Abstract
Three new tetrahydroxanthone dimers, 5-epi-asperdichrome (1), versixanthones N (2), and O (3), were isolated from the mangrove-derived fungus Aspergillus versicolor HDN1009. Their structures, including the absolute configurations, were elucidated by NMR, HRMS, and circular dichroism (CD) experiments. Among them, compound 1 was the second example of tetrahydroxanthone dimers, which dimerized by a rare diaryl ether linkage and showed promising antibacterial activities against Vibrio parahemolyticus, Bacillus subtilis, Mycobacterium phlei, and Pseudomonas aeruginosa, with MIC values ranging from 100 μM to 200 μM; whilst compounds 2 and 3 exhibited extensive cytotoxicities against five cancer cell lines (HL-60, K562, H1975, MGC803, and HO-8910), with IC50 values ranging from 1.7 μM to 16.1 μM.
Keywords: mangrove-derived fungus, Aspergillus versicolor, tetrahydroxanthone dimers, antibacterial activity, cytotoxicity
1. Introduction
Xanthones are a family of 9H-xanthen-9-one derivatives, which are widely distributed in plants, fungi, and lichens. The structures typically occur as either fully aromatized or aliphatic cyclic ketones with dihydro-, tetrahydro- or hexahydro- modifications, and poly-substitutions [1]. Among those structures, tetrahydroxanthone dimers, which were usually dimerized through biaryl C-C single bond with diverse dimeric patterns, such as 2–2′, 2–4′, 4–2′, and 4–4′ linkages [2], were frequently reported for their intriguing structures and impressive activities (antimicrobial, antiplasmodial, and antitumor bioactivities) [2,3,4]. However, tetrahydroxanthone dimers linked by diaryl ether bond were relatively rare, with only one instance reported [5].
In our previous study, the mangrove-associated fungus Aspergillus versicolor HDN1009 has been proven to be a fruitful producer of various xanthone analogues [2,6]. In a recent work, UPLC-MS and cytotoxicity directed fractionation method were employed in a new trial of chemical study on HDN1009, to track unique xanthone structures. By carefully examining the UV absorption, predicted molecular weight, and the bioactivities of the unique peaks, three new tetrahydroxanthone dimers, named 5-epi-asperdichrome (1), versixanthones N (2), and O (3), were discovered and isolated, amongst which, 5-epi-asperdichrome (1) was the second example of tetrahydroxanthone dimers linked by a diaryl ether bond, which showed mild activities against four kinds of bacterial strains. Versixanthones N (2) and O (3) showed extensive cytotoxicities against five cancer cell lines. Herein, we will report the details of isolation, structure elucidation, and bioactivity evaluation of the above compounds.
2. Results and Discussion
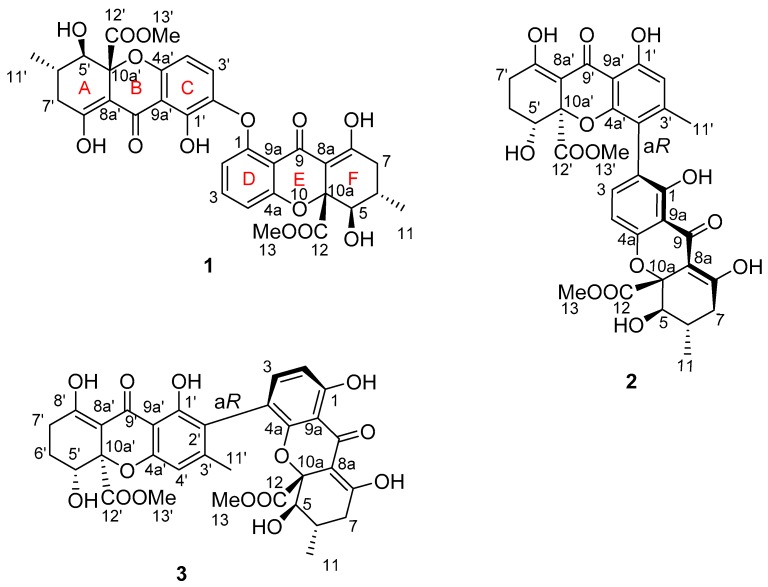
The Aspergillus versicolor HDN1009 was cultured (50 L) under static conditions at 28 °C for 4 weeks, as previous reported in Reference [2]. Guided by UPLC-MS data, the EtOAc extract (30 g) of the fermentation was fractionated by silica gel vacuum liquid chromatography, C-18 ODS column chromatography, Sephadex LH-20 column chromatography, ODS MPLC, and finally HPLC to yield compounds 1 (4.0 mg), 2 (3.0 mg), and 3 (4.0 mg) (Figure 1).
