Abstract
Immunosuppression derived after cytostatics application in cancer chemotherapy is considered as an adverse side effect that leads to deterioration of quality of life and risk of infectious diseases. A linear sulfated (1→3)-α-l-fucan M-Fuc prepared by chemical modification of a fucoidan isolated from the brown seaweed Chordaria flagelliformis, along with two structurally related synthetic sulfated oligosaccharides, were studied as stimulators of hematopoiesis on a model of cyclophosphamide immunosuppression in mice. Recombinant granulocyte colony-stimulating factor (r G-CSF), which is currently applied in medicine to treat low blood neutrophils, was used as a reference. Polysaccharide M-Fuc and sulfated difucoside DS did not demonstrate significant effect, while sulfated octasaccharide OS showed higher activity than r G-CSF, causing pronounced neutropoiesis stimulation. In addition, production of erythrocytes and platelets was enhanced after the octasaccharide administration. The assessment of populations of cells in blood and bone marrow of mice revealed the difference in mechanisms of action of OS and r G-CSF.
Keywords: granulocyte colony-stimulating factor, fucoidan, synthetic oligosaccharide, hematopoiesis, immunosuppression, cyclophosphamide
1. Introduction
Cytostatics application in cancer chemotherapy results in a number of side effects, including the suppression of different branches of hematopoiesis. Cyclophosphamide (CPh) is an alkylating agent that is currently used in the treatment of various forms of malignant neoplasms [1]. The main side effect of CPh is connected with suppression of rapidly proliferating hematopoietic stem/progenitor cells (HSPCs) and results in acute neutropenia, lymphopenia, erythropenia, and thrombocytopenia [2,3,4].
The functional activity of HSPCs has been shown to depend mainly on specific local microenvironment formed by bone marrow vascular niches [5,6]. Endothelium of bone marrow is known to express cell adhesion molecules involved in cell signaling by interaction with glycoprotein and glycolipid ligands of HSPCs [6,7,8]. Recently, it was shown that selectins and integrins play a crucial role in regulation of hematopoiesis in experimental animals with myelosuppression after chemotherapy [9,10].
Recombinant granulocyte colony-stimulating factor (r G-CSF) is currently applied in medicine to treat low blood neutrophils [11]. However, r G-CSF does not possess a significant stimulating effect on platelet and erythrocyte germs. Therefore, the development of an alternative drug that is able to stimulate several branches of the hematopoiesis is still a challenge.
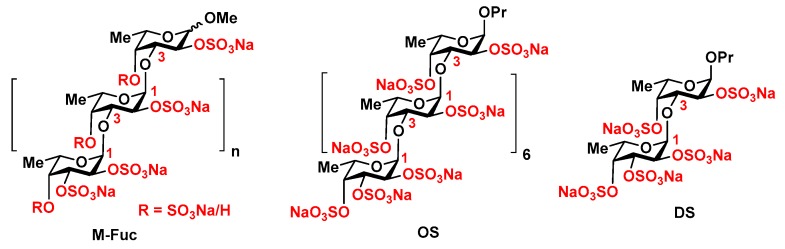
Recently, fucoidan from the brown seaweed Chordaria flagelliformis was shown to be an effective stimulator of hematopoiesis in a model of cyclophosphamide-induced mice [12]. This biopolymer demonstrated the level of activity comparable with that of r G-CSF regarding neutropoiesis stimulation. Additionally, this compound was found to be capable of stimulating erythropoiesis and thrombopoiesis. The main disadvantage of the native polysaccharide from C. flagelliformis is the very complex structure. The backbone composed of the repeating (1→3)-linked α-l-fucopyranosyl residues is decorated by numerous α-l-fucopyranosyl, α-d-glucuronyl, and more complex disaccharide branches [13]. Random sulfation of a backbone and branches significantly mask the regularity of the polysaccharide. To simplify the structure of this polysaccharide, several chemical transformations have been performed resulting in preparation of sulfated linear fucoidan M-Fuc with molecular weight ~5 kDa (Figure 1) [14].
Figure 1.
Modified fucoidan M-Fuc [14] and related synthetic octasaccharide OS [15,16] and disaccharide DS [17,18].
In this paper, we report the study of a modified fucoidan M-Fuc, along with a related synthetic octasaccharide OS [15,16] and disaccharide DS [17,18] (Figure 1), as stimulators of hematopoiesis on a model of CPh immunosuppression in mice.
