Abstract
Identification of complementary DNAs encoding the human glucocorticoid receptor predicts two protein forms, of 777 (α) and 742 (β) amino acids, which differ at their carboxy termini. The protein s contain a cysteine/lysine/arginine-rich region which may define the DNA -binding domain. Pure radiolabelled glucocorticoid receptor, synthesized in vitro, is immunoreactive and possesses intrinsic steroid-binding activity characteristic of the native glucocorticoid receptor.
TRANSCRIPTIONAL regulation of development and homeostasis is controlled in complex eukaryotes by a wide variety of regulatory substances, including steroid hormones. The latter exert potent effects on development and differentiation in phylogenetically diverse organisms and their actions are mediated as a consequence of their interactions with specific, high-affmity binding proteins referred to as receptors1–6. Many of the primary effects of steroid hormones involve increased transcription of a subset of genes in specific cell types7,8. The structural characterization of the steroid receptor protein and the definition of the molecular actions of the steroid hormone/receptor complex would help to define the biochemical events which modulate gene transcription. A series of receptor proteins, each specific for one of several known steroid hormones, are distributed in a tissue-specific fashion, although many cell types simultaneously express receptors for several steroid hormones9,10. It is postulated that steroids enter cells by facilitated diffusion and bind to the specific receptor protein, initiating an allosteric alteration of the complex. As a result of this transformation, the steroid hormone/receptor complex appears capable of binding high-affinity sites on chromatin and modulating transcription of specific genes by a mechanistically unknown process11,12.
The glucocorticoid receptor is widely distributed and expressed in many cultured cell lines, and the control of gene expression by glucocorticoids, therefore, has been widely studied as a model for transcriptional regulation. A number of glucocorticoid-responsive transcription units, including mouse mammary tumour virus (MMTV)13,14, mouse and human metal-lothionein15–16, rat α2u-globulin17 and rat and human growth hormone18–20 genes have been identified. DNA sequences mediating transcriptional stimulation of several of these genes have been localized. For MMTV, these sequences are discrete genomic regions upstream of the transcriptional start site which appear to exert their actions independently of orientation and position21,22. The steroid/receptor complex appears to bind to these regulatory sequences and purified receptor has been used to define the specific binding sites23–26. Based on the footprinting analyses of several responsive genes, a consensus DNA binding sequence sharing the core sequence 5’ TGT/CTCT 3’ has been proposed27.
The ability of the glucocorticoid-responsive element (GRE) to alter its position and orientation yet still maintain promoter inducibility suggests that it resembles the class of cis-acting regulatory sequences termed enhancers21. First discovered in viruses and subsequently in cellular genes, these sequences are necessary for efficient transcription in vivo28–31. It has been suggested that enhancers are recognized by trans- acting factors that mediate regulatory effects by tissue-specific transcriptional control. Although the enhancer factors have not been well characterized, the glucocorticoid receptor may serve as a paradigm for these putative gene activator proteins.
The availability of radiolabelled high-affinity glucocorticoid analogues such as dexamethasone and triamcinolone acetonide has led to the development of purification strategies resulting in the isolation of nearly pure rat and human receptors32,33. Although the receptor migrates as a dimer in sucrose gradients, analysis on denaturing SDS-polyacrylamide gels detects a single polypeptide of relative molecular mass (Mr) ~94,000 (94K)34,35. The native polypeptide contains intrinsic specificity for steroid binding and DNA sequence recognition. By using as probes monoclonal and polyclonal antibodies raised against the purified rat and human receptors36–38, it has been possible to identify a major immunogenic region in the receptor residing on a portion of the molecule that is distinct from the steroid- and DNA- binding regions39–41. To gain further information about the structure of this molecule and to begin an analysis of the molecular mechanisms by which it regulates gene transcription, we set out to clone receptor cDNA sequences. By using receptor-specific antibodies as probes, we and others have isolated clones containing human or rat glucocorticoid receptor cDNA inserts42,43.
Here we report the complete amino-acid sequence of the human glucocorticoid receptor (hGR), deduced from human lymphoid and fibroblast cDNA clones. The sequence reveals various structural features of the receptor, including the major immunogenic domain and a cysteine/arginine/lysine-rich region which may constitute a portion of the DNA-binding domain. We describe the use of the SP6 transcription vector system to generate analytical amounts of full-length protein, and demonstrate that the cell-free translated protein is both immunoreactive and possesses steroid-binding properties characteristic of the native glucocorticoid receptor. An accompanying paper describes the homology of the hGR sequence to that of the oncogene v-erb-A64.
Glucocorticoid receptor cDNA
A library of cDNA clones was constructed in the phage expression vector λ gt11 using poly(A)+ RNA from the human lymphoid cell line IM-9 as template, as described previously42. This library was initially screened with a rabbit polyclonal antiserum to the purified glucocorticoid receptor, resulting in the isolation of several immunopositive candidate clones from ~2.5 × 105 plaques. The β-galactosidase fusion proteins generated from these clones were used to affinity-purify receptor epitope-specific antibody, which was subsequently recovered and identified by binding to protein blots of cellular extracts. Three clones containing inserts expressing antigenic determinants of the human glucocorticoid receptor were isolated. The inserts of these clones, although of different sizes, crosshybridized, indicating that they contained a common sequence which presumably delimits the major immunogenic domain of the receptor. Together, these clones spanned 1.4 kilobase pairs (kbp) but were clearly not long enough to code for the entire receptor, which was estimated to require ~2,500 nucleotides to encode a polypeptide of Mr 94K.
