Abstract
Obesity results from the body having either high energy intake or low energy expenditure. Based on this energy equation, scientists have focused on increasing energy expenditure to prevent abnormal fat accumulation. Activating the human thermogenic system that regulates body temperature, particularly non-shivering thermogenesis in either brown or white adipose tissue, has been suggested as a promising solution to increase energy expenditure. Together with the increasing interest in understanding the mechanism by which plant-derived dietary compounds prevent obesity, flavonoids were recently shown to have the potential to regulate non-shivering thermogenesis. In this article, we review the latest research on flavonoid derivatives that increase energy expenditure through non-shivering thermogenesis.
Keywords: non-shivering thermogenesis, brown adipose tissue, beige adipocytes, obesity, flavonoids
1. Introduction
Obesity is a growing health problem worldwide [1]. Excessive body fat, which is referred to as obesity, impairs normal metabolic regulation, which increases risk factors for the development of other metabolic disorders, such as type 2 diabetes, insulin resistance, atherosclerosis, and cardiovascular diseases [1]. Given its seriousness as a health issue, various strategies have attempted to prevent and reduce obesity such as diet programs, surgery, and medications. However, these current strategies have limitations such as side effects, requirement of long-term efforts, and appropriateness for certain age ranges. Consequently, there remains a need to discover new methods to control obesity. One new way to regulate fat accumulation is to activate the non-shivering thermogenesis function of brown adipose tissue (BAT). Non-shivering thermogenesis is a cold-induced heat generating process that was initially found in newborn infants and hibernating animals against hypothermia [2,3]. Due to the re-discovery of metabolically active BAT in adult humans, which burns stored fat and diet-driven circulating fat, BAT has been re-evaluated as a potential tissue for the prevention and improvement of metabolic diseases, such as obesity, diabetes, and cardiovascular diseases [4,5,6,7].
Brown adipocytes are derived from the same precursor cells, which express myogenic factor 5 (Myf5), as muscle cells, which are interchangeable with skeletal myoblasts via positive regulatory domain containing 16 (PRDM16), a transcriptional factor [8,9]. However, the mitochondria of brown adipocytes are distinct from those of other oxidative tissues; for example, they differ from those of muscle tissues due to their expression of uncoupling protein 1 (UCP1), a BAT-specific protein in the mitochondrial inner-membrane. Mitochondria generally produce ATP by ATP synthase, which phosphorylates ADP when electron transport generates a proton gradient across the inner-membrane of mitochondria. UCP1 allows the mitochondria to dissipate heat by diminishing the proton gradient, which uncouples substrate oxidation in oxidative phosphorylation [8,9]. Unlike UCP1, which is dominantly expressed in BAT, its homologues, UCP2 and UCP3, are also expressed in the stomach, spleen, pancreas, and lung and in skeletal muscle, respectively. The physiological functions of UCP2 and UCP3 include reducing production of reactive oxygen species and decreasing insulin secretion in β-cells of the pancreas by increasing proton conductance in the mitochondrial inner-membrane [10]. Although UCP2 and UCP3 expression are increased by fatty acids, which indicates a possible relationship with energy metabolism, there is no clear understanding of whether UCP2 and UCP3 have thermogenic functions.
The development of brown adipocytes is facilitated by various transcriptional factors and proteins such as bone morphogenetic protein (BMP) 7, PRDM16, peroxisome proliferator-activated receptor gamma (PPARγ) coactivator 1 alpha (PGC1α), cell death-inducing DNA fragmentation factor alpha (DFFA)-like effector a (CIDEA), and PPARα [11]. With the activation of BMP7, brown adipocytes are developed from multipotent mesenchymal cells and then differentiated into cells that have the identity of brown adipocytes by PRDM16. PRDM16 is induced by PPARγ, a necessary transcriptional factor during white adipogenesis that is activated by CCAAT/enhancer-binding protein beta (C/EBPβ), in cooperation with C/EBPα and PPARα [11,12]. In the differentiation process of brown adipocytes, PGC1α, the thermogenic regulator is increased, which activates UCP1 and mitochondrial biogenesis [11]. PGC1α may be induced by PPARα, a transcriptional factor that regulates lipolysis and fatty acid oxidation [12]. PGC1α was also induced by increased circulating levels of fibroblast growth factor (FGF) 21, which leads to an increase in non-shivering thermogenesis [13]. Along with the differentiation process and substrate oxidation, CIDEA plays a role in developing multilocular lipid droplets in BAT, which are a characteristic of brown adipocytes [14,15,16]. Unlike brown adipocytes in BAT, some Myf5-negative adipocytes in white adipose tissue (WAT) have also shown BAT phenotypes in response to certain stimuli, e.g., cold; these are called beige adipocytes [17]. Similar to its role in BAT, PRDM16 modulates white adipocytes during adipogenesis in the cooperative system with PPARγ, C/EBPβ, and C/EBPα to have the characteristics of brown adipocytes by acquiring BAT-selective proteins, e.g., PRDM16, UCP1, PGC1α, and CIDEA, which allows the cells to display non-shivering thermogenesis activity [17]. PRDM16 is also induced by BMP7 in white progenitor cells [18]. Beyond brown-selective proteins, beige adipocytes show the increased expression of some proteins such as T-box transcription factor 1 (TBX) 1, transmembrane protein (TMEM) 26, Cbp/p300-interacting transactivator (CITED) 1, and CD137/tumor necrosis factor receptor superfamily member 9 (TNFRSF9), which are indicated as beige-selective proteins [19].
When the body is exposed to cold temperature, the hypothalamus in the sympathetic nervous system of the brain releases norepinephrine, which binds the β3-adrenergic receptor (AR) to activate cyclic AMP (cAMP). This subsequently activates protein kinase C (PKC) and PKA by phosphorylation, which increases the phosphorylation of hormone sensitive lipase (HSL) that breaks down triacylglycerols (TAGs) into glycerol and free fatty acids [20]. The free fatty acids directly activate UCP1 expression or substrate oxidation to increase the level of nicotinamide adenine dinucleotide (NADH), which eventually produce heat by UCP1-mediated uncoupled oxidative phosphorylation, called non-shivering thermogenesis [20]. Because the non-shivering thermogenesis pathway is tightly correlated with fatty acid utilization, it is also regulated by other hormones and regulators that play roles in energy metabolism, such as thyroid hormone and AMP-activated protein kinase (AMPK). Thyroid hormone plays a role in the regulation of energy metabolism, which showed a possible correlation with the regulation of non-shivering thermogenesis on BAT and the browning of WAT [21]. Thyroid hormone increases UCP1 by regulating thyroid hormone receptor or deiodinase 2 (DIO2) [22]. Hyperthyroid mice showed increased BAT activity, but this change was not found in hypothyroid mice. WAT browning was shown in both hyper- and hypothyroid mice [21]. AMPK is the main regulator in energy balance, which is activated by low energy levels (high AMP/ATP ratio) [23]. Activated AMPK, particularly by phosphorylation, inhibits acetyl-coenzyme A carboxylase (ACC) by phosphorylation, which prevents fatty acid synthesis and increases fatty acid oxidation and mitochondrial biogenesis, respectively [23]. Silent mating type information regulation 2 homolog (SIRT) 1, which is activated by a high NAD+/NADH ratio activates PGC1α by deacetylation, which continually activates UCP1-mediated non-shivering thermogenesis [23]. Non-shivering thermogenesis is positively associated with lipolysis and fatty acid oxidation, which switches the body’s metabolism to efficiently burn fat as heat [3]. Therefore, pharmaceutical studies have focused on developing drugs to activate non-shivering thermogenesis in BAT and/or WAT that improve metabolic disorders. Although cold and exercise are well-known stimuli that increase non-shivering thermogenesis, phytochemicals, such as resveratrol and curcumin have shown the potential to activate BAT and to exert a browning effect in WAT [24,25,26].
Of the known dietary compounds, flavonoids are the most popular phytochemicals found abundantly in fruits and vegetables. The general structure of flavonoids is C6-C3-C6, which consists of two phenyl rings (A and B) and one heterocyclic ring (C) (Figure 1) [27]. Based on their substitution pattern, flavonoids are classified into six categories: flavones (e.g., luteolin and apigenin), flavonols (e.g., quercetin and kaempferol), flavanones (e.g., naringenin and hesperidin), flavanols (e.g., catechin and epicatechin), anthocyanins (e.g., cyanidin and delphinidin), and isoflavones (e.g., genistein and daidzein) [27]. Although each individual flavonoid has specific health benefits; many phytochemicals that are classified as flavonoids exert an anti-obesity effect by regulating the oxidation, synthesis, uptake, and transport of fatty acids [27]. Individuals with a higher flavonoid intake show lower obesity indicators, e.g., a lower body mass index, and decreased levels of C-reactive protein, a marker of inflammation [28]. Because no previous review article specifically focused on the effects of flavonoids on BAT activation and adipocyte browning, this article reviews all subgroups of flavonoids that have shown the potential to increase energy expenditure by increasing non-shivering thermogenesis.
Figure 1.
Structure of Flavonoids.
2. Effects of Flavonoids on Non-Shivering Thermogenesis
2.1. Flavones
2.1.1. Primary Food Sources and Daily Intake
The structure of the flavone backbone is a 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one). Flavones are classified into various subgroups based on the side chains attached to the backbone molecules such as hydroxylation, methoxylation, isoprenylation, and glycosylation [29]. Flavones are commonly consumed from vegetables, fruits, and herbs, such as parsley, celery, chamomile, and marjoram, and from olives and extra virgin olive oil. Major flavones include apigenin, luteolin, tangeretin, eupatilin, jaceosidin, baicalein, and wogonin [30]. The daily flavone intake of US adults is 1.6 ± 0.2 mg/day based on the National Health and Nutrition Examination Survey (NHANES) 1999–2002 [31].
2.1.2. Effects of Flavones on Non-Shivering Thermogenesis
Luteolin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-chromenone) is found in carrots, peppers, celery, olive oil, peppermint, thyme, rosemary, and oregano [32]. A supplementation of 0.01% luteolin increased the expression of the Ucp1, Ppgc1α, Pparα, Cidea, and Sirt1 genes, which are involved in the regulation of non-shivering thermogenesis in the BAT of high-fat diet (HFD)-fed mice (45% calories from fat, 12 weeks), as was further evidenced by denser multilocular and smaller lipid droplets [33]. However, the expression of Prdm16 and the elongation of very-long chain fatty acids-like 3 (Elovl3) genes was not changed [33]. Together with markers for beige adipocytes, Tmem26, Cd137, and Cited1 genes were also induced in subcutaneous WAT (SWAT). Consistent with the increased expression of genes related to non-shivering thermogenesis in SWAT, luteolin supplementation reduced fat mass and body weight through an increase in energy metabolism, as confirmed by improvements in oxygen consumption, carbon dioxide production, and respiratory exchange rate, compared to HFD-fed mice [33]. Luteolin caused AMPK phosphorylation, which increased the activity of SIRT1 to enhance the protein expression and activity of PGC1α and increased the phosphorylation of ACC that turns on energy metabolism toward AMPK-activated energy expenditure. Because SIRT1 and PGC1α are essential regulators for mitochondrial biogenesis and function, luteolin thus increased non-shivering thermogenesis via the AMPK/SIRT1/PGC1α pathway [33,34,35].