Figure 1.
Structures of compounds 1–3.
Compound 1 was isolated as a pale, yellow powder, with a molecular formula of C32H30O14, as deduced from HREIMS peak at m/z 639.1721 [M + H]+. The 1H and 13C NMR spectra of 1 indicated four methyls (including two methoxyls); two methylenes; nine sp3 methines (including two oxygenated methines and five aromatic methines); and 17 nonprotonated carbons (including two ester carbonyls, two conjugated ketone carbonyls), which were similar to the tetrahydroxanthone dimers we reported before in Reference [2]. Careful inspection of 1D NMR spectra revealed the twin resonances indicating a dimeric structure for 1, which was quite similar to that of asperdichrome, with obvious differences only in chemical shifts and coupling constants of H-5, H-6, and H-7 (δH-5: 3.95 d (11.3) in 1 vs. 4.12 d (1.4) in asperdichrome; δH-6: 2.42 m in 1 vs. 2.14 m in asperdichrome; and δH-7: 2.72 dd (6.4, 10.6); 2.29 dd (11.0, 15.4) in 1 vs. 2.40, dd (18.8, 6.3); 2.52, dd (18.8, 11.6) in asperdichrome) (Figure 1, Table 1 and Supplementary Materials Figures S1–S21) [5]. Further interpretation of 2D NMR spectra confirmed compound 1 shared an identical planar structure with asperdichrome; however, it possessed different stereochemistry (Figure 2, Table 1 and Table 2).
Table 1.
1H NMR data of compounds 1–3 (500 MHz, CDCl3, TMS, δ ppm, J in Hz).
| No. | 1 | Asperdichrome | 2 | 3 |
|---|---|---|---|---|
| 2 | 6.36 d (8.4) | 6.39, d (8.2) | -- | 6.62 d (8.4) |
| 3 | 7.30 t (8.3) | 7.28, t (8.2) | 7.69 d (8.4) | 7.18 d (8.4) |
| 4 | 6.80 d (8.4) | 6.76, d (8.2) | 6.69 d (8.4) | -- |
| 5 | 3.95 d (11.3) | 4.12 d (1.4) | 3.94 d (11.1) | 3.82 d (11.1) |
| 6 | 2.42 m | 2.14, m | 2.46 m | 2.34 m |
| 7 | 2.72 dd (6.4, 10.6) 2.29 dd (11.0, 15.4) |
