Abstract
Cell adhesion plays a critical role in many cellular functions, such as the hemostatic/thrombotic process, inflammatory reactions, and adaptive immune response. Many cell adhesion processes involve crosstalk between multiple ligand–receptor systems through intracellular signaling. To elucidate such crosstalk requires analysis of the synergistic or antagonistic effects of binding and signalling of multi-receptor species. Current techniques for these analyses, e.g., atomic force microscopy (AFM) and biomembrane force probe (BFP) assays, are either labor-intensive, low-throughput, or limited in the types of ligands they can interrogate. Circumventing these limitations requires a technique for manipulating ligand interactions with and measuring the functional response of a population of cells. In this work, we have developed a microfluidic platform for studying binding and signaling of multi-receptor species by separating their actions in space and time. The platform directs cells through a single channel and uses sequentially presented ligands for pre-processing and stimulating cells, followed by reporting of cell activation states and functional consequences. Our method precisely patterns multiple proteins in different spatial regions without gaps. We demonstrate the utility of our method by using this platform to analyze the crosstalk between platelet receptors, glycoprotein Ib and IIb-IIIa, in the context of platelet adhesion and signaling under flow. We show the clinical utility of this platform by applying it to analyze whole blood samples and to assess differences in activation of platelets between healthy and diabetic patients.
Introduction
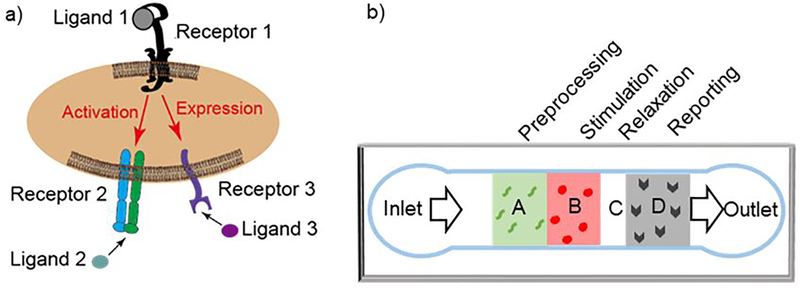
Specific interactions of a cell with another cell or with the extracellular matrix via direct physical contact usually involves multiple receptor–ligand interactions1. In some cases, these interactions are concurrent and independent, whereas in other cases, the interactions are sequential and cooperative. An example of the former case is binding of two neutrophil receptors (FcγRIIA and FcγRIIIB) to a common ligand mix (total IgG), which can be treated as concurrent and independent when the bond numbers are low enough for competition to be neglected2. Concurrent and independent interactions result in a total bond number that is equal to the sum of two bond species2. As an example of sequential and cooperative binding, moving platelets use glycoprotein (GP) Ib to transiently bind surface-immobilized von Willebrand factor (VWF) A1 domain (VWF-A1), which activates GPIIb-IIIa (integrin αIIbβ3) via mechano-signaling. The sequential process enables efficient platelet adhesion during hemostatic and thrombotic processes3, and also induces P-selectin expression4 (Fig. 1a). In some other systems, the binding of a receptor can also trigger suppression of the function of another receptor5. In many biological contexts, the exact nature of these interactions is largely unknown.
Figure 1.
Microfluidic multi-zone channel for analysis of receptor crosstalk. a) The cell interacts with its environment via multiple receptor species that may generate synergistic or antagonist effects. Ligand binding of receptor 1 may induce activation of receptor 2 and expression of receptor 3 to bind their respective ligands. b) Schematic of the multi-zone channel. Cell suspension will flow in from the inlet, across four zones, and out from the outlet. The cells are interrogated as they are allowed or prevented to sequentially interact via different receptors with different proteins patterned on different zones labelled as A: pre-processing, B: stimulation, C: relaxation, and D: reporting.
To analyze these synergistic or antagonistic effects among multi-species receptor–ligand interactions requires temporal and spatial control of when and where each receptor species can interact with their respective ligand or reporting antibody, in what sequence, and for how long. A recent study employed a newly developed dual biomembrane force probe to achieve this goal6. The study analyzed the crosstalk of GPIb with αIIbβ3 (and P-selectin) by allowing the two receptors to sequentially encounter their respective ligand (or antibody) in space and time, so as to monitor the activity change of αIIbβ3 (and surface expression of P-selectin) as a consequence of GPIb signaling. While extremely well-controlled and sensitive, this method is limited in that it requires manually moving a single cell to contact two ligand-bearing surfaces6 and do so alternatingly; these actions are technically challenging, and of low temporal resolution and low throughput. In addition, this technique cannot recapitulate the flow condition physiological for certain cell types. We hypothesize that these limitations can be overcome with the use of well-engineered microfluidic devices that use flow to deliver cells into contact with a series of protein patterns with spatiotemporal control.
In the literature, a variety of protein micropatterning techniques have been developed, which include microcontact printing7, microchannel8, and laminar flow9 coating. Microcontact printing is the most common patterning technique. It uses the relief patterns on a stamp surface to print protein patterns on the substrate. While this technique can generate patterns of complex geometry, alignment of two or more stamps is technically difficult and thus lacks accuracy and uniformity10. Microchannels have also been used to generate protein patterns. Separate channels guide different protein solutions to their desired regions on a substrate, thereby achieving automatic alignment. However, walls separating the microchannels are relatively large compared to the cell size, resulting in undesirable large gaps between patterns. Another patterning technique, laminar flow patterning9, utilizes the non-mixing property of Stokes flow to create patterns. It allows two or more layers of fluid to flow next to each other without lateral mixing except for diffusion or potential reaction. Yet, diffusion could occur and is hard to control, potentially resulting in protein contamination regions much larger than the cells11.