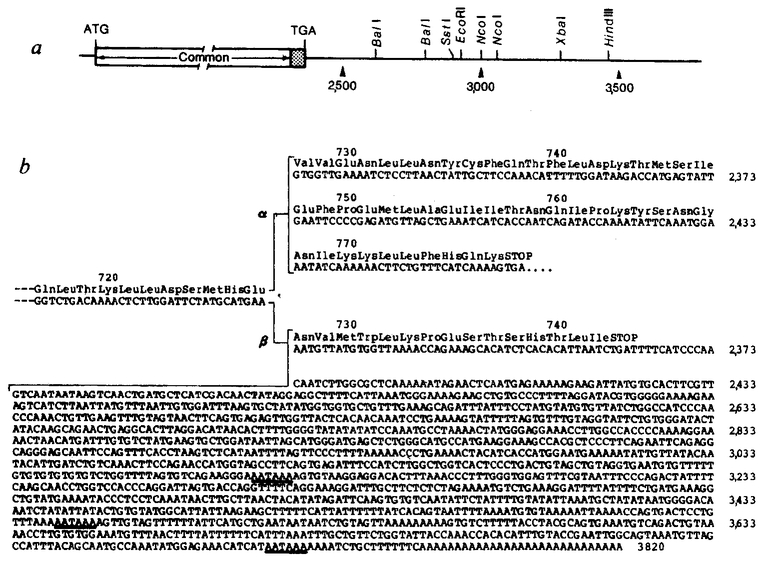
To isolate additional cDNA clones we again screened the original library and also examined a second library (given by H. Okayama) prepared with poly(A)+ RNA from human fibrob lasts in the vector described by Okayama and Berg44. Using one of the immunopositive cDNA inserts (hGR1.2) as probe, 12 clones were isolated that, together, covered more than 4.0 kbp. The nucleotide sequences of these clones were determined by the procedure of Maxam and Gilbert45 according to the strategy indicated in Fig. la. RNA blot analysis indicated that a cDNA insert of 5–7 kilobases (kb) would be necessary to obtain a full-length clone and sequence analysis indicated that the over lapping clones OB7 and hGR5.16 spanned an open reading frame of 720 amino acids, not large enough to encode the complete receptor. Therefore, a second human fibroblast cDNA library of ~ 2 × 106 transformants was screened, yielding a clone (OB10) containing a large insert that extended 150 base pairs (bp) upstream of the putative translation initiation site (see Fig. 1). Sequence analysis predicts two protein forms, termed a and β, which diverge at amino acid 727 and contain additional distinct open reading frames of 50 and 15 amino acids, respectively, at their carboxy termini (see Fig. lb). The a form, represented by clone OB7, is the predominant form of glucocorticoid receptor because eight cDNA clones isolated from various libraries contain this sequence.
Fig. 1.
Human glucocorticoid receptor cDNA sequencing strategy and schematic representation of cDNA clones, a, The composite cDNA for the α glucocorticoid receptor is represented at the top, with noncoding (lines) and coding (stippled portion) sequences indicated. Common 6-nucleotide restriction enzyme sites are shown. Overlapping cDNA inserts used to determine the sequence are shown: arrows beneath the regions sequenced show the direction and extent of sequencing. The dashed line at the 3’ end of OB10 indicates divergent sequence. Numbers refer to nucleotide positions in OB10 relative to the 5’-most transcribed sequence, b, cDNAs encoding the α and β forms of the receptor (OB7 and OB10, respectively). The 5’ end of OB7 (broken lines) is contributed by the OB10 clone. Protein-coding information is represented by wide bars; untranslated sequences are indicated by thin bars. Nucleotides and amino acids are numbered above and below the coding sequence, respectively. Common DNA sequences extend to nucleotide 2,313 (amino-acid residue 727), at which point the α- and β-receptor forms diverge, with the α cDNAs (OB12, OB7) continuing in an open reading frame for 150 nucleotides (50 amino acids) and the β cDNA (OB10) continuing for 45 nucleotides (15 amino acids; see Fig. 3). Hexanucleotide signals (AATAAA) just upstream of the poly(A) in the clones are indicated, with the first hexanucleotide in OB7 serving as poly(A) in OB12
Methods. The inserts hGR1.2, hGR2.9 and hGR5.16 were isolated from a λ gt11 IM-9 lymphoid cell cDNA library as described previously42. Two clones were isolated from cDNA libraries constructed by H. Okayama in pcD (ref. 44) using poly(A)+ mRNA from GM637 human fibroblasts (OB7) and primary human fibroblasts (OB10). Screening was performed with the hGR1.2 cDNA, radiolabelled by nick-translation with 32P-dCTP. Sequences were determined by the chemical cleavage method of Maxam and Gilbert45.