2.52, dd (18.8, 11.6) 2.40, dd (18.8, 6.3) |
2.74 dd (6.3, 19.3) 2.32 dd (10.7, 19.2) |
2.69 m |
| 11 | 1.17 d (5.9) | 1.18, d (6.3) | 1.18 d (6.4) | 1.08 d (6.3) |
| 13 | 3.72 s | 3.69 s | 3.70 s | 3.66 |
| 1-OH | -- | 11.49 s | 11.41 s | |
| 8-OH | 15.78 s | 16.0, s | 13.75 brs | 13.68 brs |
| 2′ | -- | -- | 6.51 s | -- |
| 3′ | 7.22 d (8.9) | 7.22, d (8.7) | -- | -- |
| 4′ | 6.57 d (8.9) | 6.57, d (8.7) | -- | 6.56 s |
| 5′ | 3.94 d (11.3) | 3.94, d (11.1) | 4.14 dd (5.0, 12.6) | 4.33 dd (5.0, 12.4) |
| 6′ | 2.43 m | 2.40, m | 2.12 m; 2.00, m | 2.23 m; 2.10, m |
| 7′ | 2.76 dd (6.1, 10.4); 2.32 dd (4.1, 8.2) |
2.75, dd (18.7, 5.8) 2.32, dd (18.7, 10.9) |
2.62 m | 2.66 m |
| 11′ | 1.18 d (5.7) | 1.17, d (6.8) | 2.12 s | 2.23 s |
| 13′ | 3.70 s | 3.72, s | 3.74 s | 3.73 s |
| 1′-OH | 11.35 s | 11.4, s | 11.25 brs | 11.44 brs |
| 8′-OH | 13.75 s | 13.7, s | 13.77 brs | 13.86 brs |
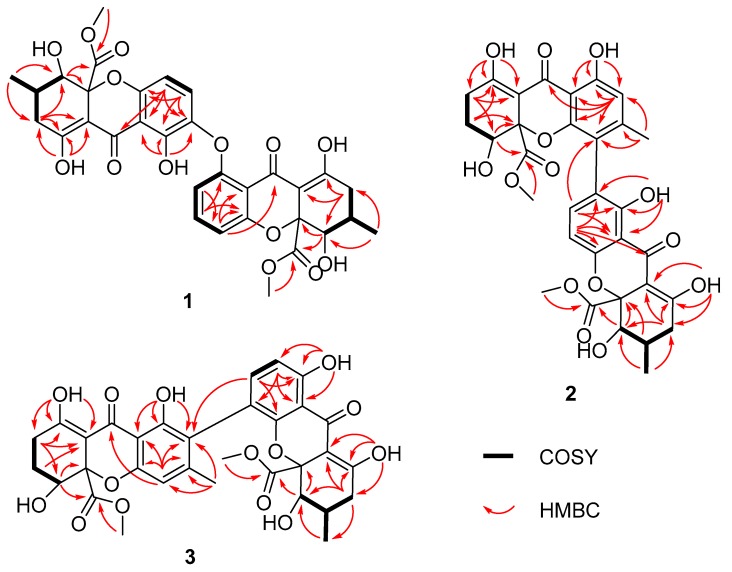
Figure 2.
Key HMBC and 1H-1H COSY correlations of 1–3.
Table 2.
13C NMR data of compounds 1–3 in CDCl3 (125 MHz, CDCl3, TMS, δ ppm).
| No. | 1 | Asperdi- chrome |
2 | 3 |
|---|---|---|---|---|
| 1 | 158.7 | 160.0 | 159.3 | 161.9 |
| 2 | 109.5 | 110.9 | 117.1 | 110.5 |
| 3 | 135.6 | 136.6 | 141.0 | 140.2 |
| 4 | 111.9 | 113.0 | 107.8 | 114.7 |
| 4a | 160.2 | 160.8 | 158.5 | 155.9 |
| 5 | 77.9 | 72.4 | 76.9 | 76.6 |
| 6 | 29.2 | 30.1 | 29.3 | 29.2 |
| 7 | 37.8 | 35.1 | 36.2 | 36.1 |
| 8 | 182.5 | 186.4 | 177.7 | 177.3 |
| 8a | 102.7 | 103.2 | 101.6 | 101.4 |
| 9 | 180.2 | 181.7 | 187.1 | 187.2 |
| 9a | 110.8 | 112.0 | 106.9 | 107.0 |
| 10a | 84.9 | 86.2 | 85.0 | 84.7 |
| 11 | 18.0 | 17.9 | 18.0 | 17.9 |
| 12 | 170.1 | 173.5 | 170.5 | 169.9 |
| 13 | 53.0 | 53.7 | 53.2 | 53.0 |
| 1′ | 158.7 | 154.1 | 159.3 | 161.9 |