To study crosstalk between two cell-surface receptor species, an ideal microfluidic device system should automatically present the stimuli followed by an immediate readout of the cell status (Fig. 1b). Specifically, upon entering the device, the cells would first be stimulated by a protein patch with a precise dosage (concentration and time) to trigger the desired level of activation, and then be interrogated by a protein patch to report any changes in the expression, conformation, or activity of the other receptor species. Other important features include pre-screening of the cells to ensure they are not already activated upon entering the device, and a non-reactive surface between the stimulating and analyzing patches to allow transient relaxation of stimulated cells to test signal reversal in the cells. To meet these specifications requires a patterning technique for making protein patches with high-precision registration, desired pattern positions, and minimum protein contamination, which are beyond the capabilities of existing technologies.
In this work, we report a patterning method that combines microcontact printing and microchannel coating to achieve gapless auto-aligned patterning of multiple protein patches (Fig. 1b). This new approach of micro-patterning allows the placement of target ligands at well-defined locations, which can be useful for studies of cell adhesion mediated by a range of receptor–ligand species12,13 and for certain biological assays such as combinatorial screening12 and cell sorting13. As a demonstration, we applied this method to interrogate the activation of platelets by GPIb—ligand binding, using αIIbβ3 activation and expression of P-selectin as readouts. Our results demonstrate the validity of this technical platform and show that the platform parameters can be designed to control the level of activation and its reversal. Finally, we show a potential utility of this platform in preclinical diagnostics by using it to assess platelet hyperreactivity in diabetic patients with a drop of whole blood.
Results
Gapless patterning process and validation
Our device design requirements are based on the initial application, which is to characterize receptor–ligand mediated platelet activation. The multiple surface zones should test the activation state of the platelets prior to controlled stimulation, stimulate the platelets and report the activation state afterwards. Several proteins are to be displayed with controllable density and good spatial registration to each other. The spatial resolution of the patterning should be on the order of the size of a platelet (which is 3-μm in diameter). This accuracy is required considering the fast signalling kinetics (seconds) and translocation velocity (a few μm/s) of platelets interacting with immobilized ligands. Furthermore, since platelet stimulation by GPIb–VWF-A1 mechanoreception alone is transient17, testing platelet activation immediate after stimulation is preferable. Hence, gaps between zones should be kept as small as possible.
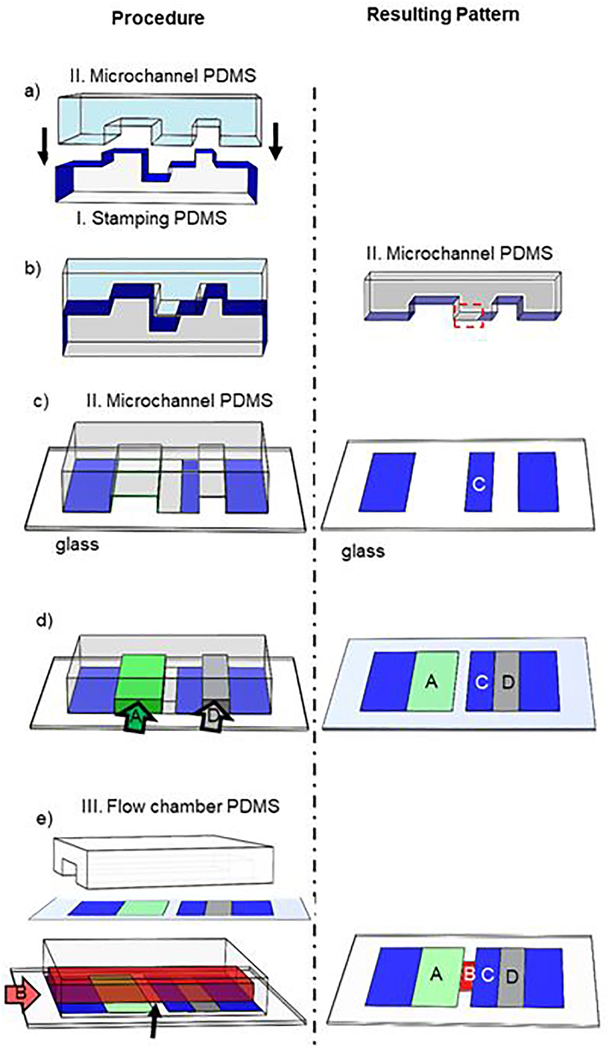
To achieve auto-alignment for surface patterning in the microchannels, we take advantage of the two existing techniques - microcontact printing and laminar flow patterning (Fig. 2). The key of the process is to protect regions using physical masking or stamping of a non-ligand protein (e.g. BSA), and sequentially pattern different proteins using a combination of stamping and flow.
Figure 2.
Process of patterning four proteins on four zones without gaps. The procedures are shown on the left. The resulting patterns are shown on the right. a) Two pieces of PDMS with nearly matched shapes to allow the stamping PDMS (I, bottom, light grey) pre-coated with BSA (blue) to print BSA on the microchannel PDMS (II, top, light blue). b) Upon contact, BSA was transferred from the upper surface of the stamping PDMS to the bottom surface of the microchannel PDMS except for the area boxed by the dashed red box. c) The microchannel PDMS stamps on and transfers the BSA onto a substrate glass, forming a protein patch on the relaxation zone C and also blocking the areas from the inlet to zone A and from zone D to the outlet. d) Two protein solutions flow through two microchannels (arrows) to coat zones A and D. e) A flow chamber is assembled by placing another piece of PDMS (III, white) on top of the substrate glass with flow direction perpendicular to the protein stripes. Another protein solution flows in to coat zone B.