2. Results
Modified fucoidan M-Fuc is a linear polysaccharide composed of the (1→3)-linked α-l-fucopyranosyl residues bearing the sulfate groups at O-2 or at both O-2 and O-4 (Figure 1). In our study, it was prepared from the high molecular weight fucoidan from the brown seaweed Chordaria flageliformis by chemical elimination of branches, partial depolymerization, and sulfation [14]. The mean molecular weight of M-Fuc was estimated as ~5 kDa. The degree of sulfation of M-Fuc was 1.7. The synthetic per-O-sulfated linear n-propyl octa-(1→3)-α-l-fucoside OS [15,16] may be regarded as a fragment of M-Fuc. Its MW was ~2.8 kDa and the degree of sulfation was 2.0. The synthetic (1→3)-linked difucoside DS [17,18] with MW ~0.5 kDa and the degree of sulfation 2.0 could be considered as a low molecular weight analogue of M-Fuc.
The effect of compounds M-Fuc, OS, and DS on hematopoiesis was studied on a model of CPh-induced immunosuppression in mice. Recombinant r G-CSF (Leicyta) was applied as a reference. Intact animals were regarded as a positive control. Active concentrations of the compounds have been determined previously [12,19]. The values of the hematological parameters in various groups of mice are presented in Table 1. The levels of white and red blood cells (WBC, RBC), platelets, and hemoglobin were also determined.
Table 1.
Hematologic parameters of mice with cyclophosphamide (CPh)-induced immunosuppression after treatment with modified fucoidan M-Fuc, octasaccharide OS, disaccharide DS, and recombinant granulocyte colony-stimulating factor (r G-CSF) (Mean ± SD).
| Groups | WBC (×103/µL) | RBC (×106/µL) | Hemoglobin (g/dL) | Platelets (×103/µL) |
|---|---|---|---|---|
| Control | 6.7 ± 1.14 | 9.4 ± 0.45 | 16.6 ± 0.64 | 571.5 ± 54.71 |
| CPh | 2.4 ± 0.36 | 5.4 ± 0.07 | 11.3 ± 1.73 | 812.3 ± 46.35 |
| CPh + M-Fuc | 1.9 ± 1.20 | 5.4 ± 2.95 | 10.2 ± 5.52 | 589.0 ± 350.06 |
| CPh + OS | 5.3 ± 0.93 | 9.1 ± 1.11 | 16.7 ± 2.33 | 1036.3 ± 104.29 |
| CPh + DS | 2.3 ± 1.82 | 4.7 ± 2.89 | 8.7 ± 5.47 | 389.6 ± 207.74 |
| CPh + r G-CSF | 3.7 ± 0.47 | 7.0 ± 0.77 | 13.0 ± 1.30 | 541.0 ± 44.20 |
These data showed that CPh injection led to a decrease in the concentration of leucocytes by 2.9 times, erythrocytes by 1.7 times, and hemoglobin by 1.5 times, while leading to an increase in the level of platelets by 1.4 times. The subsequent treatment with r G-CSF resulted in a tendency of normalization of the RBC, hemoglobin, and platelets levels. At the same time, the level of leukocytes in blood increased, but its median value did not reach the control level. Treatment with fucoidan M-Fuc and disaccharide DS did not produce significant changes in the hematologic parameters. The most pronounced effect—even exceeding that of r G-CSF—was observed in the presence of octasaccharide OS. In the CPh + OS group, the recovery of the WBC, RBC, and hemoglobin concentrations to the positive control level was observed. The level of platelets remained elevated.
An assessment of the populations of the white blood cells revealed that cyclophosphamide injection led to a decrease in levels of monocytes, lymphocytes, and neutrophils (Table 2). Neutrophils were the most sensitive to CPh impact (100 mg/kg, 1 × 4 days); their concentration in CPh-group was lower by 5 times compared to that in the intact control. After injection of M-Fuc or DS in a therapeutic regime, no significant recovery of the cells was observed, while administration of r G-CSF and OS led to a sufficient increase in the cell concentration. Notably, the effect of OS was more pronounced than that of r G-CSF in all cases of white blood cells populations.
Table 2.
