Abstract
As a nitric oxide precursor, beetroot juice (BJ) is known to enhance high-intensity exercise performance (80–100% VO2max) yet its impacts on higher intensity sprint exercise (>100% VO2max) remain to be established. This study sought to examine the effects of BJ supplementation on performance and subsequent fatigue during an all-out sprint exercise. Using a randomized cross-over, double-blind, placebo-controlled design, 15 healthy resistance-trained men (22.4 ± 1.6 years) ingested 70 mL of either BJ or placebo. Three hours later, participants undertook a 30-s all-out Wingate test. Before and after the sprint exercise and at 30 s and 180 s post-exercise, three countermovement jumps (CMJ) were performed and blood lactate samples were obtained. Compared to placebo, BJ consumption improved peak (placebo vs. BJ, 848 ± 134 vs. 881 ± 135 W; p = 0.049) and mean (641 ± 91 vs. 666 ± 100 W; p = 0.023) power output and also reduced the time taken to reach Wpeak in the Wingate test (8.9 ± 1.4 vs. 7.3 ± 0.9 s; p = 0.003). No differences were detected in the fatigue index. In addition, while over time CMJ height and power diminished (ANOVA p < 0.001) and blood lactate levels increased (ANOVA p < 0.001), no supplementation effect was observed. Our findings indicate that while BJ supplementation improved performance at the 30-s cycling sprint, this improvement was not accompanied by differences in fatigue during or after this type of exercise.
Keywords: nitric oxide, nitrates, muscle power, muscle fatigue
1. Introduction
Dietary nitrate supplementation has been described as a potential ergogenic aid for high-intensity exercise efforts (80–100% VO2max) as it reduces the oxygen cost of ATP synthesis and ATP cost of muscle contraction thus improving muscle contraction/relaxation, force and power production [1,2,3]. However, the impacts of nitrate supplementation on all-out sprint exercise performance (>100% VO2max), and particularly its effects on the fatigue induced by this mode of exercise [4,5,6] have been scarcely addressed.
Ingested nitrate (NO3−) is a well-known precursor of nitric oxide (NO) in humans [7]. Around 25% of circulating NO3− is taken up by salivary gland acinar cells in a process facilitated by sialin [8,9]. Oral microorganisms, particularly those on the posterior aspect of the tongue, initiate the reduction of NO3− into nitrite (NO2−), which subsequently in the stomach and gut, can be converted into NO and be absorbed under hypoxic conditions [8,9,10]. The majority of the remaining NO3− and NO2− molecules that reach the intestine are absorbed by this organ increasing NO levels in blood [9]. NO offers several exercise adaptation benefits [11] through its effects of inducing vasodilatation, reducing blood viscosity, and promoting muscular oxygen perfusion and gas exchange [12]. In skeletal muscle, NO reduces oxidative stressor production and promotes mitochondrial biogenesis and efficiency [13,14]. Moreover, NO it is also able to increase force and power production during muscle contraction, decreasing the cost of ATP needed as well as the oxygen required to synthesize ATP [1,2,3].
Beetroot juice (BJ) is a NO3−-rich supplement commonly used because of its high betacyanin and polyphenol contents that promote NO synthesis to a greater extent than other NO3− salts [15,16]. The ergogenic effect of NO3− supplementation was initially observed in terms of metabolic adaptations to endurance training [17]. However, despite the known impact of BJ on aerobic performance, recent data indicate a potential effect of NO3--rich supplements on anaerobic exercise [4].
Interestingly, the observed benefits of BJ only seem to affect type II muscle fibers [11]. In these fibers, NO stimulates calcium release into the sarcoplasm via calsequestrin upregulation [18] and reduces the phosphocreatine degradation rate, decreasing ATP cost across several ranges of exercise intensity [19]. During sprint exercise (>100% VO2max), type II muscle fibers are mainly recruited to satisfy the high muscle contraction demands. In these glycolytic fibers, exercise leads to a reduced pH in comparison to oxidative fibers. Intra-cell acidity also promotes the reduction of NO2− to NO [8]. In turn, the increase in NO availability may diminish the ATP and phosphocreatine required by each muscle contraction with the consequence of an ergogenic effect of NO3− supplementation in sprint exercise achieved by improving power production and attenuating the fatigue induced by this exercise mode [20,21].
However, despite acute BJ administration emerging as an effective strategy to improve different modes of exercise performed to exhaustion [22], the influence of this supplement has been scarcely explored in sprint exercise [1,2,3,20,23,24]. Two studies have shown that BJ supplementation increases peak power output in a 3–4 s [23] or 30 s cycle ergometer exercise [20,23,24]. However, the benefits of BJ on the muscle power produced in a vertical jump have not been investigated. The countermovement jump (CMJ) is a useful test to explore the muscle contractile properties and neuromuscular performance of the lower-limbs [25]. This test has been extensively used in high-intensity sports in which the stretch-shortening cycle plays a pivotal role [26]. Further, given that fatigue can be defined as a reduction in strength or power regardless of the ability to sustain a required task [27], conducting the CMJ before and after an extenuating task is an effective method of monitoring muscle fatigue [28]. In this context, the present study was designed to examine the effects of BJ, as a NO3−-rich supplement, on performance at a single 30-s all-out sprint exercise and the fatigue caused by the exercise bout. Our working hypothesis was that BJ intake would increase the peak power generated by muscle contraction and reduce the time needed to achieve this peak power output with the consequence of diminished neuromuscular fatigue after the sprint.