The process is as follows. First, a stamping PDMS piece is pre-coated with BSA (I); it is put into contact with a piece of PDMS bearing microchannels with matched geometries (II) (Fig. 2a). This allows BSA to be transferred from the stamping PDMS to the microchannel PDMS piece wherever they are in contact. There is a region, however, where the height of the stamping PDMS is smaller than the depth of the channel PDMS piece. This area in the channel between the ridges is left blank (Fig. 2b, dashed red rectangle). The microchannel PDMS is then brought into contact with a glass substrate for two simultaneous actions: (1) to transfer BSA to the non-targeted areas and relaxation zone C, and (2) to create two microchannels for protein coating of glass via physical absorption of proteins from solutions that are pumped through (Fig. 2c). This step creates protein patterns in zones A and D on the glass surface, while leaving the middle area blank because of the protective ridges (Fig. 2d). BSA is then delivered through the channels to further block any uncoated areas on zones A and D so that later procedures do not contaminate them. A third piece of PDMS (III) containing the channel for cell delivery is then sealed to the surface (Fig. 2e). Flowing another protein solution through this channel allows zone B to be coated with no impact to the other zones because they are protected by the previously coated target proteins or BSA. As a result of this process, four protein patches can be patterned on the glass surface with all other areas blocked by BSA. Because the patterns are registered by the PDMS geometries, this approach is advantageous in that the protein patches are guaranteed to align automatically.
Next, we assessed the registration of this approach by patterning the glass surface with two proteins, fibronectin (FN) and VWF-A1, and visualizing the protein patterns using their respective antibodies conjugated with two different fluorophores (FITC for FN, and PE for VWF-A1). Epifluorescent images of the patterned surface show uniform antibody staining with sharp edges in individual channels, and no overlaps when images from the two channels were registered to each other (Fig. 3a). To assess the accuracy of the registration, we quantified these images by taking fluorescence intensity line-plots perpendicular to the patterned stripes (Fig. 3b). The protein patterns have sharp and well-defined edges and relatively uniform distribution throughout the stripe. These data collectively validate the method for its ability to pattern multiple proteins with precise spatial registration and negligible cross contaminations.
Figure 3.
Assessment of protein coating quality. a) A three-zone device without zone C was coated with fibronectin (FN) in zones A and D and VWF-A1 in zone B. The two proteins were detected by their respective mAbs tagged by two different fluorophores and viewed by epi-fluorescence microscopy via the FITC (green) channel for FN (top), PE (red) channel for A1 (middle) or both channels (bottom). Scale bar is applicable to all images. b) Intensity distributions of the two fluorescent antibodies along a line in the flow direction. c) Density and uniformity assessment of VWF-A1 coating. Three independent replicate tests were performed (indicated by different colors) using different devices coated with indicated concentration of VWF-A1. Each dot indicates intensity over one 20×20 μm2 square. Two representative images were shown for VWF-A1 coating with concentration of 20 and 100 μg/ml, respectively.
To determine the consistency and robustness of the method, we measured the fluorescence intensity of the anti-VWF-A1 mAb in three independent trials using three sets of devices (Fig. 3c). Coating concentration of A1 was varied from 10 to 100 μg/ml for each set on different days. Fluorescence intensity increases with increased concentration. Under the same coating concentration and microscope setting, intensities for different devices were comparable on different days, suggesting that these procedures are quantitatively replicable. Together, these data suggest that our coating strategy can yield controlled and consistent surface density of the coated proteins.
Examine platelet rolling velocity on multiprotein patches
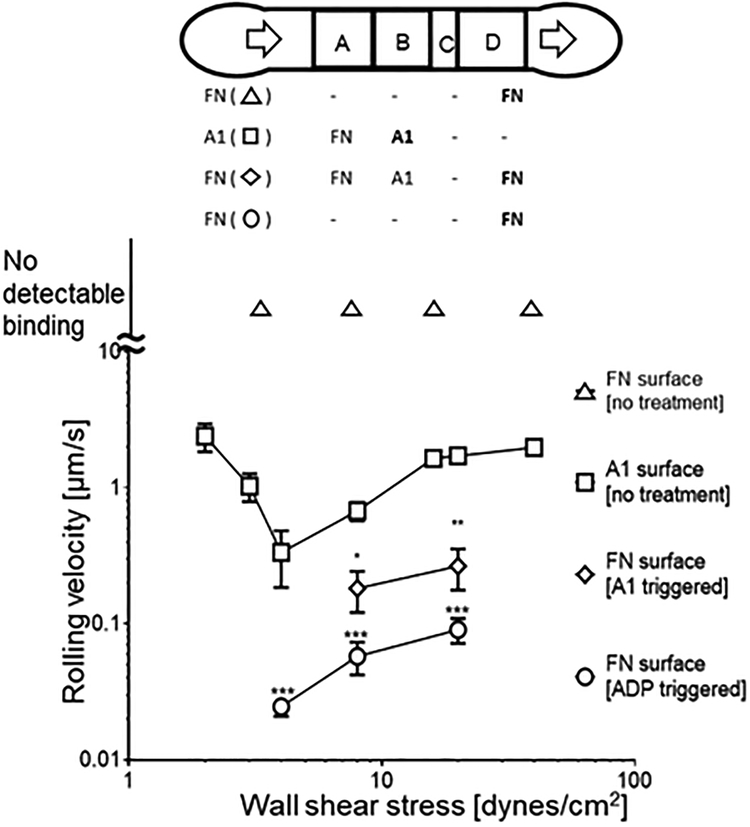
We next tested the ability of our device to separate the two pairs of receptor-ligand cooperative binding (GPIb and integrin αIIbβ3) in space and time by measuring the velocity of platelet rolling on the respective ligands. In a device coated with FN only in zone D, washed platelets flowed passed the surface showing no sign of interaction regardless of shear rate, a condition serving as negative control18 (Fig. 4, annotated as ‘do not roll’). Rolling platelets were observed over the stimulation zone, confirming the transient interaction of platelet GPIb with the surface VWF-A1 The rolling velocity first decreased with increasing wall shear stress of the flowing fluid, reached a minimum at 4 dynes/cm2, and then increased with further increase in wall shear stress (Fig. 4, squares), which is consistent with the previously reported catch-slip bond characteristic of GPIb–VWF-A1 binding19. We have also summarized the fraction of rolling under these conditions (Table 1).
