Abstract
Primary Sjögren's syndrome (pSS) is a chronic systemic inflammatory autoimmune disease characterized by lymphocytic infiltrates in exocrine glands. Current approaches do not control harmful autoimmune attacks or prevent irreversible damage and have considerable side effects. Mesenchymal stem cells (MSCs) have been effective in the treatment of several autoimmune diseases. The objective of this review is to illustrate the potential therapeutic role of MSCs in pSS. We summarize the recent advances in what is known about their immunomodulatory function and therapeutic applications in pSS. MSC transfusion can suppress autoimmunity and restore salivary gland secretory function in mouse models and patients with pSS by inducing regulatory T cells, suppressing Th1, Th17, and T follicular helper cell responses. In addition, MSCs can differentiate into salivary epithelial cells, presenting an option as a suitable alternative treatment. We also discuss current bioengineering methods which improve functions of MSCs for pSS. However, there remain many challenges to overcome before their wide clinical application.
1. Introduction
Primary Sjögren's syndrome (pSS) is a chronic, systemic autoimmune disease characterized by lymphocytic infiltrates in salivary and lacrimal glands which lead to the destruction of these glands. It affects globally 0.05–1% of people, with manifestations including xerostomia (dry mouth), dental caries, and xerophthalmia (dry eye) [1]. Activated B lymphocytes are another hallmark of the disease [2]; many antibodies appear in the circulation and tissues. Accordingly, systemic extraglandular involvement is common, including synovitis, interstitial lung disease, neuropathy, renal disease, vasculitis, and autoimmune cytopenias [3]. Furthermore, approximately 5–10% of patients may develop lymphoma, mainly the mucosa-associated lymphoid tissue non-Hodgkin lymphoma, which represents the most severe complication of the disease [4]. Although the exact etiology is unclear, it is known that adaptive and innate immune cell imbalances are involved in the pathogenesis of pSS [5–7]. Current approaches such as traditional disease-modifying antirheumatic drugs and biologic agents do not cure this disease and have considerable side and toxic effects [8]. Thus, the development of novel treatments is critically important for pSS.
Mesenchymal stem cells (MSCs), a group of mesodermal and ectodermal origin multipotent stromal cells, are first discovered by Friedenstein et al. [9]. MSCs have a capacity of self-renewal and differentiation into osteoblasts, adipocytes, and chondrocytes [10, 11]. They are of interest due to their rapid proliferation in vitro and strong immunomodulation [12]. Notably, MSCs have been successfully isolated from almost all adult tissues, including bone marrow, umbilical cord blood, adipose tissue, dental tissue, skin, and placenta [13–17]. Until now, bone marrow MSCs (BMSCs) and umbilical cord MSCs (UMSCs) have been most widely studied. Subsequently, other types of MSCs are reported, such as gingiva-derived MSCs (GMSCs) and adipose-derived MSCs (AMSCs). Unlike MSCs in bone marrow and umbilical cord blood, GMSCs and AMSCs are both abundant and easily accessible, and they can often be obtained as a discarded biological sample following dental procedures or abdominal surgery. GMSCs and AMSCs are relatively easy to isolate, homogenous and proliferate rapidly in vitro [18]. Interestingly, no tumor is observed in the mice which are injected with GMSCs. It indicated GMSCs are nontumorigenic [19]. AMSCs also show a low tendency to develop a tumor [20].
Here, we describe the therapeutic role of MSCs in pSS based on recent relevant publications. Indeed, MSCs have been effective in treating autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and type 1 diabetes mellitus. Moreover, these treatments have no significant side effects [21–27]. Several years ago, scientists summarized the preliminary studies of MSC treatment for salivary gland dysfunction and xerostomia [28, 29]. A recently published review focuses on MSCs for treating autoimmune dacryoadenitis but not the other aspects of pSS [30]. Existing evidence supports the crucial role of MSCs in the treatment of animal models and patients with pSS. MSCs may also differentiate into salivary epithelial cells, presenting an option as a suitable alternative treatment [31, 32]. In this review, we summarize the immunomodulatory effects of MSCs both in the adaptive and the innate immune responses. The defective function of MSCs in pSS is then discussed, followed by a summary of the use of MSCs in the treatment of patients with pSS or animal models. Finally, the role of bioengineering in enhancing MSC treatment is discussed.
2. Immunomodulatory Properties of MSCs on Adaptive and Innate Immune Responses
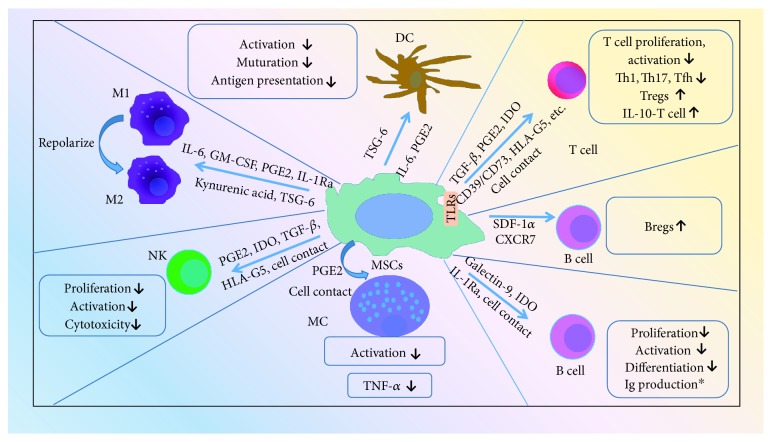
The most attractive property of MSCs is their immunosuppression on both adaptive and innate immune responses. MSCs exert major immunomodulatory effects through cell to cell contact and release of soluble factors such as prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), nitric oxide, transforming growth factor-beta (TGF-β), human leukocyte antigen-G5 (HLA-G5), CD39/CD73, hepatic growth factor (HGF), interleukin- (IL-) 10, IL-6, adenosine, kynurenic acid, TNF-α-stimulated gene 6 (TSG-6), heme oxygenase-1 (HO-1), IL-1 receptor antagonist (IL-1Ra), programmed death-1 ligand 1 (PD-L1), and galectins [33–36]. The mechanisms are illustrated (Figure 1).
Figure 1.