2. Materials and Methods
2.1. Participants
Fifteen young men (age 22.4 ± 1.6 years, height 178 ± 6 cm, weight 76.9 ± 10.3 kg) were recruited. All subjects had at least 18 months of experience with resistance exercise, training 3 sessions per week (e.g., bench press and leg press 1RM were 1.0 and 1.5-fold higher than their body mass weight, respectively) and were familiar with the 30-s all-out Wingate and CMJ tests. Subjects were instructed to refrain from taking sports supplements, medical supplements or any ergogenic aids during the 3 months before the tests and were excluded if they failed to comply. Further exclusion criteria were smoking or cardiovascular, pulmonary, metabolic or neurologic disease.
Candidate participants were first informed of the experimental protocol before giving their written consent. The study was approved by the Ethics Committee of Alfonso X University in (code 1.010.704) accordance with the latest version (7th) of the Declaration of Helsinki.
2.2. Experimental Design
The study design was randomized cross-over, placebo-controlled and double-blind. Participants reported to the laboratory on two separate days under the same experimental conditions (72 h between sessions, 0.5 h difference in test initiation). Participants were instructed to avoid any form of exercise in the 72 h leading up to each test.
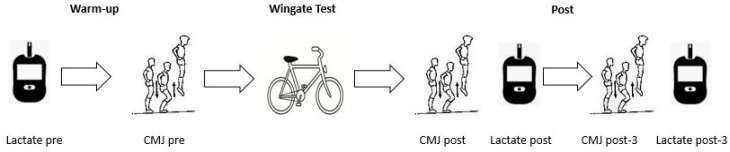
In session 1, participants were subjected to a preliminary assessment of body composition and underwent a familiarization session of the experimental protocol. Then, on two separate occasions (sessions 2 and 3) as they arrived at the laboratory, participants were provided with a supplement containing either placebo (placebo) or BJ. The trial was double-blinded such that one researcher (P.V.-H.) allocated all the participants’ drinks in a counter-balanced fashion (in each trial 50% of participants ingested placebo and 50% ingested BJ beverages) with random assignment to each supplement (using Excel, Microsoft, Washington, DC, USA) and this researcher did not take part in the subsequent experimental procedures or statistical analysis of data. Three hours after taking the supplement, all participants performed a 30 s all-out Wingate test on a Monark ergometer (Ergomedic 828E, Vansbro, Sweden), as previously described [19]. Strong verbal encouragement was provided in all the sprint tests. In addition, data were collected in three CMJ jumps and blood samples for lactate determination were obtained in duplicate before (Pre) and after the sprint exercise at 30 s (Post) and 180 s post-exercise (Post-3). The study procedure is illustrated in Figure 1.
Figure 1.
Experimental procedure.
2.3. Placebo vs. BJ Ingestion
After an overnight fast, participants reported to the laboratory 3 h before the first CMJ jump test. Upon arrival, they were provided with either 70 mL of BJ (containing 6.4 mmol of NO3−) or the same drink lacking NO3− (placebo, 0.04 mmol of NO3−) (Beet-It-Pro Elite Shot, James White Drinks Ltd., Ipswich, UK) as described elsewhere [20].
All participants were instructed to follow a diet sheet the day before each trial that consisted of 60% carbohydrates, 30% fat and 10% proteins. Dietary NO3− was limited by providing subjects a list of NO3−-rich foods (e.g., beetroot, celery or spinach) they should avoid in the 48 h before each trial. Also, in the 24 h leading up to each test, subjects were encouraged to avoid brushing their teeth or use an oral antiseptic rinse, or ingest gum, sweets or stimulants (e.g., caffeine) that could alter the oral microbiota and interfere with NO3− reduction.
2.4. Sprint Performance Variables
Power output (W) was monitored second-by-second in all sprints. Mean power output (Wmean) was calculated as the average power generated during the 30-s test. Peak power output (Wpeak) was taken as the highest W value recorded. The time (s) taken to reach Wpeak was also recorded. Minimum power output (Wmin) was considered as the lowest W value recorded during the 10 last seconds of the test. Finally, the fatigue index (FI) was calculated using the equation: FI = (Wpeak − Wmin)/Wpeak. In addition, mean power output in each Wingate test was calculated for the entire test (30 s) and at 10 s (Wmean0–10s, Wmean10–20s and Wmean20–30s) and 15 s intervals (Wmean0–15 and Wmean15–30s) as described elsewhere [19].
2.5. Neuromuscular Fatigue
Neuromuscular fatigue in the legs was measured as the loss of height and power in a CMJ test performed on a force platform (Quattro Jump model 9290AD; Kistler Instruments, Winterthur, Switzerland) [28,29,30]. Participants were highly familiarized with this vertical jump test. Two CMJ were performed before (Pre) and after the Wingate test at 30 s (Post-1) and 180 s post-exercise (Post-3). At each time-point, mean values of height (cm), mean power (CMJWmean) and peak power (CMJWpeak) were recorded.
2.6. Blood Lactate
Before the first CMJ and immediately after the subsequent vertical jumps, capillary blood samples (5 µL) were obtained from the index finger of the right-hand for lactate determination using a Lactate ProTM 2 LT-1710 Instrument (Arkray Fatory Inc., KDK Corporation, Shiga, Japan).
