead to direct fitness benefits. Flies derived from eggs that are born at a longer interval after mating will tend not to show this effect: there must be some advantage underlying the need for conditioning rather than a labelled line effect whereby cVA suppresses courtship under all circumstances. One possible explanation for such plasticity effects is that they may enable rapid phenotypic adaptation in a changing environment (Laland et al., 2015). In nature, such variation is not always relevant. Depending upon the concentration and availability of potential sex partners, D. melanogaster females lose most of the cVA that has been transferred to them after 24 h and re-mate within 10 days post mating (Singh, Singh & Hoenigsberg, 2002). However, the situation may be different if fewer conspecific males are present. In this case, there may be an advantage to not suppressing courtship when populations are small and unmated females are extremely rare —persistent courtship may eventually lead to a female re-mating (Smith et al., 2017; Yapici et al., 2008).
Conclusion
Besides its involvement in the well-known male courtship suppression phenomenon, cVA is known for its multiple roles in D. melanogaster (Ejima, 2015). Nowadays, all studies dealing with its roles in Drosophila chemical communication consider responses to this compound stereotypic and unconditional. We show that adult male courtship suppression by cVA is modulated by exposure to cVA—conjointly with some other substances—during the larval stage. Our findings, together with studies of chemical conditioning in vertebrates (Bett & Hinch, 2015; Coureaud et al., 2006; Hauser et al., 2017) and Drosophila females (Flaven-Pouchon et al., 2014), suggest that pheromonal responses may be less stereotypical than hitherto suspected, and that developmentally-determined plasticity may play an important role in naturally-occurring variation in chemical communication that may not be readily identified in the laboratory.
Next step will be to evaluate if—and how—such a conditioning effect also affects the other Drosophila behavioural responses to cVA. Furthermore, our finding opens the road to the study of how conditioning affects nervous system development, and its evolutionary significance.
Supplemental Information
Box plots indicate the amount of cVA (ng/fly) detected on virgin and recently mated male and female flies (n = 9 − 17). We used solvent extraction over two periods: the 20 min-long extraction revealed the cVA present on the fly cuticle, whereas the 24 h-long solvent extraction (at 40 °C) allowed us to extract internal cVA. For more details see Fig. 3.
Courtship frequency (A) and latency (B) were measured in focal males derived from +1D and +10D eggs in the presence of 0 or 600 ng cVA. For more details see Figs. 1 and 4.
Courtship latency was measured in focal males derived from control eggs (filled bars) or from eggs washed at different times after egg-laying (AEL; +10, +20, +48, +72 h; empty bars) with 600 ng cVA (A) or no cVA (B). For more details see Figs. 1 and 5.
Courtship latency was measured in focal males each paired with a beheaded female in the presence of 0, 350 or 600 ng cVA. Focal males were derived from +1D eggs either with no treatment (filled bars), or from washed eggs raised either on plain food (empty bars), or on food seeded with WT2 eggs (light gray bars), or on cVA-rich food. For more details see Fig. 3.
Courtship frequency (A) and latency (B) were measured in focal males dervived from control +1D eggs (filled bars), or of washed +1D eggs (empty bars), some of which were covered with a synthetic cVA solution (shaded bars). All eggs were placed on plain food and resulting males were tested with 0 or 600 ng cVA. For more details see Figs. 1 and 6.
Individual larvae produced by +1D (A) or +10D (B) control eggs were placed on an agar plate with two food sources at opposite sides. These were either plain food (PF) or PF with the addition of 1 ng –10 μg of cVA. N = 50 for each comparison. The first food source reached by each larva was noted. There were no significant differences in response to cVA, nor were there any differences between +1D or +10D larvae.
Comparison of total CHs amount on eggs laid by the females either one day (+1D) or 10 days (+10D) after mating (n = 10; 50 pooled eggs were extracted for each female). For more details, see Fig. 1.
The dataset is organised according to figures.
Acknowledgments
Jérôme Cortot, Mouhamadou Sall and Rhassane Asgassou are thanked for their help with flies.
Funding Statement
All the funding received during this study was provided in part by the Centre National de la Recherche Scientifique (CNRS, INSB), the Burgundy Regional Council (PARI 2014), and the Université de Bourgogne Franche-Comté. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Claude Everaerts conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Laurie Cazalé-Debat, Alexis Louis, Emilie Pereira, Jean-Pierre Farine performed the experiments, approved the final draft.