cDNA and protein sequences
Figure 2 shows the 4,800-nucleotide sequence encoding the human a glucocorticoid receptor, determined using clones hGR1.2, hGR5.16, OB7 and OB10. The translation initiation site was assigned to the methionine codon corresponding to nucleotides 133–135 because this is the first ATG triplet that appears downstream from the in-frame terminator TGA (nucleotides 121–123). However, in the absence of amino-terminal peptide sequence information, unequivocal determination of the initiation site is not yet possible. The codon specifying the lysine at position 777 is followed by the translation termination codon TGA. The remainder of the coding sequence is covered by multiple overlapping clones, with OB7 containing a 4.3-kb insert that continues to the poly(A) addition site and OB10 containing the putative initiator methionine. The 3’ regions of clones OB7 and OB10 diverge at nucleotide 2,314, as shown by both restriction endonuclease and DNA sequence analysis. At this junction, the α-receptor continues with a unique sequence encoding an additional 50 amino acids whereas the β-receptor continues for only 15 additional amino acids (Fig. 3). The 3’-untranslated region of OB7 is 2,325 nucleotides long, while that of OB10 is 1,433 nucleotides. There is no significant homology between these two regions, as indicated by direct sequence comparison (Figs 2, 3) or by hybridization analysis under stringent conditions (data not shown).
Fig. 2.
cDNA and predicted protein sequence of human glucocorticoid receptor. The complete α coding sequence and OB7 3’-untranslated region are shown, with the deduced amino acids given above the long open reading frame. An upstream in-frame stop codon at nucleotides 121–123 and putative additional polyadenylation signals in OB7 are underlined.
Fig. 3.
Restriction map and nucleotide sequence of the 3’ end of the human glucocorticoid receptor β cDNA. α, The common 6-nucleotide restriction enzyme sites are shown for the 3’-untranslated region of OB10. b, The cDNA sequence of the β form (OB10) from nucleotide 2,281 to 3,820 compared with the protein-coding information found in the 3’-terminal coding portion of the a form (OB7). Amino acids encoded by each of the cDNAs are presented above the nucleotide sequences. Putative polyadenylation signals (AATAAA) in the 3’-untranslated sequence of OB10 are underlined.
In addition, we have isolated from a human primary fibroblast library another cDNA clone, OB12 (data not shown), which contains sequences identical to OB7 but uses the polyadenylation signal at nucleotide 3,101 (Figs 1b, 2), giving rise to a shorter 3’-untranslated region. Use of probes specific for the 3’-untranslated region of OB7 to screen a human placenta cDNA library reveals that most clones terminate at the first poly(A) site in OB7. Thus, messenger RNA variation is the apparent consequence of both alternative polyadenylation and alternative RNA splicing (see below). The fact that the human fibroblast library contained both cDNAs suggests that both receptor forms may be present in the same cell.
Analysis of α- and β- receptor protein
Sequence analysis reveals that the a and β forms of the human glucocorticoid receptor are 777 and 742 residues long, respectively; the twoforms are identical up to residue 727, after which they diverge. To examine the receptor levels in vivo, cytoplasmic extracts from several human and mouse cell lines were probed by immunoblot analysis with a polyclonal antibody directed against the human glucocorticoid receptor37, α- And β- receptor cDNAs were inserted into the SP6 transcription vector to create synthetic mRNA for in vitro translation (Fig. 4a). The RNAs were separately added to a rabbit reticulocyte lysate system and the unlabelled products analysed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The two RNAs programme the synthesis of distinct translation products whose migration differences are consistent with the predicted polypeptide lengths of the twoforms (Fig. 4b, lanes 2, 3). Cytoplasmic extracts from untreated IM-9 cells and IM-9 cells treated with 1 μM triam cinolone acetonide serve as markers (Fig. 4b,lanes 4, 5) for the 94K receptor (the 79K form represents a putative receptor degradation product)40. Note that after steroid treatment, the intensity of the 94K band is reduced, corresponding to tighter receptor/chromatin binding and, therefore, receptor translocation to the nucleus. The a form co-migrates with the 94K band of the native receptor while the β form migrates more rapidly (Fig. 4b, compare lanes 2,3 with lanes 4,5). A comparison of cytoplasmic extracts from various human and mouse cell lines reveals the presence of only the α-receptor (Fig. 4b, lanes 6–9). Interestingly, the mouse ADR6 lymphoma cell line46, selected for resistance to steroid-induced lysis, contains no steroid-binding activity and shows no immunoreactive receptor (Fig. 4b, lane 7). Therefore, based on characterization of multiple receptor cDNA clones and receptor protein by immunoblot analysis, we conclude that the predominant physiological form of the glucocorticoid receptor is the α (94K) species.
Fig. 4.