2.7. Statistical Analysis
The Shapiro-Wilk test was first performed to assess the distribution of the data. Then paired t-tests for normally-distributed data and the Wilcoxon test for non-normally distributed variables (Time-to-Wpeak, W0–15s, W15–30s, W10–20s and W20–30s) were used to compare all sprint variables between the experimental conditions (placebo vs. BJ). A two-way ANOVA for repeated measures was also used to compare placebo vs. BJ for two between-subject conditions: supplementation (placebo vs. BJ) and time (pre-exercise, 30 s post-exercise and 180 s post-exercise). Before the ANOVA, we confirmed there was no violation of the sphericity assumption using Mauchly’s test of sphericity. Holm-Bonferroni was used as post-hoc test when significant differences were detected. Values are provided as the mean ± standard deviation (SD). Significance was set at p < 0.05. All statistical tests were performed using the software package SPSS v.18.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Sprint Performance Variables
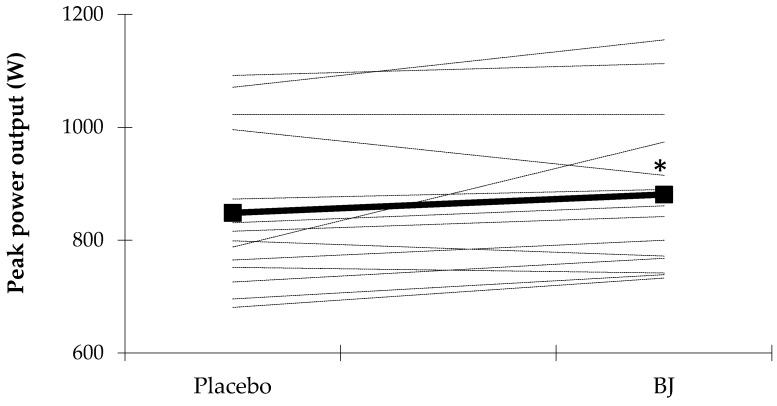
The effects of placebo and BJ on the 30-s all-out sprint test are shown in Table 1. Compared to placebo, BJ supplementation increased Wpeak (~3.8%; p = 0.049) and Wmean (~4.0%; p = 0.023), while reduced time to Wpeak (~18%; p = 0.003). In 12 of the 15 participants, Wpeak was higher after BJ administration compared to the placebo condition (Figure 2). In contrast, no significant differences were observed in Wmin (~4.4%; p = 0.064) or FI (~0.22%; p = 0.914).
Table 1.
Effects of placebo or BJ intake on performance at a 30-s sprint (Wingate) test.
| Variable | Placebo | BJ | p-Value |
|---|---|---|---|
| Wpeak (W) | 848 ± 134 | 881 ± 135 | 0.049 |
| Time to Wpeak (s) | 8.9 ± 1.4 | 7.3 ± 0.9 | 0.003 |
| Wmean (W) | 641 ± 91 | 666 ± 100 | 0.023 |
| Wmin (W) | 453 ± 64 | 472 ± 72 | 0.064 |
| Fatigue index (FI) (%) | 46 ± 8 | 46 ± 7 | 0.914 |
Values are means ± standard deviation. BJ, beetroot juice.
Figure 2.
Effects of placebo and BJ intake on Wpeak after sprint exercise. Means and individual values are shown as a bold or dotted line respectively. * p < 0.05 compared to placebo. BJ, beetroot juice.
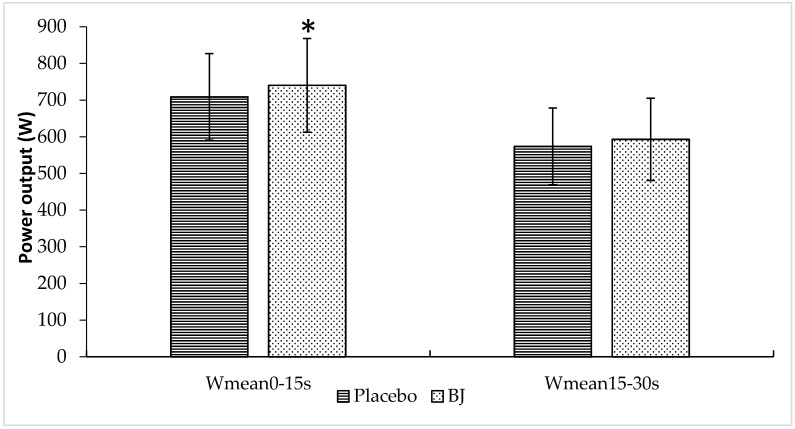
Values of Wmean were recorded in 10 and 15 s intervals. Figure 3 displays Wmean values in 15 s intervals (W0–15s and W15–30s). An increased Wmean was observed after BJ intake compared to placebo during the first 15 s of the sprint (placebo vs. BJ, 709 ± 113 vs. 740 ± 122 W0–15s; p = 0.017), while no significant differences were recorded during the last 15 s (placebo vs. BJ, 574 ± 80 vs. 593 ± 87 W15–30s; p = 0.173).
Figure 3.
Effects of placebo and BJ intake on Wmean values recorded over 15 s intervals (A, W0–15s; B, W15–30s) after the sprint. * p < 0.05 compared to placebo.
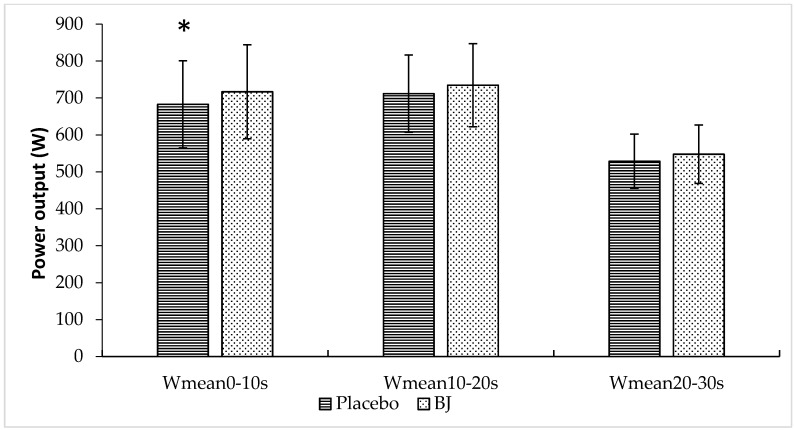
Figure 4 provides Wmean values in 10 s intervals (W0–10s, W10–20s and W20–30s). Compared to placebo, a significant increase in Wmean was observed after BJ intake during the first 10 s interval (placebo vs. BJ, 683 ± 118 vs. 717 ± 127 W0–10s; 5.0%, p = 0.043), while no significant differences emerged for the intervals 10–20 s (placebo vs. BJ, 712 ± 105 vs. 735 ± 113 W10–20s; 3.2%, p = 0.078) or 20–30 s (placebo vs. BJ, 529 ± 73 vs. 548 ± 79 W20–30s; 3.6%, p = 0.30).