| 2′ | 136.8 | 137.8 | 111.8 | 118.4 |
| 3′ | 130.3 | 131.5 | 150.2 | 149.8 |
| 4′ | 107.6 | 109.1 | 115.7 | 109.1 |
| 4a′ | 155.5 | 157.9 | 155.7 | 157.8 |
| 5′ | 77.9 | 77.6 | 71.5 | 71.9 |
| 6′ | 29.3 | 31.1 | 23.5 | 23.9 |
| 7′ | 36.3 | 37.1 | 27.5 | 27.6 |
| 8′ | 178.3 | 179.6 | 177.3 | 177.8 |
| 8a′ | 101.6 | 103.3 | 101.1 | 101.3 |
| 9′ | 186.9 | 188.7 | 186.8 | 186.7 |
| 9a′ | 107.9 | 109.1 | 105.1 | 104.5 |
| 10a′ | 84.9 | 86.8 | 84.7 | 84.5 |
| 11′ | 18.0 | 18.3 | 21.1 | 21.5 |
| 12′ | 170.2 | 172.1 | 169.8 | 170.4 |
| 13′ | 53.2 | 53.4 | 53.1 | 53.2 |
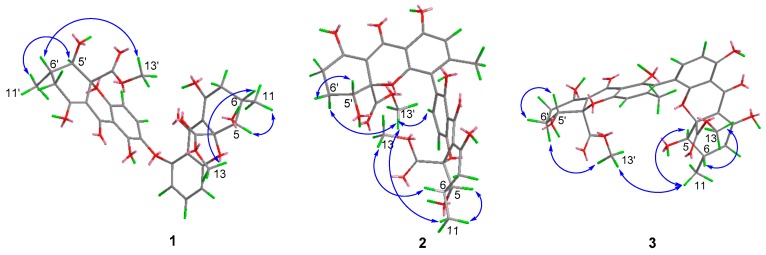
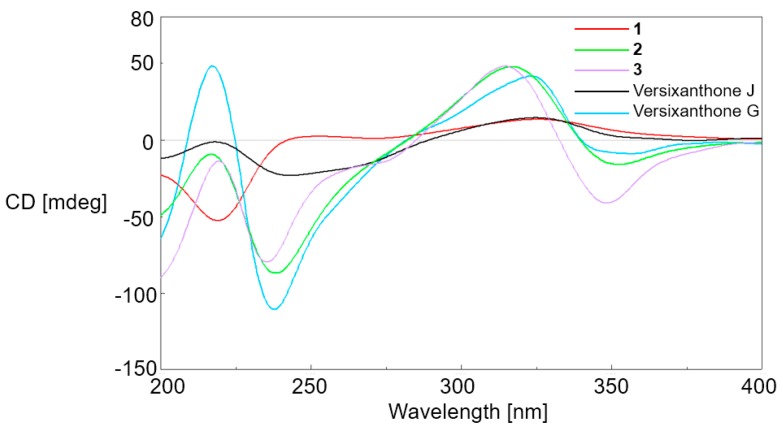
The relative configuration of 1 was established as 5′R*, 6′S*, 10a′R*, 5R*, 6S*, 10aR* by the NOE correlations of Me-13 with H-6; H-5 with Me-11; Me-13′ with H-6′; and H-5′ with Me-11′, together with the coupling constant (3JH-5, H-6 = 3J H-5′, H-6′ = 11.3 Hz). This suggested that 13-COOCH3 and OH-5 were located at the same side of ring A, whilst Me-11 was located at the other side; similarly, 13′-COOCH3 and OH-5′ were located at the same side of ring F, whilst Me-11′ was located at the other side (Figure 3). The absolute configuration of 1 was determined by CD comparison. As there was no axial chirality between two monomeric units, the ECD curves were dominated by the chirality center elements [2,5,7]. Similar to versixanthones J, which we previously discovered from this strain, the positive n→π* CD band at around 330 nm, indicated the R-configuration at C-10a/C-10a′ (Figure 4) [2,5,7]. Combined with the relative configuration, the absolute configuration of compound 1 was assigned as 5′R, 6′S, 10a′R, 5R, 6S, 10aR and proved to be the 5-epi isomer of asperdichrome, and we named it 5-epi-asperdichrome.