Immunomodulatory properties of mesenchymal stem cells. DC: dendritic cells; TSG-6: TNF-α-stimulated gene 6; PGE2: prostaglandin E2; M1: classically activated macrophages; M2: alternatively activated macrophages; GM-CSF: granulocyte-macrophage colony stimulating factor; IL-1Ra: IL-1 receptor antagonist; NK: natural killer T cells; IDO: indoleamine 2,3-dioxygenase; TGF-β: transforming growth factor-beta; HLA-G5: human leukocyte antigen-G5; MC: mast cells; MSCs: mesenchymal stem cells; Th: T helper; Tfh: T follicular helper cell; Tregs: regulatory T cells; TLRs: Toll-like receptors; SDF-1α: stromal derived factor-1α; CXCR7: CXC chemokine receptor 7; Bregs: regulatory B cells; ∗: controversial.
2.1. Immunomodulatory Functions on Adaptive Immune Cells
The essential cell populations in the adaptive immune system are effector T cells, regulatory T cells (Tregs), and B cells. Abundant evidence supports a role for MSCs to exert immunoregulatory functions on T cells by suppressing proliferation and activation or regulating differentiation [15, 16, 22, 27, 35–48]. Murine BMSCs inhibit naive and memory T cell responses to their cognate antigens [37]. Murine BMSCs markedly suppress xenogeneic graft-versus-host disease (x-GVHD)-derived T helper (Th) 1 cells through adenosine accumulation [38] and also curb experimental autoimmune encephalomyelitis- (EAE-) derived Th17 cell activation in a CC chemokine ligand 2-dependent manner [27]. Recently, it is reported that human BMSCs-derived IL-1Ra can inhibit inflammation via suppressing the Th17 differentiation [39]. Interestingly, human BMSCs and murine BMSCs themselves can produce IL-17, but IL-17+ MSC displays an impaired immunosuppressive capacity [40]. Furthermore, BMSCs could induce Tregs and IL-10-producing T cells through the release of soluble factors such as PGE2, TGF-β, and HLA-G5 or cell-cell contact [41–43]. Moreover, human BMSCs can express Toll-like receptors (TLRs). Engagement of different TLRs in MSCs enhances their immunosuppressive properties by inducing IDO or impairing Notch signaling [46, 47]. Other MSCs, for example, UMSCs and AMSCs, can also significantly suppress T cell proliferation and activation [16, 44]. AMSCs inhibit the proliferative response and the production of inflammatory cytokines by antigen-specific CD4 and CD8+ T cells. Also, the numbers of IL-10-producing T cells and monocytes are significantly augmented upon AMSC treatment [16]. It is demonstrated that GMSCs suppress T cell proliferation and activation via interferon-γ- (IFN-γ-) induced stimulation of IDO and IL-10 [15]. Moreover, GMSCs ameliorate colonic inflammation by enhancing Tregs and IL-10 expression in mice [15]. GMSCs could significantly inhibit Th1 and Th17 cells and reduce the production of inflammatory cytokines (IFN-γ, IL-17A) via CD39/CD73 signaling [22, 45].
T follicular helper cells (Tfh) are recognized as crucial effector cells for B cell maturation and immunoglobulin production. UMSCs are found to suppress the differentiation of Tfh via the secretion of IDO [26, 49]. Most importantly, BMSCs can inhibit the Tfh response in lupus-prone mice [50]. Furthermore, it is suggested that BMSCs and UMSCs could indirectly affect the maturation and immunoglobulin production of B cells by inhibiting the Tfh immune reaction.
As we know, B cell development is dependent on the interaction of B cell progenitor and stromal cells, which provides a supportive microenvironment for B cells. BMSCs can suppress B cell proliferation, differentiation toward plasmablast, and immunoglobulin production dependent on galectin-9 or IL-1Ra signaling [51, 52]. BMSCs and AMSCs both suppress activation of blood B cells by phytohemagglutinin stimulation, but UMSCs do not display an inhibitory effect [53]. Besides, BMSCs could affect chemotactic properties of B cells, because chemokine receptors (CCR) and CXC chemokine receptors (CXCR), such as CXCR4, CXCR5, and CCR7, are decreased after B cell-BMSC coculture. However, B cell costimulatory molecules CD40, CD80, and CD86 and production of various cytokines are unaffected by BMSCs [54]. Interestingly, BMSCs do not inhibit B cell proliferation but only in the presence of inflammatory cytokine IFN-γ [55]. The suppressive effect of IFN-γ is related to its ability to stimulate the release of IDO by BMSCs, which in turn inhibits the proliferation of B cells [55]. Another group finds that enhanced autoantibody production is companied by increased plasma cells after BMSC administration [56].
Several years ago, a new regulatory subset called B regulatory cells (Bregs) was identified. These cells can interact with pathogenic T cells to inhibit harmful immune responses [57]. Transfer of UMSCs ameliorates experimental colitis by inducing Bregs [58]. AMSC treatment expands the Breg population in lupus mice in vivo [59]. Further study finds that murine BMSCs promote Bregs through stromal derived factor-1α (SDF-1α) and its receptor CXCR7-mediated signaling [60].
2.2. Immunomodulatory Functions on Innate Immune Cells
The innate immune system is the first line of host defense and consists of several types of immune cells including dendritic cells (DC), macrophages, natural killer T cells (NK), and mast cells (MC). MSCs exhibit potent immunomodulatory effects on these cells which may play an important role in the pathogenesis of pSS [5]. Human BMSCs can suppress activation and maturation of DC and impair their antigen-presenting ability through the release of TSG-6 [61–63]. Other research finds that the soluble factor IL-6 is involved in the immunomodulatory mechanism mediated by murine BMSCs through partial inhibition of DC differentiation [64]. Murine BMSCs impair TLR4-induced activation of DC resulting in downregulation of antigen presentation to T cells [65]. Human GMSCs can significantly blunt the maturation and activation of DC via PGE2-dependent mechanisms [66]. Human UMSCs can facilitate the shift of monocytes toward IL-10 producing phenotypes through the production of IL-6 and HGF [67].