Different populations of leucocytes in blood of mice with CPh-induced immunosuppression after treatment with M-Fuc, OS, DS, and r G-CSF (Mean ± SD).
| Groups | Neutrophils (×103/µL) | Monocytes (×103/µL) | Lymphocytes (×103/µL) |
|---|---|---|---|
| Control | 1.9 ± 0.21 | 0.5 ± 0.01 | 4.4 ± 1.32 |
| CPh | 0.4 ± 0.25 | 0.2 ± 0.12 | 1.5 ± 0.38 |
| CPh + M-Fuc | 0.5 ± 0.39 | 0.2 ± 0.10 | 1.2 ± 0.71 |
| CPh + OS | 2.1 ± 0.88 | 0.5 ± 0.22 | 2.3 ± 0.02 |
| CPh + DS | 1.3 ± 1.22 | 0.1 ± 0.01 | 0.6 ± 0.32 |
| CPh + r G-CSF | 1.1 ± 0.74 | 0.4 ± 0.09 | 2.1 ± 0.47 |
Further elucidation of lymphocyte subpopulations revealed that after CPh treatment, a predominant depletion of the cells was observed due to the subpopulation of CD3+CD4+ cells, which was reflected by a decrease in the CD4+/CD8+ index from 1.94 ± 0.28 to 0.88 ± 0.10 (Table 3). Injection of OS recovered CD4+/CD8+ index up to a level of control group (1.93 ± 0.19), while the use of r G-CSF had a similar tendency but with lower efficiency (1.38 ± 0.05). Intergroup statistical analysis (vs. control and vs. CPh) showed no significant changes in NK content in the blood of animals of all groups.
Table 3.
Different subpopulations of lymphocytes in blood of mice with CPh-induced immunosuppression after treatment with M-Fuc, OS, DS, and r G-CSF.
| Groups | CD3+CD4+ (%) | CD3+CD8+ (%) | The Ratio CD4+/CD8+ | NK (%) |
|---|---|---|---|---|
| Control | 31 ± 0.7 | 16 ± 3.8 | 1.94 ± 0.28 | 1.3 ± 0.8 |
| CPh | 14 ± 3.4 | 16 ± 3.1 | 0.88 ± 0.10 | 2.1 ± 2.0 |
| CPh + M-Fuc | 16 ± 3.0 | 15 ± 2.2 | 1.07 ± 0.10 | 0.4 ± 1.2 |
| CPh + OS | 29 ± 2.4 | 15 ± 4.1 | 1.93 ± 0.19 | 2.3 ± 1.1 |
| CPh + DS | 24 ± 2.8 | 17 ± 1.4 | 1.41 ± 0.07 | 1.3 ± 0.2 |
| CPh + r G-CSF | 22 ± 0.8 | 16 ± 2.1 | 1.38 ± 0.50 | 3.2 ± 1.1 |
As molecules of cell adhesion selectins and integrins are known to play a key role in immune response, their levels were measured on granulocytes (Table 4). After the course of CPh, an increase in CD11c+ and CD62p+ granulocytes by ~1.5 times was observed, indicating the circulation predominantly of highly differentiated activated “old” cells. Interestingly, after r G-CSF treatment, the levels of CD11c and CD62p expression were 2 times lower than those in the case of OS. It could be supposed that in the latter case, there was a release of a large number of granulocytes capable of immune response.
Table 4.
Expression of CD11c and CD62p on the granulocytes of mice with CPh-induced immunosuppression after treatment with M-Fuc, OS, DS and r G-CSF.
| Groups | CD11c+ | CD62p+ | ||
|---|---|---|---|---|
| % | p | % | p | |
| Control | 44 ± 5.4 | - | 48 ± 2.7 | - |
| CPh | 68 ± 8.1 | 0.057 | 70 ± 6.8 | 0.029 |
| CPh + M-Fuc | 27 ± 3.1 | 0.047 | 8 ± 2.4 | 0.001 |
| CPh + OS | 34 ± 6.2 | 0.278 | 38 ± 4.1 | 0.097 |
| CPh + DS | 22 ± 3.9 | 0.021 | 23 ± 2.5 | 0.002 |
| CPh + r G-CSF | 13 ± 7.5 | 0.02 | 14 ± 8.4 | 0.012 |
Next, the effect of the tested compounds on the functional activity of the blood granulocytes was studied (Table 5). It was found that the introduction of CPh did not lead to a decrease in the functional activity of blood granulocytes released in a high rate of bacterial capture. The suppression of the anti-infectious immunity of patients after the cytostatic treatment is connected with a decrease in the number of the effector cells rather than with their reactivity. In the CPh + r G-CSF group, a slight decrease in the rate of phagocytic activity compared to the intact control was observed, which was probably due to mobilization of youth cells from the bone marrow; however, there was still a high level of reactive oxygen species (ROS) generation. By contrast, in the CPh + OS group, an increase of phagocytic activity of the granulocytes was observed compared to a control group.