Figure 3.
Key NOE correlations of 1–3.
Figure 4.
Circular dichroism (CD) spectra of 1–3, versixanthones J and G.
Compounds 2 and 3 were also isolated as pale, yellow powder, and had the same molecular formula of C32H30O14, determined by HRESIMS detected at m/z 639.1721 [M + H]+. The almost twin resonances in the 1D NMR spectra, suggested that they were heterodimers of tetrahydroxanthones. A careful comparison of the NMR data of compounds 2 and 3 with versixanthone G, which we reported previously [2], suggested they had the same monomeric units. Further analysis of the 2D NMR data, revealed that the only difference between compounds 2, 3 and versixanthone G were the linkage patterns of the two monomeric units. Compound 2 was constructed by connecting the two monomeric units via the linkage of C-2 and C-4′, based on the HMBC correlations from H-2′ to C-1′ and C-9a′; OH-1′ to C-1′; C-2′ and C-9a′; H3-11′ to C-2′; C-3′ and C-4′; OH-1 to C-1; C-2 and C-9a; and H-3 to C-4′. Based on the HMBC correlations from H-4′ to C-4a′ and C-3′; OH-1′ to C-1′; C-2′ and C-9a′; H3-11′ to C-2′; C-3′ and C-4′; OH-1 to C-1; C-2 and C-9a; and H-3 to C-2′ (Figure 2), the planar structure of compound 3 was completed by connecting the two monomeric units via the linkage between C-4 and C-2′, which was the same as purpureone, whose absolute configuration has not been reported [8,9].
The relative configurations of monomeric units in compounds 2 and 3, were established to be the same as that of versixanthone G (5R*, 6S*, 10aR*, 5′R*, 10a′R*) by the coupling constant (3JH-5, H-6 = 11.1 Hz), and the NOE correlations between Me-13 and H-6, H-5 and Me-11, Me-13′ and H-6′a (δH 2.12 for 2 and δH 2.23 for 3), H-5′ and H-6′b (δH 2.00 for 2 and δH 2.10 for 3) (Figure 3, Table 1). Similar linkage type and substitution patterns with versixanthone G, suggest compounds 2 and 3 should also possess axial chirality [2]. The axial chiralities were further deduced by ECD exciton chirality method. Based on ECD spectra, the negative exciton couplings near 230 nm and 330 nm for 2 and 3, which were similar with versixanthone G, allowing the assignment of both axial chiralities as aR [2]. Combined with the NOE correlations between two monomeric units (Me-13′ with H-3 and H-11 in 2; Me-13′ with H-11 in 3), the configurations at C-10a′ in both compounds were determined as R, and the absolute configurations of 2 and 3 were aR, 5R, 6S, 10aR, 5′R, 10a′R, accordingly.
Biological evaluation of all the compounds by an MTT method showed that 2 and 3 exhibited extensive cytotoxicity against five kinds of cancer cell lines (HL-60, K562, H1975, MGC803, and HO-8910) (Table 3), with IC50 values ranging from 1.7 μM to 16.1 μM. The results suggested that the connection type between the two monomers was very important. As with connection by oxygen atoms, the cytotoxic activity disappears completely. The antimicrobial activities of compound 1 were also evaluated and showed mild activities against Vibrio parahemolyticus, Bacillus subtilis, Mycobacterium Phlei, and Pseudomonas aeruginosa, with MIC values ranging from 100 μM to 200 μM (Table 4).
Table 3.
Inhibitory effects of 1–3 on six tumor cells.
| Comp. | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| MGC803 | HL-60 | HO-8910 | H1975 | K562 | A549 | |
| 1 | >30 | >30 | >30 | >30 | >30 | >30 |
| 2 | 1.7 | 2.7 | 8.5 | 8.8 | 9.1 | >30 |
| 3 | 1.8 | 8.1 | 6.7 | 8.5 | 16.1 | >30 |
| Dox a | 0.2 | 0.02 | 0.5 | 0.8 | 0.3 | 0.2 |
a Dox stands for doxorubicin hydrochloride, which was used as a reference drug.