Immunoblot comparison of hGR translated in vitro with in vivo hGR from cell extracts, a, The vectors constructed for in vitro transcription of the hGR cDNA sequence. The complete α (pGR107) and β (pGR108) coding sequences were placed under the transcriptional control of the SP6 promoter in pGEMl. Vector sequences, noncoding cDNA sequences and coding sequences are indicated by thin lines, thick bars and boxed regions, respectively. The poly(A) tract of ~60 nucleotides is indicated by A.,. Divergent coding sequences are indicated by striped and stippled regions, b, Western blot analysis of in vitro translation products and cell extracts. Unlabelled translation products synthesized in a rabbit reticulocyte lysate system with no added RNA (lane 1) or with RNA synthesized from pGR108 (β, lane 2) or pGR107 (α, lane 3) were fractionated on a 7.5% SDS-polyacrylamide gel. Additional lanes are: cytoplasmic extracts from IM-9 (lane 4), IM-9 treated with 1 μM triamcinolone acetonide (lane 5), HeLa (lane 6), ADR6.M1890.AD1 mouse lymphoma (lane 7), S49 mouse lymphoma (lane 8) and EL4 lymphoma (lane 9). Proteins were transferred to nitrocellulose and probed with anti-hGR antibody, followed by 125I-labelIed Staphylococcus aureus protein A as described previously42.
Methods. To construct an expression vector containing the entire α coding sequence shown in Fig. 2, the 3’ coding sequence of OB7 was fused to OB10 5’ coding information. OB7 was partially digested with EcoRI, completely digested with X baI, and the 1.2-kbp fragment was gel-purified and ligated with EcoRI/baI-digested OB10 to produce the intermediate pOB107. The entire pOBl07 cDNA sequence including the 5’ poly(G) tract (11 nucleotides, nt) and 3’ poly(A) tract (~ 6 0 nt) was excised by partial Pst I/complete Bam HI digestion. The resultant 3.5-kb fragment was gel-purified and inserted between the PstI and Bam HI sites of pGEM l (Promega Biotec) to yield pGR107. Plasmid GR108 was directly constructed from pOB10 by partial PstI/com plete Bam HI digestion and insertion of the resulting cDNA insert into the corresponding sites of pGEMl. Capped SP6 transcripts were synthesized from PvuII-linearized pGR107 and pGR108, as described by Kjieg and Melton62, with simultaneous capping effected by reduction of the GTP concentration from 400 to 100 μM and addition of m7GppG (Pharmacia) to 500 μM. Transcripts were purified by P60 chromatography and translated with micrococcal nuclease-treated rabbit reticulocyte lysate (Promega Biotec) in conditions suggested by the manufacturer. Preparation of IM-9 cytosol from steroid-treated cells was as described previously42. Size markers are phosphorylase B (97K), bovine serum albumin (66K.) and ovalbumin (45K).
Expression of hGR in vitro
To provide additional evidence that the cloned receptor is functional, we investigated the possibility that the in vitro-translated products might be able to selectively bind corticosteroids. Accordingly, the rabbit reticulocyte lysate was incubated with the radiolabelled synthetic glucocorticoid analogue 3H-triam cinolone acetonide (3H-TA) before or after addition of in vitro- synthesized a or β hGR RNA. As shown in Fig. 5, those lysates programmed with α-hGR RNA acquired selective steroidbinding capacity; unexpectedly, the β-receptor synthesized in vitro failed to bind competable 3H-TA. The in vitro-synthesized α-hGR bound radiolabelled steroid which could be competed with by addition of excess unlabelled cortisol or dexamethasone; however, binding of 3H-TA was not effectively competed with by addition of excess unlabelled oestrogen or testosterone. In contrast, excess progesterone constituted an effective competitor, consistent with the previously reported anti-glucocorticoid activities of progesterone47. To confirm these results, the competition experiments were repeated with native glucocorticoid receptor prepared from extracts of human lymphoid cells. Both the in vitro-translated receptor and the natural in vivo receptor have nearly identical properties with regard to steroid binding and competition with excess unlabelled steroid analogue (Fig. 5).
Fig. 5.
Steroid-binding of α-hGR (GR107) translated in vitro. Binding to IM-9 cytosol extract (stippled bars) and to reticulocyte lysate containing SP6-generated α-hGR RNA (GR107; open bars) are shown. Bars represent bound 3H-triamcinolone acetonide (TA) determined with a 100-fold excess of various steroid competitors; 100% competition was determined using unlabelled TA as competitor. The values represent the mean of triplicate determinations, with error bars showing P < 0.05. Steroid competitors are dexamethasone (Dex), cortisol (Cort), progesterone (Prog), testosterone (Test), and oestradiol (Oest).
Methods. Binding assays were performed in 100 μl containing 10 mM Tris-HCl pH 7.4, 100 mM NaCl, 1 mM EDTA, 10 mM sodium molybdate, 10 dithiothreitol, 150 mM 3H-TA (20 Ci mmol−1; Amersham) and 10 μl translation mixture or 100 μg fresh IM-9 cytosol. Unlabelled steroid competitor (15 μM ) was added as indicated. After 2 h at 0 °C, samples were extracted twice for 5 min each with 5 μl of 50% dextran-coated charcoal to remove unbound steroid, and counted. Uncompeted and fully competed values for the α glucocorticoid receptor (GR107) were 490 and 290 c.p.m., respectively. Reticulocyte lysate translation mixtures without added transcript or programmed with β-receptor SP6 RNA (GR108) contained no competable 3H-TA binding.
hGR sequences map to at least two genes
The human glucocorticoid receptor gene has been functionally mapped to chromosome 5. Analysis of somatic cell hybrids constructed by fusing receptor-deficient mouse T cells (EL4) with human receptor-containing T cells (CEM-C7) indicated that segregants expressing the wild-type CEM-C7 receptor maintained human chromosome 5 while dexamethasone-resis-tant segregants had lost this chromosome48.