Chrysin (5,7-dihydroxyflavone), which is present in high amounts in honey, propolis, and honeycomb, showed antidiabetic, anti-inflammatory, and anticancer effects [36,37,38]. Chrysin (50 µM) exhibited promising effects on non-shivering thermogenesis by inducing 3T3-L1 cells to exhibit the phenotype of brown adipocytes [39]. Although 3T3-L1 cells are fully committed to undergoing differentiation toward the white adipocyte phenotype, 3T3-L1 preadipocytes can also be induced to differentiate into beige-like adipocytes through prolonged treatment with beige induction cocktails (dexamethasone, 3-isobutyl-1-methylxanthine, insulin, triiodothyronine, and rosiglitazone), and partly through the manipulation of C/EBPβ [40,41]. As part of the beige cocktail treatment, chrysin induced the expression of beige specific genes such as Tbx1, Tmem26, and Cited1, and genes that are involved in the regulation of brown adipogenesis such as Ucp1 and Prdm16, Pgc1α, C/ebpβ, and Fgf 21, and the Cidea gene, which is related to the development of lipid droplets [39]. Together with the browning of 3T3-L1 adipocytes, chrysin also increased the expression of genes and proteins related to lipolysis, namely, HSL, perilipin (PLIN), acyl-coenzyme A oxidase (ACO), and carnitine palmitoyltransferase 1 alpha (CPT1α). The cells that were treated with the AMPK inhibitor, compound C, or the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide showed decreased and increased PRDM16, UCP1, and PGC1α protein levels, respectively, which indicated AMPK-mediated regulation of chrysin [39].
Olive (Olea europaea) leaf extract (OLE) contains flavones, such as luteolin and apigenin [42]. HFD-fed mice (40% calories from fat) supplemented with 0.15% OLE for 8 weeks showed the induced expression of genes involved in mitochondrial biogenesis, such as mitochondrial transcription factor A (Tfam), nuclear respiratory factor (Nrf)1, and cytochrome C oxidase, and in non-shivering thermogenesis, such as Sirt1, Pgc1α, and Ucp1 in epididymal WAT (EWAT), indicating the browning effect [43]. OLE also decreased food intake and the expression of Pparγ, C/ebpα, Cd36, and fatty acid synthase (Fas) genes that are involved in the regulation of adipogenesis and fatty acid synthesis. These changes caused a reduction in body weight, visceral fat pad (epididymal, perirenal, retroperitoneal, and mesenteric) weight, and plasma concentrations of triglyceride, total cholesterol, very-low density lipoproteins (VLDL), LDL, and free fatty acids [43]. Interestingly, the decreased white adipogenesis was associated with two mechanisms that are involved in the development of white adipocytes, the wingless type (WNT) and the galanin-mediated signaling pathway. OLE activated the WNT signaling pathway via increasing the WNT MMTV integration site family, member 10b (Wnt10b) and low-density lipoprotein receptor-related protein-5 genes and decreasing secreted frizzled-related protein 5. However, Lo et al. indicated that WNT inhibition using the WNT inhibitor C59 caused white adipocytes browning [44]. Genes that were involved in the galanin-mediated signaling pathway, such as galanin, galanin receptor 1 and 2, PKCδ, and cyclin D, were downregulated together with decreased phosphorylation of extracellular signal-regulated kinase, which further caused the reduced expression of adipogenic and lipogenic genes, such as Pparγ, C/ebpα, Cd36, and Fas [43].
Intestinal bacteria are critical for regulating the bioavailability of flavonoids, which would further affect the biological effect of flavonoids in the body [45]. Germ-free or antibiotic treatments might promote the generation of a population of bacteria that can degrade flavonoids, which would affect flavonoid-induced non-shivering thermogenesis [46,47,48]. Supplementation with a mixture of apigenin (4′,5,7-trihydroxyflavone) and naringenin (4′,5,7-trihydroxyflavanone) (80 mg/kg), which is a flavanone that is predominantly found in grapefruit, increased Ucp1 gene expression in the BAT of mice administered antibiotics during weight-cycling intervention [46]. As demonstrated through an indirect calorimetry challenge, these mice also showed a higher energy expenditure than mice that were not treated with antibiotics during this intervention and mice fed an HFD during the entire experimental period [46].
Sudachitin (5,7,4′-trihydroxy-6,8,3′-trimethoxyflavone) is a polymethoxylated flavone that is found in the Japanese citrus fruit sudachi [49]. Mice fed a HFD (40% of calories from fat) that received a daily oral administration of 5 mg/kg sudachitin for 12 weeks displayed lower body weight, improved glucose tolerance, and better insulin sensitivity compared with control mice [50]. The effects of sudachitin were also observed in ob/ob mice, but these mice did not exhibit a change in body weight. The administration of sudachitin increased energy expenditure in both mice, but this increase was not correlated with non-shivering thermogenesis, a function of BAT, as evidenced by a lack of change in UCP1 expression in BAT. Although Ucp1 gene expression was increased in SWAT, the sudachitin-induced increase in energy expenditure was supported by increased mitochondrial function in muscle, which showed upregulated expression of the Sirt1, Pgc1α, Nrf1, Nrf2, Tfam, Pparα, Ucp2, and Ucp3 genes [50], Table 1.
Table 1.
Flavonoid intake of U.S. adults aged 19+ y based on the NHANES 1999–2002 database.
| Groups | Dietary Flavonoid Intakes (mean ± SD) a |
|---|---|
| Flavone | 1.6 ± 0.2 mg/day |
| Flavonol | 12.9 ± 0.4 mg/day |
| Flavanol | 156.5 ± 11.3 mg/day |
| Flavanone | 14.4 ± 0.6 mg/day |
| Anthocyanin | 3.1 ± 0.5 mg/day |
| Isoflavone | 1.2 ± 0.2 mg/day |
a Dietary flavonoid intakes of US adults (19+ years) were estimated based on the 24-hour-dietary recall of National Health and Nutrition Examination Survey 1999–2002 (n = 8809).
2.2. Flavonols
2.2.1. Major Food Sources and Daily Intake
Flavonols are a group of flavonoids with a 3-hydroxy-2-phenylchromen-4-one backbone. Naturally occurring flavonols include quercetin (3,5,7,3′,4′-pentahydroxyflavone), myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone), kaempferol (3,5,7,4′-tetrahydroxyflavone), isorhamnetin (3,5,7,4′-tetrahydroxy-3′-methoxyflavone), and rutin (quercetin-3-O-rutinoside). The major food sources of flavonols are kale, onions, apples, teas, buckwheat, and broccoli [51]. The daily flavonol intake of US adults was 12.9 ± 0.4 mg/day based on the NHANES 1999–2002 [31].
2.2.2. Effect of Flavonols on Non-Shivering Thermogenesis
Onion peel, a byproduct of onion, contains high concentrations of quercetin, which is the most abundantly consumed flavonol. Onion peel extract (OPE) and quercetin exerted anti-obesity effects by inhibiting adipogenesis and lipogenesis [52,53]. In an in vitro study using 3T3-L1 cells, OPE (75 µg/mL and 100 µg/mL) reduced the expression of the adipocyte protein 2 (Ap2) and Acc genes and, interestingly, increased the expression of the Cpt1α gene, which encodes a rate-limiting enzyme in the beta-oxidation of fatty acids [52]. Consistent with this finding, Sprague-Dawley rats fed an HFD (40% calories from fat) supplemented with 0.72% OPE showed decreased Pparγ, C/ebpα, Fas, and Acc gene expression and increased expression of the Cpt1α and Ucp1 genes in EWAT [52]. However, 0.5% OPE significantly induced Ucp1, Prdm16, Pparγ, and Cidea expression in retroperitoneal WAT (RWAT) and did not upregulate these genes in the EWAT and SWAT of mice fed an HFD (60% calories from fat) [54]. This browning effect was in part mediated by the AMPK/SIRT1/PGC1α signaling pathway [54]. Notably, the AMPK/SIRT1/PGC1α signaling pathway was also critical for the quercetin-regulated anti-inflammatory effect [53]. Consistent with the activation of fat utilization in WAT [54], mice fed a HFD (45% calories from fat) supplemented with 0.1% quercetin showed improvements in the physiological function of WAT [53]. These changes were mediated by the amelioration of macrophage infiltration and inflammatory cytokine markers, and increased Ucp1 gene expression in BAT, which suggested increased energy expenditure [53]. Although energy expenditure was not clearly increased by quercetin, this flavonol increased the physical activity of mice by increasing the mitochondrial number in skeletal muscle and increasing the expression of genes encoding polypeptides for subunits of the mitochondrial complexes that possibly have a higher oxidative capacity [55,56]. Interestingly, isoquercetin (quercetin-3-glucoside), which is also found in onion peel, was not found to exert the adipocyte browning effect in an in vitro study [54].
Rutin (quercetin-3-rutinoside) (1 mg/mL), the other glycoside of quercetin, increased energy expenditure by inducing BAT activity and BAT- and beige adipocyte-specific genes in the BAT and WAT of both ob/ob and C57Bl/6J mice with HFD (60% calories from fat)-induced obesity [57]. To promote non-shivering thermogenesis in BAT and WAT, the expression of genes involved in the regulation of lipolysis that provides free fatty acids were also increased. Mitochondrial biogenesis, as evidenced by increased Tfam, Nrf1, and Nrf2 gene expression, was further enhanced by rutin. PGC1α is a well-known transcriptional factor that is required for the regulation of non-shivering thermogenesis [58]. PGC1α-expressing mesenchymal stem cells showed significantly increased mitochondrial biogenesis and respiration [59]. Consistent with this finding, rutin-induced non-shivering thermogenesis was attributed to SIRT1 stabilization-activated PGC1α deacetylation [57]. Based on the beneficial effect of rutin to activate thermogenesis through BAT, the therapeutic potential of rutin was studied in dehydroepiandrosterone (DHEA)-induced polycystic ovary syndrome (PCOS) rats [60]. DHEA-induced PCOS rats showed decreased expression of BAT-specific genes and genes that are involved in the regulation of lipid metabolism, such as Ucp1 and Pparα, medium-chain acyl-coenzyme A (Mcad), Pgc1α, Pgc1β, and Cpt1α genes and reduced expression of the UCP1 protein and other proteins that are involved in the regulation of oxidative phosphorylation such as ATP synthase F1 subunit alpha (ATP5α), ubiquinol-cytochrome c reductase core protein 2 (UQCRC2), and succinate dehydrogenase complex iron sulfur subunit B (SDHB), which decreased heat generation in BAT. This molecular and physiological reduction was reversed by rutin treatment to the normal level of a control group of rats that were not treated with DHEA. Consistent with increased BAT function, DHEA-induced PCOS rats supplemented with rutin showed increased insulin sensitivity, a critical factor to improve the POCS condition compared to the DHEA-induced PCOS rats [60]. Therefore, rutin may rescue PCOS infertility by increasing energy expenditure through BAT and further enhancing insulin sensitivity [60].