Table 5.
The effect of the tested compounds on the functional activity of the blood granulocytes in mice with CPh-induced immunosuppression.
| Groups | E. coli+ Cells | ROS+ Cells | ||
|---|---|---|---|---|
| % | p | % | p | |
| Control | 32 ± 3.4 | - | 31 ± 1.6 | - |
| CPh | 54 ± 4.1 | 0.009 | 36 ± 0.1 | 0.026 |
| CPh + M-Fuc | 57 ± 3.1 | 0.003 | 35 ± 1.4 | 0.119 |
| CPh + OS | 43 ± 6.2 | 0.181 | 29 ± 1.1 | 0.350 |
| CPh + DS | 52 ± 3.9 | 0.012 | 47 ± 1.8 | 0.001 |
| CPh + r G-CSF | 29 ± 3.5 | 0.056 | 51 ± 0.8 | 0.001 |
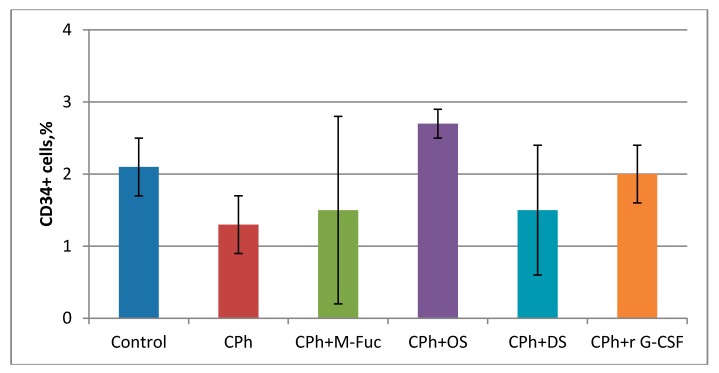
The effect of tested compounds on bone marrow cells was further elucidated. After CPh treatment, a decrease in the number of progenitor cells CD34+ was observed (Figure 2). Injection of M-Fuc or DS in therapeutic mode did not result in a significant recovery of cells, while administration of r G-CSF and OS led to a sufficient increase in the cell concentration. Notably, the effect was more pronounced in the latter case.
Figure 2.
Content of progenitor cells CD34+ in bone marrow of mice with CPh-induced immunosuppression after treatment with M-Fuc, OS, DS and r G-CSF.
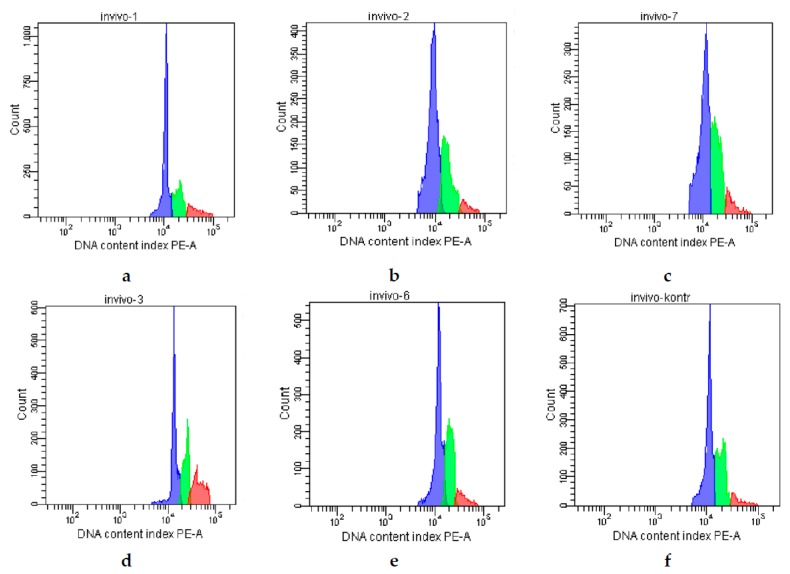
Analysis of the bone marrow cell cycle revealed that an inhibition of the proliferation was observed after CPh administration, which resulted in a decrease in the concentration of cells in the mitosis state (G2/M) from 14% to 4% (Figure 3, Table 6). After a course of all tested compounds, an increase in the proliferative index of the cells was detected. Injection of r G-CSF, M-Fuc, and DS resulted in an increase in this value by 2 times; in the CPh + OS group, the number of cells in G2/M phase reached up to 25%, which was ~3 times higher than that in the CPh + r G-CSF group.
Figure 3.