Figure 4.
Effects of placebo and BJ intake on Wmean values recorded over 10 s intervals (Wmean0–10s, Wmean10–20s and Wmean20–30s) after the sprint. Values are means ± standard deviation. * p < 0.05 compared to placebo.
3.2. Neuromuscular Fatigue and Blood Lactate Concentrations
The effects of placebo and BJ intake on neuromuscular fatigue measured through the CMJ test are shown in Table 2. The 30-s all-out Wingate test led to significant reductions in CMJheight, CMJWpeak and CMJWmean (ANOVA time effect, p < 0.001). Compared to Pre, a significant decrease was observed at Post and Post-3 in CMJheight (Pre vs. Post, ~38%; Pre vs. Post-3, ~19%; p < 0.001), CMJWpeak (Pre vs. Post, ~28%; Pre vs. Post-3, ~10%; p < 0.001) and CMJWmean (Pre vs. Post, ~21%; Pre vs. Post-3, ~14%; p < 0.001); while a significant increase was observed for Post-3 compared to Post in all variables (~24% CMJheight, ~22% CMJWpeak, ~21% CMJWmean; p < 0.001). No supplementation or interaction effects (supplement × time) were observed.
Table 2.
Effects of placebo or BJ intake in a neuromuscular fatigue (CMJ) after a 30-s all-out Wingate test.
| Variable | Placebo | BJ | Suppl. | Time | Suppl. × Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Post-3 | Pre | Post | Post-3 | ||||
| CMJheight (cm) | 30.8 ± 4.6 | 19.5 ± 5.1 a | 25.0 ± 4.3 a,b | 31.5 ± 3.4 | 19.0 ± 4.2 a | 25.3 ± 4.2 a,b | 0.863 | <0.001 | 0.864 |
| CMJWpeak (W) | 50.5 ± 4.7 | 36.9 ± 5.9 a | 45.2 ± 4.6 a,b | 51.1 ± 3.6 | 36.6 ± 4.9 a | 44.7 ± 4.5 a,b | 0.947 | <0.001 | 0.850 |
| CMJWmean (W) | 27.3 ± 3.6 | 20.0 ± 4.2 a | 24.0 ± 3.8 a,b | 27.9 ± 3.3 | 19.6 ± 3.5 a | 23.8 ± 3.6 a,b | 0.994 | <0.001 | 0.850 |
Values are means ± standard deviation. a p < 0.05 compared to Pre; b p < 0.05 compared to Post. Pre, before sprint exercise; Post, post-exercise; Post-3, 3 min post-exercise.
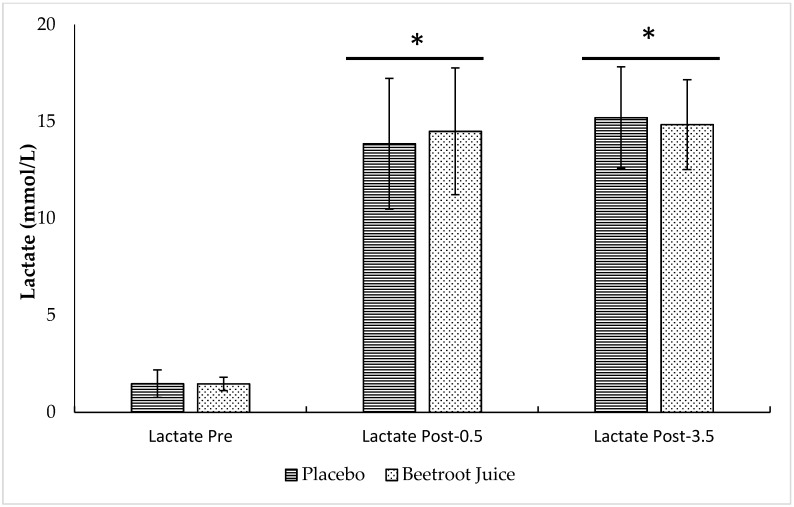
Figure 5 illustrates the blood lactate values recorded after the sprint test. Blood lactate concentration was significantly higher after the 30-s all-out Wingate test (ANOVA time effect, p < 0.001). Compared to Pre (placebo, 1.47 ± 0.71 mmol/L; BJ, 1.47 ± 0.35 mmol/L), blood lactate was significantly higher at the time points Post-0.5 (placebo, 13.86 ± 3.37 mmol/L; BJ, 14.49 ± 3.27 mmol/L; p < 0.001) and Post-3.5 (placebo, 15.20 ± 2.62 mmol/L; BJ, 14.84 ± 2.32 mmol/L; p < 0.001). No supplementation (ANOVA supplementation effect, p = 0.858) or interaction effects (ANOVA supplement × time effect, p = 0.719) were detected.
Figure 5.
Blood lactate concentrations recorded after the sprint for the placebo and BJ conditions. Values are means ± standard deviation. * p < 0.05 compared to Pre. Pre, before sprint exercise; Post-0.5, 0.5 min post-exercise; Post-3.5, 3.5 min post-exercise.