Table 4.
Inhibitory effects of 1 on six kinds of microorganisms.
| Comp. | MIC (μM) | |||||
|---|---|---|---|---|---|---|
| Vibrio parahemolyticus | Bacillus subtilis | Mycobacterium phlei | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |
| 1 | 100 | 200 | 200 | >200 | 100 | >200 |
| Positive Drug | 0.781 a | 0.391 a | 0.781 a | 0.391 a | 1.56 a | 3.13 b |
a Ciprofloxacin used as a reference drug for bacteria; b Nystatin used as a reference drug for Candida albicans.
3. Materials and Methods
3.1. General Experimental Procedures
UV spectra were measured on a Beckman DU 640 spectrophotometer (Beckman Coulter Inc., Brea, CA, USA). Specific rotations were obtained by a JASCO P-1020 digital polarimeter (JASCO Corporation, Tokyo, Japan). ESIMS were obtained on a Thermo Scientific LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) or Micromass Q-TOF ULTIMA GLOBAL GAA076 LC Mass spectrometer (Wasters Corporation, Milford, MA, USA). NMR spectra were recorded on an Agilent 500 MHz DD2 spectrometer (Agilent Technologies Inc., Santa Clara, CA, USA), using TMS as an internal standard and chemical shifts were recorded as δ-values. Semi-preparative HPLC employed an ODS column (HPLC (YMC-Pack ODS-A, 10 × 250 mm, 5 μm, 3 mL/min)) (YMC Co., Ltd., Kyoto, Japan). Medium-pressure preparation liquid chromatography (MPLC) was performed on a Bona-Agela CHEETAHTM HP100 (Beijing Agela Technologies Co., Ltd., Beijing, China) [10].
3.2. Fungal Material
As described previously in Reference [6].
3.3. Fermentation and Extraction
As described previously in Reference [6].
3.4. Isolation
The organic extract was subjected to vacuum liquid chromatography over a silica gel column, using a gradient elution with petroleum ether-CH2Cl2-MeOH to give six fractions (fractions 1–6). Guided by LC-MS-UV data, fraction 3 (6.5 g) eluted with CH2Cl2-MeOH (97:3) was applied on a C-18 ODS column using a stepped gradient elution of MeOH-H2O, yielding five subfractions (fractions 3.1–3.5). Fraction 3.4, which eluted with MeOH-H2O (75:25) was chromatographed on SephadexLH-20 with CH2Cl2-MeOH (1:1), and further separated by MPLC (C-18 ODS) using MeOH-H2O (a gradient elution, 30–100%), to furnish five subfractions (fractions 3.4.1–3.4.5). Fractions 3.4.1 was purified by semi-preparative HPLC (MeOH-H2O (55:45)), to afford compound 1 (4.0 mg, tR 18.5 min). Fraction 3.4.5 was also purified by semi-preparative HPLC, first using a gradient elution by MeOH-H2O (30–100%), and then MeCN-H2O (50–90%) to afford compounds 2 (3.0 mg, tR 22.5 min) and 3 (4.0 mg, tR 25.0 min).
5-epi-asperdichrome (1): pale yellow powder; −6.6 (c 0.1, CHCl3); ECD (MeOH) λ (nm) (Δε): 326 (+12.5), 273 (+0.4), 249 (+2.5), 220 (−53.5); UV (MeOH) λmax (log ε): 242 (2.10), 318 (3.46) nm; 1H-NMR and 13C-NMR (see Table 1 and Table 2); HR-ESI-MS [M + H]+ m/z 639.1721 (calcd. for C32H31O14, 639.1708, error = 1.9432 ppm), [M + Na] m/z 661.1538 (calcd. for C32H30O14Na, 661.1528, error = 1.6056 ppm).