Figure 4.
Platelet activation by VWF-A1 and ADP demonstrated by decreased rolling velocity on FN. Velocity of platelets rolling on surfaces patterned with indicated proteins is plotted vs. wall shear stress. Quiescent platelets (no treatment) did not interact with FN surface (0.016 μg/ml, coating concentration) in zone A (data not plotted), but rolled on VWF-A1 surface (100 μg/ml, coating concentration) in zone B (square) and FN surface (0.016 μg/ml, coating concentration) in zone D (diamond). (This device has no zone C.) Rolling velocity on FN surface after VWF-A1 stimulation was significantly lower than on VWF-A1 surface. As a control, platelets pre-treated with 50 μM ADP for 15 min rolled on zone A with significantly lower velocity (circle). Data are presented as mean ± sem of 15–20 cells. Experiment was repeated 3 times with consistent results and the representative data are shown. *, ** and *** denote p < 0.05, 0.01 and 0.001 by Student t-test.
Table 1.
Fraction of rolling cells of total interacting cells in the field of view for Fig. 4
| Shear Stress [dynes/cm2] | 2 | 4 | 8 | 20 | 40 |
| FN | no detectable binding | ||||
| A1 | 75.8% | 85.4% | 90.2% | 84.3% | 89.4% |
| FN (A1 triggered) | 77.3% | 84.2% | |||
| FN (ADP triggered) | 84.1% | 78.1% | 83.0% | ||
It is known that GPIb interaction with VWF-A1 stimulates platelets, and promotes interactions with the downstream reporting zone surface coated with FN20. High density coating of FN (0.16 μg/ml coating concentration) firmly arrested the stimulated platelets (data not shown), whereas low FN density (0.016 μg/ml coating concentration) supported their slow rolling (Fig. 4, diamond), both of which indicated the activation of platelet surface αIIbβ3 with an increased ligand binding affinity after VWF-A1 stimulus. As a positive control, washed platelets were incubated with a soluble agonist ADP (50 μM, 15 min) and perfused through a device with only low density FN coating20. Rolling velocity of ADP-treated platelets without VWF-A1 stimulation was significantly lower than platelets stimulated with VWF-A1 (Fig. 4, circle). These data confirm that platelet activation can be achieved by interacting with a surface coated with A1, and this activation is weaker than the chemical agonist stimulation. This set of experiments also demonstrates that our device allows triggering of cell activation and follow-up characterization of stimulated cells with control of the sequential timing of ligand presentation.
Platelet adhesion on ligand FN
It is reasonable to assume that the signalling amount of a cell can depend on the amount of stimulation it receives. While the stimulator dosage of a soluble agonist can easily be controlled, controlling the dosage of VWF-A1 stimulation is challenging. Indeed, how platelet activation via VWF-A1 binding is stimulation time-dependent was poorly characterized in the literature. Thus, we next characterized the stimulation time -dependency of integrin αIIbβ3 activation upon the stimulation on GPIb, which is realized via pre-programing the stimulation time in the device hardware. In our assay system, the stimulation time for platelets depends on both flow rate and the length of zone B. However, changing the flow rate would also change the wall shear stress, which would affect GPIb bond lifetimes with VWF-A1 and also platelet mechanosensing19,21. Therefore, we made a set of devices with variable zone B lengths (50–800 μm). For a given flow rate and device dimensions, the longer the zone B, the longer the potential time for platelets to be stimulated by sequential and intermittent binding of VWF-A1. Washed platelets were perfused through devices with zone B of variable lengths, zone C of zero length, and zone D coated with high coating concentration of FN (0.16 μg/m) to arrest VWF-A1 stimulated platelets.
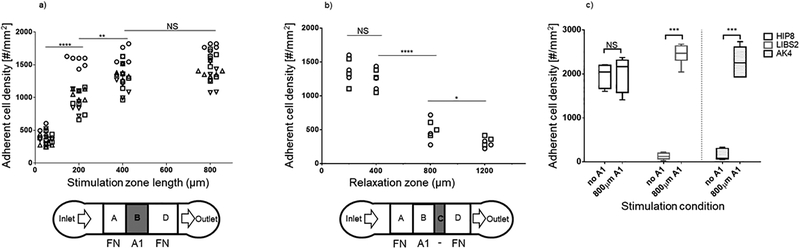
The effect of varying the stimulation time was assessed by measuring the number of adherent platelets per unit area in zone D (Fig. 5a). Increasing stimulation time when zone D is relatively short (zone B length from 50 to 200 μm) resulted in a drastic initial increment in the number of adherent cells. The activation approached saturation when zone B length reached 400 μm, because further increasing the length to 800 μm did not yield more platelet adhesion. These experimental results are robust and reproducible in independent experiments on different days. This also the first demonstration of platelet activation by VWF-A1 being stimulation time dependent.
Figure 5.