MSCs reprogram macrophages into the anti-inflammatory M2 phenotype through the production of IL-6, granulocyte-macrophage colony stimulating factor (GM-CSF), PGE2, kynurenic acid, TSG-6, or IL-1Ra [52, 68–71]. Human BMSCs are activated by TNF-α to produce an anti-inflammatory mediator, TSG-6, and thereby create a negative feedback loop that attenuates inflammation by reducing TLR2-mediated signaling in resident macrophages [72]. MSCs have been shown to interact with NK cells dependent on their activation state. When resting NK cells are exposed to IL-2, the expression levels of activating receptors such as NKG2D, NKp30, and NKp44 are increased. Human BMSCs could inhibit IL-2-induced proliferation of resting NK cells, whereas they partially affect proliferation of activated NK cells. Human BMSCs also downregulated the activation and cytotoxicity of NK cells by mechanisms involving PGE2, IDO, TGF-β, HLA-G5, adenosine, or cell contact [43, 53, 73–75]. Interestingly, human NK cells secrete NAP-2 (CXCL7), a chemokine that can induce MSC migration to repair damaged tissue [76]. And GMSCs are capable of exerting suppressive effects on mast cells through the release of PGE2 and cell-cell contact both in vitro and in vivo [66, 77].
Recently, it has been thought that the immunomodulatory capabilities of MSCs are not constitutive but rather are subject to the inflammatory milieu or different signals. MSCs primed with IFN-γ or the stimulation of TLR signaling is required to induce their immunosuppressive phenotype [47, 55]. Proinflammatory cytokines such as TNF-α and IL-1 have been shown to enhance the effect of IFN-γ on MSC priming through the production of IDO or PGE2 [55, 78, 79]. The activation of TLR3 may promote the polarization of MSCs into immune-suppressive phenotype, while TLR4 stimulation may induce polarization of MSCs toward proinflammatory phenotype [80]. Therefore, MSCs show a plastic behavior and switch into an immunosuppressive phenotype depending on microenvironmental conditions.
3. BMSCs and Salivary MSCs Are Both Defective in pSS
Some evidence shows that BMSCs are defective in immunoregulatory functions in animal models and patients with pSS [81]. BMSCs have a significantly lower proliferative capacity and less osteogenic and adipogenic differentiation potentials in nonobese diabetic (NOD) mice. BMSCs derived from NOD mice fail to suppress T cell proliferation. CD4+Foxp3+Treg cells are much lower when splenocytes are cocultured with BMSCs from NOD mice. Furthermore, BMSCs obtained from pSS patients show an impaired suppressive effect on PBMC proliferative responses. Interestingly, activated T cells can induce BMSC apoptosis via the Fas/Fas ligand pathway [82]. IFN-γ synergistically enhances TNF-α–induced BMSC apoptosis [83, 84]. Furthermore, proinflammatory T cells inhibit the ability of BMSCs to repair damaged tissue. When the concentrations of IFN-γ and TNF-α are decreased, it significantly improves the function of BMSCs in repairing the tissue [83]. As we know, Th1 (IFN-γ and TNF-α)-mediated inflammation is a key feature in the salivary gland pathology [85]. T cells are activated in patients with pSS [86]. These findings suggest that T cell-induced BMSC apoptosis may contribute to BMSC impairment in pSS. Collectively, these results demonstrate that the biological and regulatory functions of BMSCs are impaired in pSS [81].
Recently, MSCs have been also found in the salivary glands of patients with pSS. Such organ-specific MSCs may have advantages for treatment of the specific tissue of origin since they could directly act on the target cell. Jeong et al. [87] efficiently isolates and amplifies MSCs in large amounts from human parotid and submandibular salivary glands in vitro. These cells express the same characteristic MSC markers; they are negative for hematopoietic stem cell and salivary gland epithelium markers. They are able to differentiate into adipogenic, osteogenic, and chondrogenic cells and notably into amylase-expressing cells. Transplantation of SGSCs restores salivary gland function in radiation-damaged rat salivary glands [87]. However, MSCs from the labial glands of patients with pSS have a deficiency in salivary gland-like cell differentiation [88]. Taken together, BMSCs and salivary MSCs are both defective in patients with pSS disease condition, meaning that it is not ideal to treat patients using their own MSCs. We may use allogeneic MSCs for the treatment of pSS, due to nonimmunogenic characteristics and low or absent expression of non-major histocompatibility complex- (MHC-) I and MHC-II [12].
4. The Application of MSCs in the Treatment of pSS
pSS is a disease triggered by the breakdown of self–nonself discrimination and a subsequent autoreactive immune response. Salivary gland pathology is mainly a Th1-mediated immune reaction [85]. Th17 and Tfh cells are also associated with inflammation and clinical profiles in pSS [49, 86]. The Treg percentage is altered in the peripheral blood [89–91], and their suppressive function is compromised in pSS [92]. In contrast, another group reports that the Treg subset did not change in patients with pSS [93]. Therefore, the imbalance of these immune cells contributes to the pathogenesis of pSS. It is clear that MSCs could possess potent immunomodulatory functions on both adaptive and innate immune cells and especially reset the immune imbalance by upregulating Tregs and downregulating Th1, Th17, or Tfh cells. In addition, MSCs can differentiate into salivary epithelial cells, presenting an option as a suitable alternative treatment for pSS. The unique immunomodulatory and biological properties of MSCs make them candidates for cell therapy to repair tissue and organ damages caused by pSS. We have summarized the applications of MSCs in the treatment of patients with pSS or in animal models (Table 1).
Table 1.