4. Discussion
The findings of our study indicate that BJ supplementation enhances peak and mean power output, particularly during the first half of a 30-s all-out sprint test, reducing the time taken to reach peak power output. Despite this improved sprint performance, neuromuscular fatigue caused by this exercise mode was similar after the intake of BJ or placebo. These observations suggest that NO3−-rich supplements enhance sprint performance without producing cumulative impacts on fatigue levels.
NO3− supplementation has been linked to an increase in Wpeak generated by leg extension in an isokinetic machine at several angular velocities (from 0 to 6.28 rad/s) in healthy subjects (~5–6%) [2,31] and patients with heart disease (~12%) [32]. There are two reports in the literature of investigations examining the effects of an acute dose of BJ on a 30-s all-out Wingate test [19,23]. In the study by Domínguez et al. [20], a significant increase in Wpeak was observed (~6%) while Rimer et al. [23] observed no such effect. It should be mentioned that in the study by Rimer’s group [23], the 30-s Wingate test was performed after 4 series of 3–4 s all-out sprint trials and 5 min of passive rest; and despite the lack of difference in the Wingate test, the delta change in peak power output produced in the 3–4 sprints indicated an increase of ~6.0% after BJ intake compared to placebo. In the present study, a similar increase in peak power output was observed after the 30-s all-out Wingate test (~4%) and this performance improvement seems to occur during the first 15 s of the sprint and hereafter decline. These data indicate that BJ supplementation may cause a transient ergogenic elevation of peak power output during the first few seconds of sprint exercise, and that this effect could be attenuated after several doses of BJ [6].
The use of an isokinetic or isoinertial cycle ergometer for the sprint test may be a confounding factor when examining the ergogenic effect of BJ supplementation [19,23]. In this study, we used an isoinertial cycle ergometer, which measures power output based on a variable pedaling rate at a fixed load (7.5% body mass) [20]. In contrast, using an isokinetic cycle ergometer, the pedaling rate is predetermined [23]. Pedaling rate is strongly related to the angular velocity of the knee and hip, and can be used as an indicator of muscle contraction velocity [33] and type II motor unit recruitment [34]. An ergogenic effect of BJ intake has been observed not only in sprint exercise [20] but also in other tasks (e.g., leg extension) under elevated angular velocities [2,31,32]. Consistent with this idea, the present data revealed a greater effect of BJ on sprint performance (Wpeak and time to Wpeak) for the higher angular velocities.
Animal studies have shown that NO increases acetylcholine activity, particularly in type II motor units, which amplify depolarization of the muscle fibers [35] whereas BJ supplementation induces the elevation of intracellular Ca2+ concentrations accompanied by calsequestrin 1 and dihydropyridine receptor upregulation in fast-twitch muscles [18]. Although these mechanisms have not yet been proven in humans, NO3− supplementation likely increases force production by inducing type II muscle fiber depolarization and increasing myoplasm Ca2+ concentrations facilitating muscle contraction [18,36] by increasing the number of actin-myosin cross-bridges [37]. This improvement in muscle force production in response to BJ consumption has been detected as a higher rate of force development (RFD) [37] through increased peak power output, the time taken to reach that power output and a faster reaction time [4]. In effect, Time to Wpeak and reaction time are key factors in sports performance, particularly in disciplines in which acceleration determines performance [38,39]. Here, BJ supplementation led to a pronounced reduction in Time to Wpeak during a 30-s all-out Wingate test, coinciding with previous data in which the increase in Wpeak was accompanied by a shorter time needed to reach Wpeak [20]. A reduced Time to Wpeak was also found when a transient increase in Wpeak was not detected after prolonged doses of BJ supplements and repeated sprint exercise [6]. In these two previous studies [6,20], the shortened Time to Wpeak was lower (~0.7 and ~0.2 s, respectively) than the difference observed here (~1.6 s). The greater improvement in Time to Wpeak reported here may be explained by a reduced level of anaerobic training of our subjects compared to participants of the studies by Dominguez et al. [20] and Jonvik et al. [6], who were well-trained in anaerobic disciplines.
Anaerobic pathways supply ~75% of energy requirements in a 30-s all-out sprint exercise [40,41]. During the first 6 s, ready to use sources of energy are needed to produce maximal peak power output in the shortest time possible. Accordingly, free ATP and PCr stores are critical during the initial part of a sprint [42]. At this time (first 5–10 s), a marked depletion in PCr stores occurs and this compromises power output coinciding with the time at which glycolysis attains its maximum rates [43]. Along with an increased force production capacity, BJ supplementation leads to the reduced ATP cost of muscle contraction [19,44] perhaps by reducing PCr degradation rates. The reduced ATP requirements of muscle contraction together with the maintenance of free ATP and PCr stores promoted by NO3− supplementation may give rise to a higher power output during a longer period of time coinciding with the increase in mean power output produced during the first 15 s of the sprint after BJ intake.
Since BJ consumption led to elevated peak and mean power output during the first 15 s of the sprint, we could argue that the muscular fatigue that takes place during the last 15 s and at the end of the sprint will be exaggerated.
The fatigue index calculated during the sprint indicated no differences between the supplements. In addition to the mentioned maintenance of anaerobic sources of energy production, the contribution of aerobic energy production increases during the last 15 s of a Wingate test [41,43]. Since NO3− supplementation is known to reduce the oxygen cost of ATP synthesis [45] and to preserve ATP and PCr stores [19], the lack of differences between supplements (placebo vs. BJ) may be explained by a higher capacity of NO3− to induce ATP store maintenance and thus reduce the cost of its synthesis by both aerobic and anaerobic sources.