To confirm the authenticity of our cDNA clones, we mapped receptor cDNA sequences using Chinese hamster/human somatic cell hybrids containing only human chromosome 5 (HHW454). DNAs extracted from human placenta, HHW454 hybrid cells and Chinese hamster ovary (CHO) cells were digested with EcoRI or HindIII restriction endonucleases and separated on a 0.8% agarose gel. DNA fragments transferred to nitrocellulose were probed with a portion of the receptor-coding region derived from nucleotides 570–1,640 (hGR1.2A; Fig. 1). In addition to CHO-specific EcoRI bands of 6.8 and 17 kbp (Fig. 6a, lanes 2,3), DNA from the hybrid cell line also contains human-specific bands of 3.0 and 5.0 kbp. Unexpectedly, a DNA fragment of 9.5 kbp is found in total human DNA but not in the hybrid line (Fig. 6a, lane 1). Similarly, HindIII digestion revealed a 7.5-kbp band that is not present in the chromo some 5 hybrid cell DNA (Fig. 6a, lane 4). These results indicate that the receptor cDNA maps to human chromosome 5, but that there are additional receptor-related sequences elsewhere in the genome. To map these sequences, we used a dual-laser fluorescence-activated cell sorter (FACS) to sort mitotic chromosome suspensions stained with DIPI/chromomycin in conjunction with Hoechst 33258 chromomycin; this technique allows separation of the 24 human chromosome types into 22 fractions49. After the chromosomes were sorted directly onto nitrocellulose, the chromosomal DNA was denatured and hybridized to the hGR cDNA probe. In addition to confirming the chromosome 5 localization, additional sequences were found on chromosome 16 (Fig. 6b). To confirm this localization, DNAs from mouse erythroleukaemia cells and a mouse erythroleukaemia cell line containing human chromosome 16 (ref. 50) were digested with HindIII and probed with hGR cDNA (Fig. 6c); as predicted, the only DNA fragment found in the hybrid and not in the control was the 7.5-kbp DNA fragment, thus establishing the chromosome 16 assignment (Fig. 6c, lanes 1, 2).
Fig. 6.
Chromosome mapping analysis of hGR cDNA. A, 10 μg of DNA from human placenta (lanes 1, 4), CHO/human somatic cell hybrid (HHW454, lanes 2, 5) containing chromosome 5 as its only human complement, or CHO (lanes 3, 6), was digested with EcoRI (lanes 1–3) or HindIII (lanes 4–6) to completion, fractionated on a 0.8% agarose gel and transferred to nitrocellulose paper. b, Chromosomes (3 × 104) prepared from a human lymphocyte cell line, stained with 4,6-bis(2”-imadazolinyl-4H,5H)-2-phenylindole (DIPI)/chromomycin A3 and sorted using a dual-laser custom FACS IV chromosome sorter63, were denatured and neutralized on nitrocellulose paper. Note that Hoechst/chromomycin-stained chromosome 9 was sorted with chromosomes 10–12. c, 10 μg of DNA from the parental mouse cell line MEL (lane 1) or the parentally derived somatic cell hybrid carrying human chromosome 16 (ref. 50; lane 2) was digested with HindIII and separated on a 0.8% agarose gel then transferred to nitrocellulose paper. All filters were probed with the 1,100-bp insert from hGR 1.2, nick-translated to a specific activity of 3 × 108 c.p.m.μg−l and hybridized in 5 × SSPE, 1 × Denhardt’s, 0.1% SDS, 50% formamide, 100 μg ml−1 denatured salmon sperm DNA, 50% dextran sulphate at 42 °C for 18 h. Filters were washed twice (for 30 min each) in 2 × SSC at 68 °C and exposed to X-ray film at −70 °C with an intensifying screen.
Additional Southern blot analyses using the EcoRI-XbaI fragments from OB7 and OB10 3’-untranslated regions revealed hybridization only to chromosome 5 (data not shown). We conclude that both the a- and β-receptor cDNAs are probably encoded by a single gene on chromosome 5 and suggest that the two cDNA forms are generated by alternative splicing. In addition, we conclude that another gene residing on human chromosome 16 contains homology to the glucocorticoid receptor gene, at least between nucleotides 570 and 1,640. It is not clear whether these sequences on chromosome 16 represent a related steroid receptor gene, a processed gene or pseudogene, or a gene that shares a common domain with the gene for the glucocorticoid receptor. Genomic cloning and DNA sequencing may provide the answer.