Analysis of the bone marrow cells cycle in mice with CPh-induced immunosuppression after treatment by tested compounds: (a) positive control—intact mice; (b) negative control—CPh; (c) CPh + M-Fuc; (d) CPh + OS; (e) CPh + DS; (f) CPh + r G-CSF.
Table 6.
Different phases of the bone marrow cells of mice with CPh-induced immunosuppression after treatment with M-Fuc, OS, DS, and r G-CSF.
| Groups | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Control | 63 | 23 | 14 |
| CPh | 65 | 29 | 4 |
| CPh + M-Fuc | 68 | 24 | 8 |
| CPh + OS | 47 | 28 | 25 |
| CPh + DS | 58 | 34 | 8 |
| CPh + r G-CSF | 60 | 32 | 8 |
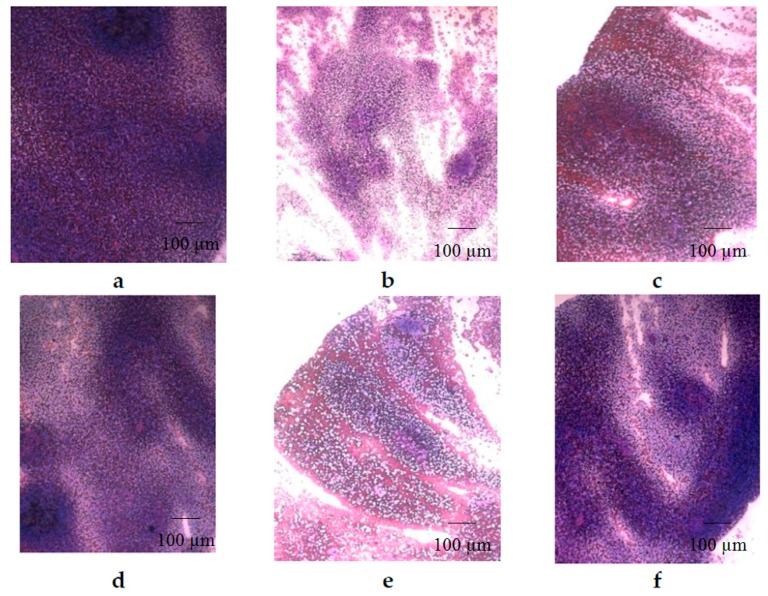
Analysis of the morphology of spleen on smear prints showed that after CPh treatment, a myelosuppression was accompanied by a depletion of the cellular composition of the white pulp (Figure 4b). Thus, the follicles (B-dependent areas) and periarteriolar sheaths (T-dependent areas) disappeared. The architecture of the spleen was destroyed, and the interstitial tissue was composed of a dense and uniform layer of lymphoid cells. After a course of OS and r G-CSF, cell recovery was observed (Figure 4d,f), while M-Fuc and DS demonstrated a moderate effect (Figure 4c,e).
Figure 4.
Morphology of the spleen of mice with CPh-induced immunosuppression after treatment with tested compounds (hematoxylin-eosin staining): (a) positive control—intact mice; (b) negative control—CPh; (c) CPh + M-Fuc; (d) CPh + OS; (e) CPh + DS; (f) CPh + r G-CSF. Original magnification ×400.
3. Discussion
Proliferation and differentiation of hematopoietic stem/progenitor cells are known to depend on specific local microenvironment formed by bone marrow vascular niches [5,6]. Endothelium of bone marrow is found to express cell adhesion molecules involved in cell signaling by interaction with glycoprotein and glycolipid ligands of HSPCs [6,7,8]. Recently, it was shown that selectins and integrins play a crucial role in regulation of hematopoiesis in experimental animals with myelosuppression after chemotherapy [9,10,20,21,22].
Polysaccharides fucoidans and fucose-enriched structures are traditionally considered as inhibitors of P- and L-selectins but not of E-selectin [23,24,25,26]. The value of the effect depends considerably on the structural features of these molecules [24]. In this study, we have demonstrated that the structure of the studied compounds related to fucoidans significantly influenced the ability to stimulate hematopoiesis in mice with CPh-induced myelosuppression. Synthetic octasacharide OS was shown to be the most active sample capable of recovering the WBC, RBC, and hemoglobin levels—as well as the absolute number of the neutrophils, monocytes, and lymphocytes—to intact control levels. At the same time, low molecular, weight-modified fucoidan M-Fuc with lower degree of sulfation and synthetic disaccharide DS did not show any significant effects in the experiments, indicating that the degree of sulfation and MW are important parameters for this type of activity.