Immediately after the sprint exercise, two CMJ jumps were performed at 30 s and 180 s. CMJ is a vertical jump test that assesses muscle contractile properties and neuromuscular performance (anaerobic power) of the lower-limbs [46,47]. Variables such as CMJ height and power have also been used as indicators of neuromuscular fatigue [48,49]. Some authors have argued that the CMJ test after extenuating exercise [28] serves to assess muscle capacity to replenish ~50% of depleted PCr stores at 30-s post-exercise [50] and to recover almost completely depleted PCr stores at 180 s post-exercise [51]. Hence a pronounced reduction in CMJ performance (height and power) after 180 s will reflect the diminished PCr store replenishment capacity of muscle fibers affecting the stretch-shortening cycle and force production [52]. The present observations are in good agreement with prior findings in which an effect of time in reducing CMJ height and mean power output was seen after a 30-s all-out Wingate test [28,29,30]. The decrease in CMJ performance was more pronounced at 30 s (~30%) compared to 180 s post-exercise (~10%). However, no differences between supplementation conditions were observed.
In our study, BJ supplementation overall did not give rise to a greater fatigue index during the second half of the test or to neuromuscular fatigue as measured in CMJ tests, after the 30-s all-out sprint test. These results indicate that the improved sprint performance induced by BJ as a NO3−-rich supplement may not be accompanied by more fatigue.
5. Limitations
Our study has several limitations. BJ is a NO3−-rich supplement known to increase circulating NO2− and NO levels [7]. However, these levels were not measured before the intake by the participants of placebo or BJ. Further, the number of subjects recruited (N = 15), although appropriate for this type of study, limits the detection of small changes that could be the consequence of BJ administration. Finally, participants were not trained cyclists and therefore the ergogenic effects produced by BJ cannot be directly transferred to this sports modality. On the up-side, however, the inclusion of resistance trained individuals was useful to explore the physiological effects of BJ supplementation on skeletal muscle power production and to examine fatigue induced by a sprint exercise to exhaustion.
6. Conclusions
In conclusion, BJ supplementation produced an ergogenic effect in a 30-s all-out Wingate test in terms of increasing Wpeak, Time to Wpeak and Wmean, particularly during the first half of the sprint, without increasing muscular fatigue accumulation during or after this extenuating sprint exercise. These findings suggest that NO3−-rich supplements could be a suitable strategy to improve performance in sports modalities in which power and acceleration largely determine performance.
Author Contributions
R.D. and S.F.d.S. designed the experiment; P.J. and P.V.-H. recruited the subjects and hosted the informative session; P.V.-H. and L.G.G.-R. checked that subjects followed the diet guidelines and the timing of supplement ingestion. R.D., E.C., P.J., P.V.-H. and L.G.G.-R. performed the experiments; E.C., A.P.-L. and S.F.d.S. analyzed the data; R.D. and S.F.d.S. conducted the statistical analysis; and R.D., E.C., P.J., P.V.-H., L.G.G.-R. A.P.-L. and S.F.d.S. wrote the manuscript.
Funding
Publication and translation costs will be met using funds from the competitive project of the VIII Announcement of the Banco Santander and Fundación UAX (project: 1010704).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Haider G., Folland G.P. Nitrate Supplementation Enhances the Contractile Properties of Human Skeletal Muscle. Med. Sci. Sports Exerc. 2014;46:2234–2243. doi: 10.1249/MSS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 2.Coggan A.R., Leibowitz J.L., Kadkhodayan A., Thomas D.P., Ramamurthy S., Spearie C.A., Waller S., Farmer M., Peterson L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide. 2015;48:16–21. doi: 10.1016/j.niox.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitfield J., Ludzki A., Heigenhauser G., Senden S., Verdijk L., Van L., Spriet L.L., Holloway G.P. Beetroot Juice Supplementation Reduces Whole Body Oxygen Consumption But Does Not Improve Indices Of Mitochondrial Efficiency in Human Skeletal Muscle. J. Physiol. 2016;594:421–435. doi: 10.1113/JP270844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson C., Vanhatalo A., Fulford J., Carter J., Nyman L., Bailey S.J., Jones A.M. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide. 2016;61:55–61. doi: 10.1016/j.niox.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Thompson C., Wylie L.J., Blackwell J.R., Fulford J., Black M.I., Kelly J., McDonagh S.T., Carter J., Bailey S.J., Vanhatalo A., et al. Influence of dietary nitrate supplementation on physiological and muscle metabolic adaptations to sprint interval training. J. Appl. Physiol. 2018;18:642–652. doi: 10.1152/japplphysiol.00909.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonvik K.L., Nyakayiru J., Van Dicj J.W., Maase K., Ballak S.B., Senden J.M.G., Van Loon L.J.C., Verdjik L.B. Repeated-sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive and elite sprint athletes. Eur. J. Sport Sci. 2018;18:524–533. doi: 10.1080/17461391.2018.1433722. [DOI] [PubMed] [Google Scholar]