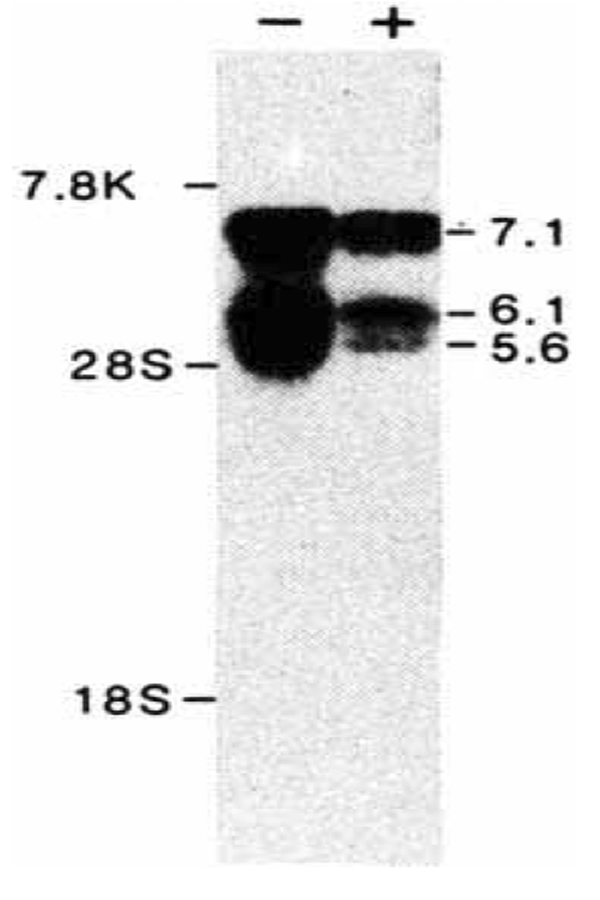
To determine the size of the mRNA encoding the glucocorticoid receptor, Northern blot hybridization51 experiments were performed using cytoplasmic mRNA isolated from a human fibroblast cell line, HT1080. Using the hGR1.2 coding sequence as probe, multiple mRNAs of 5.6, 6.1 and 7.1 kb were detected. Treatment of these cells with glucocorticoids for 24 h leads to a 2–3-fold reduction in receptor mRNAs, suggesting a potential negative feedback regulation.
Discussion
Structural analysis of the glucocorticoid receptor is a pre requisite for gaining insight into the mechanisms by which this regulatory molecule exerts its effects on gene transcription. Here, we have presented the primary sequence of the human glucocorticoid receptor deduced from nucleotide sequence analysis of cDNA clones.
Isolation of hGR cDNAs has revealed the existence of multiple mRNAs encoding at least two forms of the polypeptide. The predicted proteins differ at their carboxy termini by the substitution of 50 amino acids in the case of α-hGR and 15 amino acids in the case of β-hGR. The a glucocorticoid receptor is the major form identified in several human cell lines and cDNA libraries. However, a recent report by Northrop et al. characterizes two forms of the receptor in mouse lymphoid cells52. The relationship of a- and β-hGR to the mouse doublet species remains to be established. Also, the cellular distribution and potential function of β-hGR are unclear, although it is possible that variant receptors are used for tissue-specific functions. We are now generating antisera to synthetic peptides specific for each human receptor form to elucidate their tissue-specific expression.
Among the cDNAs selected using the immunopositive phage DNA insert hGR1.2A as probe were those containing 3’ ends similar to OB7, except that polyadenylation was signalled earlier by the use of an AATAAA at nucleotide 3,101. These clones have been isolated from both human fibroblast and placental libraries (data not shown). Alternative poly(A) site selection is a feature of many eukaryotic transcription units53. In some instances, selection of poly(A) sites specifies particular polypeptide products54–57 while in other cases, alternative poly(A) site selection produces no change in the primary structure of the polypeptide58. The selection of poly(A) sites during receptor transcription may (1) alter the stability of the mRNA in a particular tissue, (2) lead to splicing changes, or (3) be random, with no physiological consequence.
The in vitro translation studies described here provide direct evidence that the cloned molecule encodes the complete glucocorticoid receptor. First, the in vitro-translated product is identical in size to the native glucocorticoid receptor and is immunologically reactive with receptor-specific antiserum. Second, the in vitro-translated protein acts functionally as a glucocorticoid receptor in that it is capable of selectively binding the synthetic glucocorticoid triamcinolone acetonide. This binding is specifically competed with by glucocorticoids, glucocorticoid analogues and progesterone but is not competed with by the sex steroids testosterone and oestrogen. In this respect, the in vitro-translated receptor behaves identically to the in vivo receptor from human lymphoid cells, providing the first evidence for a function of the cloned molecule. The acquisition of steroid-binding properties does not appear to require any specific modifications or, if it does, these modifications can occur in the in vitro translation mix.
The results presented here provide the information necessary for studying the molecular interactions of a eukaryotic transcriptional regulatory protein with its target genes. These structural studies provide a basis from which the glucocorticoid receptor, its gene, and its RNA products can be analysed. Furthermore, the ability to express receptor in vitro provides a novel means by which the consequence of specific in vitro mutagenesis can be rapidly tested.