Application of MSCs in treating animal models and patients with pSS.
| Types of MSCs | Treatment | Recipient (human) | Recipient (mice) | Follow-up | Effect | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|
| Cell numbers, origin | Administration | |||||||
| BMSCs | 1 × 105, one dose, BALB/c or B6 mice | IV injection at 6 (prevention group) or 16 weeks age (treatment group) | NA | NOD mice | 8 weeks or 18 weeks | Inflammatory area ↓ in SG, salivary flow rate ↑, Tregs ↑, Th2 ↑, Th17 ↓, Tfh ↓ | Improvement | [81] |
| UMSCs | 1 × 106/kg, one dose, human | IV injection | Patients with pSS | NA | 12 months | SSDAI and global assessment Vas score ↓, unstimulated and stimulated salivary flow rate ↑, anti-SSA/Ro and anti-SSB/La ↓ | Improvement | |
| BMSCs | 5 × 105, four doses, mouse | IV injection | NA | NOD mice | 4 weeks | Saliva flow rate ↑, lymphocytic infiltrations ↓, serum IFN-γ ↓, IL-10 ↑, TGF-β ↑ | Improvement | [94] |
| BMSCs | 1 × 106, one dose, mouse | IP injection | NA | NOD mice | 4 weeks | Tear production ↑, aquaporin 5 mRNA ↑, lymphocytic score did not change | Improvement | [95] |
| CD45−TER119− BMSCs | 1 × 107, four doses, human | IV injection | NA | NOD mice | 14 weeks | Salivary flow rate ↑, lymphocytic infiltration in SG ↓, T and B cells ↓, Tregs ↑ in all foci | Improvement | [96] |
| UMSCs | Human | Coculture | pSS | NA | NA | Differentiation and proliferation of Tfh ↓ | Suppression | [49] |
| UMSCs microencapsulated | Human | Coculture | PBMC from 10 pSS | NA | NA | Proliferation of T cell ↓, Th1 ↓, Th17 ↓, Tregs ↑ | Suppression | [44] |
| AMSCs | 8 × 106, allogeneic, one dose, per eye, dog | Locally injected around lacrimal glands and gland of eyelid | NA | Dog with KCS | 9 months | Schirmer tear test ↑, ocular parameter score ↓ | Improvement | [97] |
MSC treatment ameliorates sialadenitis in the mouse model and in patients with pSS partly through reducing the proliferation of T cells, decreasing Th1, Th17, and Tfh cells, and increasing Tregs [44, 49, 81, 94–98]. The pSS patients tolerate the allogeneic UMSCs well, have an improvement in symptoms and a decrease in serum anti-SSA/anti-SSB antibody without significant adverse events [81]. Interestingly, infused allogeneic BMSCs could migrate toward the inflammatory regions in an SDF-1-dependent manner, as neutralization of SDF-1 ligand CXCR4 abolishes the effectiveness of BMSC treatment. Therefore, allogeneic BMSCs might target local sialadenitis and systemic inflammatory responses in pSS patients by a mechanism that is dependent on SDF-1/CXCR4 signaling [81]. Another team finds that treatment with BMSCs prevents a decline in the salivary flow rate and lymphocytic infiltrations in the salivary glands of NOD mice [94]. Furthermore, BMSCs enhance tear production in the NOD mouse model, due to decreased inflammation and increased expression of aquaporin 5 [95]. Although the number of lymphocytic foci in the lacrimal glands of treated animals did not change, the size of the foci decreased by 40.5% [95].
As we know, bone marrow mesenchymal cells can be easily contaminated with hematopoietic cells. Researchers isolate CD45−/TER119− cells from murine bone marrow by depleting CD45+ cells or TER119+ hematopoietic cells. CD45−/TER119−cells are identified as BMSCs because they are positive for stem cell surface markers and have multiple differentiation potentials. Treatment with CD45−/TER119−cells could prevent loss of saliva flow and reduce lymphocytic infiltrations in SG of NOD mice through downregulation of inflammatory cytokines such as TNF-α. Notably, the infiltrations of T and B cells are decreased in all foci, while the frequency of Tregs is increased. Investigators speculate enhanced Tregs inhibit the inflammation in SG. Meanwhile, fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF) involved in the growth, regeneration, and maintenance of salivary glands are both increased after CD45−/TER119− MSC treatment. This suggests the CD45−/TER119− BMSCs are effective in both preventing deterioration of saliva secretion and reducing lymphocytic influx in salivary glands with a systemic effect [96].
Tfh cells are recognized as crucial for B cell maturation and differentiation. Unlike BMSCs, UMSCs are found to suppress the differentiation of Tfh cells via the secretion of IDO in patients with pSS [49]. Therefore, UMSCs could provide a novel therapeutic approach for pSS by targeting Tfh cells. Other investigators find that human UMSCs inhibit proliferation of healthy T cells, but not T cells from pSS patients. Interestingly, they develop a new microencapsulation technique to make a coculture system separating UMSCs from T cells. This approach avoids a systemic immune reaction in the host. The specialized UMSCs could suppress pSS T cell proliferation and restore the Tregs/Th17 ratio, suggesting a drug delivery system able to enhance the immunomodulatory effects of UMSCs in pSS [44].
A small preclinical study regarding the application of AMSCs shows that when allogeneic AMSCs are implanted around the lacrimal glands in 12 dogs (24 eyes) with refractory keratoconjunctivitis sicca (KCS), improvement is observed in the Schirmer tear and ocular surface integrity tests during nine months follow-up. No systemic or local complications appear. However, a caveat is that no control cells are mentioned in this study [97].
Interestingly, it has been demonstrated that human BMSCs could temporarily change into salivary gland epithelial cells (SGEC) in a coculture system. BMSCs have comparable cellular structures and expressed several salivary genes such as aquaporin 5, E-cadherin, and α-amylase (α-AMY) when cocultured with SGEC. These BMSCs can secrete α-AMY. Moreover, they have comparable cellular structures to SGEC, such as tight junctions and numerous secretory granules, as shown by electron microscopy [99]. Some proteins such as ankryin-repeat-domain-containing-protein 56, high-mobility-group-protein 20B, and transcription factor E2a are the putative regulatory factors involved in the transdifferentiation of BMSCs into SGEC in an animal study [31]. These data suggest that cocultured BMSCs can generate a salivary gland acinar phenotype, meaning that this approach has potential application to treat salivary gland diseases such as pSS [31, 32, 99]. However, we still do not know whether pSS disease-derived BMSCs switch into acinar epithelial cells. Further study is needed to answer this question.