Notably, the number of neutrophils in the CPh + OS group was 2 times higher than that in the CPh + r G-CSF group (Table 2). Moreover, the phagocytic activity of these cells exceeded that of the granulocytes in the CPh + r G-CSF group (Table 5).
Analysis of the bone marrow cell cycle revealed that OS stimulated the proliferation of hematopoietic progenitor cells ~3 times more effectively than r G-CSF (Table 6). The number of these cells in the CPh + OS group was also ~1.5 times higher than that in the r G-CSF group (Figure 2). Additionally, OS was shown to effectively stimulate the reparation of spleen structure after CPh-induced myelosuppression (Figure 4).
Therefore, totally sulfated synthetic octasaccharide OS has been shown to be an effective stimulator of hematopoiesis, with its activity exceeding that of r G-CSF regarding the recovery of WBC, RBC, and hemoglobin levels, the number and activity of neutrophils in the blood, and the number of hematopoietic progenitor cells in the bone marrow. These results could be considered as a base for the development of a drug for the treatment and prevention of immunosuppression complications. In addition, OS derivatives could be applied for the construction of hybrid systems [27] with more potent biological effects.
4. Materials and Methods
4.1. General Methods
Immunophenotype and membrane-associated markers on blood and bone marrow cells were examined using anti-mouse antibodies CD11c, CD62p, CD34, CD11c, CD3, CD4, CD8, and NK1.1 (Becton Dickinson Bioscience, San Jose, CA, USA). The phagocytic activity was studied using the FagoFlow Ex Kit (Exbio, Praha, Czech Republic). BD Canto II flow cytometer (Becton Dickinson Bioscience, San Jose, CA, USA) was used for the study. Sample preparation was carried out in accordance with the manufacturer’s instructions. All measurements were carried out in triplets. To evaluate each parameter, the blood of 4 mice of each group was used. Cell cycle analysis was performed on Muse Cell Analyzer (Merck KGaA, Darmstadt, Germany) using the Muse Cell Cycle Kit (EMD Millipore Corporation, Billerica, MA, USA).
4.2. Sulfated Polysaccharides
Modified fucoidan M-Fuc was prepared by chemical modification of fucoidan from C. flageliformis as described previously [14]. Octasaccharide OS [15,16] and disaccharide DS [17,18] were synthesized from l-fucose.
4.3. Animal Model
The animal protocols used in this work were evaluated and approved by the local ethical committee of the N.N. Blokhin National Medical Research Center of Oncology (Protocol 12-2017). They are in accordance with the order 490 (5 November 2008) of the Agricultural Ministry of Russian Federation and meet National GLP Standard of Russian Federation (53434-2009) and European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, France, 18.03.1986).
Thirty-six mice of the Balb/c line (males, weight 20 ± 2 g) were divided into 6 groups with 6 animals in each group. Before and during the experiment, the animals were in standardized vivarium conditions (at 20 ± 2 °C with free access to food and water). For the inducing of myelosuppression, CPh (Endoxan, Baxter, Germany) in a dosage of 100 mg/kg was injected to animals of 5 groups once daily intraperitoneally for 4 days. Then, the following sterile solutions (0.2 mL) were administered subcutaneously to all animals for 3 days (1 time daily): 0.5 mg/mL of M-Fuc in isotonic sodium chloride solution (CPh + M-Fuc group), 0.5 mg/mL of OS in isotonic sodium chloride solution (CPh + OS group), 0.5 mg/mL of DS in isotonic sodium chloride solution (CPh + DS group), and 3 nmol/mL of r G-CSF (Leucita, Sygardis AqVida, Germany) in isotonic sodium chloride solution (CPh + r G-CSF group), sterile isotonic sodium chloride solution (CPh group). A sterile isotonic sodium chloride solution was administered to the mice of the control group in the same regime. The animals were euthanized by decapitation after 2 days. Blood of each animal was collected in the tubes with ethylenediaminetetraacetic acid (EDTA), the spleen was removed from the animals, and smears were imprinted on the polyethylene-coated glasses (Gerhard Menzei GmbH, Termo Scientific, Braunschweig, Germany). The fingerprints were fixed in May-Grunwald solution, stained with hematoxylin-eosin (HE) and analyzed by light microscopy. Hematologic parameters of blood were analyzed on an automatic analyzer, determining the concentration of WBC, platelets, and RBC. Bone marrow cells were isolated from the femurs.