In addition to the in vitro studies, the analysis of several existing rodent cell lines49,59–61 with well-characterized receptor defects in both the DNA- and steroid-binding domains should facilitate future analysis. Furthermore, the isolation of genes responsive to glucocorticoids and specific regulatory elements by both mutagenic and protein-binding studies suggests that this protein will serve as a very useful model for analysis of inducible eukaryotic gene regulation.
Fig. 7.
Northern blot analysis of hGR mRNA. 10 μg of poly(A)+ mRNA from human HT1080 fibroblast cells, collected after 24 h without ( − ) or with ( + ) treatment with 10 μM dexamethasone, was electrophoresed through a 0.8% agarose/1% formaldehyde gel, stained with acridine orange (120 μg ml−1) and transferred to nitrocellulose. The filter was hybridized overnight with nick-translated hGR1.2 (106 c.p.m. ml−1, specific activity 108 c.p.m. μg−1) and washed with 2 × SSC at 68 °C. Sizes were estimated from human fibronectin mRNA (7.8 kb), and 28 S (5.0 kb) and 18 S (2.1 kb) ribosomal RNAs.
Acknowledgments
We thank Drs Kelly Mayo, Geoffrey Wahl, Michael Wilson, Noellyn Oliver, Tony Hunter and Donald Gruol for advice and discussion; our collaborator Dr John Wasmuth for providing human/hamster somatic cell hybrids; Hiroto Okayama for providing human fibroblast cDNA libraries; Noellyn Oliver for HT1080 cells; and Kevin Struhl for suggestions on in vitro translation studies. We also thank Marijke ter Horst for preparation of artwork and secretarial assistance. S.M.H. is a predoctoral trainee, supported by a training grant to the Department of Biology, University of California, San Diego. C.W. is a fellow of the Damon Runyon-Walter Winchell Cancer Fund (DRG- 755). R.L. is an associate and M.G.R. and R.M.E. are investigators of the Howard Hughes Medical Institute. This work was supported by grants from the NIH and the Howard Hughes Medical Institute.
References
- 1.Jensen EV & De Sombre ER A. Rev. Biochem 41, 203–230 (1972). [DOI] [PubMed] [Google Scholar]
- 2.Gorski J & Gannon FA, Rev. Physiol 38, 425–450 (1976). [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto K,R & Alberts BM A. Rev. Biochem 45, 721–746 (1976). [DOI] [PubMed] [Google Scholar]
- 4.O’Malley BW, McGuire WL, Kohler PO & Komtman SG Recent Prog. Horm. Res 25, 105–160 (1969). [DOI] [PubMed] [Google Scholar]
- 5.Hayward M,A, Brock ML & Shapiro DJ Nucleic Acids Res 10, 8273–8284 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashbumer M & Berendes HD, in The Genetics and Biology of Drosophila, Vol. 2 (eds Ashburner M & Wright TRF) 315–395 (Academic, London, 1978). [Google Scholar]
- 7.Peterkofsky B & Tomkins G Proc, natn. Acad. Sci. U.S.A 60, 222–228 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKnight GS & Palmiter RDJ biol. Chem 254, 9050–9058 (1979). [PubMed] [Google Scholar]
- 9.Horwitz KB & McGuire WLJ biol. Chem 253, 2223–2228 (1978). [PubMed] [Google Scholar]
- 10.Palmiter RD, Moore PB, Mulvihill ER & Emtage S Cell 8, 557–572 (1976). [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto KR & Alberts BM Proc. natn. Acad. Sci. U.S.A 69, 2105–2109 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen EV et al. Proc. natn. Acad. Sci. U.S.A 59, 632–638 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringold GM, Yamamoto KR, Tomkins GM, Bishop JM & Varmus HE Cell 6, 299–305 (1975). [DOI] [PubMed] [Google Scholar]
- 14.Parks WP, Scolnick EM & Kozikowski EH Science 184, 158–160 (1974). [DOI] [PubMed] [Google Scholar]
- 15.Hager LJ & Palmiter RD Nature 291, 340–342 (1981). [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Anderson RD, Slater E, Smith K, & Herschman HR Nature 286,295–297 (1980). [DOI] [PubMed] [Google Scholar]
- 17.Kurtz DT & Feigelson P Proc natn. Acad. Sci. U.S.A 74, 4791–4795 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spindler SR, Mellon SH & Baxter JDJ biol. Chem 257, 11627–11632 (1982). [PubMed] [Google Scholar]
- 19.Evans RM, Birnberg NC & Rosenfeld MG Proc. natn. Acad. Sci. U.S.A 79,7659–7663 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins DM, Paek I, Seeburg PH & Axel R Cell 29, 623–631 (1982). [DOI] [PubMed] [Google Scholar]