Other flavonols, namely, myricetin and kaempferol, also showed the potential to increase energy expenditure [61,62]. Myricetin supplementation (400 mg/kg) increased BAT activity and the expression of Ucp1, Sirt1, Atp5α, and Pgc1α genes in BAT and caused browning effect on inguinal WAT (IWAT) by increasing the expression of the Tbx1, Cd137, and Tmem26 genes in db/db mice [61]. Together with increased mitochondrial DNA copy number, it indicated that myricetin-induced non-shivering thermogenesis may result from increased mitochondrial biogenesis through SIRT1-induced deacetylation and PGC1α activity. Increased energy expenditure via myricetin reduced the body weight and fat mass of db/db mice and improved the plasma lipid profile and other obesity-related metabolic alterations, such as glucose tolerance, insulin resistance, and hepatic steatosis [61]. Kaempferol increased the expression of the Pgc1α, Cpt1α, Tfam, citrate synthase, and Ucp3 genes in human skeletal muscle myocytes in vitro and increased DIO2 activity, which regulates the thyroid hormone, a major metabolic hormone, and thereby potentially increased energy expenditure [62].
2.3. Flavanones
2.3.1. Major Food Sources and Daily Intake
The flavanone structure is 2,3-dihydro-2-phenylchromen-4-one [63]. Naturally occurring flavanones include naringenin (5,7,4′-trihydroxyflavanone), hesperetin (5,7,3′-trihydroxy-4′-methoxyflavanone), eriodictyol (5,7,3′,4′-tetrahydroxyflavanone), narirutin (naringenin-7-O-rutinoside), hesperidin (hesperetin 7-O-rutinoside), and eriocitrin (eriodictyol 7-O-rutinoside) [64]. As a major food source, citrus fruits, such as satsuma mandarin orange (Citrus unshiu Marc.) and valencia orange (Citrus sinensis Valencia), contain narirutin and hesperidin [65]. The daily flavanone intake of US adults is 14.4 ± 0.6 mg/day, as demonstrated by the NHANES 1999–2002 [31].
2.3.2. Effect of Flavanones on Non-Shivering Thermogenesis
Extract from hesperidin-rich Gelidium elegans (20 and 40 µg/mL), a red seaweed, reduced lipid accumulation during 3T3-L1 cell differentiation due to decreased Pparγ and Ap2 expression [66]. Gelidium elegans (5%) caused anti-adipogenesis due to increased lipolysis in the EWAT of streptozotocin-nicotinamide-induced diabetic rats, which suggested the potential of Gelidium elegans to increase energy expenditure [67]. Consistent with accelerated lipolysis [67], the extract increased PRDM16 and UCP1 protein expression in the BAT of HFD-induced obese mice by activating the AMPK pathway [68]. Although 3T3-L1 cells treated for 8 days with 20 µM hesperidin, a major dietary compound of Gelidium elegans, showed decreased triglyceride synthesis and increased expression of the adipose triglyceride lipase gene, Gelidium elegans extract showed stronger effects on the induction of Ucp1 and Prdm16 expression [69]. The oral administration of 4G-α-glucopyranosyl hesperidin, a water-soluble form of hesperidin, also increased body temperature by activating BAT [70].
2.4. Flavanols
2.4.1. Major Food Sources and Daily Intake
The backbone structure of flavanols is 2-phenyl-3,4-dihydro-2H-chromen-3-ol, which is referred to as flavan-3-ol [71]. Interestingly, the major intake of flavonoids in the US originates from the intake of flavanols [72]. A study using data from the NHANES 1999–2002 indicated that the average daily intake of flavan-3-ols is 156.5 ± 11.3 mg/day, which accounts for 82% of the total flavonoid intake [31]. Although flavanols are commonly found in tea, wine, apples, and chocolate, black tea is a major contributor to the consumption of flavan-3-ols in the American diet [72,73]. The monomers catechin, epicatechin, epigallocatechin (EGC), epicatechin gallate (EG), and epigallocatechin gallate (EGCG), and oligomeric proanthocyanidin are classified as flavanols [74].
2.4.2. Effects of Flavanols on Non-Shivering Thermogenesis
Green tea (Camellia sinensis) is recognized as a weight loss booster by increasing energy expenditure and thermogenesis due to the presence of caffeine and catechins [75]. The administration of green tea extract (20 g/kg diet) reversed HFD-reduced energy expenditure of Sprague-Dawley rats with HFD-induced obesity to the same level of energy expenditure as observed in rats fed a normal diet [76]. This effect was inhibited by the β-AR antagonist propranolol, which indicates a critical role of β-AR in green tea-mediated energy expenditure [76]. Nomura et al. reported that 0.5% green tea catechins increased Ucp1 gene expression in the BAT of rats fed a chow diet (5% fat) but not in rats fed an HFD (35% fat) compared with their control groups [77]. Consistent with the studies using rats, mice fed both chow and an HFD supplemented with green tea catechins (100 mg/kg) showed upregulated expression of the Ucp1, Cpt1α, and Aco genes in BAT [76,77,78]. The expression of the Ucp1 gene was also induced in SWAT and visceral WAT in a manner consistent with increased and decreased Pparγ expression, respectively [78]. One of the necessary hormones for activating non-shivering thermogenesis in BAT is norepinephrine, which stimulates G-protein coupled β3-AR [79]. Catechol-P-methyltransferase (COMT) is an enzyme that degrades norepinephrine by catalyzing the addition of a methyl group to norepinephrine to generate normetanephrine [80]. EGCG and EGC acted as substrates for inhibiting COMT activity [81]. This indicated a relationship between EGCG and sympathetic activity. Green tea with high concentrations of EGCG and caffeine or EGCG independently increased the respiration rate of interscapular BAT, whereas caffeine did not affect the respiration rate [75]. This green tea-induced respiration rate was further stimulated by ephedrine, an enhancer of norepinephrine. These effects were not shown in interscapular BAT obtained from chemically sympathectomized rats. Although caffeine independently could not change the respiration rate, EGCG’s effect on the respiration rate was synergistically induced in a dose-dependent manner (50, 100, and 200 µM), together with 100 M caffeine under 0.25 µM ephedrine [75]. This boosting effect on the respiration rate by the combination of EGCG and caffeine may result from the effect of caffeine on the protection of norepinephrine-β-AR-induced cAMP by inhibiting phosphodiesterase, an enzyme that degrades cAMP [75]. Compared to green tea, oolong tea, black tea, and pu-erh tea also showed the potential to increase energy expenditure, possibly by increasing AMPK phosphorylation in the mesenteric WAT, SWAT, and BAT of mice, which would result in the reduction of adiposity [82]. However, UCP1 protein expression was only significantly increased in the WAT of mice that ingested black tea, indicating that the degrees of oxidation and fermentation and the flavanol composition of tea might be critical factors for tea-induced non-shivering thermogenesis [82].
The effect of green tea extract on energy expenditure through non-shivering thermogenesis has been further evidenced in clinical studies [83,84,85]. Healthy young men who were administered green tea extract consisting of 50 mg of caffeine and 90 mg of EGCG showed increased energy expenditure, respiratory quotient, and urinary norepinephrine excretion without any urinary excretion of nitrogen [83]. None of these changes were observed in a group that ingested caffeine alone, which suggested that green tea-induced non-shivering thermogenesis might be mediated by catechins and not by caffeine [83]. In a double-blinded design, healthy young women who drank a catechin-rich beverage (540 mg/day of catechin intake) for 12 weeks exhibited an 18.8% increased BAT density and a 17.4% lower amount of extramyocellular lipids than a placebo group, as detected by near-infrared spectroscopy [84]. When exposed to a cold water (15 °C) environment for 3 h, healthy male subjects who ingested 1600 mg of EGCG and 600 mg of caffeine from green tea showed a 10% higher energy expenditure and a 20% lower shivering intensity than subjects who ingested a placebo [85].
Another flavanol source that exerts a positive effect on non-shivering thermogenesis is chocolate, which is enriched in flavan-3-ols such as (+)-catechin, (−)-epicatechin, and B-type procyanidins [86,87,88]. Mice administered a dose of 10 mg/kg of a flavan-3-ol fraction composed of 4.56% (+)-catechin, 6.43% (−)-epicatechin, 3.93% procyanidin B2, 2.36% procyanidin C1, and 1.45% cinnamtannin A2 showed increased expression of Ucp1 and Pgc1α genes in BAT and Ucp3 and Pgc1α genes in the gastrocnemius, a higher resting energy expenditure, and increased plasma adrenaline concentrations [86]. However, these changes were not observed in mice supplemented with (−)-epicatechin [86]. Pretreatment with butaxamine, a β2-AR blocker, and/or SR59230, a β3-AR blocker, prevented all flavan-3-ol fraction-induced changes, except for the increase in AMPK phosphorylation in the gastrocnemius muscle in the presence of a β3-AR blocker [87]. This indicated that the induction of non-shivering thermogenesis by flavan-3-ol was mediated by the activation of AMPK1α through its phosphorylation.
Rabadan-Chávez et al. showed that cocoa powder (10 mg/mL) and cocoa extract (10 mg/mL) upregulated the expression of Pparα, Pgc1α, Sirt1, and Ucp1 genes, which caused browning effect on the RWAT of rats with HFD-induced obesity, but this upregulation was less effective than that induced individually by the flavanols epicatechin, catechin, and procyanidin B2 [88]. Pparγ and Cd36 genes were upregulated whereas the expression of Acc gene was downregulated by these extracts. [88]. Consistent with these changes, the HFD-induced concentrations of plasma lipids were improved by the cocoa-derived products. Although a mixture of flavan-3-ols, such as cocoa powder or cocoa extract, had a higher impact on the activation of non-shivering thermogenesis, (-)-epicatechin significantly induced adipocyte browning and improved mitochondrial function by increasing the expression of the SIRT1, PGC1α, SIRT3, UCP1, and DIO2 proteins in the EWAT of rats fed an HFD [89].