Matthew Cobb authored or reviewed drafts of the paper, approved the final draft.
Jean-François Ferveur conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Barron (2001).Barron AB. The life and death of Hopkins’ host-selection principle. Journal of Insect Behavior. 2001;14:725–737. doi: 10.1023/A:1013033332535. [DOI] [Google Scholar]
- Barron & Corbet (1999).Barron AB, Corbet SA. Preimaginal conditioning in Drosophila revisited. Animal Behaviour. 1999;58:621–628. doi: 10.1006/anbe.1999.1169. [DOI] [PubMed] [Google Scholar]
- Bartelt, Jackson & Schaner (1985a).Bartelt RJ, Jackson LL, Schaner AM. Ester components of aggregation pheromone of Drosophila-Virilis (Diptera, Drosophilidae) Journal of Chemical Ecology. 1985a;11:1197–1208. doi: 10.1007/BF01024108. [DOI] [PubMed] [Google Scholar]
- Bartelt, Schaner & Jackson (1985b).Bartelt RJ, Schaner AM, Jackson LL. Cis-vaccenyl acetate as an aggregation pheromone in Drosophila-Melanogaster. Journal of Chemical Ecology. 1985b;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- Bett & Hinch (2015).Bett NN, Hinch SG. Attraction of migrating adult sockeye salmon to conspecifics in the absence of natal chemical cues. Behavioral Ecology. 2015;26:1180–1187. doi: 10.1093/beheco/arv062. [DOI] [Google Scholar]
- Butterworth (1969).Butterworth FM. Lipids of Drosophila: a newly detected lipid in the male. Science. 1969;163:1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- Clyne et al. (1997).Clyne P, Grant A, O’Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invertebrate Neuroscience. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- Coureaud et al. (2006).Coureaud G, Moncomble AS, Montigny D, Dewas M, Perrier G, Schaal B. A pheromone that rapidly promotes learning in the newborn. Current Biology. 2006;16:1956–1961. doi: 10.1016/j.cub.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Couto, Alenius & Dickson (2005).Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Current Biology. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Das et al. (2017).Das S, Trona F, Khallaf MA, Schuh E, Knaden M, Hansson BS, Sachse S. Electrical synapses mediate synergism between pheromone and food odors in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E9962–E9971. doi: 10.1073/pnas.1712706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta et al. (2008).Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- Ejima (2015).Ejima A. Pleiotropic actions of the male pheromone cis-vaccenyl acetate in Drosophila melanogaster. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology. 2015;201:927–932. doi: 10.1007/s00359-015-1020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine et al. (2017).Farine JP, Habbachi W, Cortot J, Roche S, Ferveur JF. Maternally-transmitted microbiota affects odor emission and preference in Drosophila larva. Scientific Reports. 2017;7:6062. doi: 10.1038/s41598-017-04922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez et al. (2010).Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD, Kravitz EA. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLOS Biology. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez & Kravitz (2013).Fernandez MP, Kravitz EA. Aggression and courtship in Drosophila: pheromonal communication and sex recognition. Journal of comparative physiology A. Neuroethology, Sensory, Neural, and Behavioral Physiology. 2013;199:1065–1076. doi: 10.1007/s00359-013-0851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich et al. (2005).Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Current Biology. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Flaven-Pouchon et al. (2014).Flaven-Pouchon J, Garcia T, Abed-Vieillard D, Farine J-P, Ferveur J-F, Everaerts C. Transient and permanent experience with fatty acids changes Drosophila melanogaster preference and fitness. PLOS ONE. 2014;9(3):e92352. doi: 10.1371/journal.pone.0092352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan & Ferveur (2000).Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annual Review of Genetics. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- Guiraudie-Capraz, Pho & Jallon (2007).Guiraudie-Capraz G, Pho DB, Jallon JM. Role of the ejaculatory bulb in biosynthesis of the male pheromone cis-vaccenyl acetate in Drosophila melanogaster. Integrative Zoology. 2007;2:89–99. doi: 10.1111/j.1749-4877.2007.00047.x. [DOI] [PubMed] [Google Scholar]
- Hauser et al. (2017).Hauser WJ, Westley PAH, Kerkvliet C, Dudiak N. Homing of Pacific salmon to a marine release site: a case study of the Homer Spit Fishing Hole, Alaska. Northwest Science. 2017;91:314–323. doi: 10.3955/046.091.0310. [DOI] [Google Scholar]