- 7.Maughan R.J., Burke L.M., Dvorak J., Larson-Meyer D.E., Peeling P., Phillips S.M., Rawson E.S., Walsh N.P., Garthe I., Geyer H., et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018;52:439–455. doi: 10.1136/bjsports-2018-099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 9.Qu X.M., Wu Z.F., Pang B.X., Jin L.Y., Qin L.Z., Wang S.L. From nitrate to nitric oxide: The role of salivary glands and oral bacteria. J. Dent. Res. 2016;95:1452–1456. doi: 10.1177/0022034516673019. [DOI] [PubMed] [Google Scholar]
- 10.Tiso M., Schechter A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE. 2015;10:e0119712. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson S.K., Hirai D.M., Copp S.W., Holdsworth C.T., Allen J.D., Jones A.M., Musch T.I., Poole D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzurum S.C., Ghosh S., Janocha A.J., Xu W., Bauer S., Bryan N.S., Tejero J., Hermann C., Hille R., Stuehr D.J., et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl. Acad. Sci. USA. 2007;104:17593–17598. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dejam A., Hunter C., Schechter A., Gladwin M. Emerging role of nitrite in human biology. Blood. Cells Mol. Dis. 2004;32:423–429. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Pinna M., Roberto S., Milia R., Maronquiu E., Olla S., Loi A., Migliaccio G.M., Padulo J., Orlandi C., Tocco F., et al. Effect of beetroot juice supplementation on aerobic response during swimming. Nutrients. 2014;6:605–615. doi: 10.3390/nu6020605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peri L., Pietraforte D., Scorza G., Napolitano A., Fogliano V., Minetti M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: A new biological function for polyphenols with a catechol group? Free Radic. Biol. Med. 2005;39:668–681. doi: 10.1016/j.freeradbiomed.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Thompson C., Vanhatalo A., Kadach S., Wylie L.J., Fulford J., Ferguson S.K., Blackwell J.R., Bailey S.J., Jones A.M. Discrete physiological effects of beetroot juice and potassium nitrate supplementation following 4 weeks sprint interval training. J. Appl. Physiol. 2018;124:1519–1528. doi: 10.1152/japplphysiol.00047.2018. [DOI] [PubMed] [Google Scholar]
- 17.Domínguez R., Cuenca E., Maté-Muñoz J.L., García-Fernández P., Serra-Paya N., Estevan M.C., Herreros P.V., Garnacho-Castaño M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients. 2017;9:43. doi: 10.3390/nu9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández A., Schiffer T.A., Ivarsson N., Cheng A.J., Bruton J.D., Lundberg J.O., Weitzberg E., Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fasttwitch muscle. J. Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones A.M., Ferguson S.K., Bailey S.J., Vanhatalo A., Poole D.C. Fiber-type specific effects of dietary nitrate. Exerc. Sci. Sports Rev. 2016;44:53–60. doi: 10.1249/JES.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 20.Domínguez R., Garnacho-Castaño M.V., Cuenca E., García-Fernández P., Muñoz-González A., de Jesús F., Lozano-Estevan M.C., Fernandes da Silva S., Veiga-Herreros P., Maté-Muñoz J.L. Effects of beetroot juice supplementation on a 30-s high-intensity inertial cycle ergometer test. Nutrients. 2017;9:12. doi: 10.3390/nu9121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domínguez R., Maté-Muñoz J.L., Cuenca E., García-Fernández P., Mata-Ordoñez F., Lozano-Estevan M.C., Veiga-Herreros P., Fernandes da Silva S., Garnacho-Castaño M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018;15:2. doi: 10.1186/s12970-017-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoon M.W., Johnson N.A., Chapman P.G., Burke L.M. The Effect of Nitrate Supplementation on Exercise Performance in Healthy Individuals: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2013;23:522–532. doi: 10.1123/ijsnem.23.5.522. [DOI] [PubMed] [Google Scholar]
- 23.Rimer E.G., Peterson L.R., Coggan A.R., Martin J.C. Increase in Maximal Cycling Power with Acute Dietary Nitrate Supplementation. Int. J. Sports Physiol. Perform. 2016;11:715–720. doi: 10.1123/ijspp.2015-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer S.J., Baur D.A., Spicer M.T., Vukovich M.D., Ormsbee M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sports Nutr. 2016;3:39. doi: 10.1186/s12970-016-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobbert M.F., van Soest A.J. Why do people jump the way they do? Exerc. Sport Sci. Rev. 2001;29:95–102. doi: 10.1097/00003677-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Van Hooren B., Zolotarjova J. The difference between countermovement and squat jump performance: A review of underlying mechanisms with practical applications. J. Strength Cond. Res. 2017;31:2011–2020. doi: 10.1519/JSC.0000000000001913. [DOI] [PubMed] [Google Scholar]
- 27.Rodacki A.L., Fowler N.E., Bennett S.J. Vertical jump coordination: Fatigue effects. Med. Sci. Sports Exerc. 2002;34:105–116. doi: 10.1097/00005768-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Maté-Muñoz J.L., Lougedo J.H., Barba M., García-Fernández P., Garnacho-Castaño M.V., Domínguez R. Muscular fatigue in response to different modalities of CrossFit sessions. PLoS ONE. 2017;12:0181855. doi: 10.1371/journal.pone.0181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garnacho-Castaño M.V., Domínguez R., Maté-Muñoz J.L. Understanding the meaning of the lactate threshold in resistance exercises. Int. J. Sports Med. 2015;36:371–377. doi: 10.1055/s-0034-1398495. [DOI] [PubMed] [Google Scholar]