5. Perspectives: Learn from Bioengineering Strategies
In order to improve the function of MSCs, novel methods such as retroviral transduction, electroporation, or CRISPR-associated protein-9 nuclease (Cas9) of foreign genes have been utilized to engineer MSCs [100–103]. In cancer, MSCs have been successfully modified with a tumor suppressive gene to inhibit progress or metastasis of tumor cells. Similarly, current bioengineering strategies could be applied in pSS. For instance, IL-10 is a powerful cytokine to suppress inflammation. One team engineered mouse BMSCs with an IL-10 gene (called IL-10-BMSCs) through retroviral transduction. They demonstrated that IL-10-BMSCs, but not BMSCs alone or PBS, could modulate inflammatory arthritis and decrease the histological scores [100]. Besides, MSCs transfected with minicircles encoding CXCR4 are more likely to migrate to the injury site [102]. Moreover, it is possible to track the MSCs which are transfected with minicircles and monitor their elastic properties with noninvasive microscopy technologies. Recently, a novel gene-editing technology based on a bacterial CRISPR-associated protein-9 nuclease (Cas9) has been successfully used to target important genes in many cell lines and organisms [103]. It may be possible to develop a successful MSCs-derived therapy for pSS if we target crucial genes involved in MSC immunomodulation, such as soluble factor production and chemokine receptors using Cas9 methods.
Recently, extracellular vesicles (EVs) have been considered as a functional molecule with their potential for treatment of pSS. As we know, extracellular vesicles are secreted by many cells, including MSCs, and are classified into two types of particles: exosomes, with a size of 50 to 100 nm derived from the endosomal compartment, and microvesicles (MVs), with a size between 100 nm and 1000 nm. It has been reported that BMSCs, UMSCs, and AMSCs may be a suitable source for therapeutic EVs [104–107]. EVs can mediate cell-to-cell communication and participate in many processes including inflammation, proliferation, cell differentiation, and immune signaling [106, 108]. They can also act directly with the target cell membrane by fusion, transferring components into intracellular compartments or causing endocytosis. MVs have a role in antigen presentation and activation of endosomal receptors such as Toll-like receptors [109]. EVs can carry autoantigens, cytokines, and tissue-degrading enzymes. Collectively, EVs could repair damaged tissue or serve as agents for drug delivery in autoimmune disease [106, 108, 110]. Recently, it has been reported that MSCs derived from human-induced pluripotent stem cells (MSCs-iPSCs) can prevent the onset of sialadenitis in NOD mice. Furthermore, EVs derived from MSCs-iPSCs are shown to suppress activation of immune cells in vitro. And the infusion of these EVs at the predisease stage reduces the lymphocyte infiltration in salivary glands and serum autoantibody levels in NOD mice [111].
We focus on the therapeutic effect of GMSCs in autoimmune disease, especially in pSS. GMSCs are relatively easy to isolate, homogenous and proliferate rapidly in vitro, and have a strong immunomodulation via suppression of Th1 and Th17 cells and enhancement of Treg differentiation [22]. We further find that GMSCs suppress human T cell-mediated diseases in the x-GVHD model via CD39/CD73/adenosine and IDO signals [112]. In addition, GMSC populations existing within the inflamed gingival tissue are functionally equivalent to those derived from healthy gingival tissue, indicating the possibility of treatment with the patient's GMSCs [113]. Furthermore, our unpublished data have documented that GMSCs could inhibit the proliferation and IFN-γ production of peripheral T cells from patients with pSS in vitro. Next, we plan to study whether the patient with pSS will benefit from GMSC transfusion.
There remain many challenges to overcome for clinical application of MSCs in pSS. First, there are great variations in the MSC isolation protocols, culture systems, MSC dose, cell delivery methods, and transfusion frequency in the reported studies. Secondly, the quality control of diverse MSCs is not defined. It will be necessary to establish standardized protocols for cell culture, differentiation, and expansion, as well as recommended transfusion schedules and evaluation of responses. Thirdly, the number of enrolled patients reported in published papers is small and may not be sufficient to provide a reliable conclusion. Fourthly, another restriction in the field of clinical application of MSCs is the biosafety issues relevant to tumorigenicity [114]. Some papers report that MSCs enhance tumor angiogenesis and promote tumor formation in mice [115, 116]. Finally, whether MSC transfusion will have a long-term effect in pSS is unknown.
6. Conclusions
In summary, MSCs can be obtained from different sources and are especially abundant in adipose and dental tissues. They possess potent immunomodulatory functions and act on both adaptive and innate immune responses. They may repair damaged tissue via suppressing Th1/Th17/Tfh cell responses and upregulating Tregs. Furthermore, MSCs could induce Breg cells and modulate innate immune cells such as DC, macrophages, mast cells, and NK cells. Moreover, new bioengineering approaches, such as Cas9 methods, may improve the function of MSCs. Currently, there is no curative clinical therapy for pSS, so MSCs-based therapies show great potential in this area, with the expected capacity to significantly suppress the inflammation and preserve salivary function in pSS. As of now, no conclusive evidence to support the use of MSC-based therapies has been published. In the near future, randomized controlled trials of the therapeutic use of MSCs in pSS will be of considerable interest.