- 21.Chandler VL, Maler BA & Yamamoto KR Cell 33, 489–499 (1983). [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski MC, Huang AL, Kessel M, Woolford RG & Hager GL E M BO J 3, 1891–1899 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindan MV, Spiess E &. Majors J Proc. natn. Acad. Sci. U.S.A 79, 5157–5161 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheidereit C, Geisse S, Westphal HM & Beato M Nature 304, 749–752 (1983). [DOI] [PubMed] [Google Scholar]
- 25.Pfahl M Cell 31, 475–482 (1982). [DOI] [PubMed] [Google Scholar]
- 26.Payvar F et al. Cell 35, 381–392 (1983). [DOI] [PubMed] [Google Scholar]
- 27.Karin M et al. Nature 308, 513–519 (1984). [DOI] [PubMed] [Google Scholar]
- 28.Laimonis LA, Khoury G, Gorman C, Howard B & Gruss P Proc. natn. Acad. Sci. U.S.A 79, 6453–6457 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benoist C & Chambon P Nature 290, 304–310 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Banerji J, Olson L & Schaffner W Cell 33, 729–740 (1983), [DOI] [PubMed] [Google Scholar]
- 31.Grosschedl R & Bimstiel ML Proc. natn. Acad. Sci. U.S.A 77, 7102–7106 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simons SS & Thompson E, B. Proc. natn. Acad. Sci. U.S.A 78, 3541–3545 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gehring U & Hotz A Biochemistry 22, 4013–4018 (1983). [DOI] [PubMed] [Google Scholar]
- 34.Westphal HM, Moldenhauer G & Beato M EM BO J 1, 1467–1471 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrange O, Carlstedt-Duke J & Gustafsson JAJ biol. Chem 254, 9284–9290 (1979). [PubMed] [Google Scholar]
- 36.Okret S, Carlstedt-Duke J, Wrange O, Carlstrom K & Gustafsson J-A Biochim. biophys. Acta 677, 205–219 (1981). [DOI] [PubMed] [Google Scholar]
- 37.Harmon JM et al. Cancer Res 44, 4540–4547 (1984). [PubMed] [Google Scholar]
- 38.Gametchu B & Harrison RW Endocrinology 114, 274–279 (1984). [DOI] [PubMed] [Google Scholar]
- 39.Carlstedt-Duke J, Okret S, Wrange O & Gustafsson J-A Proc. natn. Acad. Sci. U.S.A 79, 4260–4264 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrange O, Okret S, Radojcic M, Carlstedt-Duke J & Gustafsson J-AJ biol. Chem 259, 4534–4541 (1984). [PubMed] [Google Scholar]
- 41.Dellweg HG, Hotz A, Mugele K & Gehring U EMBO J 1, 285–289 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberger C et al. Science 228, 740–742 (1985). [DOI] [PubMed] [Google Scholar]
- 43.Miesfeld R et al. Nature 312, 779–781 (1984). [DOI] [PubMed] [Google Scholar]
- 44.Okayama H & Berg P Molec. cell. Biol 3, 280–289 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxam A & Gilbert W Proc. natn. Acad. Sci. U.S.A 74, 560–564 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danielsen M & Stallcup MR Molec. cell. Biol 4, 449–453 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rousseau GG, Baxter JD & Tomkins GMJ molec. Biol 67, 99–115 (1972). [DOI] [PubMed] [Google Scholar]
- 48.Gehring U, Segnitz B, Foellmer B & Francke U Proc. natn. Acad. Sci. U.S.A 82, 3751–3755 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lebo RV et al. Science 225, 57–59 (1984). [DOI] [PubMed] [Google Scholar]
- 50.Bode U, Deisseroth A & Hendrik D Proc. natn. Acad. Sci. U.S.A 78, 2815–2819 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas PS Proc. natn. Acad. Sci. U.S.A 77, 5201–5205 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Northrop JP, Gametchu B, Harrison RW & Ringold GMJ biol. Chem 260, 6398–6403 (1985). [PubMed] [Google Scholar]
- 53.Dameli JE Nature 297, 365–371 (1982).6176879 [Google Scholar]
- 54.Amara SG ., Jonas V, Rosenfeld MG, Ong ES & Evans RM Nature 298, 240–244 (1982). [DOI] [PubMed] [Google Scholar]
- 55.Rosenfeld MG et al. Nature 304, 129–135 (1983). [DOI] [PubMed] [Google Scholar]
- 56.Alt FW et al. Cell 20, 293–301 (1980). [DOI] [PubMed] [Google Scholar]
- 57.Schwarzbauer JE, Tam kun JW, Lemischka IR & Hynes RO Cell 35,421–431 (1983). [DOI] [PubMed] [Google Scholar]
- 58.Setzer DR, McGrogan M & Schimke RTJ biol. Chem 257, 5143–5147 (1982). [PubMed] [Google Scholar]
- 59.Yamamoto KR, Stampfer MR & Tomkins GM Proc. natn. Acad. Sci. U.S.A 71, 3901–3905 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourgeois S & Newby RF Cell 11, 423–430 (1977). [DOI] [PubMed] [Google Scholar]
- 61.Grove JR, Dieckmann BS, Schroer TA & Ringold GM Cell 21, 47–56 (1980). [DOI] [PubMed] [Google Scholar]
- 62.Krieg PA & Melton DA Nucleic Acids Res 12, 7057–7070 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lebo RV & Bastian AM Cytometry 3, 213–219 (1982). [DOI] [PubMed] [Google Scholar]
- 64.W einberger C, Hollenberg SM, Rosenfeld MG & Evans RM Nature 318,670–672 (1985). [DOI] [PubMed] [Google Scholar]