Versicolone N (2): pale yellow powder; −43.0 (c 0.1, CHCl3); ECD (MeOH) λ (nm) (Δε): 360 (−16.5), 318 (+49.7), 237 (−91.5), 218 (−3.5); UV (MeOH) λmax (log ε): 238 (2.30), 313 (3.16) nm; 1H-NMR and 13C-NMR (see Table 1 and Table 2); HR-ESI-MS [M + H]+ m/z 639.1702 (calcd. for C32H31O14, 639.1708, error = −1.0639 ppm), [M + NH4] m/z 656.1976 (calcd. for C32H34O14N, 656.1974, error = 0.2826 ppm).
Versicolone O (3): pale yellow powder; −114.8 (c 0.1, CHCl3); ECD (MeOH) λ (nm) (Δε): 349 (−41.5), 315 (+48.0), 235 (−80.7), 220 (−13.0); UV (MeOH) λmax (log ε): 240 (2.25), 315 (3.26) nm; 1H-NMR and 13C-NMR (see Table 1 and Table 2); HR-ESI-MS [M + H]+ m/z 639.1703 (calcd. for C32H31O14, 639.1708, error = −0.7571 ppm), [M + Na] m/z 661.1522 (calcd. for C32H30O14Na, 661.1528, error = −0.8306 ppm).
3.5. Assay of Cytotoxicity
These biological evaluations were carried out as previously reported in Reference [11].
3.6. Assay of Antimicrobial Activity
The microorganism suspension (198 μL, 106 cfu/mL) in autoclaved growth medium (peptone (20 g/L), beef extract (3 g/L), NaCl (5 g/L) for bacteria; peptone (20 g/L), yeast extract (10 g/L), glucose (20 g/L) for Candida albicans)) was added to each well of 96-well plates. Solutions (40 mM) of the compounds and positive drugs were made up in DMSO and dispensed into 96-well plates using the 2× microdilution method, to give 16 concentrations in the range of 200–0.006 μM. Incubated at 28 °C for 24 h, and the lowest concentration that gave complete growth inhibition was recorded as the minimum inhibitory concentration (MIC).
4. Conclusions
In summary, UPLC-MS guided chemical investigation led to the discovery of three new tetrahydroxanthone dimers, 5-epi-asperdichrome (1), versixanthones N (2), and O (3), from the marine-derived fungus Aspergillus versicolor HDN1009. Compound 1 represented the second example of tetrahydroxanthone dimers with a diaryl ether bond linkage. Their absolute configurations, including the axial and central chirality elements were elucidated by NOE experiments and circular dichroism (CD) comparison. Compounds 2 and 3, exhibited extensive cytotoxicities against five cancer cell lines with IC50 values ranging from 1.7 μM to 16.1 μM, whilst compound 1 showed mild activities against four kinds of bacterial strains, with MIC values ranging from 100 μM to 200 μM.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/9/335/s1, Figures S1–S7: 1D, 2D NMR and HRESIMS spectra of 5-epi-asperdichrome (1), Figures S8–S14: 1D, 2D NMR and HRESIMS spectra of versixanthone N (2), Figures S15–S21: 1D, 2D NMR and HRESIMS spectra of versixanthone O (3).
Author Contributions
The contributions of the respective authors are as follows: G.Y. drafted the work and performed the fermentation, extraction, as well as the isolation. G.Y. and G.W. elucidated the constituents. Z.S. and X.Z. were involved in the biological evaluations. Q.C., Q.G., T.Z. and D.L. contributed to checking and confirming all the procedures of the isolation and identification. G.Z. designed the study, supervised the laboratory work, and contributed to the critical reading of the manuscript, and was also involved in structural determination and bioactivity elucidation. All the authors have read the final manuscript and approved the submission.