Acknowledgments
This study is supported in part by the grants from National Natural Science Foundation of China (81701600 and 81274161) and Natural Science Foundation of Zhejiang Province (LQ17H100001, LGF18H100001, and LY17H100006).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Maria N. I., Vogelsang P., Versnel M. A. The clinical relevance of animal models in Sjögren’s syndrome: the interferon signature from mouse to man. Arthritis Research & Therapy. 2015;17(1):p. 172. doi: 10.1186/s13075-015-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szyszko E. A., Brokstad K. A., Oijordsbakken G., Jonsson M. V., Jonsson R., Skarstein K. Salivary glands of primary Sjögren’s syndrome patients express factors vital for plasma cell survival. Arthritis Research & Therapy. 2011;13(1, article R2) doi: 10.1186/ar3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox P. C. Autoimmune diseases and Sjögren’s syndrome: an autoimmune exocrinopathy. Annals of the New York Academy of Sciences. 2007;1098(1):15–21. doi: 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 4.Nocturne G., Virone A., Ng W. F., et al. Rheumatoid factor and disease activity are independent predictors of lymphoma in primary Sjögren’s syndrome. Arthritis & Rheumatology. 2016;68(4):977–985. doi: 10.1002/art.39518. [DOI] [PubMed] [Google Scholar]
- 5.Kiripolsky J., McCabe L. G., Kramer J. M. Innate immunity in Sjögren’s syndrome. Clinical Immunology. 2017;182:4–13. doi: 10.1016/j.clim.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosi A., Wahren-Herlenius M. Update on the immunobiology of Sjögren’s syndrome. Current Opinion in Rheumatology. 2015;27(5):468–475. doi: 10.1097/BOR.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 7.Samuni Y., Baum B. J. Gene delivery in salivary glands: from the bench to the clinic. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(11):1515–1521. doi: 10.1016/j.bbadis.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraux A., Pers J.-O., Devauchelle-Pensec V. Treatment of primary Sjögren syndrome. Nature Reviews Rheumatology. 2016;12(8):456–471. doi: 10.1038/nrrheum.2016.100. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein A. J., Chailakhyan R. K., Latsinik N. V., Panasyuk A. F., Keiliss-Borok I. V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger M. F., Mackay A. M., Beck S. C., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Nam H. Y., Karunanithi P., Loo W. C., et al. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Research & Therapy. 2013;15(5, article R129) doi: 10.1186/ar4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tessier L., Bienzle D., Williams L. B., Koch T. G. Phenotypic and immunomodulatory properties of equine cord blood-derived mesenchymal stromal cells. PLoS One. 2015;10(4, article e0122954) doi: 10.1371/journal.pone.0122954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munir H., McGettrick H. M. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells and Development. 2015;24(18):2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- 14.Tomar G. B., Srivastava R. K., Gupta N., et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochemical and Biophysical Research Communications. 2010;393(3):377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q., Shi S., Liu Y., et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. The Journal of Immunology. 2009;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Rey E., Gonzalez M. A., Varela N., et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010;69(1):241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 17.Choi J. R., Yong K. W., Wan Safwani W. K. Z. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cellular and Molecular Life Sciences. 2017;74(14):2587–2600. doi: 10.1007/s00018-017-2484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatesh D., Mohan Kumar K. P., Alur J. B. Gingival mesenchymal stem cells. Journal of Oral and Maxillofacial Pathology. 2017;21(2):296–298. doi: 10.4103/jomfp.JOMFP_162_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santamaria S., Sanchez N., Sanz M., Garcia-Sanz J. A. Comparison of periodontal ligament and gingiva-derived mesenchymal stem cells for regenerative therapies. Clinical Oral Investigations. 2017;21(4):1095–1102. doi: 10.1007/s00784-016-1867-3. [DOI] [PubMed] [Google Scholar]
- 20.Choi J. R., Pingguan-Murphy B., Wan Abas W. A. B., et al. In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS One. 2015;10(1, article e0115034) doi: 10.1371/journal.pone.0115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L., Wang D., Liang J., et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis & Rheumatism. 2010;62(8):2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 22.Chen M., Su W., Lin X., et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis & Rheumatism. 2013;65(5):1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christopeit M., Schendel M., Föll J., Müller L. P., Keysser G., Behre G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. 2008;22(5):1062–1064. doi: 10.1038/sj.leu.2404996. [DOI] [PubMed] [Google Scholar]
- 24.Vija L., Farge D., Gautier J.-F., et al. Mesenchymal stem cells: stem cell therapy perspectives for type 1 diabetes. Diabetes & Metabolism. 2009;35(2):85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Mu R., Wang S., et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Research & Therapy. 2010;12(6, article R210) doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R., Li X., Zhang Z., et al. Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Scientific Reports. 2015;5(1, article 12777) doi: 10.1038/srep12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafei M., Campeau P. M., Aguilar-Mahecha A., et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. The Journal of Immunology. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 28.Sumita Y., Liu Y., Khalili S., et al. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. The International Journal of Biochemistry & Cell Biology. 2011;43(1):80–87. doi: 10.1016/j.biocel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen D. H., Oliveri R. S., Trojahn Kølle S. F., et al. Mesenchymal stem cell therapy for salivary gland dysfunction and xerostomia: a systematic review of preclinical studies. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2014;117(3):335–342.e1. doi: 10.1016/j.oooo.2013.11.496. [DOI] [PubMed] [Google Scholar]
- 30.Lu X., Wang X., Nian H., Yang D., Wei R. Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem Cell Research & Therapy. 2017;8(1):p. 126. doi: 10.1186/s13287-017-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y. J., Koh J., Gauna A. E., Chen S., Cha S. Identification of regulatory factors for mesenchymal stem cell-derived salivary epithelial cells in a co-culture system. PLoS One. 2014;9(11, article e112158) doi: 10.1371/journal.pone.0112158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y.-J., Koh J., Kwon J. T., Park Y.-S., Yang L., Cha S. Uncovering stem cell differentiation factors for salivary gland regeneration by quantitative analysis of differential proteomes. PLoS One. 2017;12(2, article e0169677) doi: 10.1371/journal.pone.0169677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao F., Chiu S. M., Motan D. A. L., et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death & Disease. 2016;7(1, article e2062) doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz L. A., DuTreil M., Fattman C., et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies L. C., Heldring N., Kadri N., Le Blanc K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells. 2017;35(3):766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gieseke F., Bohringer J., Bussolari R., Dominici M., Handgretinger R., Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116(19):3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 37.Krampera M., Glennie S., Dyson J., et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 38.Amarnath S., Foley J. E., Farthing D. E., et al. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33(4):1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee K., Park N., Jung H., et al. Mesenchymal stem cells ameliorate experimental arthritis via expression of interleukin-1 receptor antagonist. PLoS One. 2018;13(2, article e0193086) doi: 10.1371/journal.pone.0193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R., Liu Y., Kelk P., et al. A subset of IL-17+ mesenchymal stem cells possesses anti-Candida albicans effect. Cell Research. 2013;23(1):107–121. doi: 10.1038/cr.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.English K., Ryan J. M., Tobin L., Murphy M. J., Barry F. P., Mahon B. P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25highforkhead box P3+ regulatory T cells. Clinical & Experimental Immunology. 2009;156(1):149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Najar M., Raicevic G., Fayyad-Kazan H., et al. Bone marrow mesenchymal stromal cells induce proliferative, cytokinic and molecular changes during the T cell response: the importance of the IL-10/CD210 axis. Stem Cell Reviews and Reports. 2015;11(3):442–452. doi: 10.1007/s12015-014-9567-3. [DOI] [PubMed] [Google Scholar]
- 43.Selmani Z., Naji A., Zidi I., et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 44.Alunno A., Montanucci P., Bistoni O., et al. In vitro immunomodulatory effects of microencapsulated umbilical cord Wharton jelly-derived mesenchymal stem cells in primary Sjögren’s syndrome. Rheumatology. 2015;54(1):163–168. doi: 10.1093/rheumatology/keu292. [DOI] [PubMed] [Google Scholar]
- 45.Tang L., Li N., Xie H., Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. Journal of Cellular Physiology. 2011;226(3):832–842. doi: 10.1002/jcp.22405. [DOI] [PubMed] [Google Scholar]
- 46.Opitz C. A., Litzenburger U. M., Lutz C., et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-β and protein kinase R. Stem Cells. 2009;27(4):909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- 47.Liotta F., Angeli R., Cosmi L., et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing notch signaling. Stem Cells. 2008;26(1):279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 48.Chabannes D., Hill M., Merieau E., et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 49.Liu R., Su D., Zhou M., Feng X., Li X., Sun L. Umbilical cord mesenchymal stem cells inhibit the differentiation of circulating T follicular helper cells in patients with primary Sjögren’s syndrome through the secretion of indoleamine 2,3-dioxygenase. Rheumatology. 2015;54(2):332–342. doi: 10.1093/rheumatology/keu316. [DOI] [PubMed] [Google Scholar]
- 50.Yang X., Yang J., Li X., Ma W., Zou H. Bone marrow-derived mesenchymal stem cells inhibit T follicular helper cell in lupus-prone mice. Lupus. 2017;27(1):49–59. doi: 10.1177/0961203317711013. [DOI] [PubMed] [Google Scholar]
- 51.Ungerer C., Quade-Lyssy P., Radeke H. H., et al. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells and Development. 2014;23(7):755–766. doi: 10.1089/scd.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luz-Crawford P., Djouad F., Toupet K., et al. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34(2):483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 53.Ribeiro A., Laranjeira P., Mendes S., et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Research & Therapy. 2013;4(5):p. 125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corcione A., Benvenuto F., Ferretti E., et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 55.Krampera M., Cosmi L., Angeli R., et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 56.Youd M., Blickarz C., Woodworth L., et al. Allogeneic mesenchymal stem cells do not protect NZB × NZW F1 mice from developing lupus disease. Clinical & Experimental Immunology. 2010;161(1):176–186. doi: 10.1111/j.1365-2249.2010.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mauri C., Ehrenstein M. R. The ‘short’ history of regulatory B cells. Trends in Immunology. 2008;29(1):34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Chao K., Zhang S., Qiu Y., et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5+ B regulatory cells. Stem Cell Research & Therapy. 2016;7(1):p. 109. doi: 10.1186/s13287-016-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park M. J., Kwok S. K., Lee S. H., Kim E. K., Park S. H., Cho M. L. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplantation. 2015;24(11):2367–2377. doi: 10.3727/096368914X685645. [DOI] [PubMed] [Google Scholar]
- 60.Qin Y., Zhou Z., Zhang F., et al. Induction of regulatory B-cells by mesenchymal stem cells is affected by SDF-1α-CXCR7. Cellular Physiology and Biochemistry. 2015;37(1):117–130. doi: 10.1159/000430338. [DOI] [PubMed] [Google Scholar]
- 61.Ramasamy R., Fazekasova H., Lam E. W. F., Soeiro I., Lombardi G., Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Yin Z., Zhang R., et al. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochemical and Biophysical Research Communications. 2014;450(4):1409–1415. doi: 10.1016/j.bbrc.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Jiang X. X., Zhang Y., Liu B., et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 64.Djouad F., Charbonnier L. M., Bouffi C., et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 65.Chiesa S., Morbelli S., Morando S., et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su W. R., Zhang Q. Z., Shi S. H., Nguyen A. L., Le A. D. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29(11):1849–1860. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 67.Deng Y., Zhang Y., Ye L., et al. Umbilical cord-derived mesenchymal stem cells instruct monocytes towards an IL10-producing phenotype by secreting IL6 and HGF. Scientific Reports. 2016;6(1, article 37566) doi: 10.1038/srep37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Q. Z., Su W. R., Shi S. H., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasandan A. B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S. J. Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Scientific Reports. 2016;6(1, article 38308) doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang G., Cao K., Liu K., et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death & Differentiation. 2018;25(7):1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sala E., Genua M., Petti L., et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163–176.e20. doi: 10.1053/j.gastro.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Choi H., Lee R. H., Bazhanov N., Oh J. Y., Prockop D. J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spaggiari G. M., Capobianco A., Becchetti S., Mingari M. C., Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 74.Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 75.Sotiropoulou P. A., Perez S. A., Gritzapis A. D., Baxevanis C. N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 76.Almeida C. R., Caires H. R., Vasconcelos D. P., Barbosa M. A. NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Reports. 2016;6(4):466–473. doi: 10.1016/j.stemcr.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li P., Zhao Y., Ge L. Therapeutic effects of human gingiva-derived mesenchymal stromal cells on murine contact hypersensitivity via prostaglandin E2–EP3 signaling. Stem Cell Research & Therapy. 2016;7(1):p. 103. doi: 10.1186/s13287-016-0361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren G., Zhao X., Zhang L., et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. The Journal of Immunology. 2010;184(5):2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouffi C., Bony C., Courties G., Jorgensen C., Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5(12, article e14247) doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waterman R. S., Tomchuck S. L., Henkle S. L., Betancourt A. M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4, article e10088) doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J., Wang D., Liu D., et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120(15):3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaza T., Miura Y., Bi Y., et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3(7, article e2615) doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Wang L., Kikuiri T., et al. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α . Nature Medicine. 2011;17(12):1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L., Zhao Y., Liu Y., et al. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 2013;31(7):1383–1395. doi: 10.1002/stem.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh N., Cohen P. L. The T cell in Sjögren’s syndrome: force majeure, not spectateur. Journal of Autoimmunity. 2012;39(3):229–233. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katsifis G. E., Rekka S., Moutsopoulos N. M., Pillemer S., Wahl S. M. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. The American Journal of Pathology. 2009;175(3):1167–1177. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeong J., Baek H., Kim Y. J., et al. Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Experimental & Molecular Medicine. 2013;45(11, article e58) doi: 10.1038/emm.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S. Q., Wang Y. X., Hua H. Characteristics of labial gland mesenchymal stem cells of healthy individuals and patients with Sjögren’s syndrome: a preliminary study. Stem Cells and Development. 2017;26(16):1171–1185. doi: 10.1089/scd.2017.0045. [DOI] [PubMed] [Google Scholar]
- 89.Christodoulou M. I., Kapsogeorgou E. K., Moutsopoulos N. M., Moutsopoulos H. M. Foxp3+ T-regulatory cells in Sjögren’s syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. The American Journal of Pathology. 2008;173(5):1389–1396. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gottenberg J. E., Lavie F., Abbed K., et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjögren’s syndrome. Journal of Autoimmunity. 2005;24(3):235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 91.Li X., Li X., Qian L., et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjögren’s syndrome. The Journal of Rheumatology. 2007;34(12):2438–2445. [PubMed] [Google Scholar]
- 92.Coursey T. G., Bian F., Zaheer M., Pflugfelder S. C., Volpe E. A., de Paiva C. S. Age-related spontaneous lacrimal keratoconjunctivitis is accompanied by dysfunctional T regulatory cells. Mucosal Immunology. 2017;10(3):743–756. doi: 10.1038/mi.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sudzius G., Mieliauskaite D., Siaurys A., et al. Distribution of peripheral lymphocyte populations in primary Sjögren’s syndrome patients. Journal of Immunology Research. 2015;2015:10. doi: 10.1155/2015/854706.854706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruan G. F., Zheng L., Huang J. S., et al. Effect of mesenchymal stem cells on Sjögren-like mice and the microRNA expression profiles of splenic CD4+ T cells. Experimental and Therapeutic Medicine. 2017;13(6):2828–2838. doi: 10.3892/etm.2017.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aluri H. S., Samizadeh M., Edman M. C., et al. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of Sjögren’s syndrome. Stem Cells International. 2017;2017:10. doi: 10.1155/2017/3134543.3134543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khalili S., Liu Y., Kornete M., et al. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjögren’s-like disease. PLoS One. 2012;7(6, article e38615) doi: 10.1371/journal.pone.0038615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Villatoro A. J., Fernandez V., Claros S., Rico-Llanos G. A., Becerra J., Andrades J. A. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. BioMed Research International. 2015;2015:10. doi: 10.1155/2015/527926.527926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elghanam G. A., Liu Y., Khalili S., Fang D., Tran S. D. Compact bone-derived multipotent mesenchymal stromal cells (MSCS) for the treatment of Sjögren’s-like disease in NOD mice. Methods in Molecular Biology. 2017;1553:25–39. doi: 10.1007/978-1-4939-6756-8_3. [DOI] [PubMed] [Google Scholar]
- 99.Maria O. M., Tran S. D. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells and Development. 2011;20(6):959–967. doi: 10.1089/scd.2010.0214. [DOI] [PubMed] [Google Scholar]
- 100.Choi J. J., Yoo S. A., Park S. J., et al. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clinical & Experimental Immunology. 2008;153(2):269–276. doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park N., Rim Y. A., Jung H., et al. Etanercept-synthesising mesenchymal stem cells efficiently ameliorate collagen-induced arthritis. Scientific Reports. 2017;7(1, article 39593) doi: 10.1038/srep39593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mun J.-Y., Shin K. K., Kwon O., Lim Y. T., Oh D.-B. Minicircle microporation-based non-viral gene delivery improved the targeting of mesenchymal stem cells to an injury site. Biomaterials. 2016;101:310–320. doi: 10.1016/j.biomaterials.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 103.Hsu P. D., Lander E. S., Zhang F. Development and applications of CRISPR-Cas 9 for genome engineering. Cell. 2014;157(6):1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bian S., Zhang L., Duan L., Wang X., Min Y., Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of Molecular Medicine. 2014;92(4):387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 105.Franquesa M., Hoogduijn M. J., Ripoll E., et al. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Frontiers in Immunology. 2014;5:p. 525. doi: 10.3389/fimmu.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katsuda T., Kosaka N., Takeshita F., Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13(10-11):1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 107.Kilpinen L., Impola U., Sankkila L., et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. Journal of Extracellular Vesicles. 2013;2(1) doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buzas E. I., Gyorgy B., Nagy G., Falus A., Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nature Reviews Rheumatology. 2014;10(6):356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 109.Turpin D., Truchetet M.-E., Faustin B., et al. Role of extracellular vesicles in autoimmune diseases. Autoimmunity Reviews. 2016;15(2):174–183. doi: 10.1016/j.autrev.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Biancone L., Bruno S., Deregibus M. C., Tetta C., Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology Dialysis Transplantation. 2012;27(8):3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 111.Hai B., Shigemoto-Kuroda T., Zhao Q., Lee R. H., Liu F. Inhibitory effects of iPSC-MSCs and their extracellular vesicles on the onset of sialadenitis in a mouse model of Sjögren’s syndrome. Stem Cells International. 2018;2018:10. doi: 10.1155/2018/2092315.2092315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang F., Chen M., Chen W., et al. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39–CD73–adenosine and IDO signals. Frontiers in Immunology. 2017;8:p. 68. doi: 10.3389/fimmu.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ge S., Mrozik K. M., Menicanin D., Gronthos S., Bartold P. M. Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: potential use for clinical therapy. Regenerative Medicine. 2012;7(6):819–832. doi: 10.2217/rme.12.61. [DOI] [PubMed] [Google Scholar]
- 114.Yong K. W., Choi J. R., Dolbashid A. S., Wan Safwani W. K. Z. Biosafety and bioefficacy assessment of human mesenchymal stem cells: what do we know so far? Regenerative Medicine. 2018;13(2):219–232. doi: 10.2217/rme-2017-0078. [DOI] [PubMed] [Google Scholar]
- 115.Tsai K.-S., Yang S.-H., Lei Y.-P., et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141(3):1046–1056. doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y., Han Z. P., Zhang S. S., et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. The Journal of Biological Chemistry. 2011;286(28):25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]