- Hedlund et al. (1996).Hedlund K, Bartelt RJ, Dicke M, Vet LEM. Aggregation pheromones of Drosophila immigrans, D-phalerata, and D-subobscura. Journal of Chemical Ecology. 1996;22:1835–1844. doi: 10.1007/BF02028507. [DOI] [PubMed] [Google Scholar]
- Hong et al. (2017).Hong M, Ryu L, Ow MC, Kim J, Je AR, Chinta S, Huh YH, Lee KJ, Butcher RA, Choi H, Sengupta P, Hall SE, Kim K. Early pheromone experience modifies a synaptic activity to influence adult pheromone responses of C. elegans. Current Biology. 2017;27:3168–3177. doi: 10.1016/j.cub.2017.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot, Bousquet & Ferveur (2010).Houot B, Bousquet F, Ferveur JF. The consequences of regulation of desat1 expression for pheromone emission and detection in Drosophila melanogaster. Genetics. 2010;185:1297–1309. doi: 10.1534/genetics.110.117226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon, Antony & Benamar (1981).Jallon JM, Antony C, Benamar O. An anti-aphrodisiac produced by Drosophila-Melanogaster males and transferred to females during copulation. Comptes Rendus de l’Academie des Sciences Serie III Sciences de la Vie. 1981;292:1147–1149. [Google Scholar]
- Karlson & Butenandt (1959).Karlson P, Butenandt A. Pheromones (ectohormones) in insects. Annual Review of Entomology. 1959;4:39–58. doi: 10.1146/annurev.en.04.010159.000351. [DOI] [Google Scholar]
- Kohl et al. (2013).Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic, Widmer & Dickson (2007).Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Laland et al. (2015).Laland KN, Uller T, Fellman MW, Sterelny K, Muller GB, Moczek A, Jablonka E, Odling-Smee J. The extended evolutionary synthesis: its structure, assumptions and predictions. Proceedings of the Royal Society Biological Sciences Series B. 2015;282 doi: 10.1098/rspb.2015.1019. Article 20151019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasbleiz, Ferveur & Everaerts (2006).Lasbleiz C, Ferveur JF, Everaerts C. Courtship behaviour of Drosophila melanogaster revisited. Animal Behaviour. 2006;72:1001–1012. doi: 10.1016/j.anbehav.2006.01.027. [DOI] [Google Scholar]
- Laturney & Billeter (2016).Laturney M, Billeter JC. Drosophila melanogaster females restore their attractiveness after mating by removing male anti-aphrodisiac pheromones. Nature Communication. 2016;7 doi: 10.1038/ncomms12322. Article 12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton et al. (2015).Lebreton S, Trona F, Borrero-Echeverry F, Bilz F, Grabe V, Becher PG, Carlsson MA, Nässel DR, Hansson BS, Sachse S, Witzgall P. Feeding regulates sex pheromone attraction and courtship in Drosophila females. Scientific Reports. 2015;5:13132. doi: 10.1038/srep13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2011).Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nature Neuroscience. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- Manning (1967).Manning A. Pre-imaginal conditioning in Drosophila. Nature. 1967;216:338–340. doi: 10.1038/216338a0. [DOI] [PubMed] [Google Scholar]
- Peng et al. (2005).Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm-bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005;15:207–223. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Robertson & Preisler (1992).Robertson JL, Preisler HK. Pesticide bioassays with Arthropods. CRC Press/Taylor & Francis Group; Boca Raton: 1992. [Google Scholar]
- Ruta et al. (2010).Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Schaner, Bartelt & Jackson (1987).Schaner AM, Bartelt RJ, Jackson LL. (z)-ll-Octadecenyl acetate, an aggregation pheromone in Drosophila simulans. Journal of Chemical Ecology. 1987;13:1777–1786. doi: 10.1007/BF00980218. [DOI] [PubMed] [Google Scholar]
- Siegel & Hall (1979).Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, Singh & Hoenigsberg (2002).Singh SR, Singh BN, Hoenigsberg HF. Female remating, sperm competition and sexual selection in Drosophila. Genetics and Molecular Research. 2002;1:178–215. [PubMed] [Google Scholar]
- Smith et al. (2017).Smith DT, Clarke NVE, Boone JM, Fricke C, Chapman T. Sexual conflict over remating interval is modulated by the sex peptide pathway. Proceedings of the Royal Society Biological Sciences Series B. 2017;284:20162394. doi: 10.1098/rspb.2016.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetec & Ferveur (2005).Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. Journal of Experimental Biology. 2005;208:891–898. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- Symonds & Wertheim (2005).Symonds MR, Wertheim B. The mode of evolution of aggregation pheromones in Drosophila species. Journal of Evolutionary Biology. 2005;18:1253–1263. doi: 10.1111/j.1420-9101.2005.00971.x. [DOI] [PubMed] [Google Scholar]