2.5. Anthocyanins
2.5.1. Major Food Sources and Daily Intake
As a type of flavonoid, anthocyanins are water-soluble pigments that provide blue, purple, and red colors in fruits and vegetables, such as berries, grapes eggplant, and red cabbage [90]. The structure of anthocyanins contains a backbone of 2-phenylchromenylium [91]. These flavonoids are generated by the addition of various sugar molecules to the structure of the major anthocyanidins, namely, peonidin pelargonidin, malvidin, cyanidin, petunidin, and delphinidin [92]. Given their various health benefits, such as antioxidant, anti-inflammation, anti-diabetes, anti-cancer, and anti-cardiovascular disease properties, the consumption of foods containing anthocyanins is highly recommended [90]. The daily intake of anthocyanins in the US, as demonstrated by the NHANES 2001–2002, is 3.1 ± 0.5 mg/day [31].
2.5.2. Effect of Anthocyanins on Non-Shivering Thermogenesis
Cyanidin-3-glucoside, which is also called chrysanthemin, is the most abundantly ingested anthocyanin [93]. When C3H10T1/2 mesenchymal stem cells were treated with mulberry extract (ME) and mulberry wine extract (MWE) (10 µg/mL), which contained approximately 69.93 ± 1.31 and 8.50 ± 0.12 mg/g dry weight of cyanidin-3-glucoside, respectively, the expressions of the Ucp1, Pgc1α, Prdm16, and Cpt1α, genes were increased without changes in genes that are involved in the regulation of adipogenesis, e.g., Ap2, C/ebpα, C/ebpβ, and Pparγ2 during brown adipogenesis [94]. Consistent with increased expression of the Tfam and Nrf-1 genes, both ME and MWE increased the mitochondrial number and the expression of mitochondrial-specific proteins, e.g., UQCRC2 and 246 NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 8 (NDUFB8) in C3H10T1/2 cells. The cells treated with ME, but, not MWE showed increased UCP1 protein expression. The β3-AR agonist (CL316,2432) stimulated the phosphorylation of p38 mitogen-activated protein kinase (MAPK) by protein kinase A in both white and brown adipocytes, which led to an increase in non-shivering thermogenesis and mitochondria biogenesis [95]. Thus, ME-induced UCP1 expression may result from the upregulated phosphorylation of p38 mitogen-activated protein kinases, which might be critical modulators of brown adipocyte development [94]. Takikawa et al. reported that KK-Ay mice supplemented with bilberry extract (27 g/kg diet, the extract contains 375 g of anthocyanins/kg) significantly activated AMPK in WAT and skeletal muscle [96].
Consistent with the results of studies performed with whole extracts of anthocyanin-rich-foods, the treatment of 3T3-L1 cells with 50 µM or 100 µM cyanidin-3-glucoside also converted 3T3-L1 preadipocytes to beige adipocytes during differentiation [97]. A cyanidin-3-glucoside-induced browning effect was possibly due to both reduced AMP/ATP level-induced AMPK activation and enhanced brown adipogenesis by cAMP-mediated C/EBPβ, a transcriptional factor that is involved in the adipogenesis induced PGC1α-UCP1 pathway [97]. The db/db mice that were administered drinking water containing cyanidin-3-glucoside (1 mg/mL) for 16 weeks showed beige cell formation in SWAT and increased BAT activity [98]. These mice showed weight loss and decreased SWAT and EWAT weights, compared to that in a control group that was administered drinking water without the addition of cyanidin-3-glucoside [98].
2.6. Isoflavones
2.6.1. Major Food Sources and Daily Intake
Isoflavones are known as phytoestrogens, because they mimic human estrogen activity [99], and their structures have a 3-phenylchromen-4-one backbone [100]. As primary food sources of isoflavones, soy, soy products, red clover, and kudzu have high amount of isoflavones, such as daidzein, genistein, glycitein, biochanin A, and formononetin [101]. The isoflavone intake in the US is estimated as 1.2 ± 0.2 mg/day based on the NHANES 1999–2002 [31].
2.6.2. Effect of Isoflavones on Non-Shivering Thermogenesis
Estrogen is a hormone that plays a role in developing female characteristics, but it is also involved in the regulation of energy balance. Postmenopausal women and ovariectomized mice showed decreased levels of 17β-estradiol (E2), a major estrogen that is associated with weight gain and increased fat content in the body [102]. Estradiol may increase energy metabolism by increasing BAT-mediated non-shivering thermogenesis through the inhibition of AMPK in the sympathetic nervous system [103]. Although various studies have focused on the phytoestrogenic function of isoflavones, their potential to increase non-shivering thermogenesis was recently discovered [104,105,106,107,108]. The isoflavone-rich fraction of the Kudzu (Puerariae thomsonii) flower exerted anti-obesity effects by increasing lipolysis in WAT and Ucp1 expression in the BAT of mice with HFD-induced obesity [104]. Ucp1 mRNA expression was also significantly increased in the BAT of male Long-Evans rats administered a soy-based isoflavone-rich diet (600 µg/g of diet) compared to that in rats fed a low amount of isoflavones (10–15 µg/g of diet) [105].
Consistent with data from studies using isoflavone-rich food extracts, isoflavones also showed the potential to induce non-shivering thermogenesis in animal and white adipocytes [104]. Daidzein supplementation (50 mg/kg) for 14 days reduced body weight by ameliorating hepatic lipid accumulation, and this reduction was achieved by decreasing stearoyl coenzyme A desaturase 1, which is involved in the regulation of lipogenesis [106]. UCP1 protein expression in BAT was also increased [106], which suggested daidzein-induced non-shivering thermogenesis. In a study using 3T3-L1 cells, treatment with genistein (50 or 100 µM) induced a switch in the phenotypes of white adipocytes toward BAT-like adipocytes during adipogenesis [107]. These adipocytes showed the decreased expression of lipogenic and adipogenic genes and increased the expression of the Ucp1 gene and multilocular lipid droplets [107]. Accompanying this change, oxygen consumption was enhanced at both the basal and the maximal respiratory capacity. Using SIRT1 or an estrogen receptor inhibitor, Aziz et al. found that the genistein-induced browning effect was due to SIRT1 and not to an estrogen receptor-regulated pathway [107]. Formononetin (10 nM) significantly inhibited mRNA and protein expression of transcription factors that regulate adipogenesis, including PPARγ, C/EBPα, and sterol regulatory element-binding protein 1 [108]. An in vivo study using mice with HFD-induced obesity also supported the anti-adipogenesis and browning effects of formononetin [108]. PPARγ and C/EBPα protein expression was significantly reduced, whereas the expression of the UCP1, ELOVL3, and DIO2 proteins was induced in the WAT of mice supplemented with formononetin by activating the AMPK/β-catenin signaling pathway [108], Table 2 and Table 3.
Table 2.
In vitro evidence for increase of non-shivering thermogenesis by flavonoids.
| Flavonoids | Subjects | Treatments | Outcomes | Authors | |
|---|---|---|---|---|---|
| Flavone | Luteolin | Primary adipocytes from BAT and SWAT |
100 nM | ↑ Ucp1, Pgc1α and Sirt1 in BAT and SWAT ↑ AMPK phosphorylation in BAT and SWAT |
Zhang et al. [33] |
| Chrysin | 3T3-L1 | 50 µM | ↑ UCP1, Ucp1, PGC1α, Pgc1α PRDM16, Prdm16, FGF21, Fgf21, TBX1, Tbx1, TMEM26, Tmem26, CIDEA, Cidea and CITED 1, Cited1 ↑ AMPK phosphorylation |
Choi et al. [39] | |
| Sudachitin | Primary myoblasts | 30 mM | ↑ Sirt1, Pgc1α, and Ucp1 | Tsutsumi et al. [50] | |
| Flavonol | Onion peel (quercetin) | 3T3-L1 | 25-100 µg/mL | ↑ Cpt1α, Fabp4 | Moon et al. [52] |
| Quercetin | 3T3-L1 | 25–100 µM | ↑ Ucp1, Cpt1α, Tbx1, Pgc1α, Pparγ, and Prdm16 | Lee et al. [54] | |
| Rutin | C3H10T1/2 cells | 0.1–100 µM | ↑ UCP1, Ucp1, Prdm16, Pgc1α, Tfam, Nrf1, and Nrf2 ↑ PGC1α deacetylation by stabilizing SIRT1 |
Yuan et al. [57] | |
| Myricetin | C3H10T1/2 cells | 0.001–10 µM | ↑ Ucp1, UCP1, PGC1α, SIRT1, and adiponectin | Hu et al. [61] | |
| Flavanone | Gelidium elegans (Hesperidin) | 3T3-L1 | 12.5 and 50 µg/mL | ↑ UCP1 and PRDM16 | Choi et al. [69] |
| Anthocyanin | Mulberry extract (ME), mulberry wine extract (MWE) and cyanidin-3-glucoside (C3G) | C3H10T1/2 cells | ME and MWE (10 µg/mL) of C3G (1–100 µM) |
↑ UCP1, Ucp1, Pgc1α, Cpt1α, and Prdm16 by ME and MWE ↑ Phosphorylation of p38 MAPK by ME ↑ UCP1, Ucp1, Pgc1α, Pgc1β, Prdm16, Nrf1, mitochondrial copy number, and cellular oxygen respiration by C3G |
You et al. [94] |
| C3G | 3T3-L1 | 50 or 100 µM | ↑ Cellular cAMP concentration ↑ AMPK phosphorylation ↑ FABP4, UCP1, PGC1α expression ↑ Mitochondrial biogenesis ↑ C/ebpβ, Tbx1 and Cited 1 |
Matsukawa et al. [97] | |
| Isoflavone | Genistein | 3T3-L1 | 100 µM | ↑ Ucp1, Pgc1α, and Sirt1 ↑ Oxygen consumption |
Aziz et al. [107] |
| Formononetin | 3T3-L1 | 10 nM | ↑ AMPK phosphorylation and β-catenin expression | Gautam et al. [108] | |
AMPK; 5′ AMP-activated protein kinase, BAT; brown adipose tissue, C/EBPβ; CCAAT/enhancer-binding protein beta, cAMP; cyclic AMP, CIDEA; cell death-inducing DFFA-like effector a, CITED 1; Cbp/p300-interacting transactivator, CPT1α; carnitine palmitoryl transferese 1 alpha, ELOVL3; elongation of very-long chain fatty acids-like 3, FABP4; fatty acid binding protein 4, FGF21; fibroblast growth factor 21, NRF1 or 2; nuclear respiratory factor 1 or 2, PGC1α; PPARγ coactivator 1 alpha, PRDM16; positive regulatory domain containing 16, PPARα; peroxisome proliferator-activated receptor alpha, PPARγ; peroxisome proliferator-activated receptor gamma, p38 MAPK; p38 mitogen-activated protein kinase, SIRT1; silent mating type information regulation 2 homolog 1, SWAT; subcutaneous white adipose tissue, TBX1; T-box transcription factor, TFAM; mitochondrial transcription factor A, TMEM26; transmembrane protein 26, UCP1; uncoupling protein 1, ↑; an increase in the experimental group compared to the control group.