Funding
This work was financially supported by NSFC-Shandong Joint Fund for Marine Science Research Centers (U1606403), the Qingdao National Laboratory for Marine Science and Technology (2015ASKJ02 and 2016ASKJ08-02), Shandong province key research and development program (2016GSF201204), Fundamental Research Funds for the Central Universities (201562016), and the Scientific and Technological Innovation Project for Marine Science and Technology supported by Qingdao National Laboratory (2016ASKJ08-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Masters K.S., Brase S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012;112:3717–3776. doi: 10.1021/cr100446h. [DOI] [PubMed] [Google Scholar]
- 2.Wu G., Qi X., Mo X., Yu G., Wang Q., Zhu T., Gu Q., Liu M., Li J., Li D. Structure-based discovery of cytotoxic dimeric tetrahydroxanthones as potential topoisomerase I inhibitors from a marine-derived fungus. Eur. J. Med. Chem. 2018;148:268–278. doi: 10.1016/j.ejmech.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Isaka M., Palasarn S., Kocharin K., Saenboonreung J. A Cytotoxic Xanthone Dimer from the Entomopathogenic Fungus Aschersonia sp. BCC 8401. J. Nat. Prod. 2005;68:945–946. doi: 10.1021/np058028h. [DOI] [PubMed] [Google Scholar]
- 4.Chutrakul C., Boonruangprapal T., Suvannakad R., Isaka M., Sirithunya P., Toojinda T., Kirtikara K. Ascherxanthone B from Aschersonia luteola, a new antifungal compound active against rice blast pathogen Magnaporthe grisea. J. Appl. Microbiol. 2009;107:1624–1631. doi: 10.1111/j.1365-2672.2009.04349.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki H., Ukai K., Namikoshi M. Asperdichrome, an unusual dimer of tetrahydroxanthone through an ether bond, with protein tyrosine phosphatase 1B inhibitory activity, from the Okinawan freshwater Aspergillus sp. TPU1343. Tetrahedron Lett. 2016;57:732–735. doi: 10.1016/j.tetlet.2015.12.111. [DOI] [Google Scholar]
- 6.Wu G., Yu G., Kurtán T., Mándi A., Peng J., Mo X., Liu M., Li H., Sun X., Li J., et al. Versixanthones A–F, Cytotoxic Xanthone-Chromanone Dimers from the Marine-Derived Fungus Aspergillus versicolor HDN1009. J. Nat. Prod. 2015;78:2691–2698. doi: 10.1021/acs.jnatprod.5b00636. [DOI] [PubMed] [Google Scholar]
- 7.Li T., Yang M., Wang Y., Wang X., Luo J., Luo J., Kong L. Unusual dimeric tetrahydroxanthone derivatives from Aspergillus lentulus and the determination of their axial chiralities. Sci. Rep. 2016;6:38958. doi: 10.1038/srep38958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang W., Wang J., Wang J., Shi L., Li K., Lin X., Min Y., Yang B., Tang L., Liu Y., et al. Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018;81:1405–1410. doi: 10.1021/acs.jnatprod.8b00015. [DOI] [PubMed] [Google Scholar]
- 9.Lenta B.N., Ngatchou J., Frese M., Ladoh-Yemeda F., Voundi S., Nardella F., Michalek C., Wibberg D., Ngouela S., Tsamo E., et al. Purpureone, an antileishmanial ergochrome from the endophytic fungus Purpureocillium lilacinum. Z. Naturforsch. B J. Chem. Sci. 2016;71:1159–1167. doi: 10.1515/znb-2016-0128. [DOI] [Google Scholar]
- 10.Yu G., Wang S., Wang L., Che Q., Zhu T., Zhang G., Gu Q., Guo P., Li D. Lipid-Lowering Polyketides from the Fungus Penicillium steckii HDN13-279. Mar. Drugs. 2018;16:25. doi: 10.3390/md16010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du L., Zhu T.J., Liu H.B., Fang Y.C., Zhu W.M., Gu Q.Q. Cytotoxic polyketides from a marine-derived fungus, Aspergillus glaucus. J. Nat. Prod. 2008;71:1837–1842. doi: 10.1021/np800303t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.