- Tachibana, Touhara & Ejima (2015).Tachibana SI, Touhara K, Ejima A. Modification of male courtship motivation by olfactory habituation via the GABAA receptor in Drosophila melanogaster. PLOS ONE. 2015;10(8):e0135186. doi: 10.1371/journal.pone.0135186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe (1939).Thorpe WH. Further studies on pre-imaginal olfactory conditioning in insects. Proceedings of the Royal Society Biological Sciences Series B. 1939;127:424–433. doi: 10.1098/rspb.1939.0032. [DOI] [Google Scholar]
- Tukey (1977).Tukey JW. Exploratory data analysis. Addison-Wesley; Reading: 1977. [Google Scholar]
- Tully, Cambiazo & Kruse (1994).Tully T, Cambiazo V, Kruse LT. Memory through metamorphosis in normal and mutant Drosophila. Journal of Neuroscience. 1994;14:68–74. doi: 10.1523/JNEUROSCI.14-01-00068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Goes Van Naters & Carlson (2007).Van der Goes Van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Current Biology. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2011).Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nature Neuroscience. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim et al. (2005).Wertheim B, Van Baalen EJ, Dicke M, Vet LE. Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annual Review of Entomology. 2005;50:321–346. doi: 10.1146/annurev.ento.49.061802.123329. [DOI] [PubMed] [Google Scholar]
- Wyatt (2015).Wyatt TD. How animals communicate via pheromones. American Scientist. 2015;103:114–121. doi: 10.1511/2015.113.114. [DOI] [Google Scholar]
- XLSTAT (2012).XLSTAT . Data analysis and statistics with Microsoft Excel. Addinsoft; Paris: 2012. [Google Scholar]
- Yapici et al. (2008).Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- Zawistowski & Richmond (1986).Zawistowski S, Richmond RC. Inhibition of courtship and mating of Drosophila-Melanogaster by the male-produced lipid, Cis-Vaccenyl Acetate. Journal of Insect Physiology. 1986;32:189–192. doi: 10.1016/0022-1910(86)90057-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box plots indicate the amount of cVA (ng/fly) detected on virgin and recently mated male and female flies (n = 9 − 17). We used solvent extraction over two periods: the 20 min-long extraction revealed the cVA present on the fly cuticle, whereas the 24 h-long solvent extraction (at 40 °C) allowed us to extract internal cVA. For more details see Fig. 3.
Courtship frequency (A) and latency (B) were measured in focal males derived from +1D and +10D eggs in the presence of 0 or 600 ng cVA. For more details see Figs. 1 and 4.
Courtship latency was measured in focal males derived from control eggs (filled bars) or from eggs washed at different times after egg-laying (AEL; +10, +20, +48, +72 h; empty bars) with 600 ng cVA (A) or no cVA (B). For more details see Figs. 1 and 5.
Courtship latency was measured in focal males each paired with a beheaded female in the presence of 0, 350 or 600 ng cVA. Focal males were derived from +1D eggs either with no treatment (filled bars), or from washed eggs raised either on plain food (empty bars), or on food seeded with WT2 eggs (light gray bars), or on cVA-rich food. For more details see Fig. 3.
Courtship frequency (A) and latency (B) were measured in focal males dervived from control +1D eggs (filled bars), or of washed +1D eggs (empty bars), some of which were covered with a synthetic cVA solution (shaded bars). All eggs were placed on plain food and resulting males were tested with 0 or 600 ng cVA. For more details see Figs. 1 and 6.
Individual larvae produced by +1D (A) or +10D (B) control eggs were placed on an agar plate with two food sources at opposite sides. These were either plain food (PF) or PF with the addition of 1 ng –10 μg of cVA. N = 50 for each comparison. The first food source reached by each larva was noted. There were no significant differences in response to cVA, nor were there any differences between +1D or +10D larvae.
Comparison of total CHs amount on eggs laid by the females either one day (+1D) or 10 days (+10D) after mating (n = 10; 50 pooled eggs were extracted for each female). For more details, see Fig. 1.
The dataset is organised according to figures.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.