4.4. Statistical Analysis
Data in the group were presented in the format of mean and standard deviation (Mean ± SD). An analysis of the reliability of the differences was carried out using the t criterion. Differences were considered significant at p < 0.05.
5. Conclusions
Modified polysaccharide M-Fuc prepared from the fucoidan from the seaweed Chordaria flagelliformis, along with related synthetic octasaccharide OS and disaccharide DS, were studied as stimulators of hematopoiesis on a model of cyclophosphamide immunosuppression in mice. Recombinant granulocyte colony-stimulating factor (r G-CSF), which is currently applied in medicine to treat low blood neutrophils, was used as a reference. Polysaccharide M-Fuc and sulfated difucoside DS did not demonstrate significant effect in the experiments, while related sulfated octasaccharide OS was shown to be an effective stimulator of hematopoiesis, with its activity exceeding that of r G-CSF regarding the recovery of WBC, RBC, and hemoglobin levels, the number and activity of neutrophils in blood, and the number of hematopoietic progenitor cells in the bone marrow. These results could be considered as a base for development of a drug for treatment and prevention of immunosuppression complications.
Author Contributions
N.Y.A., N.E.U., M.V.K., and N.E.N. conceived and designed the experiments; N.Y.A., F.V.D., and N.A.U. performed the experiments; N.Y.A. and M.V.K. analyzed the data; M.I.B., N.E.U., and A.I.U. prepared and characterized the tested compounds; all the authors wrote the paper.
Funding
This work was supported by the Russian Foundation of Basic Research (grants nos. 17-00-00494, 17-00-00495, 17-00-00496).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Nieto Y. Pharmacodynamics of high-dose chemotherapy. Curr. Drug Metab. 2001;2:53–66. doi: 10.2174/1389200013338720. [DOI] [PubMed] [Google Scholar]
- 2.Schirmer J.H., Bremer J.P., Moosig F., Holle J.U., Lamprecht P., Wieczorek S., Haenisch S., Cascorbi I. Cyclophosphamide treatment-induced leukopenia rates in ANCA-associated vasculitis are influenced by variant CYP450 2C9 genotypes. Pharmacogenomics. 2016;17:367–374. doi: 10.2217/pgs.15.176. [DOI] [PubMed] [Google Scholar]
- 3.Im S.A., Kim K.H., Kim H.S., Lee K.H., Shin E., Do S.G., Jo T.H., Park Y.I., Lee C.K. Processed Aloe vera gel ameliorates cyclophosphamide-induced immunotoxicity. Int. J. Mol. Sci. 2014;15:19342–19354. doi: 10.3390/ijms151119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuter D.J. Managing thrombocytopenia associated with cancer chemotherapy. Oncology. 2015;29:282–294. [PubMed] [Google Scholar]
- 5.Lo Celso C., Fleming H.E., Wu J.W., Zhao C.X., Miake-Lye S., Fujisaki J., Côté D., Rowe D.W., Lin C.P., Scadden D.T. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Grandis M., Lhoumeau A.C., Mancini S.J., Aurrand-Lions M. Adhesion receptors involved in HSC and early-B cell interactions with bone marrow microenvironment. Cell. Mol. Life Sci. 2016;73:687–703. doi: 10.1007/s00018-015-2064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AbuSamra D.B., Aleisa F.A., Al-Amoodi A.S., Jalal Ahmed H.M., Chin C.J., Abuelela A.F., Bergam P., Sougrat R., Merzaban J.S. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017;1:2799–2816. doi: 10.1182/bloodadvances.2017004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabors L.K., Wang L.D., Wagers A.J., Kansas G.S. Overlapping roles for endothelial selectins in murine hematopoietic stem/progenitor cell homing to bone marrow. Exp. Hematol. 2013;41:588–596. doi: 10.1016/j.exphem.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiel M.J., Yilmaz O.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Winkler I.G., Barbier V., Nowlan B., Jacobsen R.N., Forristal C.E., Patton J.T., Magnani J.L., Lévesque J.P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 11.Crobu D., Spinetti G., Schrepfer R., Tonon G., Jotti G.S., Onali P., Dedoni S., Orsini G., Di Stefano A. Preclinical and clinical phase I studies of a new recombinant Filgrastim (BK0023) in comparison with Neupogen®. BMC Pharmacol. Toxicol. 2014;15:7. doi: 10.1186/2050-6511-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anisimova N., Ustyuzhanina N., Donenko F., Bilan M., Usov A., Nifantiev N., Kiselevskiy M. Fucoidan and fucosylated chondroitin sulfate stimulate hematopoiesis in cyclophosphamide-induced mice. Mar. Drugs. 2017;15:301. doi: 10.3390/md15100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilan M.I., Vinogradova E.V., Tsvetkova E.A., Grachev A.A., Shashkov A.S., Nifantiev N.E., Usov A.I. A sulfated glucuronofucan containing both fucofuranose and fucopyranose residues from the brown alga Chordaria flagelliformis. Carbohydr. Res. 2008;343:2605–2612. doi: 10.1016/j.carres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Ustyuzhanina N.E., Bilan M.I., Dmitrenok A.S., Tsvetkova E.A., Nifantiev N.E., Usov A.I. Modification of fucoidan from the seaweed Chordaria flagelliformis and study of anticoagulant and anti-inflammatory activity of the product. Carbohydr. Polym. under review.