Table 3.
In vivo evidence for the increase of non-shivering thermogenesis by flavonoids.
| Flavonoids | Subjects | Treatments | Outcomes | Authors | |
|---|---|---|---|---|---|
| Flavone | Luteolin | Male C57BL/6 mice | HFD with 0.01% luteolin for 12 weeks |
↑ O2 consumption and CO2 production ↑ BAT activity ↑ SWAT browning ↑ AMPK/PGC1α signaling |
Zhang et al. [33] |
| Olive leaf extract (luteolin) |
Male C57BL/6N mice | HFD with 0.15% olive leaf extract for 8 weeks |
↓ Body weight and fat pad weight ↑ Browning and mitochondrial biogenesis in EWAT |
Shen et al. [43] | |
| Apigenin mixed with naringenin | Male C57BL/6 mice | Apigenin/naringenin (80 mg/kg) for 2 weeks |
↑ UCP1 in BAT | Thaiss et al. [46] | |
| Sudachitin | C57BL/6 J mice and db/db mice |
HFD with 5 mg/kg sudachitin for 12 weeks |
↓ Body weight, subcutaneous and visceral fat contents ↑ O2 consumption and energy expenditure ↑ Ucp1 in SWAT |
Tsutsumi et al. [50] | |
| Flavonol | Onion peel Extract (quercetin) |
Male SD rats | HFD with 0.36 and 0.72% onion peel extract for 8 weeks |
↓ Body weight and weights of total visceral, retroperitoneal, and mesenteric fat fads ↑ Ucp1 and Cpt1α in EWAT |
Moon et al. [52] |
| Onion peel extract (quercetin) |
Male C57BL/6 mice | HFD with 0.5% onion peel for 8 weeks |
↑ Adipocyte browning in RWAT and SWAT | Lee et al. [54] | |
| Quercetin | Male C57BL/6 mice | HFD with 0.1% Quercetin for 12 weeks |
↓ Body weight and weights of EWAT and SWAT ↑ AMPK phosphorylation, and SIRT1 expression in EWAT ↑ Ucp1 in BAT |
Dong et al. [53] | |
| Rutin | Male C57BL/6 J mice and C57BLKS/J-(db/db) mice | HFD with rutin (1 mg/mL) in drinking water for 10 weeks |
↑ Mitochondria biogenesis and whole-body energy expenditure ↑ BAT activity and SWAT browning |
Yuan et al. [57] | |
| Rutin | Female polycystic ovary syndrome SD-rats | Rutin (100 mg/kg) in drinking water for 3 weeks |
↑ UCP1, Ucp1, Pparα, Pgc1α, Pgc1β, and Cpt1α in BAT, ↑ Body temperature |
Hu et al. [60] | |
| Myricetin | Male C57BLKS/J-(db/db) mice | HFD with myricetin (400 mg/kg) in drinking water for 14 weeks |
↓ Body weight, fat mass, and blood glucose ↑ Body temperature and oxygen consumption ↑ BAT activity ↑ IWAT browning and mitochondrial biogenesis |
Hu et al. [61] | |
|
Gelidium elegans (hesperidin rich) |
Male ICR mice | HFD with Gelidium elegans extract (50, 200 mg/kg/day) for 7 weeks |
↓ Body weight, fat mass, plasma insulin, and TG level ↑ AMPK phosphorylation in BAT and BAT activity |
Choi et al. [68] | |
| G-hesperidin | Male Wistar rats | 60 mg of G-hesperidin by acute oral administration |
↑ BAT-sympathetic nerve activity ↑ Body temperature ↓ Cutaneous sympathetic nerve activity |
Shen et al. [70] | |
| Flavanal | Green tea extract (catechin and EGCG) | Male SD rats | Chow diet with catechin and EGCG (0–200 µM) in drinking water |
↑ BAT activity and O2 uptake rate | Dulloo et al. [75] |
| Green tea (EGCG) |
Male SD rats | HFD with green tea extract (20 g/kg) | ↓ Body weight, digestibility ↑ Energy expenditure ↑ BAT density and β-adrenoceptor activity |
Choo et al. [76] | |
| Tea catechins (TC) | Male SD rats | Low fat diet (LFD) and HFD with 0.5% TC for 5 weeks | ↑ Ucp1 in BAT of LFD with TC group - No significant difference between HF and HF with TC |
Nomura et al. [77] | |
| Green tea catechins | Male SD rats | LFD and HFD with green tea catechins (100 mg/kg) for 5 weeks | ↑ PPARδ, UCP1, Ucp1, and CPT1α in WAT and BAT | Yan et al. [78] | |
| Oolong, black, and pure teas | Male ICR mice | 7 days consumption with tea boiled with 2 g tea leaves in 100 mL |
↓ Weight of WAT, ↑ AMPK phosphorylation in WAT and BAT, ↑ UCP1 in WAT |
Yamashita et al. [82] | |
| Catechin | Healthy young women | 540 mg/day; catechin for 12 weeks | ↑ BAT density | Nirengi et al. [84] | |
| Epigalo catechin gallate (EGCG) | Healthy young men | Cold exposure for 3 h after 1600 mg of EGCG and 600 mg of caffeine intake |
↑ Energy expenditure ↓ Shivering thermogenesis |
Gosselin et al. [85] | |
| Cocoa flavanols | Male ICR mice | 10 mg/kg cocoa flavonoid fraction or epicatechin. |
↑ BAT activity ↑ AMPK phosphorylation in BAT ↑ Plasma catecholamine level |
Matsumura et al. [85] | |
| Cocoa flavanols | Male Wistar rat | HFD with cocoa powder 1 g/kg, cocoa extract 100 mg/kg and (-)-epicatechin 10 mg/kg for 8 weeks |
↑ Ucp1, Pparγ, Pparα, Sirt1, and Pgc1α in BAT ↑ AMPK phosphorylation in BAT ↑ Plasma catecholamine level |
Rabadan-Chávez et al. [88] | |
| Epicatechin | Male Wistar rats | HFD for 5 weeks with (-)-epicatechin (1 mg/kg) for additional 2 weeks |
↑ EWAT browning ↓ Body weight |
Gutiérrez-Salmeán et al. [89] | |
| Anthocyanin | Bilberry Extract | Male KK-Ay mice | 27 g/kg diet for 5weeks | ↑ AMPK in SWAT and skeletal muscle | Takikawa et al. [96] |
| Cyanidin-3-glucoside (C3G) |
Male C57BLKS/J-(db/db) mice | C3G dissolved in drinking water (1 mg/mL) for 16 weeks |
↑ Energy expenditure representing oxygen consumption ↑ BAT activity ↑ Body temperature and mitochondrial biogenesis in BAT ↑ SWAT browning ↓ Body weight gain, and weight of EWAT and SWAT |
You et al. [98] | |
| Isoflavone | Puerariae flower (PFE) and isoflavone fraction (PF) | Male C57BL/6J mice | HFD with 5% PFE and PF isoflavone fraction for 6 weeks | ↑ Energy expenditure representing oxygen consumption ↑ UCP1-positive area in BAT ↓ Body weight gain, and weight of EWAT and SWAT |
Kamiya et al. [104] |
| Isoflavone mixture | Long-Evans male and female rats | 600 µg of phytoestrogens/g of diet | ↑ Core body temperature during light cycle ↑ Ucp1 in BAT |
Lephart et al. [105] | |
| Daidzein | Male Wistar rats | LFD and HFD with 50 mg/kg for 2 weeks |
↑ Ucp1 in BAT in HFD | Crespillo et al. [106] | |
| Formononetin | Male C57BL/6J mice | HFD with 0.1, 1, and 10 mg of formononetin |
↑ SWAT browning ↑ Small and multilocular lipid droplets in SWAT |
Gautam et al. [108] | |
AMPK; 5′ AMP-activated protein kinase, BAT; brown adipose tissue, CPT1α; carnitine palmitoryl transferase 1 alpha, EWAT; epididymal white adipose tissue, HFD; high fat diet, IWAT; Inguinal white adipose tissue, LFD; low fat diet, PPARγ; peroxisome proliferator-activated receptor gamma, PPARα; PPAR alpha, PPARδ; PPAR delta, PGC1α; PPARγ coactivator 1 alpha, PGC1β; PPARγ coactivator 1-beta, RWAT; retroperitoneal white adipose tissue, SD; Sprague Dawley, SIRT1; silent mating type information regulation 2 homolog 1, SWAT; subcutaneous white adipose tissue, TAG; triacylglycerol, UCP1; uncoupling protein 1, ↑; an increase in the experimental group compared to the control group, ↓; a decrease in the experimental group compared to the control group.
3. Conclusions
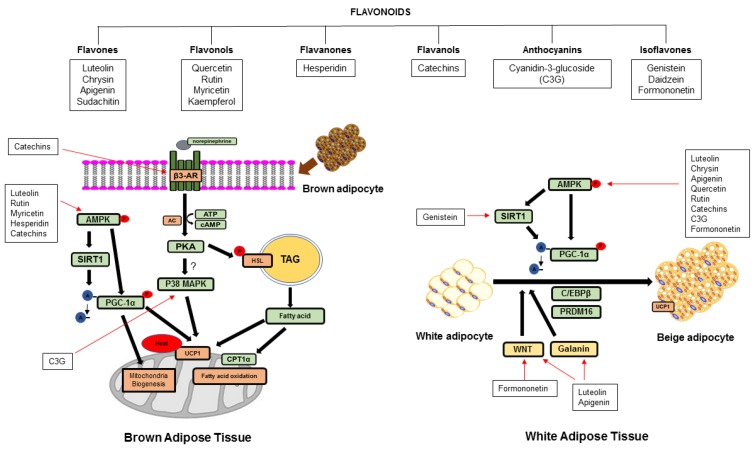
Increasing non-shivering thermogenesis by BAT activation and WAT browning is a potential approach for improving metabolic diseases. As shown in Figure 2, most dietary compounds except sudachitin increased the expression of the UCP1 gene or protein, which suggested increasing non-shivering thermogenesis. Flavonoids may regulate UCP1-mediated non-shivering thermogenesis by (i) the AMPK-activated SIRT1-PGC1α pathway, which possibly induces mitochondrial biogenesis and lipolysis and fatty acid oxidation and (ii) regulating PRDM16 in the complexed regulation with PPARγ, C/EBPα and β, PGC1α, and SIRT1 during differentiation. Fiorani et al. showed that quercetin was taken up and then stored in the mitochondria and was re-distributed into the cytosol to prevent cell damage by reactive oxygen species [109]. Therefore, flavonoids may be taken up into the mitochondria, which directly regulates thermogenic proteins. PGC1α is localized in the cytosol and nucleus depending on its activation, whereas SIRT1 is localized in the cytosol [110,111]. Various flavonoids showed increased PGC1α and deacetylation on PGC1α and SIRT1. Therefore, the stored flavonoid in mitochondria may be re-distributed into other subcellular compartments, e.g., the cytosol, which regulates the deacetylation of PGC1α by SIRT1. Flavonoids may cause SIRT1-activated deacetylated PPARγ to efficiently bind to PRDM16, which promotes brown adipogenesis and the browning effect, which is similar to the action of thiazolidinedione [112]. As uncouplers, flavonoids may regulate non-shivering thermogenesis. 2.4-dinitrophenol (DNP), an uncoupler, increases non-shivering thermogenesis by a proton leakage-diminished proton gradient that causes uncoupling oxidative phosphorylation and prevents the uptake of inorganic phosphate into mitochondria, causing reduced ATP synthesis [113]. However, some flavonoids may not function as uncouplers.