Characterization of platelet activation and relaxation. Protein patterns were made to test the effects on platelet binding to reporting zone coated with 0.16 μg/ml FN of variable length of a) stimulation zone coated with 100 μg/ml A1 and b) BSA-blocked relaxation zone. c) Antibodies (100 μg/ml) were coated on zones A and D to report the conformational change in integrin αIIbβ3 (by LIBS2) and upregulation of P-selectin (by AK4) on platelets after they were stimulated by A1 in zone B of 800 μm. The conformation-independent antibody HIP8 was used as positive control, which arrested platelets in both zones A and D. By comparison, platelets were able to bind LIBS2 and AK4 in zone D but not in zone A prior to A1 stimulation. The protein patterns used in panels a and c have no zone C. Experiments were done by perfusing 1 × 108 platelets/ml for 2 min. Each point in a and b represents measurement from one flow chamber. Different symbols indicate data obtained from different donors. Every donor donated blood twice for repeated experiments. Data in c are presented as mean ± quarter percentile and max/min of 6 experiments. *, **, *** and **** denote, p < 0.05, 0.01, 0.001 and 0.0001, respectively, by Student t-test.
The intracellular Ca2+ flux triggered by GPIb is highly transient, which can fade away within seconds3. It is therefore reasonable to question the temporal stability of the integrin αIIbβ3 activation triggered by GPIb, which is downstream of Ca2+ flux. With the same device, one is also able to investigate this temporal stability by pre-programming variable relaxation times with different zone C lengths in the device hardware. With blocking protein BSA on zone C, platelets are prevented from receiving any additional stimulation during the relaxation time, which varies from short (~5 s for 200 μm zone C) to long (~30 s for 1200 μm zone C), before entering the reporting zone. Again, zone D was coated with FN (0.16 μg/ml) and the effect of varying relaxation time was assessed by measuring the number of adherent platelet per unit area in zone D (Fig. 5b). Little decrease in adherent cell density was observed when relaxation time was short (length <400 μm). However, a steep decrease occurred when zone D length increases from 400 to 800 μm. The effect saturates beyond 800 μm. These experiments showed that platelet activation by VWF-A1 stimulation was transient (in the time scale of minute) and reversible when platelets received no further stimulation.
Platelet adhesion on integrin conformation-specific antibodies
After stimulation by VWF-A1, increased binding affinity of αIIbβ3 for FN suggests conformational change of αIIbβ3 from the inactive bent conformation to an active extended conformation, which would expose the LIBS2 epitope14. Similarly, quiescent platelets do not express P-selectin, which could be induced upon platelet activation22. To further characterize VWF-A1 stimulated platelets and demonstrate the utility of the device on other proteins, additional tests were performed using the two variations of our basic design, which replaced the coating protein FN on zones A and D with a conformation-specific mAb against integrin αIIbβ3 (LIBS2) or an anti-P-selectin mAb (AK4), respectively reporting the extending conformational change of the integrins and the upregulated expression of P-selectin as a result of GPIb signalling. The length of zone C was set to zero in these experiments. Stimulation by 800 μm A1-coated zone B resulted in high platelet binding to zone D coated with either LIBS2 or AK4 (Fig. 5c). In comparison, platelet binding to either antibody was minimal in the absence of A1 stimulation, confirming that platelets are in the resting state (Fig. 5c). As a positive control, platelet binding to HIP8, a conformation-independent mAb, in both zones A and D were indistinguishable, suggesting that the increased LIBS2 binding was due to conformational change of integrin αIIbβ3, instead of their increased expression level (Fig. 5c). These experiments confirmed our hypothesis that VWF-A1 stimulation on platelets triggers integrin conformation change and P-selectin expression.
Testing whole blood and comparing healthy with diabetic platelets
Although the use of washed platelets greatly simplifies the system for mechanistic study by removal of other blood cells and most plasma proteins, experiments using whole blood are obviously more physiological. In addition, should an assay work with whole blood, it is more likely to be usable for clinical applications. The challenge is that since whole blood is more biologically complex, experiments on whole blood are potentially prone to additional difficulties compared to assays using isolated and washed cells. We repeated the experiments as Fig. 5a on GPIb–αIIbβ3 crosstalk but with whole blood and a set of devices with variable zone B length and no zone C. As with purified platelets, increased stimulation increased adhesion (Fig. 6a). Very low numbers of platelets adhered to FN coated zone D if the coating protein to zone B was BSA instead of VWF-A1, suggesting that platelet adhesion due to potential coating of plasma proteins onto the device was negligible under our experimental conditions. A slight increase of stimulation time (50 μm zone B coated with VWF-A1) produced a significantly higher number of adherent platelets than the BSA background (p < 0.05), which was further enhanced with further increasing the zone B length (Fig. 6a). However, even when stimulation reached saturation (zone B 800 μm), the density of VWF-A1 stimulated platelets was still significantly lower (p < 0.01) than platelets pretreated with agonist Mn2+ (10 μM, 15 min), which activates platelets to fully activated state. This suggested that the platelets under VWF-A1 stimulation were not fully activated. We then made a comparison of our approach with conventional coating approach23 (surface coated with a mixture of VWF-A1 and FN) for studying two pairs of receptor-ligand crosstalk. The mixture coating also yields significantly higher adherent platelets due to additional adhesion to VWF-A1. This trend is reproduced in two separate donors (Fig. 6a).