- 15.Ustuzhanina N.E., Krylov V.B., Grachev A.A., Gerbst A.G., Nifantiev N.E. Synthesis, NMR and conformational studies of fucoidan fragments. VIII. Convergent block-wise synthesis of long chain linear and 2,3-branched oligosaccharides. Synthesis. 2006;23:4017–4031. [Google Scholar]
- 16.Krylov V.B., Kaskova Z.M., Vinnitskiy D.Z., Ustyuzhanina N.E., Grachev A.A., Chizhov A.O., Nifantiev N.E. Acid-promoted total sulfation of fucoidan fragments. Carbohydr. Res. 2011;346:540–550. doi: 10.1016/j.carres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Bilan M.I., Grachev A.A., Ustuzhanina N.E., Shashkov A.S., Nifantiev N.E., Usov A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004;339:511–517. doi: 10.1016/j.carres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Khatuntseva E.A., Ustuzhanina N.E., Zatonskii G.V., Shashkov A.S., Usov A.I., Nifant’ev N.E. Synthesis, NMR and conformational studies of fucoidan fragments 1: Desulfated 2,3-and 3,4-branched trisaccharide fragments and constituting disaccharides. J. Carbohydr. Chem. 2000;19:1151–1173. doi: 10.1080/07328300008544140. [DOI] [Google Scholar]
- 19.Anisimova N.Yu., Ustyuzhanina N.E., Donenko F.V., Bilan M.I., Ushakova N.A., Usov A.I., Nifantiev N.E., Kiselevskiy M.V. Influence of fucoidans and their derivatives on antitumor and phagocytic activity of human blood leucocytes. Biochemistry. 2015;80:925–933. doi: 10.1134/S0006297915070111. [DOI] [PubMed] [Google Scholar]
- 20.Frenette P.S., Weiss L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: Evidence for selectin-dependent and independent mechanisms. Blood. 2000;96:2460–2468. [PubMed] [Google Scholar]
- 21.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo A., Peired A.J., Weiss L.A., Katayama Y., Frenette P.S. The integrin alphaMbeta2 anchors hematopoietic progenitors in the bone marrow during enforced mobilization. Blood. 2004;104:993–1001. doi: 10.1182/blood-2003-10-3702. [DOI] [PubMed] [Google Scholar]
- 23.Pomin V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta. 2012;1820:1971–1999. doi: 10.1016/j.bbagen.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Cumashi A., Ushakova N.A., Preobrazhenskaya M.E., D’Incecco A., Piccoli A., Totani L., Tinari N., Morozevich G.E., Berman A.E., Bilan M.A., et al. Comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 25.Panagos C.G., Thomson D.S., Moss C., Hoghes A.D., Kelly M.S., Liu Y., Chai W., Venkatasamy R., Spina D., Page C.P., et al. Fucosylated chondroitin sulfates from the body wall of the sea cucumber Holothuria forskali. Conformation, selectin binding, and biological activity. J. Biol. Chem. 2014;289:28284–28298. doi: 10.1074/jbc.M114.572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes A.J., Melrose J. Glycans and glycosaminoglycans in neurobiology: Key regulators of neuronal cell function and fate. Biochem. J. 2018;475:2511–2545. doi: 10.1042/BCJ20180283. [DOI] [PubMed] [Google Scholar]
- 27.Ananikov V.P., Eremin D.B., Yakukhnov S.A., Dilman A.D., Levin V.V., Egorov M.P., Karlov S.S., Kustov L.M., Tarasov A.L., Greish A.A., et al. Organic and hybrid systems: from science to practice. Mend. Commun. 2017;27:425–438. doi: 10.1016/j.mencom.2017.09.001. [DOI] [Google Scholar]