Figure 2.
Suggested mechanistic pathway of different flavonoids in the activation of non-shivering thermogenesis in BAT and WAT. AC; adenylyl cyclase, AMPK; AMP-activated protein kinase, ATP; adenosine triphosphate, β-AR; beta-adrenergic receptor, cAMP; cyclic AMP, C/EBPβ; CCAAT/enhancer-binding protein beta CPT1α; carnitine palmitoyltransferase 1 alpha, HSL; hormone sensitive lipase, PGC1α; peroxisome proliferator-activated receptor gamma coactivator 1 alpha, PKA; protein kinase A, PRDM16; positive regulatory domain containing 16, p38 MAPK; p38 mitogen-activated protein kinase, SIRT1; silent mating type information regulation 2 homolog 1, TAG; triacylglycerol, UCP1; uncoupling protein 1, WNT; wingless type.
The current review paper has some limitations. Although the authors searched for literature that studied the effects of flavonoids, most of the literature shown in the current paper exhibited a positive effect of flavonoids on non-shivering thermogenesis. Some dietary flavonoids were effective at the clinical levels, but most of the data show flavonoids’ effects on BAT and browning WAT at pre-clinical levels using mammalian cells and animals, e.g., mice and rats. These preclinical studies may not have the same clinical effects. It may be difficult to measure BAT activity by ingested flavonoids in humans. Recently, infrared thermography showed the same results as positron emission tomography-computed tomography scan, which avoids radiation [114]. Therefore, clinical studies should be performed to confirm flavonoids’ effect on non-shivering thermogenesis using infrared thermography before the flavonoids are suggested for improving metabolic diseases. Although flavonoids have shown a positive effect on energy metabolism by regulating non-shivering thermogenesis, major concerns of flavonoids include the low bioavailability when flavonoids are ingested and their structure modification through the digestive system. On the surface of epithelial cells of the small intestine, flavonoids undergo de-glycosylation by lactase-phlorizin hydrolase [115,116]. Subsequently, de-glycosylated flavonoids are absorbed through sodium-glucose co-transporter 1 and glucose transporter (GLUT) 2 on the epithelial cells of small intestine [117,118]. The flavonoids absorbed from intestinal lumen undergo glucuronidation by UDP-glucuronosyl transferase as the detoxifying mechanism, called phase II detoxification [119,120]. The absorbed metabolites can be transferred to the liver through portal circulation. In the liver, flavonoid metabolites are further metabolized for phase II detoxification by methylation, sulfation, and glucuronidation [121]. These metabolites are circulated and distributed into several tissues. Although there is no clear evidence that ingested intact flavonoids are transferred and taken up into adipose tissues, GLUT 4, which is expressed in adipose and muscle cells, has been identified as a possible transporter for flavonoids, such as genistein, myricetin, and quercetin [122]. Some flavonoids are metabolized by intestinal bacteria in the large intestine and then absorbed into the body as well [123]. This suggests that the effects of flavonoids on non-shivering thermogenesis may be regulated by indirect signaling cascades such as the microbiome.
Taken together, future studies to understand the effects of flavonoids on non-shivering thermogenesis should be performed together with determining bioavailability of flavonoids and considering indirect regulation through the microbiome at the clinical level.
Abbreviations
| ACC | acetyl-coenzyme A carboxylase |
| ACO | acyl-coenzyme A oxidase |
| AMPK | AMP-activated protein kinase |
| AP2 | adipocyte protein 2 |
| ATP5α | ATP synthase F1 subunit alpha |
| BAT | brown adipose tissue |
| β-AR | beta-adrenergic receptor |
| BMP7 | bone morphogenetic protein 7 |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| CD137/ TNFRSF9 | tumor necrosis factor receptor superfamily member 9 |
| CIDEA | cell death-inducing DNA fragmentation factor alpha (DFFA)-like effector a |
| CITED | cbp/p300-interacting transactivator |
| COMT | catechol-O-methyltransferase |
| CPT1α | carnitine palmitoyltransferase 1 alpha |
| DHEA | dehydroepiandrosterone |
| DIO2 | deiodinase 2 |
| EG | epicatechin gallate |
| EGC | epigallocatechin |
| EGCG | epigallocatechin gallate |
| ELOVL3 | elongation of very-long chain fatty acids-like 3 |
| EWAT | epididymal white adipose tissue |
| FAS | fatty acid synthase |
| FGF 21 | fibroblast growth factor 21 |
| HFD | high-fat diet |
| HSL | hormone sensitive lipase |
| IWAT | inguinal white adipose tissue |
| LDL | low density lipoprotein |
| MAPK | mitogen-activated protein kinase |
| MCAFA | medium-chain acyl-coenzyme A |
| ME | mulberry extract |
| Myf5 | myogenic factor 5 |
| NHANES | National Health and Nutrition Examination Survey |
| NRF1 | nuclear respiratory factor 1 |
| NDUFB8 | 246 NADH Dehydrogenase (Ubiquinone) 1 Beta Subcomplex, 8 |
| OLE | olive leaf extract |
| OPE | onion peel extract |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| PLIN | perilipin |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PPARα | peroxisome proliferator-activated receptor alpha |
| PPARβ | peroxisome proliferator-activated receptor beta |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PRDM16 | positive regulatory domain containing 16 |
| PCOS | polycystic ovary syndrome |
| RWAT | retroperitoneal white adipose tissue |
| SWAT | subcutaneous white adipose tissue |
| SIRT1 | silent mating type information regulation 2 homolog 1 |
| SDHB | succinate dehydrogenase complex iron sulfur subunit B |
| TAG | triacylglycerol |
| TBX1 | T-box transcription factor 1 |
| TFAM | mitochondrial transcription factor A |
| TMEM 26 | transmembrane protein 26 |
| UCP1 | uncoupling protein 1 |
| UCP2 | uncoupling protein 2 |
| UCP3 | uncoupling protein 3 |
| UQCRC2 | ubiquinol-cytochrome c reductase core protein 2 |
| VLDL | very-low density lipoprotein |
| WAT | white adipose tissue |
| WNT | wingless type |
| WNT10b | wingless type MMTV integration site family, member 10b |
Author Contributions
H.W.K. and S.G.L. designed and wrote the draft of the manuscript. H.W.K. revised the manuscript. D.O. and K.H. supported the literature search and review and developed tables. All authors approved the final version of the manuscript.