- 30.Garnacho-Castaño M.V., Domínguez R., Ruiz-Solano P., Maté-Muñoz J.L. Acute physiological and mechanical responses during resistance exercise executed at the lactate threshold workload. J. Strength Cond. Res. 2015;29:2867–2873. doi: 10.1519/JSC.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 31.Coggan A.R., Broadstreet S.R., Mikhalkova D., Bole I., Leibowitz J.L., Kadkhodayan A., Park S., Thomas D.P., Thies D., Peterson L.R. Dietary nitrate-induced increases in human muscle power: High versus low responders. Physiol. Rev. 2018;6:2. doi: 10.14814/phy2.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coggan A.R., Leibowitz J.L., Spearie C.A., Kadkhodayan A., Thomas D.P., Ramamurthy S., Mahmood K., Park S., Waller S., Farmer M., et al. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: A double-blind, placebo-controlled, randomized trial. Circ. Heart Fail. 2015;8:914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin J.C., Brown N.A., Anderson F.C., Spirduso W.W. A governing relationship for repetitive muscular contraction. J. Biomech. 2000;33:969–974. doi: 10.1016/S0021-9290(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 34.Beelen A., Sargeant A.J. Effect of prior exercise at different pedalling frequencies on maximal power in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1993;66:102–107. doi: 10.1007/BF01427049. [DOI] [PubMed] [Google Scholar]
- 35.Petrov K.A., Malomouzh A.L., Kovyazina I.V., Kejci E., Nikitashina A.D., Prokurina S.E., Zobov V.V., Nikolsky E.E. Regulation of acetylcholinesterase activity by nitric oxide in rat neuromuscular junction via N-methyl-d-aspartate receptor activation. Eur. J. Neurosci. 2013;37:181–189. doi: 10.1111/ejn.12029. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen A.O., Kalnins V.I., Zubrycka E., Maclennan D.H. Assembly of the sarcoplasmic reticulum. Localization by immunofluorescence of sarcoplasmic reticulum proteins in differentiating rat skeletal muscle cell cultures. J. Cell Biol. 1977;74:287–298. doi: 10.1083/jcb.74.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marechal G., Gailly P. Effects of nitric oxide on the contraction of skeletal muscle. Cell. Mol. Life Sci. 1999;55:1088–1102. doi: 10.1007/s000180050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lockie R.G., Murphy A.J., Knight T.J., Janse de Jonge X.A. Factors that differentiate acceleration ability in field sport athletes. J. Strength Cond. Res. 2011;25:2704–2714. doi: 10.1519/JSC.0b013e31820d9f17. [DOI] [PubMed] [Google Scholar]
- 39.Mero A., Komi P.V., Gregor R.J. Biomechanics of sprint running. A review. Sports Med. 1992;13:376–392. doi: 10.2165/00007256-199213060-00002. [DOI] [PubMed] [Google Scholar]
- 40.Calbet J.A., Chavarren J., Dorado C. Fractional use of anaerobic capacity during a 30- and 45-s Wingate test. Eur. J. Appl. Physiol. Occup. Physiol. 1997;76:308–313. doi: 10.1007/s004210050253. [DOI] [PubMed] [Google Scholar]
- 41.Calbet J.A., De Paz J.A., Garatachea N., Cabeza de Vaca S., Chavarren J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. 2003;94:668–676. doi: 10.1152/japplphysiol.00128.2002. [DOI] [PubMed] [Google Scholar]
- 42.Gaitanos G.C., Williams C., Boobis L.H., Brooks S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 1993;75:712–719. doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- 43.Parolin M.L., Chesley A., Matsos M.P., Spriet L.L., Jones N.L., Heigenhauser G.J. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. 1999;277:890–900. doi: 10.1152/ajpendo.1999.277.5.E890. [DOI] [PubMed] [Google Scholar]
- 44.Kerley C.P. Dietary nitrate as modulator of physical performance and cardiovascular health. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:440–446. doi: 10.1097/MCO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 45.Wylie L.J., Mohr M., Krustrup P., Jackman S.R., Ermιdis G., Kelly J., Black M.I., Bailey S.J., Vanhatalo A., Jones A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013;113:1673–1684. doi: 10.1007/s00421-013-2589-8. [DOI] [PubMed] [Google Scholar]
- 46.Bosco C., Montanari G., Ribacchi R., Giovenali P., Latteri F., Iachelli G., Faina M., Colli R., Dal Monte A., La Rosa M. Relationship between the efficiency of muscular work during jumping and the energetics of running. Eur. J. Appl. Physiol. Occup. 1987;56:138–143. doi: 10.1007/BF00640636. [DOI] [PubMed] [Google Scholar]
- 47.Bosco C., Tihanyi J., Komi P.V., Fekete G., Apor P. Store and recoil of elastic energy in slow and fast types of human skeletal muscles. Acta Physiol. Scand. 1982;116:343–349. doi: 10.1111/j.1748-1716.1982.tb07152.x. [DOI] [PubMed] [Google Scholar]
- 48.Cormie P., McBridge J.M., McCaulley G.O. Power-time, force-time, and velocity-time curve analysis of the countermovement jump: Impact of training. J. Strength Cond. Res. 2009;23:177–186. doi: 10.1519/JSC.0b013e3181889324. [DOI] [PubMed] [Google Scholar]
- 49.Claudino J.G., Cronin J., Mezêncio B., McMaster D.T., McGuigan M., Tricoli V., Amadio A.C., Serrao J.C. The countermovement jump to monitor neuromuscular status: A meta-analysis. J. Sci. Med. Sport. 2017;20:397–402. doi: 10.1016/j.jsams.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Skare O.C., Skadberg O., Wisnes A.R. Creatine supplementation improves sprint performance in male sprinters. Scand. J. Med. Sci. Sports. 2001;11:96–102. doi: 10.1034/j.1600-0838.2001.011002096.x. [DOI] [PubMed] [Google Scholar]
- 51.Tomlin D.L., Wenger H.A. The Relationship between Aerobic Fitness and Recovery from High Intensity Intermittent Exercise. Sports Med. 2001;31:1–11. doi: 10.2165/00007256-200131010-00001. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa M., Dousset E., Avela J., Kyrolainen H., Kallio J., Linnamo V., Komi P.V. Changes in the soleus muscle architecture after exhausting stretch-shortening cycle exercise in humans. Eur. J. Appl. Physiol. 2016;97:298–306. doi: 10.1007/s00421-006-0180-2. [DOI] [PubMed] [Google Scholar]