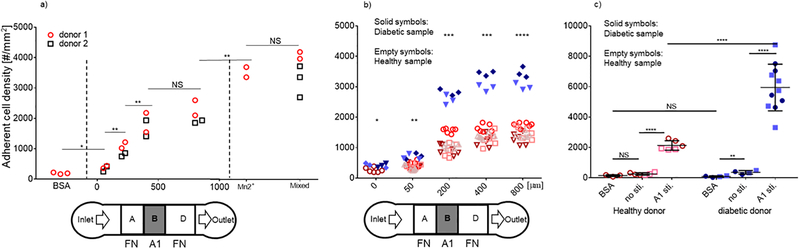
Figure 6.
Testing whole blood and comparing diabetic and healthy platelets. a) Platelets in whole blood from healthy donors respond to different lengths of VWF-A1 (100 μg/ml coating concentration) zone from none (BSA for zone B) up to 800 μm. Conditions of platelets pre-activated by Mn2+ (10 μM, 15 min) or in conventional flow chamber (a mixed of VWF-A1 and FN) were also recorded as benchmark. b) Protein patterns with varied zone B (VWF-A1 stimulus) were used to compare platelets from diabetic and healthy donors. Platelets derived from diabetic donors were statistically more responsive than those derived from healthy donors even without stimulation (p < 0.05). However, much more significant differences were observed when washed platelets were stimulated by varied length of zone B coated with VWF-A1. Experiments were done by perfusing 1 × 108 platelets/ml for 2 min. c) Comparison on platelets in whole blood from both healthy and diabetic donors were made for both conditions of no stimulation and VWF-A1 (800 μm) stimulation. Diabetic platelets in whole blood were statistically more responsive even without stimulation (p < 0.01), but much more significantly with VWF-A1 stimulation (p < 0.0001). Each point in a and b represents measurement from one flow chamber. Different symbols indicate data obtained from different donors. Different symbol shapes indicate different donors. Empty and filled symbols distinguish healthy and diabetic samples. n = 4 for healthy donors, n = 2 for diabetic sample. *, **, *** and **** denotes p < 0.05, 0.01, 0.001 and 0.0001, respectively, by Student t-test.
It is known that platelets from diabetic patients are more reactive to external stimulations than healthy donors, a phenomenon referred to as platelet hyperreactivity, which includes enhanced adhesion and activation of platelets on thrombogenic surfaces24,25. Adhesion and mechano-activation are important aspects of platelet function in mediating haemostasis and thrombosis. Disturbed shear can induce platelet aggregation and thrombus formation in vivo26. Therefore, platelet hyperreactivity to mechanical stimulations is closely related to thrombosis, a common disease that can be fatal27. To test whether platelet dysfunction can be determined by our device, we applied our device to assay the hyperactivity of platelets from patients with diabetes. We first asked whether there would be difference between diabetic and healthy samples without VWF-A1 stimulation. Washed platelets from diabetes patients and healthy donors were perfused over FN without VWF-A1 stimulation in separate devices. Significantly more (p < 0.05) diabetic platelets adhered to FN than the healthy group, indicating enhanced binding activity of the αIIbβ3 integrins on diabetic platelets before any stimulation (Fig. 6b). Notably, much more significant differences between diabetic and healthy platelets were observed when the washed platelets were stimulated with additional VWF-A1 stimulation (variable length zone B) (Fig. 6b). The significance increased with increased A1 stimulation time (zone B 200 μm, p < 0.005), and even more significant for long A1 stimulation time (p < 0.0005 for 400 μm and 0.0001 for 800 μm, respectively). These results indicate that diabetic platelets are not only pre-activated in blood, but also are more reactive upon VWF-A1 stimulation, which is in agreement with their hyperreactivity.
Additionally, we compared activity of platelets in whole blood from diabetic and healthy donors. In a device where stimulation zone is blocked, significantly more (p < 0.01) diabetic platelets in whole blood bind to FN (coating concentration 0. 16 μg/ml) than a negative control where the whole device is coated with BSA (Fig. 6c). Further, even more diabetic platelets bind to FN after VWF-A1 stimulation at 800 μm (p < 0.0001). In comparison, healthy platelets only show activity after VWF-A1 stimulation, and significantly lower than diabetic platelets upon the same stimulation condition (p < 0.0001). These experiments demonstrated the ability of our device in distinguishing diabetic platelets in whole blood. Since one drop of blood (10 to 15 μl) is enough for one test, our assay can potentially be used for preclinical applications where frequent assessment of platelet adhesion behaviour is needed.
Conclusion
We developed an approach for fabricating microfluidic devices that achieves gapless protein patterning with auto-alignment for multiple protein zones. Using this approach, our microfluidic devices achieved sequentially laid-out protein patterns with well-controlled spatial registration and protein density. This system is useful for studying multi-receptor adhesion and signalling cascades such as those seen in hemostatic/thrombotic process, inflammatory reactions, and adaptive immune response. In this work, as a demonstration, we applied devices made using this approach to study platelet activation by GPIb mechanoreception through VWF-A1 binding. We characterized that such activation was stimulation time-dependent, whereby the stimulation time needed to achieve activation at saturation was measured. The device also allowed further investigation on the reversibility of this activation, and the quantification of its time scale. Moreover, the successful application of this device to assessing whole blood samples implies its potential to test a drop of blood, which is compatible with clinical application. Furthermore, using whole blood, we detected the pre-activation of diabetic platelets and their higher activation level upon VWF-A1 stimulation, which is not easily monitored by other means. Altogether, our results indicate that this microfluidic system could serve as a promising diagnostic tool for platelet hyperactivity, which is found not only in diabetes but also many other diseases (e.g., obesity28, hypertension29, etc). Moreover, we envision that this device could be widely applied to the study of cell activation and diagnostic of cell malfunction in a diversity of biological systems, taking advantage of its precisely-controlled biochemical cues and shear environment.