Funding
This work was supported by the United States Department of Agriculture (NC.X-310-5-18-170-1).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Grundy S.M. Metabolic complications of obesity. Endocrine. 2000;13:155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- 2.Fruhbeck G., Becerril S., Sainz N., Garrastachu P., Garcia-Velloso M.J. Bat: A new target for human obesity? Trends Pharmacol. Sci. 2009;30:387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Kajimura S., Saito M. A new era in brown adipose tissue biology: Molecular control of brown fat development and energy homeostasis. Annu. Rev. Physiol. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.H., Doria A., et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.J., Enerbäck S., et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 7.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M.G., Tromsdorf U.I., Weller H., Waurisch C., et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 8.Timmons J.A., Wennmalm K., Larsson O., Walden T.B., Lassmann T., Petrovic N., Hamilton D.L., Gimeno R.E., Wahlestedt C., Baar K., et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scime A., Devarakonda S., Conroe H.M., Erdjument-Bromage H., et al. Prdm16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Seale P. Transcriptional regulatory circuits controlling brown fat development and activation. Diabetes. 2015;64:2369–2375. doi: 10.2337/db15-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hondares E., Rosell M., Diaz-Delfin J., Olmos Y., Monsalve M., Iglesias R., Villarroya F., Giralt M. PPARα induces pgc-1α gene expression and contributes to the thermogenic activation of brown fat; involvement of prdm16. J. Biol. Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanssen M.J., Broeders E., Samms R.J., Vosselman M.J., Van Der Lans A.A., Cheng C.C., Adams A.C., van Marken Lichtenbelt W.D., Schrauwen P. Serum fgf21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015;5 doi: 10.1038/srep10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L., Zhou L., Chen C., Gong J., Xu L., Ye J., Li P. Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci. China Life Sci. 2014;57:107–116. doi: 10.1007/s11427-013-4585-y. [DOI] [PubMed] [Google Scholar]
- 15.Barneda D., Planas-Iglesias J., Gaspar M.L., Mohammadyani D., Prasannan S., Dormann D., Han G.-S., Jesch S.A., Carman G.M., Kagan V., et al. The brown adipocyte protein cidea promotes lipid droplet fusion via a phosphatidic acid-binding amphipathic helix. eLife. 2015;4:e07485. doi: 10.7554/eLife.07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z., Yon Toh S., Chen Z., Guo K., Peng Ng C., Ponniah S., Lin S.-C., Hong W., Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 17.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harms M., Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 19.Garcia R.A., Roemmich J.N., Claycombe K.J. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. J. Nutr. Metab. 2016;13:24. doi: 10.1186/s12986-016-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon B., Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 21.Weiner J., Kranz M., Klöting N., Kunath A., Steinhoff K., Rijntjes E., Köhrle J., Zeisig V., Hankir M., Gebhardt C., et al. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep. 2016;6:38124. doi: 10.1038/srep38124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco A.C., McAninch E.A. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013;1:250–258. doi: 10.1016/S2213-8587(13)70069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantó C., Auwerx J. Pgc-1alpha, sirt1 and ampk, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto T., Takahashi N., Goto T., Kawada T. Dietary factors evoke thermogenesis in adipose tissues. Obes. Res. Clin. Pract. 2014;8:e533–e539. doi: 10.1016/j.orcp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Castrejon-Tellez V., Rodriguez-Perez J.M., Perez-Torres I., Perez-Hernandez N., Cruz-Lagunas A., Guarner-Lans V., Vargas-Alarcon G., Rubio-Ruiz M.E. The effect of resveratrol and quercetin treatment on ppar mediated uncoupling protein (ucp-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016;17:1069. doi: 10.3390/ijms17071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lone J., Choi J.H., Kim S.W., Yun J.W. Curcumin induces brown fat-like phenotype in 3t3-l1 and primary white adipocytes. J. Nutr. Biochem. 2016;27:193–202. doi: 10.1016/j.jnutbio.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Kawser Hossain M., Abdal Dayem A., Han J., Yin Y., Kim K., Kumar Saha S., Yang G.M., Yeon Choi H., Cho S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016;17:569. doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernarelli J.A., Lambert J.D. Flavonoid intake is inversely associated with obesity and c-reactive protein, a marker for inflammation, in us adults. Nutr. Diabetes. 2017;7:e276. doi: 10.1038/nutd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodman O.L., Meeker W.F., Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharmacol. 2005;46:302–309. doi: 10.1097/01.fjc.0000175431.62626.07. [DOI] [PubMed] [Google Scholar]
- 30.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 31.Chun O.K., Chung S.J., Song W.O. Estimated dietary flavonoid intake and major food sources of U.S. Adults. J. Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X., Zhang Q.X., Wang X., Zhang L., Qu W., Bao B., Liu C.A., Liu J. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1 α pathway-mediated mechanism. Int. J. Obes. 2016;40:1841–1849. doi: 10.1038/ijo.2016.108. [DOI] [PubMed] [Google Scholar]
- 34.Mulligan J.D., Gonzalez A.A., Stewart A.M., Carey H.V., Saupe K.W. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J. Physiol. 2007;580:677–684. doi: 10.1113/jphysiol.2007.128652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dam A.D., Kooijman S., Schilperoort M., Rensen P.C., Boon M.R. Regulation of brown fat by amp-activated protein kinase. Trends Mol. Med. 2015;21:571–579. doi: 10.1016/j.molmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Premalatha M., Parameswari C. Renoprotective effect of chrysin (5, 7 dihydroxy flavone) in streptozotocin induced diabetic nephropathy in rats. Int. J. Pharm. Pharm. Sci. 2012;4:241–247. [Google Scholar]
- 37.Hougee S., Sanders A., Faber J., Graus Y.M., van den Berg W.B., Garssen J., Smit H.F., Hoijer M.A. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem. Pharmacol. 2005;69:241–248. doi: 10.1016/j.bcp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Khoo B.Y., Chua S.L., Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J. Mol. Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J.H., Yun J.W. Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3t3-l1 adipocytes. Nutrition. 2016;32:1002–1010. doi: 10.1016/j.nut.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Asano H., Kanamori Y., Higurashi S., Nara T., Kato K., Matsui T., Funaba M. Induction of beige-like adipocytes in 3T3-L1 cells. J. Vet. Med. Sci. 2014;76:57–64. doi: 10.1292/jvms.13-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karamanlidis G., Karamitri A., Docherty K., Hazlerigg D.G., Lomax M.A. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J. Biol. Chem. 2007;282:24660–24669. doi: 10.1074/jbc.M703101200. [DOI] [PubMed] [Google Scholar]
- 42.Pereira A.P., Ferreira I.C., Marcelino F., Valentão P., Andrade P.B., Seabra R., Estevinho L., Bento A., Pereira J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y., Song S.J., Keum N., Park T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid. Based Complement. Alternat. Med. 2014;2014:971890. doi: 10.1155/2014/971890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo K.A., Ng P.Y., Kabiri Z., Virshup D., Sun L. Wnt inhibition enhances browning of mouse primary white adipocytes. Adipocyte. 2016;5:224–231. doi: 10.1080/21623945.2016.1148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D.-H., Jung E.-A., Sohng I.-S., Han J.-A., Kim T.-H., Han M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharm. Res. 1998;21:17–23. doi: 10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- 46.Thaiss C.A., Itav S., Rothschild D., Meijer M., Levy M., Moresi C., Dohnalova L., Braverman S., Rozin S., Malitsky S., et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 47.Suarez-Zamorano N., Fabbiano S., Chevalier C., Stojanovic O., Colin D.J., Stevanovic A., Veyrat-Durebex C., Tarallo V., Rigo D., Germain S., et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braune A., Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7:216–234. doi: 10.1080/19490976.2016.1158395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa H., Takaishi Y., Tanaka N., Tsuchiya K., Shibata H., Higuti T. Chemical constituents from the peels of citrus sudachi. J. Nat. Prod. 2006;69:1177–1179. doi: 10.1021/np060217s. [DOI] [PubMed] [Google Scholar]
- 50.Tsutsumi R., Yoshida T., Nii Y., Okahisa N., Iwata S., Tsukayama M., Hashimoto R., Taniguchi Y., Sakaue H., Hosaka T., et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014;11:32. doi: 10.1186/1743-7075-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhagwat S., Haytowitz D.B., Wasswa-Kintu S.I., Holden J.M. USDA develops a database for flavonoids to assess dietary intakes. Proc. Food Sci. 2013;2:81–86. doi: 10.1016/j.profoo.2013.04.013. [DOI] [Google Scholar]
- 52.Moon J., Do H.J., Kim O.Y., Shin M.J. Antiobesity effects of quercetin-rich onion peel extract on the differentiation of 3T3-L1 preadipocytes and the adipogenesis in high fat-fed rats. Food Chem. Toxicol. 2013;58:347–354. doi: 10.1016/j.fct.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Dong J., Zhang X., Zhang L., Bian H.X., Xu N., Bao B., Liu J. Quercetin reduces obesity-associated atm infiltration and inflammation in mice: A mechanism including ampkalpha1/sirt1. J. Lipid Res. 2014;55:363–374. doi: 10.1194/jlr.M038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S.G., Parks J.S., Kang H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017;42:62–71. doi: 10.1016/j.jnutbio.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Stewart L.K., Soileau J.L., Ribnicky D., Wang Z.Q., Raskin I., Poulev A., Majewski M., Cefalu W.T., Gettys T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–S46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henagan T., Cefalu W., Ribnicky D., Noland R., Dunville K., Campbell W., Stewart L., Forney L., Gettys T., Chang J. In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity. Genes Nutr. 2015;10:2. doi: 10.1007/s12263-014-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan X., Wei G., You Y., Huang Y., Lee H.J., Dong M., Lin J., Hu T., Zhang H., Zhang C., et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017;31:333–345. doi: 10.1096/fj.201600459RR. [DOI] [PubMed] [Google Scholar]
- 58.Liang H., Ward W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 59.Huang P.I., Chen Y.C., Chen L.H., Juan C.C., Ku H.H., Wang S.T., Chiou S.H., Chiou G.Y., Chi C.W., Hsu C.C., et al. PGC-1alpha mediates differentiation of mesenchymal stem cells to brown adipose cells. J. Atheroscler. Thromb. 2011;18:966–980. doi: 10.5551/jat.7401. [DOI] [PubMed] [Google Scholar]
- 60.Hu T., Yuan X., Ye R., Zhou H., Lin J., Zhang C., Zhang H., Wei G., Dong M., Huang Y., et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017;47:21–28. doi: 10.1016/j.jnutbio.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Hu T., Yuan X., Wei G., Luo H., Lee H.J., Jin W. Myricetin-induced brown adipose tissue activation prevents obesity and insulin resistance in db/db mice. Eur J. Nutr. 2017;57:391–403. doi: 10.1007/s00394-017-1433-z. [DOI] [PubMed] [Google Scholar]
- 62.Da-Silva W.S., Harney J.W., Kim B.W., Li J., Bianco S.D., Crescenzi A., Christoffolete M.A., Huang S.A., Bianco A.C. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007;56:767–776. doi: 10.2337/db06-1488. [DOI] [PubMed] [Google Scholar]
- 63.Vatkar B., Pratapwar A., Tapas A., Butle S., Tiwari B. Synthesis and antimicrobial activity of some flavanone derivatives. Int. J. ChemTech Res. 2010;2:504–508. [Google Scholar]
- 64.Klimczak I., Małecka M., Szlachta M., Gliszczyńska-Świgło A. Effect of storage on the content of polyphenols, vitamin c and the antioxidant activity of orange juices. J. Food Compos. Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- 65.Brett G.M., Hollands W., Needs P.W., Teucher B., Dainty J.R., Davis B.D., Brodbelt J.S., Kroon P.A. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br. J. Nutr. 2008;101:664–675. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo M.-J., Lee O.-H., Choi H.-S., Lee B.-Y. Extract from edible red seaweed (gelidium amansi) inhibits lipid accumulation and GOS production during differentiation in 3T3-L1 cells. Prev. Nutr. Food Sci. 2012;17:129–135. doi: 10.3746/pnf.2012.17.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang T.-H., Yao H.-T., Chiang M.-T. Red algae (gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J. Food Drug Anal. 2015;23:758–765. doi: 10.1016/j.jfda.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi J., Kim K.J., Koh E.J., Lee B.Y. Gelidium elegans regulates the ampk-prdm16-ucp-1 pathway and has a synergistic effect with orlistat on obesity-associated features in mice fed a high-fat diet. Nutrients. 2017;9:342. doi: 10.3390/nu9040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi J., Kim K.-J., Koh E.-J., Lee B.-Y. Altered gelidium elegans extract-stimulated beige-like phenotype attenuates adipogenesis in 3T3-L1 cells. J. Food Nutr. Res. 2016;4:448–453. [Google Scholar]
- 70.Shen J., Nakamura H., Fujisaki Y., Tanida M., Horii Y., Fuyuki R., Takumi H., Shiraishi K., Kometani T., Nagai K. Effect of 4G-alpha-glucopyranosyl hesperidin on brown fat adipose tissue- and cutaneous-sympathetic nerve activity and peripheral body temperature. Neurosci. Lett. 2009;461:30–35. doi: 10.1016/j.neulet.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 71.Chetia D., Rudrapal M. Structurally Diverse Bioflavonoids as Potential Source of Antimalarial Lead Molecules, 2016. [(accessed on 30 May 2018)]; Available online: http://www.old.iitbhu.ac.in/phe/pharmsociety/issue_2015/7.Chetia_and_Rudrapal.pdf.
- 72.Song W.O., Chun O.K. Tea is the major source of flavan-3-ol and flavonol in the us diet. J. Nutr. 2008;138:1543S–1547S. doi: 10.1093/jn/138.8.1543S. [DOI] [PubMed] [Google Scholar]
- 73.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 74.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dulloo A.G., Seydoux J., Girardier L., Chantre P., Vandermander J. Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J. Obes. Relat. Metab. Disord. 2000;24:252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 76.Choo J.J. Green tea reduces body fat accretion caused by high-fat diet in rats through beta-adrenoceptor activation of thermogenesis in brown adipose tissue. J. Nutr. Biochem. 2003;14:671–676. doi: 10.1016/j.jnutbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Nomura S., Ichinose T., Jinde M., Kawashima Y., Tachiyashiki K., Imaizumi K. Tea catechins enhance the mrna expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 2008;19:840–847. doi: 10.1016/j.jnutbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Yan J., Zhao Y., Zhao B. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci. China Life Sci. 2013;56:804–810. doi: 10.1007/s11427-013-4512-2. [DOI] [PubMed] [Google Scholar]
- 79.Collins S., Surwit R.S. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog. Horm. Res. 2001;56:309–328. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- 80.Gogos J.A., Morgan M., Luine V., Santha M., Ogawa S., Pfaff D., Karayiorgou M. Catechol-o-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu H., Meng X., Yang C.S. Enzymology of methylation of tea catechins and inhibition of catechol-o-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos. 2003;31:572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita Y., Wang L., Wang L., Tanaka Y., Zhang T., Ashida H. Oolong, black and pu-erh tea suppresses adiposity in mice via activation of AMP-activated protein kinase. Food Funct. 2014;5:2420–2429. doi: 10.1039/C4FO00095A. [DOI] [PubMed] [Google Scholar]
- 83.Dulloo A.G., Duret C., Rohrer D., Girardier L., Mensi N., Fathi M., Chantre P., Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 84.Nirengi S., Amagasa S., Homma T., Yoneshiro T., Matsumiya S., Kurosawa Y., Sakane N., Ebi K., Saito M., Hamaoka T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. Springerplus. 2016;5:1363. doi: 10.1186/s40064-016-3029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gosselin C., Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br. J. Nutr. 2013;110:282–288. doi: 10.1017/S0007114512005089. [DOI] [PubMed] [Google Scholar]
- 86.Matsumura Y., Nakagawa Y., Mikome K., Yamamoto H., Osakabe N. Enhancement of energy expenditure following a single oral dose of flavan-3-ols associated with an increase in catecholamine secretion. PLoS ONE. 2014;9:e112180. doi: 10.1371/journal.pone.0112180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamio N., Suzuki T., Watanabe Y., Suhara Y., Osakabe N. A single oral dose of flavan-3-ols enhances energy expenditure by sympathetic nerve stimulation in mice. Free Radic. Biol. Med. 2016;91:256–263. doi: 10.1016/j.freeradbiomed.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 88.Rabadan-Chávez G., Quevedo-Corona L., Garcia A.M., Reyes-Maldonado E., Jaramillo-Flores M.E. Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. J. Funct. Food. 2016;20:54–67. doi: 10.1016/j.jff.2015.10.016. [DOI] [Google Scholar]
- 89.Gutierrez-Salmean G., Ortiz-Vilchis P., Vacaseydel C.M., Garduno-Siciliano L., Chamorro-Cevallos G., Meaney E., Villafana S., Villarreal F., Ceballos G., Ramirez-Sanchez I. Effects of (−)-epicatechin on a diet-induced rat model of cardiometabolic risk factors. Eur. J. Pharmacol. 2014;728:24–30. doi: 10.1016/j.ejphar.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 90.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin B.W., Gong C.C., Song H.F., Cui Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Brit. J. Pharmacol. 2017;174:1226–1243. doi: 10.1111/bph.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Z., Kou X., Fugal K., McLaughlin J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004;52:688–691. doi: 10.1021/jf034596w. [DOI] [PubMed] [Google Scholar]
- 93.Yoshitama K., Hisada M., Ishikura N. Distribution pattern of anthocyanins in the polygonaceae. J. Plan. Res. 1984;97:31–38. doi: 10.1007/BF02488145. [DOI] [Google Scholar]
- 94.You Y., Yuan X., Lee H.J., Huang W., Jin W., Zhan J. Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis. Food Funct. 2015;6:401–408. doi: 10.1039/C4FO00719K. [DOI] [PubMed] [Google Scholar]
- 95.Cao W., Medvedev A.V., Daniel K.W., Collins S. Beta-adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 map kinase. J. Biol. Chem. 2001;276:27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- 96.Takikawa M., Inoue S., Horio F., Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of amp-activated protein kinase in diabetic mice. J. Nutr. 2010;140:527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 97.Matsukawa T., Villareal M.O., Motojima H., Isoda H. Increasing camp levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017;40:77–85. doi: 10.1016/j.jnutbio.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 98.You Y., Yuan X., Liu X., Liang C., Meng M., Huang Y., Han X., Guo J., Guo Y., Ren C., et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700261. [DOI] [PubMed] [Google Scholar]
- 99.Davis S.R., Dalais F.S., Simpson E.R., Murkies A.L. Phytoestrogens in health and disease. Recent prog. Horm. Res. 1999;54:185–210. [PubMed] [Google Scholar]
- 100.Tapas A.R., Sakarkar D., Kakde R. Flavonoids as nutraceuticals: A review. Trop J. Pharm. Res. 2008;7:1089–1099. doi: 10.4314/tjpr.v7i3.14693. [DOI] [Google Scholar]
- 101.Murphy P.A., Song T., Buseman G., Barua K., Beecher G.R., Trainer D., Holden J. Isoflavones in retail and institutional soy foods. J. Agric. Food Chem. 1999;47:2697–2704. doi: 10.1021/jf981144o. [DOI] [PubMed] [Google Scholar]
- 102.Rogers N.H., Perfield J.W., Strissel K.J., Obin M.S., Greenberg A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kamiya T., Nagamine R., Sameshima-Kamiya M., Tsubata M., Ikeguchi M., Takagaki K. The isoflavone-rich fraction of the crude extract of the puerariae flower increases oxygen consumption and bat UCP1 expression in high-fat diet-fed mice. Glob. J. Health Sci. 2012;4:147–155. doi: 10.5539/gjhs.v4n5p147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lephart E.D., Porter J.P., Lund T.D., Bu L., Setchell K.D., Ramoz G., Crowley W.R. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in long-Evans male rats. Nutr. Metab. (Lond) 2004;1:16. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crespillo A., Alonso M., Vida M., Pavon F.J., Serrano A., Rivera P., Romero-Zerbo Y., Fernandez-Llebrez P., Martinez A., Perez-Valero V., et al. Reduction of body weight, liver steatosis and expression of stearoyl-coa desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011;164:1899–1915. doi: 10.1111/j.1476-5381.2011.01477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aziz S.A., Wakeling L.A., Miwa S., Alberdi G., Hesketh J.E., Ford D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017;61:1600574. doi: 10.1002/mnfr.201600574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gautam J., Khedgikar V., Kushwaha P., Choudhary D., Nagar G.K., Dev K., Dixit P., Singh D., Maurya R., Trivedi R. Formononetin, an isoflavone, activates AMP-activated protein kinase/β-catenin signalling to inhibit adipogenesis and rescues C57BL/6 mice from high-fat diet-induced obesity and bone loss. Br. J. Nutr. 2017;117:645–661. doi: 10.1017/S0007114517000149. [DOI] [PubMed] [Google Scholar]
- 109.Fiorani M., Guidarelli A., Blasa M., Azzolini C., Candiracci M., Piatti E., Cantoni O. Mitochondria accumulate large amounts of quercetin: Prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 110.Anderson R.M., Barger J.L., Edwards M.G., Braun K.H., O’Connor C.E., Prolla T.A., Weindruch R. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bai W., Zhang X. Nucleus or cytoplasm? The mysterious case of sirt1′s subcellular localization. Cell Cycle. 2016;15:3337–3338. doi: 10.1080/15384101.2016.1237170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiang L., Wang L., Kon N., Zhao W., Lee S., Zhang Y., Rosenbaum M., Zhao Y., Gu W., Farmer S.R., et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grundlingh J., Dargan P.I., El-Zanfaly M., Wood D.M. 2, 4-dinitrophenol (DNP): A weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 2011;7:205. doi: 10.1007/s13181-011-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Law J., Morris D.E., Izzi-Engbeaya C., Salem V., Coello C., Robinson L., Jayasinghe M., Scott R., Gunn R., Rabiner E., et al. Thermal imaging is a noninvasive alternative to PET/CT for measurement of brown adipose tissue activity in humans. J. Nucl. Med. 2018;59:516–522. doi: 10.2967/jnumed.117.190546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilkinson A.P., Gee J.M., Dupont M.S., Needs P.W., Mellon F.A., Williamson G., Johnson I.T. Hydrolysis by lactase phlorizin hydrolase is the first step in the uptake of daidzein glucosides by rat small intestine in vitro. Xenobiotica. 2003;33:255–264. doi: 10.1080/0049825021000058088. [DOI] [PubMed] [Google Scholar]
- 116.Day A.J., Cañada F.J., Díaz J.C., Kroon P.A., Mclauchlan R., Faulds C.B., Plumb G.W., Morgan M.R., Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/S0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 117.Zou T.-B., Feng D., Song G., Li H.-W., Tang H.-W., Ling W.-H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-o-β-glucoside in caco-2 cells. Nutrients. 2014;6:4165–4177. doi: 10.3390/nu6104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen C.-H., Hsu H.-J., Huang Y.-J., Lin C.-J. Interaction of flavonoids and intestinal facilitated glucose transporters. Planta Med. 2007;73:348–354. doi: 10.1055/s-2007-967172. [DOI] [PubMed] [Google Scholar]
- 119.Xie S., You L., Zeng S. Studies on the flavonoid substrates of human UDP-glucuronosyl transferase (UGT) 2B7. Die Pharmazie. 2007;62:625–629. [PubMed] [Google Scholar]
- 120.Zhang L., Zuo Z., Lin G. Intestinal and hepatic glucuronidation of flavonoids. Mol. Pharm. 2007;4:833–845. doi: 10.1021/mp700077z. [DOI] [PubMed] [Google Scholar]
- 121.Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Brit. J. Nutr. 2006;96:107–116. doi: 10.1079/BJN20061809. [DOI] [PubMed] [Google Scholar]
- 122.Strobel P., Allard C., Perez-Acle T., Calderon R., Aldunate R., Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005;386:471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng X., Li Y., Brobbey Oppong M., Qiu F. Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 2018;16:1–14. doi: 10.1080/03602532.2018.1485691. [DOI] [PubMed] [Google Scholar]