Materials and Methods
Antibodies, proteins and chemicals
Monoclonal antibodies (mAbs) LIBS2 (ab62, Millipore, Billerica, MA), HIP8 (Thermo Fisher Scientific, Waltham, MA) and AK4 (Thermo Fisher Scientific) were used to coat protein patches. LIBS2 binds the β tail domain of integrin αIIbβ3, the epitope of which is exposed only when the integrin takes an extended conformation14. By comparison, HIP8 binds αIIbβ3 in a conformation-independent manner15. AK4 specifically recognizes P-selectin16. PE-labelled anti-A1 mAb 6G1 and FITC-labelled anti-fibronectin mAb ab2413 were from Abcam (Cambridge, UK) and used to visualize ligand coating. GPIb ligand VWF-A1 was a gift from Zaverio Ruggeri from the Scripps Research Institute (La Jolla, CA). Integrin αIIbβ3 ligand fibronectin fragment (the 9–10 domains of fibronectin III) was a gift from Andres J. Garcia from the Georgia Institute of Technology (Atlanta, GA). Biotin-PEG3500-NHS were from JenKem (Plano, TX). ADP, thrombin, dimethyl sulfoxide (DMSO), apyrase, clexane, bovine serum albumin (BSA) and other chemicals were from Sigma-Aldrich (St. Louis, MO) unless stated otherwise.
Blood samples and platelet purification
Blood samples were drawn from volunteers who signed informed consent according to protocols approved by the Institute Review Boards of the Georgia Institute of Technology and Emory University. Blood was drawn by venipuncture from healthy subjects (20–30 years of age) or patients with type 1 diabetes (8–18 years of age) by trained nurses according to procedures approved by the Institutional Review Boards of the Georgia Institute of Technology and Emory University, respectively. Blood was drawn using a 19G butterfly needle into acid citrate dextrose containing theophylline (ACD: 85 mM sodium citrate (2H2O), 72.9 mM citric acid (Anhydrous), 110 mM G-Glucose, 70 mM theophylline, diluted 7× with dH2O), mixed well, and transferred to a 10ml tube with 0.005U/ml apyrase. Whole blood was used directly. To prepare washed platelets, blood was placed at 37 °C for 15 min then centrifuged for 10 min at 200 g. Platelet rich plasma was transferred from the supernatant to fresh tubes and centrifuged again at 1700 g for 5 min. Supernatant was discarded and the platelet pellet was re-suspended in a platelet washing buffer (PWB: 43 mM K2HPO4, 43 mM Na2HPO4, 243 mM NaH2PO4, 1.13 M NaCl, 55 mM D-Glucose, 100 mM theophylline, diluted 10× with dH2O, pH 6.5, conductivity 13–15) plus clexane (20 U/ml) and apyrase (0.01 U/ml). Platelet suspension was then centrifuged at 1500 g for another 5 min. After discarding the supernatant, the platelet pellet was re-suspended at <1 × 109/ml in tyrode’s buffer (120 mM NaHCO3, 100 mM Hepes, 1.37 M NaCl, 27 mM KCl, 55 mM D-Glucose, diluted 10× with dH2O, pH 7.3, conductivity 13–15) containing BSA (5 mg/ml) and apyrase (0.02 U/ml). Platelet suspension was placed in a 37 °C water bath for 30 min. Suspension was diluted to 1 × 108 platelets/ml for use in experiments.
Device design requirements and fabrication
Standard soft lithography was used to fabricate devices in polydimethylsiloxane (PDMS, Corning Sylgard 184, Midland, MI). Two matched silicon wafers were fabricated (SU8–2025, MicroChem). The first is a one-layer mold with a 25 μm-thick single layer for microchannel PDMS. The second is a two-layer mold, 25-μm thick each for stamping PDMS. The features on the transparency mask were transferred with UV photolithography. The surface of wafers was treated with tridecafluoro 1,1,2,2-tetrahydrooctyle-1-trichlorosilane vapor from United Chemical Technologies (Lewistown, PA) to facilitate release of PDMS from the mold. A mixture of PDMS (PDMS base and crosslinker in a 10:1 ratio) was poured on the mold and the whole preparation was left for curing for 2 h at 95°C. After peeling off the PDMS, the devices were cut into shape and access holes were punched using 19 gauge needles (McMaster-Carr).
Plastic clamp holders were laser-cut with holes matching the inlets and outlets. Plastic holders and screws were applied to hold the PDMS device and glass substrate together. After assembly, the device was primed in vacuum to remove bubbles. The chip was connected to syringe pumps to deliver flow for protein coating.
Protein pattern design and preparation
Resting platelets in dynamic flow are able to bind immobilized VWF-A1 but not FN, which only binds activated platelets. In our basic design (Fig. 1b), therefore, after passing BSA blocked area, flowing platelets would first encounter a FN patch (preprocessing, zone A) to confirm the resting state of the platelets prior to stimulation (negative control). The resting platelets would travel downstream to encounter a VWF-A1 patch (stimulation, zone B) for controlled stimulation. Stimulated platelets would then travel further downstream to a relaxation zone C before encountering another FN patch (reporting, zone D) for reporting of platelet activation as assessed by ligand binding by integrin αIIbβ3. To achieve this design, BSA was stamped onto zone C by microcontact printing. FN was delivered via a microchannel to zones A and D with a concentration of 0.016 or 0.16 μg/ml, and incubated for 1 h. VWF-A1 was then delivered via another microchannel to zone B with a concentration of 100 μg/ml and incubated for 1 h.
We also used two variations of the above basic design after it was fully validated. In the first variation, a conformation reporter mAb for integrin αIIbβ3 (LIBS2) or a conformation-independent mAb (HIP8) was coated in zones A and D instead of FN. In the second variation, a mAb against P-selectin (AK4) was coated in zones A and D to detect the upregulated expression of P-selectin.
We fixed the length of zones A and D to be 800 μm, long enough for analysis of platelet binding. We varied the length of zone B to program different stimulation time in the device. We also varied the length of zone C to assess the sustainability and reversal of activation (Fig. 1b).
Quantification of cell binding and protein coating
The experiment was monitored using an inverted microscope (Leica EMIRBE) with a CMOS camera (Hamamatsu Orca) at 40X magnification. Phase-contrast mode was chosen for both video recording and image acquisition. Mean velocities of 15–20 rolling cells were measured for each condition with 9 fps video recording with frame by frame analysis via ImageJ software (ImageJ V1.48 National Institutes of Health) off-line. The experiment was repeated 3 times. The end-point assay was applied by counting the adherent cell number per mm2 over an area of 36200 μm2 after 2 min of continuous flow for 2 random fields of view. Each condition was repeated in 3 devices for each sample. Statistical comparisons were performed using the unpaired Student t-test.
Epifluorescence mode of an EMCCD camera (Hamamatsu Orca) was used to visualize the coating of FN and VWF-A1 using FITC-labelled ab2413 and PE-labelled 6G1 at saturating concentration (100 μg/ml each), respectively.
Acknowledgement
We thank Lining (Arnold) Ju for providing technical expertise in platelet adhesion, Yumiko Sakurai, Yongzhi Qiu, David R. Myers, and Karen Lindsley for helping draw blood. This work was supported by NIH grants R21EB020424 (H.L.) and R01HL132019 (C.Z.). The authors declare no competing financial interests.
Reference
- 1.Humphrey JD, Dufresne ER and Schwartz MA, Nat Rev Mol Cell Biol, 2014, 15, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams TE, Nagarajan S, Selvaraj P and Zhu C, Biophysical Journal, 2000, 79, 1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH and Jackson SP, J Biol Chem, 2002, 277, 2965–2972. [DOI] [PubMed] [Google Scholar]
- 4.Merten M and Thiagarajan P, Circulation, 2000, 102, 1931–1936. [DOI] [PubMed] [Google Scholar]
- 5.Chemnitz JM, Parry RV, Nichols KE, June CH and Riley JL, Journal of immunology (Baltimore, Md. : 1950), 2004, 173, 945–954. [DOI] [PubMed] [Google Scholar]
- 6.Ju L, Chen Y, Li K, Yuan Z, Liu B, Jackson SP and Zhu C, Sci Rep, 2017, 7, 14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A and Whiteside GM, Appl. Phys. Lett, 1993. [Google Scholar]
- 8.Kim E, Xia Y and Whitesides GM, Nature, 1995, 376, 581–584. [Google Scholar]
- 9.Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJ, Ismagilov RF and Whitesides GM, Proceedings of the National Academy of Sciences of the United States of America, 1999, 96, 5545–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perl A, Reinhoudt DN and Huskens J, Advanced Materials, 2009, 21, 2257–2268. [Google Scholar]
- 11.Berthier E, Warrick J, Casavant B and Beebe DJ, Lab on a chip, 2011, 11, 2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane R; and Whitesides GM, Biomaterials, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Bose S, Van Vliet KJ, Karp JM and Karnik R, Journal of visualized experiments : JoVE, 2011, DOI: 10.3791/2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frelinger AL, Du X, Plow EF and Ginsberg MH, J Biol Chem, 1991. [PubMed] [Google Scholar]
- 15.Bunch TA, J Biol Chem, 2010, 285, 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanley W, McCarty O, Jadhav S, Tseng Y, Wirtz D and Konstantopoulos K, J Biol Chem, 2003, 278, 10556–10561. [DOI] [PubMed] [Google Scholar]
- 17.Savage Brian, Enrique Saldívar and Ruggeri ZM, Cell, 1996. [Google Scholar]
- 18.Plow EF and Ginsberg MH, J Biol Chem, 1981. [PubMed] [Google Scholar]
- 19.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, Cruz MA, Dong JF, McIntire LV, McEver RP and Zhu C, J Clin Invest, 2008, 118, 3195–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remijn JA, Arteriosclerosis, Thrombosis, and Vascular Biology, 2002, 22, 686–691. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Juc L, Rushdi M, Ge C and Zhu C, Mol. Biol. Cell, 2017, DOI: 10.1091/mbc.E17-04-0228). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leytin V, Mody M, Semple JW, Garvey B and Freedman J, Biochemical and Biophysical Research Communications, 2000, 273, 565–570. [DOI] [PubMed] [Google Scholar]
- 23.Ju L, Chen Y, Zhou F, Lu H, Cruz MA and Zhu C, Thromb Res, 2015, 136, 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dittmar S, Polanowska-Grabowska R and Gear ARL, Thrombosis Research, 1994, 74, 273–283. [DOI] [PubMed] [Google Scholar]
- 25.Knobler H, Savion N, Shenkman B, Kotev-Emeth S and Varon D, Thrombosis Research, 1998, 90, 181–190. [DOI] [PubMed] [Google Scholar]
- 26.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A and Jackson SP, Nature medicine, 2009, 15, 665–673. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SP, Nature medicine, 2011, 17, 1423–1436. [DOI] [PubMed] [Google Scholar]
- 28.Santilli F, Vazzana N, Liani R, Guagnano MT and Davì G, Obesity Reviews, 2012, 13, 27–42. [DOI] [PubMed] [Google Scholar]
- 29.El Haouari M and Rosado JA, Blood Cells Mol Dis, 2009, 42, 38–43. [DOI] [PubMed] [Google Scholar]